Abstract
Background
Patients with obstructive jaundice have various pathophysiological changes that affect the liver, kidney, heart, and the immune system. There is considerable controversy as to whether temporary relief of biliary obstruction prior to major definitive surgery (pre‐operative biliary drainage) is of any benefit to the patient.
Objectives
To assess the benefits and harms of pre‐operative biliary drainage versus no pre‐operative biliary drainage (direct surgery) in patients with obstructive jaundice (irrespective of a benign or malignant cause).
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Clinical Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until February 2012.
Selection criteria
We included all randomised clinical trials comparing biliary drainage followed by surgery versus direct surgery, performed for obstructive jaundice, irrespective of the sample size, language, and publication status.
Data collection and analysis
Two authors independently assessed trials for inclusion and extracted data. We calculated the risk ratio (RR), rate ratio (RaR), or mean difference (MD) with 95% confidence intervals (CI) based on the available patient analyses. We assessed the risk of bias (systematic overestimation of benefit or systematic underestimation of harm) with components of the Cochrane risk of bias tool. We assessed the risk of play of chance (random errors) with trial sequential analysis.
Main results
We included six trials with 520 patients comparing pre‐operative biliary drainage (265 patients) versus no pre‐operative biliary drainage (255 patients). Four trials used percutaneous transhepatic biliary drainage and two trials used endoscopic sphincterotomy and stenting as the method of pre‐operative biliary drainage. The risk of bias was high in all trials. The proportion of patients with malignant obstruction varied between 60% and 100%. There was no significant difference in mortality (40/265, weighted proportion 14.9%) in the pre‐operative biliary drainage group versus the direct surgery group (34/255, 13.3%) (RR 1.12; 95% CI 0.73 to 1.71; P = 0.60). The overall serious morbidity was higher in the pre‐operative biliary drainage group (60 per 100 patients in the pre‐operative biliary drainage group versus 26 per 100 patients in the direct surgery group) (RaR 1.66; 95% CI 1.28 to 2.16; P = 0.0002). The proportion of patients who developed serious morbidity was significantly higher in the pre‐operative biliary drainage group (75/102, 73.5%) in the pre‐operative biliary drainage group versus the direct surgery group (37/94, 37.4%) (P < 0.001). Quality of life was not reported in any of the trials. There was no significant difference in the length of hospital stay (2 trials, 271 patients; MD 4.87 days; 95% CI ‐1.28 to 11.02; P = 0.12) between the two groups. Trial sequential analysis showed that for mortality only a small proportion of the required information size had been obtained. There seemed to be no significant differences in the subgroup of trials assessing percutaneous compared to endoscopic drainage.
Authors' conclusions
There is currently not sufficient evidence to support or refute routine pre‐operative biliary drainage for patients with obstructive jaundice. Pre‐operative biliary drainage may increase the rate of serious adverse events. So, the safety of routine pre‐operative biliary drainage has not been established. Pre‐operative biliary drainage should not be used in patients undergoing surgery for obstructive jaundice outside randomised clinical trials.
Keywords: Humans; Drainage; Drainage/adverse effects; Drainage/methods; Jaundice, Obstructive; Jaundice, Obstructive/mortality; Jaundice, Obstructive/surgery; Jaundice, Obstructive/therapy; Postoperative Complications; Randomized Controlled Trials as Topic; Stents
Plain language summary
Biliary drainage before major operations in patients with obstruction of the bile duct
The liver has various functions, including the production and storage of substances necessary for the sustenance of life. It processes toxic substances (including those that are produced within the body because of the breakdown of old red cells) and plays a role in the excretion of these processed toxic substances. It produces bile, which contains substances necessary for the digestion of food. The bile is temporarily stored in the gallbladder and reaches the small bowel via the bile duct, usually in response to a stimulus such as ingestion of fatty food. The processed toxic substances are transported in the bile. These processed toxic substances are eventually excreted when the person opens his or her bowel. When there is obstruction to the flow of bile, the breakdown products of red cells can accumulate and cause yellowish discolouration of the skin and other linings in the body such as the white of the eyeball and the undersurface of the tongue. This results in a form of jaundice called obstructive jaundice. The obstruction to the bile flow is usually caused by stones in the common bile duct. These stones can originate from the gallbladder or from common bile duct stones. The majority of such stones can be treated endoscopically. However, a small proportion of the stones require surgery for removal. Other major causes of biliary obstruction include narrowing of the bile duct resulting from inflammation caused by stones, injury to the bile duct during operations to remove the gallbladder, and cancer of the bile duct, pancreas (an organ situated behind and below the stomach that secretes the digestive juices necessary for the digestion of food in addition to containing the cells that secrete insulin in order to maintain blood sugar levels), or the upper part of the small bowel called the duodenum. Operative removal is currently the only curative treatment available for these cancers. Such operations are typically major operations. However, the presence of toxic substances because of obstruction to the bile flow can result in physiological disturbances. Some surgeons perform certain procedures to temporarily drain the bile before performing the major operation to remove biliary obstruction due to stones, inflammation, or cancer. These pre‐operative procedures can be done endoscopically (by introducing an instrument equipped with a camera through the mouth and into the small intestine and then inserting a small drainage tube through that instrument and past the obstruction in the bile duct) or under X‐ray or other forms of image guidance via the liver. However, other surgeons argue that the temporary procedures to drain the bile are not necessary and that one should perform surgery directly. We sought evidence from randomised clinical trials only regarding this controversy. Such studies, when conducted properly, provide the best evidence. Two authors independently identified the trials and obtained the information from the trials.
We included six trials involving 510 patients for this review. The number of patients included in the trials varied from 40 to 202. All trials had a high risk of bias, that is, the trials may overestimate benefits and underestimate harms. There was no significant difference in risk of death between the two groups. The rate of serious complications was higher in the patients who underwent biliary drainage prior to operation compared with those who underwent surgery directly. The quality of life was not reported in any trial. There was no significant difference in the length of hospital stay between the two groups. The costs were not reported in any of the trials. Based on the currently available best evidence, there is no justification for the use of routine drainage of bile before a major operation in patients with obstruction to the flow of bile. Routine biliary drainage should not be funded and may result in litigations. Furthermore, well designed trials with low risk of systematic errors and low risk of random errors (low risk of play of chance) may be necessary.
Summary of findings
Summary of findings for the main comparison. Pre‐operative biliary drainage versus direct surgery for obstructive jaundice.
| Pre‐operative biliary drainage versus direct surgery for obstructive jaundice | ||||||
| Patient or population: patients with obstructive jaundice. Settings: secondary or tertiary care setting. Intervention: pre‐operative biliary drainage versus direct surgery. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pre‐operative biliary drainage versus direct surgery | |||||
| Mortality | 133 per 1000 | 149 per 1000 (97 to 228) | RR 1.12 (0.73 to 1.71) | 520 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Morbidity ICH‐GCP classification | 361 per 1000 | 599 per 1000 (462 to 780) | Rate ratio 1.66 (1.28 to 2.16) | 503 (6 studies) | ⊕⊝⊝⊝ very low1,3,4 | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 All trials were of high risk of bias. 2 Overlaps 1 and 0.75 or 1.25. 3 There were few trials to assess publication bias. 4 I2 was 44%. There was also minimal overlap of confidence intervals between some of the trials.
Background
Description of the condition
Obstructive jaundice is the term used for the increased serum bilirubin levels as result of obstruction to the flow of bile due to blockage in the large bile ducts. The clinical symptoms and signs of obstructive jaundice include yellow discolouration of skin and conjunctiva, dark urine, pale stools, and pruritus. The main causes of obstructive jaundice include benign common bile duct stones or benign biliary stricture (secondary to common bile duct stones or a complication of cholecystectomy) and malignancy due to periampullary cancers (Bjornsson 2008). Periampullary cancer is the term used for cancers that form near the ampulla of Vater (NCI 2012) and includes cancer of the head and neck of the pancreas, cancer of the distal end of the bile duct, cancer of the ampulla of Vater, and cancer of the second part of the duodenum. Surgical resection is generally considered the only curative treatment for malignant obstructive jaundice. Approximately 20% of periampullary cancers presenting to surgeons are resectable (Michelassi 1989; Engelken 2003; Smith 2008).
Description of the intervention
Obstructive jaundice affects various organs and physiologic mechanisms in the body and can result in kidney failure (Wadei 2006), cardiac dysfunction (Wadei 2006), liver injury (Pauli‐Magnus 2005), haemostatic abnormalities (Papadopoulos 2007), and altered body immunity (Nehez 2002). Restoration of biliary flow prior to operation (pre‐operative biliary drainage) can be performed by percutaneous transhepatic biliary drainage under radiologic guidance or endoscopic sphincterotomy and placement of a stent across the obstruction of the biliary tract (Pisters 2001). Patients presenting with cholangitis may require pre‐operative biliary drainage to allow their condition to improve sufficiently to allow surgery. However, many physicians routinely perform pre‐operative drainage in patients with malignant obstructive jaundice (Kawasaki 2003) with the intention of relieving the biliary obstruction and reversing the pathophysiological changes caused by obstructive jaundice.
How the intervention might work
Pre‐operative drainage relieves the obstruction and may reverse the pathophysiological dysfunction caused by obstructive jaundice. As a result, the patient‐relevant outcomes may improve.
Why this review is important
A previous meta‐analysis showed that pre‐operative biliary drainage in patients with malignant obstructive jaundice carries no benefit and should not be performed routinely (Sewnath 2002). In our previous Cochrane systematic review (Wang 2008), we concluded that there is no evidence to support or refute pre‐operative biliary drainage for patients with obstructive jaundice. However, the trials included in these two reviews were old and the applicability of the two reviews in modern surgical practice can be questioned. In this updated version, which includes a new trial (van der Gaag 2010), we have revised the methods in line with the revision of the methods in Cochrane reviews (Higgins 2011), and reanalysed and revised our interpretation of the data.
Objectives
To assess the benefits and harms of pre‐operative biliary drainage in patients with obstructive jaundice (irrespective of a benign or malignant cause).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised clinical trials irrespective of language, blinding, or publication status. Quasi‐randomised studies (in which the method used to assign participants to a treatment group was not strictly random, for example, date of birth, hospital record number, and alternation) and other study designs were not included in this review due to the risk of bias.
Types of participants
Participants were patients with obstructive jaundice, irrespective of a benign or malignant cause, who were about to undergo surgery.
Types of interventions
We included trials comparing pre‐operative biliary drainage with no pre‐operative biliary drainage (direct surgery).
Co‐interventions were allowed provided that they were used equally in both groups.
Types of outcome measures
Primary outcomes
-
Survival:
hazard ratio (at maximal follow‐up);
mortality within three months (procedure or operative mortality).
-
Serious morbidity (serious complications, as classified by the authors, or grade 3 or grade 4 Clavien‐Dindo classification (Dindo 2004; Clavien 2009)). This approximately corresponded to the serious adverse events of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ‐ Good Clinical Practice (ICH‐GCP) (ICH‐GCP 1997).
Surgery‐related morbidity.
Procedure‐related morbidity.
Quality of life.
Secondary outcomes
Total length of hospital stay.
Costs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2012), Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003) until February 2012. We have given the search strategies in Appendix 1 with the time spans for the searches.
Searching other resources
We also searched the references of the identified trials and the metaRegister of Controlled Trials, which includes the ISRCTN and NIH ClinicalTrials.gov trial registers, to identify further relevant trials.
Data collection and analysis
Selection of studies
CHW, KSG, and YF independently identified the trials for inclusion. We listed the excluded studies with the reasons for the exclusion.
Data extraction and management
KSG and YF independently extracted the following data.
Year and language of publication.
Country.
Year of study.
Inclusion and exclusion criteria.
Details of intervention and control.
Co‐interventions.
Outcomes (listed above).
Risk of bias (described below).
Assessment of risk of bias in included studies
We independently assessed the risk of bias in the trials without masking the trial names. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012). Due to the risk of biased overestimation of beneficial intervention effects in randomised trials with high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), we assessed the following domains of risk of bias in the trials.
Sequence generation
Low risk of bias: the method used is either adequate (eg, computer generated random numbers, table of random numbers) or unlikely to introduce confounding.
Uncertain risk of bias: there is insufficient information to assess whether the method used is likely to introduce confounding.
High risk of bias: the method used (eg, quasi‐randomised studies) is inappropriate and likely to introduce confounding.
Allocation concealment
Low risk of bias: the method used (eg, central allocation) is unlikely to introduce bias in determining the final observed effect.
Uncertain risk of bias: there is insufficient information to assess whether the method used is likely to introduce bias for the estimate of effect.
High risk of bias: the method used (eg, open random allocation schedule) is likely to introduce bias on the final observed effect estimate.
Blinding
Low risk of bias: the patients and outcome assessors were blinded and the method of blinding was described.
Uncertain risk of bias: the patients and outcome assessors were blinded and the method of blinding was not described.
High risk of bias: the patients or outcome assessors were not blinded.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data.
Uncertain risk of bias: there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data are likely to introduce bias for the estimate of effect.
High risk of bias: the crude estimate of effects (eg, complete case estimate) will clearly be biased due to the underlying reasons for missing data and the methods used to handle missing data are unsatisfactory.
Selective outcome reporting
Low risk of bias: pre‐defined or clinically relevant and reasonably expected outcomes (such as short‐term mortality and serious adverse events either reported as number of patients with serious adverse events or total number of serious adverse events) are reported on.
Uncertain risk of bias: not all pre‐defined or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Vested interest bias
Low risk of bias: if the trial is conducted according to a publicly available protocol or if it is conducted by a party without any vested interests in the outcome of the trial.
Uncertain risk of bias: if the trial protocol is not available and if it is not clear if the trial is conducted by a party with a vested interest in the outcome of the trial.
High risk of bias: if the trial is not conducted according to a publicly available protocol, a protocol is not available, or the trial is conducted by a party with vested interests in the outcome of the trial.
We considered trials to have a low risk of bias if we assessed all the above domains as being atf low risk of bias. In all other cases, the trials were considered to have a high risk of bias.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). We also planned to report the risk difference if it was different from the RR since risk difference includes trials with zero events in both groups and allows meta‐analysis. For continuous variables, we planned to calculate the mean difference (MD) with 95% CI for hospital stay; and the standardised mean difference (SMD) with 95% CI for variables such as quality of life. For serious adverse events, we calculated the rate ratio (RaR) with 95% CI. For time‐to‐event variables, we calculated the hazard ratio (HR) with 95% CI.
Unit of analysis issues
The unit of analysis was the aggregate data on patients with benign or malignant obstructive jaundice who were about to undergo surgery according to the group they were randomised to.
Dealing with missing data
We performed an intention‐to‐treat analysis (Newell 1992) whenever possible. We imputed data for binary outcomes using various scenarios, namely best‐best analysis, worst‐worst analysis, best‐worst analysis, and worst‐best analysis (Gurusamy 2009; Gluud 2012).
For continuous outcomes, we used available‐case analysis. We imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we imputed the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation will decrease the weight of the trial for calculation of mean differences and bias the effect estimate to no effect in the case of standardised mean difference (Higgins 2011).
Assessment of heterogeneity
We examined the forest plot to visually assess heterogeneity. Overlapping of CIs was used to assess the heterogeneity visually. We explored heterogeneity by the Chi2 test with significance set at a P value of 0.10, and measured the quantity of heterogeneity by the I2 statistic (Higgins 2002).
Assessment of reporting biases
We planned to use a funnel plot to explore bias in the presence of at least 10 trials (Egger 1997; Macaskill 2001). We planned to use asymmetry in the funnel plot of trial size against treatment effect to assess this bias. We also planned to use the linear regression approach described by Egger et al (Egger 1997) to determine the funnel plot asymmetry.
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012) using the software package Review Manager 5 (RevMan 2011). We use a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In the case of a discrepancy between the two models we have reported both results; otherwise we have reported only the results from the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Trials with low risk of bias compared with trials of unclear or high risk of bias.
Method of pre‐operative biliary drainage (percutaneous transhepatic biliary drainage compared to endoscopic method).
Trials with equal to or more than 95% of patients having malignant jaundice compared to trials with less than 95% with malignant jaundice.
We were unable to perform the subgroup analysis of trials at low risk of bias versus those trials with a high risk of bias since there were no trials at low risk of bias. We were able to perform the other two subgroup analyses. We performed the Chi2 test for subgroup differences, setting a P value of 0.05 to identify any differences.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing the outcomes for binary outcomes under different scenarios, namely best‐best analysis, worst‐worst analysis, best‐worst analysis, and worst‐best analysis (Gurusamy 2009; Gluud 2012), for any significant binary outcome. We also performed a sensitivity analysis by excluding the trials in which medians or standard deviations were imputed for the continuous outcomes. Both these sensitivity analyses were planned. We also performed post hoc sensitivity analyses by excluding Lai 1994 (the severity of complications could not be assessed), Pitt 1985 (as this trial included a significant proportion of proximal obstructions), and van der Gaag 2010 (as routine antibiotic prophylaxis was not used for distal obstructions). We also performed another post hoc sensitivity analysis including all bile leaks irrespective of the way they were treated, except those leaks which clearly resolved spontaneously. Bile leaks are post‐operative complications. Some resolve spontaneously without any prolongation of hospital stay and hence will be assessed as mild adverse events. Other bile leaks need radiological or endoscopic interventions and hence will be classified as serious adverse events. If the trial authors did not report the way the bile leaks were treated then it is not possible to determine their severity. So, we performed two analyses, one excluding the bile leaks which resolved spontaneously and those for which the treatment was not reported, and another including the bile leaks for which the treatment was not reported but excluding the bile leaks which clearly resolved spontaneously.
Trial sequential analysis
We applied trial sequential analysis (CTU 2011; Thorlund 2011) using a required information size calculated form an alpha error of 0.05, a beta error of 0.20, a control event proportion obtained from the results, and a relative risk reduction of 20% for mortality to determine whether more trials are necessary on this topic (if the alpha spending boundary or the futility zone is crossed, then more trials are unnecessary) (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010). We did not perform a trial sequential analysis for serious morbidity since only one trial reported the proportion of patients with serious morbidity and since the methods for trial sequential analysis of RaR have not been established.
Results
Description of studies
Results of the search
A total of 4260 references were identified from medical journal databases. One additional reference was identified through the reference search. No additional trial was identified through the meta‐register of Current Controlled Trials. The flow of references is shown in Figure 1 (PRISMA flow diagram) (Moher 2009). In total, 14 references described six completed randomised clinical trials that fulfilled the inclusion criteria (Hatfield 1982; McPherson 1984; Pitt 1985; Lai 1994; Wig 1999; van der Gaag 2010). A total of 520 patients randomised to pre‐operative biliary drainage (265 patients) versus no pre‐operative biliary drainage (255 patients) were included in the six trials. This included one new trial (van der Gaag 2010), which increased the number of patients included in the meta‐analysis from 324 patients in the previous review to 520 patients in the updated review, that is, an increase of approximately 60%.
1.
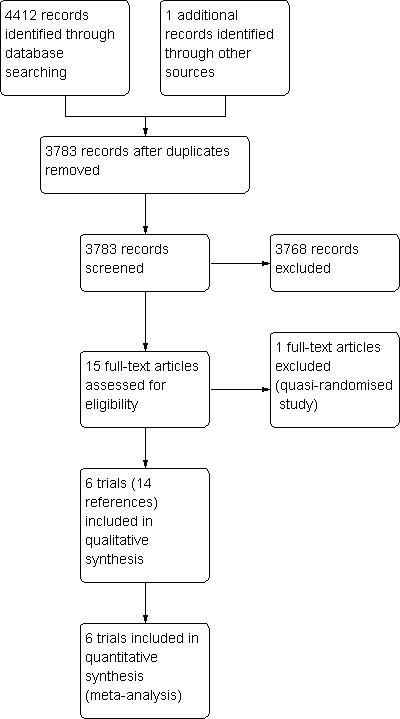
Study flow diagram.
Included studies
Four trials used percutaneous transhepatic biliary drainage as the method of drainage (Hatfield 1982; McPherson 1984; Pitt 1985; Wig 1999). Two trials used endoscopic sphincterotomy as the method of drainage (Lai 1994; van der Gaag 2010). The mean duration of drainage in the trials varied between one and six weeks (Hatfield 1982; McPherson 1984; Pitt 1985; Lai 1994; Wig 1999; van der Gaag 2010). One trial did not routinely administer antibiotic prophylaxis for drainage in cases of distal bile duct obstruction, but peri‐operative antibiotics were administered (van der Gaag 2010). Antibiotics were used routinely for pre‐operative biliary drainage and for surgery in the remaining trials.
Two trials included only patients with malignant obstructive jaundice (McPherson 1984; Lai 1994). The proportion of patients with malignant obstructive jaundice in the other trials was 95% (Hatfield 1982), 92% (van der Gaag 2010), 77% (Pitt 1985), and 60% (Wig 1999). One trial (van der Gaag 2010) included patients with bilirubin levels between 40 μmol/l and 250 μmol/l. The remaining trials included patients with minimum bilirubin levels ranging between 100 μmol/l and 172 μmol/l (Hatfield 1982; McPherson 1984; Pitt 1985; Lai 1994; Wig 1999). One trial stated that only patients in an operable condition were included (Pitt 1985). Another trial stated that the patients were fit for major surgery (van der Gaag 2010). One trial stated that patients with ongoing cholangitis were excluded (van der Gaag 2010). The other trials did not state whether they excluded such patients. Most of the patients included in the trials were patients with distal common bile duct obstruction. Further details about the included trials are shown in the table 'Characteristics of included studies'.
Excluded studies
One study was excluded because the randomisation was by hospital file numbers, that is, this was a quasi‐randomised study. This is shown in the table 'Characteristics of excluded studies'.
Risk of bias in included studies
The risk of bias is summarised in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3). All the trials were at high risk of bias.
2.
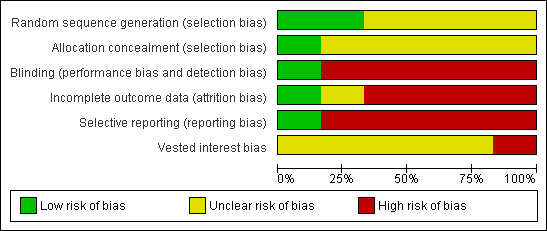
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
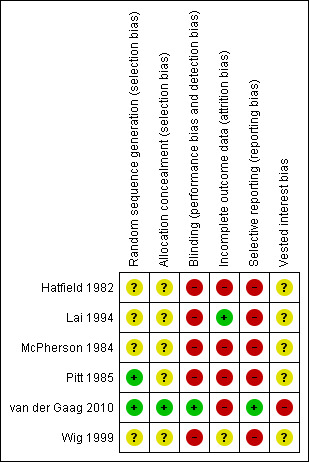
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Mortality
There was no significant difference in mortality between the two intervention groups (RR 1.12; 95% CI 0.73 to 1.71; P = 0.60) (Analysis 1.1). The mortality was 15.1% (40/265) in the pre‐operative biliary drainage group versus 13.3% (34/255) in the direct surgery group. There was no change in the results by following a random‐effects model. Heterogeneity was not statistically significant (I2 = 0%; P = 0.67). The tests for subgroup differences were not statistically significant for subgroup analyses according to method of pre‐operative biliary drainage (Analysis 2.1) or proportion of patients with malignant jaundice (equal to or more than 95% compared to less than 95%) (Analysis 3.1). Long‐term survival was reported in only one trial. There was no significant difference in the long‐term survival between the two groups in patients who underwent successful pancreatoduodenectomy for cancer in the only trial that reported this outcome (HR 0.90; 95% CI 0.65 to 1.24) (van der Gaag 2010) (Analysis 1.2). Only 520 patients of the estimated 11,130 patients required to rule out differences in mortality have been recruited so far.
1.1. Analysis.
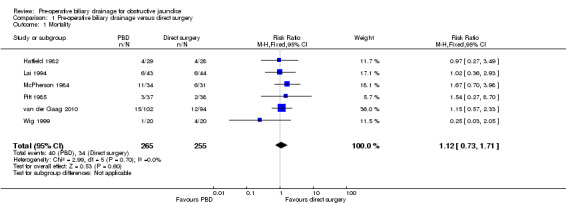
Comparison 1 Pre‐operative biliary drainage versus direct surgery, Outcome 1 Mortality.
2.1. Analysis.
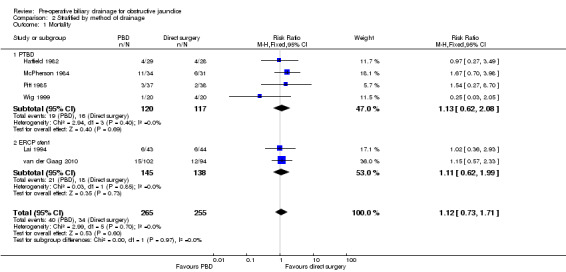
Comparison 2 Stratified by method of drainage, Outcome 1 Mortality.
3.1. Analysis.
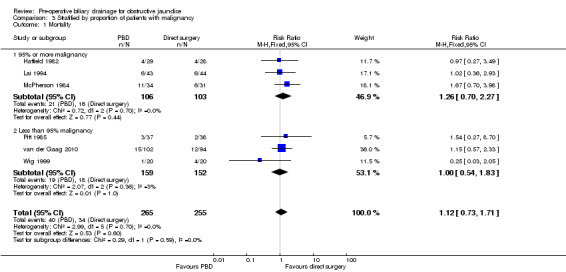
Comparison 3 Stratified by proportion of patients with malignancy, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Pre‐operative biliary drainage versus direct surgery, Outcome 2 Long‐term mortality.
| Long‐term mortality | |
|---|---|
| Study | |
| van der Gaag 2010 | Hazard ratio 0.90; 95% CI 0.65 to 1.24 |
Serious morbidity (serious adverse events)
There was a significantly higher occurrence of serious morbidity in the pre‐operative biliary drainage group than in the direct surgery group (RaR 1.66; 95% CI 1.28 to 2.16; P = 0.0002) (Analysis 1.3). There was no change in results by following a random‐effects model. Heterogeneity was not statistically significant (I2 = 44%; P = 0.11). The tests for subgroup differences were not statistically significant for the subgroup analyses (Analysis 2.2) (Analysis 3.2).
1.3. Analysis.
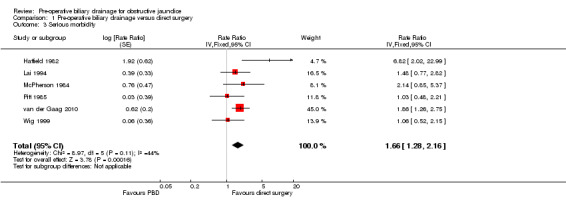
Comparison 1 Pre‐operative biliary drainage versus direct surgery, Outcome 3 Serious morbidity.
2.2. Analysis.
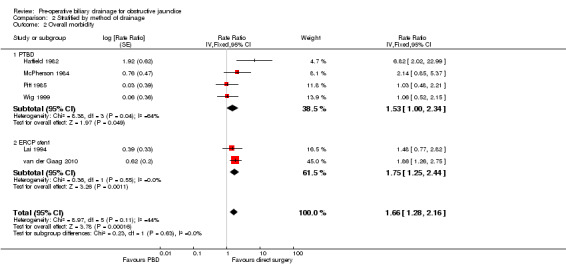
Comparison 2 Stratified by method of drainage, Outcome 2 Overall morbidity.
3.2. Analysis.
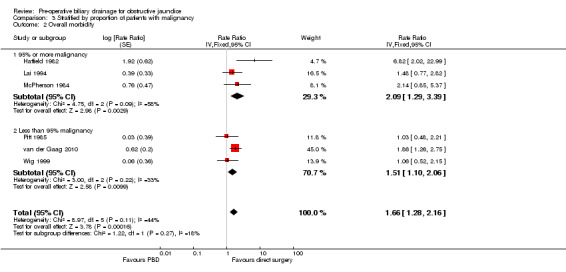
Comparison 3 Stratified by proportion of patients with malignancy, Outcome 2 Overall morbidity.
We performed post hoc sensitivity analyses by excluding the trials by Lai et al (severity of complications could not be assessed) (Lai 1994), Pitt et al (this trial included a significant proportion of proximal obstructions) (Pitt 1985), and van der Gaag et al (van der Gaag 2010) (routine antibiotic prophylaxis was not used for distal obstructions); and by including bile leaks irrespective of the management. There was no change in the results with any of these sensitivity analyses (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4).
4.1. Analysis.

Comparison 4 Sensitivity analysis of overall morbidity, Outcome 1 Serious complications without Pitt 1985 (proximal obstruction).
4.2. Analysis.
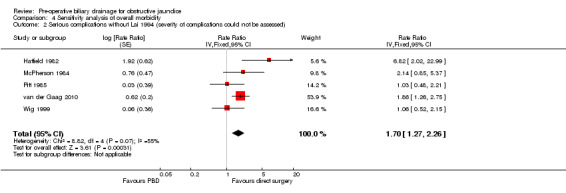
Comparison 4 Sensitivity analysis of overall morbidity, Outcome 2 Serious complications without Lai 1994 (severity of complications could not be assessed).
4.3. Analysis.

Comparison 4 Sensitivity analysis of overall morbidity, Outcome 3 Serious complications without van der Gaag 2010 (no routine antibiotic prophylaxis).
4.4. Analysis.
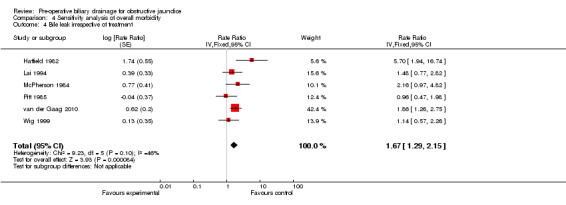
Comparison 4 Sensitivity analysis of overall morbidity, Outcome 4 Bile leak irrespective of treatment.
The proportion of patients with serious morbidity was reported in only one trial (van der Gaag 2010). The proportion of patients who developed serious morbidity was significantly higher in the group of patients who had pre‐operative biliary drainage than in the direct surgery group (75/102 (73.5%) in the pre‐operative biliary drainage group versus 37/94 (39.3%) with direct surgery; Fisher's exact test P < 0.001) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Pre‐operative biliary drainage versus direct surgery, Outcome 4 Proportion of patients with serious morbidity.
| Proportion of patients with serious morbidity | |||
|---|---|---|---|
| Study | Proportion of patients with serious morbidity in the PBD group | Proportion of patients with serious morbidity in the direct surgery group | Statistical significance by Fisher's exact test |
| van der Gaag 2010 | 75/102 (73.5%) | 37/94 (39.4%) | p<0.001 |
Quality of life
None of the trials reported this outcome.
Hospital stay
Meta‐analysis of two trials (Pitt 1985; van der Gaag 2010) showed a significantly longer hospital stay in the pre‐operative biliary drainage group using the fixed‐effect model (MD 4.26 days; 95% CI 0.92 to 7.61; P = 0.01) (Analysis 1.4) but not with the random‐effects model (MD 4.87 days; 95% CI ‐1.28 to 11.02; P = 0.12) (Analysis 1.5). Heterogeneity was statistically significant (I2 = 68%; P = 0.08). We did not perform a subgroup analysis because of the presence of only two trials for this outcome. Sensitivity analysis excluding the trial by van der Gaag et al (van der Gaag 2010), in which the mean and standard deviation was imputed from the median and P value, resulted in the inclusion of only one trial (Pitt 1985) that showed a significantly longer hospital stay in the pre‐operative biliary drainage group than in the direct surgery group.
1.5. Analysis.
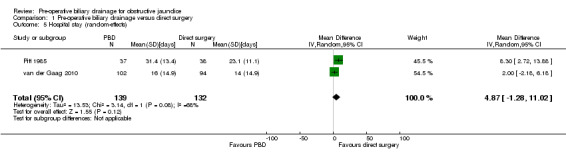
Comparison 1 Pre‐operative biliary drainage versus direct surgery, Outcome 5 Hospital stay (random‐effects).
Cost
None of the trials reported this outcome.
Reporting bias
We did not explore reporting bias because of the presence of fewer than 10 trials.
Trial sequential analysis
The alpha‐spending boundaries and the boundaries of futility were not reached (Figure 4).
4.
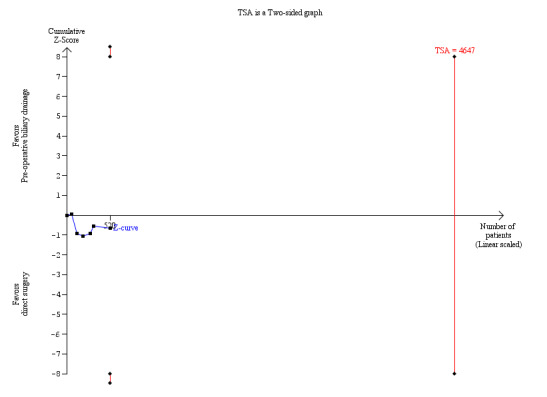
Trial sequential analysis of preoperative biliary drainage versus direct surgery for obstructive jaundice on mortality. The required information size of 11,130 patients was calculated based on a prevalence of death of 13% in the surgery group; a relative risk reduction of 20%; an alpha of 5%; and a beta of 20%. The cumulated Z‐curve shows that only a small proportion, that is 520 patients, of the required number of patients to demonstrate differences in mortality has been recruited in all the clinical trials comparing pre‐operative biliary drainage and direct surgery and that no statistical significant difference has been observed.
Discussion
Summary of main results
Our review showed that combining pre‐operative biliary drainage with surgery compared with direct surgery increased the proportion of serious adverse events without any apparent significant advantages. The overall mortality in patients with obstructive jaundice undergoing surgery was approximately 15.1% in the pre‐operative biliary drainage group and 13.3% in the control group (no statistically significant difference). One of the factors in this finding that we explored was whether this represents the current situation since many of the trials were old. So, we calculated the mortality excluding the trial by van der Gaag et al (van der Gaag 2010), which recruited patients between November 2003 and June 2008 and contributed nearly 40% of the patients in the review. In this trial, the mortality of patients in the pre‐operative biliary drainage group was 14.7% compared with 12.8% in the no pre‐operative biliary drainage group (no statistically significant difference). The overall mortality in the other trials (that is, excluding the trial by van der Gaag et al (van der Gaag 2010)) was similar (15.3% in the pre‐operative biliary drainage group and 13.7% in the no pre‐operative biliary drainage group) demonstrating no significant reduction in the mortality associated with major surgeries in patients with obstructive jaundice over time. This is in contrast to the generally quoted mortality of 2% to 4% for pancreaticoduodenectomy (Winter 2006; Diener 2011) and may reflect the severity of the jaundice or the severity of cancer in the patients included in the trials.
There was no significant difference between the two intervention groups with regards to mortality in the present review. The main question is whether this is a lack of effect or lack of evidence of an effect. We used trial sequential analysis (Wetterslev 2008; Wetterslev 2009) with an alpha error of 0.05, beta error of 0.20, control event proportion of 13%, and a relative risk reduction of 20% to identify whether the region of futility was reached. This analysis revealed that the region of futility was not yet reached, and so we cannot rule out any difference in the mortality between pre‐operative biliary drainage and direct surgery. In addition, we utilised the definition of imprecision of results used by the GRADE handbook for grading quality of evidence and strength of recommendation (Schünemann 2009)(which is currently recommended by The Cochrane Collaboration for grading the level of evidence, http://ims.cochrane.org/revman/other‐resources/gradepro). The total number of events in this review was less than 400 and the CI for mortality (RR 1.12; 95% CI 0.73 to 1.71) overlaps no effect (that is, RR of 1) and appreciable benefit or harm (25% increase, that is, RR of 1.25; and 25% reduction, that is, RR of 0.75). This supports the results of the trial sequential analysis that one cannot rule out a difference in the mortality between pre‐operative biliary drainage and direct surgery. The long‐term survival was reported in only one of the trials. There was no significant difference between the two groups. Again, the CI was wide for this outcome. So, we are unable to rule out any difference in the long‐term survival between pre‐operative biliary drainage and direct surgery.
The overall severe morbidity was higher in the pre‐operative biliary drainage group than the direct surgery group. This difference is consistent with different statistical methods used and various sensitivity analyses. There was heterogeneity in the outcome, mainly because of the magnitude of the effect rather than the direction of the effect; that is, the number of serious adverse event was higher in the pre‐operative biliary drainage group. The proportion of patients with serious adverse events was also higher in the pre‐operative biliary drainage group than the direct surgery group, although this information is only from one recent trial (van der Gaag 2010). The definitions used for serious adverse events were those used by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and are used by regulatory authorities such as the Food and Drug Administration (FDA), MDA, and Japan Society of Quality Assurance (JSQA) in US, Europe, and Japan, respectively (ICH‐GCP 1997) in determining the adverse events related to a pharmacological intervention. While it is not mandatory to report radiological, endoscopic, and surgical interventions using these definitions, we have used these definitions in the absence of any other universal definitions for complications. Serious adverse events of the ICH‐GCP classification roughly correspond to class III or class IV in the Clavien‐Dindo classification (Dindo 2004; Clavien 2009), the only validated classification system in assessing surgical interventions. Inevitably, some information will be lost by using such classifications which pool complications together. However, unless such an approach is followed, it is difficult to power trials related to surgery using any meaningful outcomes to the patients. We acknowledge that some complications are temporarily worse than others in terms of treatment required to resolve complications, prolongation of hospital stay, and the cost implications. However, the use of generic definitions for complications enables comparison of treatment across different disciplines of medicine. Until further validated definitions of serious adverse events become available, it is reasonable to use the definitions that are currently used by regulatory authorities in US, Europe, and Japan to assess pharmacological interventions on non‐pharmacological interventions.
None of the trials reported quality of life. Adequate decompression of the biliary obstruction may improve the quality of life by reducing the symptoms of biliary obstruction such as itching. On the other hand, it may produce anxiety by delaying surgery while waiting for resolution of jaundice and because of the complications related to the pre‐operative biliary drainage procedure. From a patient's perspective, long‐term survival and quality of life (which takes any permanent disability or morbidity into account) are the important determinants that should guide the healthcare provider about the use of the procedure. However, in the absence of differences in such long‐term outcomes, short‐term outcomes such as morbidity and hospital stay may be quite important. From a health funder perspective, cost per quality adjusted life year (QALY), which takes into account long‐term survival and quality of life along with short‐term outcomes such as morbidity (and the cost for management of the morbidity) and hospital stay, are important determinants to fund or not to fund the treatment. Hospital stay in the pre‐operative biliary drainage group will include the hospital stay to perform the drainage, repeat the procedure if the drainage is not adequate, and treat any complications resulting from pre‐operative biliary drainage along with the post‐operative hospital stay. Considering that the morbidity is higher in the pre‐operative biliary drainage group without any beneficial effects funding for pre‐operative biliary drainage seems inappropriate.
One of the important questions that needs to be answered was whether pre‐operative biliary drainage was performed appropriately. Pre‐operative biliary drainage is aimed at restoring the flow of bile in patients with obstructive jaundice, thereby reversing the pathophysiological changes due to obstructive jaundice. The criteria used to assess success of pre‐operative biliary drainage and the duration of pre‐operative biliary drainage varied between the trials. A short drainage time may not be enough to demonstrate benefit, but a long drainage time may increase the risk of pre‐operative biliary drainage‐related complications and allow cancer progression. There is very little information available about the duration of the drainage period required to reverse the pathophysiological changes associated with obstructive jaundice, and so the optimal duration of drainage is not clear. There is also considerable controversy about the method of drainage and the type of stent used (Moss 2007; Kloek 2010).
Overall completeness and applicability of evidence
This evidence is applicable to patients with obstructive jaundice who are fit for direct surgery, that is, do not have sepsis that precludes a major operation and those who undergo neoadjuvant therapy where an alternative treatment such as chemotherapy is carried out before definitive surgery is performed (Cheng 2006).
Quality of the evidence
As regards the quality of evidence, we assessed this under the headings described by the GRADE handbook for grading quality of evidence and the strength of the recommendation (Schünemann 2009). This included the risk of bias in the trials and consistency and directness of evidence apart from the precision of the results, which was discussed earlier. As regards the risk of bias, all the trials were at a high risk of bias. Allocation was unclear in most of the trials. Blinding of outcome assessors was not performed in most of the trials. Most of the trials had post‐randomisation drop‐outs (Characteristics of included studies). From the information available from the trials regarding post‐randomisation drop‐outs, there is no specific mechanism of drop‐out. Considering that the intervention was started pre‐operatively and that no surgery was carried out in patients who did not meet the inclusion criteria or refused the surgery, it appears that post‐randomisation drop‐outs are inevitable. While an available patient analysis may be appropriate when the drop‐outs are not related to the intervention (Gurusamy 2009), strict intention‐to‐treat analysis has to be followed in this situation since some patients could become unresectable during pre‐operative biliary drainage (while waiting for definitive surgery). Overall, the risk of bias is high and the true effect may be different from the effect obtained in this meta‐analysis. As regards consistency, as discussed previously the effect of pre‐operative biliary drainage on mortality appears to be consistent, while the magnitude of the effect on serious adverse events is not consistent across trials. As far as directness of evidence is concerned, there is not much concern about the directness of short‐term mortality and morbidity. However, there is little information available related to quality of life and to long‐term morbidity and mortality. However, in spite of all these deficiencies in the quality of evidence, this review seems to be the best evidence available on this topic.
Potential biases in the review process
We followed the Cochrane Handbook for Systematic Reviews of Interventions for this review (Higgins 2011). There were no language, publication status, or sample size restrictions. Thus, we minimised the bias due to the selection of only randomised clinical trials. We were unable to assess reporting bias because of the few trials identified. The intervention has been available for several years now, including the pre‐mandatory trial registration era, so we cannot be sure whether there are any unreported or unidentified trials. However, one has to be pragmatic and accept that the results of such trials are unlikely to be available in the near future and we have assessed the information that is available.
Agreements and disagreements with other studies or reviews
Based on the above discussions, it appears that there is no strong evidence to recommend or refute pre‐operative biliary drainage. So, should we allow individual clinicians to treat patients according to their preference? The answer is 'no', as this can result in fragmentation of care and can lead to suboptimal performance (Swensen 2010). It is necessary to standardise treatments and develop guidelines in order to improve health care (Swensen 2010). Adoption of routine pre‐operative biliary drainage into clinical practice was not evidence‐based. In spite of randomised trials repeatedly showing lack of evidence of any benefit of pre‐operative biliary drainage, clinicians continue to use routine pre‐operative biliary drainage (Kawasaki 2003). One can argue that our recommendation is based on randomised trials at high risk of bias. However, the argument for the routine use of pre‐operative biliary drainage is based on even more flawed study designs. Furthermore, randomised trials with high risk of bias usually overestimate intervention benefits and underestimate the intervention harms of the experimental intervention, that is, pre‐operative biliary drainage. As we have identified risks of significant harms in the included trials, our results suggest that the reality may be worse. One should not justify current mistakes based on past errors. Instead, it is reasonable to acknowledge past mistakes and avoid such mistakes in the future. We acknowledge that new randomised clinical trials may show results which are different from the conclusions of our systematic review. There are no new randomised clinical trials registered in the metaRegister of Controlled Trials, which includes the major clinical trials registers. So, the evidence reviewed in our review is the best evidence available currently, which is likely to remain so for the next few years. One cannot base current practice on future speculative results but it ought to be based on the currently available information. Until new evidence with low risk of bias emerges, the present systematic review is the best available evidence on this topic.
In conclusion, pre‐operative biliary drainage does not appear to be beneficial to the patients or healthcare funders in any way. It may increase serious adverse events and could add to the cost of the health care. We do not recommend routine use of pre‐operative biliary drainage in patients with obstructive jaundice who are about to undergo surgery outside well‐designed randomised clinical trials with low risk of systematic error and random error, and those trials that include long‐term survival and quality of life as important outcomes. We recommend that routine pre‐operative biliary drainage should not be funded. There may also be medico‐legal implications of these findings, making physicians vulnerable to litigations in cases of complications related to routine pre‐operative biliary drainage.
A previous meta‐analysis showed that pre‐operative biliary drainage with malignant obstructive jaundice carries no benefit and should not be performed routinely (Sewnath 2002). In our previous Cochrane review (Wang 2008), we concluded that there is no evidence to support or refute pre‐operative biliary drainage for patients with obstructive jaundice. In this review we go one important step further and state that pre‐operative biliary drainage should not be used routinely outside well designed randomised clinical trials.
Authors' conclusions
Implications for practice.
We do not recommend routine use of pre‐operative biliary drainage in patients with obstructive jaundice who are about to undergo surgery outside well designed randomised clinical trials.
Implications for research.
Well designed randomised clinical trials with low risk of systematic error and random error and those that include long‐term survival and quality of life as important outcomes may be necessary. Such trials ought to stratify patients according to the likely cause of obstruction as well as to report both short‐ and long‐term outcomes such as quality of life, non‐serious adverse events, serious adverse events, and mortality. The trials should also describe the results of the pre‐operative drainage and any delay to surgery this may have caused. Reporting of such trials according to the CONSORT statement (http://www.consort‐statement.org/consort‐statement/) will enable transparent evaluation of the intervention.
What's new
| Date | Event | Description |
|---|---|---|
| 13 April 2011 | New citation required and conclusions have changed | Conclusions changed. |
| 7 October 2010 | New search has been performed | New searches were performed, and one new trial was identified and added. |
Acknowledgements
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health.'
The entire staff of the Cochrane Hepato‐Biliary Group. In particular, we thank C Gluud, the Co‐ordinating Editor, who provided advice on the design and interpretation of the systematic review, advised on and performed trial sequential analysis. While he met all the criteria of authorship set out in the International Committee of Medical Journal Editors (ICMJE), he declined authorship in this review, informing us that this was a part of his job.
Peer Reviewers: Ehab El‐Hanafy, Egypt. Contact Editors: Ronald L Kortez, USA; Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy used |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | February 2012. | (((liver or hepat* or pancrea* or bile duct or choledoch*) and (cancer* or neoplasm* or malignan* or tumour* or tumor*)) or cholangiocarcinoma or cholesta* or icter* or jaundice) AND (drain* or stent* or cholangio* or sphincterotom* or ptc or ptbd or ERCP or endoscopic*) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 1, 2012. | #1 MeSH descriptor Jaundice, Obstructive explode all trees #2 MeSH descriptor Pancreatic Neoplasms explode all trees #3 MeSH descriptor Liver Neoplasms explode all trees #4 MeSH descriptor Bile Duct Neoplasms explode all trees #5 MeSH descriptor Ampulla of Vater explode all trees #6 MeSH descriptor Cholangiocarcinoma explode all trees #7 MeSH descriptor Cholestasis explode all trees #8 ((liver or hepat* or pancrea* or bile duct or choledoch*) and (cancer* or neoplasm* or malignan* or tumour* or tumor*)) or cholangiocarcinoma or cholesta* or icter* or jaundice #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Cholangiography explode all trees #11 MeSH descriptor Sphincterotomy, Endoscopic explode all trees #12 MeSH descriptor Sphincterotomy, Transhepatic explode all trees #13 MeSH descriptor Stents explode all trees #14 MeSH descriptor Preoperative Care explode all trees #15 MeSH descriptor Drainage explode all trees #16 drain* or stent* or cholangio* or sphincterotom* or ptc or ptbd or ERCP or endoscopic* #17 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 (#9 AND #17) |
| MEDLINE (OvidSP) | 1948 to February 2012. | 1. exp Jaundice, Obstructive/ 2. exp Pancreatic Neoplasms/ 3. exp Liver Neoplasms/ 4. exp Bile Duct Neoplasms/ 5. exp Ampulla of Vater/ 6. exp Cholangiocarcinoma/ 7. exp Cholestasis/ 8. (((liver or hepat* or pancrea* or bile duct or choledoch*) and (cancer* or neoplasm* or malignan* or tumour* or tumor*)) or cholangiocarcinoma or cholesta* or icter* or jaundice).af. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp Cholangiography/ 11. exp Sphincterotomy, Endoscopic/ 12. exp Sphincterotomy, Transhepatic/ 13. exp Stents/ 14. exp Preoperative Care/ 15. exp Drainage/ 16. (drain* or stent* or cholangio* or sphincterotom* or ptc or ptbd or ERCP or endoscopic*).af. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. 9 and 17 19. randomised controlled trial.pt. 20. controlled clinical trial.pt. 21. randomized.ab. 22. placebo.ab. 23. drug therapy.fs. 24. randomly.ab. 25. trial.ab. 26. groups.ab. 27. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. exp animals/ not humans.sh. 29. 28 not 27 30. 18 and 29 |
| EMBASE (OvidSP) | 1980 to February 2012. | 1. exp obstructive jaundice/ 2. exp pancreas tumor/ 3. exp liver tumor/ 4. exp bile duct tumor/ 5. exp Vater papilla/ 6. exp bile duct carcinoma/ 7. exp cholestasis/ 8. (((liver or hepat* or pancrea* or bile duct or choledoch*) and (cancer* or neoplasm* or malignan* or tumour* or tumor*)) or cholangiocarcinoma or cholesta* or icter* or jaundice).af. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp cholangiography/ 11. exp endoscopic sphincterotomy/ 12. exp biliary tract surgery/ 13. exp stent/ 14. exp preoperative care/ 15. exp biliary tract drainage/ 16. (drain* or stent* or cholangio* or sphincterotom* or ptc or ptbd or ERCP or endoscopic*).af. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomised controlled trial/ or single‐blind procedure/ 19. (random* or factorial* or crossover* or placebo*).af. 20. 18 or 19 21. 9 and 17 and 20 |
| Science Citation Index Expanded (ISI Web of Knowledge) | 1970 to February 2012. | # 1 TS=(((liver or hepat* or pancrea* or bile duct or choledoch*) and (cancer* or neoplasm* or malignan* or tumour* or tumor*)) or cholangiocarcinoma or cholesta* or icter* or jaundice) # 2 TS=(drain* or stent* or cholangio* or sphincterotom* or ptc or ptbd or ERCP or endoscopic*) # 4 #3 AND #2 AND #1 # 3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) |
| metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/) |
February 2012. | (icter* OR jaundice) AND (drain* OR stent*) |
Data and analyses
Comparison 1. Pre‐operative biliary drainage versus direct surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.71] |
| 2 Long‐term mortality | Other data | No numeric data | ||
| 3 Serious morbidity | 6 | Rate Ratio (Fixed, 95% CI) | 1.66 [1.28, 2.16] | |
| 4 Proportion of patients with serious morbidity | Other data | No numeric data | ||
| 5 Hospital stay (random‐effects) | 2 | 271 | Mean Difference (IV, Random, 95% CI) | 4.87 [‐1.28, 11.02] |
Comparison 2. Stratified by method of drainage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.71] |
| 1.1 PTBD | 4 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.62, 2.08] |
| 1.2 ERCP stent | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.62, 1.99] |
| 2 Overall morbidity | 6 | Rate Ratio (Fixed, 95% CI) | 1.66 [1.28, 2.16] | |
| 2.1 PTBD | 4 | Rate Ratio (Fixed, 95% CI) | 1.53 [1.00, 2.34] | |
| 2.2 ERCP stent | 2 | Rate Ratio (Fixed, 95% CI) | 1.75 [1.25, 2.44] |
Comparison 3. Stratified by proportion of patients with malignancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.71] |
| 1.1 95% or more malignancy | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.70, 2.27] |
| 1.2 Less than 95% malignancy | 3 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.83] |
| 2 Overall morbidity | 6 | Rate Ratio (Fixed, 95% CI) | 1.66 [1.28, 2.16] | |
| 2.1 95% or more malignancy | 3 | Rate Ratio (Fixed, 95% CI) | 2.09 [1.29, 3.39] | |
| 2.2 Less than 95% malignancy | 3 | Rate Ratio (Fixed, 95% CI) | 1.51 [1.10, 2.06] |
Comparison 4. Sensitivity analysis of overall morbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious complications without Pitt 1985 (proximal obstruction) | 5 | Rate Ratio (Fixed, 95% CI) | 1.77 [1.34, 2.34] | |
| 2 Serious complications without Lai 1994 (severity of complications could not be assessed) | 5 | Rate Ratio (Fixed, 95% CI) | 1.70 [1.27, 2.26] | |
| 3 Serious complications without van der Gaag 2010 (no routine antibiotic prophylaxis) | 5 | Rate Ratio (Fixed, 95% CI) | 1.51 [1.06, 2.16] | |
| 4 Bile leak irrespective of treatment | 6 | Rate Ratio (Fixed, 95% CI) | 1.67 [1.29, 2.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hatfield 1982.
| Methods | Randomised clinical trial | |
| Participants | Country: South Africa. Sample size: 57. Post‐randomisation drop‐out(s): 10 (17.5%). Revised sample size: 47. Females: not stated. Mean age: 61.8 years. Number with malignancy: 52 /55 (95%) (characteristics of 55 patients were presented in the report). Inclusion criteria: 1. Patients with obstructive jaundice (ultrasound and ERCP proven). 2. Bilirubin more than 150 micromole/litre. Exclusion criteria: Patients with hepatocellular carcinoma. | |
| Interventions | The patients were randomised to the following groups. Group 1: pre‐operative biliary drainage (n = 22). Further details: percutaneous transhepatic biliary drainage into external sealed bag system for 7 to 10 days (or longer if clinically indicated). Group 2: control (n = 25). | |
| Outcomes | The outcomes reported were mortality and overall morbidity. | |
| Notes | Reason for post‐randomisation drop‐out(s): hepatocellular carcinoma (1); cross‐over (1); technical failures (3); operations unjustified (1); pre‐operative deaths (4). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated". Comment: the trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated". Comment: the trial was described as randomised but the method used to conceal the allocation was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the trial was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: the severity of the complications was not reported. |
| Vested interest bias | Unclear risk | Quote: "Financial assistance was received from the South African Medical Reasearch Council and the Staff Research Fund,University of Cape Town". Comment: says financial assistance was received but no mention that the entire study was funded by these research charities. |
Lai 1994.
| Methods | Randomised clinical trial | |
| Participants | Country: China. Sample size: 87. Post‐randomisation drop‐outs: 0 (0%). Revised sample size: 87. Females: 28 (32.2%). Mean age: 66.4 years. Number with malignancy: 87 (100%). Inclusion criteria: 1. Patients with malignant obstructive jaundice. 2. Serum bilirubin more than 100 micromol/litre. | |
| Interventions | The patients were randomised to the following groups. Group 1: pre‐operative biliary drainage (n = 43). Further details: endoscopic drainage for at least 2 weeks. Group 2: control (n = 44). | |
| Outcomes | The outcomes reported were mortality and overall morbidity. | |
| Notes | Numbers of patients with complications rather than the number of complications were reported. The severity of the complications could not be determined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised". Comment: the trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were randomised by drawing consecutively numbered envelopes that contained the assigned treatment". Comment: further details were not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the trial was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: the severity of the complications was not reported. |
| Vested interest bias | Unclear risk | Comment: the trial did not report the source of funding. |
McPherson 1984.
| Methods | Randomised clinical trial | |
| Participants | Country: United Kingdom. Sample size: 70. Post‐randomisation drop‐out(s): 5 (7.1%). Revised sample size: 65. Females: not stated. Median age: 61.8 years. Number with malignancy: 65/65 (100%). Inclusion criteria: 1. Patients with malignant obstructive jaundice (confirmed by ultrasound and PTC). Exclusion criteria: 1. Patients with recurrent disease. 2. Patients with biliary obstruction due to secondary hilar deposits. | |
| Interventions | The patients were randomised to the following groups. Group 1: pre‐operative biliary drainage (n = 34). Further details: percutaneous transhepatic biliary drainage for 14 days or until bilirubin reached half its original value, into a closed drain system or using an antiseptic barrier. Group 2: control (n = 31). | |
| Outcomes | The outcomes reported were mortality and morbidity. | |
| Notes | Reason for post‐randomisation drop‐out(s): benign disease (4); metastatic deposit (1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were matched first. Matched patients were then randomised to either drainage group or surgery group". "Toss of coin was used". Comment: the trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Toss of coin used". Comment: the trial was described as randomised but the method used to conceal the allocation was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the trial was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: the severity of the complications was not reported. |
| Vested interest bias | Unclear risk | Quote: "This work was generously supported by a Cancer Research Campaign Grant". Comment: does not rule out additional support. |
Pitt 1985.
| Methods | Randomised clinical trial | |
| Participants | Country: United Stated of America.
Sample size: 79.
Post‐randomisation drop‐out(s): 4 (5.1%).
Revised sample size: 75.
Females: 29 (36.7%).
Mean age: 60.7 years. Number with malignancy: 58/75 (77.3%). Inclusion criteria: 1. Patients with obstructive jaundice. 2. Bilirubin greater than 171 micromoles/litre. 3. Patient was an operative candidate according to the attending physician's judgement. 4. Five or more previously described risk factors. |
|
| Interventions | The patients were randomised to the following groups. Group 1: pre‐operative biliary drainage (n = 37). Further details: percutaneous transhepatic biliary drainage into a closed drainage system until the bilirubin dropped below 171 micromoles/litre or the number of risk factors decreased to two. Group 2: control (n = 38). | |
| Outcomes | The outcomes reported were mortality, morbidity, and hospital stay. | |
| Notes | Reason for post‐randomisation drop‐outs was not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "From a list of random numbers at each institution, patients were randomised to receive percutaneous transhepatic drainage ….". Comment: sequence generation was achieved using random number generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated". Comment: the trial was described as randomised but the method used to conceal the allocation was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the trial was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: the severity of the complications was not reported. |
| Vested interest bias | Unclear risk | Comment: the trial did not report the source of funding. |
van der Gaag 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Netherlands.
Sample size: 202.
Post‐randomisation drop‐out(s): 6(3%).
Revised sample size:196.
Females: 77/196(39.3%).
Mean age: 64.7 years. Number with malignancy: 181/196 (92.3%). Inclusion criteria: 1. Patients with obstructive jaundice due to cancer of the pancreatic head. 2. Bilirubin level of 40 to 250 μmol per litre. 3. Age 18 to 85 years. 4. No evidence of distant metastasis or local vascular involvement. Exclusion criteria: 1. Patients with serious co‐existing illness. 2. Contra‐indications for major surgery. 3. Ongoing cholangitis. 4. Previous pre‐operative biliary drainage. 5. Current recipient of neoadjuvant chemotherapy. 6. Presence of serious gastric outlet obstruction. |
|
| Interventions | The patients were randomised to the following groups. Group 1: pre‐operative biliary drainage (n = 102). Further details: endoscopic drainage for 4 to 6 weeks. Group 2: control (n = 94). | |
| Outcomes | The outcomes reported were mortality, morbidity, and hospital stay. | |
| Notes | Total number of patients with complications rather than total number of complications were used. Reason for post‐randomisation drop‐out(s): did not meet inclusion criteria (4); withdrew consent (2) after allocation to the two groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, which was stratified according to study center, was performed with a computer program at the coordinating trial center". Comment: sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization, which was stratified according to study center, was performed with a computer program at the coordinating trial center". Comment: allocation was controlled by a central and independent randomisation unit. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: an adjudication committee reviewed all events in a blinded fashion. Comment: the trial was described as blinded, the assessor blinding was described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs after allocation of patients. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and severe complications were reported. |
| Vested interest bias | High risk | Quote: "Dr. Bruno reports receiving lecture fees from Boston Scientific; and Dr. Greve, consulting fees from GI Dynamics and lecture fees from Covidien and Johnson & Johnson". |
Wig 1999.
| Methods | Randomised clinical trial | |
| Participants | Country: India. Number randomised: 40. Post‐randomisation drop‐out(s): not stated. Revised sample size: 40. Mean age: 48.9 years. Females: 20 (50%). Number with malignancy: 24/40 (60%). Inclusion criteria: 1. Patients with obstructive jaundice. 2. Bilirubin greater than 172 micromoles per litre. | |
| Interventions | The patients were randomised to the following groups. Group 1: pre‐operative biliary drainage (n = 20). Further details: percutaneous transhepatic biliary drainage into a sealed bag containing povidone iodine continued until bilirubin drops below 102.85 micromoles per litre. Group 2: control (n = 20). | |
| Outcomes | The outcomes reported were mortality and morbidity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated". Comment: the trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated". Comment: the trial was described as randomised but the method used to conceal the allocation was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the trial was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: we did not know if there were post‐randomisation drop‐outs or withdrawals. |
| Selective reporting (reporting bias) | High risk | Comment: the severity of the complications was not reported. |
| Vested interest bias | Unclear risk | Comment: the trial did not report the source of funding. |
PTC = percutaneous transhepatic cholangiography. ERCP = endoscopic retrograde cholangio pancreatography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Smith 1985 | Randomisation by hospital file numbers, ie, quasi‐randomised study. |
Differences between protocol and review
The protocol had the title 'Preoperative biliary drainage for malignant obstructive jaundice' (Gurusamy KS, Wang C. Preoperative biliary drainage for malignant obstructive jaundice (Protocol). Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No.: CD005444. DOI: 10.1002/14651858.CD005444.).
We have modified the title of the review to read: 'Pre‐operative biliary drainage for obstructive jaundice' as we felt that the purpose of the preoperative biliary drainage is the same whether it was done for benign causes or malignant causes. We have modified the inclusion and exclusion criteria accordingly. However, we have performed a subgroup analysis to evaluate the beneficial and harmful effects of pre‐operative biliary drainage in malignant obstructive jaundice.
Differences between the previous review version and this updated review version
We have revised the methods according to the currently available version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We have revised the discussion in line with the GRADE handbook for grading quality of evidence and the strength of a recommendation (Schünemann 2009).
Contributions of authors
Y Fang: update of the present review; searching for trials, quality assessment of trials, data extraction, data analyses, contributed to the results and discussion, and developed the draft of the review. KS Gurusamy: lead author of the protocol of the present review; searching for trials, risk of bias assessment of trials, data extraction, data analyses, revised the review and wrote the discussion for this version of the review. Q Wang: data analysis, contributed to discussion, and modified the draft of the previous version of the review to reach Cochrane standards. BR Davidson: critically commented on the review and contributed to discussion. L He: searching for trials, contributed to discussion. X Xie: searching for trials, risk of bias assessment of the trials. C Wang: protocol and review development; searching for trials, risk of bias assessment of trials, data extraction, data analyses, and review development.
Sources of support
Internal sources
None, Not specified.
External sources
-
National Institute for Health Research, UK.
Department of Health disclaimer: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.'
Declarations of interest
The authors who have been involved in this review have done so without any known conflicts of interest. They are not involved in any of the primary trials included in this systematic review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Hatfield 1982 {published data only}
- Hatfield AR, Tobias R, Terblanche J, Girdwood AH, Fataar S, Harries‐Jones R, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet 1982;2:896‐9. [DOI] [PubMed] [Google Scholar]
Lai 1994 {published data only}
- Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. British Journal of Surgery 1994;81:1195‐8. [DOI] [PubMed] [Google Scholar]
- Lai EC, Mok FP, Fan ST, You KT, Tan ES, Choi TK, et al. A randomized controlled trial of preoperative endoscopic drainage for malignant obstructive jaundice: an interim report. Journal of Gastroenterology and Hepatology 1990;5(Suppl 2):4. [Google Scholar]
McPherson 1984 {published data only}
- McPherson GA, Benjamin IS, Blumgart LH. Improving renal function in obstructive jaundice without preoperative drainage. Lancet 1984;1(8375):511‐2. [DOI] [PubMed] [Google Scholar]
- McPherson GA, Benjamin IS, Blumgart LH. Percutaneous drainage in biliary obstruction. Lancet 1982;2(8308):1155‐6. [DOI] [PubMed] [Google Scholar]
- McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Pre‐operative percutaneous transhepatic biliary drainage: the results of a controlled trial. British Journal of Surgery 1984;71:371‐5. [DOI] [PubMed] [Google Scholar]
Pitt 1985 {published data only}
- Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP Jr. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost?. Annals of Surgery 1985;201:545‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Gaag 2010 {published data only}
- Eshuis WJ, Gaag NA, Rauws EAJ, Eijck CHJ, Bruno MJ, Kuipers EJ, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Annals of Surgery 2010;252(5):840‐8. [DOI] [PubMed] [Google Scholar]
- Kloek JJ, Heger M, Gaag NA, Beuers U, Gulik TM, Gouma DJ, et al. Effect of preoperative biliary drainage on coagulation and fibrinolysis in severe obstructive cholestasis. Journal of Clinical Gastroenterology 2010;44(9):646‐52. [DOI] [PubMed] [Google Scholar]
- Gaag NA, Gouma DJ. A study of the value of preoperative biliary‐tract drainage in the treatment of periampullary tumours: The DROP‐trial. Nederlands Tijdschrift voor Geneeskunde 2006;150(9):509‐11. [PubMed] [Google Scholar]
- Gaag NA, Rauws E, Eijck CH, Bruno M, Harst E, Gerritsen JJ, et al. Preoperative biliary drainage versus direct operation for pancreatic tumors causing obstructive jaundice. Gastroenterology 2009;136 Suppl 1(5):A127. [70151149] [Google Scholar]
- Gaag NA, Rauws EA, Eijck CH, Bruno MJ, Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. New England Journal of Medicine 2010;362(2):129‐37. [DOI] [PubMed] [Google Scholar]
- Gaag NA, Castro SM, Rauws EA, Bruno MJ, Eijck CH, Kuipers EJ. Preoperative biliary drainage for periampullary tumors causing obstructive jaundice; DRainage vs. (direct) OPeration (DROP‐trial). BMC Surgery 2007;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wig 1999 {published data only}
- Wig JD, Kumar H, Suri S, Gupta NM. Usefulness of percutaneous transhepatic biliary drainage in patients with surgical jaundice. A prospective randomised study. Journal of Association of Physicians of India 1999;47(3):271‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
Smith 1985 {published data only}
- Smith RC, Faithful GR, George CR. The effect of percutaneous transhepatic biliary drainage (PTBD) on post‐operative morbidity and renal function. Australian and New Zealand Journal of Medicine 1984;14 Suppl 1(3):323. [Google Scholar]
- Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery 1985;97:641‐8. [PubMed] [Google Scholar]
Additional references
Bjornsson 2008
- Bjornsson E, Gustafsson J, Borkman J, Kilander A. Fate of patients with obstructive jaundice. Journal of Hospital Medicine 2008;3(2):117‐23. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Cheng 2006
- Cheng TY, Sheth K, White RR, Ueno T, Hung CF, Clary BM, et al. Effect of neoadjuvant chemoradiation on operative mortality and morbidity for pancreaticoduodenectomy. Annals of Surgical Oncology 2006;13(1):66‐74. [DOI] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ (accessed 8 February 2012).
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Diener 2011
- Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, et al. Pylorus‐preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD006053] [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engelken 2003
- Engelken FJ, Bettschart V, Rahman MQ, Parks RW, Garden OJ. Prognostic factors in the palliation of pancreatic cancer. European Journal of Surgical Oncology 2003;29(4):368‐73. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 2. Art. No.: LIVER.
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Kawasaki 2003
- Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: Application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Annals of Surgery 2003;238(1):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kloek 2010
- Kloek JJ, Gaag NA, Aziz Y, Rauws EA, Delden OM, Lameris JS, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. Journal of Gastrointestinal Surgery 2010;14(1):119‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Michelassi 1989
- Michelassi F, Erroi F, Dawson PJ, Pietrabissa A, Noda S, Handcock M, et al. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Annals of Surgery 1989;210(4):544‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The prisma statement. PLoS Medicine 2009;6(7):e1000097. Epub 2009 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moss 2007
- Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta‐analysis of trials comparing endoscopic stents for malignant biliary obstruction. European Journal of Gastroenterology and Hepatology 2007;19(12):1119‐24. [DOI] [PubMed] [Google Scholar]
NCI 2012
- National Cancer Institute (U.S. National Institute of Health). Dictionary of Cancer terms. Periampullary cancer. http://www.cancer.gov/dictionary/?CdrID=543930 (accessed on 8 August 2012).
Nehez 2002
- Nehez L, Andersson R. Compromise of immune function in obstructive jaundice. European Journal of Surgery 2002;168(6):315‐28. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Papadopoulos 2007
- Papadopoulos V, Filippou D, Manolis E, Mimidis K. Haemostasis impairment in patients with obstructive jaundice. Journal of Gastrointestinal and Liver Diseases 2007;16(2):177‐86. [17592568] [PubMed] [Google Scholar]
Pauli‐Magnus 2005
- Pauli‐Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. Journal of Clinical Gastroenterology 2005;39 Suppl 2(4):103‐10. [DOI] [PubMed] [Google Scholar]
Pisters 2001
- Pisters PW, Hudec WA, Hess KR, Lee JE, Vauthey JN, Lahoti S, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy‐associated morbidity in 300 consecutive patients. Annals of Surgery 2001;234(1):47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Oxman A, editors. Grade handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The Grade Working Group, 2009. Available from http://www.Cc‐ims.Net/gradepro (accessed 8 August 2012).
Sewnath 2002
- Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta‐analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Annals of Surgery 2002;236:17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2008
- Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet‐lymphocyte ratio improves the predictive value of serum CA19‐9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery 2008;143(5):658‐66. [DOI] [PubMed] [Google Scholar]
Swensen 2010
- Swensen SJ, Meyer GS, Nelson EC, Hunt GC Jr, Pryor DB, Weissberg JI, et al. Cottage industry to postindustrial care ‐ the revolution in health care delivery. New England Journal of Medicine 2010;362(5):e12. Epub 2010 Jan 20. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 8 August 2012).
Wadei 2006
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: Pathophysiology and management. Clinical Journal of the American Society of Nephrology 2006;1(5):1066‐79. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winter 2006
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single‐institution experience. Journal of Gastrointestinal Surgery 2006;10(9):1199‐210. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wang 2008
- Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005444] [DOI] [PubMed] [Google Scholar]


