Abstract
Background
Weight gain is common for people with schizophrenia and this has serious implications for health and well being.
Objectives
To determine the effects of both pharmacological (excluding medication switching) and non pharmacological strategies for reducing or preventing weight gain in people with schizophrenia.
Search methods
We searched key databases and the Cochrane Schizophrenia Group’s trials register (April 2006), reference sections within relevant papers, hand searched key journals, and contacted the first author of each relevant study and other experts to collect further information.
Selection criteria
We included all clinical randomised controlled trials comparing any pharmacological or non pharmacological intervention for weight gain (diet and exercise counselling) with standard care or other treatments for people with schizophrenia or schizophrenia-like illnesses.
Data collection and analysis
We reliably selected, quality assessed and extracted data from studies. As weight is a continuous outcome measurement, weighted mean differences (WMD) of the change from baseline were calculated. The primary outcome measure was weight loss.
Main results
Twenty-three randomised controlled trials met the inclusion criteria for this review. Five trials assessed a cognitive/behavioural intervention and eighteen assessed a pharmacological adjunct. In terms of prevention, two cognitive/behavioural trials showed significant treatment effect (mean weight change) at end of treatment (n=104, 2 RCTs, WMD −3.38 kg CI −4.2 to −2.0). Pharmacological adjunct treatments were significant with a modest prevention of weight gain (n=274, 6 RCTs, WMD − 1.16 kg CI −1.9 to −0.4). In terms of treatments for weight loss, we found significantly greater weight reduction in the cognitive behavioural intervention group (n=129, 3 RCTs, WMD −1.69 kg CI −2.8 to −0.6) compared with standard care.
Authors’ conclusions
Modest weight loss can be achieved with selective pharmacological and non pharmacological interventions. However, interpretation is limited by the small number of studies, small sample size, short study duration and by variability of the interventions themselves, their intensity and duration. Future studies adequately powered, with longer treatment duration and rigorous methodology will be needed in further evaluating the efficacy and safety of weight loss interventions for moderating weight gain. At this stage, there is insufficient evidence to support the general use of pharmacological interventions for weight management in people with schizophrenia.
Medical Subject Headings (MeSH): *Weight Gain, *Weight Loss, Obesity [diet therapy; *therapy], Randomized Controlled Trials as Topic, Schizophrenia [*complications; drug therapy]
MeSH check words: Humans
BACKGROUND
Obesity and schizophrenia
Obesity is a common problem for people with schizophrenia, a problem that has been exacerbated more recently with the increased use of second generation antipsychotics, many of which are associated with the risk of weight gain and metabolic disturbance (Allison 1999; Homel 2002; Casey 2004). The prevalence of obesity in people with schizophrenia has been reported to be anywhere from one and a half to four times higher than the general population (ADA/APA 2004; Coodin 2001; Silverstone 1988). For people with schizophrenia, there is a marked increase in standardized mortality ratios for both natural and unnatural causes of death and much of this increment may be attributed to the increased prevalence of coronary heart disease risk (Cohn 2004; Goff 2005; Henderson 2005a; Mackin 2005; Saari 2005), and related obesity in this population (Coodin 2001; Daumit 2003; Susce 2005). The significance and recognition of this prevalence and its impact on premature mortality and morbidity has led to the development of recent consensus statements on its management (ADA/APA 2004; De Nayer 2005).
Mechanisms of weight gain in schizophrenia
It is difficult to identify the relative contributions of disease-specific factors such as genetics, the side effects of medications, and lifestyle factors such as diet and physical inactivity on the prevalence of obesity in schizophrenia. In a meta-analysis, every antipsychotic medication except ziprasidone and molindone was associated with some degree of weight gain after just ten weeks of treat ment (Allison 1999). The effects were greatest with olanzapine and clozapine which increased body weight by approximately 4 to 4.5 kilograms. To date, there is no consensus on what pharmacological factors may be involved in this weight gain particularly regarding the newer antipsychotics. Ananth and colleagues (Ananth 2004) have reviewed a range of potential weight-inducing mechanisms such as dopaminergic blockage; increased appetite due to the interaction of antipsychotic medication with dopamine, serotonin, and histamine neuronal receptors; increased leptin; and increases in systemic levels of various cytokines and soluble cytokine receptors. The relative receptor affinities of the novel antipsychotics for histamine H1 appear to be a consistent correlate of antipsychotic-related weight gain (Kroeze 2003; Wirshing 1999). It is important to note however that obesity was commonly reported before antipsychotics were widely introduced (Baptista 2002). In terms of lifestyle factors, physical activity is one important component of weight management and research consistently demonstrates that people with schizophrenia are less physically active than the general population (Brown 1999; Cohn 2004; Daumit 2005). Similarly, research suggests that people with schizophrenia have a diet high in fat and low in fibre and vitamins (McCreadie 1998), and consume more calories than population controls (Strassnig 2003a). As with the general population, the aetiology of obesity appears complex and multifactorial. Consequently, intervention strategies must also target a broad range of factors that may contribute to weight gain in this population.
Health effects of obesity
Obesity doubles the risk of all-cause mortality, coronary heart disease, stroke and type 2 diabetes, increases the risk of some cancers, musculoskeletal problems and loss of function, and carries negative psychological consequences (DoH 2004). Being an obese or overweight adult is associated with large decreases in life expectancy and increases in early mortality in recent US population data, and these decreases are similar to those seen with smoking (Peeters 2003). The burden of obesity in schizophrenia will be at least comparable in terms of premature mortality and morbidity, and is likely to have important deleterious effects on mortality and health (Fontaine 2001).
Quality of life is further reduced for people with schizophrenia with high body mass index (Strassnig 2003b; Kurzthaler 2001) and those gaining weight (Allison 2003). Furthermore, Weiden 2004 reported a significant, positive association between obesity, subjective distress from weight gain and medication non-compliance in a sample of people with schizophrenia. People with schizophrenia face the combined challenges of living with the illness, and for many, obesity and related illnesses. This combination is a major public health problem (Wirshing 2004) and carries considerable human cost. Recognition of this has lead to growing concern with how best to intervene (Green 2000; Le Fevre 2001; Osborn 2001; Birt 2003; Catapana 2004).
Treatment of obesity in the general population
The treatment of obesity consists of non-pharmacological and pharmacological interventions. Current guidelines state that non-pharmacological interventions should always be used before, and then in conjunction with the latter (Snow 2005). In terms of non-pharmacological interventions, strategies should combine diet, exercise and psychological/behavioural components. In a recent Cochrane review (Shaw 2005), cognitive-behaviour therapy, when combined with a diet/exercise intervention, was found to increase weight loss compared with diet/exercise alone by 4.9 kg (CI −7.3 to −2.4). Behaviour therapy alone was found to result in significantly greater weight loss (−2.5 kg) than placebo when assessed as a stand-alone weight loss strategy (CI −1.7 to −3.3). Bariatric surgery could be considered as a treatment option for patients with a BMI of 40 kg/m2 or greater, and who have failed an adequate lifestyle modification programme (with or without adjunctive pharmacological therapy) (see Hamoui 2004 in the context of schizophrenia).
Pharmacological interventions can be divided into two broad categories (Padwal 2004). The first includes inhibitors of intestinal fat absorption (orlistat). The second acts to suppress appetite, increase satiety, or increase thermogenesis by modifying central nervous system neurotransmission of norepinephrine, dopamine and serotonin (sibutramine). One meta-analysis of anti-obesity agents found that average weight losses compared to placebo were modest and never exceeded four kilograms for any one agent (Haddock 2002). A recent Cochrane review (Padwal 2004) of long term interventions reported that orlistat treated patients lost 2.7 kilograms (CI −2.3 kg to −3.1 kg) more weight than placebo while sibutramine-treated patients lost 4.3 kilograms (CI −3.6 kg to −4.9 kg) more weight than placebo.
Overall, existing evidence suggests that even effective treatments for adult obesity only produce modest weight loss (approximately 2-5 kg) compared to no treatment or usual care. One implication of this finding is that the best treatment of obesity is its prevention. However, modest weight loss is a worthwhile outcome of interventions. There is evidence that loss of body weight by as little as 5-10% may reduce some of the health risks associated with adult obesity (Wilding 1997). Furthermore, sustained changes in health behaviours as a result of such interventions, e.g., increased levels of physical activity, may reduce risk of mortality and morbidity independent of any weight loss (Wei 1999). Increasing levels of physical activity are also associated with a range of mental health benefits in this population (Faulkner 1999).
Prevention and treatment of obesity in schizophrenia
Given such modest weight loss in studies in the adult population, it might be expected that interventions may be difficult for people with schizophrenia given the range of social and cognitive difficulties associated with the illness. There are at least four systematic reviews examining behavioural interventions (Werneke 2003; Loh 2006), pharmacological interventions (Werneke 2002), or both (Faulkner 2003). This Cochrane review updates and extends these recent reviews by focusing on evidence from randomised controlled trials.
OBJECTIVES
To determine the effects of both pharmacological (excluding antipsychotic medication switching) and non-pharmacological strategies (diet/exercise) for reducing or preventing weight gain in people with schizophrenia.
METHODS
Criteria for considering studies for this review
Types of studies
We sought all relevant randomised controlled trials. Where a trial was described as ‘double-blind’, but it was only implied that the study was randomised, these trials were included in a sensitivity analysis. If there was no substantive difference within primary outcomes (see types of outcome measures) when these ‘implied randomisation’ studies were added, then they were included in the final analysis. If there was a substantive difference, only clearly randomised trials were used and the results of the sensitivity analysis described in the text. Quasi-randomised studies, such as those allocating by using alternate days of the week, were excluded.
Types of participants
We included people diagnosed with schizophrenia or schizophrenia-like illnesses, using any criteria. Trials were included where it was implied that the majority (>50%) of the participants had a severe mental illness likely to be schizophrenia. Trials were not excluded due to age, nationality or sex of participants. Trials were included with participants with any length of illness who were being treated in any treatment setting.
Types of interventions
Weight loss (treatment) and weight maintenance (prevention) studies evaluating pharmacologic or nonpharmacologic adjunctive interventions were included in this review. To be included in the review, the primary outcome of the trial had to be weight loss or maintenance. In this review we do not focus on interventions examining the switching of antipsychotic medication.
1. Prevention of weight gain
1.1 Non pharmacological interventions
All types of non pharmacological interventions were considered for inclusion. Typically, interventions incorporate dietary and/or exercise components. Additionally, some studies may include cognitive/behavioural components. These treatments attempt to enhance dietary restraint by providing adaptive dietary strategies and by discouraging maladaptive dietary practices, and by increasing motivation to be more physically active (Shaw 2005). Studies were considered based on the following subcategories:
1.1.1 Cognitive/behavioural intervention versus standard care. These referred to studies promoting changes in diet and/or physical activity including elements of cognitive and/or behavioural modification;
1.1.2. Exercise/dietary intervention versus standard care. These referred to studies promoting changes in diet and/or physical activity without elements of cognitive and/or behavioural modification.
1.2 Pharmacological interventions
All types of adjunctive pharmacological interventions were considered for inclusion. At this stage, we have included:
1.2.1 Pharmacological adjunctive treatments - currently licensed for use as a weight loss agent (sibutramine; orlistat);
1.2.2 Pharmacological adjunctive treatments - off-label therapy (fluoxetine, topiramate, metformin);
1.2.3 Pharmacological adjunctive treatments - withdrawn from the market (fenfluramine, phenylpropanolamine).
1.3 Standard care
We defined this as care that a person would normally receive had they not been included in the research trial.
2. Treatment of weight gain
2.1 Non pharmacological interventions
2.1.1 Cognitive/Behavioural intervention versus standard care. These referred to studies promoting changes in diet and/or physical activity including elements of cognitive and/or behavioural modification;
2.1.2. Exercise/dietary intervention versus standard care. These referred to studies promoting changes in diet and/or physical activity without elements of cognitive and/or behavioural modification.
2.2 Pharmacological interventions
2.2.1 Pharmacological adjunctive treatments - currently licensed somewhere for use as weight loss agents (sibutramine, orlistat);
2.2.2 Pharmacological adjunctive treatments - off-label therapy (fluoxetine, topiramate, metformin);
2.2.3 Pharmacological adjunctive treatments - withdrawn from the market (fenfluramine, phenylpropanolamine).
2.3 Standard care
We defined this as care that a person would normally receive had they not been included in the research trial.
Types of outcome measures
1. Weight or another indicator of body mass (e.g. body mass index, waist measurement, waist-to-hip ratio)
-
1.1
Total body weight (lbs/kg)
-
1.2
Change in weight
-
1.3
Total BMI
-
1.4
Change in BMI
-
1.5
Total waist circumference
-
1.6
Change in waist circumference
-
1.7
Total waist-to-hip circumference ratio
-
1.8
Change in waist-to-hip circumference ratio
-
1.9
Total percent body fat
-
1.10
Change in percent body fat
-
1.11
Any change in weight (as defined by individual studies)*
-
1.12
No clinically important change in body mass index (BMI) (as defined by individual studies)
-
1.13
No clinically important change in waist circumference (as defined by individual studies)
-
1.14
No clinically important change in waist-to-hip circumference ratio (as defined by individual studies)
-
1.15
No clinically important change in total percent body fat (as defined by individual studies)
2. Global measures
-
2.1
No clinically important change in global measure
-
2.2
Continuous measures of global state
3. Mental state (with particular reference to the positive and negative symptoms of schizophrenia)
-
3.1
No clinically important change in general mental state
-
3.2
Average endpoint general mental state score
-
3.3
Average change in general mental state scores
-
3.4
No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania)
-
3.5
Average endpoint specific symptom score
-
3.6
Average change in specific symptom scores
4. Well-being and quality of life measures
-
4.1
No clinically important change in quality of life
-
4.2
Average endpoint quality of life score
-
4.3
Average change in quality of life scores
-
4.4
No clinically important change in specific aspects of quality of life
-
4.5
Average endpoint specific aspects of quality of life
-
4.6
Average change in specific aspects of quality of life
5. Adverse effects - general and specific
-
5.1
Clinically important general adverse effects
-
5.2
Average endpoint general adverse effect score
-
5.3
Average change in general adverse effect scores
-
5.4
Clinically important specific adverse effects
-
5.5
Average endpoint specific adverse effects
-
5.6
Average change in specific adverse effects
-
5.7
Death - suicide and natural causes
6. Leaving the study early
7. Other outcomes measures
-
7.1
Cardiovascular measures
-
7.2
Laboratory measures
-
7.3
Compliance
-
7.4
Economic outcomes
-
7.5
Other extractable outcomes
We analysed data according to three time points. Short term follow up (less than 12 weeks), medium term follow up (12 to 52 weeks) and long term follow up (longer than one year).
Search methods for identification of studies
1. Electronic search
We searched the Cochrane Schizophrenia Group’s study based register (April 2006) using the phrase: [(*weight* or *body mass* or * bmi* or *obes* or * eat* or *fat* or *exercise* or *diet* or *sport* or *physical therap* or *physical activit* or *cog* and (*beh* or *therap*)) in REFERENCE or (*diet* or *nutrition* or *exercise* in STUDY INTERVENTION) or (*weight* or obes* or body mass* or *diet* or eat* or waist* in STUDY OUTCOME)]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings see below.
1.1 We searched the Cochrane Schizophrenia Group’s Register using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.2 We searched MEDLINE (1966-4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.3 We searched CINAHL (1982-4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.4 We searched EMBASE (1974-4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.5 We searched PsycINFO (1872 to 4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.6 We searched UMI ProQuest Digital Dissertations (1861 to 4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.7 We searched HealthSTAR (1990-4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
1.8 We searched Sports Discus (1975-4/2006) using the phrase
[schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy]
2. We searched registers of ongoing clinical trials
3. Reference searching
We inspected reference list of all identified studies, including existing reviews for relevant citations. Additionally, we hand searched copies of the following journals (from January 2000 to April 2006): Journal of Clinical Psychiatry; British Journal of Psychiatry; Schizophrenia Bulletin; Schizophrenia Research; Journal of Clinical Psychopharmacology; Psychiatric Services and the American Journal of Psychiatry. Finally, conference proceedings of the American Psychiatric Association (personal records), Society of Biological Psychiatry (published in Biological Psychiatry), the European College of Neuropsychopharmacology (published in European Neuropsychopharmacology), the Collegium Internationale Neuro-psychopharmacologicum (published in the International Journal of Neuropsychopharmacology) and the International Congress on Schizophrenia Research (published in Schizophrenia Research) were hand searched.
4. Personal contact
We contacted the first author of each relevant study for information on unpublished trials. Experts in the area of schizophrenia and weight gain were also consulted. Authors and experts were contacted by e-mail or post to establish missing details in the methods and results sections of the written reports and to determine their knowledge of or involvement in any current work in the area. Contacts at major pharmaceutical companies were also asked if they have conducted or were currently undertaking any weight-related interventions in relation to schizophrenia (including representatives from Janssen Pharmaceuticals, Pfizer Inc, Eli Lilly and Company, Astra Zeneca, and Bristol-Myers Squibb).
Data collection and analysis
1. Selection of trials
We (GF, TC, GR) independently assessed the abstracts from the trial searches. Any disagreements were discussed and reported and if consensus could not be reached we obtained the full report and repeated the assessment process until agreement was reached.
2. Assessment of methodological quality
Two reviewers (GF and TC) independently assessed the methodological quality of included trials in this review using the criteria described in the Cochrane Handbook (Higgins 2005) and the Jadad Scale (Jadad 1996). The former is based on the evidence of a strong relationship between allocation concealment and direction of effect (Schulz 1995). The categories are defined below:
Low risk of bias (adequate allocation concealment)
Moderate risk of bias (some doubt about the results)
High risk of bias (inadequate allocation concealment).
The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
Was the study described as randomised?
Was the study described as double-blind?
Was there a description of withdrawals and drop outs?
Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomisation or the blinding/masking procedures described are inadequate. For this review we used a cut-off of two points on the Jadad scale to check the assessment made by the Handbook criteria. However, we did not use the Jadad Scale to exclude trials.
This classification was planned as the basis of a sensitivity analysis.
3. Data management
3.1 Data extraction
GF independently extracted data from selected trials, while TC separately re-extracted information from two different samples (10%). When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
4. Data synthesis
4.1 Data types
We assessed outcomes using continuous (for example, changes in weight), categorical (for example, one of three categories on a behaviour scale, such as ‘little change’, ‘moderate change’ or ‘much change’) or dichotomous measures (for example, either ‘no important changes’ or ‘important changes’ in a person’s weight). Currently RevMan does not support categorical data so we were unable to analyse these.
4.2 Intention to treat analysis
We assumed that participants who left before study completion e.g. withdrawn by an investigator or left of their own volition, for binary outcomes, to have had a negative outcome. We tested the effects of this assignment in a sensitivity analysis. For continuous data it is impossible to manage the data in this way therefore we presented ‘completer’ data.
4.3 Binary data
When summation was appropriate, with binary outcomes such as improved/not improved, we calculated the relative risk (RR) statistic with a 95% confidence interval (CI) and used a fixed effects model.
4.2 Continuous data
4.2.1 Normally distributed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non-parametric data, we applied the following standards to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); (c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S-Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them.
For change data (endpoint minus baseline), the situation is even more problematic. In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALLSTAT electronic statistics mailing list, we presented change data in order to summarise available information. In doing this, it was assumed either that data were not skewed or that the analyses could cope with the unknown degree of skew. Again, without individual patient data it is impossible to test this assumption.
4.2.2 Summary statistic
For continuous outcomes we estimated a weighted mean difference (WMD) between groups. Again this was based on the random effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We did not consider continuous data presented without use of summary statistics (i.e. mean, SD, SE, median, interquartile range), although we noted the existence of these data in the description of included studies.
4.2.3 Rating scales
Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore continuous data from rating scales were included only if the measuring instrument had been described in a peer-reviewed journal. Furthermore, we stipulated that the instrument should either be a self report or be completed by an independent rater or relative (not the therapist), and that the instrument could be considered a global assessment of an area of functioning.
4.2.4 Endpoint versus change data
Where possible we presented endpoint data and if both endpoint and change data were available for the same outcomes then we only reported endpoint data.
4.3 Cluster trials
Studies increasingly employ ‘cluster randomisation’ (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a ‘unit of analysis’ error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra-class correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non-cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ‘design effect’. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co-efficient (ICC) [Design effect = 1+(m-1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intra-class correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
5. Investigation for heterogeneity
Firstly, we undertook consideration of all the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. This was supplemented using, primarily, the I-squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I-squared estimate was greater than or equal to 75%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency was high, we did not summate data, but presented the data separately and investigated the reasons for heterogeneity.
6. Addressing publication bias
We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias.
7. Sensitivity analyses
We compared the results for high doses (however ‘high’ was defined in the study) to those for lower doses with regard to the primary outcome of weight loss or maintenance.
8. General
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the weight management treatment.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
For substantive descriptions of studies please see Included and Excluded Studies table.
1. Excluded studies
We excluded six studies from the review and reasons for this are presented in the ‘characteristics of excluded studies’ table (Archie 2003; Harmatz 1968; McCreadie 2005; Morrison 2002; Nickel 2005; Rotatori 1980).
2. Awaiting assessment
Four studies await assessment. We have sought further information from Ganguli 2005 but authors have not responded to our requests and that study remains in awaiting assessment. We identified three studies after completion of this version of the review, and we will incorporate these into following reviews (Alvarez-Jimenez 2006; Brown 2006; McKibbin 2006).
3. Ongoing studies
Two trials are ongoing (Ritchie 2003; Bristol-Meyer Squib, see table of Ongoing studies).
4. Included studies
We included twenty-three randomised controlled trials. Five assessed a cognitive/behavioural intervention and eighteen a pharmacological adjunct. All but two studies were published from 2002 onward which reflects the recent recognition of weight gain as a significant clinical concern.
4.1 Methods
All trials were randomised. The five cognitive/behavioural studies were not double-blind due to the inherent difficulties involved in disguising psychosocial interventions. All pharmacological interventions were double-blind except for two studies which were open (Fluvoxamine 2004; Topirimate 2006). The duration of the trials ranged between six weeks (Famotidine 2004; Reboxetine 2003) and twenty-four weeks (Amantadine 2005). The average duration was approximately twelve weeks.
4.2 Participants and setting
All trials included people diagnosed with schizophrenia, schizoaffective or schizophreniform psychosis and the majority employed DSM-IV criteria for diagnoses. Diagnostic criteria was not reported in two studies (Cog/Behav 2005b; Metformin 2006) while diagnosis was obtained by chart records in one study (Dextroamphetam 1965) and detailed ‘psychiatric interview’ in another (D-Fenfluramine 1988). Two studies explicitly targeted first-episode patients (Fluoxetine 2002; Famotidine 2004). Over 1000 participants were included with slightly more male than female participants included (where described). Participants were aged between 18 and 64 with a mean age of approximately 36 years. When data were provided (in fourteen studies), the mean BMI of the participants at baseline was approximately 29 kg/m2. When data were provided (in fifteen studies), the mean weight of the participants at baseline was approximately 80 kg.
4.3 Setting
Eleven trials included only outpatients (Amantadine 2005b; Cog/Behav 2003; Cog/Behav 2005b; Cog/Behav 2006a; Cog/Behav 2006b; D-Fenfluramine 1988; Fluoxetine 2003; Phenylpropanol 2002; Sibutramine 2005a; Sibutramine 2005b; Topirimate 2006), seven trials only inpatients (Dextroamphetam 1965; Famotidine 2004; Fluoxetine 2002; Fluvoxamine 2004; Metformin 2006; Reboxetine 2003; Topiramate 2005), and five involved both in-patients and outpatients (Amantadine 2005; Cog/Behav 2005a; Nizatidine 2003a; Nizatidine 2003b; Nizatidine 2004).
4.4 Study size
Most studies were small (mean number randomised 45) and ranged between 14 and 171 participants.
4.5 Interventions - Prevention
Nine trials were preventive interventions i.e. the attenuation of weight gain.
4.5.1 Non pharmacological Interventions
Two trials included a cognitive/behavioural intervention (Cog/Behav 2003; Cog/Behav 2005b). In the first trial, the intervention group attended a weekly, one-hour psychoeducation class using “Solutions of Wellness” modules incorporating nutrition and exercise material for 16 weeks (Cog/Behav 2003). Modules involved individual and group work with written exercises, tests and discussions. Learning objectives of the material included setting realistic goals, learning appropriate serving sizes, developing support systems, initiating and maintaining an exercise program and learning low-cost and no-cost exercise strategies. In the second trial (Cog/Behav 2005b), the intervention group received six one-on-one individual nutrition education sessions over a three month period. Each session lasted one hour and included discussions on healthy eating, exercise, label reading, energy density, high fibre diets, non-hungry eating and maintenance of healthy eating and changes in activity levels. Goals for healthy eating and lifestyle change were set, reviewed and revised on a fortnightly basis.
4.5.2 Pharmacological Interventions
The remaining trials focused on a range of pharmacological adjuncts for attenuating weight gain including famotidine, nizatidine, fluoxetine, reboxetine, metformin, topiramate, and fluvoxamine.
Famotidine is an H2 antagonist that has been associated with the attenuation of weight gain. Nizatidine is another H2 receptor antagonist which has been implicated in weight loss. Fluoxetine induces stimulation of the 5-HT2C receptor, implicated in weight gain, which may compensate for olanzapine’s antagonistic activity at these same receptors. Reboxetine is a selective norepinephrine reuptake inhibitor. Metformin is an anti-diabetic agent that has been found to be beneficial in decreasing weight and improving insulin sensitivity. Topiramate is an anticonvulsant medication that has been used in a variety of other conditions, including bipolar affective disorder, migraine and neuropathic pain it has been reported to induce weight loss, although the mechanism is not well understood. Fluvoxamine is an add-on selective serotonin reuptake inhibitor (SSRIs) which has been used adjunctively with clozapine to enhance therapeutic response with regards to depressive or negative symptomatology. The co-administration of this agent may accelerate clozapine-induced increases in plasma leptin levels and decrease plasma norclozapine/clozapine ratios. Norclozapine may be primarily responsible for weight gain.
Poyurovsky and colleagues (Famotidine 2004) examined the effect of famotidine on preventing weight gain in first episode people. In a small study of fourteen patients taking olanzapine (10 mg/day), participants were randomised to receive either famotidine (40 mg/day) or placebo for six weeks. Cavazonni and colleagues (Nizatidine 2003a) evaluated the efficacy of two fixed doses (150 mg b.i.d. and 300 mg b.i.d.) of nizatidine for sixteen weeks. Participants received open label olanzapine (5 to 20 mg/day). Poyurovsky and colleagues (Fluoxetine 2002) examined whether the co administration of fluoxetine (20 mg/day) prevented or attenuated olanzapine-induced (10 mg/day) weight gain in people with first-episode schizophrenia. Poyurovsky and colleagues (Reboxetine 2003) examined its addition for preventing weight gain in participants during six weeks of olanzapine treatment (10 mg/day). Participants were randomly allocated to receive reboxetine (4 mg/day) or placebo. Baptista and colleagues (Metformin 2006) randomised patients taking olanzapine (10 mg/day) to a treatment group which received 850-1750 mg/day metformin or a placebo group for fourteen weeks. Kim and colleagues (Topirimate 2006) examined its addition for olanzapine treated patients (~ 12 mg/day). The treatment group received topiramate (25 mg b.i.d for eight days then a fixed dose to 50 mg b.i.d. to 12 weeks). Lu and colleagues (Fluvoxamine 2004) randomly assigned patients to a 12 week treatment group which received fluvoxamine (50 mg/day) plus low-dose clozapine (< 250 mg/day), or a monotherapy group which received clozapine (< 600 mg/day).
4.5.3 Standard Care
In all trials, standard care included treatment with antipsychotic medication. In one study, the standard care group received passive nutritional education from a booklet (Cog/Behav 2005b). In the metformin study, both groups received a balanced diet (2500-3000 KCal daily) during the fourteen-week trial (Metformin 2006).
4.6 Interventions - Treatment
Fourteen trials involved treatments for weight loss.
4.6.1 Non pharmacological Interventions
Three trials examined cognitive/behavioural interventions. In the trial reported by Ganguli and colleagues (Cog/Behav 2005a), participants were treated with olanzapine and randomly assigned to a 14-week behavioural weight loss treatment group or standard care. The treatment group received two therapy sessions per week for six weeks and then one session per week for eight weeks (a total of 20 sessions). In these sessions, a range of weight loss techniques were taught, including self-monitoring of weight and food consumed, modifying urges to overeat, slowing the pace of eating, and changing snacking habits. Only two sessions covered exercise. Techniques were rehearsed by the participants, and were demonstrated with food models when appropriate. In a pilot study of a group intervention modified after the Diabetes Prevention Project (DPP), Weber and Wyne (Cog/Behav 2006a) randomly assigned patients to a cognitive/behavioural intervention group or standard care. The treatment group consisted of one-hour group sessions each week for sixteen weeks. Each session included activities such as role play, goal setting, problem solving, discussion of barriers to change, presentations on low-fat diets and developing plans to increase activities such as walking. A food and activity diary was kept and reviewed at each session. In the study reported by Kwon and colleagues (Cog/Behav 2006b), olanzapine-treated patients were randomly allocated to a weight management program or standard care. Individual sessions were held once a week for the first four weeks and then every two weeks up to the end of treatment at week 12. Diet management included a food diary and nutritional education. A dietician reviewed and discussed the food diaries and helped with diet planning. Patients were also educated about nutrition concepts using food models including healthy snacking, low-calorie cooking preparation and food shopping. Exercise management included the keeping of an exercise diary and education regarding lifestyle modification. An exercise coordinator reviewed and discussed the diaries with the patients and helped patients plan exercise. Education included explanations of calorie consumption in daily activities, choosing suitable exercise, and using the community health centre.
4.6.2 Pharmacological interventions
The remaining eleven trials focused on a range of pharmacological adjuncts for attenuating weight gain including amantadine, dfenfluramine, dextroamphetamine sulfate, fluoxetine, nizatidine, phenylpropanolamine, sibutramine and topiramate.
Amantadine is a dopamine agonist that is used for the treatment of extrapyramidal side-effects associated with antipsychotic drugs. Its ability to modify dopamine and serotonin neurotransmission may in turn produce weight-reducing effects. Two trials examined the adjunctive use of amantadine. In the trial reported by Deberdt and colleagues (Amantadine 2005), olanzapine (5-20 mg/day) was coadminstered with 100-300 mg/day amantadine or placebo. Graham and colleagues (Amantadine 2005b) similarly assigned olanzapine treated patients to up to 300 mg/day amantadine or placebo for 12 weeks.
Goodall (D-Fenfluramine 1988) examined the efficacy of d-fenfluramine, an anorectic drug (since removed from the market), in treating weight gain during a 12-week trial. All patients were receiving a ‘regular’ dose of depot antipsychotic medication - fluphenazine decanoate, flupenthixol decanoate, or clopenthixol decanoate, and those with a BMI 3 27 were randomised to receive 30 mg/day of d-fenfluramine or placebo. All participants received dietary advice throughout the 12-week trial.
Dextroamphetamine sulfate is an isomer of amphetamine which has been used as a central nervous system stimulant to help treat narcolepsy and Attention Deficit Hyperactivity Disorder. Interest in its potential anorectic effect lead Model and Hussar (Dextroamphetam 1965) to examine whether 5 mg/daily of this agent promoted weight loss over eight weeks compared to a placebo.
Bustillo and colleagues (Fluoxetine 2003) examined 60 mg/day doses of fluoxetine in treating patients who had gained > 3% of their baseline weight in the first 8 weeks of olanzapine treatment. In two studies, Atmaca and colleagues investigated the efficacy of 150 mg/day nizatidine on the treatment of olanzapine-induced (mean daily dose = 15.3 mg/day) weight gain (Nizatidine 2003b) and quetiapine-induced (mean daily dose = 504.4 mg/day) weight gain (Nizatidine 2004).
Phenylpropanolamine is a synthetic catecholamine appetite suppressant which has been removed from the market. Borovicka and colleagues (Phenylpropanol 2002) conducted a 12-week trial of PPA in treating outpatients who were stable on clozapine treatment (PPA, clozapine mean dosage = 506 ± 115 mg; placebo, clozapine mean dosage = 431 ± 187 kg) for at least four months and had gained > 10% of their baseline body weight since starting treatment on this antipsychotic. Participants were randomised to receive PPA 75 mg/daily or placebo. All patients also attended mandatory monthly sessions of dietary counselling.
Sibutramine, approved for the long-term treatment of obesity, inhibits reuptake of serotonin and norepinephrine and was introduced into the US market in 1997. Henderson and colleagues (Sibutramine 2005a) conducted a 12-week trial of sibutramine addition (up to 15mg) in patients treated on a stable dose of olanzapine. In an unpublished study (Sibutramine 2005b), Weiden and colleagues conducted a 16-week trial of sibutramine (up to 15 mg) in outpatients maintained on a stable dose of an antipsychotic for at least three months. Patients were randomised to sibutramine treatment or placebo in a 2:1 ratio and all received dietary counselling weekly for four weeks and then every other week.
Ko and colleagues (Topiramate 2005), in a 12-week trial, added topiramate to the participants daily dose of atypical antipsychotics. Patients were randomised to receive topiramate, titrated to two dosage endpoints (100 mg or 200 mg) over 12 weeks, or placebo.
4.6.3 Standard Care
In all trials, standard care included treatment with antipsychotic medication. There was wide variation in the described nature of the standard care received by patients. All patients received nutritional counselling in three trials (Amantadine 2005; Phenylpropanol 2002; Sibutramine 2005b) or access to a healthy lifestyle education program and a three month membership to a gym or commercial weight-loss program in one (Amantadine 2005b). Standard care could also consist of no advice on weight reduction (Cog/Behav 2005a) or verbal recommendations regarding exercise and diet (Cog/Behav 2006b). In one study, all patients participated in weekly group support consisting of weight education and behaviour modification for the first 8 weeks of the trial (Sibutramine 2005a).
4.7 Outcomes
Much data were collected in studies and reported in ways that made them unusable for the purpose of this review. Much data were lost due to studies failing to report appropriate measures of central tendency and deviation. Several studies presented findings in graphs which prevented data extraction. No data were reported regarding cost. Most studies reported on some type of continuous data for weight assessment. Final total body weight (kg) was extracted in twelve trials (Cog/Behav 2003; Famotidine 2004; Fluoxetine 2002; Fluoxetine 2003; Fluvoxamine 2004; Metformin 2006; Nizatidine 2003a; Nizatidine 2003b; Nizatidine 2004; Phenylpropanol 2002; Reboxetine 2003; Sibutramine 2005a). Change in weight from baseline to endpoint was extracted in all trials except Phenylpropanol 2002. Final BMI was extracted in thirteen trials (Cog/Behav 2003; Cog/Behav 2005a; Cog/Behav 2005b; Cog/Behav 2006a; Cog/Behav 2006b; Famotidine 2004; Fluoxetine 2002; Fluvoxamine 2004; Metformin 2006; Nizatidine 2003b; Nizatidine 2004; Reboxetine 2003; Sibutramine 2005a). Change in BMI from baseline to endpoint was extracted in ten trials (Amantadine 2005b; Cog/Behav 2005a; Cog/Behav 2006a; Cog/Behav 2006b; Famotidine 2004; Nizatidine 2003b; Nizatidine 2004; Reboxetine 2003; Sibutramine 2005a; Sibutramine 2005b). Total waist circumference was reported in two trials at endpoint (Metformin 2006; Sibutramine 2005a). Two trials reported change in waist circumference from baseline to endpoint (Cog/Behav 2005b; Sibutramine 2005a). Two trials reported waist-to-hip ratio at endpoint (Cog/Behav 2006a; Sibutramine 2005a). Two trials reported change in waist-to-hip ration from baseline to endpoint (Cog/Behav 2006a; Sibutramine 2005a). Two trials reported total percent body fat at endpoint (Fluoxetine 2003; Sibutramine 2005a). No studies reported change in percent body fat.
In terms of binary data, trials reported the number of patients with >5% weight loss (Cog/Behav 2005a), or > 7% weight loss (Amantadine 2005) or > 10% weight loss (Cog/Behav 2006b). Alternatively, trials reported the number of patients gaining >7% of initial weight (Amantadine 2005; Cog/Behav 2005b; Famotidine 2004; Fluvoxamine 2004; Reboxetine 2003) or the number of patients with weight stabilization or weight loss (Amantadine 2005b; Cog/Behav 2005b).
4.8. Outcome scales: details of the only scales that provided usable data are shown below. Reasons for exclusions of data are given under ‘Outcomes’ in the ‘Included studies’ section
4.8.1 Global state
4.8.1.1 Clinical Global Impression Scale - CGI (Guy 1976)
Trialists used this to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven-point scoring system is usually used with low scores showing decreased severity and/or overall improvement. Five trials reported data using this instrument (Cog/Behav 2005a; Cog/Behav 2005b; Famotidine 2004; Fluvoxamine 2004; Reboxetine 2003).
4.8.1.2 Global Assessment of Functioning - GAF (Frances 1995)
The Global Assessment of Functioning, or GAF scale, is a numeric scale (0 through 100) used to rate the social, occupational and psychological functioning of adults and was reported in one study (Fluvoxamine 2004).
4.8.1.3 Global Assessment Scale - GAS (Endicott 1976)
This scale rates people from 0-100 on a continuum from psychological or psychiatric sickness to health (high = good). One trial reported data using this instrument (Sibutramine 2005a).
4.8.2 Satisfaction with care
4.8.2.1 Client Satisfaction Questionnaire - CSQ-8 (Attkisson 1982)
This is an eight-item self-rated questionnaire for patients to assess their satisfaction with a provided service. One study reported data using this scale (Cog/Behav 2005a).
4.8.3 Mental state
4.8.3.1 Brief Psychiatric Rating Scale - BPRS (Overall 1962)
This is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18-item scale is commonly used. Each item is defined on a seven-point scale varying from ‘not present’ to ‘extremely severe’, scoring from 0-6 or 1-7. Scores can range from 0-126, with high scores indicating more severe symptoms. Three trials reported data using this instrument (Amantadine 2005; Metformin 2006; Nizatidine 2003a).
4.8.3.2 Hamilton Rating Scale for Depression - HDRS (Hamilton 1960)
This instrument is designed to be used only on patients already diagnosed as suffering from affective disorder of depressive type. It is used for quantifying the results of an interview, and its value depends entirely on the skill of the interviewer in eliciting the necessary information. The scale contains 17 variables measured on either a five or a three-point rating scale, the latter being used where quantification of the variable is either difficult or impossible. Among the variables are: depressed mood, suicide, work and loss of interest, retardation, agitation, gastro-intestinal symptoms, general somatic symptoms, hypochondria, loss of insight, and loss of weight. It is useful to have two raters independently scoring a patient at the same interview. The scores of the patient are obtained by summing the scores of the two physicians. High scores indicate greater severity of depressive symptoms. Three trials reported data using this instrument (Fluoxetine 2002; Fluoxetine 2003; Reboxetine 2003).
4.8.3.3 Positive and Negative Syndrome Scale - PANSS (Kay 1986)
This schizophrenia scale has 30 items, each of which can be defined on a seven-point scoring system varying from 1 - absent to 7 - extreme. This scale can be divided into three sub-scales for measuring the severity of general psychopathology, positive symptoms (PANSS-P), and negative symptoms (PANSS-N). A lower score indicates lesser severity. Six trials reported data using this instrument (Cog/Behav 2005a; Fluoxetine 2003; Nizatidine 2003b; Nizatidine 2004; Phenylpropanol 2002; Sibutramine 2005a).
4.8.3.4 Scale for the Assessment of Negative Symptoms - SANS (Andreasen 1983)
This six-point scale gives a global rating of the following negative symptoms; alogia, affective blunting, avolition-apathy, anhedonia-asociality and attention impairment. Higher scores indicate more symptoms. Four studies reported data using this instrument (Famotidine 2004; Fluoxetine 2002; Reboxetine 2003; Sibutramine 2005a).
4.8.3.5 Scale for the Assessment of Positive Symptoms - SAPS (Andreasen 1984)
This scale measures the positive symptoms of psychosis and usable data was reported in three trials (Fluoxetine 2002; Fluoxetine 2003; Reboxetine 2003).
4.8.3.6 Montgomery-Asberg Depression Rating Scale - MADRS (Montgomery 1979)
A 65-item comprehensive psychopathology scale was used to identify the 17 most commonly occurring symptoms in primary depressive illness. Ratings are based on 10 items, with higher scores indicating more symptoms. This depression rating scale is designed to be sensitive to change. Maximum score is 30. One study reported data using this scale (Amantadine 2005).
4.8.4 Adverse effects scales
4.8.4.1 Barnes Akathisia Scale - BAS (Barnes 1989)
The scale comprises items rating the observed, restless movements that characterise akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from 0 - normal to 3 - severe. In addition, there is an item for rating global severity (from 0 - absent to 5 - severe). A low score indicates low levels of akathisia. Two studies reported data using this scale (Fluoxetine 2003; Reboxetine 2003).
4.8.4.2 Simpson Angus Scale - SAS (Simpson 1970)
This ten-item scale, with a scoring system of 0-4 for each item, measures drug-induced parkinsonism, a short-term drug-induced movement disorder. A low score indicates low levels of parkinsonism. Four studies reported data using this scale (Famotidine 2004; Fluoxetine 2003; Nizatidine 2003a; Reboxetine 2003).
4.8.4.3 Abnormal Involuntary Movements Scale - AIMS (Munetz 1988)
AIMS is a 12-item instrument assessing abnormal involuntary movements associated with antipsychotic drugs, such as tardive dystonia and chronic akathisia, as well as ‘spontaneous’ motor disturbance related to the illness itself. Scoring the AIMS consists of rating the severity of movement in three main anatomic areas (facial/oral, extremities, and trunk), based on a five-point scale (0=none, 4=severe). Two trials reported data using this scale (Fluoxetine 2003; Sibutramine 2005a).
4.8.4.4 Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale (Lingjaerde 1987)
The UKU rates four major topics: psychological side effects (10 items), neurological side effects (eight items), autonomic side effects (11 items) and other side effects (19 items). Each item is defined by means of a four-point scale where zero means not/doubtfully present. Scoring range is 0-144. One trial reported data using this scale (Fluvoxamine 2004).
4.8.4.5 Hillside Akathisia Scale (Fleischhacker 1989)
The Hillside Akathisia Scale (HAS) has two subjective and three objective items for which anchored rating points are provided. One trial reported data using this scale (Sibutramine 2005a).
4.8.5 Leaving the study early
The majority of trials reported number of people leaving the trial early. This excluded studies where there was no dropout reported (Cog/Behav 2003; Dextroamphetam 1965; Famotidine 2004; Fluvoxamine 2004). One trial reported this data in graph form (no extractable data) (Amantadine 2005) and another trial did not distinguish between treatment and placebo patients who withdrew from the study (Sibutramine 2005b).
4.8.6 Other outcome measures
4.8.6.1 Cardiovascular measures
Several trials reported blood pressure (Cog/Behav 2005a; Sibutramine 2005a), heart rate (Sibutramine 2005a) and pulse (Dextroamphetam 1965).
4.8.6.2 Laboratory measures
Metabolic-related laboratory measures were of particular interest. Extractable data regarding laboratory measures included abnormal values of high cholesterol, trigylcerides and glucose (Amantadine 2005); fasting serum glucose, total cholesterol and triglycerides (Fluvoxamine 2004); glucose, insulin, triglycerides and cholesterol (Metformin 2006), serum leptin (Nizatidine 2003b; Nizatidine 2004) and lipids, glucose, uric acid, cortisol and haemoglobin A1c(Sibutramine 2005a).
4.8.6.3 Compliance
Three cognitive/behavioural intervention trials reported data for compliance with the weight management programme (Cog/Behav 2003; Cog/Behav 2005a; Cog/Behav 2006b).
4.8.6.4 Sleep/appetite
Data were extracted regarding sleep and appetite outcomes in one trial (Dextroamphetam 1965).
4.8.7 Unusable data
Much data were collected in studies and reported in ways that made them unusable for the purpose of this review. Much data were lost due to studies failing to report appropriate measures of central tendency and deviation. Several studies presented findings in graphs which prevented data extraction.
4.8.8 Missing outcomes
We found no usable outcomes for specific quality of life measures. One study reported assessing this outcome but numerical data were not presented (Cog/Behav 2006b).
Risk of bias in included studies
1. Randomisation
All included studies were reported as randomised. However most of the included studies did not explicitly describe the methods used. Only four trials gave some indication of the randomisation process used. For example, a computer-generated randomisation code was used in two trials (Metformin 2006; Topiramate 2005) while two trials reported allocating according to entries of a table of random numbers (Dextroamphetam 1965; Reboxetine 2003). Concealment of allocation has repeatedly been shown to be of key importance in excluding selection biases (Jüni 2001). Given that at least one quality criterion was not met or unclear in all studies, we classified all studies as at least having a moderate risk of bias (category B, see methods section). Analyses based on methodological quality were not conducted.
2. Blindness
Five cognitive/behavioural studies were not double-blind due to the inherent difficulties involved in disguising psychosocial interventions. This may exaggerate estimates of treatment effect (Boutron 2004). All pharmacological interventions were double-blind except for two studies which were open (Fluvoxamine 2004; Topirimate 2006). None of the studies reported any test of blinding.
3. Loss to follow up
Four trials reported no loss (Cog/Behav 2003; Dextroamphetam 1965; Famotidine 2004; Fluvoxamine 2004). Eight trials reported using intention to treat analysis (ITT) (Amantadine 2005; Amantadine 2005b; Cog/Behav 2005a; Fluoxetine 2002; Fluoxetine 2003; Sibutramine 2005a; Sibutramine 2005a; Topirimate 2006) with two reporting last observation carried forwards (LOCF) analysis (Cog/Behav 2006b; Sibutramine 2005b) and four trials also conducting an OC analysis (observed cases, defined as those completing the trial) (Cog/Behav 2005a; Fluoxetine 2002; Fluoxetine 2003; Sibutramine 2005b). Of note, nine trials only analysed completers (Cog/Behav 2005b; Cog/Behav 2005b; Cog/Behav 2006a; D-Fenfluramine 1988; Metformin 2006; Reboxetine 2003; Nizatidine 2003b; Nizatidine 2004; Topiramate 2005).
Patient attrition was classified as too great for inclusion of other outcomes in this meta-analysis: Cog/Behav 2005a (30% dropout);Cog/Behav 2005b (33% dropout at end of trial; 63% at six months); D-Fenfluramine 1988 (52% dropout); Fluoxetine 2003 (35% dropout); Nizatidine 2003a (40% dropout); Sibutramine 2005b (48% dropout). Caution is clearly required in interpreting the findings reported in the two trials that did not use ITT or LOCF analyses (Cog/Behav 2005b; D-Fenfluramine 1988). At this stage, we have included this latter trial with marginally greater than 50% dropout but note that this trial is prone to multiple biases.
4. Data reporting
Data were often poorly reported and could not be used. Outcomes were often presented as graphs, in percentiles or just reported as inexact p-values which we could not include. Many studies failed to provide standard deviations when reporting mean changes. We are seeking further data from the first authors of relevant trials.
Effects of interventions
1. The search
The search strategy identified 57 abstracts for review. Of these 33 potentially relevant trials were selected. We were able to include 23 studies and six studies were excluded.
2. PREVENTION COMPARISON 1: COGNITIVE/BEHAVIOURAL INTERVENTION versus STANDARD CARE
Two trials were included in this comparison (Cog/Behav 2003; Cog/Behav 2005b) both of medium term duration.
2.1 Weight
2.1.1 Total body weight (kg)
For the total body weight at end of treatment (16 weeks) (n=70, WMD −5.17 CI −13.6 to 3.3) and final body weight at six month follow up (Cog/Behav 2003) we found no significant differences (n=70, WMD −3.69 kg CI −11.9 to 4.5).
2.1.2 Change in weight
In terms of change in weight, two trials (Cog/Behav 2003,Cog/Behav 2005b) favoured cognitive behavioural intervention at medium term (n=104, WMD −3.38 kg, CI −4.8 to −2.0) and end of treatment follow up ~ six months (n=89, 2 RCTs, WMD −4.87 kg CI −7.1 to −2.6) compared with standard care.
2.1.3 Total BMI
We found data from one study (Cog/Behav 2003) reporting final BMI did not reveal any significant difference at 16 weeks (n=70, WMD −2.27 CI −4.6 to 0.1) or during follow up at 24 weeks (n= 70, WMD −1.79 CI −4.0 to 0.5).
2.1.4 Change in BMI
Two studies reported outcomes for BMI change scores (Cog/Behav 2003, Cog/Behav 2005b) and we found the cognitive behavioural intervention group had small increases in BMI at medium term assessment (n=104, WMD −1.06 CI −1.5 to −0.6) and at up to six months follow-up (n=89, WMD −1.52 CI −2.3 to −0.8) compared with standard care.
2.1.5 Change in waist circumference
We found participants receiving cognitive behavioural intervention experienced smaller increases in waist circumference than standard care by six months follow up (Cog/Behav 2005b, n=34, WMD −5.50 cm CI −8.2 to −2.8).
2.1.6 Any change in weight (as defined by individual studies)
One trial reported the number of participants who had increased their initial body weight by 7% at endpoint 14 weeks. We found more participants in the standard care group to have gained weight (Cog/Behav 2005b, n=34, RR 0.20 CI 0.1 to 0.6, NNH 2 CI 2 to 5).
2.2 Global state
Based on the Clinical Global Impressions Scale, there were no significant differences between treatment and standard care groups in terms of subjective improvements in quality of life and overall health Cog/Behav 2005b), although body image did reach statistical significance (n=34, WMD 1.10 CI 0.3 to 1.9) in favour of standard care.
2.3 Leaving the study early
There were no dropouts in one trial (Cog/Behav 2003). In the remaining trial (Cog/Behav 2005b), we found significantly fewer participants left in the treatment group (n=51, RR 0.41 CI 0.2 to 1.0, NNT 4 CI 3 to 7), but by six months follow up no significant differences were found (n=34, RR 1.91 CI 0.7 to 5.4, NNT 5 CI).
2.4 Compliance
The authors report a compliance rate of 92% to the program sessions in one trial (Cog/Behav 2003). Compliance was not reported in the remaining trial (Cog/Behav 2005b).
3. PREVENTION COMPARISON 2: H2 ANTAGONISTS versus PLACEBO
Two trials were included in this comparison (Famotidine 2004 who reported short term data at six weeks, and Nizatidine 2003a who reported medium term data at 16 weeks).
3.1 Weight
3.1.1 Total body weight (kg)
For final body weight we found no significant differences at short term (n=14, RR 2.40 kg CI −6.1 to 10.9), or medium term assessment (n=113, WMD −2.40 CI −7.8 to 3.0) between H2 antagonists and placebo groups.
3.1.2 Change in weight
We also found no significant differences for mean change in weight (kg) at short term (n=14, WMD −0.10 CI −2.8 to 2.6), and medium term (n=113, WMD −0.89 CI −2.7 to 0.9) assessments between the H2 antagonist and placebo groups.
3.1.3 Total BMI
In the one trial reporting BMI (Famotidine 2004), we found no significant difference between groups by six weeks assessment (n= 14, WMD −0.90 CI −3.9 to 2.1).
3.1.4 Change in BMI
In the one trial reporting change in Body Mass Index (Famotidine 2004) we found no significant difference at end of treatment, six weeks (n=14, WMD −0.10 CI −0.7 to 0.5).
3.1.5 Any change in weight (as defined by individual studies)
We found no significant difference in the number of patients increasing their initial weight by 7% (Famotidine 2004, n=14, RR 1.33 CI 0.5 to 3.9) compared with placebo.
3.2 Global state
3.2.1 Clinical Global Impression
We found no significant differences in CGI change scores at 6 weeks between famotidine and placebo (Famotidine 2004, n=14, WMD 0.10 CI −0.7 to 0.9).
3.3 Mental state
3.3.1 Average BPRS change score
We found BPRS endpoint change scores at 16 weeks were equivocal (Nizatidine 2003a, n=113, WMD 2.45 CI −1.5 to 6.4) between treatment groups.
3.3.2 Average SAPS/SANS change score
We found no significant differences in SAPS (Famotidine 2004, n=14, WMD −0.10 CI −4.1 to 3.9), or SANS endpoint change scores at six weeks (Famotidine 2004, n=14, WMD −1.90 CI −5.0 to 1.2).
3.4 Adverse effects
3.4.1 Simpson and Angus Scale
We found short term SAS endpoint change scores favoured the H2 antagonist (famotidine) compared with placebo (n=14, WMD −2.00 CI −3.8 to −0.2). Medium term assessment at 16 weeks were equivocal (Nizatidine 2003a, n=113, WMD 0.39 CI −1.0 to 1.8).
3.4.2 Somnolence
We found no significant difference in daytime somnolence between treatment and control group by six weeks (n=14, RR 080 CI 0.4 to 1.8).
3.5 Leaving the study early
There were no dropouts in one trial (Famotidine 2004). In the remaining trial (Nizatidine 2003a), we found no significant difference in the number of participants leaving the study early (n= 118, RR 1.12 CI 0.7 to 1.7).
4. PREVENTION COMPARISON 3: H2 ANTAGONISTS -HIGHER DOSE versus H2 ANTAGONISTS - LOWER DOSE
One trial was included in this comparison (Nizatidine 2003a) reporting data at 16 weeks.
4.1 Weight
4.1.1 Total body weight (kg)
We found no significant difference at 16 weeks for final body weight (Nizatidine 2003a, n=112, WMD −4.58 kg CI −10.0 to 0.7).
4.1.2 Change in weight
There were no significant differences in terms of mean change in weight at 16 weeks assessment (n=112, WMD −0.27 CI −2.2 to 1.6) between groups allocated to high and low dosages of nizatidine.
4.2 Mental state - BPRS
There were no significant differences between high and low dosage nizatidine groups for BPRS change scores at 16 weeks (n=113, WMD 0.25 CI −3.8 to 4.3).
4.3 Adverse effects - Simpson and Angus Scale
We found no significant difference between the two groups in terms of SAS endpoint change scores (n=113, WMD 0.40 CI −1.0 to 1.8) at 16 weeks.
4.4 Leaving the study early
We found that participants allocated to either high or low dosages of nizatidine were left the study in similar numbers with no significant differences between groups (n=115, RR 1.12 CI 0.7 to 1.7).
5. PREVENTION COMPARISON 4: 5HT REUPTAKE BLOCKER versus PLACEBO
We were able to include one short term trial (eight weeks) in this comparison (Fluoxetine 2002).
5.1 Weight
5.1.1 Total body weight (kg)
For final body weight, we found no significant difference at end of treatment (n=24, WMD 6.40 kg CI −7.6 to 20.4).
5.1.2 Change in weight
We found no significant difference in terms of mean change in weight by eight weeks evaluation (n=30, WMD 0.20 kg CI −3.4 to 3.8).
5.1.3 Total BMI
There were no significant differences in terms of final Body Mass Index at 8 weeks assessment (n= 24, WMD 1.10 CI −2.4 to 4.6).
5.2 Mental state SANS/SAPS and HAM-D
We found no significant differences in SANS (n=24, WMD 1.24 CI −2.5 to 5.0) or SAPS (n=24, WMD 1.09 CI −0.4 to 2.5) change scores between 5HT reuptake blocker and placebo. Hamilton and Montgomery Depression Scale endpoint change scores were also equivocal at eight weeks (n=24, WMD 1.66 CI −0.8 to 4.1).
5.3 Adverse effects
There were no significant differences between the treatment and placebo groups in terms of ‘any’ reported adverse effects.
5.4 Leaving the study early
The number of participants leaving the study early by eight weeks were not significantly different (n=30, RR 2.00 CI 0.4 to 9.3).
6. PREVENTION COMPARISON 5: SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
One trial was included in this comparison (Reboxetine 2003) reported short term data by six weeks.
6.1 Weight
6.1.1 Total body weight (kg)
We found no significant differences in total body weight by six week assessment (n=20, WMD 3.00 kg CI −9.6 to 15.6).
6.1.2 Change in weight
Mean weight change favoured the treatment group (n=20, WMD −3.00 kg CI −5.6 to −0.5) compared with placebo.
6.1.3 Total BMI
Body Mass Index data were equivocal by six weeks (n=20, WMD 1.47 CI −1.3 to 4.3).
6.1.4 Change in BMI
Body Mass Index change scores by six weeks favoured the treatment group (n=20, WMD −0.98 CI −1.8 to −0.2) compared with the control group.
6.1.5 Any change in weight (as defined by individual studies)
The number of participants who had gained at least 7% of their initial weight did not reveal any significant differences between groups (n=20, RR 0.29 CI 0.1 to 1.1).
6.2 Global state
6.2.1 Clinical Global Impression
We found CGI (psychosis) scores were not significantly different between treatment groups by six week assessment (n=20, WMD 0.10 CI −1.0 to 1.2).
6.3 Mental state
6.3.1 SAPS/SANS and HAM-D
We found no significant differences for positive symptom scores (SAPS), or negative symptoms scores (SANS) by six weeks evaluation. Also, depression data (HAM-D) did not reveal any significant differences between treatment groups (n=20, WMD −1.00 CI −3.4 to 1.4).
6.4 Leaving the study early
Equal numbers of patients left the study early by six weeks (3 from each group, n=20, RR 1.00 CI 0.3 to 4.1).
7. PREVENTION COMPARISON 6: ANTIDIABETIC AGENTS versus PLACEBO
We were able to include one trial in this comparison (Metformin 2006) with outcomes reported at 14 weeks.
7.1 Weight
7.1.1 Total body weight (kg)
We found no significant difference between participants receiving antidiabetic agents and the placebo group after 14 weeks of treatment (n=37, WMD −1.80 kg CI −7.8 to 4.2).
7.1.2 Change in weight
No significant differences were found in terms of mean weight change at the end of treatment (n=37, WMD −0.80 kg CI −2.6 to 1.0).
7.1.3 Total BMI
Body Mass Index data did not reveal any significant difference between antidiabetic agents and the placebo group after 14 weeks of treatment (n=37, WMD −0.20 CI −2.4 to 2.0).
7.1.4 Waist circumference
No significant differences were found between groups in terms of final waist circumference (n=37, WMD 3.40 cm CI −2.0 to 8.8).
7.2 Mental state - BPRS
We found data at 14 weeks to be non-significant in terms of final BPRS change scores (n=37, WMD −1.80 CI −6.5 to 2.9) between antidiabetic agents and the placebo group.
7.3 Laboratory data
We found no significant differences between groups in terms of any of the final laboratory measurements (glucose, basal and post-load, total cholesterol, LDL cholesterol, HDL cholesterol. Other physiological measurements (basal insulin, triglycerides, Homa-IR and VLDL cholesterol) were also equivocal, containing wide confidence intervals (skewed data) and are reported in other data tables.
7.4 Leaving the study early
The number of people leaving early in the antidiabetic agents and placebo group by 14 weeks were not significantly different (Metformin 2006, n=40, RR 0.50 CI 0.1 to 5.1).
8. PREVENTION COMPARISON 7: ANTICONVULSANT AGENT versus CONTROL
We were able to include one trial (Topirimate 2006) in this medium term comparison of 12 weeks.
8.1 Weight
8.1.1 Change in weight
We found participants in the anticonvulsant group had significantly smaller increases in weight than the control group (n=60, WMD −1.36 kg CI −2.5 to −0.3).
8.2 Adverse effects
No significant differences were found between groups for the outcome insomnia by 12 weeks assessment (n=48, RR 0.69 CI 0.2 to 2.8).
8.3 Leaving the study early
The number of participants who left the study by 12 weeks did not reveal any significant differences (n=60, RR 0.71 CI 0.3 to 2.0).
9. PREVENTION COMPARISON 8: SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
We were able to include one trial (Fluvoxamine 2004) in this medium term comparison of 12 weeks.
9.1 Weight
9.1.1 Total body weight
We found no significant difference in final body weight between treatment and control groups at the end of 12 weeks of treatment (n=68, WMD 0.30 CI −5.8 to 6.4).
9.1.2 Total BMI
Body Mass Index data were not significantly different between groups by 12 weeks assessment (n=68, WMD −0.30 CI −2.1 to 1.5).
9.1.3 Any change in weight (as defined by individual studies)
Significantly fewer patients in the SSRI’s plus low dose clozapine increased their initial weight by 7% (n=68, RR 0.27 CI 0.1 to 0.9, NNT 5 CI 4 to 29) compared with the high dose clozapine group.
9.2 Global state - GAF
Global Assessment of Functioning (n=68, WMD 0.70 CI −0.9 to 2.3) and Clinical Global Impression scores (n=68, WMD 0.06 CI −0.1 to 0.2) were not significantly different between groups by 12 weeks assessment.
9.3 Adverse effects
Several adverse events outcomes were recorded (see comparison tables) but all data were equivocal.
9.4 Laboratory data
We found the physiological measures serum glucose (n=68, WMD −3.30 CI −6.3 to −0.3) and triglycerides (n=68, WMD −22.7 CI −44.6 to −0.8) were significantly lower in the SSRI’s plus low dose clozapine compared with high dose clozapine by 12 weeks assessment. Total cholesterol did not reveal any significant differences between groups.
10. TREATMENT COMPARISON 1: COGNITIVE/BEHAVIOURAL INTERVENTION versus STANDARD CARE
Three trials were included in this comparison (Cog/Behav 2005a; Cog/Behav 2006a; Cog/Behav 2006b) all medium term studies between 12 and 16 weeks duration.
10.1 Weight
10.1.1 Change in weight
We found change in weight to be significantly more reduced in the treatment group (n=129, 3 RCTs, WMD −1.69 kg CI −2.8 to −0.6) compared with standard care.
10.1.2 Change in BMI
Similarly, we found the treatment group had a greater reduction in BMI (n=129, 3 RCTs, WMD −0.66 CI −1.1 to −0.3) compared with the standard care group.
10.1.3 Change in waist-to-hip circumference ratio
One trial reported mean change in waist-hip circumference ratio (Cog/Behav 2006a) and we found no significant difference at the end of treatment (n=15, WMD −0.02 CI −0.1 to 0.02).
10.1.4 Any weight change
One trial reported the number of participants with >10% weight loss at end of treatment (Cog/Behav 2006b). We found significantly more participants in the treatment group achieved this amount of weight loss (n=43, RR 0.78 CI 0.6 to 1.0, NNT about 5 CI 3 to 36). Another trial reported number of participants with >5% weight loss and we found no significant difference between groups (n=72, RR 0.83 CI 0.7 to 1.0).
10.2 Satisfaction (Satisfaction with service)
One trial assessed patient satisfaction (CSQ-8) related to the treatment (Cog/Behav 2005a) and we found the treatment group were overall more satisfied with their treatment (n=71, WMD 2.30 CI 0.9 to 3.7) than the standard care group.
10.3 Mental state - PANSS
We found PANSS total endpoint scores were not significantly different (Cog/Behav 2005a, n=71, WMD 3.00 CI −6.2 to 12.2) between cognitive behavioural intervention and standard care
10.4 Global state - CGI
We found Clinical Global Impression scores ‘not improved’ were equivocal (Cog/Behav 2005a, n=72, RR 0.86 CI 0.6 to 1.2).
10.5 Physiological
Sitting and standing systolic blood pressure were reported by Cog/Behav 2005a and we found data were equivocal.
10.6 Laboratory data
Increases in the ratio of LDL to HDL cholesterol (Cog/Behav 2006b) were equivocal.
10.7 Leaving the study early
The number of participants leaving the study early did not reveal any significant differences between cognitive behavioural interventions and standard care (n=137, 3 RCTs, RR 0.13 CI −0.01 to 0.3) during medium term assessment.
11. TREATMENT COMPARISON 2: ANTI OBESITY AGENTS +/− LIFESTYLE MANAGEMENT versus PLACEBO +/− LIFESTYLE MANAGEMENT
Two medium term trials were included in this comparison (Sibutramine 2005a; Sibutramine 2005b).
11.1 Weight
11.1.1 Total body weight
We found data favoured the treatment group who had a significantly lower mean weight (Sibutramine 2005a, n=37, WMD −16.96 CI −27.0 to −6.9) compared with participants given placebo and lifestyle management.
11.1.2 Change in weight
We found change in weight favoured the treatment group, but data are heterogeneous and we analysed the trials separately. We found data from Sibutramine 2005a favoured the treatment group who lost significantly more weight (n=37, WMD −4.58, CI −5.2 to −4.0). We found no significant difference in weight change between the treatment and placebo groups in the Sibutramine 2005b study.
11.1.3 Total BMI
We found Body Mass Index to be significantly lower in the treatment group (Sibutramine 2005a, n=37, WMD −9.00 CI −13.5 to −4.5) compared with the placebo group.
11.1.3 Change in BMI
No significant differences were found in BMI change scores at end of treatment (n=41, 2 RCTs, WMD −0.17 CI −1.3 to 1.0)
11.1.4 Waist circumference
Waist circumference measurements were significantly lower in the treatment group (Sibutramine 2005a, n=37, WMD −11.50 CI −17.8 to −5.2) compared with placebo.
11.1.5 Change in waist circumference
We found change scores in waist circumference were not significantly different between groups (Sibutramine 2005a, n=22, WMD −7.60 CI −18.9 to 3.7).
11.1.6 Waist-hip ratio
There were no significant differences in final waist-hip ratio between groups (Sibutramine 2005a, n=37, WMD 0.04 CI −0.02 to 0.10) compared with placebo.
11.1.7 Change in waist-hip ratio
We found change in waist-hip ratio at during medium term assessment (Sibutramine 2005a) were almost statistically significant in favour of the treatment group (n=22, WMD 0.13 CI 0.00 to 0.3, p=0.05).
11.1.8 Hip circumference
We found the treatment group to have a significantly lower hip circumference than the placebo group (Sibutramine 2005a, n=37, WMD −8.60 CI −15.2 to −2.0) by three months assessment.
11.1.9 Body fat (%)
At the end of treatment, we found no significant differences between groups in terms of body fat (%) (Sibutramine 2005a, n=37, WMD 0.10 CI −3.4 to 3.6).
11.2 Global state - GAS
We found data from the Global Assessment Scale were equivocal (Sibutramine 2005a, n=37, RR-1.50 CI −6.2 to 3.2)
11.3 Mental state - PANSS/SANS
The PANSS total endpoint scores at 12 weeks assessment were equivocal (Sibutramine 2005a, n=37, RR 1.10 CI −4.5 to 6.7). Similarly, we found negative symptom scores (SANS) (Sibutramine 2005a) were not significantly different.
11.4 Extrapyramidal symptoms
The Hillside Akathisia Scale and the Abnormal Involuntary Move ment Scale were used by Sibutramine 2005a to measure extrapyramidal symptoms but data are skewed.
11.5 Adverse effects
A greater number of adverse events were reported by patients taking sibutramine (Sibutramine 2005a) (see analyses figures) but these differences were not significant. In terms of satiety, sibutramine patients were not more likely to report excessive appetite (n=36, RR 0.45 CI 0.1 to 1.5) or excessive thirst (n=36, RR 2.24 CI 0.5 to 10.1). Regarding adverse effects related to sleep, no significant differences emerged in terms of difficulty falling asleep (n=36, RR 1.25 CI 0.5 to 3.2) or interrupted sleep (n=36, RR 4.47 CI 0.6 to 34.6). Similarly reports of blurred vision (n=36, RR 2.68 CI 0.3 to 23.4), constipation (n=36, RR 2.24 CI 0.5 to 10.1), dry mouth (n=36, RR 3.58 CI 0.9 to 14.6) and rapid heart rate (n=36, RR 1.79 CI 0.2 to 18.0) were non-significant.
11.6 Cardiovascular measures
We found systolic (n=37, WMD 9.90 mm Hg, CI 1.2 to 18.6) and diastolic (n=37, WMD 6.10 mm Hg CI 0.1 to 12.1) blood pressure favoured the control group compared with anti-obesity drugs (Sibutramine 2005a). No significant differences were found in heart rate (n=37, WMD 2.90 bpm CI −4.8 to 10.6).
11.7 Laboratory data
There were few clinically significant changes in haematological and biochemical laboratory measures from one trial (Sibutramine 2005a - see comparison tables). At end of treatment, there were significant differences between sibutramine and placebo groups in terms of lipids - LDL (n=37, WMD −33.80 CI −60.4 to −7.2) and a significant decrease in haemoglobin (n=37, WMD −0.70 CI −1.3 to −0.1) suggesting an improvement in glucose metabolism.
11.8 Leaving the study early
Dropout rates in one trial (Sibutramine 2005a) in the treatment and placebo groups were similar at the end of treatment (n=37, RR 0.83 CI 0.4 to 1.8) and again were non-significant by six months follow up (n=37, RR 0.95 CI 0.2 to 4.1) in one trial (Sibutramine 2005a).
12. TREATMENT COMPARISON 3: H2 ANTAGONISTS versus PLACEBO
There were two trials included in this comparison (Nizatidine 2003b; Nizatidine 2004) both reporting short term data by eight weeks assessment.
12.1 Weight
12.1.1 Total body weight (kg)
For final body weight, we found a significant difference favouring the treatment group at the end of eight weeks treatment (n=59, 2 RCTs, WMD −5.30 kg CI −8.4 to −2.20).
12.1.2 Change in weight
Due to heterogeneity (I2 = 97.7%), results from the two trials were not summated for changes in weight. Heterogeneity may result from using different antipsychotics - olanzapine (Nizatidine 2003b) and quetiapine (Nizatidine 2004). Both trials did demonstrate a significant reduction in weight for the treatment groups (Nizatidine 2003b, n=34, WMD −6.80 kg CI −7.9 to −5.7;Nizatidine 2004, n=25, WMD −2.20 kg CI −3.0 to −1.5).
12.1.3 Total BMI
We found Body Mass Index favoured the H2 antagonists (n=59, 2 RCTs, WMD −1.51 CI −2.5 to −0.6) compared with placebo at eight weeks assessment.
12.1.4 Change in BMI
Due to heterogeneity (I2 = 96%), results from the two trials were not summated for changes in BMI. Individually, each trail demonstrated a significant reduction in BMI in the treatment groups (Nizatidine 2003b: n=34, WMD −2.90 CI −3.5 to −2.3; Nizatidine 2004, n=25, WMD −1.10 CI −1.4 to −0.8).
12.2 Mental state - PANSS
There were no significant differences between groups on the PANSS scale at the end of treatment (n=59, 2 RCTs, WMD −2.03 CI −4.1 to 0.01).
12.3 Adverse effects
No adverse effects were reported in one trial (Nizatidine 2004). We found reports of ‘any adverse effects’ were equivocal between H2 antagonists and placebo (Nizatidine 2003b, n=34, RR 1.33 CI 0.4 to 5.1).
12.4 Laboratory data
Both trials examined whether leptin levels were associated with reductions in weight. At the end of treatment, we found final leptin levels to favour the treatment group (n=59, 2 RCTs, WMD −3.83 ng/ml CI −5.5 to −2.2). Changes in serum leptin levels were reported in Nizatidine 2004 and we found data favoured the H2 antagonist nizatidine (n=23, WMD −1.60 ng/ml CI −2.2 to −1.0) compared with placebo.
5 Leaving the study early
Dropout rates were similar in both trials with no significant differences between groups (n=63, 2 RCTs RR 0.98 CI 0.2 to 5.3).
13. TREATMENT COMPARISON 4: APPETITE SUPPRESSANT versus PLACEBO
Three trials were included in this comparison (D-Fenfluramine 1988; Dextroamphetam 1965; Phenylpropanol 2002) although variations in reported data restrict substantive comparisons.Dextroamphetam 1965 reported short term data by 8 weeks, andD-Fenfluramine 1988 and Phenylpropanol 2002 were medium term, three months.
13.1 Weight
13.1.1 Total body weight (kg)
We found data favoured the placebo group (Phenylpropanol 2002, n=16, WMD 4.99 kg CI 2.0 to 8.0), with total body weight by 3 months being lower compared with appetite suppressants.
13.1.2 Change in weight
Weight change from short term assessment data (8 weeks) were equivocal (Dextroamphetam 1965, n=20, WMD 0.82 CI −2.5 to 4.1). We found medium term data (D-Fenfluramine 1988) favoured the treatment group in terms of mean weight loss (n=16, WMD −2.60 CI −5.1 to −0.1). In one of these trials (D-Fenfluramine 1988), the dropout rate was high with 44% in the D-Fenfluramine group and 46% in the placebo group not completing the study. Analyses included completers only.
13.2 Mental state - PANSS
We found no significant differences between groups on the PANSS scale at three months assessment (Phenylpropanol 2002, n=16, WMD −4.80 CI −11.6 to 2.0).
13.3 Adverse effects
In one trial (D-Fenfluramine 1988) no significant differences were found for the outcomes, lethargy/fatigue (n=16, RR 4.00, CI 0.2 to 72.0), diarrhoea (n=16, RR 1.56 CI 0.2 to 13.9), sleepiness (n= 16, RR 0.78 CI 0.1 to 10.4) and agitation (n=16, RR 0.78, CI 0.1 to 10.4). We found no significant differences between groups in terms of sleep related adverse effects (Dextroamphetam 1965) by short term assessment.
13.4 Other outcomes
We found no difference between groups in terms of appetite disruption (Dextroamphetam 1965, n=20, RR 2.25 CI 1.0 to 4.9). In this trial, no significant differenced were found between the two groups in terms of pulse rates.
13.5 Leaving the study early
There were no withdrawals in one trial (Dextroamphetam 1965). In the remaining trials, there were no significant differences between groups (n=49, 2 RCTs, RR 0.96 CI 0.5 to 2.0) during three months of evaluation.
14. TREATMENT COMPARISON 5: 5HT REUPTAKE BLOCKER versus PLACEBO
We were able to include one trial (Fluoxetine 2003) in this comparison which reported medium term data at 4 months.
14.1 Weight
14.1.1 Total body weight (kg)
We found no significant difference between groups in terms of final body weight (n=30, WMD 4.70 kg CI −6.7 to 16.1).
14.1.2 Body fat (%)
There were no significant differences between groups in terms of percentage body fat (n=30, WMD 0.30 CI −7.3 to 7.9).
14.2 Mental state - PANSS, HAM-D
There were no significant differences between groups on any of the mental state measures (PANSS +ve/-ve, and HAM-D).
14.3 Extrapyramidal symptoms
There were no significant differences between groups at the end of treatment as measured by the AIMS or BAS scales. Simpson and Angus Scale data almost reached statistical significance (n= 30, WMD 2.40 CI −0.02 to 4.8, p=0.05).
14.4 Leaving the study early
We found no significant differences between groups (n=30, RR 0.67 CI 0.2 to 1.9) at endpoint - four months.
15. TREATMENT COMPARISON 6: ANTIPARKINSONIAN DRUG versus PLACEBO
We were able to include two trials in this comparison (Amantadine 2005; Amantadine 2005b) both of medium term duration.
15.1 Weight
15.1.1 Change in weight
We found the antiparkinsonian group had significantly more weight loss (n=89, 2 RCTs, WMD −2.30 kg CI −4.2 to −0.4) compared with the placebo group.
15.1.2 Change in BMI
In the one trial reporting change in BMI, we found data favoured the treatment group (Amantadine 2005b, n=21, WMD −1.31 CI −2.6 to −0.1).
15.1.3 No gain in weight
One trial reported the number of patients not gaining weight and we found no significant differences (Amantadine 2005b, n=21, RR 3.00 CI 0.8 to 10.9).
15.1.4 Increase in initial weight
Amantadine 2005 reported the number of patients increasing their initial weight by 7% and also decreasing their initial weight by 7%. We found no significant difference for both outcomes.
15.2 Mental state - BPRS, MADRS
We found no significant differences between groups on the BPRS or MADRS at the end of treatment.
15.3 Adverse effects
We found reports of ‘worsening of schizophrenia’ to be equivocal (n=89, 2 RCTs, RR 2.04 0.3 to 13.2). Lipid abnormalities (elevated glucose, triglycerides and cholesterol) were not significantly different. We found insomnia to be more frequent in the treatment group compared with placebo (Amantadine 2005, n= 125, RR 3.52 CI 1.2 to 10.2). Upper abdominal pain (n=125, RR 14.07 CI 0.8 to 244.5) and fatigue (n=125, RR 0.08 CI 0.0 to 1.5) were not significantly different between groups (Amantadine
2005).
15.4 Leaving the study early
We found the number of participants leaving the study early were similar, with no statistical significance differences between groups (n=146, 2 RCTs RR 1.80 CI 0.7 to 4.5).
16. TREATMENT COMPARISON 7: ANTICONVULSANTS versus PLACEBO
We were able to include one trial in this comparison (Topiramate 2005) comparing 100 mg/day topiramate with placebo over 3 months. Most data were reported in graphical form and were not extractable.
16.1 Change in weight
No significant differences were found for change in weight between anticonvulsants and placebo by three months (Topiramate 2005, n=36, WMD −1.38 kg CI −4.2 to 1.5).
16.2. Adverse events
There were no significant differences between groups in terms of frequency of adverse events such as paraesthesia (Topiramate 2005, n=36, RR 2.50 CI 0.5 to 12.0), fatigue (n=36, RR 2.50 CI 0.3 to 25.2), headaches (n=36, RR 3.75 CI 0.4 to 32.7), or anorexia (n=31, RR 2.50 CI 0.3 to 25.2).
16.3 Leaving the study early
The number of people leaving the study were not significantly different between groups (Topiramate 2005, n=44, RR 3.00 0.7 to 13.3) by three months.
17. TREATMENT COMPARISON 8: ANTICONVULSANTS HIGHER DOSE versus ANTICONVULSANTS LOWER DOSE
We were able to include one trial in this comparison (Topiramate 2005) comparing 200 mg/day topiramate with 100 mg/day topiramate at three months. Most data were reported in graphical form and was not extractable.
17.1 Change in weight
We found a significant difference favouring higher dose anticonvulsants compared with the lower dosage group (Topiramate 2005, n=33, WMD −3.67 kg CI −7.0 to −0.4). Overall, there was greater weight loss in the 200mg/day topiramate group (5.35 kg), compared with the 100mg/day group (1.68 kg), and with placebo (0.3 kg - see treatment comparison 7).
17.2 Adverse events
There were no significant differences between groups in terms of frequency of adverse events such as paraesthesia (Topiramate 2005) (n=33, RR 2.35 CI 0.9 to 6.0), diarrhoea (n=33, RR 2.82 CI 0.3 to 24.4) or anorexia (n=33, RR 1.88 CI 0.4 to 8.9). There were no reports of serious side effects such as metabolic acidosis, narrow angle glaucoma or nephrolithiasis.
17.3 Leaving the study early
We found no significant difference in attrition rates between groups (Topiramate 2005, n=44, RR 0.83 CI 0.3 to 2.3).
18. PREVENTION (PHARMACOLOGICAL) MEAN WEIGHT CHANGE - TREATMENT versus CONTROL
18.1 Weight change at end of treatment
We found the treatment group had significantly less gain in weight than the control group (n=274, 6 RCTs, WMD −1.16 CI −1.9 to −0.4).
19. PREVENTION (NON-PHARMACOLOGICAL) MEAN WEIGHT CHANGE - TREATMENT versus CONTROL
19.1 Weight change at end of treatment
We found the treatment group had significantly less gain in weight than the control group (n=104, 2 RCTs, WMD −3.38 CI −4.8 to −2.0).
20. SYNTHESIZING PREVENTION COMPARISONS (ALL POOLED PHARMACOLOGICAL TRIALS) MEAN WEIGHT CHANGE - TREATMENT versus CONTROL
20.1 Weight change at end of treatment
We found the treatment group had significantly less gain in weight than the control group (n=129, 3 RCTs, WMD −1.69 CI −2.8 to −0.6).
21. SYNTHESIZING TREATMENT COMPARISONS (ALL POOLED PHARMACOLOGICAL TRIALS) MEAN WEIGHT CHANGE - TREATMENT versus CONTROL
21.1 Weight change at end of treatment
We found the treatment group had significantly less gain in weight than the control group but data contained significant heterogeneity (I2 =88%).
DISCUSSION
1 Applicability of results
The included studies involved people with schizophrenia from a wide range of settings including both inpatient and outpatients from the USA, Europe, Asia and Australia. Overall, both pharmacological and non pharmacological interventions showed modest efficacy in reducing weight gain confirming that weight loss is possible in people with schizophrenia (Faulkner 2003). However, there were marked heterogeneity across the studies, which limits reliability in drawing substantive conclusions.
Interpretation is limited by a number of factors including: The small number of studies; for many pharmacological comparisons there was only a single study. There was variability in the nature and intensity (dosage) of the interventions. There was variability in study duration and follow-up. There was variability in study methodology. Most studies did not describe adequate randomisation and blinding procedures, and not all studies included an intention-to-treat analysis. While some studies reported metabolic endpoints apart from weight change (such as leptin, fasting glucose and fasting lipids), there was not enough consistency to allow for comparison between studies. Side effects and tolerability were not evaluated systematically by using structured scales. In addition, while clinical guidelines suggest that weight loss medications should only be used in combination with lifestyle counselling, the majority of pharmacological studies did not include, or did not describe, such a component. As with the general obesity literature, RCTs of pharmacological therapy rarely incorporate intensive exercise and behaviour therapy programmes (Poston 2001). Finally, there was an absence of long-term studies that would allow for evaluation of longer-term effectiveness and safety of the interventions. Given the rapid increase in publication of studies examining interventions for weight gain in this population since 2002, we expect that future studies will address these weaknesses and provide greater insight into what works best and for whom.
2. General
2.1 Pharmacological interventions
There was considerable variability in weight loss efficacy between pharmacological agents, as well as between studies of the same agent. At the present time, no single agent emerges as consistently superior in terms of weight loss efficacy. With the use of adjunctive pharmacological agents, there is always a concern regarding exacerbation of psychiatric symptomatology and adverse physical adverse effects. Generally, pharmacological agents were described as well tolerated. Clearly, the safety and tolerability of adjunctive pharmacological treatments need to be evaluated along with the effectiveness of these agents in moderating weight gain. Of the weight loss drugs currently in development, rimonabant, a selective cannabinoid receptor 1 antagonist, submitted to the FDA for approval, may have special applicability in the psychiatric population and deserves future research attention (Faulkner 2006). Given that antipsychotic drugs most implicated in weight gain have in common antagonism at the H1 receptor, H1 agonists such as betahistine should be explored as potential pharmacological interventions for weight gain.
2.2 Non pharmacological Interventions
In terms of non pharmacological intervention, the five studies reported here suggest that such interventions are at least possible in this population with acceptable compliance although conclusions regarding the intensity of the intervention cannot be made at this stage. There is a need for further randomised controlled trials with long term follow-up to determine how best to package behavioural interventions, identify patient characteristics associated with adherence and successful outcome, and to examine how sustainable any outcomes are. Such interventions will probably need to set realistic goals, be highly structured, provide intensive support initially, and offer reduced but continued support over time, if not indefinitely. Adopting a stepwise approach of incremental weight management interventions implemented as early as possible and maintained throughout treatment, could be considered (Chue 2004). Intervention components should probably focus on reducing caloric intake (changing types of food consumed and reducing portion sizes) rather than complex dietary change (Cog/Behav 2005a), and support people in gradually increasing their levels of physical activity to 30 - 60 minutes of moderate physical activity most days of the week (Faulkner 2003). Cognitive-behavioural strategies to facilitate these changes that are tailored to this population need to be developed and evaluated. Finally, further research will need to examine whether the costs of providing such intensive programs are less than the cost associated with not intervening at all (see Loh 2006). While not assessed within the included studies, we also suggest that future interventions should also include broader environmental manipulations to increase habitual levels of physical activity (for example, greater use of stairs instead of lifts or elevators; incorporating short bouts of physical activity during the day) and to decrease the amount of time spent sitting or engaged in sedentary behaviours such as television viewing, and to assist and reinforce small dietary changes (removal of ‘junk food’ vending machines; access to fruit and vegetables). Despite the low number of studies reported, the role of physical inactivity and poor diet as independent risk factors for cardiovascular disease infers the need for non pharmacological or lifestyle intervention regardless of weight loss per se.
Given the modest weight loss reported in both pharmacological and non pharmacological studies, the first strategy in preventing or alleviating weight gain remains identifying an appropriate choice of antipsychotic which has a lower liability of weight gain. Switching patients to an antipsychotic with a lower liability of weight gain might also be considered. However, a recent review suggests that available evidence does not yet support the clinical superiority of switching antipsychotics on various outcome measures such as weight gain (Remington 2005). In addition, reviewing concomitant medications with additional weight gain liability (e.g. antidepressants and mood stabilizers) should also be considered.
3. PREVENTION COMPARISON 1: COGNITIVE/BEHAVIOURAL INTERVENTION versus STANDARD CARE
3.1 Weight
Differences between groups for final total body weight were not significant, although when evaluated by measuring change scores, the cognitive behavioural intervention (CBI) group did achieve a significant improvement. Body Mass Index total scores were not significant, but again when assessed in terms of change from baseline the CBI group showed a significant advantage compared with standard care. Similarly, change scores derived from waistline measurements favoured the CBI group. More participants in the standard care group gained at least 7% of their initial weight than the CBI group. Overall, most available data, which was limited to small studies, did indicate that the intervention group gained less weight, but more robust studies of longer duration are needed to determine whether these differences are reliable and sustained.
3.2 Global state
Clinical Global Impression scores (quality of life, and overall improvement) were equivocal. Body image did favour CBI, although the data appear to be derived from another scale based around the CGI scale and no conclusion can be made.
3.3 Leaving the study early
One small study (Cog/Behav 2003) managed to retain all its participants. Whilst (Cog/Behav 2005b) initially indicating better retention in the CBI group but this was not sustained during further follow up at six months after the study began.
4. PREVENTION COMPARISON 2: H2 ANTAGONISTS versus PLACEBO
4.1 Weight
All outcomes reported for weight gain, total scores, mean change in weight, body mass index or predefined cut off points (7%) did not reveal any significant differences between H2 antagonists and placebo. Only 14 participants were included in one (Famotidine 2004) study. Studies of this size are unlikely to detect small treatment effects, and much larger studies of longer duration are needed before we can determine whether these interventions are of value in the prevention of weight gain.
4.2 Global state
From limited data (n=14) we did not find any differences in Clinical Global Impression Scores from this short term study byFamotidine 2004.
4.3 Mental state
We found all mental state outcomes, positive and negative symptoms scores and BPRS total scores, were equivocal.
4.5 Adverse effects
From limited data, adjunctive H2 antagonist treatment did not appear to worsen or improve movement disorders in either short (Famotidine 2004) or medium term (Nizatidine 2003a) studies.
4.6. Leaving the study early
The adjunctive H2 antagonist treatment did not improve or reduce attrition relative to the control group in either short or medium term studies.
5. PREVENTION COMPARISON 3: H2 ANTAGONISTS -HIGHER DOSE versus H2 ANTAGONISTS - LOWER DOSE
5.1 All outcomes
All outcome measures (weight, mental state, adverse effects, leaving the study early) did not reveal any significant differences between high and lower dose H2 antagonists.
6. PREVENTION COMPARISON 4: 5HT REUPTAKE BLOCKER versus PLACEBO
6.1 Weight
We did not find any significant outcomes to suggest that 5HT reuptake blockers prevent or reduce weight gain. All data came from one study Fluoxetine 2002, and with just 30 participants detecting treatment effects was improbable.
6.2 Mental state
None of the outcomes measuring positive and negative symptoms domains and also depression were significant. Larger studies are needed to determine whether such effects exist.
6.3 Adverse effects
Only one outcome was reported for side effects categorised as ‘any adverse effects’ and we did not find 5HT reuptake blockers more likely to produce adverse effects compared with the control group.
6.4 Leaving the study early
Adjunctive 5HT reuptake blockers did not improve or reduce attrition rates relative to the control group in this short term study.
7. PREVENTION COMPARISON 5: SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
7.1 Weight
Outcomes measures were not consistent in the Reboxetine 2003 study. No differences were found in total body weight, but mean weight change did favour the selective norepinephrine reuptake inhibitor group. Similarly, total body mass index data were equivocal, but mean change in body mass index favoured the treatment group. Weight gain predefined at 7% or more gain in initial body weight failed to show any differences between groups. With just 20 participants included this study is underpowered and more data are needed to determine whether a genuine treatment effect is present.
7.2 Global state
We did not find Clinical Global Impression scores were worse of better in the treatment group, based on limited data.
7.3 Mental state
Adjunctive treatment did not appear to affect positive (SAPS) and negative (SANS, HAM-D) symptoms domains compared with the control group, and was unlikely with both groups receiving antipsychotics (olanzapine).
7.4 Leaving the study early
Adjunctive selective norepinephrine reuptake inhibitor did not improve or reduce attrition rates relative to the control group in this short term (six week) study.
8. PREVENTION COMPARISON 6: ANTIDIABETIC AGENTS versus PLACEBO
8.1 Weight
All available data from just one (Metformin 2006) small study (n= 37) indicated that antidiabetic agents did not prevent of reduce weight gain compared with the control group. Again, this study is underpowered and larger and longer studies are needed to determine whether a genuine treatment effect exists.
8.2 Mental state
Limited data did not indicate metformin affected mental state in terms of BPRS change scores.
8.3 Physiological
Adjunctive antidiabetic treatment did not worsen or improve glucose and lipids levels.
8.4 Leaving the study early
Adjunctive antidiabetic treatment did not improve or reduce attrition rates relative to the control group in this 14 weeks study.
9. PREVENTION COMPARISON 7: ANTICONVULSANT AGENT versus CONTROL
9.1 Weight
We found that participants given anticonvulsants had significantly smaller mean increase in weight (kg) compared with the control group. No other measures were available to assess changes in weight, and this results is based on 60 participants. More supporting data are needed to determine whether this is a genuine treatment effect.
9.2 Adverse effects
Only insomnia was reported as an adverse effect and anticonvulsants did not appear from limited data to worsen sleep compared with the control group.
9.3 Leaving the study early
Adjunctive anticonvulsants (toprimate) did not improve or reduce attrition rates relative to the control group in this 12 week study.
10. PREVENTION COMPARISON 8: SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
10.1 Weight
No advantages were apparent for those participants given SSRI’s in terms of total body weight and total body mass index values. When weight was assessed using the criteria of 7% or more gain in their initial weight the result did favour adjunctive SSRI’s. Clinicians will need more consistent data to support this single positive result.
10.2 Global state
Adjunctive SSRIs do not appear to improve or worsen global state measures (GAF, CGI) compared with the control group.
10.3 Adverse effects
Many adverse effects outcomes were recorded but all data were equivocal. Two physiological measures (serum glucose and triglycerides) favoured adjunctive SSRIs, however this group were given low dose clozapine whilst the control were given higher doses of clozapine.
11. SYNTHESIZING PHARMACOLOGICAL COMPARISONS (PREVENTION)
11.1 Pharmacological
Although caution is required in summating trials examining different pharmacological interventions, there was a significant treatment effect demonstrating modest prevention of weight gain (n= 274, 6 RCTs, WMD − 1.16 CI −1.9 to −0.4).
12 TREATMENT COMPARISONS 1: COGNITIVE/BEHAVIOURAL INTERVENTION versus STANDARD CARE
12.1 Weight
Overall, results indicated that participants given cognitive behavioural intervention (CBI) lost more weight than the standard care group. This was consistent for most measures such as total weight score, mean weight change, body mass index, or >10% weight reduction. The three included studies were small and most outcomes would have been equivocal had the data been analysed without being pooled. Larger studies are needed to add weight to these initial favourable finding.
12.2 Satisfaction
Only one study (Cog/Behav 2005a) assessed satisfaction with care and overall more participants were satisfied in the CBI group than those receiving standard care.
12.3 Global state
No differences were found in terms of Clinical Global Impression scores.
12.4 Mental state
Mental state was not affected by adjunctive CBI compared with participants receiving only antipsychotics.
12.5 Laboratory measure
The addition of CBI did not affect blood pressure or lipids levels.
12.6 Leaving the study early
The additional intervention of CBI does not improve or reduce attrition rates relative to the control group during medium term.
13. TREATMENT COMPARISON 2: ANTI OBESITY AGENTS +/− LIFESTYLE MANAGEMENT versus PLACEBO +/− LIFESTYLE MANAGEMENT
13.1 Weight
Weight loss outcomes were inconsistent. Anti obesity agents did significantly reduce weight in terms of total body weight, as did change scores but the two pooled studies were heterogeneous. Also total body mass index favoured anti obesity agents but BMI change scores did not support this finding. The two studies providing data were underpowered and much larger studies are needed to determine whether such treatments are effective for weight loss.
13.2 Global state
Anti obesity agents did not worsen or improve global state (GAF).
13.3 Mental state
Outcome measures (PANSS, SANS) did not indicate that anti obesity agents improved or worsened mental state.
13.4 Adverse effects
Many adverse effects were recorded but the addition of anti obesity agents did not increase the frequency of such side effects compared with the control group. Systolic and diastolic blood pressure were significantly lower in the control group, but this is based on a sample of just 37. Most physiological measures were equivocal and impossible to draw firm conclusion from the few data which favoured the control group.
13.5 Leaving the study early
The additional intervention of anti obesity agents did not improve or reduce attrition rates relative to the control group during medium term evaluation.
14. TREATMENT COMPARISON 3: H2 ANTAGONISTS versus PLACEBO
14.1 Weight
Outcomes were inconsistent and the advantages of the H2 antagonist (nizatidine) are unclear. Final body weight did significantly favour H2 antagonists, as did mean change in weight, but this latter data contained substantial heterogeneity. Similarly, total body mass index favoured H2 antagonists but when reported as change scores these outcomes were heterogeneous. The available data are inconclusive and more robust data are needed before firm conclusion can be drawn.
14.2 Mental state
The addition of nizatidine did not improve or worsen mental state (PANSS) scores.
14.3 Adverse effects
Participants receiving the H2 antagonists did not experience any more adverse effects than the standard care group, although data were only available from one of the two studies in this comparison. Leptins were, however, lower in the treatment group.
14.4 Leaving the study early
The data did not suggest that the addition of nizatidine worsened or improved attrition in the treatment group compared with the control group.
15 TREATMENT COMPARISON 4: APPETITE SUPPRESSANT versus PLACEBO
15.1 Weight
Data did not reveal a consistent pattern. Total body weight at end of study was significantly lower in the control group (Phenylpropanol 2002). Short term change scores, however, were equivocal but medium term data did favour appetite suppressants. The results were based on small data sets (n=16 to 20) and no conclusion can be drawn as to the effects of appetite suppressants on weight loss.
15.2 Mental state
Limited data (PANSS) were unremarkable and no significant differences were found.
15.3 Adverse effects
All data were equivocal and no significant differences were apparent from limited data.
15.4 Leaving the study early
Appetite suppressants did not appear to worsen or improve study compliance.
16. TREATMENT COMPARISON 5: 5HT REUPTAKE BLOCKER versus PLACEBO
16.1 Weight
The limited data (n=30) from just one study (Fluoxetine 2003) did not indicate any benefit gained in weight loss whilst using adjunctive 5HT reuptake blockers compared with the control group. This study is however underpowered and larger and longer trials are needed before any firm conclusion can be drawn.
16.2 Mental state
Positive and negative symptom (PANSS +ve, PANSS −ve, and HAM-D) domain scores were not improved or worsened with the addition of fluoxetine.
16.3 Extrapyramidal symptoms
Movement disorders were equivocal whether measured with the SAS, BAS scales or AIMS checklist.
16.4 Leaving the study early
The addition of fluoxetine did not improve or worsen attrition rates relative to the control group.
17. TREATMENT COMPARISON 6: ANTIPARKINSONIAN DRUGS versus PLACEBO
17.1 Weight
Most outcomes were not significantly different and not clear conclusion can be made from three small underpowered studies.
17.2 Mental state
All outcomes were equivocal (BPRS, MADRS) from limited data.
17.3 Adverse effects
All outcomes, except insomnia, were not significantly different between the antiparkinsonian group and control and no trend emerged to suggest amantadine increased the likelihood of experiencing such side effects.
17. 4 Leaving the study early
The antiparkinsonian drug (amantadine) did not appear to worsen or improve study attrition.
18. TREATMENT COMPARISON 7: ANTICONVULSANTS versus PLACEBO
18.1 Weight change
Only one study (Topiramate 2005) reported on weight with just 20 participants included. Participants given the anticonvulsant topiramate lost weight but no more than the placebo group. Larger and longer trials are needed before we can determine whether anticonvulsants are an effective intervention for the control of weight in people with schizophrenia.
18.2 Adverse effects
All data (n=20) were equivocal.
18.3 Leaving the study early
The addition of topiramate did not improve or worsen study attrition during medium term evaluation.
19. TREATMENT COMPARISON 7: ANTICONVULSANTS HIGHER DOSE versus PLACEBO LOWER DOSE
19.1 Weight
Higher dosage topiramate did have a greater reduction in weight than the lower dose group, but this is based on only 33 participants and no firm conclusions can be made from such limited data.
19.2 Adverse effects
Higher dose topiramate did not worsen or improve side effects compared with the lower dose group.
19.3 Leaving the study early
Neither high nor low doses of adjunctive anticonvulsants affected attrition rates.
20. SYNTHESIZING PHARMACOLOGICAL COMPARISONS (TREATMENT)
20.1 Weight
There was an overall treatment effect (mean weight change) for the pharmacological interventions (n=273, 9 RCTs, WMD −3.85 CI −4.3 to −3.4) but there is also substantial, and understandable, heterogeneity (I2=89%).
20.1.1 Pharmacological Interventions
There was considerable variability in weight loss efficacy between pharmacological agents, as well as between studies of the same agent. Of drugs currently on the market, reboxetine, and topiramate were effective at weight prevention and topiramate (at 200mg) was effective for established weight gain, although there was only a single study for each. There were mixed results with sibutramine, nizatidine and amantadine and negative results for fluoxetine (treatment and prevention study), and famotadine and phenylpropinolamine. At the present time, no single agent emerges as consistently superior in terms of weight loss efficacy. With the use of adjunctive pharmacological agents, there is always a concern regarding exacerbation of psychiatric symptomatology and adverse physical side-effects. Generally, pharmacological agents were described as well tolerated. Clearly, the safety and tolerability of adjunctive pharmacological treatments need to be evaluated along with the effectiveness of these agents in moderating weight gain. Of the weight loss drugs currently in development, rimonabant, a selective cannabinoid receptor 1 antagonist, submitted to the FDA for approval, may have special applicability in the psychiatric population and deserves future research attention (Faulkner 2006). Given that antipsychotic drugs most implicated in weight gain have in common antagonism at the H1 receptor, H1 agonists such as betahistine should be explored as potential pharmacological interventions for weight gain.
20.1.2 Non pharmacological interventions
In terms of non pharmacological intervention, the five studies reported here suggest that such interventions are at least possible in this population with acceptable compliance although conclusions regarding the intensity of the intervention cannot be made at this stage. There is a need for further randomised controlled trials with long term follow-up to determine how best to package behavioural interventions, identify patient characteristics associated with adherence and successful outcome, and to examine how sustainable any outcomes are. Such interventions will probably need to set realistic goals, be highly structured, provide intensive support initially, and offer reduced but continued support over time, if not indefinitely. Adopting a stepwise approach of incremental weight management interventions implemented as early as possible and maintained throughout treatment, could be considered (Chue 2004). Intervention components should probably focus on reducing caloric intake (changing types of food consumed and reducing portion sizes) rather than complex dietary change (Cog/Behav 2005a), and support people in gradually increasing their levels of physical activity to 30 - 60 minutes of moderate physical activity most days of the week (Faulkner 2003). Cognitive-behavioural strategies to facilitate these changes that are tailored to this population need to be developed and evaluated. Finally, further research will need to examine whether the costs of providing such intensive programs are less than the cost associated with not intervening at all (see Loh 2006). While not assessed within the included studies, we also suggest that future interventions should also include broader environmental manipulations to increase habitual levels of physical activity (for example, greater use of stairs instead of lifts or elevators; incorporating short bouts of physical activity during the day) and to decrease the amount of time spent sitting or engaged in sedentary behaviours such as television viewing, and to assist and reinforce small dietary changes (removal of ‘junk food’ vending machines; access to fruit and vegetables). Despite the low number of studies reported, the role of physical inactivity and poor diet as independent risk factors for cardiovascular disease infers the need for non pharmacological or lifestyle intervention regardless of weight loss per se.
Given the modest weight loss reported in both pharmacological and non pharmacological studies, the first strategy in preventing or alleviating weight gain remains identifying an appropriate choice of antipsychotic which has a lower liability of weight gain. Switching patients to an antipsychotic with a lower liability of weight gain might also be considered. However, a recent review suggests that available evidence does not yet support the clinical superiority of switching antipsychotics on various outcome measures such as weight gain (Remington 2005). In addition, reviewing concomitant medications with additional weight gain liability (e.g. antidepressants and mood stabilizers) should also be considered.
AUTHORS’ CONCLUSIONS
Implications for practice
1. For people with schizophrenia
Weight gain is common for people with schizophrenia. Modest short-term weight loss can be achieved with some pharmacological and non pharmacological interventions (that promote physical activity and changes in diet). However, clear guidance regarding what works best is limited by the small number of studies and the variability of the interventions themselves as well as their intensity and duration. People with schizophrenia should ask their clinician for support and advice regarding weight management, appraise the evidence in this review, and come to some mutually acceptable approach to prevention or treatment. It may be that the best path is for people with schizophrenia to work with researchers and clinicians to help build better evidence than is seen in half a decade of trials.
2. For clinicians
There is currently limited information in terms of randomised controlled trials to evaluate the effects of interventions to moderate weight gain. Existing data suggest that short term modest weight loss is possible with non-pharmacological and selective pharmacological interventions. There is, however, insufficient evidence to support the general use of pharmacological interventions for weight management in people with schizophrenia. The first strategy in preventing or alleviating weight gain or metabolic disturbance is to appraise metabolic risk when prescribing antipsychotic and other psychotropic medications, although priority should be given to achieving good control of the mental illness (Faulkner 2006; Gough 2004). Patients, family, and caregivers should be educated about metabolic risks and receive lifestyle advice regarding diet and physical activity. Baseline screening and a monitoring plan must be initiated on commencement of antipsychotic treatment (Cohn 2006). During monitoring, if diabetes develops in close temporal relation to the new antipsychotic prescription, serious consideration must be given to discontinuing or switching medication. With significant weight gain or emerging metabolic effects (for example, impaired fasting glucose or dyslipidemia), the risk and benefit of antipsychotic choice and concomitant medications should be re-evaluated from both a psychiatric and a metabolic perspective, and at this stage, patients should be referred to a more structured and supervised lifestyle intervention where available. Adjunctive pharmacotherapy for weight loss should be reserved for patients who do not respond adequately to lifestyle interventions alone.
3. For managers and policy makers
Managers and policy makers should be aware that the weight gain commonly seen in people with schizophrenia is a serious clinical problem that is related to premature morbidity and mortality, and reduced quality of life. The long-term impact of addressing the high prevalence of obesity in this population will provide a significant economic burden. However, current strategies for preventing or treating weight gain in people with schizophrenia only produce modest reductions in weight and these changes have only been found in the short-term. There is merit to incorporating lifestyle interventions within the skill set of the case management system, as opposed to referring people to a primary care physician or other health care provider for management of cardiovascular disease risk factors (Faulkner 2006). People with serious mental illness have frequent contact with their mental health service providers. Changing health behaviours can be difficult, and frequent reinforcement may play a critical role in successful long-term adoption of regular physical activity and diet modification. Additionally, there are mental health-specific barriers to physical activity and dietary change that can be more appropriately addressed by people trained to be sensitive and supportive in regard to these issues (Richardson 2005). At this stage, there is no evidence that such strategies will be cost-effective.
Implications for research
1. General
All future studies should respect standards of measuring outcomes and of reporting data in order to enhance the comparability of study results (Begg 1996; Moher 2001). In the included studies data reporting was inconsistent.
2. Specific
Further randomised controlled trials with larger samples and longer treatment duration are needed to evaluate the effectiveness and safety of both pharmacological and non pharmacological interventions in preventing weight gain and effecting weight loss in schizophrenia patients treated with antipsychotic medications. To assist interpretation, trialists should report baseline and final outcomes including means difference (including standard deviations). Binary outcomes (number of patients losing > 7% initial body weight) should also be reported as they are easier to interpret and clinically relevant. Pharmacological studies should include behavioural lifestyle interventions (diet and physical activity) in all study arms. Side effects and tolerability should be evaluated more systematically using structured rating scales. Participants and interventions should be described in detail and results should include intention-to-treat analysis and document characteristics of participants lost to follow-up and ideally their outcome. Generation of allocation sequence and allocation concealment should be clearly described. As reported elsewhere, most studies to date have not used adequate methods or failed to describe how they concealed the allocation (separating the process of randomisation from the recruitment of patients)(Hewitt 2005). This may influence the degree of effect found. While double-blind evaluation of the outcome of cognitive/behavioural interventions may be impossible, researchers should take every effort to minimise biases by using blind or independent raters. Finally, access to such interventions may be less likely in standard clinical practice. Including economic analyses regarding the delivery of these interventions will be necessary to enable clinicians and purchasers to manage service provision and make best use of resources.
Studies predominantly used weight or body mass index (BMI) as the outcome measure. Other measures such as waist-to-hip ratio and waist circumference should be included. For example, BMI does not recognise fat distribution and the accumulation of fat in and around the abdominal region associated with obesity. In particular, abdominal visceral adipose tissue increases with age in both sexes. Therefore, measures of waist circumference should also be included, as it is a simple measurement that indicates accumulation of abdominal fat. Such a measure may also be the best single indicator of cardiovascular risk factors and a simpler measure for identifying the need for weight management. Studies should carefully monitor and report adherence to physical activity programs and dietary intake. Further research is required examining interventions during the initial period of antipsychotic treatment when weight gain appears to be most rapid. Finally, distinguishing between weight gain prevention and weight gain reversal needs closer attention. We suggest an outline for a trial in Table 1.
Table 1.
Suggested design of study
| Type of study | Patients | Interventions | Outcomes | Notes |
|---|---|---|---|---|
| Allocation: the randomisation process should be clearly described. Double-blind evaluation of the outcomes of a lifestyle intervention is extremely difficult, and probably impossible. Trialists should, take every precaution to minimise the effect of biases by using blind or independent raters. Intention-to-treat analysis is preferable. Trialists should describe from which groups withdrawals came, why they occurred and what was their outcome. Duration: One year follow-up at minimum. |
Diagnosis: people with schizophrenia or related disorders. Age: all ages . Sex: men & women. N=300.* Ethnicity: should be reported. History: people in their first episode reported separately. Distinction between weight gain prevention and treatment should be clear |
Pharmacological interventions should include behavioral lifestyle interventions in all study arms. Lifestyle interventions should incorporate both dietary and exercise counselling set within a behavioural modification programme |
Binary outcomes (e.g., number of patients losing > 7% initial body weight) should be reported. Incorporate multiple measures of adiposity such as waist circumference, %body fat, waist-to-hip ratio. Adherence to dietary and physical activity components of lifestyle interventions. Behavioral change (physical activity and diet) in lifestyle interventions. Serious adverse effects: any, list. Satisfaction: (binary outcome). Quality of life: binary outcome. Economic outcomes. |
* Size of study with sufficient power to highlight ~10% difference between groups for primary outcome |
PLAIN LANGUAGE SUMMARY.
Interventions to reduce weight gain in schizophrenia
Weight gain and obesity is a common problem for people with schizophrenia and both pharmacological (medication) and non pharmacological (diet/exercise) interventions have been tried to treat this problem. In this review we are able to show that small weight loss is possible with selective pharmacological or non-pharmacological interventions but it is difficult to be sure of the results because the studies were small and compared different interventions over different time periods.
ACKNOWLEDGEMENTS
We would also like to thank Clive Adams, Tessa Grant and Gill Rizzello of the Cochrane Schizophrenia Group for their help and support with this review.
SOURCES OF SUPPORT
Internal sources
University of Toronto, Canada.
External sources
Ontario Mental Health Foundation, Canada.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 16 + 8 weeks concurrent with olanzapine treatment. Setting: inpatients/outpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=68. Age: range 28-53 yrs, mean 40 yrs. Sex: male and female. History: chronic illness mean length 18.5 yrs. Mean BMI = 31.1 (91 kg); weight increase of > 5% during the first 9 months of olanzapine treatment; n.b.demographic data drawn from entire sample which included individuals with bipolar disorder |
|
| Interventions | 1. Olanzapine: dose 5-20 mg/day (mean 11.6 mg/day) + amantadine 100-300 mg/day (mean 235.6 mg/day). N=35 2) Olanzapine: dose 5-20 mg/day (mean 13.3 mg/day) + placebo. N=33 All received nutritional counseling, but this did not include any behavioural program or diet |
|
| Outcomes | Weight. BMI. Mental state: BPRS, MADRS. Abnormal values: high cholesterol; high triglycerides; high glucose. Adverse effects. Unable to use - Adverse events: SAS, BAS, AIMS (no usable data). Physical: blood pressure, heart rate, and ECG (no usable data). Leaving study early: no usable data. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate. % participants in analysis: 125/125 (LOCF). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 12 weeks concurrent with olanzapine treatment (5-30 mg/day; treatment duration: median of 7 months). Setting: outpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV) or schizoaffective disorder; 3 individuals with bipolar disorder included in analyses. N=21. Age: not reported. Sex: 12 M, 9 F. Ethnicity: 76% white. Mean BMI: 32.35. |
|
| Interventions | 1. Amantadine: dose up to 300 mg/day. N=12. 2. Placebo. N=9. Both groups received 12 sessions of a healthy lifestyle education program and a 3-month membership to a gym or a commercial weight-loss program |
|
| Outcomes | Weight. BMI. Leaving the study early. Unable to use - Mental state: PANSS (no usable data). Laboratory analyses: fasting glucose, insulin, prolactin. total cholesterol, HDL cholesterol LDL cholesterol, triglycerides (no usable data). |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate (3/21). % participants in analysis: 100% (ITT/LOCF). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 16 weeks treatments, 8 weeks follow up. Setting: outpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=70. Age: range 24-35 yrs, mean ~34 yrs. Sex: 42 M, 27 F. Ethnicity: 52 caucasian, 18 African American. History: duration ill >10 yrs, age of onset ~20 yrs. Mean BMI at start = 26.7 (81.53 kg). |
|
| Interventions | 1. Psychoeducation class: dose 1 hour diet & exercise educational modules & weekly reminder letters, weekly sessions for 16 weeks + standard care. N=35 2. Standard care: not defined. N=35. All participants began treatment with olanzapine at entry into the study with use of a stepped-initiation conversion process. Dosage ranged between 5-20 mg/day |
|
| Outcomes | Weight or body mass: weight - absolute & change, BMI - absolute & change. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Open. Patient attrition: Adequate (no dropouts reported). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 14 weeks. Setting: outpatients or stable long-term inpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV) or schizoaffective disorder. N=72. Age: range 21-64 yrs, mean 40 yrs. Sex: 29 M, 42 F. Ethnicity: 50% white; 35% black. Mean BMI: > 26 and motivated to lose weight; mean weight=101.2 kg |
|
| Interventions | 1. Calorie intake reduction techniques: dose 20 group sessions, two sessions/week for 6 weeks, then one for 8 weeks; two sessions on exercise. N=35 2. Control: participants asked to lose weight on their own without advice on weight reduction. N=37 |
|
| Outcomes | Weight or body mass: weight change, weight loss (> 5%).* Satisfaction: CSQ-8. Mental state: PANSS. Global state: CGI-C. Other physiological measures: BP. Leaving the study early. Unable to use - Weight or body mass: BMI, slenderness index (no numerical data reported). Adverse events. (no data). Laboratory tests: haematology, biochemistry (no data reported) |
|
| Notes | Generation of allocation sequence: Unclear Groups comparable at baseline: Yes Open Patient attrition: Inadequate (30% dropout) % participants included in the analysis: 100% (ITT) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 12 weeks with follow up at 24 weeks. Setting: outpatients (Australia). |
|
| Participants | Diagnosis: schizophrenia, schizoaffective or schizophreniform psychosis (25% of baseline sample identified as having bipolar/depression). N=51. Age: mean 34 yrs. Sex: 22 M, 29 F. Mean BMI: 28. |
|
| Interventions | 1. Passive nutritional education (booklet): dose + six one-to-one individual nutrition education sessions with a dietician over 3 months; goal setting of healthy eating and lifestyle goals; mean dose of olanzapine 15.54 mg/day. N=29 2. Passive nutritional education (booklet): dose of olanzapine 14.29 mg/day. N=22 |
|
| Outcomes | Weight. Waist circumference. BMI. Global state: CGI - quality of life, health and body image. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear Groups comparable at baseline: Yes Open Patient attrition: Inadequate (33% dropout at 3 months; 63% at 6 months) % participants included in the analysis: 67% and 27% |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 16 weeks. Setting: outpatients (USA). |
|
| Participants | Diagnosis: DSM-IV schizophrenia or schizoaffective disorder. N=17. Age: not reported. Sex: 5 M, 12 F. Ethnicity: 9 afro-american; 3 hispanic; 5 caucasian. Mean BMI: 33. |
|
| Interventions | 1. Cognitive/behavioral group intervention: dose 1 one-hour group session/week for 16 weeks; food and activity diary; sessions included role play, goal setting, problem solving, discussions on barriers to change, presentations on low-fat diets, and plans to increase activities such as walking. N=8 2. Standard care: weighed and measured at regular four week intervals. N=7 |
|
| Outcomes | Weight. BMI. Waist-to-hip ratio. Leaving the study early. Unable to use - Blood sugar - not analysed. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Open. Patient attrition: Adequate (12% dropout - all from control group). % participants included in the analysis: 88%. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 12 weeks concurrent with olanzapine treatment (5-20 mg/day). Setting: outpatients (Korea). |
|
| Participants | Diagnosis: schizophrenia (DSM IV) or schizoaffective disorder. N=48 Age: range 19-64 yrs, mean 30 yrs. Sex: 15 M, 33 F. History: mean duration of illness 2.15 yrs. BMI =27.4; >7% gain in body weight since receiving olanzapine |
|
| Interventions | 1. Weight management program (diet and exercise based on cognitive/behavioral therapy). Individual format 1session per week for 4 weeks and then every two weeks; diet: primarily education and use of a food diary; exercise: exercise diary and education. N=33 2. Standard care: verbal recommendations regarding exercise and eating behavior. N=15 |
|
| Outcomes | Weight. BMI. Compliance. Leaving the study early. Unable to use - Mental state: PANSS (no usable data). Adverse events: AIMS, vital signs (no usable data). Laboratory data: hematology, clinical chemistry, lipid profile, high density lipoprotein, and urinalysis (no usable data). QoL: WHO-QOL-BREF (no usable data). |
|
| Notes | Generation of allocation sequence: Unclear - ratio of 2 (intervention) to 1 (control) Groups comparable at baseline: Yes Open Patient attrition: Adequate (25%) % participants included in the analysis: 43/48. Last observation carried forward (LOCF) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 12 weeks. Setting: outpatients (England). |
|
| Participants | Diagnosis: schizophrenia (psychiatric interview and BPRS). N=29. Age: range 19-50, mean 38 yrs. Sex: 16 M, 17 F. Mean BMI at start: 35.15. |
|
| Interventions | 1. Depot antipsychotic medication: dose not stated + 30 mg/day d-fenfluramine. N=16 2. Depot antipsychotic medication: dose not stated + placebo. N=13 All patients receiving a regular dose of fluphenazine decanoate, flupenthixol decanoate, or clopenthixol decanoate; all patients received dietary advice (not described) |
|
| Outcomes | Weight. Adverse effects. Leaving the study early. Unable to use - Mental state: BPRS (no extractable data). Drug plasma levels (no extractable data). |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Inadequate: 52% dropout. % participants in analysis: 48% (16/33). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 8 weeks. Setting: inpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (chart records). N=20. Age: range 32-54 yrs, mean 42 yrs. Sex: all male. Mean weight: 91.85 kg. |
|
| Interventions | 1. Dextroamphetamine sulfate: dose 5 mg/day. N=10. 2. Placebo: dose daily. N=10. Patients taking thioridazine hydrochloride, chlorpromazine hydrochloride, imipramine hydrochloride, or chlordiazepoxide hydrochloride |
|
| Outcomes | Weight change. Appetite. Sleep. Pulse. |
|
| Notes | Generation of allocation sequence: Table of random numbers. Groups comparable at baseline: Not clear. Patient attrition: Adequate: No dropout. % participants in analysis: 100%. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 6 weeks. Setting: inpatients (Israel). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=14. Age: range 22-23 yrs, mean 22 yrs. Sex: 9 M, 5 F. History: first episode of acute psychosis. Mean weight at start: 65.95 kg BMI range at start: 18.5-24.9 |
|
| Interventions | 1. Olanzapine: dose 10 mg/day + 40 mg/day famotidine. N=7. 2. Olanzapine: dose 10 mg/day + placebo. N=7. |
|
| Outcomes | Weight. BMI. Mental state: SAPS/SANS. Global state: CGI. Adverse effects: SAS, adverse effects. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate (no dropout). % participants in analysis: 14/14. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 8 weeks. Setting: inpatients (Israel). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=30. Age: range 19-34 yrs, mean 25 yrs. Sex: 21 M, 9 F. History: chronic illness, mean length 26.4 months. Mean BMI=21.3 (64.45 kg). |
|
| Interventions | 1. Olanzapine: dose 10 mg/day + 20 mg/day fluoxetine. N=15. 2. Olanzapine: dose 10 mg/day + placebo. N=15. Less than 4 wks antipsychotic exposure. |
|
| Outcomes | Weight. BMI. Mental state: SANS/SAPS; Hamilton Depression Rating Scale. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Unclear Patient attrition: Adequate: 20% dropout % participants in analysis: 30/30 (ITT). Completer analysis also reported (24/30) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 4 months (preceded by 4-8 weeks olanzapine treatment). Setting: outpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=30. Age: range 33-36 yrs, mean 34 yrs. Sex: 24 M, 6 F. Mean weight =78.8 kg (patients gaining >3% of baseline weight during inital 8 week clozapine treatment) |
|
| Interventions | 1. Olanzapine: dose 15 +/− 4.8 mg/day + 60 mg/day fluoxetine. N=15 2. Olanzapine: dose 15 +/− 4.2 mg/day + placebo. N=15. |
|
| Outcomes | Weight. Percent body fat. Mental state: PANSS; Hamilton Depression Scale. Adverse events: AIMS, SAS, BAS. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Inadequate (35% dropout). % participants included in analysis: 100% (ITT). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 12 weeks. Setting: inpatients (Taiwan). |
|
| Participants | Diagnosis: DSM-IV schizophrenia. N=68. Age: range 18-60 yrs, mean age 34 yrs. Sex: 20 M, 48 F. History: treatment resistant to typical antipsychotics, mean age at onset 21 yrs. Mean BMI = 24.55 (66.6 kg). |
|
| Interventions | 1. Fluvoxamine-clozapine coadministration: dose fluvoxamine (50 mg/day) + low dose clozapine (up to 250 mg/day). N=34 2. Clozapine: dose high (up to 250 mg/day). N=34. |
|
| Outcomes | Weight. BMI. Global state: CGI, GAF. Adverse events: UUKU, adverse effects. Fasting serum glucose. Total cholesterol. Triglycerides. |
|
| Notes | Generation of allocation sequence: Not specified. Groups comparable at baseline: Yes . Open. Patient attrition: Adequate (no dropout). % participants in analysis: 68/68. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 14 weeks. Setting: inpatients (USA). |
|
| Participants | Diagnosis: severe schizophrenia or schizoaffective disorder (diagnostics tool not reported) N=40. Age: mean 47 yrs. Sex: 22 M, 15 F. Mean BMI = 23.05. |
|
| Interventions | 1. Olanzapine: dose 10 mg + 850-1750 mg/day metformin. N=20. 2. Olanzapine: dose 10 mg + placebo. N=20. Both groups received a balanced diet (2500-3000 Kcal daily); switched from typical antipsychotics to olanzapine |
|
| Outcomes | Weight. BMI. Waist circumference. Mental state: BPRS. Laboratory data: glucose, insulin, homa-IR, triglycerides, cholesterol. Adverse effects. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Computer based. Groups comparable at baseline: Yes. Patient attrition: Adequate. % participants in analysis: 37/40. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 16 weeks concurrent with olanzapine treatment. Setting: Inpatient. |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=171. Age:range 20-64, mean 40 yrs. Sex: 121 M, 54 F. History: chronic illness, mean length ill ~ 14 yrs. Mean weight 77.64 kg. |
|
| Interventions | 1. Olanzapine: dose mean 12.6 mg +/− 4.1 + 300 mg nizatidine. N=58 2. Olanzapine: dose mean 11.8 mg +/− 4.5 + 150 mg nizatidine. N=57 3. Olanzapine: dose mean 11.2 mg +/− 4.1 + placebo. N=56. |
|
| Outcomes | Weight. Mental state: BPRS. Adverse events: SAS, adverse effects. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Inadequate: 40% dropout. % participants in analysis: 169/175 (ITT reported). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 8 weeks (preceded by 3 month open-label screening period). Setting: Inpatients or outpatients (Turkey). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=34 Age: range 19-37, mean 27 yrs. Sex: 14 F, 21 M. Mean weight = 67.1 kg. |
|
| Interventions | 1. Olanzapine: dose mean 15.3 mg/day + 150 mg/bid nizatidine. N=17 2. Olanzapine: dose mean 15.3 mg/day + placebo. N=17. |
|
| Outcomes | Weight. BMI. Mental state: PANSS. Laboratory data: serum leptin. Adverse effects. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate (1/35). % participants included in final analysis: 34/35. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 8 weeks (preceded by 2.5-4 month open-label screening phase). Setting: inpatients or outpatients (Turkey). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=28. Age: range 21-41 yrs, mean 30 yrs. Sex: 13 M, 12 F. Mean weight: 70.65 kg. |
|
| Interventions | 1. Quetiapine: dose mean 504.4 mg/day + 150 mg/bid nizatidine. N=14 2. Quetiapine: dose mean 504.4 mg/day + placebo (one pill bid). N=14 |
|
| Outcomes | Body weight. BMI. Mental state: PANSS. Laboratory data: serum leptin. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate (3/28). % participants included in analysis: 25/28. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 12 weeks. Setting: outpatients (USA). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N=16. Age: range 42-46 yrs, mean 44 yrs. Sex: 14 M, 2 F. Mean weight=102.29 kg (weight history: had gained > 10% of baseline body weight since starting clozapine) |
|
| Interventions | 1. Clozapine: dose mean 506 +/− 115 mg/day + phenylpropanolamine 75 mg (7 day quantities sustained release). N=8 2. Clozapine: dose mean 431 +/− 187 mg/day + placebo. N=8. Both groups received monthly dietary counseling. |
|
| Outcomes | Body weight. Mental state: PANSS. Leaving the study early. Unable to use - Adverse events: AIMS, SAS (no usable data). |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate (25% dropout). % participants included in analysis: 100%. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 6 weeks. Setting: inpatients (Israel). |
|
| Participants | Diagnosis: schizophrenia (DSM-IV) (best estimate diagnosis). N=26. Sex:17 M, 9 F. Age: range 19-47 yrs, mean 30 yrs. History: chronic illness, years since onset: 2.95 yrs. Mean BMI=21.53 (62.75 kg). |
|
| Interventions | 1. Olanzapine: dose 10 mg + 4 mg/day reboxetine. N=13. 2. Olanzapine: dose 10 mg + placebo. N=13. |
|
| Outcomes | Weight. BMI. Mental state: SAPS/SANS; Hamilton Depression Rating Scale. Global state: CGI. Adverse events: BAS, SAS, adverse effects. Leaving the study early. |
|
| Notes | Generation of allocation sequence: Allocated according to entries of a table of random numbers. Groups comparable at baseline: Yes. Nonsignificant difference in terms of weight - treatment group heavier than placebo. Patient attrition: Adequate: 24% dropout. % participants in analysis: 20/26. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 12 weeks + 3 month follow-up concurrent with olanzapine treatment (stable for at least 4 months). Setting: outpatients (USA). |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM-IV) . N=37. Age: range 30-43 yrs, mean 41 yrs. Sex: 23 M, 14 F. Mean BMI at start=36.35 (105.9 kg); BMI = > 30 or = > 27 plus at least 1 cardiovascular risk factor |
|
| Interventions | 1. Olanzapine: dose not stated + sibutramine (up to 15 mg/day). N=19 2. Olanzapine: dose not stated + placebo. N=18. For the first 8 weeks, all participants attended a weekly 1-hour group meeting that incorporated weight education, behavior modification, and group support to facilitate healthy dietary changes; weekly goals for meal planning, portion control, food preparation, healthy snacking, and exercise were discussed in group sessions with individual follow-ups |
|
| Outcomes | Weight. BMI. Waist circumference, waist hip ratio; body fat (%). Heart rate, blood pressure. Laboratory: lipids; glucose; uric acid, cortisol, haemoglobin. Mental state: PANSS, SANS. Global state: GAS. Adverse events: Hillside Akathisia Scale, AIMS. Leaving the study early. Unable to use - Complete blood count, creatinine, and liver function tests; olanzapine serum levels (no usable data) |
|
| Notes | Varying patterns of missing data Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Patient attrition: Adequate: 16% dropout. % participants included in analysis: 100% at end of treatment (ITT) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration:16 weeks. Setting: outpatients (USA) |
|
| Participants | Diagnosis: schizophrenia or schizo-affective (DSM IV). N=21. Age: mean 36 yrs. Sex: 7 M, 14 F. Mean BMI at baseline =37.2. |
|
| Interventions | 1. Typical or atypical oral antipsychotic + sibutramine 5-10 mg for 4 wks, then titration up to 15 mg + dietary counseling. N=14 2. Placebo + dietary counseling. N=7. |
|
| Outcomes | Weight. BMI. Unable to use - Mental state: BPRS (no usable data). Global state: CGI (no usable data). Leaving the study early (no usable data). |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Unclear. Patient attrition: Inadequate (48% dropout). % participants in analysis: 19/19 (LOCF). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: double. Duration: 12 weeks. Setting: inpatients (Korea). |
|
| Participants | Diagnosis: schizophrenia (DSM IV). N=66*. Age: mean 35 yrs. Sex: male and female. Mean BMI=28.1 (74.7 kg). |
|
| Interventions | 1. Antipsychotic: dose mean risperidone equivalent 5.7 mg + Topiramate 100 mg/day. N=22 2. Antipsychotic: dose mean risperidone equivalent 5.4 mg + Topiramate 200 mg/day. N=22 3. Antipsychotic: dose mean risperidone equivalent 5.1 mg + Placebo. N=22 |
|
| Outcomes | Weight. Adverse effects. Leaving the study early. Unable to use - BMI (no usable data). Waist circumference (no usable data). Hip measurement (no usable data). Waist-to-hip ratio (no usable data). Mental state: BPRS (no usable data) Global state: CGI (no usable data). |
|
| Notes | *Exact number randomised into groups unclear. Generation of allocation sequence: computer-generated randomisation code. Groups comparable at baseline: Yes. Patient attrition: Adequate (20% dropout). % participants in analysis: 80% (53/66). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation: randomised (no further details). Blinding: open. Duration: 12 weeks. Setting: outpatients (Korea). |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM-IV). N=60*. Age: not reported. Sex: all male. |
|
| Interventions | 1. Olanzapine: dose mean 12.43 +/− 4.45 mg/day + topiramate (25 mg bid 50 mg D.O.D. on day 8 then fixed to 12 weeks). N=30 2. Olanzapine: dose mean 12.29 +/− 4.26 mg/day. N=30. |
|
| Outcomes | Weight. Leaving the study early. Unable to use: Mental state: PANSS (no usable data). |
|
| Notes | Generation of allocation sequence: Unclear. Groups comparable at baseline: Yes. Open. Patient attrition: Adequate (20%). % participants included in the analysis: 100% (ITT reported) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
BMI - body mass index
CGI-C - Clinical Global Impressions - change
CGI-S - Clinical Global Impressions - severity of Illness
CSQ-8 Client Satisfaction Questionnaire
DSM-IV-
Global state:
CGI - Clinical Global Impression
GAF - Global Assessment of Functioning
GAS - Global Assessment Scale
Mental state:
BPRS - Brief Psychiatric Rating Scale.
MADRS - Montgomery-Asberg Depression Rating Scale.
PANSS - The Positive and Negative Syndrome Scale.
SANS - Scale for the assessment of Ngative symptoms.
SAPS - Scale for the assessment of Positive symptoms.
Adverse events:
AIMS - Abnormal Involuntary Movement Scale
BAS - Barnes Akathisia Scale
SAS - Simpson Angus Scale
UUKU - Udvalg for Kliniske Undersogelser
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Archie 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: free membership to a YMCA health club for six months (no comparison group reported) |
| Harmatz 1968 | Allocation: randomised. Participants: diagnostic criteria not clear. Interventions: behavior modification versus group therapy versus diet only. Outcomes: no usable data. |
| McCreadie 2005 | Allocation: randomised (by houses not individuals to receive free fruit & vegetables). Participants: people with schizophrenia. Intervention: free fruit and vegetables with instructions versus free fruit and vegetables with no instructions versus no intervention. Outcomes: no usable data. |
| Morrison 2002 | Allocation: not randomised, case series. |
| Nickel 2005 | Allocation: randomised. Participants: diagnostic criteria unclear; majority (53%) classified as unipolar and bipolar disorder (author contacted for further details - no response) |
| Rotatori 1980 | Allocation: randomised. Participants: people with schizophrenia and those with “adjustment reaction to adult life”, diagnostic criteria not clear (no further information) |
Characteristics of ongoing studies [ordered by study ID]
Bristol-Meyer Squib
| Trial name or title | Effects of aripiprazole in overweight patients treated with olanzapine for schizophrenia or schizoaffective disorder |
|---|---|
| Methods | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. N=300. Age: Adults (18-65 years). History: taking olanzapine. |
| Interventions | 1. Aripiprazole. 2. Olanzapine. |
| Outcomes | Primary outcomes: Comparison of weight change from baseline to Week 16. Secondary outcomes: Assessment of metabolic laboratory measures |
| Starting date | Finished recruiting. |
| Contact information | Bristol-Myers Squibb, Otsuka America Pharmaceutical Local Institution, Little Rock, Arkansas ClinicalTrials.gov Identifier: NCT00095524 |
| Notes | Allocation: randomised. Blinding: double. |
| Trial name or title | The efficacy of pindolol in reducing weight gain associated with the use of olanzapine |
|---|---|
| Methods | |
| Participants | Diagnosis: schizophrenia. Age: Adults N=36. |
| Interventions | 1. Olanzapine treatment in combination with pinadolol. 2. Olazapine treatment alone. |
| Outcomes | Weight gain. |
| Starting date | End date identified as 01 September 2003 |
| Contact information | Dr Craig Ritchie Metabolic and Clinical Trials Unit Royal Free and University College Medical School Royal Free Campus Rowland Hill Street London NW3 2PF Telephone: 020 7794 0500 x33951 Fax: 020 783032808 c.richie@medsch.ucl.ac.uk |
| Notes | Allocation: randomised. |
DATA AND ANALYSES
Comparison 1.
PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 by 16 weeks | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | −5.17 [−13.63, 3.29] |
| 1.2 by follow up 24 weeks | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | −3.69 [−11.87, 4.49] |
| 2 Weight: 2. Change (kgs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 2 | 104 | Mean Difference (IV, Fixed, 95% CI) | −3.38 [−4.81, −1.96] |
| 2.2 end of treatment - follow up, upto 6 months | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | −4.87 [−7.11, −2.64] |
| 3 Weight: 3. Body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 by 16 weeks | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | −2.27 [−4.62, 0.08] |
| 3.2 follow up 24 weeks | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | −1.79 [−4.04, 0.46] |
| 4 Weight: 4. Change in body mass index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 medium term | 2 | 104 | Mean Difference (IV, Fixed, 95% CI) | −1.06 [−1.54, −0.59] |
| 4.2 end of treatment - follow up, upto 6 months | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | −1.52 [−2.25, −0.79] |
| 5 Weight: 5. Change in waist circumference | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 by 6 months follow up | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | −5.5 [−8.21, −2.79] |
| 6 Weight: 6. Increase initial weight by7% | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 by 12 weeks | 1 | 34 | Risk Ratio (M-H, Fixed, 95% CI) | 0.20 [0.07, 0.64] |
| 7 Global state: 1. Improvement in quality of life, mean change score - 3 months (CGI, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 by 12 weeks | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [−0.04, 1.84] |
| 8 Global state: 2. Improvement in overall health, mean change score - 3 months (CGI, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 by 12 weeks | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [−0.01, 1.81] |
| 9 Global state: 3. Improvement in body image, mean change score - 3 months (CGI, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 by 12 weeks | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.29, 1.91] |
| 10 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 by 12 weeks | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 0.41 [0.18, 0.95] |
| 10.2 by 6 month follow up | 1 | 34 | Risk Ratio (M-H, Fixed, 95% CI) | 1.91 [0.68, 5.42] |
Comparison 2.
PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [−6.09, 10.89] |
| 1.2 medium term | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | −2.40 [−7.81, 3.01] |
| 2 Weight: 2. Change (kgs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−2.75, 2.55] |
| 2.2 medium term | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | −0.89 [−2.68, 0.90] |
| 3 Weight: 3. Body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | −0.90 [−3.86, 2.06] |
| 4 Weight: 4. Change in body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−0.73, 0.53] |
| 5 Weight: 5. Increase initial weight by 7% | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 short term | 1 | 14 | Risk Ratio (M-H, Fixed, 95% CI) | 1.33 [0.46, 3.88] |
| 6 Global state: 1. Average endpoint change scores - 6 weeks (CGI, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [−0.74, 0.94] |
| 7 Mental state: 1. Average endpoint change score - 16 weeks (BPRS 6 items, high score=worse) | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 2.45 [−1.50, 6.40] |
| 8 Mental state: 2. Average endpoint change score - 6 weeks (SAPS, worse=poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−4.09, 3.89] |
| 9 Mental state: 3. Average endpoint change score - 6 weeks (SANS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | −1.90 [−4.97, 1.17] |
| 10 Adverse effects: 1. Movement disorders - average endpoint change scores (SAS, high=worse) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 short term | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | −2.0 [−3.84, −0.16] |
| 10.2 medium term | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [−0.97, 1.75] |
| 11 Adverse effects: 2. Daytime somnolence | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 short term | 1 | 14 | Risk Ratio (M-H, Fixed, 95% CI) | 0.8 [0.36, 1.77] |
| 12 Leaving the study early | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 12.1 short term | 1 | 14 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12.2 medium term | 1 | 118 | Risk Ratio (M-H, Fixed, 95% CI) | 1.12 [0.73, 1.74] |
Comparison 3.
PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | −4.58 [−9.88, 0.72] |
| 2 Weight: 2. Change (kgs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | −0.27 [−2.17, 1.63] |
| 3 Mental state: 1. Average endpoint change score - 16 weeks (BPRS, high=worse) | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [−3.81, 4.31] |
| 4 Adverse effects: 1. Movement disorders - average endpoint change score - 16 weeks (SAS, high=worse) | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [−0.98, 1.78] |
| 5 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 1 | 115 | Risk Ratio (M-H, Fixed, 95% CI) | 1.12 [0.72, 1.74] |
Comparison 4.
PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) (short term) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 6.40 [−7.56, 20.36] |
| 2 Weight: 2. Change (kgs) (short term) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−3.35, 3.75] |
| 3 Weight: 3. Body mass index (short term) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [−2.35,4.55] |
| 4 Mental state: 1. Average endpoint change score - 8 weeks (SANS, high=worse) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [−2.54, 5.02] |
| 5 Mental state: 2. Average endpoint change score - 8 weeks (SAPS, high=worse) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.09 [−0.36, 2.54] |
| 6 Mental state: 3. Average endpoint change score - 8 weeks (HAM-D, high=worse) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [−0.75, 4.07] |
| 7 Adverse effects: 1. Any (short term) | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.16, 6.20] |
| 8 Leaving the study early (short term) | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 2.0 [0.43, 9.32] |
Comparison 5.
PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [−9.57, 15.57] |
| 2 Weight: 2. Change (kgs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −3.0 [−5.55, −0.45] |
| 3 Weight: 3. Body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [−1.31,4.25] |
| 4 Weight: 4. BMI change | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −0.98 [−1.80, −0.16] |
| 5 Weight: 5. Increase initial weight by 7% | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 short term | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 0.29 [0.08, 1.05] |
| 6 Global state: 1. Average endpoint change scores - 6 weeks (CGI, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [−0.96, 1.16] |
| 7 Mental state: 1. Average endpoint change score - 6 weeks (SAPS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −7.00 [−14.94, 0.94] |
| 8 Mental state: 2. Average endpoint change score - 6 weeks (SANS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −4.30 [−18.61, 10. 01] |
| 9 Mental state: 3. Average endpoint change score - 6 weeks (HAM-D, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 short term | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [−3.44, 1.44] |
| 10 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 short term | 1 | 26 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.25, 4.07] |
Comparison 6.
PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||
| 1.1 medium term | 37 | Mean Difference (IV, Fixed, 95% CI) | −1.80 [−7.84, 4.24] | |
| 2 Weight: 2. Change (kgs) | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||
| 2.1 medium term | 37 | Mean Difference (IV, Fixed, 95% CI) | −0.80 [−2.63, 1.03] | |
| 3 Weight: 3. Body mass index | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||
| 3.1 medium term | 37 | Mean Difference (IV, Fixed, 95% CI) | −0.20 [−2.42, 2.02] | |
| 4 Weight: 4. Waist circumference | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||
| 4.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [−1.99, 8.79] |
| 5 Mental state: 1. Average endpoint change scores - 14 weeks (BPRS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −1.80 [−6.50, 2.90] |
| 6 Physiological | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 glucose, basal | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −0.20 [−0.73, 0.33] |
| 6.2 glucose, postload | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [−1.13, 2.13] |
| 6.3 total cholesterol | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −24.0 [−59.16, 11. 16] |
| 6.4 LDL cholesterol | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −26.00 [−61.35, 9. 35] |
| 6.5 HDL cholesterol | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [−0.20, 12.40] |
| 7 Physiological (skewed data) | Other data | No numeric data | ||
| 7.1 insulin, basal | Other data | No numeric data | ||
| 7.2 homa-IR | Other data | No numeric data | ||
| 7.3 triglycerides | Other data | No numeric data | ||
| 7.4 vldl cholesterol | Other data | No numeric data | ||
| 8 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 medium term | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.05, 5.08] |
Comparison 7.
PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Change (kgs) | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||
| 1.1 medium term | 60 | Mean Difference (IV, Fixed, 95% CI) | −1.36 [−2.47, −0.25] | |
| 2 Adverse effects: 1. Insomnia | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 1 | 48 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.17, 2.76] |
| 3 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.25, 2.00] |
Comparison 8.
PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [−5.77, 6.37] |
| 2 Weight: 2. Body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−2.10, 1.50] |
| 3 Weight: 3. Increase initial weight by 7% | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.27 [0.08, 0.89] |
| 4 Global state: 1. Average total endpoint score - 12 weeks (GAF, high=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 medium term | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [−0.89, 2.29] |
| 5 Global state: 2. Average total endpoint score - 12 weeks (CGI, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [−0.06, 0.18] |
| 6 Adverse effects: 1. Physical (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 hypertriglyceridemia | 1 | 61 | Risk Ratio (M-H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 6.2 hypercholesterolemia | 1 | 58 | Risk Ratio (M-H, Fixed, 95% CI) | 0.31 [0.01, 7.35] |
| 7 Adverse effects: 2. Side effects (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 7.1 sedation | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.42, 1.66] |
| 7.2 hypersalivation | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.27, 1.67] |
| 7.3 constipation | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 1.33 [0.52, 3.43] |
| 7.4 postural hypotension | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.18, 1.77] |
| 7.5 tachycardia | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.8 [0.23, 2.73] |
| 7.6 accommodation disturbances | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.4 [0.08, 1.92] |
| 7.7 nausea | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.25, 100.43] |
| 8 Physiological (medium term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Glucose | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | −3.30 [−6.32, −0.28] |
| 8.2 Triglycerides | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | −22.70 [−44.59, −0.81] |
| 8.3 Total cholesterol | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | −10.20 [−21.01, 0.61] |
Comparison 9.
TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Change (kgs) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 3 | 129 | Mean Difference (IV, Fixed, 95% CI) | −1.69 [−2.77, −0.61] |
| 2 Weight: 2. BMI change | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 3 | 129 | Mean Difference (IV, Fixed, 95% CI) | −0.66 [−1.06, −0.25] |
| 3 Weight: 3. Waist-hip change | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | −0.02 [−0.06, 0.02] |
| 4 Weight: 4. No loss (only >10% loss data) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 medium term | 1 | 43 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.62, 0.97] |
| 5 Weight: 5. No loss (only >5% loss data) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.67, 1.04] |
| 6 Satisfaction: 1. Average total endpoint score - 14 weeks (CSQ-8, high=positive) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 medium term | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [0.90, 3.70] |
| 7 Mental state: 1. Average endpoint score - 14 weeks (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 medium term | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [−6.23, 12.23] |
| 8 Global state: 1. Not much improved (CGI-C) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 medium term | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.62, 1.19] |
| 9 Physiological: Blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 medium term - sitting systolic | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | −2.80 [−8.43, 2.83] |
| 9.2 medium term - standing systolic | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | −3.70 [−8.85, 1.45] |
| 10 Laboratory data: Number increasing ratio of LDL to HDL | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 medium term | 1 | 38 | Risk Ratio (M-H, Fixed, 95% CI) | 0.58 [0.26, 1.32] |
| 11 Leaving the study early | 3 | Risk Difference (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 medium term | 3 | 137 | Risk Difference (M-H, Fixed, 95% CI) | 0.13 [−0.01, 0.27] |
Comparison 10.
TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −16.96 [−27.01, −6.91] |
| 2 Weight: 2a. Change (kgs) (heterogeneous data) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | −4.41 [−3.00, −3.83] |
| 3 Weight: 2b. Body weigh (kg) studies analysed seperately | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −4.58 [−5.17, −3.99] |
| 3.2 medium term | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [−2.76, 3.66] |
| 4 Weight: 3. Body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −9.0 [−13.51, −4.49] |
| 5 Weight: 4. Change in body mass index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | −0.17 [−1.31, 0.98] |
| 6 Weight: 5. Waist circumference | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −11.5 [−17.76, −5.24] |
| 7 Weight: 6. Change in waist circumference (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 medium term | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | −7.6 [−18.90, 3.70] |
| 8 Weight: 7. Waist-hip ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [−0.02, 0.10] |
| 9 Weight: 8. Change in waist-hip ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 medium term | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.00, 0.26] |
| 10 Weight: 9. Hip circumference | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −8.60 [−15.24, −1.9 |
| 11 Weight: 10. Body fat (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [−3.38, 3.58] |
| 12 Global state: 1. Average total endpoint score - medium term (GAS, high=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −1.50 [−6.21, 3.21] |
| 13 Mental state: 1. Average total endpoint score - medium term (PANSS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [−4.46, 6.66] |
| 14 Mental state: 2. Average total endpoint score - medium term (SANS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [−4.64, 9.44] |
| 15 Extrapyramidal symptoms: (skewed data) | Other data | No numeric data | ||
| 15.1 hillside akathisia scale | Other data | No numeric data | ||
| 15.2 abnormal involuntary movement scale | Other data | No numeric data | ||
| 16 Adverse effects: 1. Related to satiety (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 16.1 appetite - decrease | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.60, 1.60] |
| 16.2 appetite - excessive | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.45 [0.13, 1.52] |
| 16.3 thirst - excessive | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.24 [0.50, 10.06] |
| 17 Adverse effects: 2. Related to sleep (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 17.1 dificulty falling asleep | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.49, 3.22] |
| 17.2 sleep - interrupted | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 4.47 [0.58, 34.57] |
| 17.3 sleep - shortened | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.24 [0.50, 10.06] |
| 17.4 early waking | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.21, 3.85] |
| 18 Adverse effects: 3. Other (medium term) | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 18.1 anticholinergic - blurred vision | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.68 [0.31, 23.43] |
| 18.2 anticholinergic - constipation | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.24 [0.50, 10.06] |
| 18.3 anticholinergic - dry mouth | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 3.58 [0.88, 14.58] |
| 18.4 cardiovascular - rapid heart rate | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.79 [0.18, 18.02] |
| 18.5 other - dizziness | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.30 [0.03, 2.60] |
| 18.6 other - headache | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.23, 2.24] |
| 18.7 other - rhinitis | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.26, 3.04] |
| 18.8 other-hypertension | 1 | 19 | Risk Ratio (M-H, Fixed, 95% CI) | 1.2 [0.06, 25.53] |
| 19 Cardiovascular (medium term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 Blood pressure - systolic | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [1.20, 18.60] |
| 19.2 Blood pressure - dystolic | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [0.12, 12.08] |
| 19.3 Heart rate while seated (bpm) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [−4.80, 10.60] |
| 20 Laboratory data (medium term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 Lipids - total (mg/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −30.10 [−60.54, 0.34] |
| 20.2 Lipids - HDL (mg/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [−6.21, 8.81] |
| 20.3 Lipds - LDL (mg/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −33.80 [−60.41, −7.19] |
| 20.4 Triglycerides (mg/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 11.90 [−151.50, 175.30] |
| 20.5 Glucose (mg/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [−14.30, 21.90] |
| 20.6 Uric acid (mg/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −0.5 [−1.53, 0.53] |
| 20.7 Cortisol (ug/dl) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [−2.26, 3.86] |
| 20.8 Hemoglobin A1C (%) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | −0.70 [−1.26, −0.14] |
| 21 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 21.1 medium term (3months) | 1 | 37 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.38, 1.81] |
| 21.2 follow up at 6 months | 1 | 37 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.22, 4.10] |
Comparison 11.
TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 short term - 8 weeks | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | −5.30 [−8.39, −2.20] |
| 2 Weight: 2. Change (kgs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term - 8 weeks | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | −3.62 [−4.24, −2.99] |
| 3 Weight: 3. Body mass index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 short term - 8 weeks | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | −1.51 [−2.45,-0.58] |
| 4 Weight: 4. Change in body mass index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 short term - 8 weeks | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | −1.45 [−1.74, −1.17] |
| 5 Mental state: 1. Average endpoint scores - 8 weeks (PANSS total, high=worse) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 short term | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | −2.03 [−4.08, 0.01] |
| 6 Adverse effects: 1. Any | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 short term | 2 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 1.33 [0.35, 5.08] |
| 7 Other physiological: 1a. Leptin levels | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 short term - 8 weeks | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | −3.83 [−5.47, −2.19] |
| 8 Other physiological: 1b. Leptin change | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 short term - 8 weeks | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | −1.6 [−2.20, 1.00] |
| 9 Leaving the study early | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 short term - 8 weeks | 2 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.18, 5.34] |
Comparison 12.
TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 4.99 [2.01,7.97] |
| 2 Weight: 2. Change (kgs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term - 8 weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [−2.49, 4.13] |
| 2.2 medium term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | −2.60 [−5.14, −0.06] |
| 3 Mental state: Average endpoint scores - 12 weeks (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | −4.80 [−11.63, 2.03] |
| 4 Adverse effects: 1. Side-effects (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Lethargy/fatigue | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 4.0 [0.22, 72.01] |
| 4.2 Sleepiness | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.06, 10.37] |
| 4.3 diarrhoea | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 1.56 [0.17, 13.87] |
| 4.4 Agitation/tremor/sweating/tachycardia | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.06, 10.37] |
| 5 Adverse effects: 2. Related to sleep (short term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Observed Incidents of napping during day | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −0.90 [−38.64, 36.84] |
| 5.2 Observed incidents of awake during night | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −8.90 [−26.37, 8.57] |
| 6 Adverse effects: 3. Appetite (short term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Number of patients refusing meal/lower consumption | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 2.25 [1.02, 4.94] |
| 7 Adverse effects: 4. Physical (short term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pulse | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 4.80 [−2.51, 12.11] |
| 8 Leaving the study early | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 medium term | 2 | 45 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.46, 2.00] |
Comparison 13.
TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [−6.67, 16.07] |
| 2 Weight: 2. Percent body fat | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [−7.25, 7.85] |
| 3 Mental state: 1a. Positive symptoms, average change score - 4 months (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [−4.53, 4.73] |
| 4 Mental state: 1b. Negative symptoms, average change score - 4 months (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [−1.61, 7.21] |
| 5 Mental state: 2. Depressive symptoms, average change score - 4 months (HAM-D, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [−1.23, 4.83] |
| 6 Extrapyramidal symptoms: 1. Tardive dyskinesia, average change score - 4 months (AIMS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [−0.75, 1.75] |
| 7 Extrapyramidal symptoms: 2. Parkinsonism, average total endpoint score - 4 months (SAS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [−0.02, 4.82] |
| 8 Extrapyramidal symptoms: 3. Akathisia, average change score - 4 months (BAS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 medium term | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [−0.05, 1.65] |
| 9 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 medium term | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.23, 1.89] |
Comparison 14.
TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Change (kgs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | −2.30 [−4.22, −0.38] |
| 2 Weight: 2. Change in body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 medium term | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | −1.31 [−2.55,-0.07] |
| 3 Weight: 3. No gain | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 21 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.83, 10.86] |
| 4 Weight: 4. Increase initial weight by 7% | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 medium term | 1 | 123 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.29, 2.81] |
| 5 Weight: 5. Decrease initial weight by 7% | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 medium term | 1 | 123 | Risk Ratio (M-H, Fixed, 95% CI) | 2.53 [0.69, 9.34] |
| 6 Mental state: 1. Average change scores - medium term (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 medium term | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [−2.84, 4.44] |
| 7 Mental state: 2. Average change scores - medium term (MADRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 medium term | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | −1.80 [−4.75, 1.15] |
| 8 Adverse effects: 1. Worsening of schizophrenia | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 medium term | 2 | 89 | Risk Ratio (M-H, Fixed, 95% CI) | 2.04 [0.31, 13.22] |
| 9 Adverse effects: 2. Lipid abnormalities (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 High cholesterol (> 6.21 mmol/l) | 1 | 43 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.04, 3.67] |
| 9.2 High triglycerides (> 2.26 mEq/l) | 1 | 45 | Risk Ratio (M-H, Fixed, 95% CI) | 0.48 [0.17, 1.36] |
| 9.3 High glucose (> 6.99 mmol/l) | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.46 [0.12, 1.74] |
| 10 Adverse effects: 3. Other (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Insomnia | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 3.52 [1.21, 10.21] |
| 10.2 Upper abdominal pain | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 14.07 [0.81, 244.45] |
| 10.3 Fatigue | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.08 [0.00, 1.45] |
| 11 Leaving the study early | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 medium term | 2 | 146 | Risk Ratio (M-H, Fixed, 95% CI) | 1.80 [0.71, 4.54] |
Comparison 15.
TREATMENT: 7. ANTICONVULSANTS versus PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: Change (kgs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | −1.38 [−4.21, 1.45] |
| 2 Adverse events (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 paresthesia | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.50 [0.52, 11.96] |
| 2.2 psychomotor slowing | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.08, 18.46] |
| 2.3 fatigue | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.50 [0.25, 25.15] |
| 2.4 dizziness | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.08, 18.46] |
| 2.5 nausea | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 [0.05, 3.63] |
| 2.6 diarrhea | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.06, 6.29] |
| 2.7 headache | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 3.75 [0.43, 32.70] |
| 2.8 dyspepsia | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.16, 4.40] |
| 2.9 anorexia | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.50 [0.25, 25.15] |
| 3 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 44 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.68, 13.27] |
Comparison 16.
TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: Change (kgs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 medium term | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | −3.67 [−6.99, −0.35] |
| 2 Adverse events (medium term) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 paresthesia | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 2.35 [0.92, 6.01] |
| 2.2 psychomotor slowing | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 1.88 [0.19, 18.80] |
| 2.3 fatigue | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.06, 13.82] |
| 2.4 dizziness | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 1.88 [0.19, 18.80] |
| 2.5 nausea | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 1.88 [0.19, 18.80] |
| 2.6 diarrhea | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 2.82 [0.33, 24.43] |
| 2.7 headache | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 0.13 [0.01, 2.42] |
| 2.8 dyspepsia | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 1.41 [0.27, 7.38] |
| 2.9 anorexia | 1 | 33 | Risk Ratio (M-H, Fixed, 95% CI) | 1.88 [0.40, 8.90] |
| 3 Leaving the study early | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 44 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.30, 2.33] |
Comparison 17.
PREVENTION: 9. (PHARMACOLOGICAL) MEAN WEIGHT CHANGE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of treatment | 6 | 274 | Mean Difference (IV, Fixed, 95% CI) | −1.16 [−1.90, −0.41] |
Comparison 18.
TREATMENT: 10. (PHARMACOLOGICAL) MEAN WEIGHT CHANGE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of treatment | 9 | 273 | Mean Difference (IV, Fixed, 95% CI) | −3.85 [−4.25, −3.44] |
Analysis 1.1. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 1.2. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 1.3. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 3 Weight: 3. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 3 Weight: 3. Body mass index
 |
Analysis 1.4. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 4 Weight: 4. Change in body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 4 Weight: 4. Change in body mass index
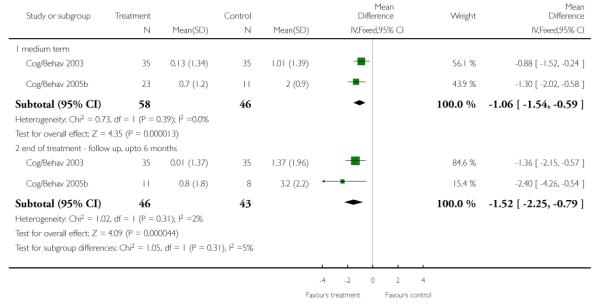 |
Analysis 1.5. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 5 Weight: 5. Change in waist circumference
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 5 Weight: 5. Change in waist circumference
 |
Analysis 1.6. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus
STANDARD CARE, Outcome 6 Weight: 6. Increase initial weight by 7%.
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 6 Weight: 6. Increase initial weight by 7%
 |
Analysis 1.7. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 7 Global state: 1. Improvement in quality of life, mean change score - 3 months (CGI, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 7 Global state: 1. Improvement in quality of life, mean change score - 3 months (CGI, high=worse)
 |
Analysis 1.8. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 8 Global state: 2. Improvement in overall health, mean change score - 3 months (CGI, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 8 Global state: 2. Improvement in overall health, mean change score - 3 months (CGI, high=worse)
 |
Analysis 1.9. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 9 Global state: 3. Improvement in body image, mean change score - 3 months (CGI, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 9 Global state: 3. Improvement in body image, mean change score - 3 months (CGI, high=worse)
 |
Analysis 1.10. Comparison 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 10 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 1 PREVENTION: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 10 Leaving the study early
 |
Analysis 2.1. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 2.2. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 2.3. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 3 Weight: 3. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 3 Weight: 3. Body mass index
 |
Analysis 2.4. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 4 Weight: 4. Change in body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 4 Weight: 4. Change in body mass index
 |
Analysis 2.5. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 5 Weight: 5. Increase initial weight by 7%
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 5 Weight: 5. Increase initial weight by 7%
 |
Analysis 2.6. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 6 Global state: 1. Average endpoint change scores - 6 weeks (CGI, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 6 Global state: 1. Average endpoint change scores - 6 weeks (CGI, high=worse)
 |
Analysis 2.7. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 7 Mental state: 1. Average endpoint change score - 16 weeks (BPRS 6 items, high score=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 7 Mental state: 1. Average endpoint change score - 16 weeks (BPRS 6 items, high score=worse)
 |
Analysis 2.8. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 8 Mental state: 2. Average endpoint change score - 6 weeks (SAPS, worse=poor)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 8 Mental state: 2. Average endpoint change score - 6 weeks (SAPS, worse=poor)
 |
Analysis 2.9. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 9 Mental state: 3. Average endpoint change score - 6 weeks (SANS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 9 Mental state: 3. Average endpoint change score - 6 weeks (SANS, high=worse)
 |
Analysis 2.10. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 10 Adverse effects: 1. Movement disorders - average endpoint change scores (SAS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 10 Adverse effects: 1. Movement disorders - average endpoint change scores (SAS, high=worse)
 |
Analysis 2.11. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 11 Adverse effects: 2. Daytime somnolence
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 11 Adverse effects: 2. Daytime somnolence
 |
Analysis 2.12. Comparison 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO, Outcome 12 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 2 PREVENTION: 2. H2 ANTAGONISTS versus PLACEBO
Outcome: 12 Leaving the study early
 |
Analysis 3.1. Comparison 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 3.2. Comparison 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 3.3. Comparison 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE, Outcome 3 Mental state: 1. Average endpoint change score - 16 weeks (BPRS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE
Outcome: 3 Mental state: 1. Average endpoint change score - 16 weeks (BPRS, high=worse)
 |
Analysis 3.4. Comparison 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE, Outcome 4 Adverse effects: 1. Movement disorders - average endpoint change score - 16 weeks (SAS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE
Outcome: 4 Adverse effects: 1. Movement disorders - average endpoint change score - 16 weeks (SAS, high=worse)
 |
Analysis 3.5. Comparison 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE, Outcome 5 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 3 PREVENTION: 3. H2 ANTAGONISTS - HIGHER DOSE versus LOWER DOSE
Outcome: 5 Leaving the study early
 |
Analysis 4.1. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg) (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg) (short term)
 |
Analysis 4.2. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 2 Weight: 2. Change (kgs) (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 2 Weight: 2. Change (kgs) (short term)
 |
Analysis 4.3. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 3 Weight: 3. Body mass index (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 3 Weight: 3. Body mass index (short term)
 |
Analysis 4.4. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 4 Mental state: 1. Average endpoint change score - 8 weeks (SANS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 4 Mental state: 1. Average endpoint change score - 8 weeks (SANS, high=worse)
 |
Analysis 4.5. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 5 Mental state: 2. Average endpoint change score - 8 weeks (SAPS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 5 Mental state: 2. Average endpoint change score - 8 weeks (SAPS, high=worse)
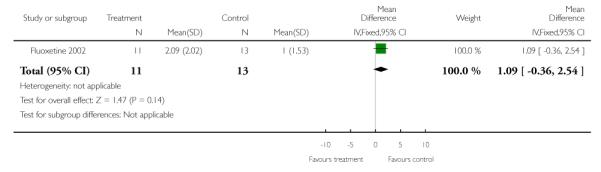 |
Analysis 4.6. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 6 Mental state: 3. Average endpoint change score - 8 weeks (HAM-D, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 6 Mental state: 3. Average endpoint change score - 8 weeks (HAM-D, high=worse)
 |
Analysis 4.7. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 7 Adverse effects: 1. Any (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 7 Adverse effects: 1. Any (short term)
 |
Analysis 4.8. Comparison 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 8 Leaving the study early (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 4 PREVENTION: 4. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 8 Leaving the study early (short term)
 |
Analysis 5.1. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 5.2. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 5.3. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 3 Weight: 3. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 3 Weight: 3. Body mass index
 |
Analysis 5.4. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 4 Weight: 4. BMI change
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 4 Weight: 4. BMI change
 |
Analysis 5.5. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 5 Weight: 5. Increase initial weight by 7%
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 5 Weight: 5. Increase initial weight by 7%
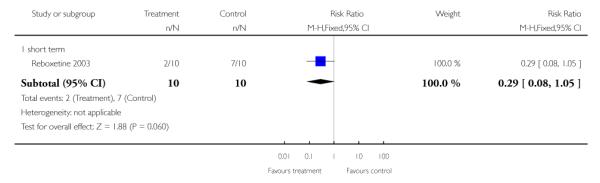 |
Analysis 5.6. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 6 Global state: 1. Average endpoint change scores - 6 weeks (CGI, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 6 Global state: 1. Average endpoint change scores - 6 weeks (CGI, high=worse)
 |
Analysis 5.7. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 7 Mental state: 1. Average endpoint change score - 6 weeks (SAPS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 7 Mental state: 1. Average endpoint change score - 6 weeks (SAPS, high=worse)
 |
Analysis 5.8. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 8 Mental state: 2. Average endpoint change score - 6 weeks (SANS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 8 Mental state: 2. Average endpoint change score - 6 weeks (SANS, high=worse)
 |
Analysis 5.9. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 9 Mental state: 3. Average endpoint change score - 6 weeks (HAM-D, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 9 Mental state: 3. Average endpoint change score - 6 weeks (HAM-D, high=worse)
 |
Analysis 5.10. Comparison 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO, Outcome 10 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 5 PREVENTION: 5. SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR versus PLACEBO
Outcome: 10 Leaving the study early
 |
Analysis 6.1. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 6.2. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 6.3. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 3 Weight: 3. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 3 Weight: 3. Body mass index
 |
Analysis 6.4. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 4 Weight: 4. Waist circumference
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 4 Weight: 4. Waist circumference
 |
Analysis 6.5. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 5 Mental state: 1. Average endpoint change scores - 14 weeks (BPRS total, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 5 Mental state: 1. Average endpoint change scores - 14 weeks (BPRS total, high=worse)
 |
Analysis 6.6. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 6 Physiological
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 6 Physiological
 |
Analysis 6.7. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 7 Physiological (skewed data)
| Study | Intervention | Mean | SD | N |
|---|---|---|---|---|
| insulin, basal | ||||
| Metformin 2006 | Antidiabetic agents | 15.30 | 8.20 | 19 |
| Metformin 2006 | Placebo | 14.4 | 5.10 | 18 |
| homa-IR | ||||
| Metformin 2006 | Antidiabetic agents | 2.90 | 1.30 | 19 |
| Metformin 2006 | Placebo | 3.10 | 1.70 | 18 |
| triglycerides | ||||
| Metformin 2006 | Antidiabetic agents | 123.0 | 64.7 | 19 |
| Metformin 2006 | Placebo | 153.0 | 86.8 | 18 |
| vldl cholesterol | ||||
| Metformin 2006 | Antidiabetic agents | 24.60 | 12.9 | 19 |
| Metformin 2006 | Placebo | 30.5 | 17.4 | 18 |
Analysis 6.8. Comparison 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO, Outcome 8 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 6 PREVENTION: 6. ANTIDIABETIC AGENTS versus PLACEBO
Outcome: 8 Leaving the study early
 |
Analysis 7.1. Comparison 7 PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL, Outcome 1 Weight: 1. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 7 PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL
Outcome: 1 Weight: 1. Change (kgs)
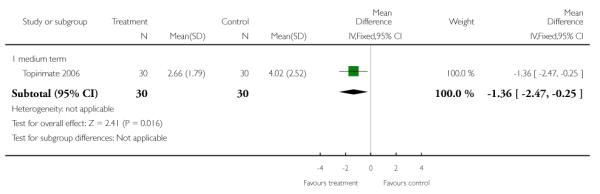 |
Analysis 7.2. Comparison 7 PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL, Outcome 2 Adverse effects: 1. Insomnia
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 7 PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL
Outcome: 2 Adverse effects: 1. Insomnia
 |
Analysis 7.3. Comparison 7 PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL, Outcome 3 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 7 PREVENTION: 7. ANTICONVULSANT AGENT versus CONTROL
Outcome: 3 Leaving the study early
 |
Analysis 8.1. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 8.2. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 2 Weight: 2. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 2 Weight: 2. Body mass index
 |
Analysis 8.3. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 3 Weight: 3. Increase initial weight by 7%
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 3 Weight: 3. Increase initial weight by 7%
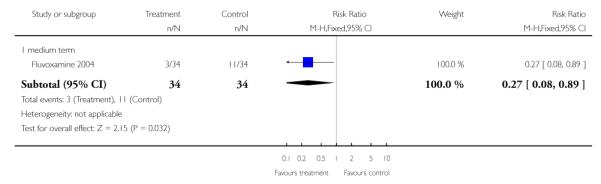 |
Analysis 8.4. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 4 Global state: 1. Average total endpoint score - 12 weeks (GAF, high=good)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 4 Global state: 1. Average total endpoint score - 12 weeks (GAF, high=good)
 |
Analysis 8.5. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 5 Global state: 2. Average total endpoint score - 12 weeks (CGI, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 5 Global state: 2. Average total endpoint score - 12 weeks (CGI, high=worse)
 |
Analysis 8.6. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 6 Adverse effects: 1. Physical (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 6 Adverse effects: 1. Physical (medium term)
 |
Analysis 8.7. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 7 Adverse effects: 2. Side effects (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 7 Adverse effects: 2. Side effects (medium term)
 |
 |
Analysis 8.8. Comparison 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE, Outcome 8 Physiological (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 8 PREVENTION: 8. SSRI’s + LOW DOSE CLOZAPINE versus HIGH DOSE CLOZAPINE
Outcome: 8 Physiological (medium term)
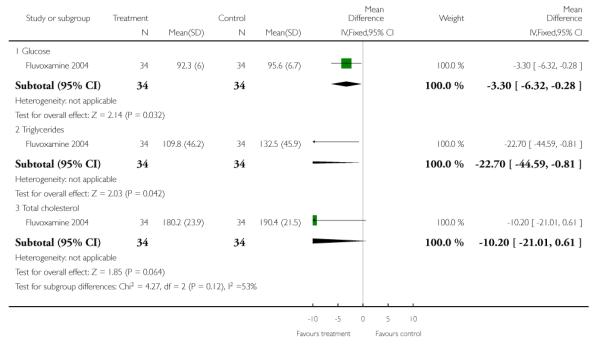 |
Analysis 9.1. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 1 Weight: 1. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 1 Weight: 1. Change (kgs)
 |
Analysis 9.2. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 2 Weight: 2. BMI change
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 2 Weight: 2. BMI change
 |
Analysis 9.3. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 3 Weight: 3. Waist-hip change
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 3 Weight: 3. Waist-hip change
 |
Analysis 9.4. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 4 Weight: 4. No loss (only >10% loss data)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 4 Weight: 4. No loss (only >10% loss data)
 |
Analysis 9.5. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 5 Weight: 5. No loss (only >5% loss data)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 5 Weight: 5. No loss (only >5% loss data)
 |
Analysis 9.6. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 6 Satisfaction: 1. Average total endpoint score - 14 weeks (CSQ-8, high=positive)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 6 Satisfaction: 1. Average total endpoint score - 14 weeks (CSQ-8, high=positive)
 |
Analysis 9.7. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 7 Mental state: 1. Average endpoint score - 14 weeks (PANSS total, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 7 Mental state: 1. Average endpoint score - 14 weeks (PANSS total, high=worse)
 |
Analysis 9.8. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 8 Global state: 1. Not much improved (CGI-C)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 8 Global state: 1. Not much improved (CGI-C)
 |
Analysis 9.9. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 9 Physiological: Blood pressure
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 9 Physiological: Blood pressure
 |
Analysis 9.10. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 10 Laboratory data: Number increasing ratio of LDL to HDL
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 10 Laboratory data: Number increasing ratio of LDL to HDL
 |
Analysis 9.11. Comparison 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE, Outcome 11 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 9 TREATMENT: 1. COGNITIVE/BEHAVIORAL INTERVENTION versus STANDARD CARE
Outcome: 11 Leaving the study early
 |
Analysis 10.1. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 10.2. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 2 Weight: 2a. Change (kgs) (heterogeneous data)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 2 Weight: 2a. Change (kgs) (heterogeneous data)
 |
Analysis 10.3. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 3 Weight: 2b. Body weigh (kg) studies analysed seperately
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 3 Weight: 2b. Body weigh (kg) studies analysed seperately
 |
Analysis 10.4. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 4 Weight: 3. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 4 Weight: 3. Body mass index
 |
Analysis 10.5. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 5 Weight: 4. Change in body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 5 Weight: 4. Change in body mass index
 |
Analysis 10.6. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 6 Weight: 5. Waist circumference
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 6 Weight: 5. Waist circumference
 |
Analysis 10.7. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 7 Weight: 6. Change in waist circumference (cm)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 7 Weight: 6. Change in waist circumference (cm)
 |
Analysis 10.8. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 8 Weight: 7. Waist-hip ratio
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 8 Weight: 7. Waist-hip ratio
 |
Analysis 10.9. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 9 Weight: 8. Change in waist-hip ratio
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 9 Weight: 8. Change in waist-hip ratio
 |
Analysis 10.10. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 10 Weight: 9. Hip circumference
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 10 Weight: 9. Hip circumference
 |
Analysis 10.11. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 11 Weight: 10. Body fat (%)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 11 Weight: 10. Body fat (%)
 |
Analysis 10.12. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 12 Global state: 1. Average total endpoint score - medium term (GAS, high=good)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 12 Global state: 1. Average total endpoint score - medium term (GAS, high=good)
 |
Analysis 10.13. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 13 Mental state: 1. Average total endpoint score - medium term (PANSS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 13 Mental state: 1. Average total endpoint score - medium term (PANSS, high=worse)
 |
Analysis 10.14. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 14 Mental state: 2. Average total endpoint score - medium term (SANS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 14 Mental state: 2. Average total endpoint score - medium term (SANS, high=worse)
 |
Analysis 10.15. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 15 Extrapyramidal symptoms: (skewed data)
Extrapyramidal symptoms: (skewed data)
| Study | Intervention | Mean | SD | N |
|---|---|---|---|---|
| hillside akathisia scale | ||||
| Sibutramine 2005a | Anti-obesity drugs | 1.10 | 2.40 | 19 |
| Sibutramine 2005a | Placebo | 0.80 | 1.80 | 18 |
| abnormal involuntary movement scale | ||||
| Sibutramine 2005a | Anti-obesity drugs | 2.20 | 1.50 | 19 |
| Sibutramine 2005a | Placebo | 0.90 | 1.20 | 18 |
Analysis 10.16. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 16 Adverse effects: 1. Related to satiety (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 16 Adverse effects: 1. Related to satiety (medium term)
 |
Analysis 10.17. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 17 Adverse effects: 2. Related to sleep (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 17 Adverse effects: 2. Related to sleep (medium term)
 |
Analysis 10.18. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 18 Adverse effects: 3. Other (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 18 Adverse effects: 3. Other (medium term)
 |
 |
Analysis 10.19. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 19 Cardiovascular (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 19 Cardiovascular (medium term)
 |
Analysis 10.20. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 20 Laboratory data (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 20 Laboratory data (medium term)
 |
 |
Analysis 10.21. Comparison 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO, Outcome 21 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 10 TREATMENT: 2. ANTI OBESITY DRUGS +/− LIFE STYLE MANAGEMENT versus PLACEBO
Outcome: 21 Leaving the study early
 |
Analysis 11.1. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 11.2. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 11.3. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 3 Weight: 3. Body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 3 Weight: 3. Body mass index
 |
Analysis 11.4. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 4 Weight: 4. Change in body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 4 Weight: 4. Change in body mass index
 |
Analysis 11.5. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 5 Mental state: 1. Average endpoint scores - 8 weeks (PANSS total, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 5 Mental state: 1. Average endpoint scores - 8 weeks (PANSS total, high=worse)
 |
Analysis 11.6. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 6 Adverse effects: 1. Any
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 6 Adverse effects: 1. Any
 |
Analysis 11.7. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 7 Other physiological: 1a. Leptin levels
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 7 Other physiological: 1a. Leptin levels
 |
Analysis 11.8. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 8 Other physiological: 1b. Leptin change
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 8 Other physiological: 1b. Leptin change
 |
Analysis 11.9. Comparison 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO, Outcome 9 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 11 TREATMENT: 3. H2 ANTAGONISTS versus PLACEBO
Outcome: 9 Leaving the study early
 |
Analysis 12.1. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 12.2. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 2 Weight: 2. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 2 Weight: 2. Change (kgs)
 |
Analysis 12.3. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 3 Mental state: Average endpoint scores - 12 weeks (PANSS total, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 3 Mental state: Average endpoint scores - 12 weeks (PANSS total, high=worse)
 |
Analysis 12.4. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 4 Adverse effects: 1. Side-effects (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 4 Adverse effects: 1. Side-effects (medium term)
 |
 |
Analysis 12.5. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 5 Adverse effects: 2. Related to sleep (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 5 Adverse effects: 2. Related to sleep (short term)
 |
Analysis 12.6. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 6 Adverse effects: 3. Appetite (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 6 Adverse effects: 3. Appetite (short term)
 |
Analysis 12.7. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 7 Adverse effects: 4. Physical (short term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 7 Adverse effects: 4. Physical (short term)
 |
Analysis 12.8. Comparison 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO, Outcome 8 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 12 TREATMENT: 4. APPETITE SUPPRESSANT versus PLACEBO
Outcome: 8 Leaving the study early
 |
Analysis 13.1. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 1 Weight: 1. Body weight (kg)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 1 Weight: 1. Body weight (kg)
 |
Analysis 13.2. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 2 Weight: 2. Percent body fat
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 2 Weight: 2. Percent body fat
 |
Analysis 13.3. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 3 Mental state: 1a. Positive symptoms, average change score - 4 months (PANSS total, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 3 Mental state: 1a. Positive symptoms, average change score - 4 months (PANSS total, high=worse)
 |
Analysis 13.4. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 4 Mental state: 1b. Negative symptoms, average change score - 4 months (PANSS total, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 4 Mental state: 1b. Negative symptoms, average change score - 4 months (PANSS total, high=worse)
 |
Analysis 13.5. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 5 Mental state: 2. Depressive symptoms, average change score - 4 months (HAM-D, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 5 Mental state: 2. Depressive symptoms, average change score - 4 months (HAM-D, high=worse)
 |
Analysis 13.6. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 6 Extrapyramidal symptoms: 1. Tardive dyskinesia, average change score - 4 months (AIMS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 6 Extrapyramidal symptoms: 1. Tardive dyskinesia, average change score - 4 months (AIMS, high=worse)
 |
Analysis 13.7. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 7 Extrapyramidal symptoms: 2. Parkinsonism, average total endpoint score - 4 months (SAS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 7 Extrapyramidal symptoms: 2. Parkinsonism, average total endpoint score - 4 months (SAS, high=worse)
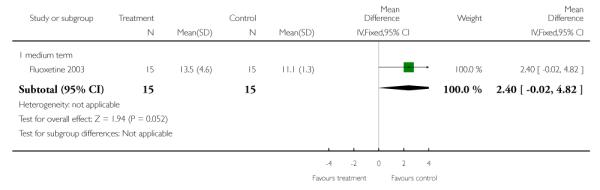 |
Analysis 13.8. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 8 Extrapyramidal symptoms: 3. Akathisia, average change score - 4 months (BAS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 8 Extrapyramidal symptoms: 3. Akathisia, average change score - 4 months (BAS, high=worse)
 |
Analysis 13.9. Comparison 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO, Outcome 9 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 13 TREATMENT: 5. 5HT REUPTAKE BLOCKER versus PLACEBO
Outcome: 9 Leaving the study early
 |
Analysis 14.1. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 1 Weight: 1. Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 1 Weight: 1. Change (kgs)
 |
Analysis 14.2. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 2 Weight: 2. Change in body mass index
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 2 Weight: 2. Change in body mass index
 |
Analysis 14.3. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 3 Weight: 3. No gain
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 3 Weight: 3. No gain
 |
Analysis 14.4. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 4 Weight: 4. Increase initial weight by 7%
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 4 Weight: 4. Increase initial weight by 7%
 |
Analysis 14.5. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 5 Weight: 5. Decrease initial weight by 7%
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 5 Weight: 5. Decrease initial weight by 7%
 |
Analysis 14.6. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 6 Mental state: 1. Average change scores - medium term (BPRS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 6 Mental state: 1. Average change scores - medium term (BPRS, high=worse)
 |
Analysis 14.7. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 7 Mental state: 2. Average change scores - medium term (MADRS, high=worse)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 7 Mental state: 2. Average change scores - medium term (MADRS, high=worse)
 |
Analysis 14.8. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 8 Adverse effects: 1. Worsening of schizophrenia
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 8 Adverse effects: 1. Worsening of schizophrenia
 |
Analysis 14.9. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 9 Adverse effects: 2. Lipid abnormalities (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 9 Adverse effects: 2. Lipid abnormalities (medium term)
 |
Analysis 14.10. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 10 Adverse effects: 3. Other (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 10 Adverse effects: 3. Other (medium term)
 |
Analysis 14.11. Comparison 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO, Outcome 11 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 14 TREATMENT: 6. ANTIPARKINSONIAN DRUG versus PLACEBO
Outcome: 11 Leaving the study early
 |
Analysis 15.1. Comparison 15 TREATMENT: 7. ANTICONVULSANTS versus PLACEBO, Outcome 1 Weight: Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 15 TREATMENT: 7. ANTICONVULSANTS versus PLACEBO
Outcome: 1 Weight: Change (kgs)
 |
Analysis 15.2. Comparison 15 TREATMENT: 7. ANTICONVULSANTS versus PLACEBO, Outcome 2 Adverse events (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 15 TREATMENT: 7. ANTICONVULSANTS versus PLACEBO
Outcome: 2 Adverse events (medium term)
 |
 |
Analysis 15.3. Comparison 15 TREATMENT: 7. ANTICONVULSANTS versus PLACEBO, Outcome 3 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 15 TREATMENT: 7. ANTICONVULSANTS versus PLACEBO
Outcome: 3 Leaving the study early
 |
Analysis 16.1. Comparison 16 TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose, Outcome 1 Weight: Change (kgs)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 16 TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose
Outcome: 1 Weight: Change (kgs)
 |
Analysis 16.2. Comparison 16 TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose, Outcome 2 Adverse events (medium term)
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 16 TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose
Outcome: 2 Adverse events (medium term)
 |
 |
Analysis 16.3. Comparison 16 TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose, Outcome 3 Leaving the study early
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 16 TREATMENT: 8. ANTICONVULSANTS - higher dose versus ANTICONVULSANTS - lower dose
Outcome: 3 Leaving the study early
 |
Analysis 17.1. Comparison 17 PREVENTION: 9. (PHARMACOLOGICAL) MEAN WEIGHT CHANGE, Outcome 1 End of treatment
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 17 PREVENTION: 9. (PHARMACOLOGICAL) MEAN WEIGHT CHANGE
Outcome: 1 End of treatment
 |
Analysis 18.1. Comparison 18 TREATMENT: 10. (PHARMACOLOGICAL) MEAN WEIGHT CHANGE, Outcome 1 End of treatment
Review: Interventions to reduce weight gain in schizophrenia
Comparison: 18 TREATMENT: 10. (PHARMACOLOGICAL) MEAN WEIGHT CHANGE
Outcome: 1 End of treatment
 |
HISTORY
Protocol first published: Issue 4, 2004
Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 26 April 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment |
WHAT’S NEW
Last assessed as up-to-date: 13 November 2006.
| Date | Event | Description |
|---|---|---|
| 15 February 2010 | Amended | Contact details updated. |
Footnotes
DECLARATIONS OF INTEREST In the past year Tony Cohn has received grant funding and accepted speaker fees from Novartis, Canada
References to studies included in this review
- Amantadine 2005 {published and unpublished data} .Deberdt W, Winokur A, Cavazzoni PA, Trzaskoma QN, Carlson CD, Bymaster FP, Wiener K, Floris M, Breier A. Amantadine for weight gain associated with olanzapine treatment. European Neuropsychopharmacology. 2005;15:13–21. doi: 10.1016/j.euroneuro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Amantadine 2005b {published data only} .Graham KA, Gu H, Lieberman JA, Harp JB, Perkins DO. Double-blind, placebo-controlled investigation of amantadine for weight loss in subjects who gained weight with olanzapine. American Journal of Psychiatry. 2005;162:1744–6. doi: 10.1176/appi.ajp.162.9.1744. [DOI] [PubMed] [Google Scholar]
- Cog/Behav 2003 {published data only} .Littrell KH, Hilligoss NM, Kirshner CD, Petty RG, Johnson CG. The effects of an educational intervention on antipsychotic-induced weight gain. Journal of Nursing Scholarship. 2003;35(3):237–41. doi: 10.1111/j.1547-5069.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- Cog/Behav 2005a {published data only} .Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry. 2005;66:205–12. doi: 10.4088/jcp.v66n0208. [DOI] [PubMed] [Google Scholar]
- Cog/Behav 2005b {published data only} .Evans S, Newton R, Higgins S. Nutritional intervention to prevent weight gain in patients commenced on olanzapine: A randomized controlled trial. Australian and New Zealand Journal of Psychiatry. 2005;39:479–86. doi: 10.1080/j.1440-1614.2005.01607.x. [DOI] [PubMed] [Google Scholar]
- Cog/Behav 2006a {published data only} .Weber M, Wyne K. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophrenia Research. 2006;83:95–101. doi: 10.1016/j.schres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Cog/Behav 2006b {published data only} .Kwon JS, Choi JS, Bahk WM, Kim CY, Kim CH, Shin YC, Park BJ, Oh CG. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. Journal of Clinical Psychiatry. 2006;67:547–53. doi: 10.4088/jcp.v67n0405. [DOI] [PubMed] [Google Scholar]
- D-Fenfluramine 1988 {published data only} .Goodall E, Oxtoby C, Richards R, Watkinson G, Brown D, Silverstone T. A clinical trial of the efficacy and acceptability of D-Fenfluramine in the treatment of neuroleptic-induced obesity. British Journal of Psychiatry. 1988;153:208–13. doi: 10.1192/bjp.153.2.208. [DOI] [PubMed] [Google Scholar]
- Dextroamphetam 1965 {published data only} .Modell W, Hussar AE. Failure of dextroamphetamine sulfate to influence eating and sleeping patterns in obese schizophrenic patients. Journal of the American Medical Association. 1965;193:95–8. doi: 10.1001/jama.1965.03090040019006. [DOI] [PubMed] [Google Scholar]
- Famotidine 2004 {published data only} .Poyurovsky M, Tal V, Maayan R, Gil-Ad I, Fuchs C, Weizman A. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: A double-blind placebo-controlled pilot study. European Neuropsychopharmacology. 2004;14:332–6. doi: 10.1016/j.euroneuro.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fluoxetine 2002 {published data only} .Poyurovsky M, Pashinian A, Gil-Ad I, Maayan R, Schneidman M, Fuchs C, Weizman A. Olanzapine-induced weight gain in patients with first-episode schizophrenia: A double-blind, placebo-controlled study of fluoxetine addition. American Journal of Psychiatry. 2002;159:1058–60. doi: 10.1176/appi.ajp.159.6.1058. [DOI] [PubMed] [Google Scholar]
- Fluoxetine 2003 {published data only} .Bustillo JR, Lauriello J, Parker K, Hammond R, Rowland L, Bogenschutz M, Keith S. Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Neuropsychopharmacology. 2003;28:527–9. doi: 10.1038/sj.npp.1300089. [DOI] [PubMed] [Google Scholar]
- Fluvoxamine 2004 {published data only} .Lu ML, Lane HY, Lin SK, Chen KP, Chang WH. Adjunctive fluvoxamine inhibits clozapine-related weight gain and metabolic disturbances. Journal of Clinical Psychiatry. 2004;65:766–71. doi: 10.4088/jcp.v65n0607. [DOI] [PubMed] [Google Scholar]
- Metformin 2006 {published data only} .Baptista T, Martinez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, Arape Y, Martinez M, de Mendoza S, Teneud L, Hernandez L. Metformin for prevention of weight gain and insulin resistance with olanzapine: A double-blind placebo-controlled trial. Canadian Journal of Psychiatry. 2006;51:192–6. doi: 10.1177/070674370605100310. [DOI] [PubMed] [Google Scholar]
- Nizatidine 2003a {published and unpublished data} .Cavazzoni P, Tanaka Y, Roychowdhury SM, Breier A, Allison DB. Nizatidine for prevention of weight gain with olanzapine: A double-blind placebo-controlled trial. European Neuropsychopharmacology. 2003;13:81–5. doi: 10.1016/s0924-977x(02)00127-x. [DOI] [PubMed] [Google Scholar]
- Nizatidine 2003b {published data only} .Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Nizatidine treatment and its relationship with leptin levels in patients with olanzapine-induced weight gain. Human Psychopharmacology. 2003;18:457–61. doi: 10.1002/hup.514. [DOI] [PubMed] [Google Scholar]
- Nizatidine 2004 {published data only} .Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Kilic N. Nizatidine for the treatment of patients with quetiapine-induced weight gain. Human Psychopharmacology. 2004;19:37–40. doi: 10.1002/hup.477. [DOI] [PubMed] [Google Scholar]
- Phenylpropanol 2002 {published data only} .Borovicka MC, Fuller MA, Konicki E, White JC, Steele V, Jaskiw GE. Phenylpropanolamine appears not to promote weight loss in patients with schizophrenia who have gained weight during clozapine treatment. Journal of Clinical Psychiatry. 2002;63(4):345–48. doi: 10.4088/jcp.v63n0412. [DOI] [PubMed] [Google Scholar]
- Reboxetine 2003 {published data only} .Poyurovsky M, Isaacs I, Fuchs C, Schneidman M, Faragian S, Weizman R, Weizman A. Attenuation of olanzapine-induced weight gain with reboxetine in patients withschizophrenia: A double-blind, placebo-controlled study. American Journal of Psychiatry. 2003;160:297–302. doi: 10.1176/appi.ajp.160.2.297. [DOI] [PubMed] [Google Scholar]
- Sibutramine 2005a {published data only} .Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD, Louie PM, Evins AE, Freudenreich MD, Hayden D, Goff DC. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. American Journal of Psychiatry. 2005;162:954–62. doi: 10.1176/appi.ajp.162.5.954. [DOI] [PubMed] [Google Scholar]
- Sibutramine 2005b {published and unpublished data} .Weiden P, Radulovic L, Wang C, Allison D. Sibutramine for the treatment of obesity in schizophrenia: Randomized, placebo controlled pilot study. Unpublished.
- Topiramate 2005 {published data only} .Ko Y-H, Joe S-H, Kim S-H. Topiramate as an adjunctive treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clinical Neuropharmacology. 2005;28(4):169–75. doi: 10.1097/01.wnf.0000172994.56028.c3. [DOI] [PubMed] [Google Scholar]
- Topirimate 2006 {published data only} .Kim JH, Yim SJ, Nam JH. A 12-week, randomized, open-label, parallel-group trial of topiramate in limiting weight gain during olanzaoine in patients with scizophrenia. Schizophrenia Research. 2006;82(1):115–7. doi: 10.1016/j.schres.2005.10.001. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Archie 2003 {published data only} .Archie S, Wilson JW, Osborne S, Hobbs H, McNiven J. Pilot study: Access to fitness facility and exercise levels in olanzapine-treated patients. Candian Journal of Psychiatry. 2003;48:628–32. doi: 10.1177/070674370304800910. [DOI] [PubMed] [Google Scholar]
- Harmatz 1968 {published data only} .Harmatz MG, Lapuc P. Behavior modification of overeating in a psychiatric population. Journal of Consulting and Clinical Psychology. 1968;32(5):583–87. doi: 10.1037/h0026402. [DOI] [PubMed] [Google Scholar]
- McCreadie 2005 {published data only} .McCreadie RG, Kelly C, Connolly M, Williams S, Baxter G, Lean M, Paterson JR. Dietary improvement in people with schizophrenia. British Journal of Psychiatry. 2005;187:346–51. doi: 10.1192/bjp.187.4.346. [DOI] [PubMed] [Google Scholar]
- Morrison 2002 {published data only} .Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. American Journal of Psychiatry. 2002;159:655–57. doi: 10.1176/appi.ajp.159.4.655. [DOI] [PubMed] [Google Scholar]
- Nickel 2005 {published data only} .Nickel MK, Nickel C, Muehlbacher M, Leiberich PK, Kaplan P, Lahmann C, Tritt K, Krawczyk J, Kettler C, Egger C, Rother WK, Loew TH. Influence of topiramate on olanzapine-related adiposity in women: A random, double-blind, placebo-controlled study. Journal of Clinical Psychopharmacology. 2005;25:211–17. doi: 10.1097/01.jcp.0000162806.46453.38. [DOI] [PubMed] [Google Scholar]
- Rotatori 1980 {published data only} .Rotatori AF, Fox R, Wicks A. Weight loss with psychiatric residents in a behavioral self-control program. Psychological Reports. 1980;46:483–86. doi: 10.2466/pr0.1980.46.2.483. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Alvarez-Jimenez 2006 {published data only} .Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, Perez-Iglesias R, Martinez-Garcia O, Perez-Pardal T, Ramirez-Bonilla ML, Crespo-Facorro B. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: A randomized controlled trial. Journal of Clinical Psychiatry. 2006;67:1253–260. doi: 10.4088/jcp.v67n0812. [DOI] [PubMed] [Google Scholar]
- Brown 2006 {published data only} .Brown S, Chan K. A randomized controlled trial of a brief health promotion intervention in a population with serious mental illness. Journal of Mental Health. 2006;15:543–49. [Google Scholar]
- Ganguli 2005 {published data only} .Ganguli R, Brar JS. Prevention of weight gain by behavioral interventions in patients starting novel antipsychotics. Schizophrenia Bulletin. 2005;Vol. 31:561–62. [Google Scholar]
- McKibbin 2006 {published data only} .McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, Mudalier S, Barrio C, O’Hanlon K, Griver K, Sirkin A, Jeste DV. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: A randomized controlled trial. Schizophrenia Research. 2006;86:36–44. doi: 10.1016/j.schres.2006.05.010. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Bristol-Meyer Squib {published data only (unpublished sought but not used)} .Bristol-Meyer Squibb. Otsuka America Pharmaceutical. Effects of aripiprazole in overweight patients treated with olanzapine for schizophrenia or schizoaffective disorder. Clinical Trials.gov. 2004.
- Ritchie 2003 {published data only (unpublished sought but not used)} .Ritchie C. The efficacy of pindolol in reducing weight gain associated with the use of olanzapine. NHS Trusts Clinical Trials Register. 2003.
Additional references
- ADA/APA 2004 .American Diabetes Association. the American Psychiatric Association. the American Association of Clinical Endocrinologists. the North American Association for the Study of Obesity Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Allison 1999 .Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: A comprehensive research synthesis. American Journal of Psychiatry. 1999;156:1686–9. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Allison 2003 .Allison DB, Mackell JA, McDonnell DD. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatric Services. 2003;54:565–67. doi: 10.1176/appi.ps.54.4.565. [DOI] [PubMed] [Google Scholar]
- Altman 1996 .Altman DG, Bland JM. Detecing skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [: OLZ020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth 2004 .Ananth J, Venkatesh R, Burgoyne K, Gadasalli R, Binford R, Gunatilake S. Atypical antipsychotic induced weight gain: pathophysiology and management. Annals of Clinical Psychiatry. 2004;16:75–85. doi: 10.1080/10401230490453293. [DOI] [PubMed] [Google Scholar]
- Andreasen 1983 .Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry. 1982;39:784–8. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen 1984 .Andreasson NC. Scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City: 1984. [Google Scholar]
- Attkisson 1982 .Attkisson CC, Zwick R. The client satisfaction questionnaire: psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning. 1982;5:233–7. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Baptista 2002 .Baptista T, Kin NM, Beaulieu S, de Baptista EA. Obesity and related metabolic abnormalities during antipsychotic drug administration : Mechanisms, management and research perspectives. Pharmacopsychiatry. 2002;35:205–19. doi: 10.1055/s-2002-36391. [DOI] [PubMed] [Google Scholar]
- Barnes 1989 .Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Begg 1996 .Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of randomized controlled trials: The CONSORT statement. JAMA. 1996;276:637–9. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- Birt 2003 .Birt J. Management of weight gain associated with antipsychotics. Annals of Clinical Psychiatry. 2003;15:49–58. doi: 10.1023/a:1023280610379. [DOI] [PubMed] [Google Scholar]
- Bland 1997 .Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutron 2004 .Boutron I, Tubach F, Giraudeau B, Ravaud P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. Journal of Clinical Epidemiology. 2004;57:543–50. doi: 10.1016/j.jclinepi.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Brown 1999 .Brown S, Birtwhistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychological Medicine. 1999;29:697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Casey 2004 .Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, Lindenmayer JP, Manoukian SV, Banerji MA, Lebovitz HE, Hennekens CH. Antipsychotic-induced weight gain and metabolic abnormalities: Implications for increased mortality in patients with schizophrenia. Journal of Clinical Psychiatry. 2004;65(Suppl 7):4–18. [PubMed] [Google Scholar]
- Catapana 2004 .Catapana L, Castle D. Obesity in schizophrenia: What can be done about it? Australasian Psychiatry. 2004;12:23–5. doi: 10.1046/j.1039-8562.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- Chue 2004 .Chue P. The assessment and management of antipsychotic-associated metabolic disturbances from a psychiatric perspective. Canadian Journal of Psychiatry. 2004;49:200–7. doi: 10.1177/070674370404900308. [DOI] [PubMed] [Google Scholar]
- Cohn 2004 .Cohn T, Prud’homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: High prevalence of the metabolic syndrome. Canadian Journal of Psychiatry. 2004;49:753–60. doi: 10.1177/070674370404901106. [DOI] [PubMed] [Google Scholar]
- Cohn 2006 .Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Canadian Journal of Psychiatry. 2006;51:492–501. doi: 10.1177/070674370605100804. [DOI] [PubMed] [Google Scholar]
- Coodin 2001 .Coodin S. Body mass index in persons with schizophrenia. Canadian Journal of Psychiatry. 2001;46:549–55. doi: 10.1177/070674370104600610. [DOI] [PubMed] [Google Scholar]
- Daumit 2003 .Daumit GL, Clark JM, Steinwachs DM, Graham CM, Lehman A, Ford DE. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. Journal of Nervous and Mental Disease. 2003;191:799–805. doi: 10.1097/01.nmd.0000100923.20188.2d. [DOI] [PubMed] [Google Scholar]
- Daumit 2005 .Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J, Wohlheiter K, Dixon LB. Physical activity patterns in adults with severe mental illness. The Journal of Nervous and Mental Disease. 2005;193:641–6. doi: 10.1097/01.nmd.0000180737.85895.60. [DOI] [PubMed] [Google Scholar]
- De Nayer 2005 .De Nayer A, De Hert M, Scheen A, Van Gaal L, Peuskens J, on behalf of the Consensus Group Belgian consensus on metabolic problems associated with atypical antipsychotics. International Journal of Psychiatry in Clinical Practice. 2005;9:130–7. doi: 10.1080/13651500510018310. [DOI] [PubMed] [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7:623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- DoH 2004 .Department of Health . At least five a week. A report from the Chief Medical Officer. HMSO; London: 2004. [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- Endicott 1976 .Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale. Archives of General Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Faulkner 1999 .Faulkner G, Biddle S. Exercise and schizophrenia: A review. Journal of Mental Health. 1999;8:441–7. [Google Scholar]
- Faulkner 2003 .Faulkner G, Soundy A, Lloyd K. Weight control and schizophrenia: A systematic review. Acta Psychiatrica Scandinavica. 2003;108:324–32. doi: 10.1034/j.1600-0447.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- Faulkner 2006 .Faulkner G, Cohn TA. Pharmacologic and nonpharmacologic strategies for weight gain and metabolic disturbance in patients treated with antipsychotic medications PPharmacologic and Nonpharmacologic Strategies for Weight Gain and Metabolic Disturbance inatients Treated With Antipsychotic Medications. Canadian Journal of Psychiatry. 2006;51:502–11. doi: 10.1177/070674370605100805. [DOI] [PubMed] [Google Scholar]
- Fleischhacker 1989 .Fleischhacker WW, Bergmann KJ, Perovich R, Pestreich LK, Borenstein M, Lieberman JA, Kane JM. The Hillside Akathisia Scale: A new rating instrument for neuroleptic-induced akathisia. Psychopharmacology Bulletin. 1989;25:222–6. [PubMed] [Google Scholar]
- Fontaine 2001 .Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Research. 2001;101:277–88. doi: 10.1016/s0165-1781(01)00234-7. [DOI] [PubMed] [Google Scholar]
- Frances 1995 .Frances A, Pincus HA, First MB. The Global Assessment of Functioning Scale (GAF). Diagnostic and Statistical Manual of Mental Disorder - IV. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- Goff 2005 .Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah NA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Research. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Gough 2004 .Gough S, Peveler R. Diabetes and its prevention: Pragmatic solutions for people with schizoprhenia. British Journal of Psychiatry. 2004;47:S106–S11. doi: 10.1192/bjp.184.47.s106. [DOI] [PubMed] [Google Scholar]
- Green 2000 .Green A, Patel J, Goisman R. Weight gain from novel antipsychotic drugs: Need for action. General Hospital Psychiatry. 2000;22:224–35. doi: 10.1016/s0163-8343(00)00081-5. [DOI] [PubMed] [Google Scholar]
- Gulliford 1999 .Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Guy 1976 .Guy U. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health; Rockville: 1976. Revised. [Google Scholar]
- Haddock 2002 .Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M, Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. International Journal of Obesity and Related Metabolic Disorders. 2002;26:262–73. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- Hamilton 1960 .Hamilton M. A rating scale of depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoui 2004 .Hamoui N, Kingsbury S, Anthone GJ, Crookes PF. Surgical treatment of obesity in schizophrenic patients. Obesity Surgery. 2004;14:349–52. doi: 10.1381/096089204322917873. [DOI] [PubMed] [Google Scholar]
- Henderson 2005a .Henderson DC, Nguyen DD, Copeland PM, Hayden DL, Borba CP, Louie PM, Freudenreich O, Eden Evins A, Cather C, Goff DC. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: Results of a 10-year naturalistic study. Journal of Clinical Psychiatry. 2005;66:1116–21. doi: 10.4088/jcp.v66n0905. [DOI] [PubMed] [Google Scholar]
- Hewitt 2005 .Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: Review of recent trials published in four general medical journals. BMJ. 2005;330:1057–8. doi: 10.1136/bmj.38413.576713.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2005 .Higgins JPT, Green S, editors. The Cochrane Library. Issue 3. John Wiley & Sons, Ltd; Chichester: [updated May 2005]. 2005. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. issue. [Google Scholar]
- Homel 2002 .Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia. Schizophrenia Research. 2002;55:277–84. doi: 10.1016/s0920-9964(01)00256-0. [DOI] [PubMed] [Google Scholar]
- Jadad 1996 .Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of randomized trials: Is blinding necessary? Control Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jüni 2001 .Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–6. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay 1986 .Kay SR. Positive and negative symptom (PANNS) scale manual. Schizophrenia Bulletin. 1987;13:261–78. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kroeze 2003 .Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–26. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- Kurzthaler 2001 .Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. Journal of Clinical Psychiatry. 2001;62(Supple 7):32–7. [PubMed] [Google Scholar]
- Le Fevre 2001 .Le Fevre PD. Improving the physical health of patients with schizophrenia: Therapeutic nihilism or realism? Scottish Medical Journal. 2001;46:11–31. doi: 10.1177/003693300104600105. [DOI] [PubMed] [Google Scholar]
- Lingjaerde 1987 .Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU Side Effect Rating Scale: A new comprehensive rating scale for psychotropic drugs and corss-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatrica Scandinavica. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Loh 2006 .Loh C, Meyer JM, Leckband SG. A comprehensive review of behavioral interventions for weight management in schizophrenia. Annals of Clinical Psychiatry. 2006;18:23–31. doi: 10.1080/10401230500464646. [DOI] [PubMed] [Google Scholar]
- Mackin 2005 .Mackin P, Watkinson HM, Young AH. Prevalence of obesity, glucose homeostasis disorders and metabolic syndrome in psychiatric patients taking typical or atypical antipsychotic drugs: A cross-sectional study. Diabetologia. 2005;48:215–21. doi: 10.1007/s00125-004-1641-y. [DOI] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales - a major source of bias in randomised controlled trials of treatments for schizophrenia? British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- McCreadie 1998 .McCreadie R, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: Case-control study. BMJ. 1998;317:784–5. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher 2001 .Moher D, Schulz KF, Altman D, CONSORT Group The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–91. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Montgomery 1979 .Montgomery S, Asberg M. A new depression scaled designed to be sensitive to change. British Journal of Psychiatry. 1979;154:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Munetz 1988 .Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hospital and Community Psychiatry. 1988;39:1172–7. doi: 10.1176/ps.39.11.1172. [DOI] [PubMed] [Google Scholar]
- Osborn 2001 .Osborn DPJ. The poor physical health of people with mental illness. Western Journal of Medicine. 2001;175:329–32. doi: 10.1136/ewjm.175.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Padwal 2004 .Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database of Systematic Reviews. 2004;(Issue 3) doi: 10.1002/14651858.CD004094.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters 2003 .Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Annual of Internal Medicine. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- Poston 2001 .Poston WSC, Haddock CK, Dill PL, Thayer B, Foreyt JP. Lifestyle treatments in randomised clinical trials of pharmacotherapies for obesity. Obesity Research. 2001;9:552–63. doi: 10.1038/oby.2001.72. [DOI] [PubMed] [Google Scholar]
- Remington 2005 .Remington G, Chue P, Stip E, Kopala L, Girard T, Christensen B. The crossover approach to switching antipsychotics: What is the evidence? Schizophrenia Research. 2005;76:267–72. doi: 10.1016/j.schres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Richardson 2005 .Richardson CR, Faulkner G, McDevitt J, Skrinar GS, Hutchinson DS, Piette JD. Integrating physical activity into mental health services for persons with serious mental illness. Psychiatric Services. 2005;56:324–31. doi: 10.1176/appi.ps.56.3.324. [DOI] [PubMed] [Google Scholar]
- Saari 2005 .Saari KM, Lindeman SM, Viilo KM, Isohanni MK, Jarvelin MR, Lauren LH, Savolainen MJ, Koponen HJ. A 4-fold risk of metabolic syndrome in patients with schizophrenia: The Northern Finland 1966 birth cohort study. Journal of Clinical Psychiatry. 2005;66:559–63. doi: 10.4088/jcp.v66n0503. [DOI] [PubMed] [Google Scholar]
- Schulz 1995 .Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Shaw 2005 .Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD003818.pub2. [DOI] [PubMed] [Google Scholar]
- Silverstone 1988 .Silverstone T, Smith G, Goodall E. Prevalence of obesity in patients receiving depot antipsychotics. British Journal of Psychiatry. 1988;153:214–7. doi: 10.1192/bjp.153.2.214. [DOI] [PubMed] [Google Scholar]
- Simpson 1970 .Simpson GM, Angus JWS. A rating scale for extrpyramidal side-effects. Acta Psychiatrica Scandinavica Supplementum. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Snow 2005 .Snow V, Barry P, Fitterman N, Qaseem A, Weiss K. Pharmacologic and surgical management of obesity in primary care: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine. 2005;142:525–31. doi: 10.7326/0003-4819-142-7-200504050-00011. [DOI] [PubMed] [Google Scholar]
- Strassnig 2003a .Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophrenia Bulletin. 2003;29:393–7. doi: 10.1093/oxfordjournals.schbul.a007013. [DOI] [PubMed] [Google Scholar]
- Strassnig 2003b .Strassnig M, Brar JS, Ganguli R. Body mass index and quality of life in community-dwelling patients with schizophrenia. Schizophrenia Research. 2003;62:73–6. doi: 10.1016/s0920-9964(02)00441-3. [DOI] [PubMed] [Google Scholar]
- Susce 2005 .Susce MT, Villanueva N, Diaz FJ, de Leon J. Obesity and associated complications in patients with severe mental illnesses: A cross-sectional survey. Journal of Clinical Psychiatry. 2005;66:167–73. doi: 10.4088/jcp.v66n0203. [DOI] [PubMed] [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment. 1999;3(5):iii–92. [MEDLINE: 10982317] [PubMed] [Google Scholar]
- Wei 1999 .Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, Jr, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal weight, overweight and obese men. JAMA. 1999;282:1547–53. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- Weiden 2004 .Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophrenia Research. 2004;66:51–7. doi: 10.1016/s0920-9964(02)00498-x. [DOI] [PubMed] [Google Scholar]
- Werneke 2002 .Werneke U, Taylor D, Sanders TAB. Options for pharmacological management of obesity in patients treated with atypical antipsychotics. International Clinical Psychopharmacology. 2002;17:145–60. doi: 10.1097/00004850-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Werneke 2003 .Werneke U, Taylor D, Sanders TAB, Wessely S. Behavioural management of antipsychotic-induced weight gain: A review. Acta Psychiatrica Scandinavica. 2003;108:252–9. doi: 10.1034/j.1600-0447.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- Wilding 1997 .Wilding J. Science, medicine, and the future: Obesity treatment. BMJ. 1997;315:997–1000. doi: 10.1136/bmj.315.7114.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirshing 1999 .Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR. Novel antipsychotics: comparison of weight gain liabilities. Journal of Clinical Psychiatry. 1999;60:358–63. [PubMed] [Google Scholar]
- Wirshing 2004 .Wirshing DA. Schizophrenia and obesity: impact of antipsychotic medications. Journal of Clinical Psychiatry. 2004;65(Suppl 18):13–26. [PubMed] [Google Scholar]
- * Indicates the major publication for the study


