Abstract
Epigenetic mechanisms are proposed to link maternal concentrations of methyl group donor nutrients with the risk of low birth weight. However, empirical data are lacking. We have examined the association between maternal folate and birth weight and assessed the mediating role of DNA methylation at nine differentially methylated regions (DMRs) of genomically imprinted genes in these associations. Compared with newborns of women with folate levels in the lowest quartile, birth weight was higher in newborns of mothers in the second (β = 143.2, se = 63.2, P = 0.02), third (β = 117.3, se = 64.0, P = 0.07), and fourth (β = 133.9, se = 65.2, P = 0.04) quartiles, consistent with a threshold effect. This pattern of association did not vary by race/ethnicity but was more apparent in newborns of non-obese women. DNA methylation at the PLAGL1, SGCE, DLK1/MEG3 and IGF2/H19 DMRs was associated with maternal folate levels and also birth weight, suggestive of threshold effects. MEG3 DMR methylation mediated the association between maternal folate levels and birth weight (P =0.06). While the small sample size and partial scope of examined DMRs limit our conclusions, our data suggest that, with respect to birth weight, no additional benefits may be derived from increased maternal folate concentrations, especially in non-obese women. These data also support epigenetic plasticity as a key mechanistic response to folate availability during early fetal development.
Keywords: birth weight, DNA methylation, imprinted genes, folate, epidemiology, epigenetics
Introduction
Folate is a water-soluble vitamin that decreases the risk of neural tube defects when taken periconceptionally. This has led to recommendations that women at risk of pregnancy supplement their daily diet with 400 µg of folic acid (FA), a synthetic form of folate.1-6 Because approximately half of US pregnancies are unplanned, FA fortification of milled grain was instituted in the late 1990s. A commensurate increase in erythrocyte folate concentrations was reported in women of reproductive age within two years.7 What is described as “supra-physiological” levels of circulating folate8-10 has been reported in a quarter of the US population since FA fortification began.7 Approximately 10% of pregnant women report taking supplements containing FA in doses exceeding 1000 µg per day, the maximum tolerable limit recommended by the Institute of Medicine.11 The long-term effects of high folate levels are unknown and remain an active topic of investigation.
Recent animal data demonstrate that high periconceptional and prenatal FA intake result in growth retardation that is partly rescued by maternal methylenetetrahydrofolate reductase (Mthfr) deficiency,12 an enzyme central to folate metabolism.13 In humans, evidence of associations between maternal folate concentrations and fetal growth is conflicting, as high levels have been linked to both low and high birth weight,14-24 while other studies found no associations.25-31 Extremes of the birth weight distribution have been associated with a wide range of childhood and adult-onset chronic diseases and conditions including obesity, impaired glucose tolerance, type 2 diabetes, coronary artery disease, congestive heart failure and some cancers.32-36 Periconceptional and perinatal exposure to FA supplements has also been linked to wheezing in mice37 and humans,38 as well as childhood asthma.39 While these early studies support the developmental origins of adult disease susceptibility hypothesis,40,41 low and high birth weight are used as an approximation for a wide range of adverse prenatal exposures, for which targeted interventions and monitoring will be difficult without mechanistic insights.
Epigenetic shifts in response to restricted or excessive prenatal nutrition have been hypothesized to mediate the associations between low birth weight and adult disease susceptibility, and could serve as intermediate endpoints for early monitoring42-44; however, empirical human data remain limited. In viable yellow agouti (Avy) mice, supplementing pregnant dams with one carbon cycle nutrients, including FA, resulted in shifts in the coat color distribution in the offspring, from yellow to brown.45 The shift to brown coat color was directly attributed to increased DNA methylation at a cryptic promoter in an intracisternal A particle (IAP) upstream of the Avy locus, which, when unmethylated, drives ectopic Agouti expression. In humans, differences in DNA methylation of the differentially methylated regions (DMRs) regulating the imprinted expression of insulin like growth factor-2 (IGF2), were reported in individuals exposed to FA during the periconceptional46 and prenatal periods.47,48 However, historical reports of self-reported FA intake may be fraught with recall concerns and do not account for individual variation in diet and metabolism. Higher IGF2 and lower PEG3 DMR methylation levels were recently reported in DNA from umbilical cord leukocytes of infants born to women with higher prenatal erythrocyte folate.49 Herein, we expand on the repertoire of imprinted genes for which we examined DNA methylation (IGF2/H19, DLK1/MEG3, PEG1/MEST, PEG3, PEG10/SGCE, PLAGL1, and NNAT) in relation to maternal folate levels in early pregnancy, and assess the extent to which DNA methylation changes at regulatory regions of these genes may influence birth weight.
Results
Study participants
Characteristics of pregnant women and their offspring previously shown to influence birth weight and/or DNA methylation are presented for each race/ethnicity in Table 1. Non-Hispanic Blacks comprised 38%, Hispanics 31%, Non-Hispanic Whites 27%, and other ethnicities including Asians and Native Americans 4% of the study population. Advanced maternal age (36+ years) was reported by 11% of pregnant women, comparable among all ethnic groups, although Non-Hispanic Blacks (56%) and Hispanics (40%) were more likely to be younger (< 25 y) at enrollment than Non-Hispanic Whites (17%) (P < 0.0001). Mean maternal BMI was 28 Kg/m2, which was comparable in Hispanics and Non-Hispanic Blacks, but significantly lower in Non-Hispanic Whites (P < 0.0001). Birth weight was lowest among infants born to Non-Hispanic Black women and highest in Non-Hispanic White women, despite similar proportions of infants born ≤ 37 wk gestation (16.9% of Non-Hispanic Blacks, vs. 12.8% Non-Hispanic Whites and 12.3% of Hispanics, P = 0.5). Lower socio-economic correlates, including living without a partner and lower educational attainment, were also more frequent among Hispanics and Non-Hispanic Blacks (P < 0.0001). Parity was higher in ethnic minorities, compared with Non-Hispanic White women, with approximately one-sixth of Non-Hispanic Black and Hispanic women reporting parity of three or more compared with one-fifth of Non-Hispanic Whites (P = 0.04). Gestational age at enrollment was 12.7 wk (sd = 4.8 wk) and was comparable among ethnic groups as were gestational age at delivery, delivery mode (vaginal vs. caesarean section) and sex distribution of infants (P > 0.05). Average maternal erythrocyte folate concentrations were 217.8 ng/ml (interquartile range 167–278) and it was highest in Non-Hispanic White women, moderate in Hispanics and lowest in Non-Hispanic Black women. We accounted for these differences in multivariable models and by restriction.
Table 1. Characteristics of 496 study participants by maternal race/ethnicity.
| Non-Hispanic Black (n = 189) |
Non-Hispanic White (n = 133) |
Hispanic (n = 154) |
Others (n = 20) |
p-values | |
|---|---|---|---|---|---|
| Characteristic of study participants | #(%) | #(%) | #(%) | #(%) | |
|
Maternal age 18–20 y 21–25 y 25–30 y 31–35 y 36+years |
33 (17.5) 73 (38.6) 42 (22.2) 21 (11.1) 20 (10.6) |
3 (2.3) 19 (14.3) 45 (33.8) 54 (40.6) 12 (9.0) |
16 (10.4) 46 (29.9) 42 (27.3) 35 (22.7) 15 (9.7) |
2 (10.0) 3 (15.0) 6 (30.0) 5 (25.0) 4 (20.0) |
< 0.0001 |
|
Maternal Marital Status Not partnered (never married/ divorced) Living with partner (cohabit/married) Other Missing |
106 (56.1) 71 (37.6) 7 (3.7) 5 (2.6) |
12 (9.0) 120 (90.2) 1 (0.8) 0 (0.0) |
37 (24.0) 114 (74.0) 3 (2.0) 0 (0.0) |
4 (20.0) 16 (80.0) 0 (0.0) 0 (0.0) |
< 0..0001* |
|
Maternal Education < High School High School - < college College graduate Graduate/professional school Missing |
5 (2.7) 99 (52.7) 69 (36.7) 15 (7.9) 1 (0.5) |
3 (2.3) 19 (14.3) 55 (41.4) 55 (41.4) 1 (0.8) |
62 (40.3) 75 (48.7) 10 (6.5) 7 (4.5) 0 (0.0) |
2 (10.0) 7 (35.0) 4 (20.0) 7 (35.0) 0 (0.0) |
< 0.0001 |
|
Parity None One Two Three or more |
50 (26.5) 44 (23.2) 34 (18.0) 61 (32.3) |
43 (32.3) 44 (33.1) 25 (18.8) 21 (15.8) |
33 (21.4) 32 (20.8) 36 (23.4) 53 (34.4) |
6 (30.0) 6 (30.0) 3 (15.0) 5 (25.0) |
0.02 |
|
Maternal BMI pre-pregnancy < 25 25- < 30 30- < 35 35- < 40 40+ Missing |
60 (31.6) 50 (26.5) 33 (17.5) 21 (11.1) 19 (10.1) 6 (3.2) |
75 (56.4) 26 (19.6) 22 (16.5) 4 (3.0) 4 (3.0) 2 (1.5% |
37 (24.0) 62 (40.3) 21 (13.6) 10 (6.5) 5 (3.3) 19 (12.3) |
12 (60.0) 6 (30.0) 1 (5.0) 0 (0.00) 0 (0.0) 1 (5.0) |
< 0.0001 |
| Mean BMI (sd) | 29.4 (8.2) | 25.2 (5.8) | 27.5 (5.5) | 23.4 (3.2) | < 0.0001 |
|
Maternal cigarette smoking Ever smoked Quit sometime during pregnancy Never Missing |
16 (8.5) 48 (25.4) 125 (66.1) 0 (0.0) |
29 (68.2) 18 (13.5) 85 (63.9) 1 (0.8) |
8 (5.2) 6 (3.9) 139 (90.2) 1 (0.7) |
4 (20.0) 2 (10.0) 14 (70.0) 0 (0.0) |
< 0.0001 |
|
Gestational age a delivery > 37 wk < = 37weeks |
157 (83.1) 32 (16.9) |
116 (87.2) 17 (12.8) |
135 (87.7) 19 (12.3) |
16 (80.0) 4 (20.0) |
0.5 |
|
Sex of offspring Male Female |
93 (49.2) 96 (50.8) |
75 (56.4) 58 (43.6) |
72 (46.8) 82 (53.2) |
9 (45.0) 11 (55.0) |
0.4 |
|
Prenatal physical activity No Yes |
130 (68.8) 59 (31.2) |
119 (89.5) 14 (10.5) |
114 (74.0) 40 (26.0) |
17 (85.0) 3 (15.0) |
0.0002 |
|
Gestational/Type II diabetes mellitus Yes No |
14 (7.4) 174 (92.1) |
5 (3.8) 128 (96.2) |
6 (4.5) 146 (95.5) |
0 20 (100) |
0.001* |
| Missing | 1 (0.5) | 0 | 2 (1.3) | 0 | |
| Birth weight (mean, sd) | 3164.0 (535.1) | 3452.9 (548.7) | 3342.1 (498.2) | 3126.1 (512.2) | < 0.0001 |
| Maternal folate levels (µg/L) in all | 194.6 (83.2) | 247.6 (66.6) | 222.3 (64.1) | 204.4 (83.6) | < 0.0001 |
| 1st trimester blood draw (n = 326) | 191.3 (79.9) | 249.3 (66.1) | 215.5 (61.2) | 214.7 (55.1) | < 0.0001 |
| 2nd trimester blood draw (n = 160) | 198.2 (92.9) | 243.8 (69.5) | 228.9 (69.4) | 175.5 (119.7) | 0.02 |
| 3rd trimester blood draw (n = 6) | 208.3 (57.4) | 188.0 (NA) | 229.0 (35.2) | 283.5 (NA) | 0.4 |
P values based on Fisher’s exact test. White pregnant mothers are older, more likely to be married, have a higher educational attainment, have a lower pre-pregnancy BMI > 30 or diabetes . NA-no variability.
Associations between maternal folate concentrations and birth weight
The average birth weight (3295 g) was higher in males (3350 g) than females (3237 g) and higher in infants born to Hispanics and Non-Hispanic Whites (3342 g and 3453 g, respectively) than Non-Hispanic Blacks (3164 g). After adjusting for sex, ethnicity/race, gestational age at delivery, delivery mode, cigarette smoking, prenatal physical activity and maternal pre-pregnancy BMI, we observed a relationship between maternal folate levels and birth weight consistent with a threshold effect (Table 2). Compared with infants born to women with folate levels in the lowest quartile, birth weight increased among newborns of women with folate levels in the second quartile (β2nd quartile = 143.18, se = 63.24, P = 0.02); however, the magnitude of this relationship did not significantly change when folate levels in the third and fourth were examined (β3rd quartile = 117.32, se = 63.99 and β4th quartile = 133.88, se = 65.2). Despite large differences in folate levels and birth weight among ethnic groups, this pattern of association did not vary significantly by race/ethnicity. Repeating these analyses stratified by maternal obesity (BMI > 30 vs. BMI ≤ 30) further adjusted for race/ethnicity revealed a pattern of association consistent with a threshold effect only in the subgroup of infants born to women with BMI ≤ 30kg/m2, whereas a non-statistically significant U-shaped relationship was observed between maternal folate levels and birth weight when pre-pregnancy BMI was > 30kg/m2 (Fig. 1). However, none of the cross-product terms for maternal folate quartiles and continuous BMI in statistical models for the association between maternal folate and birth weight were statistically significant (P = 0.19). Repeating these analyses in those with gestational age ≥ 37 wk and those with folate measured from blood obtained at gestational age < 13 wk, did not alter our findings, suggesting our results were unlikely to be influenced by preterm birth or gestational age at blood draw.
Table 2. Regression coefficients for the association between maternal folate and offspring birth weight.
| Erythrocyte folate concentrations | |
|---|---|
| Characteristic | Regression coefficient (SE) p-value |
| Intercept | 3044.68 (179.08) |
|
Maternal folate concentrations 1st Quartile |
Referents |
| 2nd Quartile | 143.18 (63.24) 0.024 |
| 3rd Quartile | 117.32 (63.99) 0.067 |
| 4th Quartile | 133.88 (65.20) 0.041 |
| Male sex | 140.82 (43.55) 0.001 |
| Gestational age < 37 wk | -620.70 (61.84) 0.000 |
|
Race/ethnicity Non-Hispanic Black |
-243.63 (60.96) 0.000 |
| Hispanic | -121.74 (62.43) 0.052 |
| Other | -213.85 (117.48) 0.069 |
| Non-Hispanic White | Referents |
| Pre-pregnancy BMI | 11.88 (3.40) 0.001 |
After adjusting for maternal race/ethnicity, offspring sex, cigarette smoking, gestational age at delivery, gestational age at blood draw, physical activity, maternal pre-pregnancy body mass index (BMI) and delivery route, we note a relationship between maternal folate concentrations and birth weight consistent with a threshold effect.
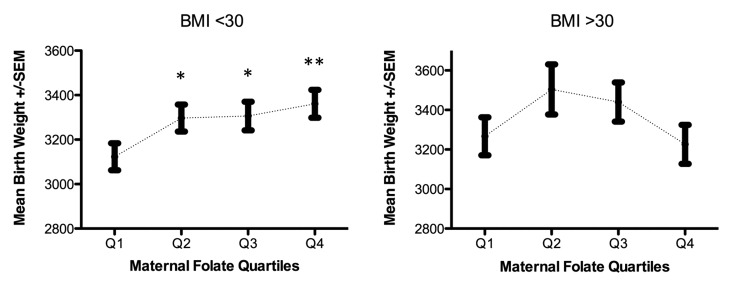
Figure 1. Maternal folate and birth weight by pre-pregnancy BMI < 30 and BMI ≥ 30Kg/m2. Adjusted for race/ethnicity, gestational age, cigarette smoking, gestational age at delivery, pre-pregnancy BMI and physical activity during pregnancy. *p-value < 0.01, ** p-value < 0.001
Association between maternal folate concentrations and DNA methylation
We evaluated the extent to which epigenetic mechanisms may mediate the association between maternal folate levels and birth weight by examining associations between maternal folate and DNA methylation levels at each of the nine DMRs measured (Table 3). We also examined associations between DMR methylation and birth weight, overall and stratified by pre-pregnancy BMI (Table 4), prior to evaluating the subset of DMRs associated with both maternal folate levels and birth weight for potential mediation. Mean DNA methylation levels at DMRs regulating MEG3 was 67%, se = 0.64. Mean DNA methylation levels at DMRs were also estimated for MEG3-IG (mean = 49.9%, se = 0.69), NNAT (mean = 57%, se = 0.84), PEG10/SGCE (mean = 45%, se = 0.37), PLAGL1 (mean = 50%, se = 0.46), MEST (mean = 44%, se = 0.48), H19 (mean = 46%, se = 0.37), IGF2 (mean = 51.0, se = 0.49), and PEG3 (mean = 36%, se = 0.26).
Table 3. Regression coefficients and standard errors for associations between maternal folate levels in quartiles and DNA methylation fractions at 9 DMRs regulating imprinted genes.
| All | BMI < 30 | BMI ≥ 30 | ||||
|---|---|---|---|---|---|---|
| DMR | Mean, β, SE | p-value | β, SE | p-value | β, SE | p-value |
| MEG3 (mean, se) | 67.2% (0.64) | |||||
| Quartile 2 | -1.16 (0.56) | 0.041 | -0.77 (0.64) | 0.237 | -2.02 (1.17) | 0.092 |
| Quartile 3 | -1.93 (0.57) | 0.001 | -1.47 (0.69) | 0.037 | -2.72 (1.05) | 0.013 |
| Quartile 4 | -2.02 (0.58) | 0.001 | -1.68 (0.68) | 0.017 | -2.47 (1.16) | 0.039 |
| NNAT (mean, se) | 56.7% (0.84) | |||||
| Quartile 2 | -0.83 (0.71) | 0.241 | -0.85 (0.84) | 0.314 | -1.02 (1.32) | 0.441 |
| Quartile 3 | -1.34 (0.73) | 0.068 | -1.90 (0.92) | 0.039 | -0.21 (1.21) | 0.866 |
| Quartile 4 | -1.15 (0.74) | 0.119 | -2.00 (0.91) | 0.028 | 1.16 (1.27) | 0.361 |
| PEG10/SGCE (mean, se) | 44.8% (0.37) | |||||
| Quartile 2 | -0.22 (0.32) | 0.489 | -0.08 (0.34) | 0.820 | -0.83 (0.61) | 0.174 |
| Quartile 3 | -0.44 (0.32) | 0.178 | 0.23 (0.35) | 0.518 | -2.08 (0.56) | 0.000 |
| Quartile 4 | -0.14 (0.33) | 0.661 | -0.11 (0.36) | 0.769 | -1.12 (0.58) | 0.054 |
| MEG3-IG (mean, se) | 49.9% (0.69) | |||||
| Quartile 2 | -0.54 (0.60) | 0.372 | 0.04 (0.71) | 0.953 | -2.36 (1.19) | 0.048 |
| Quartile 3 | -0.67 (0.60) | 0.263 | -0.43 (0.75) | 0.566 | -1.31 (1.04) | 0.206 |
| Quartile 4 | -0.68 (0.61) | 0.269 | -0.50 (0.75) | 0.504 | -0.92 (1.16) | 0.426 |
| PLAGL1 (mean, se) | 50.0% (0.46) | |||||
| Quartile 2 | 0.20 (0.39) | 0.607 | 0.62 (0.50) | 0.209 | -0.44 (0.69) | 0.521 |
| Quartile 3 | -1.01 (0.40) | 0.011 | 0.01 (0.53) | 0.988 | -1.86 (0.62) | 0.003 |
| Quartile 4 | -0.08 (0.41) | 0.851 | 0.55 (0.53) | 0.305 | -1.04 (0.64) | 0.105 |
| PEG3 (mean, se) | 36.0% (0.26) | |||||
| Quartile 2 | 0.43 (0.22) | 0.031 | -0.12 (0.28) | 0.679 | 1.66 (0.38) | 0.000 |
| Quartile 3 | 0.22 (0.23) | 0.294 | -0.24 (0.30) | 0.428 | 1.01 (0.34) | 0.003 |
| Quartile 4 | 0.06 (0.23) | 0.602 | -0.28 (0.29) | 0.339 | 0.63 (0.37) | 0.089 |
| PEG1/MEST (mean, se) | 43.7% (0.48) | |||||
| Quartile 2 | -0.57 (0.42) | 0.177 | -0.13 (0.55) | 0.816 | -1.51 (0.60) | 0.013 |
| Quartile 3 | -0.09 (0.43) | 0.835 | 0.40 (0.57) | 0.483 | -1.38 (0.58) | 0.017 |
| Quartile 4 | 0.39 (0.44) | 0.367 | 0.56 (0.58) | 0.333 | 0.01 (0.60) | 0.984 |
| H19 (mean, se) | 46.0% (0.37) | |||||
| Quartile 2 | -0.03 (0.32) | 0.920 | 0.37 (0.35) | 0.287 | -2.17 (0.74) | 0.003 |
| Quartile 3 | -0.20 (0.33) | 0.545 | 0.04 (0.37) | 0.908 | -0.99 (0.67) | 0.138 |
| Quartile 4 | 0.09 (0.33) | 0.777 | 0.26 (0.37) | 0.484 | -0.77 (0.71) | 0.278 |
| IGF2 (mean, se) | 51.0% (0.49) | |||||
| Quartile 2 | -1.22 (0.42) | 0.004 | -1.17 (0.50) | 0.020 | -1.39 (0.80) | 0.083 |
| Quartile 3 | -0.47 (0.43) | 0.271 | -1.06 (0.52) | 0.041 | 0.64 (0.74) | 0.388 |
| Quartile 4 | -0.04 (0.43) | 0.929 | 0.07 (0.52) | 0.886 | -0.43 (0.78) | 0.586 |
Relationships adjusted for maternal race/ethnicity, cigarette smoking, delivery route, gestational age at blood draw, gestational age and delivery, physical activity, pre-pregnancy BMI. Referents are infants born to women with folate levels in the lowest quartiles. Maternal folate concentrations are associated with DNA methylation at all DMRs for either all subjects, those born to obese or non-obese women-associations are nonlinear.
Table 4. Regression coefficients for associations between offspring DMR methylation at the 9 regions and birth weight.
| DMR | β SE | p-value |
|---|---|---|
| *MEG3 | -10.92 (4.12) | 0.008 |
| NNAT | -7.57 (4.04) | 0.062 |
| *PEG10/SGCE | 18.11 (5.77) | 0.002 |
| MEG3-IG | 0.42 (9.13) | 0.963 |
| *PLAGL1 | 12.33 (3.78) | 0.001 |
| PEG3 | 1.36 (8.97) | 0.879 |
| PEG1/MEST | -5.60 (5.68) | 0.325 |
| *H19 | 20.25 (6.76) | 0.003 |
| IGF2 | 6.10 (5.71) | 0.286 |
Referents are infants with methylation levels in the fourth quartile. Models are adjusted for maternal race, sex, cigarette smoking, gestational age at delivery, gestational age at blood draw, physical activity, pre-pregnancy BMI, and delivery route. *Significant associations are noted between birth weight and methylation fractions at DMRs regulating SGCE, and PLAGL1, and one of two DMRs regulating the IGF2/H19 and DLK1/MEG3 imprinted domains.
After adjusting for maternal race/ethnicity, cigarette smoking, delivery route, gestational age at blood draw, gestational age at delivery, physical activity and maternal pre-pregnancy obesity among all participants, we observed decreases in offspring DNA methylation levels with increasing maternal folate levels in at least one quartile, which were consistent with a threshold effect at the MEG3, NNAT, PEG10/SGCE, MEG3-IG, PLAGL1, PEG3 and the H19 DMRs. Linear increases in methylation levels with increasing maternal folate levels were noted at the IGF2 and PEG1/MEST DMRs. These associations were statistically significant only for MEG3 and PLAGL1, where the magnitude of associations increased with increasing quartile of maternal folate (β2nd quartile = -1.16, P = 0.041, β3rd quartile = -1.93, P = 0.001, β4th quartile = -2.02, P = 0.001 for MEG3 and β2nd quartile = 0.20, P = 0.607, β3rd quartile = -1.01, P = 0.011, β4th quartile = -0.08, P = 0.851 for PLAGL1), and for the PEG3 and IGF2 DMRs, where the magnitude of associations weakened with increasing quartile (β2nd quartile = 0.43, P = 0.031, β3rd quartile = 0.22, P = 0.294, β4th quartile = 0.06, P = 0.602 for PEG3 and β2nd quartile = -1.22, P = 0.004, β3rd quartile = -0.47, P = 0.271, β4th quartile = -0.04, P = 0.929 for the IGF2 DMR). Remarkably, repeating these analyses in infants born to women with pre-pregnancy BMI ≤ 30 and BMI > 30 revealed that, with the exception of the NNAT and IGF2 DMRs, these patterns of association were stronger in newborns of women with pre-pregnancy BMI > 30 (P < 0.05). Nonetheless, the cross-product terms for the interaction of maternal pre-pregnancy BMI and maternal folate were not statistically significant at any DMR (P > 0.59), presumably because we were underpowered.
Association between DNA methylation and birth weight
Table 4 shows that five of the DMRs associated with maternal folate were also associated with birth weight. Higher birth weight was associated with higher DNA methylation levels at the PEG10/SGCE (β = 18.1, P = 0.002), PLAGL1 (β = 12.3, P = 0.001), and H19 (β = 20.2, P = 0.003) DMRs, and lower methylation levels at the MEG3 (β = -10.9, P = 0.008) and NNAT (β = -7.6, P = 0.062) DMRs. To evaluate the potential for confounding by gestational age at blood draw (folate levels decrease with increasing gestational age) and preterm deliveries, we repeated these analyses among the 320 women with blood draws at gestational age < 120 d, and gestational age at birth > 37 wk and our findings remained essentially unaltered. Inclusion of DMRs associated with both maternal folate and birth weight into refined models attenuated these associations, suggesting that DNA methylation at the MEG3, H19 and PLAGL1 DMRs may mediate these associations.
Structural equation models using Mplus version 7.1 with bootstrapping were used to test for a significant mediated effect of MEG3, PLAGL1, and H19 DMRs in the relationship between folate measured on a continuous scale and birth weight adjusting for sex, ethnicity/race, prenatal cigarette smoking, gestational age at delivery, delivery mode, cigarette smoking, prenatal physical activity and maternal pre-pregnancy BMI. We found that the indirect effect of DNA methylation at the MEG3 DMR approached significance (estimate = 0.09, s.e. = 0.05, P = 0.06), while that of PLAGL1 and H19 were not statistically significant (estimate = -0.007, s.e. = 0.04, P = 0.87; estimate = -0.02, s.e = 0.03, P = 0.64, respectively).
Discussion
We have examined maternal folate concentrations in early pregnancy, representing folate from all sources, in relation to birth weight in a multiethnic cohort, and the extent to which DNA methylation at multiple regulatory sequences of genomically imprinted genes mediates these associations, accounting for differences in maternal race/ethnicity, offspring sex, cigarette smoking, gestational age at blood draw or delivery, maternal pre-pregnancy BMI, physical activity and delivery route. We found associations consistent with a nonlinear relationship between maternal folate levels at median 12 wk gestation, and birth weight that was consistent with a threshold effect. Restricting these analyses by maternal obesity status revealed that this pattern of association was limited to newborns of women with BMI < 30. Birth weight is a robust, albeit non-specific indicator of fetal wellbeing, and both low and high birth weight are consistently associated with a wide range of common adult onset diseases. We also found nonlinear relationships largely consistent with a threshold effect between maternal folate and DNA methylation levels of newborns of women with BMI ≥ 30, at seven of nine DMRs examined; methylation levels in five of these DMRs were also linearly associated with birth weight and MEG3 DMR methylation may mediate the relationship between maternal folate and birth weight.
Associations between maternal folate and birth weight
While clinical trials have demonstrated that folate deficiency increases risk of neural tube defects, evidence for excess folate availability, altering birth weight is inconsistent, regardless of whether folate is measured in plasma or erythrocytes, or is self-reported as diet rich in one carbon cycle nutrients. Our findings that moderate folate levels in early pregnancy are associated with higher birth weight regardless of race/ethnicity are consistent with previous studies,14-24,50 although an equally large number of studies from Europe, Asia and the US25-31 have not confirmed these associations. Reasons for discordant findings are unclear. Some of the discordant findings could be due to population differences in the prevalence of competing risk factors for low birth weight, including maternal obesity, nutritional status, cigarette and alcohol use and genetic susceptibility. In general, maternal obesity is higher and periconceptional FA use is lower in the present study.11,51
Discordant findings could also be due to differences in the gestational age at folate measurement; maternal folate concentrations measured in peripheral blood decrease with increasing gestational age, as folate demands increase during fetal growth and development.52 The majority of studies with null findings collected blood specimens during the second or third trimester. In our analysis, restricted to 326 infant-mother pairs in whom maternal specimens were collected at gestational age < 12 wk, the magnitude of associations was somewhat higher than when all women were included. If the influence of folate supply on birth weight differs by gestational age, weaker associations in previous studies could be due, in part, to wide variability in gestational age in which folate measurements were made. Nonetheless, this multiethnic cohort reveals a pattern of association consistent with a threshold, interpretable as a lack of additional benefit to birth weight related to high folate levels beyond the second quartile; a pattern that may vary by maternal obesity status. Because up to 20% of American women enter their pregnancies at BMI > 30,53 the seeming U-shaped relationship between maternal folate levels and birth weight among obese women requires urgent clarification in larger studies.
Associations between maternal folate levels, DMR methylation and birth weight
We also found inverse relationships between maternal folate levels and DNA methylation in all participants are apparent for at least one folate quartile for MEG3, MEG3-IG, NNAT, PEG10/SGCE, PLAGL1, PEG3 and the H19 DMRs, while positive associations were found in relation to the IGF2 and PEG1/MEST DMRs. These findings are consistent with the central role of folate in the conversion of methionine to S-adenosylmethionine, which transfers methyl groups to proteins and nucleic acids, including for DNA methylation. These findings also support that DNA methylation variation at regulatory sequences of imprinted genes may provide important epigenetic readouts for early prenatal nutrient adequacy and/or excess regardless of race/ethnicity. However, only nine of the more than 65 known imprinted genes are examined here, therefore a cautious interpretation of these findings is warranted, at least until more DMRs are examined in relation to folate.
DNA methylation at four DMRs was also associated with birth weight; higher birth weight was associated with higher methylation levels at the H19, PEG10/SGCE and PLAGL1 DMRs and lower MEG3 methylation levels. Although we found statistical evidence for mediation of the relationship between maternal folate levels and birth weight by the MEG3 DMR, due to limited statistical power and the knowledge that imprinted genes are over-selected for growth effectors, we cannot exclude the possibility that DNA methylation at other DMRs including H19 and PLAGL1 DMRs may also mediate the association between maternal folate concentration and birth weight. The IGF2/H19 domain, one of the best-characterized imprinted regions, is located in chromosome 11p15.5, and was originally associated with Beckwith-Wiedemann syndrome, an overgrowth disorder that increases the risk of Wilms tumor and hepatoblastoma.54 Higher DNA methylation levels at the IGF2/H19 imprinting center (IC) in a diploid cell results from increased methylation of the normally unmethylated maternally-derived allele, and can result in biallelic expression of IGF2 by inhibiting binding the CTCF insulator protein.55 CTCF normally binds in a methylation-sensitive manner to the maternal allele at this region, blocking interaction between downstream enhancers and IGF2.56,57 The H19 DMR analyzed here encompasses one of the six CTCF binding motifs within the IC region of IGF2/H19. Hypermethylation of the H19 DMR has been linked to relaxation of imprint controls, increased transcription and translation of the potent IGF2 growth mitogen,58 and higher birth weight.59 Our findings are consistent with those of previous studies in which FA intake before or/and during pregnancy or folate levels were associated with DNA methylation differences of a similar magnitude at this imprinted domain46-49,60 and early childhood obesity.61-63
Similar to the IGF2/H19 imprinted domain where IGF2 and H19 are reciprocally imprinted, the DLK1 and MEG3 genes, located on chromosome 14q32.2, are also reciprocally imprinted.64 Methylation at the MEG3 DMR is established post-fertilization on the paternally derived allele, while methylation of the other DMRs is established in the gametes. While the gametic DMRs must resist demethylation during post-fertilization epigenetic reprogramming, the MEG3 DMR is first established during this time. It remains unclear if this temporal difference in imprint mark establishment renders the MEG3 DMR somehow differentially responsive to periconceptional folate levels. Nonetheless, aberrant methylation of the DMRs in the DLK1/MEG3 domain leads to widespread disruption of imprinting and gene expression.65,66 Higher methylation of the MEG3 DMR has been associated with fetal distress, associated with famine in utero67; in the present study, lower methylation levels at the MEG3 DMR were associated with moderate folate levels. PLAGL1 is located at chromosome 6q24.2, and encodes a developmentally regulated zinc-finger transcription factor thought to be involved in tumor development and growth via IGF2 signaling.29 Differences in DNA methylation at the PLAGL1 DMR have been associated with chorioamnionitis and funisitis68 as well as lower birth weight.63 Aberrant epigenetic marks at this site are also associated with transient neonatal diabetes mellitus (TNDM), a disorder of growth restriction and hyperglycemia.69,70 The PLAGL1 gene product is proposed to function as a major regulatory “hub” that coordinates the expression of a network of genes, including imprinted IGF2, H19, DLK1, MEG3 and MEST.71 Collectively, DNA methylation at networked DMRs are thought to have additive effects.72
Our study strengths include the prospective design, the multiethnic composition of the cohort and examination of erythrocyte folate levels with the majority of specimens collected in early pregnancy, demonstrating that the steady-state folate concentrations during the periconceptional period have similar epigenetic and physiological effects regardless of race/ethnicity. We also consider measurement of erythrocyte folate, which accounts for folate derived from all sources, a distinct strength of this study which improves upon numerous prior studies in which maternal folate was estimated by FA supplementation. DNA methylation examined here was measured from unfractionated umbilical cord blood specimens containing multiple cell types. Thus, one concern is that findings here may be a function of the major cell types. However, methylation profiles at these DMRs in fractionated polymorphonuclear and mononuclear cells were comparable between these cell fractions, suggesting that DMR methylation profiles measured using unfractionated umbilical cord blood may not sizably differ by cell type.47,73 Furthermore, DNA methylation in the regions examined was comparable in multiple tissues representing the three germ layers in human conceptal tissues.73 Lastly, at the well-characterized IGF2/H19 IC, inverse associations have been reported for DMR methylation and in utero FA exposure using DNA methylation measurements from umbilical cord endothelial cells,48 placenta48 and umbilical cord leukocytes.47-49
The main limitation of this study is the relatively small sample size for maternal pre-pregnancy obesity- and race/ethnic-specific analyses to disentangle the role of maternal obesity from that of race/ethnicity in the associations between maternal folate, DMR methylation and birth weight. In addition, although the goal was to determine the extent to which periconceptional folate availability altered DNA methylation levels, some maternal blood from which folate levels were measured, was collected after 120 d gestation, the average life span of a red blood cell. However, the majority of specimens (>95%) were collected prior to 120 d gestation and should have included red blood cells that were present during the periconceptional period, a potential window of vulnerability for epigenetic alterations due to the methylation reprogramming that occurs during this time. Moreover, our findings remained unaltered after repeating the analysis in the subgroup in which maternal folate was measured from maternal blood obtained in the first trimester. Another study limitation is that we examined only nine imprint regulatory sequences, a small fraction of the 65 imprinted genes that are currently known.74 Moreover, epigenetic changes resulting from variable maternal folate concentrations in early pregnancy are likely not restricted to imprinted domains. Nonetheless, while these data clearly require replication in larger studies, they support the contention that at least for birth weight, excessively high folate concentrations may have no added benefit, and that epigenetic plasticity, measurable at some genomically imprinted DMRs, may mediate these relationships. Such plasticity could be developed as biomarkers to improve monitoring folate adequacy or inadequacy much earlier than is currently possible. These findings also raise questions about potential links between declining birth weight in the US63 and high FA intake.
In summary, we found a relationship between maternal folate and birth weight consistent with a threshold effect, an association stronger in newborns of non-obese women. We also found DNA methylation at some imprinted loci may mediate these associations. Larger studies are required to confirm these findings and clarify the pattern of associations in children born to obese women.
Methods
Study participants
Study participants were enrolled as part of the Newborn Epigenetics STudy (NEST). A description of study enrollment procedures is detailed elsewhere.75 Briefly, between 2009 and 2011, English or Spanish speaking pregnant women aged 18 y and older were identified from clinic logs of five prenatal clinics in Durham County, NC, USA. Women intending to relinquish custody of the offspring resulting from the index pregnancy were excluded, as were those positive for human immunodeficiency virus, due to the limited research on the relationships between HIV, its treatment, and DNA methylation in the offspring. Of 2,548 women who met eligibility criteria, 1700 (66.6%) consented and were enrolled. The 848 women who declined were similar to those who consented in age (P = 0.70) although more likely to be Asian and Native American (P < 0.001). Of the 1304 (76.7%) women who remain enrolled in the study, erythrocyte folate concentrations were measured from maternal venous blood among the first 528, 63% (n = 330) of which was drawn during the first trimester. These analyses are restricted to 496 singleton infant-mother pairs in whom birth weight, pre-pregnancy weight and height data were available. The study protocol was approved by the Duke University and Durham Regional Hospital Institutional Review Boards (IRB).
Data collection
Maternal age, marital status, parity, weight and height at last menstrual period, sex of offspring, prenatal obesity, gestational diabetes and ethnicity/race were self-reported and also verified with abstracted medical records. Maternal age at delivery was computed as the difference between date of birth and delivery date. General health during pregnancy, cigarette smoking, alcohol use, and maternal education were self-reported via a questionnaire. Parturition data including birth weight, gestational age at birth, infant sex, delivery mode and any infection present during delivery were abstracted by trained personnel from medical records after delivery. Gestational age at birth was divided into two categories: preterm < 37 wk gestation and term ≥ 37 wk gestation. Delivery mode was defined as either vaginal delivery or Caesarian section (C-section).
Specimen collection and handling
Following enrollment at the first prenatal visit, pregnant women donated peripheral blood specimens in a 10 ml lavender top vacutainer tube from which 1 ml was removed and frozen for erythrocyte folate measurements. Infant cord blood specimens were collected at birth, also in EDTA-treated vacutainer tubes. The samples were centrifuged using standard protocols to allow for collection of plasma and buffy coat for DNA extraction (Qiagen). All samples were stored at -80 °C prior to use.
DNA methylation analysis
DNA was extracted using Puregene reagents (Qiagen) and quantified using a Nanodrop 1000 Spectrophotometer (Thermo Scientific). 800 ng of genomic DNA was treated with sodium bisulfite using the Zymo EZ DNA Methylation kit (Zymo Research), to convert unmethylated cytosines to uracils, leaving methylated cytosines unchanged. Pyrosequencing was performed using a Pyromark Q96 MD pyrosequencer (Qiagen) to measure DNA methylation at nine imprint regulatory regions in mothers and newborns. Pyrosequencing assay design, genomic coordinates, assay conditions and assay validation have been previously described.73,76 Percent methylation for each CpG cytosine was determined using Pyro Q-CpG Software (Qiagen). DMRs in which DNA methylation was measured include two regions regulating the IGF2/H19 imprinted domain (one upstream of the imprinted IGF2 promoters and one upstream of the H19 locus at the imprint center), two regions regulating the DLK1/MEG3 imprinted domain (the imprint center intergenic to DLK1 and MEG3 and the DMR within the MEG3 promoter region, the maternally methylated PEG3 promoter region, the maternally methylated MEST promoter region, the maternally methylated PEG10 promoter region from which SGCE is also transcribed in the opposite direction, the maternally methylated NNAT promoter and the maternally methylated PLAGL1 promoter.
Measurement of erythrocyte folate concentrations
Maternal whole blood samples were sent to Craft Technologies for measurement of erythrocyte folate using a commercial kit, ID-Vit Folic acid (Immundiagnostic-ALPCO; Salem, NH, Ref KIF005) which uses the folate dependent strain Lactobacillus rhamnosus (taxon id 47715) in a 96 well format.77 Erythrocyte folate levels were calculated using the following formula: RBC folate = (whole blood folate(ng/ml))-(plasma folate ng/ml)(1-hemotocrit/100))/ [ hematocrit levels] as previously described.78
Statistical analysis
Birth weight was normally distributed, and folate concentrations were slightly right-skewed. Folate was therefore categorized into quartiles. Maternal obesity was treated as a continuous variable in adjustment, but also was dichotomized at BMI ≤ 30 to determine if associations varied by maternal obesity. Self-reported maternal physical activity was used as a continuous variable. The following variables were treated as categorical variables: maternal age 18–20, 21–30, 31–39 and 40+ years, maternal education as less than high school; high school to less than college, college and graduate school education, parity as none, one, two and three or more, delivery mode as vaginal or Cesarean section, and finally, gestational age at birth stratified as less than, equal to, or more than 37 wk. Because DNA methylation fractions at some CpG dinucleotides within the PLAGL1, PEG3, MEG3, and PEG10/SGCE DMRs were not normally distributed as assessed via Kolmogorov-Smirnov tests, analyses were also confirmed using quartiles of DNA methylation levels and Wilcoxon rank sum tests.
Mean differences in birth weight were compared within each folate quartile using generalized linear models to adjust for potential confounding by maternal age, gestational age at blood draw, gestational age at delivery, cigarette smoking (smoked before pregnancy, during pregnancy and none), sex of offspring, education, and maternal race/ethnicity. Because the functional significance of DMR methylation marks at imprinted loci and folate concentrations vary considerably by pre-pregnancy obesity and ethnicity/race,59 we also explored associations between folate and birth weight, restricted to Non-Hispanic Whites, Non-Hispanic Blacks and Hispanics and BMI < 30 vs. BMI ≥ 30 kg/m2. Folate concentrations were measured in blood that was collected < 120 d gestation (approximating the average lifespan of a red blood cell) in 80% of women, 15% were measured in blood collected between 120–170 d, and the remainder in blood samples collected after 180 d gestation. Hence, gestational age at blood draw, computed as the difference between date of delivery and date of blood draw, was included in multivariable analyses. In exploratory analyses, we also restricted refined models to specific trimester of maternal blood draw.
To examine whether DNA methylation at sequences regulating imprinted genes may explain some of the observed association between folate and birth weight, we identified DMRs that were associated with maternal folate levels using mixed models to allow for unconstrained model entry of individual CpGs, adjusting for factors previously shown to influence DNA methylation.11,46,79 DMRs significantly associated with folate were then examined for associations with birth weight; the subset statistically significantly associated with maternal folate levels and birth weight (P ≤ 0.05) were included in refined models to determine the extent to which the main effects were attenuated. Finally, structural equation models were then used to evaluate mediation of the relationship between maternal folate and BW by the subset of DMRs that attenuated the main effects. These models quantify mediation as the product of the regression coefficients for folate, relevant DMR and birth weight.80
Acknowledgments
We are grateful to the participants of the NEST project, without whom this work would not have been possible. We are also grateful to Stacy Murray, Kennetra Irby, Siobhan Greene and Anna Tsent for their recruiting efforts and Carole Grenier, Erin Erginer, Cara Davis and Allison Barratt for excellent technical assistance. This work was funded by NIH R01-ES016772.
References
- 1.Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–14. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 2.Merchant AT, Msamanga G, Villamor E, Saathoff E, O’brien M, Hertzmark E, Hunter DJ, Fawzi WW. Multivitamin supplementation of HIV-positive women during pregnancy reduces hypertension. J Nutr. 2005;135:1776–81. doi: 10.1093/jn/135.7.1776. [DOI] [PubMed] [Google Scholar]
- 3.Villamor E, Msamanga G, Saathoff E, Fataki M, Manji K, Fawzi WW. Effects of maternal vitamin supplements on malaria in children born to HIV-infected women. Am J Trop Med Hyg. 2007;76:1066–71. [PubMed] [Google Scholar]
- 4.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, et al. Collaborative Project for Neural Tube Defect Prevention Prevention of neural-tube defects with folic acid in China. China-U.S. N Engl J Med. 1999;341:1485–90. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 5.Effectiveness in Disease and Injury Prevention Use of Folic Acid for Prevention of Spina Bifida and Other Neural Tube Defects–1983-1991. Centers for Disease Control, 1991. [PubMed]
- 6.Osório-Costa F, Rocha GZ, Dias MM, Carvalheira JB. Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq Bras Endocrinol Metabol. 2009;53:213–26. doi: 10.1590/S0004-27302009000200013. [DOI] [PubMed] [Google Scholar]
- 7.McDowell MA, Lacher DA, Pfeiffer CM, Mulinare J, Picciano MF, Rader JI, Yetley EA, Kennedy-Stephenson J, Johnson CL. Blood folate levels: the latest NHANES results. NCHS Data Brief. 2008:1–8. [PubMed] [Google Scholar]
- 8.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–33. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 9.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123–8. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr. 2007;86:271–3. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 11.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, Kurtzberg J, Jirtle RL, Murphy SK. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST) BMC Public Health. 2011;11:46. doi: 10.1186/1471-2458-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickell L, Wu Q, Wang XL, Leclerc D, Friedman H, Peterson AC, Rozen R. Targeted insertion of two Mthfr promoters in mice reveals temporal- and tissue-specific regulation. Mamm Genome. 2011;22:635–47. doi: 10.1007/s00335-011-9351-5. [DOI] [PubMed] [Google Scholar]
- 13.Pickell L, Brown K, Li D, Wang XL, Deng L, Wu Q, Selhub J, Luo L, Jerome-Majewska L, Rozen R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res A Clin Mol Teratol. 2011;91:8–19. doi: 10.1002/bdra.20754. [DOI] [PubMed] [Google Scholar]
- 14.Rao S, Yajnik CS, Kanade A, Fall CH, Margetts BM, Jackson AA, Shier R, Joshi S, Rege S, Lubree H, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr. 2001;131:1217–24. doi: 10.1093/jn/131.4.1217. [DOI] [PubMed] [Google Scholar]
- 15.Siega-Riz AM, Savitz DA, Zeisel SH, Thorp JM, Herring A. Second trimester folate status and preterm birth. Am J Obstet Gynecol. 2004;191:1851–7. doi: 10.1016/j.ajog.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 16.Lindblad B, Zaman S, Malik A, Martin H, Ekström AM, Amu S, Holmgren A, Norman M. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet Gynecol Scand. 2005;84:1055–61. doi: 10.1111/j.0001-6349.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 17.Relton CL, Pearce MS, Parker L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br J Nutr. 2005;93:593–9. doi: 10.1079/BJN20041395. [DOI] [PubMed] [Google Scholar]
- 18.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr. 1996;63:520–5. doi: 10.1093/ajcn/63.4.520. [DOI] [PubMed] [Google Scholar]
- 19.Neggers YH, Goldenberg RL, Tamura T, Cliver SP, Hoffman HJ. The relationship between maternal dietary intake and infant birthweight. Acta Obstet Gynecol Scand Suppl. 1997;165:71–5. [PubMed] [Google Scholar]
- 20.Furness DL, Yasin N, Dekker GA, Thompson SD, Roberts CT. Maternal red blood cell folate concentration at 10-12 weeks gestation and pregnancy outcome. J Matern Fetal Neonatal Med. 2012;25:1423–7. doi: 10.3109/14767058.2011.636463. [DOI] [PubMed] [Google Scholar]
- 21.Furness D, Fenech M, Dekker G, Khong TY, Roberts C, Hague W. Folate, vitamin B12, vitamin B6 and homocysteine: impact on pregnancy outcome. Matern Child Nutr. 2013;9:155–66. doi: 10.1111/j.1740-8709.2011.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiefte-de Jong JC, Timmermans S, Jaddoe VW, Hofman A, Tiemeier H, Steegers EA, de Jongste JC, Moll HA. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr. 2012;142:731–8. doi: 10.3945/jn.111.154948. [DOI] [PubMed] [Google Scholar]
- 23.Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, Tiemeier H. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr. 2012;95:1413–21. doi: 10.3945/ajcn.111.030791. [DOI] [PubMed] [Google Scholar]
- 24.Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Muthayya S, Kurpad AV, Yajnik CS, Fall CH. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J Nutr. 2010;140:1014–22. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsen RM, Vollset SE, Monsen AL, Ulvik A, Haugen M, Meltzer HM, Magnus P, Ueland PM. Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. J Nutr. 2010;140:572–9. doi: 10.3945/jn.109.118158. [DOI] [PubMed] [Google Scholar]
- 26.Ronnenberg AG, Goldman MB, Chen D, Aitken IW, Willett WC, Selhub J, Xu X. Preconception folate and vitamin B(6) status and clinical spontaneous abortion in Chinese women. Obstet Gynecol. 2002;100:107–13. doi: 10.1016/S0029-7844(02)01978-6. [DOI] [PubMed] [Google Scholar]
- 27.Takimoto H, Mito N, Umegaki K, Ishiwaki A, Kusama K, Abe S, Yamawaki M, Fukuoka H, Ohta C, Yoshiike N. Relationship between dietary folate intakes, maternal plasma total homocysteine and B-vitamins during pregnancy and fetal growth in Japan. Eur J Nutr. 2007;46:300–6. doi: 10.1007/s00394-007-0667-6. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, Fukuoka H, Sugiyama T, Nagai Y, Ogasawara K, Yoshiike N. Dietary folate intake during pregnancy and birth weight in Japan. Eur J Nutr. 2008;47:341–7. doi: 10.1007/s00394-008-0733-8. [DOI] [PubMed] [Google Scholar]
- 29.Vollset SE, Gjessing HK, Tandberg A, Rønning T, Irgens LM, Baste V, Nilsen RM, Daltveit AK. Folate supplementation and twin pregnancies. Epidemiology. 2005;16:201–5. doi: 10.1097/01.ede.0000152914.84962.13. [DOI] [PubMed] [Google Scholar]
- 30.Alwan NA, Greenwood DC, Simpson NA, McArdle HJ, Cade JE. The relationship between dietary supplement use in late pregnancy and birth outcomes: a cohort study in British women. BJOG. 2010;117:821–9. doi: 10.1111/j.1471-0528.2010.02549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogeveen M, Blom HJ, van der Heijden EH, Semmekrot BA, Sporken JM, Ueland PM, den Heijer M. Maternal homocysteine and related B vitamins as risk factors for low birthweight. Am J Obstet Gynecol. 2010;202:e1–6. doi: 10.1016/j.ajog.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 32.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yajnik CS, Deshpande SS, Panchanadikar AV, Naik SS, Deshpande JA, Coyaji KJ, Fall C, Refsum H. Maternal total homocysteine concentration and neonatal size in India. Asia Pac J Clin Nutr. 2005;14:179–81. [PubMed] [Google Scholar]
- 34.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–6. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 37.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Håberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–4. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–93. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 40.Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–5. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 42.Hoyo C, Murphy SK, Jirtle RL. Imprint regulatory elements as epigenetic biosensors of exposure in epidemiological studies. J Epidemiol Community Health. 2009;63:683–4. doi: 10.1136/jech.2009.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics. 2009;4:526–31. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- 44.Dolinoy DC. Epigenetic gene regulation: early environmental exposures. Pharmacogenomics. 2007;8:5–10. doi: 10.2217/14622416.8.1.5. [DOI] [PubMed] [Google Scholar]
- 45.Waterland RA. Do maternal methyl supplements in mice affect DNA methylation of offspring? J Nutr. 2003;133:238–, author reply 239. doi: 10.1093/jn/133.1.238. [DOI] [PubMed] [Google Scholar]
- 46.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoyo C, Murtha AP, Schildkraut JM, Jirtle RL, Demark-Wahnefried W, Forman MR, Iversen ES, Kurtzberg J, Overcash F, Huang Z, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6:928–36. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loke YJ, Galati JC, Morley R, Joo EJ, Novakovic B, Li X, Weinrich B, Carson N, Ollikainen M, Ng HK, et al. Association of maternal and nutrient supply line factors with DNA methylation at the imprinted IGF2/H19 locus in multiple tissues of newborn twins. Epigenetics. 2013;8:1069–79. doi: 10.4161/epi.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am J Clin Nutr. 2013;97:94–9. doi: 10.3945/ajcn.112.042572. [DOI] [PubMed] [Google Scholar]
- 50.Haggarty P, Hoad G, Horgan GW, Campbell DM. DNA methyltransferase candidate polymorphisms, imprinting methylation, and birth outcome. PLoS One. 2013;8:e68896. doi: 10.1371/journal.pone.0068896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, Overcash F, Kurtzberg J, Jirtle R, Iversen ES, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7:735–46. doi: 10.4161/epi.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ubeda N, Reyes L, González-Medina A, Alonso-Aperte E, Varela-Moreiras G. Physiologic changes in homocysteine metabolism in pregnancy: a longitudinal study in Spain. Nutrition. 2011;27:925–30. doi: 10.1016/j.nut.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2009-2010. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engel JR, Smallwood A, Harper A, Higgins MJ, Oshimura M, Reik W, Schofield PN, Maher ER. Epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome. J Med Genet. 2000;37:921–6. doi: 10.1136/jmg.37.12.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakagawa H, Chadwick RB, Peltomaki P, Plass C, Nakamura Y, de La Chapelle A. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:591–6. doi: 10.1073/pnas.98.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–9. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- 57.Kanduri C, Pant V, Loukinov D, Pugacheva E, Qi CF, Wolffe A, Ohlsson R, Lobanenkov VV. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol. 2000;10:853–6. doi: 10.1016/S0960-9822(00)00597-2. [DOI] [PubMed] [Google Scholar]
- 58.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–5. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 59.Hoyo C, Fortner K, Murtha AP, Schildkraut JM, Soubry A, Demark-Wahnefried W, Jirtle RL, Kurtzberg J, Forman MR, Overcash F, et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control. 2012;23:635–45. doi: 10.1007/s10552-012-9932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timmermans S, Jaddoe VW, Hofman A, Steegers-Theunissen RP, Steegers EA. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the Generation R Study. Br J Nutr. 2009;102:777–85. doi: 10.1017/S0007114509288994. [DOI] [PubMed] [Google Scholar]
- 61.St-Pierre J, Hivert MF, Perron P, Poirier P, Guay SP, Brisson D, Bouchard L. IGF2 DNA methylation is a modulator of newborn’s fetal growth and development. Epigenetics. 2012;7:1125–32. doi: 10.4161/epi.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perkins E, Murphy SK, Murtha AP, Schildkraut J, Jirtle RL, Demark-Wahnefried W, Forman MR, Kurtzberg J, Overcash F, Huang Z, et al. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J Pediatr. 2012;161:31–9. doi: 10.1016/j.jpeds.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vidal AC, Murphy SK, Murtha AP, Schildkraut JM, Soubry A, Huang Z, Neelon SE, Fuemmeler B, Iversen E, Wang F, et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes (Lond) 2013;37:907–13. doi: 10.1038/ijo.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wylie AA, Murphy SK, Orton TC, Jirtle RL. Novel imprinted DLK1/GTL2 domain on human chromosome 14 contains motifs that mimic those implicated in IGF2/H19 regulation. Genome Res. 2000;10:1711–8. doi: 10.1101/gr.161600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kagami M, O’Sullivan MJ, Green AJ, Watabe Y, Arisaka O, Masawa N, Matsuoka K, Fukami M, Matsubara K, Kato F, et al. The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet. 2010;6:e1000992. doi: 10.1371/journal.pgen.1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Cheunsuchon P, Nakayama Y, Lawlor MW, Zhong Y, Rice KA, Zhang L, Zhang X, Gordon FE, Lidov HG, et al. Activation of paternally expressed genes and perinatal death caused by deletion of the Gtl2 gene. Development. 2010;137:2643–52. doi: 10.1242/dev.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–53. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Hoyo C, Murphy S, Huang Z, Overcash F, Thompson J, Brown H, Murtha AP. DNA methylation at imprint regulatory regions in preterm birth and infection. Am J Obstet Gynecol. 2013;208:e1–7. doi: 10.1016/j.ajog.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boonen SE, Mackay DJ, Hahnemann JM, Docherty L, Grønskov K, Lehmann A, Larsen LG, Haemers AP, Kockaerts Y, Dooms L, et al. Transient neonatal diabetes, ZFP57, and hypomethylation of multiple imprinted loci: a detailed follow-up. Diabetes Care. 2013;36:505–12. doi: 10.2337/dc12-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Temple IK, Mackay DJG, Docherty LE. Diabetes Mellitus, 6q24-Related Transient Neonatal. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, eds. GeneReviews. Seattle WA: University of Washington, Seattle; 1993. [Google Scholar]
- 71.Varrault A, Gueydan C, Delalbre A, Bellmann A, Houssami S, Aknin C, Severac D, Chotard L, Kahli M, Le Digarcher A, et al. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell. 2006;11:711–22. doi: 10.1016/j.devcel.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Heijmans BT, Mill J. Commentary: The seven plagues of epigenetic epidemiology. Int J Epidemiol. 2012;41:74–8. doi: 10.1093/ije/dyr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy SK, Huang Z, Hoyo C. Differentially methylated regions of imprinted genes in prenatal, perinatal and postnatal human tissues. PLoS One. 2012;7:e40924. doi: 10.1371/journal.pone.0040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodfine K, Huddleston JE, Murrell A. Quantitative analysis of DNA methylation at all human imprinted regions reveals preservation of epigenetic stability in adult somatic tissue. Epigenetics Chromatin. 2011;4:1. doi: 10.1186/1756-8935-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, Overcash F, Kurtzberg J, Jirtle R, Iversen ES, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7:735–46. doi: 10.4161/epi.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nye MD, Hoyo C, Huang Z, Vidal AC, Wang F, Overcash F, Smith JS, Vasquez B, Hernandez B, Swai B, et al. Associations between methylation of paternally expressed gene 3 (PEG3), cervical intraepithelial neoplasia and invasive cervical cancer. PLoS One. 2013;8:e56325. doi: 10.1371/journal.pone.0056325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34:2357–9. [PubMed] [Google Scholar]
- 78.Piyathilake CJ, Robinson CB, Cornwell P. A practical approach to red blood cell folate analysis. Anal Chem Insights. 2007;2:107–10. [PMC free article] [PubMed] [Google Scholar]
- 79.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muthén LKMB. Mplus: The comprehensive modeling program for applied researchers. Computer software 1998;Los Angeles. [Google Scholar]


