Abstract
Aims
To investigate risk factors for metabolic syndrome in prepubertal boys with Klinefelter syndrome.
Methods
Eighty-nine boys with Klinefelter syndrome, ages 4–12.9 years, and 34 age-matched control boys had height, weight, waist circumference and blood pressure measured and their parents completed a questionnaire about physical activity. The boys with Klinefelter syndrome also had measurement of lipids, fasting glucose and insulin. Insulin-glucose homeostasis model assessment was calculated, and the boys were evaluated for childhood metabolic syndrome.
Results
The Klinefelter syndrome and control groups were similar ages (7.5 ± 2.4 vs. 8.1 ± 2.3 years). Body mass index measurements were similar, but waist circumference was >90‰ in 30% of boys with Klinefelter syndrome versus 21% of controls. The mean daily time spent running was 42 min less in the Klinefelter syndrome versus control groups (p < 0.01). About 37% of the boys with Klinefelter syndrome had elevated LDL cholesterol, 24% had insulin resistance, and 7% met the three criteria for diagnosis of metabolic syndrome.
Conclusions
Truncal obesity, insulin resistance and metabolic syndrome are present in boys as young as 4–12 years with Klinefelter syndrome, and these occur in association with reduced running-type activity.
Keywords: 47, XXY, Insulin resistance, Karyotype, Klinefelter syndrome, Metabolic syndrome, Testicular failure
INTRODUCTION
Klinefelter syndrome (KS), 47,XXY, was first described in association with infertility and androgen deficiency. Adults with KS are at increased risk for abdominal obesity, metabolic syndrome (1–4) and type 2 diabetes (5–7), with associated increased morbidity and mortality. Left ventricular dysfunction has been associated with insulin resistance in men with KS (2), and there is an increase in mortality associated with diabetes in adults with KS (1).
It is unclear what factors increase the risk for insulin resistance and diabetes in adult men with KS and whether this risk begins during childhood. Bojesen has posed the question: ‘does increased total body fat mass/waist circumference (WC) precede the hypogonadal state? Or does the hypogonadal state predispose to increased total body fat? Or are they separate and independent risk factors?’ (2). Insulin resistance and the metabolic syndrome are associated with lower testosterone levels in adults with KS (3,4). Hypogonadism is also an independent risk factor for metabolic syndrome in other populations (2,5–7). Testosterone treatment has been associated with decreased fat mass (7,8). It does appear that there is increased body fat mass in KS in childhood and adolescence (9). A recent study by Aksglaede et al. described 24 boys, ages 4.3–18.6 years, who were examined and underwent DEXA evaluation. Despite the finding that the boys with KS had weight and body mass index (BMI) similar to control boys, they had increased body fat mass and body fat per cent on DEXA scanning, with no apparent androgen treatment effect. Aside from this study, there is a paucity of data regarding insulin resistance and the metabolic syndrome during childhood and adolescence in KS and whether increased body fat precedes the development of hypogonadism or vice versa.
The objective of this study was to compare auxologic measures and truncal obesity in prepubertal boys with KS versus controls, and to investigate risk factors for metabolic syndrome in boys with KS.
METHODS
Subjects
Subjects were generally referred to the paediatric endocrine clinic at Thomas Jefferson University. The control group was recruited from families participating in other studies and through posting in the paediatric clinics. The control boys had no known medical diagnoses. No blood samples were obtained from controls. All boys with KS had postnatal karyotypes confirming the diagnosis of KS. All boys, both KS and controls, were of ages 4–12 years and had testicular volume ≤4 mL. KS boys had prepubertal gonadotropin and morning testosterone levels. The study was approved by the Human Studies Committee at Thomas Jefferson University. Informed consent/assent was obtained in all cases. The clinical evaluation was performed at Thomas Jefferson University.
Physical features
The clinical assessment included conversion of measurements to SD scores using age- and gender-specific norms of height (by stadiometer) (10), weight (10), WC (11), BMI (10), body fat measurement (using a calliper) and blood pressure (BP) (12). Body fat measurements were taken using the Skyndex/System I, which is an electronic body fat calculator. Skin fold measurements were taken from the right triceps and calf, after which the instrument calculated the per cent body fat using the Slaughter/Lohman formula for boys (% body fat = 0.735 (triceps + calf) + 1.0) (13). Blood pressure was measured in the seated position after a meal and rest by auscultation using an aneroid sphygmomanometer and appropriate size cuff on either the right or left arm. Testicular size was measured by palpation using the Prader orchidometer and converted to standard deviation scores using published normative data (14), and pubic hair was assessed according to Tanner staging (15).
Exercise
Physical activity was measured with a validated parental questionnaire (16) that assesses the child’s activities on a typical school day in a typical week. Parents were asked how many minutes the child spends on school days on each of a list of specific activities, based on the ‘Yesterday Activity Checklist’ of the Physical Activity Checklist Interview Protocol. The list includes sports, active games, and individual activities such as walking, running and skating.
Laboratory testing
Plasma insulin concentration was determined with a solid-phase radioimmunoassay (Coat-a-Count; Diagnostic Products Corp, Los Angeles, CA, USA). Coefficients of variation for intra- and interassay variability for glucose and insulin assays were <5%. Homeostasis model assessment (HOMA) was calculated as a measure of insulin resistance as follows: [fasting blood glucose [FBG] (mmol/L) × fasting insulin (μU/mL)]/22.5 (17). Fasting lipid values (low-density lipoprotein [LDL], total cholesterol, triglycerides [TG], high-density lipoprotein [HDL]) were measured using commercial assays.
Definitions
Elevated LDL ≥110 mg/dL, elevated total cholesterol ≥170 mg/dL, HOMA consistent with insulin resistance ≥2.5; childhood metabolic syndrome was defined as ≥3 of the following: fasting TG ≥100 mg/dL; HDL <50 mg/dL; WC >75‰ for age; systolic/diastolic BP >90‰ and FBG ≥100 mg/dL) (11).
Statistics
Results are presented as mean SD scores ±SD. Statistical comparisons included t-tests, the Pearson correlations and Fisher’s exact test comparisons. Results were considered statistically significant at p ≤ 0.05. The data were normally distributed.
RESULTS
The KS (n = 89) and control (n = 34) boys were similar in age: range of 4–12 years (Tables 1–3), mean 7.5 ± 2.4 vs. 8.1 ± 2.3 years. The karyotype of boys with KS was 47,XXY (84), 48,XXYY (1), 47,XXY/46,XY (2), 46,XX translocation (1).
Table 1.
Physical exam features of boys with KS compared with controls
| KS (n = 89) | Controls (n = 34) | p Value | |
|---|---|---|---|
| Mean age (years) | 7.5 ± 2.4 | 8.1 ± 2.3 | 0.3 |
| % Pubic Hair Tanner 1 | 91 | 97 | 0.1 |
| Mean height (SDS) | 0.6 ± 1.0 | 0.1 ± 1.0 | 0.02* |
| Mean weight (SDS) | 0.6 ± 1.1 | 0.6 ± 1.2 | 0.8 |
| Mean BMI (SDS) | 0.5 ± 1.2 | 0.7 ± 1.2 | 0.4 |
| % Body Fat | 25.6 ± 9.3 | 27.8 ± 12.0 | 0.5 |
| % waist circumference > 75‰ | 49 | 56 | 0.6 |
| % waist circumference > 90‰ | 30 | 21 | 0.4 |
| % elevated blood pressure | 0 | 0 |
BMI = body mass index; KS, Klinefelter syndrome; SDS = standard deviation scores.
Table 3.
Boys with Klinefelter syndrome meeting criteria for metabolic syndrome
| No. criteria met | n (%) | Which criteria (n) |
|---|---|---|
| 3 (diagnosis metabolic syndrome) | 7 (8) | HDL, WC, Trig (7) |
| 2 | 32 (36) | HDL, WC (26) |
| HDL, Trig (5) | ||
| WC, Trig (1) | ||
| 1 | 30 (34) | HDL (20) |
| WC (10) | ||
| TG (0) |
HDL = high-density lipoprotein; TG = triglycerides; WC = waist circumference.
Physical features
Weight, BMI and per cent body fat were similar, but WC was >90‰ in 30% of boys with KS and 21% of controls (Table 1). The boys with KS were significantly taller than the controls and none had elevated BP. All boys had testicular volume ≤4 mL, and the KS boys had, on average, smaller testicular volume than the controls (1.1 ± 0.7 vs. 1.7 ± 0.7 mL, p < 0.01). Most were Tanner I pubic hair. Pubic hair Tanner staging was not different between the groups.
Exercise
The mean daily time spent running or playing on a sports team on a school day was 42 min less in the KS versus control group: 72 ± 62 min vs. 114 ± 61 min (p < 0.01). In the KS group, the per cent of time spent running was negatively correlated with HOMA scores (p < 0.001), BMI SDS (p < 0.001), systolic BP (p = 0.03) and triglyceride values (p = 0.04). Less time running was associated with older age (p < 0.001) and with more boys having a WC >75‰. There was no association with time spent running and per cent body fat (p = 0.8) or HDL levels (p = 0.14).
Lipids in KS
For lipids (measured in the KS group only), 37% had elevated LDL and 40% had elevated total cholesterol levels (Table 2).
Table 2.
Fasting lipid and glucose values in boys with Klinefelter syndrome compared with normative data
| Lab and cut-off value | % who met cut-off | Mean ± SD | Mean 5–18-year-olds |
|---|---|---|---|
| Low-density lipoprotein ≥ 2.9 mmol/L (≥110 mg/dL) | 37 | 2.7 ± 0.7 (105 ± 25 mg/dL) | 2.4–2.6 (92–100 mg/dL) |
| T cholesterol ≥ 4.4 mmol/L (≥170 mg/dL) | 40 | 4.3 ± 0.8 (165 ± 28 mg/dL) | 4.1–4.2 (157–162 mg/dL) |
| Triglycerides ≥ 1.1 mmol/L (≥100 mg/dL) | 15 | 0.8 ± 0.5 (71 ± 46 mg/dL) | 0.60–0.90 (53–80 mg/dL) |
| High-density lipoprotein < 1.3 mmol/L (<50 mg/dL) | 65 | 1.2 ± 0.3 (46 ± 10 mg/dL) | 1.2–1.4 (48–55 mg/dL) |
| Glucose ≥ 6.1 mmol/L (≥110 mg/dL) | 1 | 4.7 ± 0.4 (85 ± 8 mg/dL) | 3.3–5.8 (60–105 mg/dL) |
Hormones in KS
Morning gonadotropins and total testosterone levels were in the prepubertal range in 89 boys with KS.
Insulin resistance in KS
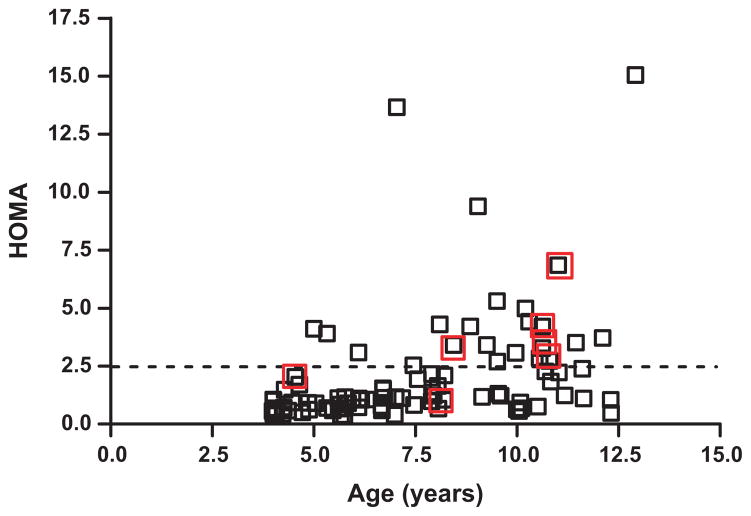
Insulin resistance was common (Fig. 1). Three boys did not have insulin levels drawn. Twenty boys (24%) had a HOMA ≥2.5. A higher HOMA score was associated with a higher systolic BP (p = 0.02), older age (p = 0.007) and increased BMI (p = 0.01). Despite the positive correlation between age and insulin resistance, boys as young as 5 years old already were exhibiting insulin resistance (Fig. 1). Eight boys (9.3%) had fasting insulin levels ≥20 μU/mL.
Figure 1.
Age (years) versus homeostasis model assessment (HOMA) in boys with Klinefelter syndrome. Red boxes indicate subjects who fit criteria for the metabolic syndrome. Horizontal line marks cut-off for insulin resistance: HOMA ≥2.5 consistent with insulin resistance.
Metabolic syndrome in KS
Of the five criteria for metabolic syndrome, 65% of the boys with KS had decreased HDL levels, 15% had elevated TG levels, and 49% had a WC >75‰ (Table 3). None had elevated BP or FBG. Eight of 86 (9.3%) had fasting insulin levels >20 μU/mL and 20 of 85 (24%) had a HOMA consistent with insulin resistance. Seven (8%) KS boys met the three criteria required for diagnosis of metabolic syndrome (low HDL, increased WC, elevated TG) and 32 of 89 (36%) met two criteria for metabolic syndrome.
Ascertainment
Ascertainment was prenatal in 56 (screening karyotype) and postnatal in 37 (developmental/motor/speech delay, behaviour problem, dysmorphic features). The boys diagnosed prenatally were slightly younger, but not significantly so (7.2 ± 2.3 vs. 7.6 ± 2.6 years, p = 0.09). They had similar physical features (BMI SDS [p = 0.3], WC >75% [p = 0.8], body fat% [p = 0.4]) and were similar metabolically (HOMA [p = 0.1], HDL [47 ± 10 vs. 45 ± 11, p = 0.06]).
Karyotype
Exclusion of boys with higher grade aneuploidy and mosaicism did not change the results.
DISCUSSION FOLLOWING THE PRESENTATION
In a large cohort of prepubertal boys as young as 4–12 years with KS, 24% had insulin resistance, 36% met two criteria for metabolic syndrome and 7% had the metabolic syndrome. The boys with KS also had decreased activity levels compared with age-matched controls, which strongly correlated with risk factors for metabolic syndrome. There are very few data regarding the prevalence of metabolic syndrome in the general paediatric population. In one survey of 1960 children older than 12 years, the US prevalence was 9% (11), and another study examining 1192 girls ages 9–10 noted a prevalence of 0.2% (18). Few studies have attempted to identify metabolic syndrome in a cohort as young as our study population or in solely prepubertal children. Comparison data are also lacking, as childhood metabolic syndrome is not yet consistently defined. Risk factors for metabolic syndrome identified in childhood include obesity (19), increased WC, increased triglyceride levels (18), and decreased activity levels (20), all of which were seen in this KS study population. Thus, it appears that in this young prepubertal cohort of boys with KS, the risk for metabolic syndrome is already increased. We also found that more than one-third of the boys had elevated total cholesterol and LDL levels, additional risk factors for later cardiovascular morbidity.
By looking at a prepubertal population, we attempted to answer the question: Does increased total body fat mass/WC precede the hypogonadal state or does the hypogonadal state predispose to increased total body fat? With the available current assays for measuring sex steroids, no difference in androgen concentrations, or subtle differences, between KS boys and healthy boys has been found so far (21,22). In the present study, the boys were all prepubertal by exam, yet appeared to have increased WC and an increased risk for metabolic syndrome already, which would suggest that the increased total body fat mass/WC may precede the hypogonadal state or that subtle hypogonadism early in life has later effect so on total body fat mass. Limitations of the study were that hormone levels were not measured in control boys. With the use of more sensitive assays, it may turn out that KS boys have lower androgen concentrations already in childhood when compared with healthy boys. Without these sensitive assays, the question of the impact of early and mild testosterone deficiency on the risk for insulin resistance in childhood is not yet fully answered. A prospective study evaluating pubertal aged-boys with KS who are replaced with testosterone versus those who are not replaced and compared with controls would help to answer this question. Clearly, general factors related to sex chromosome trisomy may also impact this phenotype.
The boys with KS had decreased running-type activity compared with their peers, which appeared to be significantly correlated with this early increased risk for insulin resistance. Possible explanations for the decreased running-type activity include decreased tone, strength and running speed (23), or personality traits such as anxiety or shyness that may interfere with willingness to participate in team sports.
Clarifying the risk for MS during childhood is important, as features of MS that present during childhood may track into adulthood, MS is associated with adverse cardiovascular outcomes and diabetes, and lifestyle interventions in childhood are effective. Boys with KS may already be at greater risk for type 2 diabetes and cardiovascular disease. The findings of this study suggest that a potential mediator is reduced running-type physical activity. Early intervention with recommendations for increased exercise may be helpful. The role of early androgen deficiency and treatment requires further study.
Acknowledgments
We thank all of the families who participated in this study. Supporting Grants by the NIH RO1NS #050597 and in part by a grant from the Pennsylvania Department of Health. The Department of Health disclaims responsibility for any analyses, interpretations or conclusions.
Abbreviations
- BMI
body mass index
- BP
blood pressure
- FBG
fasting blood glucose
- HDL
high-density lipoprotein
- HOMA
homeostasis model assessment
- KS
klinefelter syndrome
- LDL
low-density lipoprotein
- TG
triglycerides
- WC
waist circumference
APPENDIX: DISCUSSION FOLLOWING MARTHA BRADSLEY’S PRESENTATION
Insulin resistance and metabolic syndrome in prepubertal boys with KS
Michael Zitzmann (Muenster, Germany)
The metabolic syndrome and insulin resistance occur generally in association with hypogonadism, but more so in Klinefelter syndrome (KS). The HOMA test for testing glucose and insulin is very useful for patients with diabetes, but did you consider using the Quicky index which might be better for the normal population?
Martha Bradsley (Philadelphia, USA)
The QUICKI model gave similar results to the HOMA test in our studies.
Gary Butler (London, UK)
If you analysed these boys blindly without knowing their karyotype but only looked at boys based on their metabolic profile and level of obesity, would you see nullification of the karyotype? Are the insulin results due purely to their status, that is, are the results explicable because of their size and obesity, and not their karyotype?
Martha Bradsley
That is difficult to assess because we have no controls for the laboratory data. There is an epidemic of obesity in childhood, and we used normal laboratory reference values rather than comparing KS boys with other obese non-KS controls.
Fred Wu (Manchester, UK)
What data do you have on your control boy population?
Martha Bradsley
We just have physical measurements and no laboratory data.
Anders Juul (Copenhagen, Denmark)
In your KS population, 10–11% of boys had entered puberty and had elevated testosterone levels. Puberty is a time when individuals do develop metabolic syndrome and insulin resistance. Did you extract data excluding pubertal boys to see if a similar proportion had insulin resistance?
Martha Bradsley
I extracted all cases with Turner II pubic hair and elevated testosterone levels, and found the same results.
Robert McLachlan (Melbourne, Australia)
The sensitivity of your androgen and testosterone assay was very high and you had no control data. It is possible that there are no differences in testosterone levels between KS boys and controls. As the concept of androgen deficiency in childhood is new to me, are you really sure that androgen levels are low in KS boys.
Martha Bradsley
The results are controversial. We used radioimmunoassay commercially available in USA. It is possible that there is no difference between KS boys and controls.
References
- 1.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–22. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- 2.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591–8. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 3.Pei D, Sheu WH, Jeng CY, Liao WK, Fuh MM. Insulin resistance in patients with Klinefelter’s syndrome and idiopathic gonadotropin deficiency. J Formos Med Assoc. 1998;97:534–40. [PubMed] [Google Scholar]
- 4.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 5.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–4. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 6.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 7.Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–7. [PubMed] [Google Scholar]
- 8.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 9.Aksglaede L, Molgaard C, Skakkebaek NE, Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child. 2008;93:30–4. doi: 10.1136/adc.2007.120675. [DOI] [PubMed] [Google Scholar]
- 10.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–29. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 11.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 12.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 13.Slaughter M, Lohman TG, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- 14.Hall JG, Froster-Iskenius Ursula G, Allanson Judith E. Handbook of normal physical measurements. Oxford: Oxford University Press; 1995. [Google Scholar]
- 15.Marshall WATJ. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1993;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35:1196–202. doi: 10.1249/01.MSS.0000074446.02192.14. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Morrison JA, Friedman LA, Harlan WR, Harlan LC, Barton BA, Schreiber GB, et al. Development of the metabolic syndrome in black and white adolescent girls: a longitudinal assessment. Pediatrics. 2005;116:1178–82. doi: 10.1542/peds.2004-2358. [DOI] [PubMed] [Google Scholar]
- 19.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 20.Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89:2526–39. doi: 10.1210/jc.2004-0276. [DOI] [PubMed] [Google Scholar]
- 21.Wikstrom AM, Dunkel L, Wickman S, Norjavaara E, Ankarberg-Lindgren C, Raivio T. Are adolescent boys with Klinefelter syndrome androgen deficient? A longitudinal study of Finnish 47,XXY boys. Pediatr Res. 2006;59:854–9. doi: 10.1203/01.pdr.0000219386.31398.c3. [DOI] [PubMed] [Google Scholar]
- 22.Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:1864–8. doi: 10.1210/jc.2003-031624. [DOI] [PubMed] [Google Scholar]
- 23.Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MP, Kushner H, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A:708–19. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]