Abstract
Varicocele is the main cause of male infertility. Treatment stops continuous damage to spermatogenesis, thereby potentially improving fertility. Among all the available procedures, the ante-grade scrotal sclerotherapy (ASS), a combined radiological-surgical approach first introduced by Tauber, is gaining more popularity due to its minimal invasiveness. We report the case of a 35-year old man who was subjected to a colonic resection after antegrade scrotal sclerotherapy for varicocele. The procedure was necessary due to the embolization of venous anastomosis between the spermatic and mesenteric veins, which were not detectable at the preoperative phlebography.
Introduction
Varicocele represents the main cause of male infertility and leads to changes in testicular spermatogenesis in 60% to 70% of cases. Treatment prevents continuous damage to spermatogenesis, thereby potentially improving fertility. Successful treatment of varicocele improves semen quality in 40% to 60% of patients and recovers fertility in 10% to 40%.1 Among all the available procedures, the antegrade scrotal sclerotherapy (ASS) is a combined radiological-surgical approach first introduced by Tauber in 19882 and is usually performed under local anesthesia. The procedure starts with a short longitudinal scrotal incision at the base of the scrotum, isolation of the funiculum and identification of the most enlarged vein. Thereafter, a small incision of the vein allows for the insertion of a 23-gauge needle to perform a venogram by iodine contrast. In the end atoxysclerol mixed with air is injected.2–5 This technique is well-established to treat varicocele. It is easier, faster and less invasive than open surgical and laparoscopic treatments.5 It results in a persistence rate as high as 9% in adults and 3% in children.6 An analysis of seminal parameters showed a statistically significant improvement in the rate of fast progressive spermatozoa and reduction of immotile spermatozoa in patients who underwent ASS compared to open surgery.5 Reported complication rates are low and they include scrotal hematoma, sterile epididymitis (due to paravascular application of the sclerosing agent), testicular atrophy (accidental sclerotherapy of the testicular artery), as well as intestinal7 and abdominal wall necrosis (accidental sclerotherapy of the cremasteric artery).2
Case report
A 35-year-old man presented with severe oligoasthenospermia and diagnosed with third grade varicocele on ultrasound. After we explained the therapeutic options to the patient, he agreed to undergo antegrade scrotal sclerotherapy. Incising the base of the scrotum, the funiculum was identified and the most enlarged vein was isolated and suspended between two slack sutures. A little incision of the vein was performed to insert a 23-gauge needle. The right position of the needle was checked by washing the vessel lumen with saline solution; the iodine contrast was then injected to perform a venogram (Fig. 1). Finally, during the Valsalva manoeuvre, 1 mL of air was injected, followed by 3 mL of 3% ethoxysclerol (air-block technique).
Fig. 1.
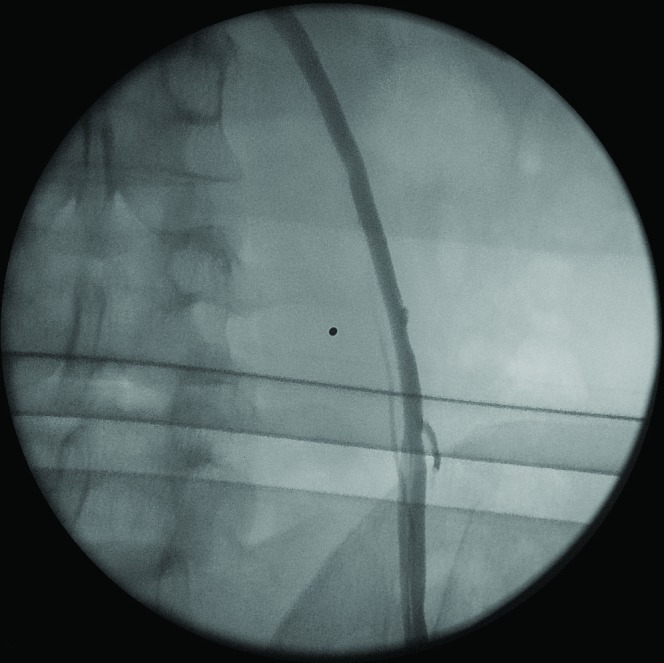
Iodine contrast injected to perform a venogram (not during the Valsalva manoeuvre).
Immediately after the injection of atoxysclerol, the patient reported intense pain in the left iliac fossa and flank and so the procedure was interrupted. Physical examination revealed an acute abdomen with positive Blumberg, abdominal distension and torpid peristalsis. A rectal probe was inserted and the patient was given prokinetics to facilitate intestinal peristalsis and steroids to reduce the inflammation.
A computed tomography (CT) scan revealed small aerial bubbles into mesenteric circulation of the sigmoid and descending colon (Fig. 2, Fig. 3). This finding was consistent with pneumatisation of the mesenteric venous plexus as a complication of the sclerotherapy and the tissues were minimally thickened with modest flogistic reaction of the loose tissue nearby. We decided to start anticoagulant therapy with low-molecular-weight heparin (LMWH) to prevent a potential venous thrombosis of the mesenteric circle secondary to chemical injuries.
Fig. 2.
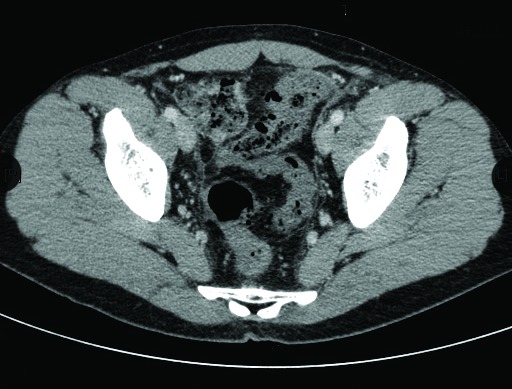
Small aerial bubbles into mesenteric circulation of the sigmoid and descending colon.
Fig. 3.
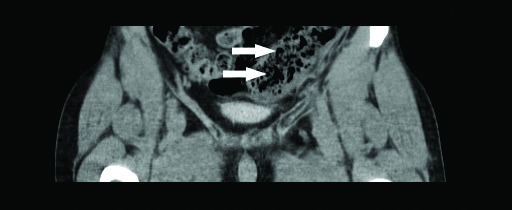
Small aerial bubbles into mesenteric circulation of the sigmoid and descending colon.
Further CT scans were inconclusive, while the patient’s pain continued to increase. The white blood cell count rose to 19 × 103 uL the day after the procedure and continued to increase during the following days with a percentage of neutrophil granulocytes between 86% and 88%.
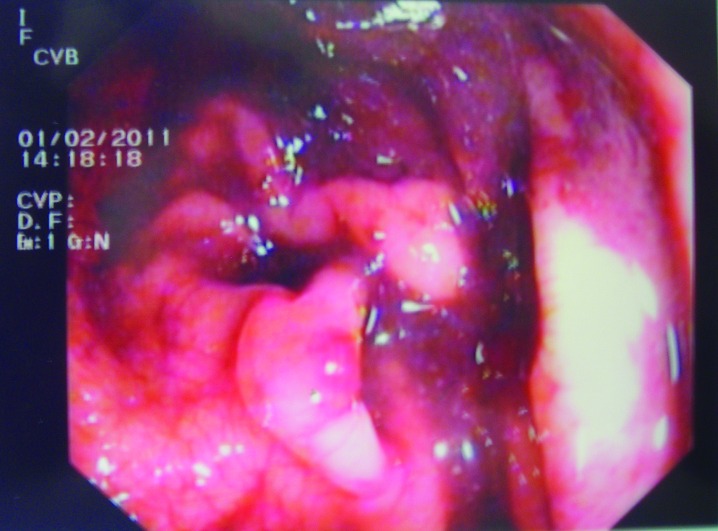
As recommended by the general surgeon, a rectoscopy was performed, but it was inconclusive due to the presence of coagulated blood and mucus (Fig. 4). A new CT scan revealed a considerable aerial distension of the ileal and colic loops, mainly of the transverse and descending colon, with no radiocontrast outside the rectal ampulla.
Fig. 4.
The rectoscopy showing coagulated blood and mucus.
Eight days after the procedure, the patient was immediately transferred to a general surgery unit due to severe pain resistant to morphine and benzodiazepine and concomitant septic shock. At this time, the CT revealed necrosis of the descending and sigmoid colon with faeces and air in the abdomen. A colic resection with end colostomy was carried out, with the rectum sewn shut and embeded in the pelvis for subsequent anastomosis (among transverse colon and rectum), which was performed 6 months later with complete fecal continence.
Discussion
Although the existence of an anastomotic circle between the spermatic and mesenteric veins is not well-documented in the literature, Salerno and colleagues8 suggest considering this finding as a risk for varicocele recurrence. Despite this, many authors do not routinely perform a preoperative phlebography9 before proceeding with sclerotherapy. Considered that on the phlebography the presence of communications between spermatic vein and visceral veins is reported in about 11% of patients,8 a complete angiographic study may represent a safer, if not compulsory, approach to guide the procedure, before antegrade sclerotherapy.
Indeed, the presence of an anastomotic circle should prompt a halting of the antegrade scrotal sclerotherapy to avoid injuries to the visceral vessels and should indicate surgical treatment. However, as previously reported by Fulcoli and colleagues,7 performing a phlebography is not devoid of possible false negative findings.
In the present experience, on the phlebography, internal spermatic vein was correctly visualized and there was no evidence of such anastomosis. Iodine contrast was injected to perform a venogram (not during the Valsalva manoeuvre) (Fig. 1).2 The injection of 1 mL of air followed by 3 mL of 3% ethoxysclerol (air-block technique) during the Valsalva manoeuvre caused an increase in intravascular pressure due to the presence of air and to the Valsalva manoeuvre. These injections may have opened the venous passages between the spermatic and the inferior mesenteric veins that were not detectable by the phlebography.
Moreover, the injection of air followed by the sclerosing agent has the ability to better identify anastomotic circles between the spermatic and the inferior mesenteric veins compared to radiocontrast, due to the different densities of these 2 substances. Therefore, the cause leading to the necrosis of the descending and sigmoid colon was thought to be a bowel infarct secondary to venous thrombosis of the inferior mesenteric vein. On the other hand, given the small calibre of these vessels, no interventional radiology procedure could be performed to treat venous thrombosis. No further imaging or interventional radiology may have helped in the diagnosis, due to the variability of the vasculature and the technical difficulty.10
Conclusion
We found a complete angiographic study, mainly an ante-grade phlebography, may represent a safer approach to guide the procedure before antegrade sclerotherapy. Therefore, performing a preoperative venogram during the Valsalva manoeuvre becomes it is imperative to reveal unknown anastomosis before there is more vascular damage. Although the Tauber approach represents a minimally invasive, well-tolerated and easy to perform technique to treat varicocele, surgeons must be aware of the difficulties in determining possible anastomotic vessels between the intestinal and spermatic veins and possible severe side effects that may arise from its embolization.
Footnotes
Competing interests: Dr. Vicini, Dr. Di Pierro, Dr. Grande, Dr. Voria, Dr. Antonini, Dr. De Marco, Dr. Nicola and Dr. Gentile all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Green KF, Turner TT, Howards SS. Varicocele reversal of the testicular blood flow and temperature effects by varicocele repair. J Urol. 1984;131:1208–11. doi: 10.1016/s0022-5347(17)50874-5. [DOI] [PubMed] [Google Scholar]
- 2.Tauber R, Pfeiffer D. Surgical atlas varicocele: Antegrade scrotal sclerotherapy. BJU Int. 2006;98:1333–44. doi: 10.1111/j.1464-410X.2006.06579.x. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra V, Porcaro AB, Righetti R, et al. Antegrade scrotal sclerotherapy in the treatment of varicocele: A prospective study. BJU Int. 2002;89:264–8. doi: 10.1046/j.1464-4096.2001.02418.x. [DOI] [PubMed] [Google Scholar]
- 4.Zaupa P, Mayr J, Höllwarth ME. Antegrade scrotal sclerotherapy for treating primary varicocele in children. BJU Int. 2006;97:809–12. doi: 10.1111/j.1464-410X.2006.06033.x. [DOI] [PubMed] [Google Scholar]
- 5.Zucchi A, Mearini L, Mearini E, et al. Treatment of varicocele: Randomized prospective study on open surgery versus Tauber antegrade sclerotherapy. J Androl. 2005;26:328–32. doi: 10.2164/jandrol.04143. [DOI] [PubMed] [Google Scholar]
- 6.Johnsen N, Johnsen I, Tauber R. Spermiogram findings following antegrade sclerosing of a varicocele. WMW. 1997;147:81–3. [PubMed] [Google Scholar]
- 7.Fulcoli V, Costa G, Gigli F, et al. Ischemic necrosis of the sigmoid colon after antegrade sclerotherapy of idiopathic varicocele: a case report. Urologia. 2013;80:162–4. doi: 10.5301/RU.2013.10722. [DOI] [PubMed] [Google Scholar]
- 8.Salerno S, Cannizzaro F, Lo Casto A, et al. Anastomosis between the left internal spermatic and splanchnic veins. Retrospective analysis of 305 patients. Radiol Med. 2000;99:347–51. [PubMed] [Google Scholar]
- 9.Pecoraro G, Motta L, Olivio G, et al. Is phlebography crucial in Tauber varicocele sclerotherapy? Urologia. 2008;75:105–7. [PubMed] [Google Scholar]
- 10.Artyukhin AA. Anatomy and microanatomy of the venous system of scrotal organs and spermatic cord. Bull Exp Biol Med. 2007;143:99–104. doi: 10.1007/s10517-007-0027-9. [DOI] [PubMed] [Google Scholar]