Abstract
The Simple View of reading emphasizes the critical role of two factors in normal reading skills: word recognition and reading comprehension. The current study aims to identify the anatomical support for aspects of reading performance that fall within these two components. Fractional anisotropy (FA) values were obtained from Diffusion Tensor images in twenty-one typical adolescents and young adults using the Tract Based Spatial Statistics (TBSS) method. We focused on the Arcuate Fasciculus (AF) and Inferior Longitudinal Fasciculus (ILF) as fiber tracts that connect regions already implicated in the distributed cortical network for reading. Our results demonstrate dissociation between word-level and narrative-level reading skills: the FA values for both left and right ILF were correlated with measures of word reading, while only the left ILF correlated with reading comprehension scores. FA in the AF, however, correlated only with reading comprehension scores, bilaterally. Correlations with the right AF were particularly robust, emphasizing the contribution of the right hemisphere, especially the frontal lobe, to reading comprehension performance on the particular passage comprehension test used in this study. The anatomical dissociation between these reading skills is supported by the Simple View theory and may shed light on why these two skills dissociate in those with reading disorders.
Keywords: Arcuate fasciculus, Inferior longitudinal fasciculus, Diffusion tensor imaging, Reading, Reading comprehension
1. Introduction
The Simple View of reading (Gough & Tunmer, 1986; Hoover & Gough, 1990; Tunmer & Chapman, 2012) holds that normal reading can be predicted by two key factors: word recognition and language comprehension. In competent readers, both are thought to be equally important. This simple construct has enjoyed considerable support. Behavioral studies of reading indicate that both word recognition and comprehension independently account for variance in reading (Aaron et al., 2008; Hoover & Gough, 1990; Protopapas, Simos, Sideridis, & Mouzaki, 2012; Sabatini, Sawaki, Shore, & Scarborough, 2010; Vellutino et al., 2007). Support for the separate contribution that word recognition and comprehension have on reading ability came from genetic studies. Twin studies suggest that there is a separate genetic influence for word recognition and listening comprehension on reading ability (Keenan et al., 2006) and the genetic contribution of these two factors is stable over time (Betjemann et al., 2008). However, since reading is a learned skill, other cognitive and environmental elements are clearly involved (Byrne et al., 2009; Gayan & Olsen 2003 Petrill et al., 2006).
Word recognition within the Simple View model may include word decoding as well as the ability to recognize words as holistic units (Gough & Tunmer, 1986). Although word recognition and in particular, decoding skills, have garnered considerable attention in the reading literature, comprehension skills have been relatively under-studied. Language comprehension in the Simple View model is thought to encompass multiple aspects of receptive language skills from word- to discourse-level language. This very broad conceptualization may be why the relationship between word reading and reading comprehension has proven to be test-dependent (Keenan, Betjemann, & Olson, 2008; Nation & Snowling, 1997) and why word-level vs. sentence- or discourse-level aspects of comprehension relate to different biological factors (Betjemann et al., 2011). Neuroimaging studies clearly show that tasks that rely on word-level vs. discourse-level processing of spoken language show very different patterns of activation (Holland et al., 2007; Plante et al., 2006). Therefore, it is important to clearly identify which aspects of language comprehension are tapped by a task in order to make neurological predictions.
Prior imaging studies of reading comprehension have largely focused on word- or sentence-level processing (Booth et al., 1999; Caplan et al., 2001; Cutting et al., 2006; Keller et al., 2003; Mason & Just, 2007; Meyer et al., 2000; Ni et al., 2000; Rimrodt et al., 2009). In general, reading sentences activates the same regions as reading words, with more spread and bilateral activation occurring specifically in the middle and superior temporal gyri, bilateral temporal poles, and the left frontal and parietal regions. However, there is reason to believe that right hemisphere activation also characterizes some aspects of language comprehension. Studies in which participants were asked to listen to stories reported larger regions of right temporal lobe activity than when the sentences of these stories were arranged in a conceptually unrelated manner (e.g., Plante et al., 2006; Robertson et al., 2000). These studies focused on the precise role of the right hemisphere in language comprehension. Evidence from patients with right hemisphere brain damage suggests several possibilities. Deficits comprehending nonliteral and indirect language such as metaphors, humor, and sarcasm have been associated with right hemisphere injury (e.g., see Johns et al., 2008 for a review). There has also been increasing interest in the ability of individuals with right hemisphere damage to utilize narrative context when drawing conclusions. Despite evidence suggesting an understanding of the main ideas contained within a narrative, these individuals show impaired use of semantic context in interpreting new information (Roman, Brownell, Potter, & Seibold, 1987; Wapner et al., 1981). This may be due to an overarching problem in the organization of narrative information into a coherent mental model (Delis et al., 1983; Hough, 1990). The failure to construct a mental model inhibits the ability to make predictions concerning anticipated novel information (Blake, 2009). George et al. (1999) performed a similar study on typically developing readers in which they demonstrated that greater right hemisphere activation was associated with reading comprehension following the reading of untitled versus titled narrative texts. This suggests that the presence of a title, which provides the initial context for a narrative, engages cognitive processes that influence the way in which the reader processes the subsequent text.
This evidence suggests that the right hemisphere most likely contributes to reading comprehension through the production of contextual understanding which allows an individual to predict future information in a story context. The Woodcock-Johnson III Reading Comprehension subtest items serve as a means to evaluate this particular reading skill in typical and impaired readers (Woodcock, McGrew, & Mather, 2001). For this subtest, test takers are asked to read individual sentences or a short passage and use the semantic context to fill in the omitted word. Although word reading is a prerequisite to performance of this task, this subtest requires comprehension skills that go beyond word recognition and comprehension of individual words. Readers must accumulate sufficient semantic representation at the narrative level in order to predict missing words within the text. As such, this particular comprehension measure taps the ability to integrate semantic cues into a mental model of comprehension. This skill specifically relies on connections between right hemisphere structures. Therefore, variation in this skill should correspond to an underlying variation in the right hemisphere pathways that contribute to the larger narrative comprehension network.
The other component of the Simple View model is word recognition. The majority of previous studies of word reading have focused largely on the cortical contributions, mainly in the left hemisphere, to the reading network with fewer studies examining the fiber pathways between cortical regions within the network (see Vandermosten, Boets, Wouters, & Ghesquière, 2012 for a review). Although the specific regions implicated may vary, multiple studies have shown an association between word recognition skills (i.e., decoding, single word identification) and white matter in the left temporal-parietal regions (Deutsch et al., 2005; Klingberg et al., 2000; Niogi et al., 2006; Odegard et al., 2009). However, a more recent study showed more correlations for measures of word reading, decoding, and fluency with white matter microstructure, which was diffusely and bilaterally distributed (Lebel et al., 2013). It is worth noting that these studies have used subject samples that combined reading disabled and typical readers. Steinbrink et al. (2008) also found correlations between left parietal white matter measures and word recognition measures, but only among participants with dyslexia. No such correlation was apparent among typically developing children. In contrast, Yeatman et al., (2011) and Saygin et al. (2013) indicated that the left arcuate fasciculus microstructure correlated with phonological awareness (a word-recognition sub-skill) in typically developing children. This fiber bundle runs from posterior temporal-parietal regions to anterior frontal lobe regions. This leaves open the question of the degree to which posterior white matter findings characterize poor reading in those with reading disabilities versus normal variance in reading skills. Furthermore, we note that all of these studies focused on skills related to word recognition only. Since the Simple View model includes both word recognition and comprehension as equal contributors to reading efficiency, we aim to examine the neural circuits related to each of the abilities in healthy, typically developing adolescents. This will enable us to understand the underlying biological mechanism that contributes to several language disorders from reading disorders due to either word recognition deficit (i.e., dyslexia, see Olulade et al., 2013,) or due to reading comprehension (i.e., Specific Language Impairment, see Spencer, Quinn, & Wagner, 2014).
Despite its equal role in the Simple View of reading, we are aware of only one study which has examined the correspondence between reading comprehension and white matter metrics. Hasan et al. (2011) examined the corpus callosum in children with and without reading disabilities. In this study, children with reading disabilities were subdivided into those with classic dyslexia (poor word recognition scores) and those with poor performance on measures of comprehension or fluency. The latter group showed differences relative to typical readers in the microstructure of the genu of the corpus callosum. Although these differences could be attributed in part to poor performance in fluency, these results may likely suggest that connections between the right and left hemisphere may play a role in reading comprehension. If so, by extension, this would implicate a right hemisphere role in reading comprehension.
In this study, we aimed to provide a neuroanatomical support to the distinct neural-correlates to the two sub-components of the Simple View model: word recognition and comprehension. To achieve that, we used a region-of-interest (ROI)-based diffusion tensor imaging (DTI) analysis, as well as a tract-based spatial statistics (TBSS) method (Smith et al., 2006) to explore neuroanatomical substrate of normal reading in two fiber tracts in the right and left hemispheres. We specifically focused on two white matter tracts which have been associated with reading abilities: the Arcuate Fasciculus (AF) (Qiu et al., 2011; Yeatman et al., 2012; for review see Vandermosten et al., 2012) and the Inferior Longitudinal Fasciculus (ILF) (Epelbaum et al., 2008; Yeatman et al., 2012; Rauschecker et al., 2009). The AF tract is involved in reading through its connections between semantic and syntactic language regions in the inferior frontal gyrus and phonological and language association areas in the temporal-parietal areas (Qui et al., 2011; Yeatman et al., 2012). The ILF connects these temporal lobe regions (Superior-temporal gyrus/Medial-temporal gyrus) with the visual word form area in the occipital lobe (Occipito-temporal sulcus, BA 37), which is considered to be the region in the brain for rapid word processing (Yeatman et al., 2012) and visual memory (Catani et al., 2003). The ROI-based analysis offers us global mean values of Fractional Anisotropy (or FA; a measure of white matter microstructure) whereas the voxel-wise TBSS within the two ROIs provides us regional characteristics of FA related to reading skills within the AF and ILF. TBSS combines the strength of both voxel-based and tractography-based analyses and overcomes the limitations of conventional methods, including partial voluming, spatial smoothing, and arbitrary thresholds (Smith & Nichols, 2009; Wang et al., 2012). TBSS improves the normalization and alignment for group analysis of DTI data. Therefore, it increases sensitivity to detect white matter microstructure changes associated with behavior measures.
In line with previous studies, we predict that reading measures that focus on word-level reading skills will correlate with left hemisphere tracts. Word-level skills, particularly those requiring rapid identification of words, will be associated with greater FA in the ILF and AF, consistent with prior research. In addition, we make the unique prediction that a measure of comprehension that requires readers to use narrative context to predict a missing word in the text will be associated with both the left and right hemisphere pathways, specifically the AF, given the bilateral contributions to narrative comprehension.
2. Results
2.1. Behavioral results
All participants had average to above average full-scale IQ (IQ range=93-133, mean standard score=112±10.38). Reading testing verified that all participants had normal to above average scores in automatic word reading as measured by the subtest of sight word efficiency from the TOWRE-II tests (range=87-130, mean standard score=104.27±12.52, normative standard score: 100 ±15) as well as intact reading comprehension scores as measured by the Woodcock-Johnson III (range=88-120, mean standard score=106.68±10, normative standard score: 100 ±15). The Pearson correlation between word reading standard scores (as measured by the TOWRE-II) and reading comprehension standard scores (as measured by the WJ-III) did not result in a statistically significant correlation (p>.05).
2.2. Imaging data
Following the generation of the FA maps from each participant, we used TBSS for voxel-based group analysis and aligned each subject's FA image onto the cohort mean FA skeleton. Voxel-wise analysis within the two tracts of interest were performed (Inferior Longitudinal Fasciculus and Arcuate Fasciculus) to find a regional correlation between reading measures and the FA values at each voxel, as well as the global mean FA in the ROIs
2.2.1. ROI: Inferior Longitudinal Fasciculus
Based on the voxel-wise TBSS results, we found a significant positive correlation between the regional FA in bilateral anterior temporal portion of the ILF and the sight word efficiency scores from the TOWRE-II word reading measure (see Figure 1 and 2a). Moreover, we also found a significant positive correlation between the regional FA in the left middle temporal portion of the ILF and the WJ-III passage comprehension measures (see Figure 2b).
Figure 1.
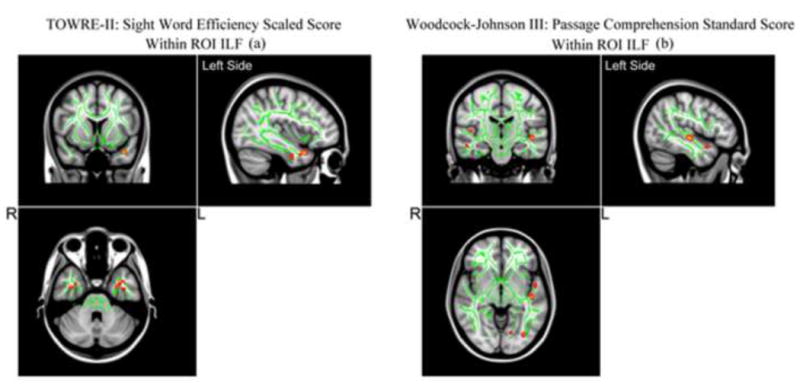
TBSS results of significant positive correlations between FA in ILF and the Sight Word Reading Efficiency standard score (TOWRE-II) (a), and the Woodcock-Johnson III: Passage Comprehension Standard Score (WJ-III) (b) are superimposed on the fiber skeleton (Green) and overlaid on the MNI152 T1 template (p <0.002, uncorrected). Images are in radiological orientation.
Figure 2.
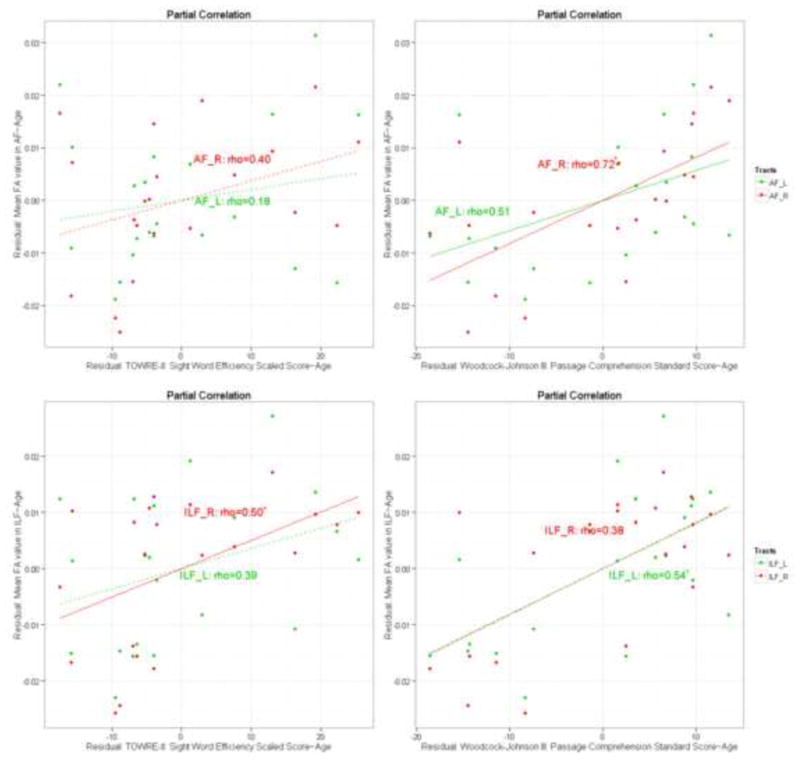
Partial correlation plots (controlling for age) for mean FA values from two atlas-based fiber tracts within the TBSS skeleton: [A-1]: Mean FA values in the AF correlates with the TOWRE-II; [A-2]: Mean FA values in the AF correlates with WJ-III; [I-1]: Mean FA values in the ILF correlates with the TOWRE-II: [I-2]: Mean FA values in the ILF correlates with WJ-III.
Further examining the mean FA within the ILF, Figure 2 shows significant positive correlation between the mean FA in the right ILF and the sight word efficiency scores from the TOWRE-II word reading measure [0.50 (p <0.05, FDR corrected)] as indicated by the solid red line in the bottom left panel (Figure 2). In addition, we see a significant positive correlation between the mean FA in the left ILF and the WJ-III passage comprehension measures as indicated by the solid green line in Figure 2 bottom right panel [0.54 (p < 0.05, FDR corrected)].
2.2.2. ROI: Arctuate Fasciculus
Based on the voxel-wise TBSS results, there was no significant positive correlation between the regional FA in the AF and the sight word efficiency scores from the TOWRE-II word reading measure. However we did find significant positive correlation between the regional FA in the left frontal portion of the AF and the WJ-III passage comprehension measures, as well as positive correlation between the regional FA in the bilateral parietal portion of the AF and the WJ-III passage comprehension measures as shown in Figure 3.
Figure 3.
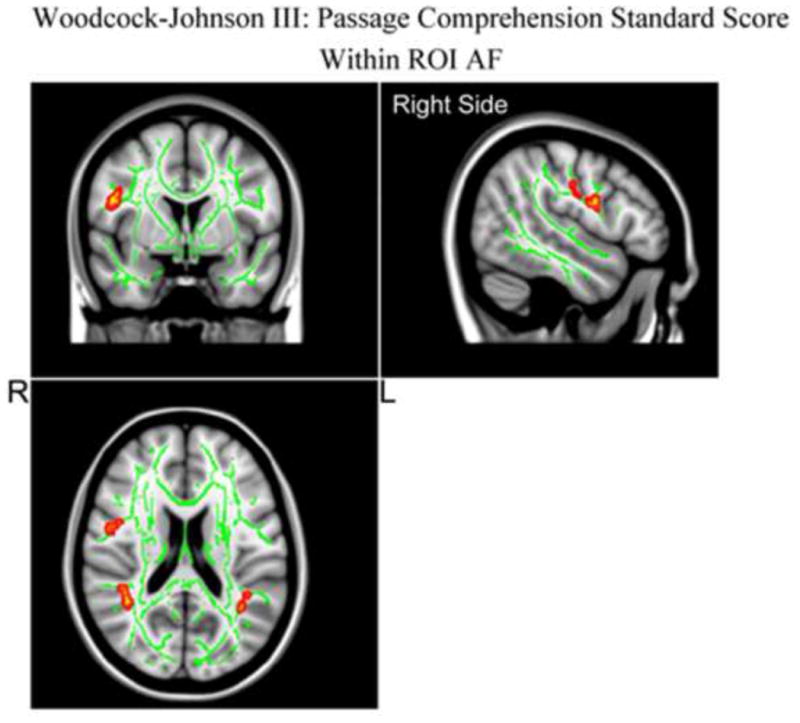
TBSS results of significant positive correlations between FA in AF and the Woodcock-Johnson III: Passage Comprehension Standard Score (WJ-III) are superimposed on the fiber skeleton (Green) and overlaid on the MNI152 T1 template (p <0.02, uncorrected). Images are in radiological orientation.
Examining the correlations between the mean FA in the AF (shown in Figure 3 in more detail), Figure 1 shows a significant positive correlation between the mean FA in bilateral AF and the WJ-III passage comprehension measures (Figure 2, top right panel). But the linear regression between the mean FA in the AF and the sight word efficiency scores from the TOWRE-II word reading measure (Figure 2, top left panel) do not reach significance as indicated by the dashed lines. For the mean FA in the AF, the partial correlation with the WJ-III passage comprehension measures, controlling for age, for the right side was 0.72 (p < 0.003, FDR corrected) and that for the left side was 0.51 (p < 0.05, FDR corrected) (see Figure 2).
3. Discussion
The Simple View of reading postulates that two separate factors contribute to variance in reading ability. Here we demonstrate differences in the association between two specific skills that reflect the word recognition and the language comprehension components of this model and fiber pathways thought to be central to the reading network. Our measure of word recognition (sight word efficiency scores from the TOWRE-II test) correlated with the right ILF, whereas our measure of language comprehension via the WJ-III Passage Comprehension Subtest, correlated with the left ILF and the AF bilaterally. These findings suggest a different contribution for each of these anatomical tracts to these two theoretically important components of reading. Furthermore, the predicted association between the right AF and passage comprehension performance supports the role of the right hemisphere in reading comprehension (cf., Kintch, 1989). This is consistent with the prediction of independence of these two skills under the Simple View of reading.
The AF tract connects the inferior frontal gyrus (IFG), the parietal portion of the supramarginal gyrus, and the lateral temporal lobe (Catani, Jones, & Ffytche, 2005; Saygin et al., 2013; Yeatman et al., 2012). Both cortical regions connected by the AF play an important role in language comprehension, and the AF itself is associated with oral language comprehension (Rolheiser, Stamatakis, & Tyler, 2011). Therefore, we expected an association between FA values for left hemisphere pathways and language skills. We predicted a correlation between the Passage Comprehension subtest scores and the left AF based on the functional imaging evidence of left-lateralized activation for story comprehension (e.g., Roberts et al., 2000; Holland et al., 2007; Horowitz-Kraus, Vannest & Holland, in press; Plante et al., 2006). Saygin and colleagues (2013) reported a correlation between FA values in the left AF and phonological awareness in kindergarten-aged children, which may support a relationship between listening/oral comprehension and reading comprehension. Based on the association between oral and reading comprehension (Aaron, 1991), it is possible that phonological awareness in children, which supports oral comprehension, lays the foundation for future reading comprehension through the continued development of a shared anatomical source. A future study which employs both oral and reading comprehension should be conducted to verify this point.
In addition, we predicted that Passage Comprehension scores would correlate with the AF in the right hemisphere based on prior evidence of a right hemisphere role in narrative processing (Delis et al., 1983; Hough, 1990; Roman et al., 1987; Wapner et al., 1981). Specifically, the use of semantic context required by the Passage Comprehension subtest made it an appropriate measure to test this hypothesis. Indeed, the correlation between the Passage Comprehension scores and the right AF was robust, and stronger for the right than left AF or the left ILF. We caution, however, that this right lateralized finding might be reversed if other measures of language comprehension are tested. As conceptualized by the Simple View, language comprehension encompasses everything from word meaning to passage comprehension (Tunmer & Chapman, 2012), and it is likely that different relations between these various skills and brain structures will exist. However, it is unlikely that single word reading, which is prerequisite to passage reading, accounts for this brain-behavior relation given that our single word reading measure did not correlate with the AF, and our two reading measures did not correlate with one another.
The association between the right AF and reading comprehension scores in our group of readers has potential implications for the nature of reading disabilities. One interpretation of our findings is that regions of the right frontal lobe connected to posterior temporal parietal language areas are an inherent part of an effective reading comprehension circuit in typical reading but does not play a critical role in dyslexia, given that comprehension is typically intact for this group of poor readers. Conversely, poor readers with weak comprehension may not effectively engage cortical areas in the right hemisphere or may have inefficient connections between them. Differences in the particular aspects of reading associated with various anatomical pathways might help to explain the variety of clinical subtypes of reading difficulties (e.g., individuals with reading comprehension problems and intact word recognition or and vice versa) (see also Cutting & Scarborough, 2013; Fletcher et al., 2013). Interestingly, Cutting and colleagues have recently demonstrated a unique brain activation pattern for individuals who have reading comprehension problems in spite of having intact reading abilities using a functional MRI task (Cutting & Scarborough, 2013). This particular study suggested a decreased activation of the left inferior frontal gyrus in this population as compared to dyslexics and age-matched controls as a result of difficulties in semantic access (Cutting et al., 2013). It is possible that because Cutting and colleagues focused on words as opposed to contextual reading, the decreased activation associated with semantic retrieval during word reading was localized primarily in the left hemisphere. Additionally, it is possible that in the same population, semantic retrieval associated with contextual reading will result in decreased activation of the right hemisphere homologue.
Right hemisphere activation for word recognition has been reported for individuals with reading impairment but it has been viewed as a possible compensatory mechanism. In a seminal outcome study of dyslexia, Hoeft and colleagues (2011) reported that right hemisphere activation during a word recognition task and white matter in the right Superior Longitudinal Fasciculus (which includes the AF that departs from the SLF tract to branch into temporal cortices and connects Broca's and Wernicke's areas; Saygin et al., 2013) predicted reading improvement for children with dyslexia. However, our results with non-impaired readers suggest the possible importance of right hemisphere pathways in normal reading as well. This may have implications for the interpretation of right hemisphere findings in dyslexia. Individuals with dyslexia, as classically defined (IDA, 2011), have poor word-decoding skills despite relatively good language comprehension (Peterson & Pennington, 2012; Fletcher et al., 2013; Cutting & Scarborough, 2013). It may be that the frontal activation and white matter results reported by Hoeft et al. (2011) actually reflect contributions to different aspects of reading. Our results suggest that this frontal activation and white matter results may account for variance in decoding, whereas the AF may reflect the contribution of normal variance in language comprehension. Since the current study did not measure decoding, future research should examine this possibility in depth.
In contrast to the predicted right hemisphere association with passage comprehension, we hypothesized that only the left ILF would correlate with the sight word efficiency scores from the TOWRE-II test. Therefore, the relationship between the right hemisphere pathway and word recognition was a new and unanticipated finding. If confirmed by future studies, this finding would serve to focus further attention on the right hemisphere's role in word recognition as an aspect of reading. The ILF connects the temporal lobe to the occipital lobe and projects to the visual word form area (Yeatman et al., 2012). The correlation for the right ILF was slightly weaker than for the left, mirroring the well-established left lateralization in word recognition. We suggest that since the participants in the current study were typical readers who had already constructed a stable mental lexicon, they likely employed automatic word retrieval (for the mental lexicon theory, see Share, 2008). This is also consistent with the proposed posterior reading circuits that control word recognition skills, particularly for recognition of known words (Pugh et al., 2001).
The following limitations should be taken into account when reviewing the results for the current study. Here, we interpreted our DTI results in the framework of the Simple View theory since we were interested in the relative contribution of two skills that directly reflect a comprehension and automatic word recognition reading model. However, it is important to note that while this model is helpful in conceptualizing different common forms of reading disorders and in reading instruction (Kirby & Savage 2008), the traditional Simple View theory does not account for all phenomena known to be important in typical and disordered reading. Additional studies are needed to address both alternate measures of comprehension and word reading (e.g., untimed word reading, word rather than sentence comprehension), as well as broader skill areas not addressed by the Simple View theory. Such studies qualify the Simple View theory by accounting for additional aspects of reading such as fluency which, although not considered here, is also considered important in describing skilled reading (Breznitz, 2006; Kirby & Savage 2008; Katzir et al., 2006). Since reading fluency connects the two “edges” of word recognition and comprehension, a future study that examines aspects of fluency in concert with DTI data may indicate that fluency involves neural tracts supporting both basic and higher-level reading proficiency. This hypothesis should be explored empirically. Third, although reading and oral comprehension are highly correlated in later grades (Aaron, 1991) and are supported by similar neural circuits (Horowitz-Kraus, Vannest & Holland, 2013), they demonstrate some differences and may vary in performance among reading-disabled populations or between older and younger age groups (Badian, 1999). Oral comprehension is a natural linguistic ability that emerges early in life and relies on speech perception, auditory word recognition, syntactic processing, and discourse coherence (Vannest et al., 2009). On the other hand, reading comprehension is a complex and less natural ability. It relies on transcription of a grapheme into corresponding phoneme and fluent word recognition (Frith, 1985) in addition to higher order abilities such as working memory, integration, inferences, and monitoring (Cane et al., 2004). Since the participants in the current study were teens and young adults who were typical readers, additional studies are needed to verify whether the findings here also apply to impaired readers and younger children.
To conclude, the association between word reading and reading comprehension with white matter tracts connecting key reading and language brain regions, provides neurobiological support for the Simple View model of reading. Further studies looking at changes of FA in these tracts following intervention might help us in understanding the effect of different types of reading therapies on neuroplasticity in AF and ILF and the influence of the integrity of these tracts, particularly in the right hemisphere, on reading ability. Note that the relatively small number of participants precluded separating males and females in the analysis, although we know that sex influences white matter developmental trajectories in general (Wang et al., 2012). Future studies should consider the differences between genders as well as testing our hypotheses about the role of the right hemisphere by enrolling a clinical population as well.
4. Materials and Methods
4.1 Participants
Twenty-one healthy, right handed, native English speaking adolescents and young adults (age range 15-19 years, average age of 18.1±1.4 years-old 11 males, 10 females) were drawn from a longitudinal subgroup recruited from a larger cross-sectional sample of participants previously included in our fMRI studies of language development (Holland et al., 2007). The entire eligible sample within the age range of 15 and older were included in the analysis. Informed consent and assent was given by all parents and participants. This study was approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center. None of the participants had any neurological impairment or neurological trauma.
4.2 Neurocognitive testing
All participants received the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) for full-scale IQ (FSIQ) scores. Word reading ability was assessed using the Test of Word Reading Efficiency (TOWRE-II) reading measures (Torgesen, Wagner & Rashotte, 2012). We used the sight word efficiency subtest of the TOWRE-II, which assesses word recognition automaticity, by reading a list of words in 45 seconds. The list of words increased in length and decreased in frequency as participants progressed through the task. Reading comprehension was measured using the reading comprehension subtest from the Woodcock Johnson-III battery (Woodcock, McGrew, & Mather, 2001). For this test, the reader is asked to read a sentence or paragraph and to supply a missing word that is implied by the context of the text. All participants completed the same test forms for both the sight word efficiency subtest from the TOWRE-II and the Woodcock Johnson-III tests (form A in both cases).
4.3 Image acquisition
DTI data were acquired using a single-shot spin-echo, echo-planar imaging (SE-EPI) sequence on a Philips Achieva 3T MRI scanner with Dual Quasar gradients and transmit/receive quadrature head coil (Philips Medical Systems, Best, The Netherlands). Acquisition parameters were: TR/TE = 12000/89 ms, acquisition matrix = 92 × 89, field of view = 180 × 180 (in-plane resolution = 2 × 2 mm), and slice thickness = 2 mm with no gap. Diffusion images were comprised of 32 diffusion weighted volumes with gradient encoding applied in 32 non-collinear directions and b = 1000 s/mm2, and one non-diffusion weighted (T2-weighted, b = 0 s/mm2 ) reference image, denoted b0.
4.4 Image processing
Images were pre-processed in the FSL software package (FMRIB software Library, FMRIB, Oxford, UK (Smith et al., 2004) including correction for eddy current induced distortion and participant's head motion (Jenkinson & Smith, 2001) and generation of a brain mask from b0 images (Smith, 2002, see ‘Supplementary data’ for the masks' display). Following these initial pre-processing steps, DTI data from all twenty-one participants yielded images of sufficient quality for further processing and the pre-processed images from all participants (N=21) were then subjected to tensor decomposition for generating fractional anisotropy (FA) maps using FDT (FMRIB's Diffusion Toolbox) (Behrens, 2003). Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006) was then used to prepare the individual diffusion maps for voxel-based group analysis by performing the following steps (see Figure 4): all subject FA images were aligned to a template of averaged FA images (FMRIB-58) in Montreal Neurological Institute (MNI) space using a non-linear registration algorithm implemented in FNIRT (FMRIB's Non-linear Registration Tool) (Rueckert et al., 1998). We have previously found that the MNI adult framework is adequate for co-registration of DTI data from adolescent brains (Wilke, Schmithorst, & Holland, 2003; Wang et al., 2012 ). After transformation into MNI space, a cohort mean FA image was created and thinned to generate a cohort mean FA skeleton of the white matter tracts. Each subject's aligned FA image was then projected onto the cohort mean FA skeleton by filling the cohort mean FA skeleton with FA values from the nearest relevant tract center, which was achieved by searching perpendicular to the local skeleton structure for maximum value. This second local co-registration step can alleviate alignment problems and reduce significant inter-subject variability.
Figure 4.
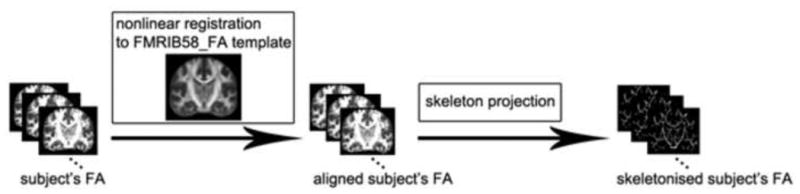
TBSS processing pipeline. Images are in radiological orientation.
4.5 Statistical Analysis
The Pearson correlation coefficient was used to check the correlation between sight word efficiency scores from the TOWRE-II test and reading comprehension standard scores as measured by the WJ-III. By using the JHU white-matter tractography atlas built in FSL (Hua et al., 2008; Wakana et al., 2007), two major white matter fiber tracts, including Arcuate Fasciculus (AF) and the Inferior Longitudinal Fasciculus (ILF), were selected as masks to extract mean FA from voxels with FA > 0.2 on the skeleton space from TBSS (see Figure 4). Controlling for age, we used Spearman's rank correlation coefficient (p<0.05, family-wise error (FWE) corrected using false discovery rate (FDR), see Benjamini & Hochberg (1995)) to compute the partial correlations between the mean FA of each ROI and the reading measures: Sight Word Efficiency subtest (TOWRE-II), (Torgesen et al., 2012) and the Passage Comprehension test [WJ-III, (Woodcock, McGrew, & Mather, 2001)]. Further, for voxel-wise analysis within the two fiber tracts, we used a non-parametric permutation-based statistical analysis (with 5000 permutations) provided by “randomize” (Nichols et al., 2002) with the Threshold-Free Cluster Enhancement (TFCE) (Smith, et al., 2007) approach (p < 0.001, uncorrected).
Supplementary Material
Highlights.
The FA in the left and right Inferior Longitudinal Fasciculus was correlated with word reading scores
The FA in the right Arcuate Fasciculus was correlated with reading comprehension scores.
The anatomical dissociation in reading and reading comprehension provide support to the Simple View theory
The results may explain separate difficulties in reading comprehension or in technical reading in different populations
Acknowledgments
This study was supported by a grant from the U.S. National Institute of Health NIH grant R01-HD38578
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron PG. Can reading disability be diagnosed using intelligence tests? Journal of Learning Disabilities. 1991;24:178–186. doi: 10.1177/002221949102400306. [DOI] [PubMed] [Google Scholar]
- Aaron PG, Joshi RM, Gooden R, Bentum KE. Diagnosis and treatment of reading disabilities based on the component model of reading: An alternative to the discrepancymodel of LD. Journal of Learning Disabilities. 2008;41:67–84. doi: 10.1177/0022219407310838. [DOI] [PubMed] [Google Scholar]
- Badian NA. Reading disability defined as a discrepancy between listening and reading comprehension: a longitudinal study of stability, gender differences, and prevalence. Journal of Learning Disabilities. 1999;32:138–148. doi: 10.1177/002221949903200204. [DOI] [PubMed] [Google Scholar]
- Beenman M. Coarse semantic coding and discourse comprehension. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: perspectives from cognitive neuroscience. Mahwah (NJ): Lawrence Erlbaum; 1998. pp. 255–284. [Google Scholar]
- Beenman M, Friedman RB, Grafman J, Perez E, Diamond S, Lindsay MB. Summation priming and coarse semantic coding in the right hemisphere. Journal of Cognitive Neuroscience. 1994;6:26–45. doi: 10.1162/jocn.1994.6.1.26. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance Medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Betjemann RS, Keenan JM, Olson RK, DeFries JC. Choice of Reading Comprehension Test Influences the Outcomes of Genetic Analyses Scientific Studies of Reading. 2011;15:363–382. doi: 10.1080/10888438.2010.493965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betjemann RS, Willcutt EG, Olson RK, Keenan JM, DeFries JC, Wadsworth SJ. Word reading and reading comprehension: stability, overlap and independence. Reading and Writing. 2008;21:538–558. [Google Scholar]
- Blake ML. Inferencing processes after right hemisphere brain damage: Maintenance of inferences. Journal of Speech, Language, and Hearing Research. 2009;52:359–372. doi: 10.1044/1092-4388(2009/07-0012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic J, Feldman HM. Functional organization of activation patterns in children: whole brain fMRI imaging during three different cognitive tasks. Prog Neuropsychopharmacol Biolological Psychiatry. 1999;23:669–682. doi: 10.1016/s0278-5846(99)00025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznitz Z. Fluency in Reading: Synchronization of Processes. Mahwah, NJ: Lawrence Erlbaum and Associates; 2006. [Google Scholar]
- Byrne B, Coventry WL, Olson RK, Corley R, Willicutt EG, et al. Genetic and environmental influences on aspects of literacy and language in early childhood, Continuity and change from preschool to Grade 2. Journal of Neurolinguistics. 2009;22:219–236. doi: 10.1016/j.jneuroling.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Vijayan S, Kuperberh G, West C, Waters G, Greve D, Dale AM. Vascular responses to syntactic processing: event-related fMRI study of relative causes. Human Brain Mapping. 2001;15:26–38. doi: 10.1002/hbm.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Cain K, Oakhill J, Bryant P. Children's reading comprehension ability: Concurrent prediction by working memory, verb alability, and component skills. Journal of Educational Psychology. 2004;96:31–42. [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehéricy S, Samson Y, Obadia M, et al. Visual word recognition in the left and right hemispheres: Anatomical and functional correlates of peripheral alexias. Cerebral Cortex. 2003;13:1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Scarborough HS. Multiple Bases for Comprehension Difficulties: The Potential of Cognitive and Neurobiological Profiling for Validation of Subtypes and Development of Assessments. In: Sabatini JP, O'Reily T, Albro ER, editors. In Reaching and Understanding: Innovations in How We View Reading Assessment. 2012. [Google Scholar]
- Cutting LE, Clements AM, Courtney S, Rimrodt SR, Schafer JGB, Wilkins J, Pekar JJ, Pugh KR. Differential components of sentence comprehension: beyond single word reading and memory. Neuroimage. 2006;29:429–438. doi: 10.1016/j.neuroimage.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Clements-Stephens A, Pugh KR, Burns S, Cao A, Pekar JJ, Davis N, Rimrodt SL. Brain Connectivity. 2013;3:199–211. doi: 10.1089/brain.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Wapner W, Gardner H, Moses JA. The contribution of the right hemisphere to the organization of paragraphs. Cortex. 1983;19:43–50. doi: 10.1016/s0010-9452(83)80049-5. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L. Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex. 2008;44:962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Morris R, Lyon GR, Stuebing KK, Shaywitz S, Shankweiler DP, Katz L, Shaywitz B. Subtypes of dyslexia: An old problem revisited. In: Blachman B, editor. Foundations of Reading Acquisition and Dyslexia: Implications of Early Intervention. 2013. [Google Scholar]
- Frith U. Beneath the surface of developmental dyslexia. In: Patterson KE, Marshall JC, Coltheart M, editors. Surface dyslexia. Hillsdale, NJ: Erlbaum; 1985. pp. 301–330. [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clémenceau S, Volle E, Hasboun D, et al. Direct intracranial FMRI and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gayan J, Olson RK. Genetic and envoiremental influences on individual differences in pronted word recognition. Journal of Experimental Child Psychology. 2003;84:97–123. doi: 10.1016/s0022-0965(02)00181-9. [DOI] [PubMed] [Google Scholar]
- George M, Kutas M, Martinez A, Sereno MI. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain. 1999;122:1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Gough PB, Tunmer WE. Decoding, reading, and reading disability. Remedial and Special Education. 1986;7:6–10. [Google Scholar]
- Hasan KM, Molfese DL, Walimuni IS, Stuebing KK, Papanicolaou AC, Narayana PA, Fletcher JM. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR in Biomedicine. 2012;25:1263–1270. doi: 10.1002/nbm.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK, Vannest JJ, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, Schmithorst VJ, Yuan W, Plante E, Byars AW. Functional MRI of language lateralization during development in children. International Journal of Audiology. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Vannest JJ, Holland SK. Overlapping Neural Circuitry for Narrative Comprehension and Proficient Reading in Children and Adolescents. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Hoover WA, Gough PB. The simple view of reading. Reading and Writing. 1990;2:127–160. [Google Scholar]
- Hough MS. Narrative comprehension in adults with right and left hemisphere brain damage: theme organization. Brain and Language. 1990;38:253–77. doi: 10.1016/0093-934x(90)90114-v. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Li X, Reich DS, Calabresi PA, Pekar JJ, vam Ziji PCM, Mori S. Tract probability maps in stereotaxic spaces: analysis of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Dyslexia Association. Definition of dyslexia. http://interdys.org/DyslexiaDefinition.htm, 2011. Based in the initial definition of the Research Committee of the Orton Dyslexia Society, former name of the IDA, done in 1994.
- Jenkinson M, Smith SA. Global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johns CL, Tooley KM, Traxler MJ. Discourse impairments following right hemisphere brain damage: A critical review. Language and Linguistics Compass. 2008;2:1038–1062. doi: 10.1111/j.1749-818X.2008.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir T, Kim Y, Wolf M, O'Brien B, Kennedy B, Lovett M, Morris R. Reading fluency: the whole is more than the parts. Annals of Dyslexia. 2006;56:51–82. doi: 10.1007/s11881-006-0003-5. [DOI] [PubMed] [Google Scholar]
- Keenan JM, Betjemann RS. Genetic and environmental influences on reading and listening. Journal of Research in Reading. 2006;29:75–91. [Google Scholar]
- Keenan JM, Betjemann RS, Olson RK. Reading comprehension tests vary in the skills they assess: Differential dependence on decoding and oral comprehension. Scientific Studies of Reading. 2008;12:281–300. [Google Scholar]
- Keller TA, Carpenter PA, Just MA. Brain imaging of tongue-twister sentence comprehension: twisting the tongue and the brain. Brain and. Language. 2003;84:189–203. doi: 10.1016/s0093-934x(02)00506-0. [DOI] [PubMed] [Google Scholar]
- Kintsch W. The role of knowledge in discourse comprehension: A construction– integration model. Psychology Review. 1998;95:163–182. doi: 10.1037/0033-295x.95.2.163. [DOI] [PubMed] [Google Scholar]
- Kirby JR, Savage RS. Can the Simple View deal with the complexities of reading? Literacy. 2008;42:75–82. [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Landi N, Frost SJ, Menc WE, Sandak R, Pugh K. Neurobiological bases of reading comprehension: insights from neuroimaging studies of word level and text level processing in skilled and impaired readers. Reading and Writing. 2013;29:145–167. doi: 10.1080/10573569.2013.758566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping. 2009;30:3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Shaywitz B, Holahan J, Shaywitz S, Marchione K, Beaulieu C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain and Language. 2013;125:215–222. doi: 10.1016/j.bandl.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. Lexical ambiguity in sentence comprehension. Brain Research. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY. Neurocognition of auditory sentence comprehension: event related fMRI reveals sensitivity to syntactic violations and task demands. Cognitive Brain Research. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, Van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Oga T, Okada T, Sadato N, Takayama Y, Wydell T, et al. Hemispheric asymmetry emerges at distinct parts of the occipitotemporal cortex for objects, logograms and phonograms: a functional MRI study. Neuroimage. 2005;28:521–528. doi: 10.1016/j.neuroimage.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Nation K, Snowling M. Assessing reading difficulties: The validity and utility of current measures of reading skill. British Journal of Educational Psychology. 1997;67:359–370. doi: 10.1111/j.2044-8279.1997.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. Journal of Cognitive Neuroscience. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in nonreading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Flowers DL, Napoliello EM, Eden G. Developmental differences for word processing in the ventral stream. Brain and Language. 2013;125:134–145. doi: 10.1016/j.bandl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. Developmental dyslexia. The Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, Dearter-Deckard K, Thompson LA, Schatschneider LS. Reading skills in early readers: Genetic and shared environmental influences. Journal of Learning Disabilities. 2006;39:48–55. doi: 10.1177/00222194060390010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante E, Ramage AE, Magloire J. Processing narratives for verbatim and gist information by adults with language learning disabilities: A functional neuroimaging study. Learning Disabilities Research & Practice. 2006;21:61–76. [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex Differences in the Activation of Language Cortex During Childhood. Neuropsychologia. 2006;44:1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Protopapas A, Simos PG, Sideridis GD, Mouzaki A. The Components of the Simple View of Reading: A Confirmatory Factor Analysis. Reading Psychology. 2012;33(3):217–240. [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Siok WT, Zhou K, Khong PL. Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: A diffusion tensor tractography study in a Chinese cohort. Human Brain Mapping. 2011;32:2054–2063. doi: 10.1002/hbm.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker AM, Deutsch GK, Ben-Shachar M, Schwartzman A, Perry LM, Dougherty RF. Reading impairment in a patient with missing arcuate fasciculus. Neuropsychologia. 2009;47:180–194. doi: 10.1016/j.neuropsychologia.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE. Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cerebral Cortex. 2009;19:402–413. doi: 10.1093/cercor/bhn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DA, Gernsbacher MA, Guidotti SJ, Robertson RRW, Irwin W, Mock BJ, et al. Functional neuroanatomy of the cognitive processes of mapping during discourse comprehension. Psychological Science. 2000;11:255–260. doi: 10.1111/1467-9280.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: Synergy between the arcuate fascicle and extreme capsule. Journal of Neuroscience. 2011;31:16949–16957. doi: 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M, Brownell HH, Potter HH, Seibold MS. Script knowledge in right hemisphere-damaged and in normal elderly adults. Brain and Language. 1987;31:151–170. doi: 10.1016/0093-934x(87)90066-6. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20:1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Registration using free-form deformations: application to breast MR images. IEEE Transaction Medical Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sabatini JP, Sawaki Y, Shore JR, Scarborough HS. Relationships among reading skills of adults with low literacy. Journal of Learning Disabilities. 2010;43:122–138. doi: 10.1177/0022219409359343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Pakchik O, Yendiki A, Fischl B, Gaab N, Gabrieli JDE. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. The journal of Neuroscience. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Share DL. On the Anglocentricities of current reading research and practice: the perils of overreliance on an “outer” orthography. Psychology Bullitin. 2008;134:584–615. doi: 10.1037/0033-2909.134.4.584. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, et al. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castilo EM, et al. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as. FSL Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with Tract- Based Spatial Statistics Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Spencer M, Quinn JM, Wagner RK. Specific Reading Comprehension Disability: Major Problem, Myth, or Misnomer? Learning Disabilities Research & Practice. 2014;29:3–9. doi: 10.1111/ldrp.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubeek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Storch SA, Whitehurst GJ. Oral language and code-related precursors to reading: evidence from a longitudinal structural model. Developmental Psychology. 2002;38:934–947. [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency (TOWRE) Austin, TX: Pro-Ed; 19992012. [Google Scholar]
- Tunmer WE, Chapman JW. The simple view of reading redux: Vocabulary knowledge and the independent components hypothesis. Journal of Learning Disabilities. 2012;45:453–466. doi: 10.1177/0022219411432685. [DOI] [PubMed] [Google Scholar]
- Van der Leij A, Van Daal VHP. Automatization aspects of dyslexia: Speed limitations in word identification, sensitivity to increasing task demands, and orthographic compensation. Journal of Learning Disabilities. 1999;32:417–428. doi: 10.1177/002221949903200507. [DOI] [PubMed] [Google Scholar]
- Van Ettinger-Veenstra HM, Regnehed M, McAllister A, Lundberg A, Engstrom M. Right-hemispheric cortical contributions to language ability in healthy adults. Brain and Language. 2012;120:395–400. doi: 10.1016/j.bandl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Vannest JJ, Karunanayaka PR, Altaye M, Schmithorst VJ, Plante EM, Eaton KJ, Rasmussen JM, Holland SK. Comparison of fMRI data from passive Listening and active-response story processing tasks in children. Journal of Magnetic Resonance Imaging. 2009;29:971–976. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Tunmer WE, Jaccard J, Chen S. Components of reading ability: Multivariate evidence for a convergent skills model of reading development. Scientific Studies of Reading. 2007;11:3–32. [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the Visual Word Form Area? Neuroimage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Wahl M, Li Y, Ng J, LaHue SC, Cooper SR, Sherr EH, et al. Microstructural correlations of white matter tracts in the human brain. Neuroimage. 2010;51:531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallo JH, Perry M, Gollub RL, Hua K, Zhang J, Jing H, Dubey P, Blitz A, van Ziji P, Mor S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Adamson C, Yuan W, Altaye M, Rajagopal A, Byars AW, Holland SK. Sex differences in white matter development during adolescence: A DTI study. Brain research. 2012;1478:1–15. doi: 10.1016/j.brainres.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapner W, Hamby S, Gardner H. The role of the right hemisphere in the apprehension of complex linguistic materials. Brain and Language. 1981;14:15–33. doi: 10.1016/0093-934x(81)90061-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence: Psychological Corporation 1999 [Google Scholar]
- Wilke M, Schmithorst V, Holland SK. Normative pediatric brain data for spatial normalization and segmentation differs from standard adult data. Magnetic Resonanace Medicine. 2003;50(4):749–57. doi: 10.1002/mrm.10606. [DOI] [PubMed] [Google Scholar]
- Woodcock R, McGrew KS, Mather N. Woodcock-Johnson Psychoeducational Battery-Third Edition. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shchar M, Wandell BA. Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, et al. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience. 2011;23:3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: Adjacent cortical circuits and long-range white matter connections. Brain and Language. 2012 doi: 10.1016/j.bandl.2012.04.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


