Abstract
The habenular complex, encompassing medial (MHb) and lateral (LHb) divisions, is a highly conserved epithalamic structure involved in the dorsal diencephalic conduction system (DDC). These brain nuclei regulate information flow between the limbic forebrain and the mid- and hindbrain, integrating cognitive with emotional and sensory processes. The MHb is also one of the strongest expression sites for mu opioid receptors (MORs), which mediate analgesic and rewarding properties of opiates. At present however, anatomical distribution and function of these receptors have been poorly studied in MHb pathways. Here we took advantage of a newly generated MOR-mcherry knock-in mouse line to characterize MOR expression sites in the DDC.
MOR-mcherry fluorescent signal is weak in the lateral habenula, but strong expression is visible in the medial habenula, fasciculus retroflexus and interpeduncular nucleus (IPN), indicating that MOR is mainly present in the MHb-IPN pathway. MOR-mcherry cell bodies are detected both in basolateral and apical parts of MHb, where the receptor co-localizes with cholinergic and Substance P (SP) neurons, respectively, representing two main MHb neuronal populations. MOR-mcherry is expressed in most MHb-SP neurons, and is present in only a subpopulation of MHb-cholinergic neurons. Intense diffuse fluorescence detected in lateral and rostral parts of the IPN further suggests that MOR-mcherry is transported to terminals of these SP and cholinergic neurons. Finally, MOR-mcherry is present in septal regions projecting to the MHb, and in neurons of the central and intermediate IPN.
Together, this study describes MOR expression in several compartments of the MHb-IPN circuitry. The remarkably high MOR density in the MHb-IPN pathway suggests that these receptors are in a unique position to mediate analgesic, autonomic and reward responses.
Keywords: Medial habenula, Mu opioid receptor, knock-in mice, interpeduncular nucleus, Substance P, Acetylcholine
The dorsal diencephalic conduction (DDC) is a highly conserved pathway present in all vertebrates, which interconnects limbic forebrain structures (septum, pallidum, striatum, lateral hypothalamus) to mid- and hindbrain regions including raphe and tegmental nuclei, locus coeruleus, ventral tegmental area (VTA) and the interpeduncular nucleus (IPN). The habenular complex (Hb) is central to DDC (Sutherland, 1982, Bianco and Wilson, 2009). The key role of this highly conserved epithalamic structure in integrating cognitive with emotional and sensory processing has raised increasing interest (Klemm, 2004, Lecourtier and Kelly, 2007, Hikosaka et al., 2008, Ikemoto, 2010, Darcq et al., 2011, Goutagny et al., 2013, Lee and Goto, 2013), and Hb contribution to motivational processes and value-based decisionmaking has been established (Hikosaka, 2010). The Hb comprises two nuclei, the medial habenula (MHb) and the lateral habenula (LHb), which show distinct anatomy and connectivity within brain networks, and there is some evidence of interconnections between the two nuclei (Sutherland, 1982, Kim and Chang, 2005). The LHb, which has pallidal and hypothalamic afferences and mainly projects to midbrain and hindbrain structures such as the raphe nuclei and VTA (Lecourtier and Kelly, 2007, Jhou et al., 2009, Kaufling et al., 2009, Kim, 2009), was shown involved in aversion and behavioral avoidance (Lammel et al., 2012, Stamatakis and Stuber, 2012, Ilango et al., 2013). The MHb receives septal inputs and projects primarily to the IPN, and has been less studied (Viswanath et al., 2013). This nucleus was proposed to regulate inhibitory controls, cognition-dependent executive functions, place aversion learning (Darcq et al., 2011, Kobayashi et al., 2013) and the nicotine withdrawal syndrome (Dani and De Biasi, 2013, Kobayashi et al., 2013, Dao et al., 2014). A recent report showed that post-natal ablation of MHb cells in transgenic mice induce an abnormal phenotype, characterized by impulsive and compulsive behavior, environmental maladaptation and learning deficits (Kobayashi et al., 2013), underscoring functional implication of this structure in a broad range of behaviors.
MHb expresses remarkably high levels of mu opioid receptors (MORs). This G protein coupled receptor belongs to the opioid system, which plays a major role in pain control and autonomic functions, and modulates affective behavior and neuroendocrine physiology (Kieffer and Evans, 2009, Lutz and Kieffer, 2013). The MOR has unambiguously been established as the molecular target for opiate drugs, and mediates all the biological activities of morphine including both therapeutic and adverse effects (Matthes et al., 1996, Charbogne et al., 2014). Further, this receptor mediates reinforcing properties of non-opioid drugs of abuse including alcohol, cannabinoids, and nicotine (Kieffer and Gaveriaux-Ruff, 2002), as well as motivation for food (Papaleo et al., 2007) and social interactions (Moles et al., 2004), indicating a major role in reward processing. The broad MOR distribution throughout the brain supports all these roles (Erbs et al., 2014). Studies of this receptor have traditionally focused on the well described nociceptive and reward circuitries, but there is also some evidence for MOR-mediated morphine analgesia at the level of MHb (Terenzi et al., 1990, Terenzi and Prado, 1990, Darcq et al., 2012). Neuroanatomical distribution and function of MOR in the MHb, however, have been little explored despite strongest expression levels compared to all other brain areas (Kitchen et al., 1997) (reviews in (Le Merrer et al., 2009, Lutz and Kieffer, 2013)).
MOR distribution throughout the nervous system has been reported at RNA and protein levels (for a review Le Merrer et al., 2009). Low MOR mRNA abundance has rendered in situ hybridization experiments on mouse brain truly challenging, and data have been mainly reported from rat (George et al., 1994, Mansour et al., 1994). Also, protein detection at neuron level has been difficult due to poor availability of specific antibodies in tissue sections. MOR protein levels have otherwise been mapped and quantified using ligand autoradiography (Kitchen et al., 1997, Slowe et al., 1999, Goody et al., 2002), however this approach does not allow cellular resolution and the precise MOR distribution within MHb and associated circuitry is unknown. Our laboratory has recently generated knock-in mice expressing MOR in fusion with the red fluorescent protein mcherry (MOR-mcherry) in place of the native receptor. This mouse has enabled characterizing MOR distribution throughout the entire brain, as well as MOR colocalization with the delta opioid receptor with cellular resolution (Erbs et al., 2014). In the present study, we used this unique tool to investigate MOR distribution in neurons of the two Hb nuclei, and characterized MOR-expressing neuronal populations in the MHb-IPN pathway. Our data demonstrate prominent MOR expression in different subregions of MHb and IPN, and provides a basis for MOR-mediated mechanisms operating at the level of both substance P-ergic and cholinergic systems, in interaction with glutamatergic transmission in the MHb.
Experimental Procedures
Animals
MOR-mcherry knock-in mice were generated by homologous recombination, as done earlier by our laboratory to generate DOR-eGFP knock-in mice (Scherrer et al., 2006). In these mice, the mcherry cDNA was introduced into exon 4 of the mu opioid receptor gene, in frame and 5’ of the stop codon, as described in Erbs et al., 2014. This C-terminal construct was designed to allow correct native-like MOR expression at sub-cellular level (see (Erbs et al., 2014) and leads to visualize the best described MOR protein population. Proteins that may arise from alternative splicing lacking exon 4 would not be visualized in these mice (Pasternak and Pan, 2013). The genetic background of all mice was C57/BL6J;129svPas (50:50 %). Functional properties of MOR are maintained in MOR-mcherry mice both in vitro and in vivo (Erbs et al., 2014), as previously observed for DOR-eGFP mice (Scherrer et al., 2006, Pradhan et al., 2009).
Mice were housed in a temperature- and humidity-controlled animal facility (21±2°C, 45±5% humidity) on a 12 h dark-light cycle with food and water ad libitum. Male (n=2) and female (n=3) mice aged 8 to 12 weeks were used. All experiments were performed in accordance with the European Communities Council Directive of 26 May 2010 and approved by the local ethical committee (Com’Eth 2010-003).
Tissue preparation and immunohistochemistry
Mice were anaesthetized with ketamine (Virbac, Carros, France) / xylazine (Rompun, Kiel, Germany) (100/10 mg/kg, i.p.) and perfused intracardiacally with 100 ml of 4% paraformaldehyde (at 2–4°C) in PB 0.1M (Sigma, St Louis, MO, USA) pH 7.4. Brains were post-fixed for 24 hours at 4°C in the 4% PFA solution, cryoprotected at 4°C in a 30% sucrose (Sigma, St Louis, MO, USA), PB 0.1M pH 7.4 solution and finally embedded in OCT (Optimal Cutting Temperature medium, Thermo Scientific) frozen and kept at −80°C. Frozen brains were sliced coronally into 30 µm-thick serial sections by using a cryostat (CM3050, Leica) and kept floating in PB 0.1M pH 7.4.
Immunohistochemistry was performed according to standard protocols. Briefly, 30 µm-thick sections were incubated in blocking solution (PB 0.1M pH 7.4, 0.5% Triton X100 (Sigma, St Louis, MO, USA), 5% normal goat or donkey serum (Invitrogen, Paisley, UK) (depending on the secondary antibody) for 1 hour at room temperature (RT). Sections were incubated overnight at 4°C in the blocking solution with appropriate primary antibodies (see the section below for characterization of the primary antibodies used). After three washes in PB 0.1M pH 7.4, 0.5% Triton X100, sections were incubated for 2 hours at RT with appropriate AlexaFluor conjugated secondary antibodies. Sections were washed three times in PB 0.1 M and mounted on SuperfrostTM glass (Menzel-Glaser) with Mowiol (Calbiochem, Darmstadt, Germany) and 4’, 6-diamidino-2-phenylindole (DAPI) (Roche Diagnostic, Mannheim, Germany) (0.5 µg/ml).
Antibody characterization
Antibodies used in the present study are commercially available and have been tested in previous studies and ours as follows: Rabbit anti-DsRed polyclonal antibody (Cat. Nr 632496, Ozyme, Saint-Quentin-en-Yvelines, France, dilution 1:1000) was used for mcherry fluorescent protein detection when indicated, as in previous studies (Erbs et al., 2014). Guinea pig anti-vesicular glutamate transporter type 2 (VGLUT2) (Cat. Nr AB2251, Millipore, Billerica, MA, USA, dilution 1:2000) and goat anti-choline acetyltransferase (ChAT) (Cat. Nr AB144, Millipore, Billerica, MA, USA, dilution 1:400) polyclonal antibodies have been largely used in the literature and in our laboratory (Erbs et al., 2012). Rat anti-Substance P (SP) monoclonal antibody (Cat. Nr MAB356, Millipore, Billerica, MA, USA, dilution 1:500) reacts with the COOH-terminal end of Substance P. Radioimmunoassay studies showed the absence of cross-reactivity with other brain peptides, including β-endorphin, somatostatin, leu-enkephalin, or met-enkephalin (Cuello et al., 1979). No labeling was found when the antibody was preadsorbed with the synthetic substance P (Cuello et al., 1980). No labeling was found in Tachykinin 1 (encoding for Substance P peptides) knock-out mice (Zimmer et al., 1998). In our study, this antibody stained MHb and other areas including the ventral pallidum, the preoptic area and the hypothalamus in the same manner as in rat studies (Aizawa et al., 2012) and as other antibodies (Larsen, 1992, Root et al., 2013). Rabbit anti-Met Enkephalin polyclonal antibodies (Cat Nr AB5026, Millipore, Billerica, MA, USA, dilution 1:1000) have low cross-reactivity with leu-enkephalin, beta-endorphin and beta-lipotropin according to the manufacturer. We observed no labeling in Pre-proenkephalin knockout mice brain (generously provided by Dr A. Zimmer). Labeling observed in our study is relevant with previous studies observations (Kalivas, 1985, Konig et al., 1996, Fusco et al., 2003, Porteous et al., 2011).
The following AlexaFluor conjugated secondary antibodies (Molecular Probes, Paisley, UK) were used: goat anti rat AlexaFluor 488 conjugated (Cat. Nr A11006, dilution 1:500), goat anti rabbit AlexaFluor 488 conjugated (Cat. Nr A11034, dilution 1:2000), goat anti guinea pig AlexaFluor 488 conjugated (Cat. Nr A11073, dilution 1:500), donkey anti goat AlexaFluor 488 conjugated (Cat. Nr A11055, dilution 1:500), and goat anti rabbit IgG AlexaFluor 594 conjugated (Cat. Nr A11012, dilution 1:2000). Immunohistochemistry was also performed without primary antibodies to verify absence of non-specific staining by the secondary antibody alone. Absence of cross-reactivity between secondary antibodies was systematically checked in control experiments.
Image acquisition
Samples were observed with an epifluorescence microscope (DM4000, Leica) using 10x (numerical aperture (NA): 0.25), 20x (NA:0.7), 40x (NA:0.75) or 63x (NA:1.32) objectives, zoom 1 or 0.55 with a CCD camera CoolSnap or with a confocal microscope (SP2RS, Leica) using 40x (NA:1.25) and 63x (NA:1.4) objectives zoom 1 or 4 with the LCS (Leica) software for image acquisition. Image acquisition was also performed with the slide scanner NanoZoomer 2 HT equipped with the fluorescence module L11600-21 (Hamamatsu Photonics, Japan).
Quantification of regional fluorescence density
Fluorescence densities were measured using FIJI software in n = 4 animals, on 10x-objective magnification 8-bit images after delimiting areas of interest with the ROI manager. For each Hb images (n=11) the same region presenting no MOR-mcherry fluorescence was used to define background level. A similar operation was done for IPN images (n=9). Ratio between fluorescence density of the region of interest and fluorescence density of background was obtained for each image. Mean fluorescence density ratio (Mean fluo ratio) were compared per area, Hb or IPN.
Statistical analysis
Statistical analysis was performed with Graph-Pad Prism v4 (GraphPad). Fluorescence densities were analyzed using a Student t-test and a one way analysis of variance (ANOVA). Multiple comparisons were made using Newman-Keuls test for post hoc analysis.
Results
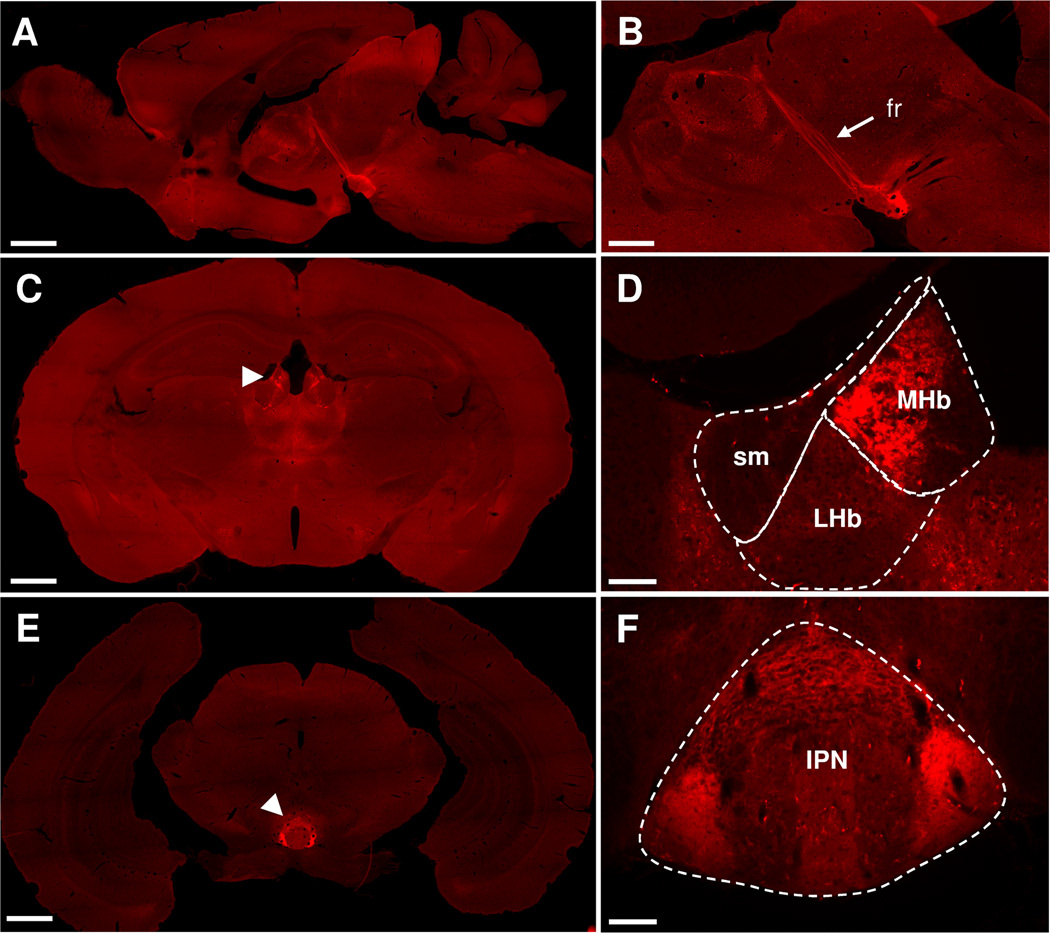
The MOR-mcherry fluorescent signal is widely present throughout the brain (Fig. 1A, for detailed distribution see Erbs et al., 2014). In the dorsal diencephalic conduction pathway, intense fluorescence was observed at the level of the Hb (Fig. 1C, D), efferent fibers forming the fasciculus retroflexus (fr, Fig. 1A, B), and the interpeduncular nucleus (IPN, Fig. 1E, F) representing the main projection area of the medial Hb. Notably, the red fluorescent signal was detectable with three different patterns. The signal may appear as intense fluorescence within cell bodies, revealing MOR-mcherry-expressing cells, such as in the MHb (Fig. 1D). Fluorescence may also show a fibrous aspect, which presumably reflects MOR-positive passing fibers, such as in the fr (Fig. 1B). Finally diffuse MOR expression was also observed at many sites, and potentially arose from MOR expressed at the level of afferent terminals, such as in the lateral parts of the IPN (Fig. 1F).
Figure 1. MOR-mcherry is strongly expressed in the habenulo-interpeduncular pathway.
A & B. Sagittal brain sections from MOR-mcherry knock-in mice where the fasciculus retroflexus (fr) is most visible as a strong red fluorescent fiber track (mcherry protein fluorescence). C. Coronal brain section at the level of habenula nuclei (white arrowhead). D. Higher magnification image of habenula nuclei with the stria medullaris (sm), medial (MHb) and lateral (LHb) habenula delimited. E. Coronal brain section at the level of the interpeduncular nucleus (IPN) (white arrowhead). F. Higher magnification image of the IPN. Scale bar = 1 mm (A, C & E), 500 µm (B), and 100 µm (D & F).
1. Expression of MOR-mcherry in the MHb-IPN pathway
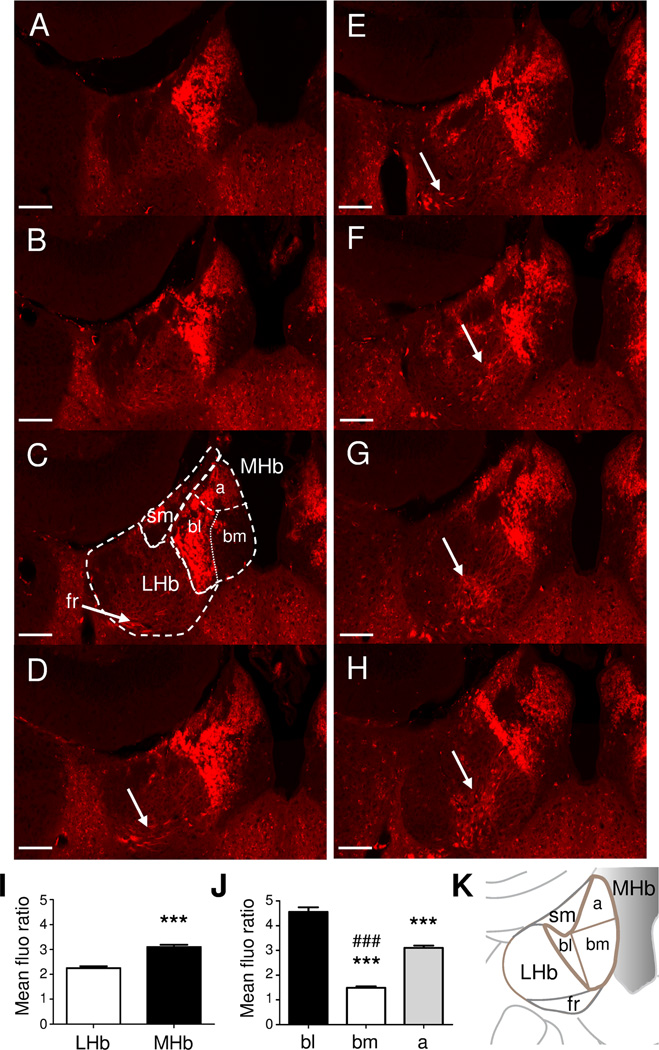
The two main Hb nuclei (LHb and MHb) and their connections have been characterized in the past years (Lecourtier and Kelly, 2007, Hikosaka et al., 2008). Both LHb and MHb have been subdivided in smaller regions according to their neuronal populations, as well as inputs and outputs (Aizawa et al., 2012). Serial coronal sections from MOR-mcherry mice at the level of Hb (Fig. 2) showed that red fluorescence was almost exclusively present in MHb, compared to the LHb, where only few fluorescent neurons were observed (Fig. 2A–I). Predominant MOR expression in MHb is coherent with previous studies of the mouse brain showing that MHb is the primary binding site for the MOR antagonist naloxone (Gackenheimer et al., 2005), and the brain site with densest DAMGO binding (Kitchen et al., 1997, Slowe et al., 1999, Goody et al., 2002). Main subregions of the MHb are represented in Fig. 2C and K. In MHb, MOR-mcherry were mainly expressed in cell bodies of the basolateral part (bl) alongside the LHb, and to a lower extent, in cell bodies of the apical part (a) (Fig. 2J). Homogenous red fluorescence was also observed in this apical region. Finally, strong fluorescence was visible in areas corresponding to the fr, with a stringy aspect reflecting high MOR-mcherry protein density along neuronal fibers (Fig. 2C–H and Fig. 3A), consistent with the observed signal in sagittal sections (Fig. 1B).
Figure 2. MOR-mcherry expression in medial habenula.
A–H. Images of habenular nuclei in coronal brain sections of MOR-mcherry knock-in mice from Bregma −1.22 to Bregma −2.18 mm (Paxinos and Franklin, 2001). Numerous cell bodies expressing MOR-mcherry proteins are visible in MHb. The fasciculus retroflexus (fr) is visible from Fig. 2C to 2H (white arrows). Scale bar= 100 µm. I. Quantitative analysis of MOR-mcherry fluorescence in mean fluorescence density ratio (versus background). ***: p<0.001 student t test compared to LHb. J. Comparison of fluorescence density ratio between the three main sub-regions of the MHb. ***: p<0.001 One-way ANOVA compared to the basolateral part of MHb, ###: p<0.001 One-way ANOVA compared to the apical part of MHb. K. Schematic representation of the right hemisphere habenula nuclei at Bregma: −1.58 mm (comparable to Fig 2C). a: apical MHb, bm: baso-medial MHb, bl: baso-lateral MHb, LHb: lateral habenula, fr: fasciculus retroflexus, sm: stria medullaris.
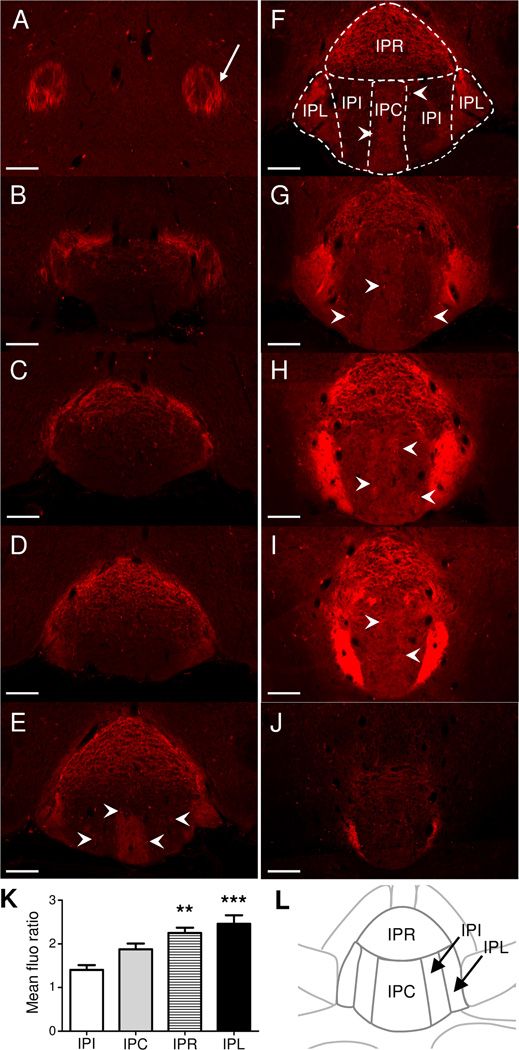
Figure 3. MOR-mcherry expression in the interpeduncular nucleus.
A–J. Images of the Interpeduncular nucleus in coronal brain sections of MOR-mcherry knock-in mice from Bregma −3.08 to Bregma −4.04 mm (Paxinos and Franklin, 2001). A. An important part of the fasciculus retroflexus (fr) axons expresses MOR-mcherry proteins (white arrow). B. Fusion between the fr and the IPN. C–J. The IPR and IPL show MOR-mcherry expression throughout the rostro-caudal axis of the IPN. IPI and IPC appear in Fig 3E. Small cell bodies expressing mu opioid receptor are visible in the IPC and IPI (white arrowheads). Homogenous red fluorescence, presumably in terminals, is visible in IPL, IPC and IPR. J. IPL is the only nucleus showing strong MOR-mcherry expression in the caudal part of the IPN. Scale bar= 100 µm. K. Comparison of MOR-mcherry fluorescence between the different nuclei of the IPN. Fluorescence densities are expressed as a mean fluorescence density ratio (versus background). One-way ANOVA, **: p<0.01, ***: p<0.001 compared to the IPI. L. Schematic representation of the interpeduncular nucleus at Bregma: −3.80 mm (comparable to Fig 3F). IPC: central nucleus, IPI: intermediate nuclei, IPL: lateral nuclei, IPR: rostral nucleus.
The IPN (Fig. 3) is the main MHb-fr projection area, and has been divided into several subnuclei (Groenewegen et al., 1986, Kawaja et al., 1991) that include central (IPC), intermediate (IPI), lateral (IPL) and rostral (IPR) nuclei (Fig. 3F and L). Projections from MHb to these IPN subnuclei are well characterized (Kawaja et al., 1988, Qin and Luo, 2009). MOR-mcherry expressing cell bodies were visible in IPC and IPI (Fig. 3E–I) and few cells were also detected in the IPR. IPL and IPR, and to a lower extend IPC, also showed homogenous mcherry fluorescence, potentially reflecting MOR-mcherry neuron terminals from MHb neurons (Fig. 3K).
Finally, among other areas, we observed the presence of numerous MOR-mcherry-expressing cell bodies throughout several subregions of the posterior septum, raphe and tegmental nuclei (Fig. 4) (for detailed distribution see Erbs et al., 2014). The posterior septum, including the triangular septum (TS), septo-fimbrial septum (SFi) and bed nucleus of the anterior commissure (BAC), together with the diagonal band are described as main afferent regions to the MHb (Sutherland, 1982, Qin and Luo, 2009, Yamaguchi et al., 2013). The median (MnR) and dorsal (DRN) raphe, and the dorsal tegmental region (DTR) including the laterodorsal tegmental nucleus (LDTg), have been shown to receive main afferences from the IPN (Groenewegen et al., 1986). Altogether therefore, the analysis of MOR-mcherry expression demonstrates the presence of MOR all along the MHb-IPN pathway and its known afferent and efferent sites.
Figure 4. MOR-mcherry expressing neurons are present in both afferent and efferent areas of the MHb-IPN pathway.
A & B. Large cells expressing MOR-mcherry are present in the triangular septum (TS) and in the septo-fimbrial septum (SFi). C. MOR-mcherry expression is also detected in small size cell bodies of the bed nucleus of the anterior commissure (BAC). D. Numerous cells expressing MOR-mcherry are observed in the median raphe (MnR). E & F. Strong expression is also observed in the caudo-dorsal part of the raphe (DRC). The LDTg identified by its large cholinergic cells (green; immunostaining for choline acetyltransferase (ChAT) present small size MOR-mcherry expressing cells. ac: anterior commissure, Aq: aqueduct, cc: corpus callosum, DRC: dorsal raphe nucleus, caudal part, DTg: dorsal tegmental nucleus, f: fornix, LDTg: laterodorsal tegmental nucleus, MnR: median raphe nucleus, xscp: decussation of the superior cerebellar peduncle. Anatomical coordinates for each coronal brain section are as follows; Bregma: −0.46 (A), +0.02 (B), −0.10 (C), −4.48 (D), and −5.02 mm (E). Drawings are adapted from The mouse brain atlas in stereotaxic coordinates (Paxinos and Franklin, 2001). Scale bar= 200 µm (A–D), 500 µm (E), 100 µm (F).
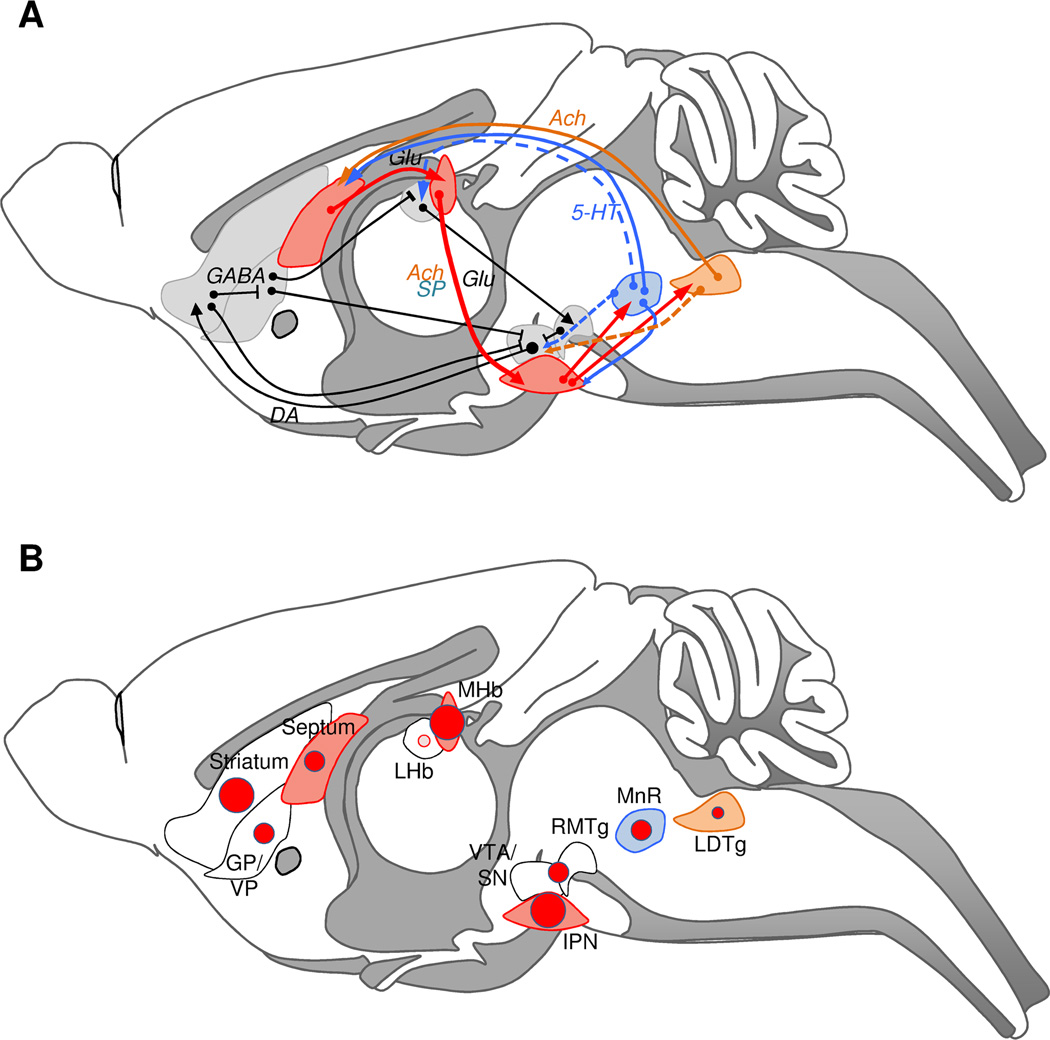
Figure 5 shows a schematic representation of MOR expression sites in habenular circuits (this study and Erbs et al., 2014) together with associated monoaminergic pathways (Sutherland, 1982, Groenewegen et al., 1986, Lecourtier and Kelly, 2007, Geisler and Trimble, 2008, Hikosaka et al., 2008, Qin and Luo, 2009, Brinschwitz et al., 2010).
Figure 5. Habenula nuclei connectivities with dopamine and serotonin systems, and mu opioid receptor expression at these sites.
A. The MHb-IPN pathway is shown in red. MHb receives glutamatergic projections from different nuclei of the posterior septum. IPI and IPC send projections to the medial raphe nucleus (MnR) and the dorsal tegmental nuclei including the LDTg. Both the MnR and the LDTg send serotoninergic (blue) and cholinergic (orange) projections, respectively, to the septum allowing feed-back loop regulation of this circuitry. The mesolimbic dopaminergic pathway is represented in black, and includes VTA projections to the striatum/pallidum and GABAergic feedback loops. The LHb is part of this well-described dopaminergic circuitry. LHb receives inhibitory afferences from the pallidal region (GP/VP), and projects excitatory neurons onto GABAergic neurons of the RMTg (or tVTA), which in turn exert an inhibitory control on VTA dopamine neuron firing. Dashed lines show known projections from the MnR and LDTg, which potentially create interconnections between MHb (red) and LHb (black) circuits. Known GABAergic inhibitory projections in the meso-limbic system are symbolized without arrowheads. See (Sutherland, 1982, Groenewegen et al., 1986, Lecourtier and Kelly, 2007, Geisler and Trimble, 2008, Hikosaka et al., 2008, Qin and Luo, 2009, Brinschwitz et al., 2010) for references. B. Relative distribution of the MOR-mcherry signal in MHb (red) and LHb (black)-associated brain regions. MnR and LDTg are highlighted in blue and orange respectively. The size of the red circles is correlated to receptor density in each given area as described in Erbs et al (Erbs et al., 2014) and quantified by Kitchen and coll (Kitchen et al., 1997). MOR expression is rare in LHb (pink circle). The 3 sizes of circles correspond to (in ascending order): moderate, dense, and very dense expression. For more details, see Erbs et al. 2014. Ach: acetylcholine, DA: dopamine, Glut: glutamate, GP/VP: Globus Pallidus/Ventral Pallidum, IPN: Interpeduncular nucleus, LDTg: Laterodorsal Tegmental Nucleus, LHb: Lateral Habenula, MHb: Medial Habenula, MnR: Median Raphe Nucleus, RMTg: Rostromedial Tegmental Nucleus, SP: Substance P, VTA/SN: Ventral Tegmental Area/Substantia Nigra, 5-HT: serotonin.
2. Characterization of MOR-mcherry expressing cells in the MHb-IPN pathway
The MHb is composed of two major cell types, acetylcholine and substance P neurons (Contestabile et al., 1987, Aizawa et al., 2012), and also contains glutamate-expressing neurons (Qin and Luo, 2009, Ren et al., 2011, Aizawa et al., 2012). We therefore characterized MOR expression in these different types of neurons.
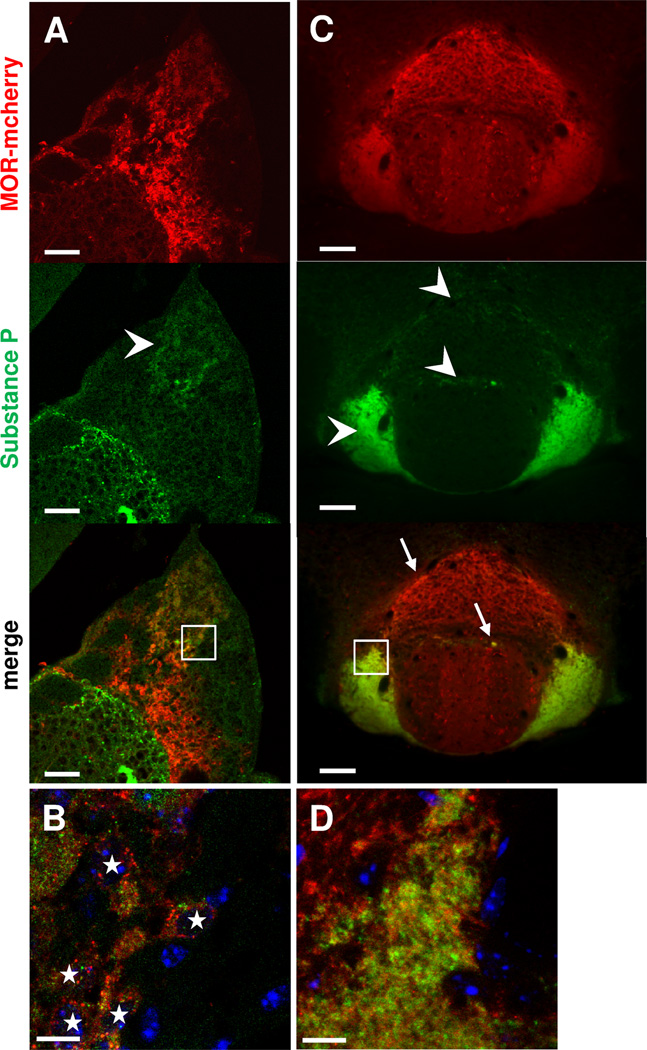
In the brain of MOR-mcherry mice, and as expected from the literature (Kawaja et al., 1991, Yang et al., 2014), substance P immunostaining (Fig. 6) was restricted to apical neurons of the MHb and also found within LHb, along the MHb (Fig. 6A). The MOR-mcherry signal colocalized with substance P staining in the apical MHb region (Fig. 6A, B) and further, most substance P positive cell bodies of apical MHb expressed MOR-mcherry. Substance P neurons of the MHb are known to massively project to the IPL and, to a smaller extend, to the IPR, as shown in Fig. 6C. Strikingly, MOR-mcherry expression was also detected in most substance P projection terminals of the IPN (Fig. 6C, D). These data together suggest that MOR is expressed in most SP neurons along the MHb-IPN pathway.
Figure 6. MOR-mcherry is expressed in most IPL-projecting Substance P neurons in the apical MHb.
Photomicrographs of the medial habenula MHb (A–B) and the interpeduncular nucleus IPN (C–D) of MOR-mcherry knock-in mice. Substance P immunostaining (green) is observed in cell bodies of the apical part of MHb (arrowhead) and in fibers at the border of LHb. B. Higher magnification image of the apical region of the MHb (white square in A) reveals neurons co-expressing MOR-mcherry and substance P (white stars). C. In the IPN, substance P immunostaining is mainly found in the IPL. Substance P staining is also visible in the IPR (arrowheads). Co-expression of MOR-mcherry and Substance P is found in both IPR (arrows) and IPL. D. Higher magnification image of the IPL showing co-expression of MOR-mcherry and substance P. DAPI in blue. Scale bar= 50 µm (A & C), 10 µm (B & D).
Choline acetytransferase (ChAT) immunostaining showed cholinergic neurons in the basal subdivision of MHb (Fig. 7A), consistent with previous reports (Kawaja et al., 1991, Aizawa et al., 2012). Their axons terminated with a cistern shape in IPI, IPC and IPR subdivisions of the IPN (Fig. 7C). Few of these MHb cholinergic neurons co-expressed MOR-mcherry proteins, and colocalization was only detected in the basolateral part of MHb (Fig. 7A, B), as reported recently by in situ hybridization (Aizawa et al., 2012). Notably, almost all MOR-mcherry-positive neurons colocalized with the ChAT signal in this part of MHb. At the level of IPN, MOR-mcherry and ChAT colocalization was restricted to cholinergic terminals projecting to the rostral subnucleus of the IPN (Fig. 7C, D). Thus, MOR expression is restricted to a subpopulation of cholinergic neurons in the MHb-IPN pathway.
Figure 7. MOR-mcherry is expressed in IPR-projecting cholinergic neurons of the baso-lateral part of MHb.
Photomicrographs of the medial habenula MHb (A–B) and the interpeduncular nucleus IPN (C–D) of MOR-mcherry knock-in mice. A. Antibodies directed against choline acetyltransferase (ChAT), specific to cholinergic neurons, massively label neurons of the basal MHb (arrowhead). No staining is found in the LHb. B. A higher magnification image of the area delimited by a white square in A reveals co-expression between ChAT (green) and MOR-mcherry (red) (white stars) in cell bodies of the basolateral part of the MHb. C. Cholinergic axons (green) of the MHb terminate in a cistern shape in the medial part of the IPN, through the IPI, IPC and IPR. Solely the IPR area presents both ChAT staining and MOR-mcherry fluorescence. D. Higher magnification image of the area delimited in C (white square) reveals colocalization (yellow dots labeled with white arrows) between ChAT (green) and MOR-mcherry (red) in IPR axon terminals. DAPI in blue. Scale bar= 50 µm (A & C), 10 µm (B & D).
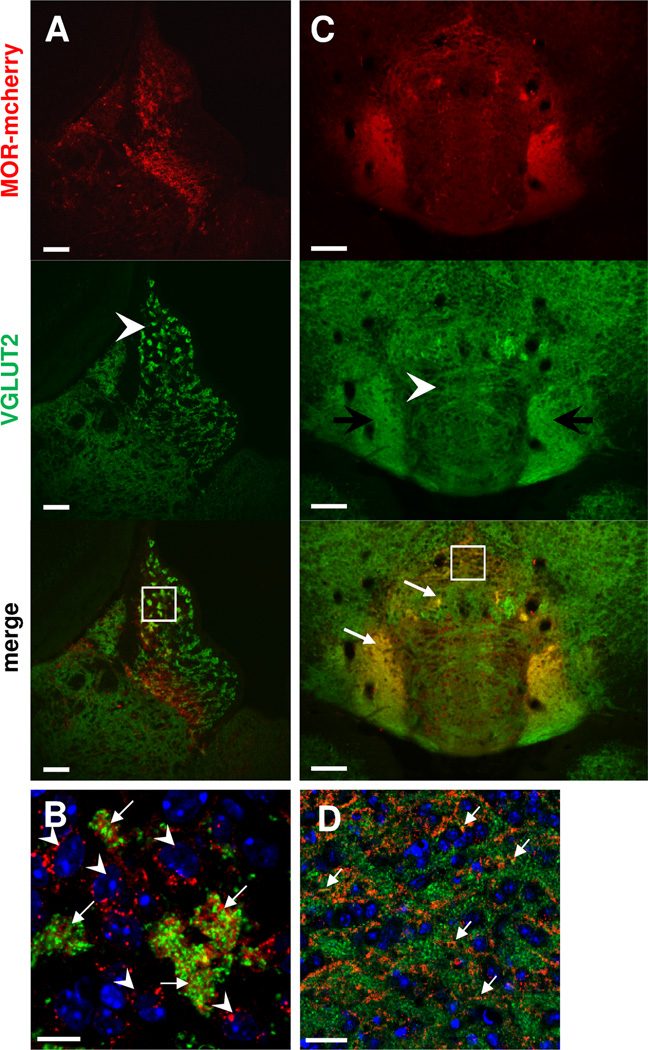
We also tested type 2 vesicular glutamatergic transporter (VGLUT2) immunostaining, known to detect axon terminals, but not somata and dendrites, of glutamatergic neurons (Herzog et al., 2001). In MOR-mcherry mice, the VGLUT2 signal was observed throughout MHb, and was undetectable in LHb (Fig. 8A) as expected from the literature (Qin and Luo, 2009). Within MHb, strong VGLUT2 staining was observed in the apical region. A part of these terminals showed expression of both VGLUT2 and MOR-mcherry, and were surrounded by neurons expressing MOR-mcherry alone (Fig. 8B). In the IPN, VGLUT2 immunostaining revealed an excitatory axonic cistern in the upper part of IPI and IPC, and punctuate VGLUT2 expression in IPR (Fig. 8C). This expression pattern overlapped in part with choline acetyltransferase (ChAT) staining observed in Figure 7C. VGLUT2 immunostaining was also detected in the IPL, where MHb Substance P neurons project massively. Finally, we observed MOR-mcherry colocalization with VGLUT2 markers in the IPL and IPR (Fig. 8C, D). Together, these observations suggest that MOR could regulate glutamate transmission at several levels of the MHb–IPN pathway.
Figure 8. MOR-mcherry colocalize with VGLUT2 in the apical part of the MHb, and in the IPR and IPL.
Photomicrographs of medial habenula MHb (A–B) and interpeduncular nucleus IPN (C–D) of MOR-mcherry knock-in mice. A. Staining with antibodies directed against the vesicular glutamate transporter 2 (VGLUT2, green) indicates the presence of glutamatergic terminals in the MHb, with stronger staining in the apical part (arrowhead). B. Higher magnification image (white square in A) reveals co-occurrence of VGLUT2 (green) and MOR-mcherry (red) immunostaining in in-between somata spaces of the apical MHb (arrows), surrounded by MOR-mcherry expressing neurons (arrowheads). C. In the IPN, VGLUT2 is located in axons forming a cistern in the upper part of the IPI and IPC (arrowhead). Immunostaining is also found in the IPL and IPR. Colocalization with MOR-mcherry is detected in the IPL and IPR (white arrows). D. Enlargement of the IPR area delimited by a white square in C. Colocalization between VGLUT2 (green) and MOR-mcherry (red) is visible in the IPR by the presence of yellow dots (white arrows). DAPI in blue. Scale bar= 50 µm (A & C), 10 µm (B & D).
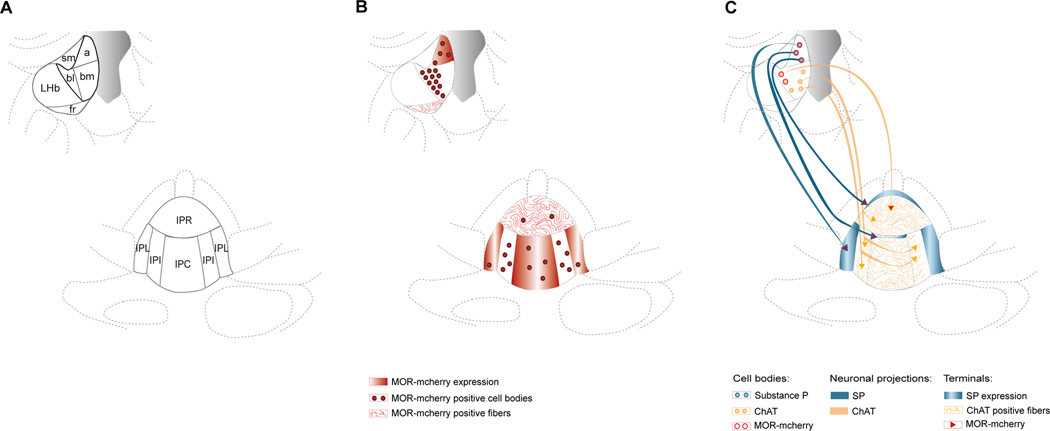
Figure 9 shows a schematic diagram summarizing MOR-mcherry expression and colocalization in neurons of the MHb-IPN pathway.
Figure 9. Distribution and characterization of MOR-mcherry expression in the MHb-IPN pathway.
A. Scheme representing habenular nuclei at Bregma −1.58 mm and the interpeduncular nucleus at Bregma −3.80 mm. For nomenclature of sub-regions, see legends of Figure 2 and 3. B. MOR-mcherry red fluorescence is detectable with three different patterns. Intense fluorescence within cell bodies reveals MOR-mcherry expressing cells (red circles). MOR-positive cell bodies are seen in apical and basolateral parts of the MHb, as well as IPC and IPL. Few cells are also observed in the IPL and IPR. Fluorescence presenting a fibrous aspect reflects MOR-positive passing fibers (red lines). Fibers presenting MOR-mcherry expression are observed in the fr and IPR. Finally, diffuse fluorescence potentially arises from MOR expression at the level of afferent terminals (plain red areas) and is found in the apical part of MHb, and in both IPL and IPC. C. Representation of the MHb cholinergic (orange circles) and Substance P expressing neurons (blue circles) projecting to the IPN and colocalization with MOR-mcherry. Projection sites are symbolized with orange lines for cholinergic axons in the IPN and plain blue areas for substance P terminals. MOR-mcherry co-expression at the level of MHb neurons and IPN terminal sites is represented with red.
3. MOR-mcherry expressing cells in the MHb-IPN pathway and PEnk expression
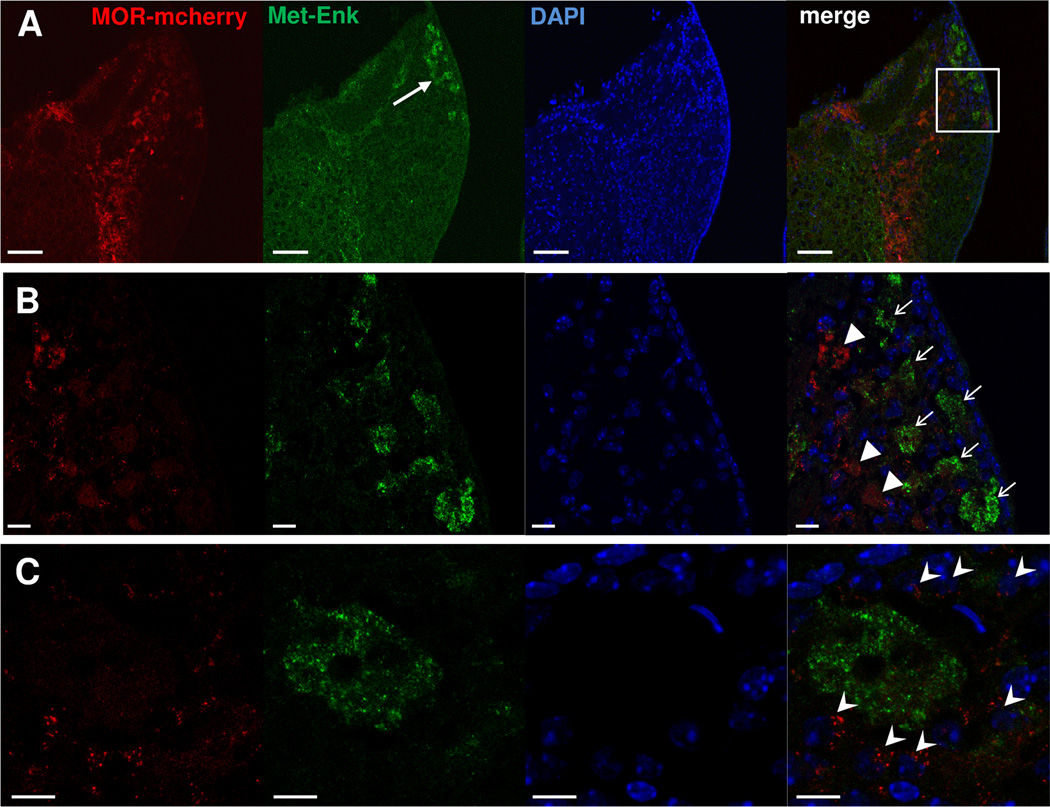
To correlate MOR-mcherry distribution with expression of a main MOR endogenous ligand, we co-stained brain sections using antibodies directed against MOR-mcherry and Met-Enkephalin (Met-enk). Met-Enk immunostaining was mainly present in the apical area of the MHb (Fig. 10A, B). There was no detectable colocalization of Met-Enk staining with MOR-mcherry in MHb (Fig. 10B). In fact, areas showing Met-Enk signal were most often surrounded by small-size cell bodies expressing MOR-mcherry proteins (Fig. 10C). In apical MHb, therefore, MOR-mcherry is ideally located for activation by endogenous opioid peptides. In the IPN, Met-Enk was seen in IPR, IPC, and surrounding IPL. At all these locations, Met-Enk immunostaining was in close vicinity to the MOR-mcherry signal (Fig. 11). MORs could thus also be activated by Met-Enkephalin in the IPN.
Figure 10. Met-Enkephalin is mostly expressed in the apical part of the MHb in close vicinity to MOR-mcherry-expressing neurons.
A. Met-Enkephalin immunolabeling in the MHb of a MOR-mcherry mouse. Met-Enkephalin is located in the apical part of MHb (white arrow). B. Enlargement of this area (white square in A). Terminals presenting Met-Enkephalin expression (white arrows) are different from those expressing MOR-mcherry (white arrowheads). C. Higher magnification image shows that MOR-mcherry expressing neurons (white arrowheads) are located all around Met-Enkephalin releasing areas. DAPI in blue. Scale bar= 50 µm (A) and 10 µm (B & C).
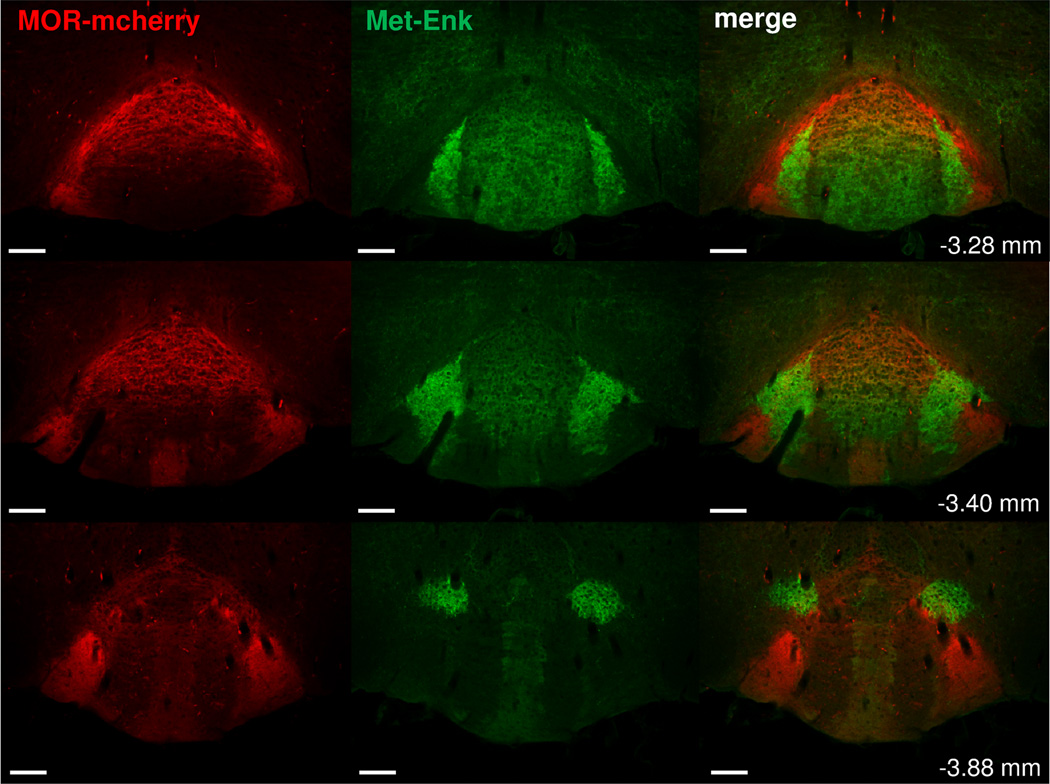
Figure 11. Met-Enkephalin and MOR-mcherry expression in the interpeduncular nucleus.
Immunolabeling obtained with a primary antibody directed against Met-Enkephalin (Met-Enk) peptides using a secondary antibody coupled to an AlexaFluor488 (green) in brain sections of MOR-mcherry mice from Bregma: −3.28 to −3.88 mm. Met-Enkephalin immunostaining is present in the IPR, IPC and IPL. Rostrally, IPL are the nuclei presenting the strongest Met-Enkephalin expression. Caudally, highest fluorescent signal is found in the dorso-lateral nuclei of the IPN (IPDL). At all these sites, MOR-mcherry is found in close vicinity to the Met-Enkephalin immunostaining. Scale bar= 50 µm.
Discussion
The Hb is an important relay structure between forebrain septal and pallidal regions, and mid/hindbrain areas that include ventral tegmental area, interpeduncular nucleus, raphe and tegmental nuclei. LHb and MHb nuclei, the two Hb nuclei, regulate monoamine and cholinergic transmission (Lee and Goto, 2011, Lammel et al., 2012, Kobayashi et al., 2013), and are key structures in the modulation of affective and cognitive functions. LHb has been most extensively studied but little is known about MHb. Interest for the medial nucleus, however, is growing. Using the newly generated MOR-mcherry knock-in mice, we show here that MOR expression is mainly restricted to MHb, concordant with previous autoradiographic binding (Kitchen et al., 1997, Slowe et al., 1999, Goody et al., 2002) experiments. We further characterize sites for MOR expression in the distinct MHb cell populations, as well as afferents and projections sites (summary in Fig. 9).
Mu opioid receptor in the MHb-IPN pathway – a site for interaction with Substance P
In the apical part of MHb, we found important MOR-mcherry co-localization with substance P, and further, MOR-mcherry expression was detected in most substance P-positive axon terminals in the IPL. These observations together suggest that most substance P neurons of apical MHb also express MOR. Previous literature has documented functional interactions between opioid and substance P systems. At the level of the spinal cord, dorsal root ganglia and primary afferents, opiates acting at MORs inhibit Substance P release and alleviate pain transmission (Beaudry et al., 2011, Suzuki et al., 2012, Chen et al., 2014, Mizoguchi et al., 2014). Several studies also correlate substance P release and concentration with the severity of morphine withdrawal symptoms (Johnston and Chahl, 1991, Maldonado et al., 1993, Buccafusco and Shuster, 1997, Zhou et al., 1998, Trang et al., 2002, Michaud and Couture, 2003, Gu et al., 2005). Knockout mice for the tachykinin 1 gene, encoding substance P, show increased analgesic response to morphine, and also reduced respiratory depression, signs of withdrawal, sensitization and tolerance to morphine (Bilkei-Gorzo et al., 2010). Finally, rewarding properties of morphine are suppressed in mice deficient for the Substance P receptor NK1 (Murtra et al., 2000, Ripley et al., 2002). These data demonstrate that substance P modulates many aspects of morphine activities. Our findings of MOR expression in substance P neurons in apical MHb suggest that some of the mu opioid-substance P interactions may occur within this particular neuronal population. These may regulate analgesic, autonomic or reward responses, which all are processed at least partly through Hb networks (Hikosaka et al., 2008, Hikosaka, 2010, Kobayashi et al., 2013).
Mu opioid receptor in the MHb-IPN pathway – a site for interaction with the cholinergic system
Our study reveals that MOR-mcherry is expressed in a sub-population of MHb cholinergic neurons restricted to the basolateral part of MHb, and in cholinergic terminals located in the upper part of the IPR. A known feature of the MHb-IPN pathway is the predominant expression of α3 and β4 subunits of the nicotinic receptors (Grady et al., 2009). Also, systemic administration of 18-Methoxyroconaridine, a α3β4 antagonist, as well as local injection of this compound in MHb or IPN, both decrease various signs of naltrexone precipitated-withdrawal (Panchal et al., 2005), suggesting that nicotinic receptors in the MHb-IPN pathway contribute to the expression of opioid withdrawal via cross-talk within cholinergic neurons. Further, habenular cholinergic neurons also play an essential role in nicotine withdrawal (Dani and De Biasi, 2013). MORs expressed in these neurons, therefore, could be involved in somatic, vegetative or motivational withdrawal signs for several drugs of abuse, a hypothesis that deserves further investigation.
Mu opioid receptor in the MHb-IPN pathway – Expression in a glutamate coreleasing neuronal populations?
Recent observations showed vglut2 and vglut1 mRNAs in MHb, VGLUT1 positive MHb terminals in the IPN, and co-release of glutamate and acetylcholine by MHb neurons in the IPN (Qin and Luo, 2009, Ren et al., 2011, Aizawa et al., 2012), indicating that MHb contains glutamate-expressing neurons. In our study, MOR-mcherry expression is detected in glutamate terminals of the IPL and IPR subnuclei. IPL receiving massive projections from MHb-Substance P neurons, our data raise the possibility of MOR-mcherry expression in a neuronal population coreleasing glutamate and Substance P, a hypothesis that would deserve further investigation.
Corelease of neurotransmitters has been intensively studied in recent years, with the notion that glutamate corelease with another neurotransmitter encodes distinct information depending on activation levels (Hnasko and Edwards, 2012), and can convey temporal-specific information (Lapish et al., 2006, Lapish et al., 2007). MOR expression in these particular neuronal populations could thus fine-tune information processing.
Mu opioid receptor expression upstream and downstream the MHb-IPN pathway – the bigger picture
We showed that MOR-mcherry is strongly expressed in both MHb and IPN. In addition, our observation of the MOR-mcherry fluorescent signal indicates that MOR is also expressed in afferent neurons projecting to MHb, as well as IPN neurons projecting to brainstem nuclei, and therefore contributes to medial habenular activity both upstream and downstream of MHb-IPN pathway.
The strong punctuate MOR-mcherry fluorescence in MHb mainly reflects MOR-expressing cell bodies, which primarily project to IPN. MHb receives main inputs from the posterior septum, consisting of the triangular septum (TS), the septo-fimbrial septum (SFi) and the bed nucleus of the anterior commissure (BAC), and also from the diagonal band (DB). The observation of numerous MOR-mcherry expressing cell bodies in most of these regions (see Fig. 4 and Erbs et al., 2014) suggests that MORs also likely control MHb activity from upstream pathways.
Diagonal band projections to the MHb were reported as mostly GABAergic, whereas septal projections are mainly glutamatergic (Qin and Luo, 2009). Thus, the co-expression of MOR-mcherry with VGLUT2 transporters in terminals located in apical MHb (see Fig. 8) suggests that MORs are present in MHb-projecting posterior septum glutamate neurons. Septum is a critical region in the modulation of anxiety-related behaviors (Menard and Treit, 1996). Also, the specific study of septal-MHb projections showed their role in anxiety-related behavior as well as fear-directed behavior (Yamaguchi et al., 2013). MOR expression in the posterior septum-MHb pathway could thus be involved in the negative emotional state developed during drug addiction.
Most fluorescence detected in the IPN is diffuse or shows a stringy pattern. This signal likely arises from MOR expression along MHb-fr projecting neurons. Part of the MOR-mcherry signal in IPN could also reflect MOR expression in IPN terminals from other projecting neurons, such as lateral hypothalamus, diagonal band, median raphe nucleus or dorsal tegmental region neurons (Groenewegen et al., 1986), which also show MOR-mcherry cell bodies (see Fig. 4 and Erbs et al., 2014). MOR-mcherry-positive cell bodies were also found in the IPN, mainly in central and intermediate subnuclei of the IPN, with only rare MOR-mcherry expressing neurons in IPL and IPR. Former tracing experiments have shown that IPC and IPI mainly project to the median raphe nucleus (MnR) and to the dorsal tegmental area (Groenewegen et al., 1986). These MOR-expressing neurons could thus play a role in the regulation of serotonin and dopamine transmission. Indeed the laterodorsal tegmental area (LDTg) and the pedunculopontine tegmental area (PPTg), both part of the dorsal tegmental area, send projections to the VTA dopaminergic neurons and influence their firing (Lodge and Grace, 2006, Changeux, 2010, Norton et al., 2011). Also, anterograde tracing experiments have shown that MnR serotoninergic neurons send important back projections to the IPN and the posterior septum, and also massive projections to the VTA and to the LHb, which in turn projects to the rostromedial tegmental nucleus RMTg (also called tail of the VTA; tVTA) (Morin and Meyer-Bernstein, 1999). The LDTg was also shown to send cholinergic projections back to septal areas (Cornwall et al., 1990).
Conclusion
Altogether, this report demonstrates that MOR is distributed all along the MHb-IPN pathway and associated circuitry. In addition to the well-described expression of this receptor in dopaminergic mesolimbic circuits, associated to LHb, MOR should therefore also be considered a prominent player in MHb-related networks. Fig. 5 summarizes the anatomy of MOR expression sites within MHb and LHb circuitries and their main connections as reported by other reports (Sutherland, 1982, Groenewegen et al., 1986, Lecourtier and Kelly, 2007, Geisler and Trimble, 2008, Hikosaka et al., 2008, Qin and Luo, 2009, Brinschwitz et al., 2010) and this study.
Early reports have demonstrated that substance P and acetylcholine neurotransmission of MHb-IPN are implicated in morphine analgesia, negative emotional states and somatic signs of drug withdrawal (Panchal et al., 2005, Bilkei-Gorzo et al., 2010). The strong MOR expression in the MHb-IPN pathways pleads for a key role of this opioid receptor in the control of positive and negative properties of drugs of abuse. Future functional studies will likely confirm this hypothesis and perhaps reveal a broader contribution of MHb MORs in the integration of emotional and cognitive functions.
Highlights.
-
-
Mu opioid receptors (MORs) are most dense and understudied in the MHb-IPN pathway
-
-
MOR-mcherry knock-in mice reveal receptor expression with cellular resolution
-
-
Most substance P neurons of MHb express MOR-mcherry in both cell bodies and terminals
-
-
Only a subset of cholinergic neurons in MHb expresses MOR-mcherry
-
-
MOR-mcherry is also strongly expressed upstream and downstream the MHb-IPN pathway
Acknowledgments
We thank E. Darcq for critical reading of the manuscript. We thank our funding sources including the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université de Strasbourg, The Agence Nationale pour la Recherche (IMOP), the National Institutes of Health (NIDA DA-05010) and the Stefan and Shirley Hatos Center for Neuropharmacology. O. Gardon was supported by the National Institute on Alcohol Abuse and Alcoholism (grant #16658), and L. Faget was supported by a region Alsace fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:Spc1. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci. 2011;31:13068–13077. doi: 10.1523/JNEUROSCI.1817-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Berner J, Zimmermann J, Wickstrom R, Racz I, Zimmer A. Increased morphine analgesia and reduced side effects in mice lacking the tac1 gene. Br J Pharmacol. 2010;160:1443–1452. doi: 10.1111/j.1476-5381.2010.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Shuster LC. Effect of intrathecal pretreatment with the neurokinin receptor antagonist CP-99994 on the expression of naloxone-precipitated morphine withdrawal symptoms. Brain Res Bull. 1997;43:321–326. doi: 10.1016/s0361-9230(97)00013-0. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–217. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, McRoberts JA, Marvizon JC. mu-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Mesolimbic dopamine and habenulo-interpeduncular pathways in nicotine withdrawal. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DQ, Perez EE, Teng Y, Dani JA, De Biasi M. Nicotine Enhances Excitability of Medial Habenular Neurons via Facilitation of Neurokinin Signaling. J Neurosci. 2014;34:4273–4284. doi: 10.1523/JNEUROSCI.2736-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Befort K, Koebel P, Pannetier S, Mahoney MK, Gaveriaux-Ruff C, Hanauer A, Kieffer BL. RSK2 signaling in medial habenula contributes to acute morphine analgesia. Neuropsychopharmacology. 2012;37:1288–1296. doi: 10.1038/npp.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Koebel P, Del Boca C, Pannetier S, Kirstetter AS, Garnier JM, Hanauer A, Befort K, Kieffer BL. RSK2 signaling in brain habenula contributes to place aversion learning. Learn Mem. 2011;18:574–578. doi: 10.1101/lm.2221011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Kessler P, Hentsch D, Vonesch JL, Matifas A, Kieffer BL, Massotte D. Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience. 2012;221:203–213. doi: 10.1016/j.neuroscience.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D. A -delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco FR, Martorana A, De March Z, Viscomi MT, Sancesario G, Bernardi G. Huntingtin distribution among striatal output neurons of normal rat brain. Neurosci Lett. 2003;339:53–56. doi: 10.1016/s0304-3940(02)01474-x. [DOI] [PubMed] [Google Scholar]
- Gackenheimer SL, Suter TM, Pintar JE, Quimby SJ, Wheeler WJ, Mitch CH, Gehlert DR, Statnick MA. Localization of opioid receptor antagonist [3H]-LY255582 binding sites in mouse brain: comparison with the distribution of mu, delta and kappa binding sites. Neuropeptides. 2005;39:559–567. doi: 10.1016/j.npep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Geisler S, Trimble M. The lateral habenula: no longer neglected. CNS Spectr. 2008;13:484–489. doi: 10.1017/s1092852900016710. [DOI] [PubMed] [Google Scholar]
- George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, Kouvelas A, Chan AS, O’Dowd BF. Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem Biophys Res Commun. 1994;205:1438–1444. doi: 10.1006/bbrc.1994.2826. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Goutagny R, Loureiro M, Jackson J, Chaumont J, Williams S, Isope P, Kelche C, Cassel JC, Lecourtier L. Interactions between the Lateral Habenula and the Hippocampus: Implication for Spatial Memory Processes. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Gu G, Kondo I, Hua XY, Yaksh TL. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J Pharmacol Exp Ther. 2005;314:1362–1369. doi: 10.1124/jpet.105.087718. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Shumake J, Wetzel W, Scheich H, Ohl FW. Electrical stimulation of lateral habenula during learning: frequency-dependent effects on acquisition but not retrieval of a two-way active avoidance response. PLoS One. 2013;8:e65684. doi: 10.1371/journal.pone.0065684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PA, Chahl LA. Tachykinin antagonists inhibit the morphine withdrawal response in guinea-pigs. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:283–288. doi: 10.1007/BF00251127. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Interactions between neuropeptides and dopamine neurons in the ventromedial mesencephalon. Neurosci Biobehav Rev. 1985;9:573–587. doi: 10.1016/0149-7634(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hrycyshyn AW. Topographical and ultrastructural investigation of the habenulo-interpeduncular pathway in the rat: a wheat germ agglutinin-horseradish peroxidase anterograde study. J Comp Neurol. 1988;275:117–127. doi: 10.1002/cne.902750110. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hunt SP, Hrycyshyn AW. Substance P immunoreactivity in the rat interpeduncular nucleus: synaptic interactions between substance P-positive profiles and choline acetyltransferase- or glutamate decarboxylase-immunoreactive structures. Neuroscience. 1991;42:739–755. doi: 10.1016/0306-4522(91)90042-m. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 56 Suppl. 2009;1:205–212. doi: 10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–273. [PubMed] [Google Scholar]
- Kobayashi Y, Sano Y, Vannoni E, Goto H, Suzuki H, Oba A, Kawasaki H, Kanba S, Lipp HP, Murphy NP, Wolfer DP, Itohara S. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Larsen PJ. Distribution of substance P-immunoreactive elements in the preoptic area and the hypothalamus of the rat. J Comp Neurol. 1992;316:287–313. doi: 10.1002/cne.903160304. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lee YA, Goto Y. Neurodevelopmental disruption of cortico-striatal function caused by degeneration of habenula neurons. PLoS One. 2011;6:e19450. doi: 10.1371/journal.pone.0019450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Goto Y. Habenula and ADHD: Convergence on time. Neurosci Biobehav Rev. 2013;37:1801–1809. doi: 10.1016/j.neubiorev.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Girdlestone D, Roques BP. RP 67580, a selective antagonist of neurokinin-1 receptors, modifies some of the naloxone-precipitated morphine withdrawal signs in rats. Neurosci Lett. 1993;156:135–140. doi: 10.1016/0304-3940(93)90457-v. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. Lateral and medial septal lesions reduce anxiety in the plus-maze and probe-burying tests. Physiol Behav. 1996;60:845–853. doi: 10.1016/0031-9384(96)00138-2. [DOI] [PubMed] [Google Scholar]
- Michaud N, Couture R. Cardiovascular and behavioural effects induced by naloxone-precipitated morphine withdrawal in rat: characterization with tachykinin antagonists. Neuropeptides. 2003;37:345–354. doi: 10.1016/j.npep.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Takagi H, Watanabe C, Yonezawa A, Sato T, Sakurada T, Sakurada S. Involvement of multiple micro-opioid receptor subtypes on the presynaptic or postsynaptic inhibition of spinal pain transmission. Peptides. 2014;51:15–25. doi: 10.1016/j.peptides.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Morin LP, Meyer-Bernstein EL. The ascending serotonergic system in the hamster: comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/s0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci. 2011;33:1885–1896. doi: 10.1111/j.1460-9568.2011.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal V, Taraschenko OD, Maisonneuve IM, Glick SD. Attenuation of morphine withdrawal signs by intracerebral administration of 18-methoxycoronaridine. Eur J Pharmacol. 2005;525:98–104. doi: 10.1016/j.ejphar.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Porteous R, Petersen SL, Yeo SH, Bhattarai JP, Ciofi P, de Tassigny XD, Colledge WH, Caraty A, Herbison AE. Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J Comp Neurol. 2011;519:3456–3469. doi: 10.1002/cne.22716. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology. 2002;43:1258–1268. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Root DH, Ma S, Barker DJ, Megehee L, Striano BM, Ralston CM, Fabbricatore AT, West MO. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J Comp Neurol. 2013;521:558–588. doi: 10.1002/cne.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of mu-,delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Narita M, Hasegawa M, Furuta S, Kawamata T, Ashikawa M, Miyano K, Yanagihara K, Chiwaki F, Ochiya T, Suzuki T, Matoba M, Sasaki H, Uezono Y. Sensation of abdominal pain induced by peritoneal carcinomatosis is accompanied by changes in the expression of substance P and mu-opioid receptors in the spinal cord of mice. Anesthesiology. 2012;117:847–856. doi: 10.1097/ALN.0b013e31826a4ac8. [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Guimaraes FS, Prado WA. Antinociception induced by stimulation of the habenular complex of the rat. Brain Res. 1990;524:213–218. doi: 10.1016/0006-8993(90)90693-6. [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Prado WA. Antinociception elicited by electrical or chemical stimulation of the rat habenular complex and its sensitivity to systemic antagonists. Brain Res. 1990;535:18–24. doi: 10.1016/0006-8993(90)91818-2. [DOI] [PubMed] [Google Scholar]
- Trang T, Sutak M, Quirion R, Jhamandas K. The role of spinal neuropeptides and prostaglandins in opioid physical dependence. Br J Pharmacol. 2002;136:37–48. doi: 10.1038/sj.bjp.0704681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R. The medial habenula: still neglected. Front Hum Neurosci. 2013;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–544. doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LM, Yu L, Jin HJ, Zhao H. Substance P receptor antagonist in lateral habenula improves rat depression-like behavior. Brain Res Bull. 2014;100:22–28. doi: 10.1016/j.brainresbull.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liu Z, Ray A, Huang W, Karlsson K, Nyberg F. Alteration in the brain content of substance P (1–7) during withdrawal in morphine-dependent rats. Neuropharmacology. 1998;37:1545–1552. doi: 10.1016/s0028-3908(98)00128-2. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Baffi J, Usdin T, Reynolds K, Konig M, Palkovits M, Mezey E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci U S A. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]