Abstract
The ability to make advantageous decisions under circumstances in which there is a risk of adverse consequences is an important component of adaptive behavior; however, extremes in risk taking (either high or low) can be maladaptive and are characteristic of a number of neuropsychiatric disorders. To better understand the contributions of various affective and cognitive factors to risky decision making, cohorts of male Long-Evans rats were trained in a “Risky Decision making Task” (RDT), in which they made discrete trial choices between a small, “safe” food reward and a large, “risky” food reward accompanied by varying probabilities of footshock. Experiment 1 evaluated the relative contributions of the affective stimuli (i.e., punishment vs. reward) to RDT performance by parametrically varying the magnitudes of the footshock and large reward. Varying the shock magnitude had a significant impact on choice of the large, “risky” reward, such that greater magnitudes were associated with reduced choice of the large reward. In contrast, varying the large, “risky” reward magnitude had minimal influence on reward choice. Experiment 2 compared individual variability in RDT performance with performance in an attentional set shifting task (assessing cognitive flexibility), a delayed response task (assessing working memory), and a delay discounting task (assessing impulsive choice). Rats characterized as risk averse in the RDT made more perseverative errors on the set shifting task than did their risk taking counterparts, whereas RDT performance was not related to working memory abilities or impulsive choice. In addition, rats that showed greater delay discounting (greater impulsive choice) showed corresponding poorer performance in the working memory task. Together, these results suggest that reward-related decision making under risk of punishment is more strongly influenced by the punishment than by the reward, and that risky and impulsive decision making are associated with distinct components of executive function.
1. Introduction
Decisions among options that vary in both their payoffs and their potential for adverse consequences are a consistent feature of everyday life. When faced with such choices, most individuals can weigh the relative risks and rewards associated with the competing options and decide adaptively; however, such choice behavior (henceforth referred to as “risky decision making”) may be altered in several neuropsychiatric conditions, such that choices are strongly biased toward or away from “risky” options. For example, high levels of risk taking are present in ADHD and addiction, where they may contribute to some of the adverse outcomes associated with these conditions (Bechara et al., 2001; Drechsler, Rizzo, & Steinhausen, 2008; Ernst et al., 2003; Kagan, 1987). In contrast, abnormally low levels of risk taking (risk aversion) are found in anorexia nervosa and social anxiety ((Butler & Mathews, 1987; Kaye, Wierenga, Bailer, Simmons, & Bischoff-Grethe, 2013; Stanley, 2002) although see (Reynolds et al., 2013)). Hence, a better understanding of the neurobehavioral mechanisms underlying risky decision making may yield benefits across the clinical spectrum.
The current study employed a rat model of risky decision making in which rats make discrete trial choices between a small, “safe” food reward and a large “risky” food reward accompanied by varying probabilities of mild footshock (the “Risky Decision making Task”, or RDT). Previous work has shown that male Long-Evans rats display marked individual variability in their preference for the large, risky reward in this task. Some rats show a strong preference for the large reward even with a high probability of shock (i.e., “risk takers”), whereas other rats show a strong preference for the small reward even when there is a low probability of shock (i.e., “risk averse”) (Simon et al., 2011). These differences in performance are not associated with variability in reward motivation, anxiety, or shock reactivity (Simon, Gilbert, Mayse, Bizon, & Setlow, 2009; Simon, et al., 2011). However, rats with a high preference for risk taking acquire cocaine self-administration more rapidly and have lower striatal dopamine D2 receptor mRNA expression than rats with a low preference for risk taking (Mitchell et al., 2014); Simon et al., 2011). Notably, elevated risk taking in humans is associated with both addiction and reduced striatal D2 receptor availability (Bechara, et al., 2001; Goldstein et al., 2009; Rogers et al., 1999; Volkow, Fowler, Wang, & Swanson, 2004), supporting the validity of the RDT as a model of human risk taking behavior. The aim of the current study was to assess affective and cognitive mechanisms that may mediate RDT performance, by manipulating the affective value of the RDT task parameters, and by determining associations between risk taking and several measures of executive function. A first cohort of rats was exposed to varying magnitudes of footshock to determine the effects of punishment magnitude on RDT performance. A second cohort was presented with variable numbers of food pellets upon choice of the “risky” option, to determine the effects of reward magnitude on RDT performance. Finally, a third cohort of rats was trained on a set shifting task, a working memory task, the RDT, and a delay discounting task to determine relationships among these different aspects of cognition and decision making.
2. Materials and Methods
2.1 Experiment 1: Effects of varying shock or reward magnitude on risky decision making task performance
2.1.1 Subjects
Two cohorts of male Long-Evans rats (n=16 for Experiment 1A, and n=8 for Experiment 1B, 275–300 g on arrival, Charles River Laboratories, Raleigh, NC) were individually housed and kept on a 12 h light/dark cycle (lights on at 0800 hours) with free access to food and water except as noted. Prior to the start of behavioral testing, rats were reduced to 85% of their free feeding weights over the course of five days, and maintained at this weight for the duration of the experiment, with allowances for growth. Animal procedures were conducted from 0800 to 1700, and were approved by the University of Florida Institutional Animal Care and Use Committee and followed NIH guidelines.
2.1.2 Apparatus
Testing was conducted in standard behavioral test chambers (Coulbourn Instruments, Whitehall, PA) housed within sound-attenuating isolation cubicles. Each chamber was equipped with a recessed food pellet delivery trough fitted with a photobeam to detect head entries and a 1.12 W lamp to illuminate the food trough, which was located 2 cm above the floor in the center of the front wall. Forty-five mg grain-based food pellets (PJAI, Test Diet, Richmond, IN) could be delivered into the food trough. Two retractable levers were located to the left and right of the food trough, 11 cm above the floor. A 1.12 W house light was mounted on the rear wall of the isolation cubicle. The floor of the test chamber was composed of steel rods connected to a shock generator that delivered scrambled footshocks. Locomotor activity could be assessed throughout each session with an infrared activity monitor mounted on the ceiling of the test chamber. This monitor consisted of an array of infrared detectors focused over the entire test chamber. Movement in the test chamber (in x, y, or z planes) was defined as a relative change in the infrared energy falling on the different detectors in the array. Test chambers were interfaced with a computer running Graphic State 3 software (Coulbourn Instruments), which controlled task event delivery and data collection.
2.1.3 Behavioral Procedures
2.1.3.1 Shaping
On the day prior to shaping, each rat was given five 45 mg food pellets in its home cage to reduce neophobia to the food reward used in the task. Shaping procedures followed those used previously (Cardinal, Robbins, & Everitt, 2000; Simon, et al., 2009; Simon, Mendez, & Setlow, 2007). Following magazine training, rats were trained to press a single lever (either the left or the right, balanced across rats; the other lever was retracted during this phase of training) to receive a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were then trained on the opposite lever under the same criterion. This was followed by further shaping sessions in which both levers were retracted and rats were shaped to nose poke into the food trough during simultaneous illumination of the trough and house lights. When a nose poke occurred, a single lever was extended (left or right), and a lever press resulted in immediate delivery of a single food pellet. Immediately following the lever press, the trough light was extinguished and the lever was retracted. Rats were trained to a criterion of 30 presses on each lever within 60 min.
2.1.3.2 Risky Decision Making Task
In the RDT, rats made discrete trial choices between two response levers, one which delivered a small reward, and the other which delivered a large reward accompanied by varying risks of footshock. Testing procedures were identical to Simon et al. (2009) and Mitchell et al. (2011). In brief, sessions were 60 min in duration and consisted of 5 blocks of trials. Each 40 s trial began with a 10 s illumination of the food trough and house lights. A nose poke into the food trough extinguished the trough light and triggered extension of either a single lever (forced choice trials) or both levers simultaneously (free choice trials). If rats failed to nose poke within the 10 s time window, the lights were extinguished and the trial was scored as an omission. A press on one of the levers (either left or right, balanced across rats) resulted in one food pellet (the small safe reward) delivered immediately following the lever press. A press on the other lever resulted in immediate delivery of 3 food pellets (the large reward). Selection of this lever was also accompanied by a possible 1 s footshock (0.30 mA).
Risk of footshock was contingent on a preset probability specific to each trial block. The “risky” reward was delivered following every choice of this reward lever, regardless of whether or not the footshock occurred. The probability of footshock accompanying the large reward was set at 0% during the first block of trials. In subsequent blocks of trials, the probability of footshock increased to 25, 50, 75, and 100%. Each trial block began with 8 forced choice trials (4 for each lever, used to establish the punishment contingencies in effect for that block) followed by 10 free choice trials. Once either lever was pressed, both levers were immediately retracted. Food delivery was accompanied by re-illumination of both the food trough and house lights, which were extinguished upon entry to the food trough to collect the food or after 10 s, whichever occurred sooner. On the forced choice trials (in which only one lever was present) the probability of shock following a press on the large reward lever was dependent across the four trials in each block. For example, in the 25% risk block, one and only one of the four large reward forced choice trials (randomly selected) always resulted in shock, and in the 75% risk block, three and only three of the four large reward forced choice trials always resulted in shock. In contrast, the probability of shock on the free choice trials (in which both levers were present) was independent, such that the probability of shock on each trial was the same, irrespective of shock delivery on previous trials in that block.
Raw data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). Statistical analyses were conducted in SPSS 21. The primary measure of interest was the percentage of choice trials in each block on which rats chose the large, risky reward. To assess stability of RDT performance, repeated measures ANOVAs (session X trial block) were conducted on group data averaged across 5 consecutive sessions. Stable performance was defined by the absence of a main effect of session, the absence of an interaction between session and trial block, and the presence of a main effect of trial block. Baseline locomotor activity was measured by averaging activity across all inter-trial interval (ITI) segments (in which no lights or levers were present). Shock reactivity was measured by averaging locomotor activity during the 1 s shock delivery periods. In all cases, p values less than 0.05 were considered significant.
2.1.4 Experiment 1A. Effects of varying shock magnitude on risky decision-making task performance
Upon reaching stable performance in the RDT as described in section 2.1.3.2 (following 25 sessions), the effects of different shock magnitudes on choice performance were assessed using a counterbalanced, within-subjects design, such that each rat was tested for a single session with each magnitude (0.2, 0.25, 0.35, and 0.4 mA) in a randomized order, with 1 session of testing under baseline shock conditions (0.3 mA) interposed between each shock magnitude test session.
Data Analyses
Choice data were analyzed using a repeated measures ANOVA (shock magnitude X trial block). In addition, as a measure of incentive motivation to obtain the two rewards (small, safe vs. large, risky), rats’ latencies to press the levers (time between lever extension and lever press) associated with each of these rewards were assessed on forced choice trials. Forced choice were used to assess response latency because of insufficient data from free choice trials in some rats (i.e., some rats chose one lever exclusively on some blocks of free choice trials). In addition, the forced choice trials provided a measure of incentive motivation for the two rewards that was relatively uncontaminated by comparative reward values or decision processes. Response latency data were analyzed using a repeated measures ANOVA (shock magnitude X trial block X lever identity).
2.1.5 Experiment 1B. Effects of varying reward magnitude on risky decision-making task performance
Upon reaching stable performance in the RDT as described in section 2.1.3.2 (following 20 sessions), the task was modified such that the number of pellets delivered upon choice of the “risky” lever varied from 1, 2, 4 and 5 pellets during separate sessions in order to assess the effect of reward magnitude on choice behavior. Each rat was subjected to each of the 4 conditions (i.e. 1 pellet vs. 1 pellet, 1 pellet vs. 2 pellets, etc.) in a randomized and counterbalanced fashion. Pilot experiments suggested that a single test session with modified large reward magnitudes was insufficient to shift choice preference. Hence, each condition was repeated daily for 5 consecutive days, with 2 days off between reward magnitude shifts.
2.1.5.1 Data Analyses
Choice data were analyzed using a repeated measures ANOVA (reward magnitude X trial block) on performance averaged across the final 2 days of testing with each reward magnitude condition. Lever press response latency data were analyzed in the same way as in Experiment 1A (repeated measures ANOVA, reward magnitude X trial block X lever identity).
2.2 Experiment 2: Relationships between risky decision making, executive function, and impulsive decision making
2.2.1 Subjects
Male Long-Evans rats (n=16, 275–300 g on arrival, Charles River Laboratories, Raleigh, NC) were housed as in Experiment 1. Behavioral testing took place in test chambers identical to those used in Experiment 1, with the addition of 1.12 W cue lamps above each lever for the set shifting task. The behavioral tasks are described below in the order in which they were conducted.
2.2.2 Set shifting task procedures
The ability to adapt behavior in response to changing environmental contingencies (i.e., cognitive flexibility) can take several forms which depend on different but overlapping neural circuitry (Brown & Bowman, 2002; Dias, Robbins, & Roberts, 1996). Experiment 2 focused on one form of cognitive flexibility – set-shifting – which involves the same prefrontal cortical-striatal circuitry implicated in risky decision making (Floresco, Magyar, Ghods-Sharifi, Vexelman, & Tse, 2006; Mitchell, et al., 2014; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002; Simon, et al., 2011; Stefani, Groth, & Moghaddam, 2003) and is impaired in the same neuropsychiatric conditions in which alterations in risky decision making are prominent (Danner et al., 2012; Ernst, et al., 2003; Floresco, et al., 2006; Shott et al., 2012).
2.2.2.1 Shaping
The design of the set shifting task was based on that used by Floresco et al. (2008). Prior to the start of behavioral testing, rats were reduced to 85% of their free feeding weight over the course of five days and maintained at this weight for the duration of the experiment, with allowances for growth. Rats progressed through four stages of shaping prior to the start of the set shifting task, with new stages beginning on the day immediately following completion of the previous stage. On the day prior to Shaping Stage 1, each rat was given five 45 mg food pellets in its home cage to reduce neophobia to the food reward used in the task. Shaping Stage 1 consisted of a 64 min session of magazine training, involving 38 deliveries of a single food pellet with an inter-trial interval (ITI) of 100 ± 40 s. Shaping Stage 2 consisted of lever press training, in which a single lever (left or right, balanced across rats) was extended and a press resulted in delivery of a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were then trained on the opposite lever using the same procedures.
Shaping Stage 3 was designed to train rats to press the levers after their insertion into the test chamber. Each session had 90 trials, and each 20 s trial began with illumination of the house light and insertion of a single lever (either left or right, randomly selected within each pair of trials) into the test chamber, where it remained for a maximum of 10 s. A response on the lever within this time window resulted in retraction of the lever, delivery of a single food pellet, and continued illumination of the house light for an additional 4 s. If a rat failed to respond on the lever within 10 s, the lever was retracted and the house light turned off, and the trial was scored as an omission. Rats received at least 4 daily sessions in this stage, and were trained until reaching criterion performance of fewer than 10 omissions out of the 90 trials.
Shaping Stage 4 was designed to determine each rat’s side bias (i.e., preference for one lever over the other). Each trial in this stage consisted of multiple phases. In the first phase of a trial, the house light was illuminated and both levers were inserted into the test chamber. A response on either lever resulted in retraction of both levers and delivery of a single food pellet. In the second phase of a trial, both levers were again inserted, but only a response on the lever opposite to the one chosen in the first phase resulted in food delivery. A response on the same lever chosen in the first phase (i.e., “incorrect”) resulted in the levers being retracted and the houselight being extinguished. After a “correct” response on this second phase of a trial, a new trial was initiated, whereas after an “incorrect” response, the second phase of the trial was repeated. The second phase was repeated until rats made a “correct” response. In cases in which 5 or more of the initial 7 first phase choices were confined to a single side, that side was considered the rat’s biased side. However, in cases in which neither side was initially chosen a disproportionate number of times (<5), the side associated with the greatest number of total responses across this stage of testing was considered the rat’s biased side.
2.2.2.2 Visual Cue Discrimination
Following the side bias determination session, rats were trained to press the lever associated with a visual cue (light). In this discrimination, illumination of a cue light over a lever signaled the correct response, irrespective of the location (left or right) of the cue. Each 20 s trial began with illumination of one of the cue lights (left or right, randomly selected in each pair of trials). After 3 s, the house light was illuminated and both levers were inserted into the chamber (the cue light remained illuminated while the levers were extended). A response on the lever corresponding to the cue light (a correct response) resulted in the house light remaining on for 4 s, during which time the levers were retracted, the cue light was extinguished, and a single food pellet was delivered. A response on the opposite lever (an incorrect response) or failure to respond within 10 s (omission) resulted in retraction of both levers and all lights being extinguished. Rats were considered to have acquired the task upon reaching criterion performance of 8 consecutive correct trials (and at least 30 total trials, excluding omissions), with the maximum number of trials per session set at 120. If rats failed to acquire the task within a single session, they received additional sessions on subsequent days.
2.2.2.3 Left/Right Discrimination (Set Shift)
After reaching criterion performance on the visual discrimination, rats were tested the next day in the set shift condition, in which the task contingencies were altered. On the set shift, rats were required to ignore the light cue and instead to consistently choose the left or right lever (whichever was not their biased side as determined in Shaping Stage 4). Hence, accurate performance required rats to “shift” their attention away from the visual cue and toward the left/right position of the lever. Beyond the shift in reward contingencies, trials were identical in presentation to those in the visual cue discrimination (i.e., on each trial, both levers were presented, with the cue light illuminated over one lever). As in the visual cue discrimination, the location of the illuminated cue light was randomized (left or right) in each pair of trials. Rats were considered to have acquired the task upon reaching criterion performance of 8 consecutive correct trials, excluding omissions. The maximum number of trials per session was set at 120 and if rats failed to acquire the task within a single session, they received additional sessions on subsequent days.
2.2.2.4 Set Shifting Data Analyses
The total numbers of trials and errors required to achieve criterion on the visual discrimination and on the set shift were used as the indices of performance. Given that the task design involved explicit presentation of the same set of stimuli during both the initial discrimination learning and the set shift, the types of errors were also examined. Specifically, analyses were performed separately for errors that involved responses corresponding to previously reinforced choices (the cue light was incongruent with the correct lever location and the rat chose based on the previous visual discrimination rule) and for errors that had never been reinforced (the cue light and spatial location were congruent and the rat’s choice was not correct according to the rule in either type of discrimination (Floresco, Block, & Tse, 2008; Ragozzino, et al., 2002). For each measure, comparisons between risk subgroups as defined by RDT performance (see below) were conducted using unpaired t-tests.
2.2.3 Delayed response working memory task procedures
Following the set shifting task, rats immediately began testing on a delayed response working memory task in the same testing chambers. Because the rats were already familiar with pressing the retractable levers to earn food rewards, they did not require additional shaping procedures. The design of the task was based on Sloan et al. (2006). Each session was 40 min in duration, and the house light was illuminated throughout the entire session except during timeout periods (see below). Each trial began with insertion of a single lever (the “sample” lever) into the chamber. The left/right position of this lever was randomly selected within each pair of trials, and a lever press caused it to retract and started the delay period timer. During the delay, rats were required to nosepoke into the food trough, and the first nosepoke emitted after the delay timer expired initiated the “choice” phase of the trial. During the choice phase, both levers were extended, and a response on the same lever pressed during the sample phase (a correct response) resulted in both levers being retracted and delivery of a single food pellet. Entry into the food trough to collect the food pellet initiated a 5 s inter-trial interval, after which the next trial was initiated. A response on the opposite lever from that chosen during the sample phase (an incorrect response) resulted in both levers being retracted and initiation of a 5 s “timeout” period during which the house light was extinguished, followed immediately by the start of the next trial.
During initial sessions in this task, there were no delays between the sample and choice phases, and a correction procedure was employed such that the sample lever was repeated on the same side following an incorrect response to prevent development of side biases. Once rats reached a criterion of 80% correct choices across a session for two consecutive sessions, this correction procedure was discontinued and a set of seven delays was introduced. The presentation of delay durations was randomized within each block of seven trials, such that each delay was presented once. Upon establishing greater than 80% correct performance across two consecutive sessions, delays were systematically increased (Set 1: 0, 1, 2, 3, 4, 5, 6 s; Set 2: 0, 2, 4, 8, 12, 16 s; Set 3: 0, 2, 4, 8, 12, 18, and 24 s). Rats were tested for 10 consecutive sessions on the delays in Set 3.
2.2.3.1 Delayed Response Data Analyses
Performance (percentage of correct choices) was averaged across the 10 sessions of Set 3 delays to provide a measure of mean accuracy at each delay. Group comparisons in the working memory task were conducted using a two-factor (risk subgroup X delay) repeated measures ANOVA. Comparisons of individual performance across the set-shifting (trials and errors to criterion) and working memory tasks (mean % accuracy at each delay) were conducted using bivariate correlations.
2.2.4 Risky Decision-Making Task
The RDT was performed identically to Experiment 1, in test chambers identical to those used for set-shifting and working memory but without the cue lamps.
2.2.5 Delay Discounting Task
This task was conducted in the same test chambers used for the RDT. No additional shaping was needed prior to testing in the delay-discounting task, as it was similar in design to the RDT. A detailed description of this task is provided in Mendez et al. (2012). Each 60 min session consisted of 5 blocks of 12 trials each. Each 60 s trial began with a 10 s illumination of the food trough and house lights. A nosepoke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced choice trials) or of both levers simultaneously (free choice trials). Trials on which rats failed to nosepoke during this 10 s window were scored as omissions. Each block consisted of 2 forced choice trials followed by 10 free choice trials. A press on one lever (either left or right, balanced across subjects) resulted in one food pellet (the small reward) delivered immediately. A press on the other lever resulted in 3 food pellets (the large reward) delivered after a variable delay. Failure to press either lever within 10 s of their extension resulted in the levers being retracted and lights extinguished, and the trial was scored as an omission. Once either lever was pressed, both levers were retracted for the remainder of the trial. The delay duration increased between each block of trials (0, 4, 8, 16, 32 s), but remained constant within each block (Cardinal, et al., 2000; Evenden & Ryan, 1996; Simon, Mendez, et al., 2007; Winstanley, Dalley, Theobald, & Robbins, 2003).
2.2.5.1 Delay Discounting Data Analyses
Stability in the delay discounting task was assessed as described for the RDT in Experiment 1. Group comparisons in the delay discounting task were conducted using a two-factor (risk subgroup X delay) repeated measures ANOVA. Comparisons between delay discounting and other task performance were conducted using bivariate correlations.
3. Results
3.1 Experiment 1A
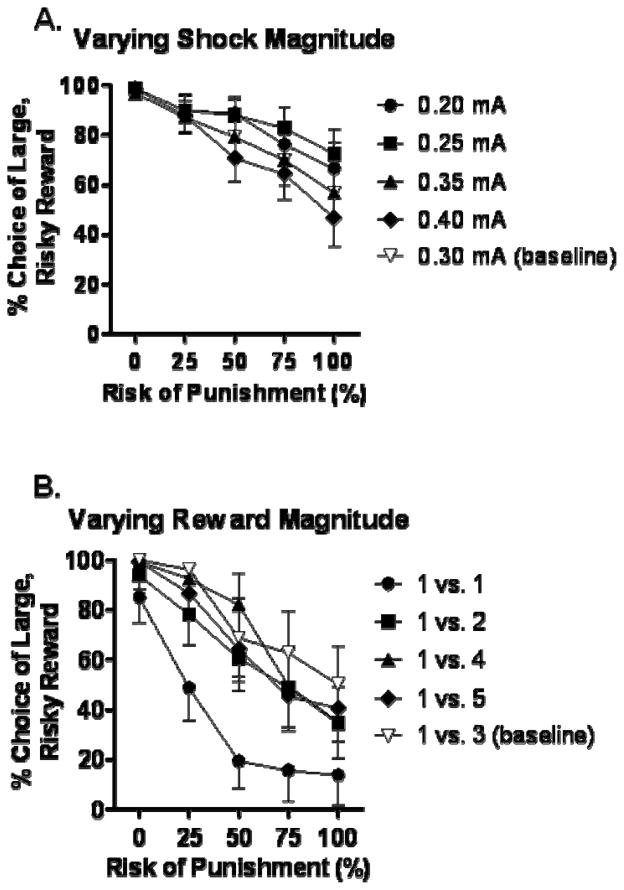
To determine the contribution of the shock to RDT performance, the shock magnitude was varied using the standard RDT reward magnitudes (1 v. 3 food pellets). Comparisons of choice performance at different shock magnitudes using a repeated measures ANOVA revealed a main effect of both trial block [F(4, 60)=11.93, p<0.05] and shock magnitude [F(3, 45)=4.96, p<0.05] on choice of the large, risky reward, as well as a significant interaction between these two factors [F(12, 180)=2.56, p<0.05] (Figure 1A). Post hoc comparisons between pairs of different shock magnitudes revealed that there was no significant difference in choice behavior between 0.2 and 0.25 mA, 0.2 and 0.35 mA, or 0.35 and 0.4 mA [Fs<3.38, ps>.08]. However, comparisons among all other magnitudes revealed significant differences (0.2 vs. 0.4 mA [F(1, 15)=5.79, p<0.05]; 0.25 vs. 0.35 mA [F(1, 15)=7.6, p<0.05]; 0.25 vs. 0.4 mA [F(1,15)=7.78, p<0.05]).
Figure 1.
Effects of variation in RDT parameters on choice behavior. (A) Effects of varying shock magnitude on preference for the large, risky reward. As shock magnitude increased, rats shifted their preference toward the small, safe reward. Stable (baseline) performance in the 5 sessions of standard RDT testing prior to shock magnitude variation is shown for reference (gray triangles). (B) Effects of varying the number of food pellets associated with the risky lever. Only when both levers yielded equivalent rewards (1 pellet) did rats significantly shift their choice to the small, safe lever. For all other conditions (2, 4, and 5 pellets associated with the large, risky lever), rats performed similarly. Stable (baseline) performance in the 5 sessions of standard RDT testing prior to reward magnitude variation is shown for reference (gray triangles). Data represent means +/− SEM.
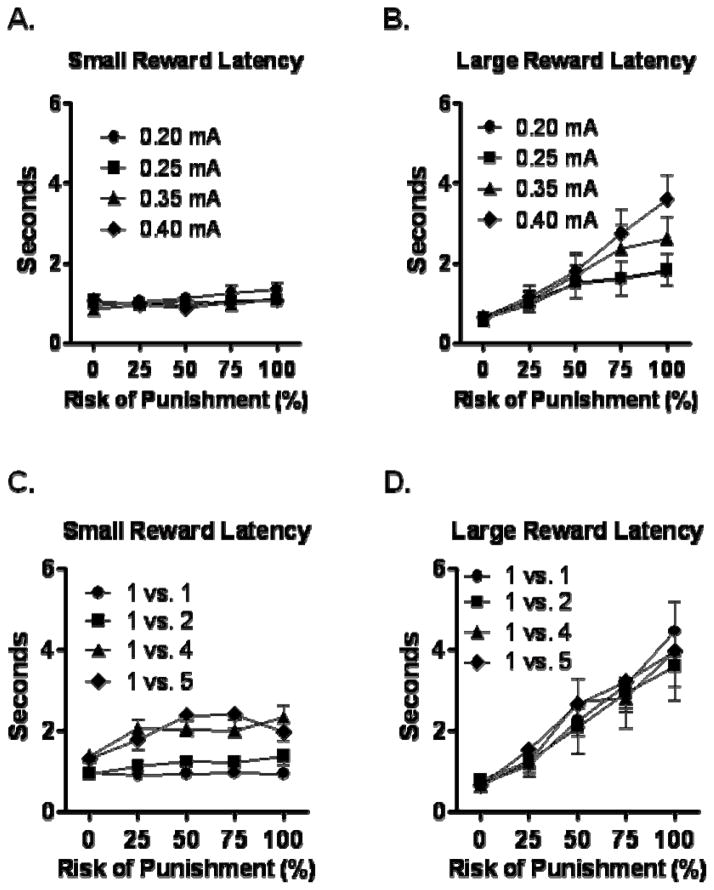
As an additional measure of how shock magnitude contributes to the value of the large, risky reward (i.e., whether greater shock magnitudes reduced the value of this reward), latency to respond at each lever on forced choice trials was compared across shock magnitudes and blocks (Figure 2A & B). A three-factor repeated measures ANOVA (lever (safe vs. risky) X shock magnitude X trial block) revealed significant main effects of both shock magnitude [F(3, 45)=8.23, p<0.05] and block [F(4, 60)=14.07, p<0.05], such that response latencies were longer as both shock magnitude and risk of shock increased. There were also significant interactions between lever and shock magnitude [F(3, 45)=7.96, p<0.05], lever and trial block [F(4, 60)=9.17, p<0.05], shock magnitude and trial block [F(12, 180)=4.36, p<0.05], and among lever, shock magnitude, and trial block [F(12, 180)=4.17, p<0.05]. These interactions indicated that the increase in response latencies across trial blocks was greater on the risky vs. the safe reward lever, and that it was more robust at greater shock magnitudes.
Figure 2.
Response latencies on the small and large reward levers in Experiment 1. (A) and (B) show latencies to press the small, safe and large, risky reward levers during forced choice trials with different shock magnitudes (Experiment 1A). Latencies to press the large, risky reward lever increased as a function of both risk of shock and shock magnitude. (C) and (D) show latencies to press the small, safe and large, risky reward levers during forced choice trials with different large, risky reward magnitudes (Experiment 1B). Latencies to press the large, risky reward lever increased as a function of risk of shock, but were unaffected by large reward magnitude. Data represent means +/− SEM.
3.2 Experiment 1B
To determine the contribution of the reward to RDT performance, rats characterized in the standard RDT (0.3 mA) were shifted to a modified protocol which implemented varying magnitudes of the large reward while the small reward was held constant at 1 food pellet (e.g. 1 vs. 1 pellet, 1 vs. 2 pellets, etc.) as outlined in Methods. For statistical analyses, the mean choice of the risky reward on the final 2 days of testing in each condition was used, as choice performance stabilized at these time points. Comparisons of choice performance at different reward magnitudes using a two-factor repeated measures ANOVA revealed a main effect of trial block [F(4, 28)=21.78, p<0.05], as well as a main effect of reward magnitude [F (3, 21)=7.74, p < 0.05], although no interaction between reward and trial block [F(12, 84)=1.57, p=.12] (Figure 1B). These results suggest that RDT performance is sensitive to the large reward magnitude to some degree; however, the effect of reward magnitude on choice performance was largely carried by the 1 vs. 1 pellet condition, as post hoc comparisons showed significant differences between this and all other magnitude conditions (Fs>9.22, ps<0.05), but no differences among the other magnitudes (Fs<2.31, ps>0.17).
As an additional measure of how reward magnitude contributes to the value of the large, risky (i.e., whether greater reward magnitudes increased the value of this reward), latency to respond at each lever on forced choice trials was compared across reward magnitudes and blocks (Figure 2C & D). A three-factor repeated measures ANOVA (lever (safe vs. risky) X reward magnitude X trial block) revealed a significant effect of trial block [F(4, 28)=27.01, p<0.05], such that response latencies increased with increasing risk of shock, but no main effects of lever [F(1, 7)=3.06, p=0.12] or reward magnitude [F(3, 21)=2.55, p=0.08], and no interactions between lever and reward magnitude [F(3, 21)=2.42, p=0.10], reward and trial block [F(12, 84)=0.62, p=0.82], or lever, reward, and trial block [F(12, 84)=0.53, p=0.89]. The interaction between lever and trial block was significant, however [F(4, 28)=14.44, p<0.05], indicating that response latencies increased with increasing risk of shock to a greater extent on the risky vs. the safe reward lever.
3.3 Experiment 2
The purpose of this experiment was to determine relationships between risky decision making and other cognitive processes. Two aspects of executive function were assessed: cognitive flexibility (the ability to modify behavioral strategies in accordance with changing environmental contingencies or task rules), and working memory (the ability to hold information “in mind” for short time periods). Additionally, because risk taking and impulsivity covary in disorders such as ADHD, addiction, and anorexia, impulsive choice was measured using a delay discounting task. Because the primary focus of this experiment was on relationships between risk taking and other behavioral measures, the RDT data are reported first.
3.3.1 Risky decision making task
Rats were tested in the RDT until reaching stable performance (in 25 sessions). Consistent with previous work in this task (Simon, et al., 2009; Simon, et al., 2011), there was considerable individual variability in performance (Figure 3). Because choice performance in this cohort fell in a strongly bimodal distribution, rats were divided on the basis of mean percent choice of the large, risky reward (averaged across all five blocks of trials) into “risk taking” (greater than 75% mean choice of the large, risky reward, n=9) and “risk averse” (less than 25% mean choice of the large, risky reward, n=7) subgroups. These designations were used for subsequent comparisons between RDT performance and the other tasks. Consistent with previous findings (Simon, et al., 2011), there were no differences between the two subgroups in locomotor activity [t(14)=0.008; p=0.89], body weight [t(14)=0.04, p=0.97], or nosepokes into the food trough [t(14)=2.16; p=0.38] during the inter-trial intervals. The two subgroups also did not differ in shock reactivity (locomotor activity during the shock delivery period; [t(12)=2.24, p=0.64]; note that two rats were excluded from this latter analysis due to the absence of choices of the large, risky lever). For this latter measure, there is no evidence that it reflects “freezing” behavior as is typically observed in fear conditioning tasks. This is likely due to the relatively low shock intensities and the voluntary nature of the shock delivery (i.e., rats can avoid the shock by pressing the small, safe reward lever).
Figure 3.
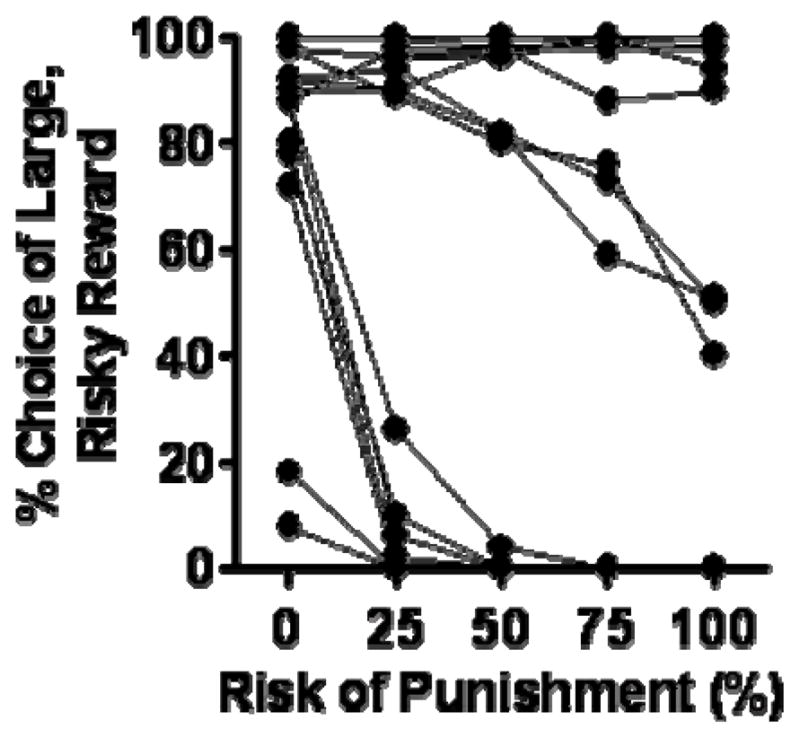
Individual rats’ performance in the RDT in Experiment 2. Each line represents data from an individual rat (averaged across five consecutive sessions of stable performance). Within this cohort there was a bi-modal distribution of performance into risk taking and risk averse subgroups.
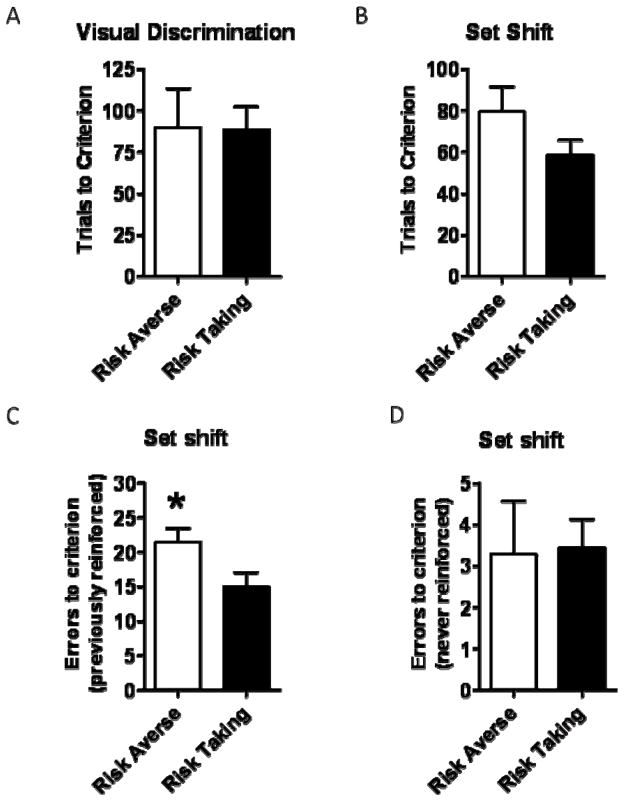
3.3.2 Set shifting
There was no difference in the number of trials to reach criterion on the initial (visual) discrimination between risk averse and risk taking subgroups [t(14)=0.04, p=0.96] (Figure 4A). On the set shift itself, there were more previously reinforced errors in the risk averse compared to the risk taking subgroup [t(14)=2.15, p<0.05] (Figure 4B). This difference also approached significance on the trials to criterion measure [t(14)=1.56, p=0.10] (Figure 4C) but not on the never reinforced errors measure [t(14)=0.12, p=0.31] (Figure 4D).
Figure 4.
Relationships between risk subgroup and set shifting performance. Neither trials to criterion on the initial discrimination (A) nor set shift (B) were significantly different between the subgroups. However, the risk averse subgroup committed significantly more previously-reinforced errors than the risk taking subgroup (C). There was no relationship with never-reinforced errors (D). Data represent means +/− SEM. *p<0.05
3.3.3 Delayed response task
The measure of performance on the delayed response task was the percentage of correct choices across the different delays. A repeated measures ANOVA (delay X risk subgroup) revealed a significant main effect of delay [F(6, 84)=28.37, p<0.05], but no main effect or interaction involving risk subgroup [Fs<0.14, ps>0.71] (Figure 5). A previous study with the same delayed response and set shifting tasks used here found a positive relationship between working memory accuracy at short delays and set shifting performance in young adult Fischer 344 rats (Beas, Setlow, & Bizon, 2013). In the current dataset, however, bivariate correlations between delayed response and set shifting performance revealed no relationships between the two tasks at any delays (rs<0.31, ps>0.23).
Figure 5.
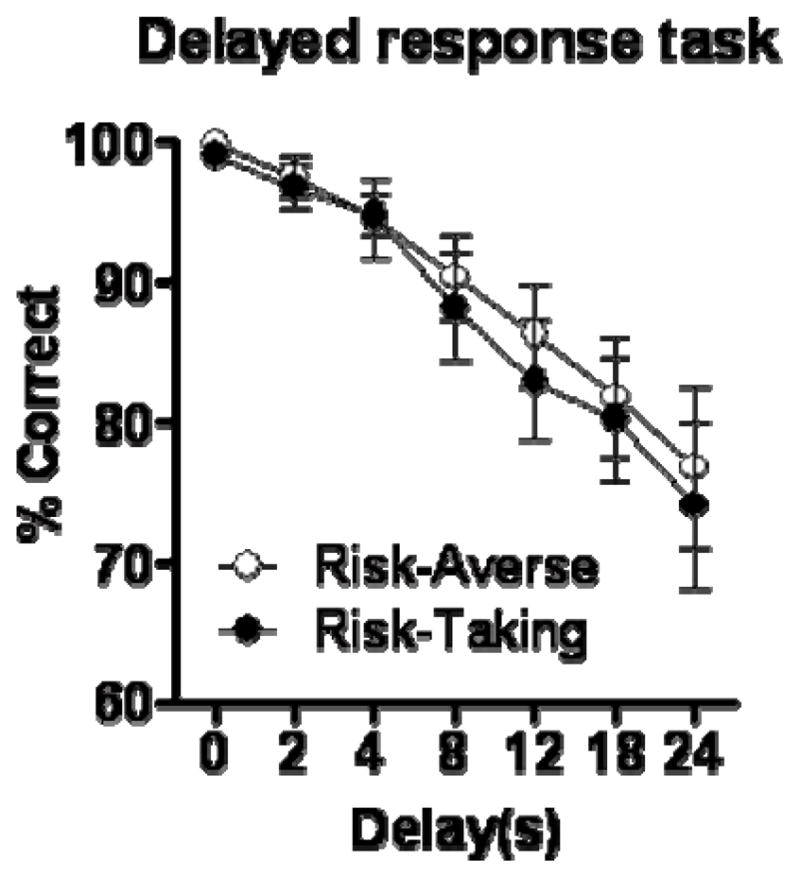
Relationship between risk subgroup and working memory performance. The risk taking and risk averse subgroups did not differ in performance on the delayed response task. Data represent means +/− SEM.
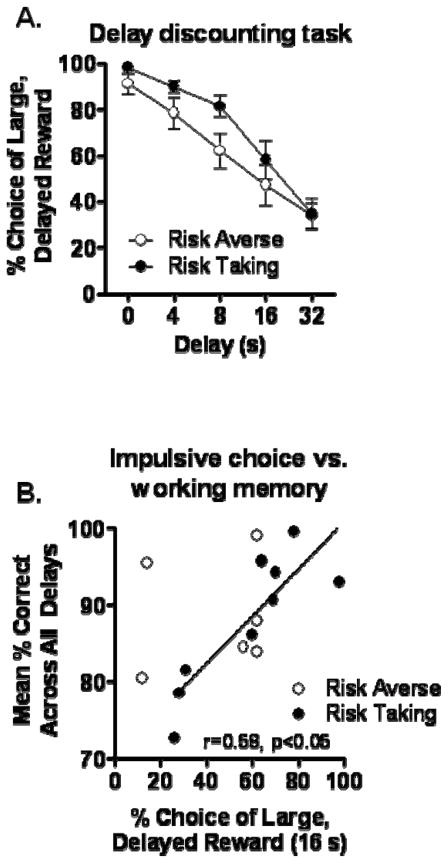
3.3.4 Delay discounting task
The measure of performance on the delay discounting task was the percent choice of the large, delayed reward across the different delays. A two-factor ANOVA (delay block X risk subgroup) conducted on data averaged across the 5 sessions of stable performance revealed a main effect of delay [F(4, 56)=73.31, p<0.05], but neither a main effect nor an interaction involving risk subgroup [Fs<2.06, ps>0.17] (Figure 6A). There were also no relationships between performance on the delay discounting task (at any delay) and the set shifting task (rs<0.29, ps>0.27). Interestingly, however, there was a significant correlation between delay discounting performance and mean percent accuracy in the delayed response task, such that better working memory (mean % accuracy across all delays) was associated with greater choice of the large, delayed reward in the delay discounting task at both 16 s (r=0.58, p<0.05) and 32 s (r=0.50, p<0.05) delays (Figure 6B). Separate correlations conducted between delay discounting and working memory performance in each risk subgroup revealed significant relationships between the two tasks in the risk taking but not the risk averse subgroup (risk taking: 16 s, r=0.74, p<0.05; 32 s, r=0.66, p=0.06: risk averse: 16 s, r=0.04, n.s.; 32 s, r=0.14, n.s.) however, it is difficult to draw strong conclusions from these analyses given the small sizes of the subgroups.
Figure 6.
Relationships among delay discounting, risk subgroup, and working memory. The two risk subgroups did not differ in delay discounting task performance (A). However, delay discounting and working memory performance were significantly related, such that rats with steeper delay discounting (greater impulsive choice) were less accurate in the delayed response task (B). Open and closed circles indicate the rats in the risk taking and risk averse subgroups in the RDT.
4. Discussion
The goal of these experiments was to determine the contributions of affective and cognitive factors to risk taking behavior in a rat model of risky decision making. Experiment 1 varied the magnitude of the shock associated with the large reward in one cohort of rats, and the magnitude of the large reward in another cohort of rats. Varying the shock produced magnitude-dependent shifts in choice of the large reward (i.e., larger shock magnitudes produced less choice of the large reward), consistent with previous findings from a smaller cohort of rats (Simon, et al., 2009). In contrast, choice of the large reward remained stable when its magnitude was varied, with the exception of the condition in which both the “risky” and “safe” levers yielded equivalent outcomes (the 1 vs. 1 pellet condition). In this latter case, rats reliably shifted their preference to the “safe” lever. A similar pattern was observed with the response latency measures, for which varying the shock magnitude altered latencies to press the large reward lever, whereas varying the large reward magnitude had no such effect. Together, these data suggest that choice behavior in the RDT is more strongly influenced by the adverse consequence (the “risk”) associated with the large reward than by the magnitude of the large reward itself (i.e., choice was significantly altered by small shifts in shock magnitude, whereas choice was only altered by shifts in the large reward magnitude when there was no advantage to choosing the risky reward). Consistent with this interpretation, only a single test session was necessary for changes in shock magnitude to produce significant shifts in choice behavior, whereas 5 sessions were required for changes in large reward magnitude to have similar effects (but only when the risky reward was changed to 1 food pellet).
The relative insensitivity of RDT performance to variation in reward magnitude is in agreement with previous findings that altering the value of the food reward via pre-feeding had no effect on choice behavior (Simon, et al., 2009). However, it is also possible that the limited sensitivity to shifts in reward magnitude reflects the fact that the specific reward magnitudes employed in Experiment 1.2 were less salient to the rats than the shock magnitudes employed in Experiment 1.1 (there were also fewer rats in Experiment 1.2, yielding less statistical power to detect differences between reward magnitudes). Although the different modalities of the two stimuli render them difficult to compare directly, it is important to note that rats were not completely insensitive to the value of the risky reward, as rendering it equivalent to the small reward still significantly altered choice behavior. More importantly, unpublished work from our laboratory using a delay discounting task similar to that in Experiment 2 showed that decreasing the magnitude of the large, delayed reward from 4 to 2 pellets produced a robust decrease in choice of this reward (Simon, Busch, Richardson, Walls, & Setlow, 2007). These data indicate that under parameters similar to those used in the risky decision making task, rats are able to detect and respond to a change in reward magnitude that is comparable to those in Experiment 1.2. These data further suggest that it is the specifics of the task contingencies that determine the degree to which rats are sensitive to the affective components of the task. However, it will be important in future work to address this issue with a similar (and wider) range of reward magnitudes across multiple types of decision making tasks.
In Experiment 2, rats were divided into two groups on the basis of their “risk preference” (high or low levels of choice of the large, risky reward) in the RDT. The term “risk” can be used in a number of ways, including economically (e.g., the quantifiable probability of a particular outcome) and more informally (“the possibility that something unpleasant or unwelcome will happen”; Oxford English Dictionary). Although the design of the RDT does incorporate “risk” in the economic sense of the word, the task likely better captures the informal sense of “risk”, in that it involves a possibility of an unpleasant outcome (shock delivery). The combination of both shock and reward in the same context makes it difficult to compute the economic values of the outcomes of the two choices in the same way in which they could be computed if the “risk” involved reward omission, and hence it is not always clear how decision making under different types of risks are related (e.g., Mitchell, et al., 2011; St Onge & Floresco, 2009). Arguably, however, the increased complexity of the RDT models many “real world” decision making conditions, in which the risks cannot be easily quantified on the same scale as the rewards. For example, for an addicted individual, drug use may produce a reliably larger reward than drug non-use (at least in the short term), but each episode of drug use is also accompanied by some probability of adverse consequences (e.g., incarceration or overdose). The expected values of drug use and non-use cannot be readily computed on the same scale, but the relative values of each choice can nevertheless be determined empirically. In this context, “risk averse” rats in the RDT are those for which the possibility of shock (modulated by its magnitude) outweighs the attraction of the large relative to the small reward, whereas “risk taking” rats are those for which the possibility of shock is insufficient to have this effect. Importantly, we showed recently that high levels of risk taking in the RDT are predictive of subsequently greater cocaine self-administration, supporting the validity of this risk taking characterization (Mitchell, et al., 2014).
The goal of Experiment 2 was to determine the relationship between risk taking and two forms of executive function (set shifting and working memory), as well as impulsive choice (preference for immediate over delayed gratification). Risk taking (choice of the large, risky reward in the RDT) was unrelated to working memory performance (% accuracy) in the delayed response task. In contrast, risk taking was positively associated with performance in the set shifting task, with the risk taking subgroup showing better performance (fewer perseverative errors on the set shift) than the risk averse subgroup. This finding is somewhat surprising in light of the pattern of choice behavior in the risk averse subgroup, which showed a greater shift in choice behavior across blocks than the risk taking subgroup (Figure 3), as well as with data showing that greater risk taking in the RDT is associated with lower levels of striatal D2 dopamine receptors, which have been associated with impaired cognitive flexibility under some conditions (Groman et al., 2012; Simon, et al., 2011; Volkow et al., 1998). However, the observed pattern of results is consistent with several other lines of research which have examined relationships between risk taking and cognitive flexibility. A neuropsychological evaluation of British entrepreneurs revealed that these individuals were both more likely to take risks and displayed greater cognitive flexibility compared to matched controls, indicating that these two aspects of cognition are associated in some individuals (Lawrence, Clark, Labuzetta, Sahakian, & Vyakarnum, 2008). A similar association is evident in patients with anorexia nervosa, who display both low levels of risk taking and cognitive inflexibility as evidenced by perseverative errors on set shifting tasks (Danner, et al., 2012; Kaye, et al., 2013; Shott, et al., 2012). Interestingly, the fact that anorexia nervosa is associated with high levels of striatal D2 receptor availability is consistent with the neurobiological profile of rats characterized as risk averse in the RDT (Mitchell, et al., 2014; Simon, et al., 2011), suggesting that this subgroup of rats may be useful for modeling some aspects of this disorder.
Experiment 2 assessed relationships between risk taking and only one form of cognitive flexibility (set shifting); however, cognitive flexibility can take several forms, including reversal learning, which is dependent upon neural circuitry partially distinct from that involved in set shifting (Birrell & Brown, 2000; Dias, et al., 1996; McAlonan & Brown, 2003). Damage to orbitofrontal cortex in rodents and primates (including humans) results in impaired reversal learning and elevated risk taking in laboratory decision making tasks, and chronic administration of drugs of abuse causes a similar pattern of deficits (Bechara, et al., 2001; Dias, et al., 1996; Ghods-Sharifi, Haluk, & Floresco, 2008; Mitchell, et al., 2014; Schoenbaum, Saddoris, Ramus, Shaham, & Setlow, 2004; Stopper, Green, & Floresco, 2014). Direct assessments of relationships between risk taking and reversal learning (as well as the mechanisms of any such relationships) will be an important avenue of future research.
Several studies in human subjects have shown associations between measures of working memory and delay discounting, with better working memory predicting less discounting of delayed rewards (less impulsive choice) in both normal and patient populations (Gunn & Finn, 2013; Huckans et al., 2011; Shamosh et al., 2008). In addition, manipulations that increase working memory load result in increased discounting of delayed rewards (i.e. greater impulsive choice) (Hinson, Jameson, & Whitney, 2003), and cognitive training to improve working memory leads to reduced discounting of delayed rewards (Bickel, Yi, Landes, Hill, & Baxter, 2011). The results of Experiment 2 are consistent with these findings, in that more accurate performance in the delayed response task (better working memory) was associated with less discounting of the delayed reward. Indeed, such a relationship is not unexpected, as the ability to delay gratification (particularly in tasks such as that used here, in which the delays are actually experienced) presumably requires maintenance of a stable internal representation of the delayed reward (Simon, Mendez, et al., 2007). In combination with the finding that greater risk taking is associated with greater cognitive flexibility, these results indicate the presence of a double dissociation between the influence of two distinct aspects of executive function (working memory and cognitive flexibility) on two independent forms of cost-benefit decision making (delay- and risk-based).
Both risk taking and impulsive choice are elevated in addiction and ADHD (Chamorro et al., 2012; DeVito et al., 2008; Drechsler, Rizzo, & Steinhausen, 2010; Ersche, Turton, Pradhan, Bullmore, & Robbins, 2010), whereas both are reduced in anorexia (Kaye, et al., 2013; Steinglass et al., 2012)). Given this covariance, it may be viewed as surprising that risk taking in the RDT was unrelated to impulsive choice in the delay discounting task (although we have observed this lack of relationship in two previous studies; (Mitchell et al., 2012; Simon, et al., 2009)). However, despite their emergence in similar clinical contexts and the similarities in the design of the tasks used to assess them in Experiment 2, there are important differences between risky and impulsive decision making. Most obviously, the “costs” associated with each type of decision making (risk of punishment vs. delay to reward delivery) may be computed and weighted against rewards differently, resulting in orthogonal variation in choice behavior. In support of such differences, available data regarding the neural basis of performance on the two tasks suggest both overlapping and distinct neural mechanisms. For example, although damage to the basolateral nucleus of the amygdala causes an increase in both risky and impulsive decision making (Orsini, Trotta, Bizon, & Setlow, submitted; Winstanley, Theobald, Cardinal, & Robbins, 2004), expression of D2 receptor mRNA in the striatum is inversely related to risky decision making, but appears to be unrelated to impulsive decision making (Loos et al., 2010; Mitchell, et al., 2014; Simon et al., 2013; Simon, et al., 2011). In light of these differences, a likely account for the fact that risky and impulsive decision making covary in neuropsychiatric conditions is that mechanisms common to both are altered (e.g., computations of outcome value). Importantly, there was no evidence that the variation in performance on any of the tasks in Experiment 2 was due to differences in learning ability. For example, the risk taking and risk averse subgroups (which differed dramatically in their RDT performance – see Figure 3) did not differ in the delay discounting task (as well as the delayed response task and visual discrimination performance in the set shifting task). In addition, the similarities in the design of the delay discounting task and RDT suggest that individual differences in RDT performance are not due to differential acquisition of general task contingencies, but instead to task-specific factors (i.e., the influence of risk of shock vs. delays on large reward preference).
The results of these studies shed light on both affective and cognitive factors supporting choice behavior under conditions of risk of adverse consequences. The results of Experiment 1 suggest that (at least under the conditions tested) the adverse consequence (footshock) plays a greater role in driving choice behavior than does the reward associated with the adverse consequence. The results of Experiment 2 suggest that cognitive flexibility plays a role in risk taking, in that greater risk taking was associated with greater flexibility. In combination with ongoing studies of the neural basis of performance in the RDT, these data help to provide a more comprehensive understanding of risk taking behavior, which may ultimately allow better treatments for conditions in which disordered risk taking is prominent.
Highlights.
Rats chose between small, “safe” rewards and large rewards with risks of shock
Choice was more sensitive to shock intensity than reward magnitude
Risk averse rats showed less cognitive flexibility than risk taking rats
Rats with greater impulsive choice showed worse working memory
Risky and impulsive choice are associated with distinct executive functions
Acknowledgments
We thank Dominique Ouimet-Erlacher, Ryan Gilbert, and Bonnie McLaurin for technical assistance. Supported by DA024671 (BS), DA033074 (MRM), and a NSF Graduate Research Fellowship (BSB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging. 2013;34(9):2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25(7):340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Butler Gillian, Mathews Andrew. Anticipatory anxiety and risk perception. Cognitive Therapy and Research. 1987;11(5):551–565. doi: 10.1007/bf01183858. [DOI] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152(4):362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Chamorro J, Bernardi S, Potenza MN, Grant JE, Marsh R, Wang S, Blanco C. Impulsivity in the general population: a national study. J Psychiatr Res. 2012;46(8):994–1001. doi: 10.1016/j.jpsychires.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner UN, Sanders N, Smeets PA, van Meer F, Adan RA, Hoek HW, van Elburg AA. Neuropsychological weaknesses in anorexia nervosa: set-shifting, central coherence, and decision making in currently ill and recovered women. Int J Eat Disord. 2012;45(5):685–694. doi: 10.1002/eat.22007. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Kent L, Ersche KD, Clark L, Salmond CH, Sahakian BJ. The effects of methylphenidate on decision making in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64(7):636–639. doi: 10.1016/j.biopsych.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, Steinhausen HC. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J Neural Transm. 2008;115(2):201–209. doi: 10.1007/s00702-007-0814-5. [DOI] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, Steinhausen HC. Decision making with uncertain reinforcement in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2010;16(2):145–161. doi: 10.1080/09297040903190774. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Bolla K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160(6):1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68(8):770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128(2):161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. [Research Support, Non-U.S. Gov’t] Behavioural brain research. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89(4):567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, Jentsch JD. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32(17):5843–5852. doi: 10.1523/jneurosci.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RL, Finn PR. Impulsivity partially mediates the association between reduced working memory capacity and alcohol problems. Alcohol. 2013;47(1):3–8. doi: 10.1016/j.alcohol.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Hoffman W. Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. J Clin Exp Neuropsychol. 2011;33(2):176–186. doi: 10.1080/13803395.2010.499355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan DM. Addictive personality factors. J Psychol. 1987;121(6):533–538. doi: 10.1080/00223980.1987.9712681. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36(2):110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A, Clark L, Labuzetta JN, Sahakian B, Vyakarnum S. The innovative brain. Nature. 2008;456(7219):168–169. doi: 10.1038/456168a. [DOI] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, van Gaalen MM. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex. 2010;20(5):1064–1070. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146(1–2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology (Berl) 2012;224(4):489–499. doi: 10.1007/s00213-012-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B. Effects of developmental nicotine exposure in rats on decision-making in adulthood. Behav Pharmacol. 2012;23(1):34–42. doi: 10.1097/FBP.0b013e32834eb04a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology (Berl) 2011;218(4):703–712. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology. 2014;39(4):955–962. doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta R, Bizon JL, Setlow B. Adaptive risk-taking in the face of punishment requires the integrity of the basolateral amygdala submitted. [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral neuroscience. 2002;116(1):105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds EK, Schreiber WM, Geisel K, MacPherson L, Ernst M, Lejuez CW. Influence of social stress on risk-taking behavior in adolescents. J Anxiety Disord. 2013;27(3):272–277. doi: 10.1016/j.janxdis.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–339. doi: 10.1016/s0893-133x(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19(7):1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, Gray JR. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shott ME, Filoteo JV, Bhatnagar KA, Peak NJ, Hagman JO, Rockwell R, Frank GK. Cognitive set-shifting in anorexia nervosa. Eur Eat Disord Rev. 2012;20(5):343–349. doi: 10.1002/erv.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37(11):1779–1788. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Busch TN, Richardson AJ, Walls KJ, Setlow B. Neuroscience Meeting Planner. Society for Neuroscience. Online. 2007. Cocaine exposure and impulsive choice: a parametric analys. Program No. 933.934. [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34(10):2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121(3):543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Setlow B. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31(48):17460–17470. doi: 10.1523/jneurosci.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behavioural brain research. 2006;171(1):116–126. doi: 10.1016/j.bbr.2006.03.030. S0166-4328(06)00184-7 [pii] [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stanley Robb. Anxiety and its Disorders—The Nature and Treatment of Anxiety and Panic. In: Barlow David H., editor. Stress and Health. 4. Vol. 18. The Guilford Press; New York: 2002. pp. 704pp. 193–194. 2002. [DOI] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117(4):728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc. 2012;18(4):773–780. doi: 10.1017/s1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB. Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb Cortex. 2014;24(1):154–162. doi: 10.1093/cercor/bhs297. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155(3):344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170(3):320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24(20):4718–4722. doi: 10.1523/jneurosci.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]