Abstract
Aseptic loosening of Total Knee Arthroplasty (TKA) components is the foremost cause of implant failure in the long term. While tibial component loosening is of primary concern, femoral loosening may become a clinical problem due to younger, more active patients seeking TKA, and also high-flexion designs. In this study, we analyzed the fixation for 19 non-revised, postmortem retrieved, femoral components of TKA with time in service ranging from 1–22 years. We found that average total contact fraction for cemented components was 9.5% and had a power law response with years in service. The average initial interdigitation depth was 0.7mm, and the average current interdigitation depth was 0.13mm. Loss of interdigitation was 81%. Over all, minimal fixation seems necessary for long term success of TKA.
Keywords: Total Knee Arthroplasty, fixation, aseptic loosening, cementation, interdigitation depth
INTRODUCTION
Total Knee Arthroplasty (TKA) is an established and reliable procedure that seeks to restore joint mobility and relieve pain associated with rheumatoid or osteoarthritis [1]. A clinically successful TKA has a lifetime of nearly 20 years with in vivo use before revision surgery becomes a necessity due to wear, loosening, and/or migration of implant components [2–5]. However, failure of TKA occurs at rates approximating 10% at 15 years depending on the implant design [2], most commonly caused by long term aseptic loosening of components [2,3,5,6]. Aseptic loosening results from the loss of fixation between the cement and bone in cemented fixation and metal and bone in press-fit fixation. Without bony fixation, the implant can become unstable and migrate. In general, the tibial component loosens 2–3 times more frequently than the femoral component, but femoral component loosening is still a clinical concern, especially in younger populations [7] and with some high-flexion knees [8–11].
Clinically, it is difficult to assess detailed changes in fixation at the bone-implant interface, as analysis is generally limited to assessment of radiographic changes based on plain x-rays. Enbloc postmortem retrievals, obtained from functioning TKAs, could be used to assess the fixation morphology and the changes that occur due to in vivo service. Our group recently assessed the fixation between trabecular bone and cement in tibial components with time in service ranging from 0–20 years [12]. We quantified the amount of bone in contact with the cement layer, as well as the loss of interdigitation, due to in vivo service. The latter measure required quantifying the penetration of trabecular bone into the cement layer at time of death, as well as extrapolating the initial penetration of bone into the cement at the time of surgery. It was possible to estimate the initial penetration of bone using a ‘trace fossil’ approach: similar to formation of trace fossils such a dinosaur footprint, an impression of the trabecular bone structure is preserved in the cured bone cement immediately after implantation. When bone resorbs from this interface, interconnected cavities remain in the cement. For cemented implants, this approach allows for the assessment of initial fixation at the time of surgery and the current fixation due to in vivo service.
The goal of the present study was to assess the morphology of the fixation interfaces for a series of postmortem retrieved femoral components from TKAs. We asked three research questions: 1) What is the amount of fixation between bone and cement, and bone and metal? 2) What is the regional distribution of fixation? 3) Do implants from donors with greater age and longer time in service have less fixation? In contrast to revision retrievals, which may be clinically loose and cannot be removed with the interface intact, the implants analyzed in this study were obtained postmortem, and would likely represent the fixation status of functioning total knee replacements.
METHODS
Procurement and Radiographic Assessment of Loosening
Nineteen fresh-frozen knees with Total Knee Arthroplasties (TKAs) were obtained postmortem from the SUNY Upstate Anatomical Gift Program. There were 14 total donors and 5 had bilateral implants. Sixteen of the femoral components were cemented, two were cementless press-fits, and one was a partially-cemented press-fit design. Donor age, weight, height, time in service and BMI were documented (Table 1). Plain radiographs of the TKA retrievals were reviewed and classified according to standard radiographic techniques of assessment for loosening [13, 14].
Table 1.
Donor information in order of increasing age.
| Donor | Years in Service | Sex | Age* | weight (kg) | height (cm) | BMI (kg/m2) | Implant Type | Radiographic Score§ | Manufacturer | Model | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 10 | M | 54 | 98 | 185 | 28.6 | PS | Well-fixed | Biomet | Maxim | infarct/gangrene |
| 1B | 12.4 | M | 54 | 98 | 185 | 28.6 | CR | Well-fixed | Biomet | Maxim | infarct/gangrene |
| 2 | 5 | M | 61 | 103 | 177 | 32.9 | CR | Well-fixed | Stryker | Triathlon | cardiopulmonary arrest |
| 3A | 6.5 | M | 72 | 100 | 186 | 28.9 | PS | Possibly loose | Zimmer | Nexgen LPS 7° | small cell lung cancer |
| 3B | 4 | M | 72 | 100 | 186 | 28.9 | CR | Well-fixed | Zimmer | Nexgen LPS 7° | small cell lung cancer |
| 4 | 1 | F | 73 | 61 | 164 | 22.7 | CR | Well-fixed | Biomet | Vanguard | cardiopulmonary arrest |
| 5A | 17 | M | 78 | 65 | 188 | 18.4 | CR/Press-fit** | Well-fixed | Howmedica | PCA | Pick’s Disease |
| 5B | 10 | M | 78 | 65 | 188 | 18.4 | PS | Possibly loose | Depuy | AMK | Pick’s Disease |
| 6 | 2.5 | F | 82 | 108 | 172 | 36.5 | PS | Well-fixed | Depuy | PFC Sigma | acute CHF |
| 7 | 22 | F | 85 | 85 | 153 | 36.3 | CR/Press-fit | Well-fixed | Richards | RMC | cardiopulmonary arrest/ARF |
| 8 | 11 | M | 85 | 98 | 174 | 32.4 | CR | Well-fixed | Richards | S&N Genesis II Gender | pneumonia, septic shock |
| 9 | 18 | F | 86 | 78 | 166 | 28.3 | PS | Well-fixed | Zimmer | IB II | COPD/pneumo mia |
| 10A | 3 | F | 87 | 67.5 | 168 | 23.9 | PS | Well-fixed | Zimmer | Nexgen 7° | pulmonary infection |
| 10B | 3 | F | 87 | 67.5 | 168 | 23.9 | PS | Well-fixed | Zimmer | Nexgen 7° | pulmonary infection |
| 11A | 5 | F | 87 | 75 | 160 | 29.3 | CR | Well-fixed | Zimmer | Nexgen Precoat | metastatic ovarian cancer |
| 11B | 13.5 | F | 87 | 75 | 160 | 29.3 | CR/Press-fit | Well-fixed | Zimmer | Pegged Nexgen Porous | metastatic ovarian cancer |
| 12 | 14.5 | F | 88 | 96 | 173 | 32.1 | PS | Well-fixed | J&J | PFC Modular Pegged | cardiopulmonary arrest |
| 13 | 10 | M | 90 | 65.5 | 170 | 22.7 | CR | Well-fixed | Zimmer | Nexgen Precoat | Parkinson’s Disease |
| 14 | 11 | F | 91 | 58 | 162 | 22.1 | CR | Well-fixed | Richard s | S&N Genesis Pegged II | heart attack |
Age at death.
Donor 5A was a partially-cemented press-fit with cementation in the anterior (zones 1, 2, and C) only.
Rated as loose, possibly loose, probably loose, or well-fixed. PS=posterior stabilizing.
CR=cruciate retaining. CHF=congestive failure. ARF=acute renal failure.
Sectioning and Imaging
The femoral component and distal femur were sectioned in the sagittal plane (Figure 1A) using a water-irrigated silicon carbide blade (IsoMet 2000; Buehler Inc, Lake Bluff, IL, USA). The initial cut bisected the intercondylar notch in the sagittal plane, and following cuts were made medial and lateral to the initial midline cut in 10mm intervals. Pulsatile lavage was used to clean debris from the cutting operation out of the trabecular bone and interface after sectioning. The surfaces of the midline-facing sections were then polished to 600-grit using a water-irrigated polisher (EcoMet 6; Buehler Inc). High resolution, white-light images (5.7um/pixel) of the entire implant section were obtained using a CCD (Charge Coupled Device) camera with macro lens attached to a custom x-y stage.
Figure 1.
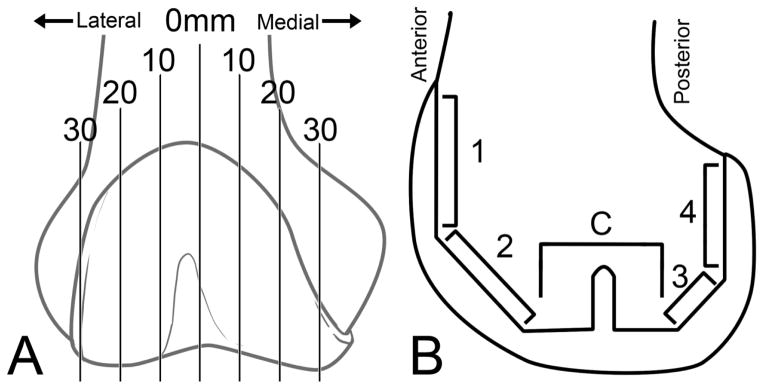
Schematic of sagittal section locations and distance in millimeters from the midline of the knee (A). A modified zone system (B) was used where Zone Central, or “C,” includes zones 5–7 as described by the Knee Society Scoring System [17].
Cement-Bone and Bone-Metal Contact Fraction
For each implant section, five regional zones were established to document interface morphology of the femoral component, based on the Knee Society Scoring System (KSSS) [15]. KSSS Zones 5–7 were grouped together as Zone C and describe the distal, center cut region, with or without pegs (Figure 1B).
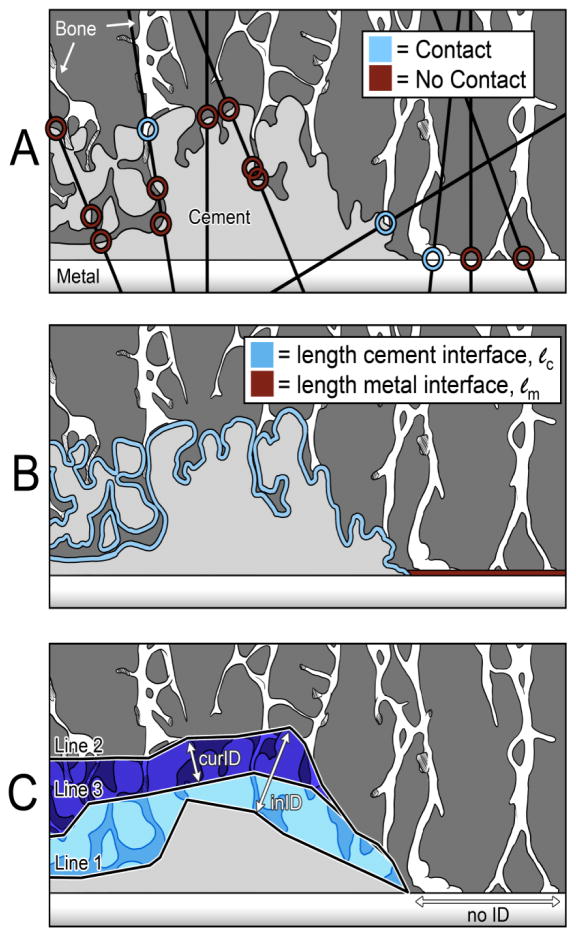
Random line stereology was used to determine the contact fraction between bone and cement (cement-bone contact fraction, CFc-b) and bone and metal (bone-metal contact fraction, CFb-m) where there was no cement layer at the metal surface. One hundred lines were projected in random orientation over each zone (Image-Pro Plus, Media Cybernetics, Rockville, MD, USA). The CFc-b was calculated as the number of points where there was cement in direct contact with bone divided by the total number of points where lines crossed the cement interface (Figure 2A). Similarly, the CFb-m was calculated as the total number points where bone was in direct contact with metal divided by the total number of points where lines crossed the metal interface. To allow inter-specimen comparison, the CFc-b was normalized to the undulating length of the cement interface for each region of interest (ROI), and CFb-m was normalized to the flat length of any cementless, interior, bare metal for each ROI (Figure 2B). Together, these measurements were normalized the entire length of cement+bare metal interface for each ROI, to give a measurement of Total Contact Fraction, or CFT. This was done to account for the fact that not all ROIs were the same length and had different local morphologies. See Appendix for more details.
Figure 2. Contact Fraction and Interdigitation Depth measurements.
On the left side of each image is the cement-bone interface, where cement is present. On the right side of each image is the bone-metal interface, where there is no cement and bone may directly contact metal. A demonstration of random line stereology is shown (A). Lines of random orientation overlay the image, and may contact cement only, cement in contact with bone, metal only, or metal in contact with bone. Lines may also cross circuitous borders of cement multiple times, and each intersection is recorded as a separate data point. One hundred lines were counted for the cement-bone and bone-metal interfaces of each zone, separately (i.e., 200 lines total for each zone). The length of the cement-bone interface was measured as the circuitous length of the cement, and the length of bare metal in each zone (B). Initial (inID) and current interdigitation depth (curID) was determined using average distance between Line 1 and Line 2, and Line 2 and Line 3, respectively, using a local minimum point-to-point measurement algorithm in Image-Pro Plus (C).
Cement coverage was defined as the fraction of the metal surface that was covered with cement for each implant. The Fraction of Fixation provided by cement-bone contact (FFc-b) as opposed to bone-metal contact was determined using a length-weighted normalization procedure (see Appendix). The Fraction of Fixation provided by the bone-metal interface (FFb-m) was 1-FFc-b.
Cement-Bone Interdigitation Depth
The initial interdigitation depth (inID) was used to estimate the initial extent of interlock between cement and bone at the completion of surgery [12]. A line was drawn at the furthest initial extent of bone into cement, as documented by the cement mold (Line 1, Figure 2C). Another line was drawn at the extent of cement that penetrated into trabecular bone (Line 2). The inID was calculated as the average distance between these lines using a local minimum point-to-point measurement algorithm (Image-Pro Plus). The current interdigitation depth (curID) was used to define the current amount of interlock between cement and bone at the time of death. A third line (Line 3) was drawn at the current extent of trabecular bone into the cement layer. The curID was calculated as the average distance between Line 2 and Line 3 along the interface (Figure 2C). The percent difference between inID and curID (inID-curID/inID) defined the loss of interdigitation depth (lossID) and represented an estimate of how much trabecular bone was resorbed during in vivo service. The estimated error in determining the inID has been reported to be 0.06mm [12] using lab-prepared constructs where there was no bony resorption.
Statistical Methods
For research question #1, descriptive statistics were used to characterize the quantity of fixation at the cement-bone and bone-metal interfaces for the 19 postmortem retrieved TKA femoral components. For research question #2, one-way Analysis of Variance (ANOVA) was used with contact fraction and interdigitation depth as dependent variables and medial-lateral section and anterior-posterior zone as independent variables. Tukey-Kramer post hoc tests were used to compare means between groups. For research question #3, a two-parameter linear regression model with total contact fraction as the dependent variable, and donor age and years in service as independent variables was used. All statistical analyses were conducted using JMP 9.0 (SAS Institute, Cary, NC, USA).
RESULTS
Femoral Component Fixation with In vivo Service: Contact Fraction
The contact fraction between cement and bone (CFc-b) was used as one of the primary outcome measures of implant fixation for the cemented femoral components (n=16). We found that CFc-b averaged 9.5% (sd=4.1) and ranged from 1.8 to 17.1% (Table 2). All these implants showed evidence of resorption at the cement-bone interface; examples of high and low CFc-b can be seen in Figure 3A and 3B, respectively. In Figure 3B, the bone resorption and remaining cavities (trace fossils) in the PMMA were particularly evident. The press-fit component that was cemented in place had a cement-bone contact fraction (12%) that was similar to the cemented implants. Since CFc-b required the presence of cement, the two cementless press-fits were not included.
Table 2.
Descriptive statistics for outcome measures of implant fixation for the femoral component of TKA. Mean, standard deviation (SD) and range are show for the cemented implants (n=16), the partially cemented-press fit component (n=1), and the press fit components without cement (n=2).
| Cemented (n=16) | Cemented-Press Fit (n=1) | Press Fit (n=2) | ||||
|---|---|---|---|---|---|---|
| Outcome Measure | Mean | SD | Range | Mean | Mean | Range |
| Cement-bone contact fraction, CFc-b (%) | 9.5 | 4.1 | 1.8–17.9 | 12.02 | ||
| Bone-metal contact fraction, CFb-m (%) | 18.0 | 19.6 | 0–73 | 7.56 | 6.5 | 2.2–10.9 |
| Total contact fraction, CFT (%) | 10.3 | 4.6 | 1.7–17.7 | 10.65 | 6.5 | 2.2–10.9 |
| Cement coverage (%) | 87.6 | 6.69 | 68–96 | 66.4 | ||
| Cement-Bone fixation fraction, FFc-b (%) | 86 | 14 | 56–100 | 78 | ||
| Bone-metal fixation fraction, FFb-m (%) | 14 | 14 | 0–44 | 22 | ||
| Initial interdigitation depth, inID (mm) | 0.70 | 0.15 | 0.4–0.92 | 0.42 | ||
| Current interdigitation depth, curID (mm) | 0.13 | 0.08 | 0.03–0.31 | 0.06 | ||
| Loss of interdigitation depth, lossID (%) | 80.8 | 12.2 | 54.5–94.7 | 85.7 | ||
Figure 3. Contact Fraction.
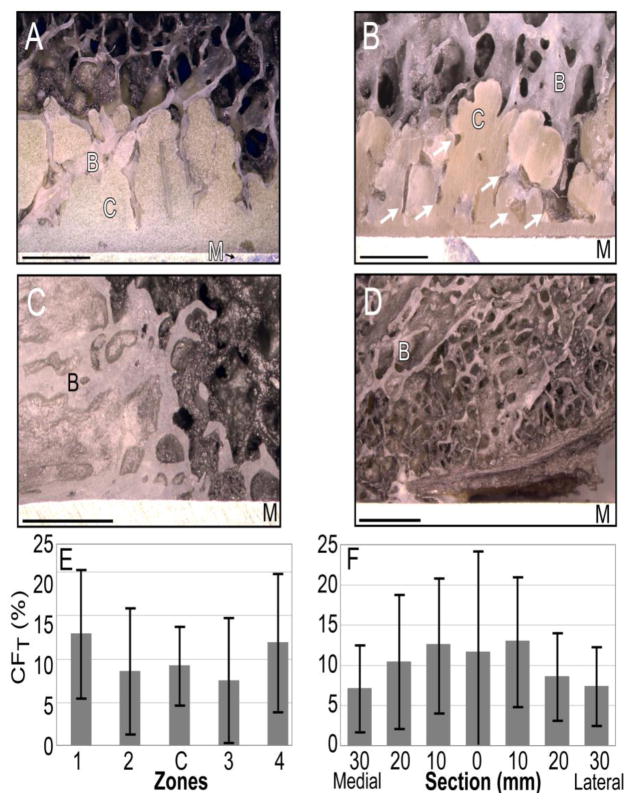
Donor 3A shows well-interdigitated bone in contact with the cement (A). Donor 5A (B) shows evidence of trabecular resorption by the presence of empty spaces in the cement that were once filled with bone (white arrows), demonstrating the trace fossil concept. Bone is in direct apposition with metal in (C), for Donor 10A. In this region for Donor 5A (D), there is no cement, and bone has either remodeled away from the implant or was never in contact with it. B=bone, C=cement, M=metal. Bars represent 2mm. Total contact fraction (CFT) by zone (E) and by section (F) shows there was no significant pattern of the spatial distribution of contact fraction (n=19, p>0.05).
In multiple samples, we observed a paucity or even complete absence of cement at the implant surface in the proximal regions of Zone 1 and Zone 4. There was also limited cement coverage in the posterior-stabilized implants surrounding the central box. The total cement coverage of the femoral component implant surface was on average 88%, with a range of 68 to 98% (Table 2). The large range in cement coverage suggests that the cement techniques used were not all successful in achieving ideal 100% cement coverage.
Despite the lack of full cement coverage in many specimens, we observed that bone sometimes remodeled into direct apposition with the metal component (Figure 3C). Regions of the implant surface lacking cement coverage could also have a gap between the implant and bone (Figure 3D) suggesting that the bone either 1) remodeled away from the metal surface, or 2) bone was never in apposition with the metal surface. The bone-metal contact fraction (CFb-m) was used to quantify the amount of the exposed metal surface in contact with bone. The average CFb-m for the cemented implants was 18% (range: 0–73%), which was comparable with the CFb-m for the cemented press-fit component (7.6%), and the two press-fit components (6.5%) (Table 2).
In order to combine bony fixation for both cement-bone and metal-bone interfaces, a length-weighted approach was used to create a total contact fraction measure (CFT). The average CFT for cemented components (n=16) was 10.3% (sd=4.6) with a range of 1.7 to 17.7%. The CFT for the single cemented press-fit (10.7%) and the two press-fit (2.2 to 10.9%) components fell within the range of the cemented components. The radiograph rated as possibly loose (Table 1) did not coincide with very low CFT, though without post-operative or pre-mortem radiographs for comparison, progressive radiolucency was not possible to identify.
To determine the portion of CFT provided by either cement-bone or bone-metal fixation, a fraction of fixation outcome measure was used. For example, if the CFc-b was 10% over a cement length of 50mm, but the CFb-m was 50% across a length of 10mm, the fraction of fixation (FF) provided by each is 0.5 (see Appendix). For the cemented specimens, the fraction of fixation provided by the cement-bone interface (FFc-b) was 86% (sd=14%), ranging from 56 to 100%, while the fraction of fixation provided by bone-metal contact (FFb-m) was 14% (sd=14%), ranging from 0 to 44% (Table 2). Again, the cemented press-fit (n=1) was comparable to the cemented components (Table 2). The press-fit components (n=2) by default could only have bone-metal fixation. These findings show that most of the cemented component fixation was provided by the cement-bone interface, but a sizeable fraction was provided by the bone-metal interface in some cases.
Femoral Component Fixation with In Vivo Service: Interdigitation Depth
The initial interdigitation depth (inID) of cement into the cut trabecular bone surface provides a direct measure of the PMMA cement infiltration achieved during surgical cementation. The current interdigitation depth (curID) is a measure of the current interlock between trabeculae and the cement layer after bony resorption that occurs with in vivo service. The average inID for the 16 cemented implants was 0.7mm (sd=0.15mm), ranging from 0.4–0.92mm, and the average current interdigitation depth (curID) was 0.13mm (sd=0.08mm), ranging from 0.03–0.31mm (Table 2). The average loss of interdigitation depth (lossID) was 80.9%, ranging from 54.5–94.7% (sd=12.2%). The cemented press-fit component had inID (0.42 mm) and curID (0.06mm) that were near the lower range of the cemented components (Table 2). Because cement is a requirement to estimate initial interdigitation depth, cementless press-fits could not be analyzed using this method.
Regional Distribution of Fixation
We found no consistent trends relating to spatial pattern of fixation when all samples were considered as a single group, or when specimens were grouped according to type (i.e., cemented or press-fit; posterior-stabilized versus cruciate retaining). Each implant had a different, unique distribution of contact fraction. Because contact fraction magnitudes for the cemented and press-fit TKAs were in the same range, they were grouped together for analysis of CFT based on zone and section. Using one-way ANOVA, CFT did not depend on zone (p=0.10) (Figure 3E) or section (p=0.26) (Figure 3F).
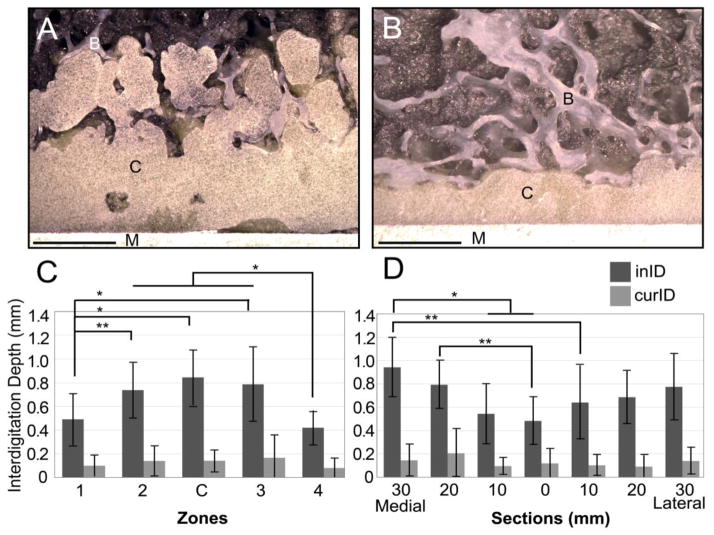
In contrast to CFT, the initial interdigitation depth (inID) was dependent on the zone and section. For example, there was a thicker cement layer and more evidence of cement pressurization into trabecular bone in Zone C (Figure 4A) compared to Zone 4 (Figure 4B) in the same specimen. InID was significantly (p<0.05) different based on zone as tested using a one-way ANOVA (Figure 4C). The anterior and posterior zones of the implant, which were parallel to the longitudinal axis of the femur (Zones 1 and 4), had significantly (p<0.03) less inID compared to the central zones (Zones 2, C and 3).
Figure 4. Interdigitation Depth.
Example of large initial interdigitation depth from Zone C (A) and limited initial interdigitation in Zone 4 (B) of the 20mm medial (20M) section for Donor 3A. A 2mm scale is shown. Initial Interdigitation Depth (inID) and current interdigitation depth (curID) are shown as a function of Zone (C) and Section (D). Significance levels indicated (*=p<0.005, **=p<0.05).
InID was also significantly (p<0.04) different based on section (Figure 4D). Overall, there was a trend for lower inID for sections near the mid-line (0 M/L). The most medial slices (20M and 30M) had greater inID compared to the central section. In contrast, curID was not different between zones (p=0.277) or sections (p=0.142).
Age and Years in Service
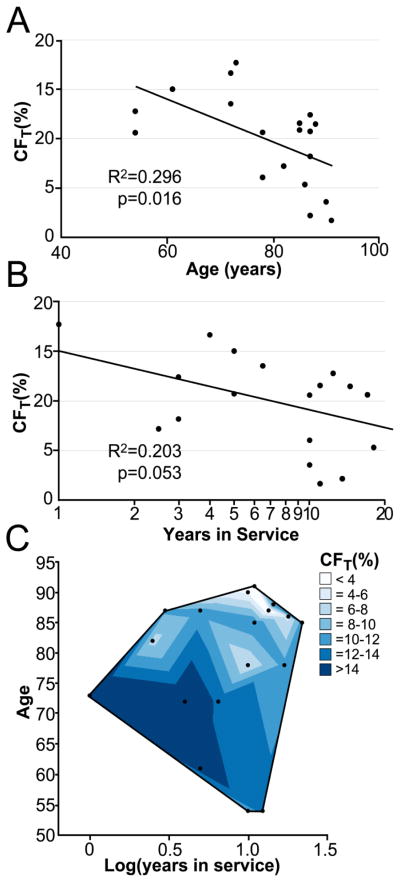
Since the process of aseptic loosening may progress with loss of bone mass with aging and longer time in service, we asked how increasing patient age and years in service was related to fixation. Grouping all 19 implants, we found that CFT decreased linearly with greater donor age (r2=0.30, p=0.016) (Figure 5A). Relating CFT to years in service was more complex: CFT decreased with greater years in service (r2=0.20, p=0.053) (Figure 5B), but without accounting for donor age, this was not statistically significant. Using a two-parameter regression model (r2=0.46, p=0.007) (Figure 5C), both age (p=0.0146) and years in service (p=0.044) were significant parameters in the estimate of CFT. Younger donors and those with fewer years in service had the greatest amount of fixation as measured using contact fraction.
Figure 5. Fixation as a function of age and years in service.
Total Contact Fraction declines with increasing age (A), as well as with increasing years in service (B, log scale). When a two parameter linear regression in performed (C), the R2 value increases to 0.457, page=0.0146 and plog(years)=0.0444.
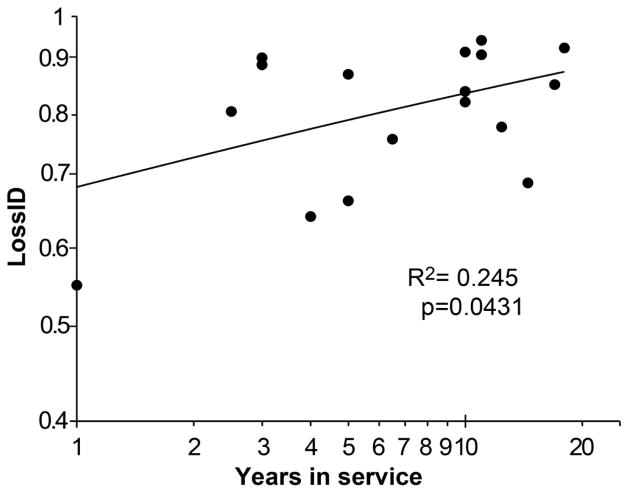
The loss of interdigitation depth (lossID) increased with greater years in service (r2=0.25, p=0.043), and had a power law response. The lossID mainly occurred in the short term and then leveled off after about five years at 80% (sd=12%) (Figure 6).
Figure 6. Loss of Interdigitation Depth.
ID is lost early after implantation. Both axes are presented on a log scale.
DISCUSSION
The goal of this study was to examine the quantity and distribution of fixation between implant components and donor bone in a series of functioning TKA femoral components that had experienced in vivo service. While traditional TKAs rarely exhibit femoral component loosening, some groups have raised concerns about loosening with younger, more active patients undergoing this procedure [3,4,7], as well as with high-flexion knees [8–11]. The disparate findings of the high-flexion knee studies suggest that component design, initial fixation at the time of surgery, and loss of fixation with in vivo service are the likely contributors to the range of loosening rates reported clinically. While this study does not examine fixation of these supposed higher risk populations, ours is the first we know of to directly quantify the fixation status between implant and bone in femoral components using postmortem retrieved TKAs.
This study had several limitations. First, we did not have access to medical records of the deceased. Knowledge of donor mobility, mechanical loading of the joint, and satisfaction with the TKA could add a functional meaning to our measurements of fixation. How these patient factors might affect fixation status could best be answered in a prospective cohort study with patient-reported outcomes [16], followed by access to the joint replacement at the time of death.
A second potential limitation in this study is that the 19 TKAs came from the SUNY Upstate Anatomical Gift Program, and this may not represent the entire population of TKA recipients. However, we found our 19 donors to be comparable to Kaiser Permanente Total Joint Replacement Registry demographics for donor age and sex [6].
A third limitation in this study is that different implant designs were used, and that surgical and cementation techniques, and rehabilitation procedures were not documented. It is possible that the aforementioned factors may also impact the quantity and distribution of fixation. However, we found that despite all the differences, the contact fractions and interdigitation depths were not very dissimilar across the series of specimens.
Amount of Implant-Bone Contact
There appears to be no comparable data in the literature for femoral component fixation, but the CFc-b for post-mortem retrieved cemented tibial components averaged 10.2% [12] (Table 3). A difference between tibial and femoral component fixation was the amount of fibrous tissue found at the interface between cement and bone. Very little fibrous tissue was observed at the femoral component cement-bone interface. In contrast, there was often an organized fibrous tissue interposed between bone and cement beneath the tibial tray, especially at the periphery of the component [12]. Because fibrous tissue tends to form and organize in regions where there is substantial micro-motion [17], the lack of fibrous tissue for the femoral components suggests that they are not subject to substantial micro-motion.
Table 3.
Descriptive statistics for outcome measures between the tibial study performed by Miller et al, and the present femoral study, comparing only cemented components.
| Miller et al (n=12) | Current Study (n=16) | |||
|---|---|---|---|---|
| Parameter | mean (sd) | range | mean (sd) | range |
| Time in service (years) | 8.7 (6.5) | 1–20 | 9.4 (5.9) | 1–20 |
| Age (years) | 76.8 (9.2) | 61–90 | 70.2 (11.4) | 54–91 |
| CFc-b (%) | 10.2 (7.6) | 4.2–32.2 | 9.5 (4.1) | 1.8–17.9 |
| inID (mm) | 1.45 (0.68) | 0.63–3.0 | 0.70 (0.15) | 0.4–0.92 |
| curID* (mm) | 0.63 (0.54) | 0.23–2.1 | 0.13 (0.08) | 0.03–0.31 |
| lossID (%) | 56.2 (22.9) | 0.24–0.86 | 80.8 (12.2) | 54.5–94.7 |
called curCF in Miller et al 2014.
Regarding cement coverage of implant interior metal surfaces, we found an average of 88% coverage, and even in regions without cement, there was occasional bone in contact with the metal femoral component. The magnitude of the functional stability provided by the bone-metal contact was not addressed in this study. While there was a trend for specimens with less cement coverage to have more Total Contact Fraction (CFT), poor cement coverage has long been associated with loosening [18]. Radiolucency of the posterior region—the most difficult area to adequately cement [19, 20]—is theorized to be the first indicator of progressive radiolucency [18], which is associated with aseptic loosening.
Regarding the amount of cement interdigitation created and maintained for femoral TKA with in vivo service, we found a stark difference in the initial interdigitation depth (inID) and current interdigitation depth (curID) compared to those reported for retrieval tibial components [12] (Table 3). These differences may be largely attributed to the shape and surgical approach used with the components; bone cement is more easily pressurized into trabecular bone of the flat and exposed tibial plateau compared to the multiple cuts necessary for the distal femur. This can result in poor cement coverage of the posterior cuts and shallow cement interdigitation for both the anterior and posterior cuts.
Pattern of Fixation
Concerning our second research question, we found no discernable spatial pattern of contact fraction in these specimens, perhaps due to the different implant designs, initial cement distribution and amount of interlock, as well as the surgical technique. The regional distribution of inID is likely due to the application of the component, where sliding displacements during implantation in Zones 1 and 4 will not pressurize cement into bone, whereas compression in the distal aspect will force cement into the bone surface.
Multiple laboratory studies have noted the lack of cement and cement penetration into Zones 1 and 4 [19, 20], though our study is the first we know of to quantify TKA femoral component cementation depth established in an operating room setting. A depth of cement penetration of 2mm or greater is preferred for long-term fixation, as this depth is likely to interlock with at least one transverse trabeculae [21]. Vaninbroukx et al [20] compared four cementation techniques and found application of cement to the anterior and distal surfaces of the bone, with additional application of cement on the posterior surfaces of the implant, to be the most successful in terms of cement penetration. LaButti et al [19] showed the benefit of pressure-injecting cement to achieve the goal of >1.5mm of penetration in Zone 4. It was noted that without proper cementation, the normally compressive forces acting on the posterior condyles during flexion instead become shear forces, which could contribute to loosening.
For Zone 1 beneath the anterior flange, high shear stresses are also a concern, particularly in high-flexion designs [8]. Van de Groes et al [22] recommended removal of the anterior periosteum, roughening of the cortex and drilling of holes into the cortex prior to cementation in order to improve the mechanical strength of the cement-bone interface. A follow up to this study by the same group showed this reduced interface failure from 31.3% to 2.6% in a FE model of high flexion knees [10]. In a traditional (non high-flexion) implant design, Shi et al [23] demonstrated a stress concentration immediately adjacent and proximal to the anterior flange that could initiate fracture as loads rapidly transfer to the flange. Therefore it is likely that improved anterior and posterior fixation would reduce the risk of peri-implant fracture as well (regardless of traditional vs. high-flexion design), considering that improved fixation would result in more peripheral load transfer and retention of bone stock.
Years in Service and Age
Regarding our third research question, we found that increased donor age and a greater number of years in service were associated with reduced fixation of the femoral components. There is a reduction in bone mineral density (BMD) with aging [24], and there is a loss of distal femoral BMD with in vivo service for patients with TKAs [25–27]. Both of these factors likely reduce the amount of trabecular bone available for fixation at the interface.
Loss of fixation may be of concern, but may also represent adaptation as load transfer is accomplished proximally through the stiff, metal implant instead of through the bone in the distal condylar regions (i.e., Zones 2 and C). Finite element remodeling simulations [23, 28] have been used to explain the loss of distal femur BMD seen after TKR implantation. The addition of a well-bonded femoral prosthesis to the distal femur resulted in over 50% loss of BMD in areas analogous to Zone 2 and Zone C within two years of implantation in the simulations. These results coincide with where we often observed extensive bone resorption, as evidenced by lack of trabecular bone in Zones 2, C, and 3. Overall, it would appear that greater age and more time in service would therefore seem to increase risk of loosening as the amount of fixation decreases, both by loss of contact between bone and the implant and loss of trabecular supporting bone likely due to stress shielding.
In conclusion, our findings indicate the fixation of the femoral component of TKA with in vivo service is relatively scant, but is likely sufficient for long term stability and function in most patients. Improving the interdigitation of cement in the anterior and posterior zones of the femoral component could improve load transfer along the anterior and posterior flanges which may help decrease the risk of peri-implant fracture of the distal femur and decrease the risk of loosening for more active/high-flexion implant recipients. More work is needed to determine if the loss of fixation with in vivo service documented here results in increased risk of mechanical loosening of the TKA femoral component.
Supplementary Material
Acknowledgments
The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR42017. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge the assistance of Dan Jaeger for providing the postmortem retrievals and tissue from the SUNY Upstate Anatomical Gift Program.
Appendix
To calculate a contact fraction based on zone, sagittal section, and for each donor implant in total, an interface length normalization procedure was used. The length of the cement interface (lc) was defined as the length of the circuitous contour of the cement; the length of the metal interface (lm) was defined as any flat, metal region lacking cement in the interior aspect of the implant (Figure 2B). These lengths were determined using ImageJ (U. S. National Institutes of Health, Bethesda, Maryland, USA) analysis of the high resolution interface images. The contact fraction of each zone/section was multiplied by the length of the interface for the zone/section. These length-weighted values were then summed and divided by the sum of the lengths of zone/section.
For example, to determine the cement-bone contact fraction for Zone 1 ( ):
| (Eqn 1) |
where summation (Σ) would occur over seven possible sections. This procedure was performed for all zones and all sections for the cement-bone interface and bone-metal interface.
To determine the total contact fraction for the implant (CFT) which incorporated cement-bone and bone-metal components, we used:
| (Eqn 2) |
The fixation fraction provided by the cement-bone interface (FFc-b) was used to describe the total amount of fixation provided by cement-bone contact and was calculated using:
| (Eqn 3) |
The remaining fixation fraction was provided by the bone-metal interface (FFb-m) and was calculated using:
| (Eqn 4) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callahan CM, Drake BG, Heck DA, Dittus RS. Patient Outcomes Following Tricompartmental Total Knee Replacement. A Meta-Analysis. JAMA: The Journal of the American Medical Association. 1994 May 4;271(17):1349–1357. [PubMed] [Google Scholar]
- 2.Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A Randomised Controlled Trial of Cemented versus Cementless Press-Fit Condylar Total Knee Replacement: 15-Year Survival Analysis. The Journal of Bone and Joint Surgery British Volume. 2007 Dec;89(12):1608–1614. doi: 10.1302/0301-620X.89B12.19363. [DOI] [PubMed] [Google Scholar]
- 3.Cristofolini L, Affatato S, Erani P, Leardini W, Tigani D, Viceconti M. Long-Term Implant-Bone Fixation of the Femoral Component in Total Knee Replacement. Proceedings of the Institution of Mechanical Engineers Part H, Journal of Engineering in Medicine. 2008 Apr;222(3):319–331. doi: 10.1243/09544119JEIM328. [DOI] [PubMed] [Google Scholar]
- 4.Henricson Anders. Total Knee Arthroplasty: Aspects on Improved Fixation in the Younger Patient. 2008 http://umu.diva-portal.org/smash/get/diva2:141692/FULLTEXT01.
- 5.Sundfeldt Mikael, Carlsson Lars V, Johansson Carina B, Thomsen Peter, Gretzer Christina. Aseptic Loosening, Not Only a Question of Wear: A Review of Different Theories. Acta Orthopaedica. 2006 Apr;77(2):177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 6.Paxton Elizabeth W, Furnes Ove, Namba Robert S, Inacio Maria CS, Fenstad Anne M, Havelin Leif I. Comparison of the Norwegian Knee Arthroplasty Register and a United States Arthroplasty Registry. The Journal of Bone & Joint Surgery. 2011 Dec 21;93(Supplement_3):20–30. doi: 10.2106/JBJS.K.01045. [DOI] [PubMed] [Google Scholar]
- 7.Bozic Kevin J, Kurtz Steven M, Lau Edmund, Ong Kevin, Chiu Vanessa, Vail Thomas P, Rubash Harry E, Berry Daniel J. The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clinical Orthopaedics and Related Research. 2010 Jan;468(1):45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zelle Jorrit, Janssen Dennis, Van Eijden Jolanda, De Waal Malefijt Maarten, Verdonschot Nico. Does High-Flexion Total Knee Arthroplasty Promote Early Loosening of the Femoral Component? Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2011 Jul;29(7):976–983. doi: 10.1002/jor.21363. [DOI] [PubMed] [Google Scholar]
- 9.Han HS, Kang S-B, Yoon KS. High Incidence of Loosening of the Femoral Component in Legacy Posterior Stabilised-Flex Total Knee Replacement. The Journal of Bone and Joint Surgery British Volume. 2007 Nov;89(11):1457–1461. doi: 10.1302/0301-620X.89B11.19840. [DOI] [PubMed] [Google Scholar]
- 10.Van de Groes S, de Waal-Malefijt M, Verdonschot N. Probability of Mechanical Loosening of the Femoral Component in High Flexion Total Knee Arthroplasty Can Be Reduced by Rather Simple Surgical Techniques. The Knee. 2013 May 31; doi: 10.1016/j.knee.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwenhuijse Marc J, van der Voort Paul, Kaptein Bart L, van der Linden-van der Zwaag HMJ, Valstar Edward R, Nelissen Rob GHH. Fixation of High-Flexion Total Knee Prostheses: Five-Year Follow-up Results of a Four-Arm Randomized Controlled Clinical and Roentgen Stereophotogrammetric Analysis Study. The Journal of Bone and Joint Surgery American Volume. 2013 Oct 2;95(19):e1411–1411. doi: 10.2106/JBJS.L.01523. [DOI] [PubMed] [Google Scholar]
- 12.Miller Mark A, Goodheart Jacklyn R, Izant Timothy H, Rimnac Clare M, Cleary Richard J, Mann Kenneth A. Loss of Cement-Bone Interlock in Retrieved Tibial Components from Total Knee Arthroplasties. Clinical Orthopaedics and Related Research. 2014 Jan;472(1):304–313. doi: 10.1007/s11999-013-3248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx A, Saxler G, Landgraeber S, Loer F, Holland-Letz T, von Knoch M. Comparison of subtraction arthrography, radionuclide arthrography and conventional plain radiography to assess loosening of total knee arthroplasty. Biomed Tech (Berl) 2005;50(5):143–147. doi: 10.1515/BMT.2005.021. [DOI] [PubMed] [Google Scholar]
- 14.Math KR, Zaidi SF, Petchprapa C, Harwin SF. Imaging of total knee arthroplasty. Semin Musculoskelet Radiol. 2006;10(1):47–63. doi: 10.1055/s-2006-934216. [DOI] [PubMed] [Google Scholar]
- 15.Ewald FC. The Knee Society Total Knee Arthroplasty Roentgenographic Evaluation and Scoring System. Clinical Orthopaedics and Related Research. 1989 Nov;(248):9–12. [PubMed] [Google Scholar]
- 16.Franklin PD, Allison JJ, Ayers DC. Beyond Joint Implant Registries: A Patient-Centered Research Consortium for Comparative Effectiveness in Total Joint Replacement. JAMA. 2012 Sep 26;308(12):1217–1218. doi: 10.1001/jama.2012.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman SB. The Effects of Micromotion and Particulate Materials on Tissue Differentiation. Bone Chamber Studies in Rabbits. Acta Orthopaedica Scandinavica Supplementum. 1994 Jun;258:1–43. doi: 10.3109/17453679409155227. [DOI] [PubMed] [Google Scholar]
- 18.King TV, Scott RD. Femoral Component Loosening in Total Knee Arthroplasty. Clinical Orthopaedics and Related Research. 1985 Apr;(194):285–290. [PubMed] [Google Scholar]
- 19.Labutti Ronald S, Bayers-Thering Mary, Krackow Kenneth A. Enhancing Femoral Cement Fixation in Total Knee Arthroplasty. The Journal of Arthroplasty. 2003 Dec;18(8):979–983. doi: 10.1016/s0883-5403(03)00450-9. [DOI] [PubMed] [Google Scholar]
- 20.Vaninbroukx Michaël, Labey Luc, Innocenti Bernardo, Bellemans Johan. Cementing the Femoral Component in Total Knee Arthroplasty: Which Technique Is the Best? The Knee. 2009 Aug;16(4):265–268. doi: 10.1016/j.knee.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Walker PS, Soudry M, Ewald FC, McVickar H. Control of Cement Penetration in Total Knee Arthroplasty. Clinical Orthopaedics and Related Research. 1984 May;(185):155–164. [PubMed] [Google Scholar]
- 22.Van de Groes SAW, de Waal Malefijt MC, Verdonschot N. Influence of Preparation Techniques to the Strength of the Bone-Cement Interface behind the Flange in Total Knee Arthroplasty. The Knee. 2013 Jun;20(3):186–190. doi: 10.1016/j.knee.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Shi JF, Wang CJ, Laoui T, Hart W, Hall R. A Dynamic Model of Simulating Stress Distribution in the Distal Femur after Total Knee Replacement. Proceedings of the Institution of Mechanical Engineers Part H, Journal of Engineering in Medicine. 2007 Nov;221(8):903–912. doi: 10.1243/09544119JEIM256. [DOI] [PubMed] [Google Scholar]
- 24.Daly Robin M, Rosengren Bjorn E, Alwis Gayani, Ahlborg Henrik G, Sernbo Ingemar, Karlsson Magnus K. Gender Specific Age-Related Changes in Bone Density, Muscle Strength and Functional Performance in the Elderly: A-10 Year Prospective Population-Based Study. BMC Geriatrics. 2013 Jul 6;13:71. doi: 10.1186/1471-2318-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Rajab RB, Watson WS, Walker B, Roberts J, Gallacher SJ, Meek RMD. Peri-Prosthetic Bone Mineral Density after Total Knee Arthroplasty. Cemented versus Cementless Fixation. The Journal of Bone and Joint Surgery British Volume. 2006 May;88(5):606–613. doi: 10.1302/0301-620X.88B5.16893. [DOI] [PubMed] [Google Scholar]
- 26.Soininvaara Tarja A, Miettinen Hannu JA, Jurvelin Jukka S, Suomalainen Olavi T, Alhava Esko M, Kröger Heikki PJ. Periprosthetic Femoral Bone Loss after Total Knee Arthroplasty: 1-Year Follow-up Study of 69 Patients. The Knee. 2004 Aug;11(4):297–302. doi: 10.1016/j.knee.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Mintzer CM, Robertson DD, Rackemann S, Ewald FC, Scott RD, Spector M. Bone Loss in the Distal Anterior Femur after Total Knee Arthroplasty. Clinical Orthopaedics and Related Research no. 1990 Nov;260:135–143. [PubMed] [Google Scholar]
- 28.Van Lenthe GH, de Waal Malefijt MC, Huiskes R. Stress Shielding after Total Knee Replacement May Cause Bone Resorption in the Distal Femur. The Journal of Bone and Joint Surgery British Volume. 1997 Jan;79(1):117–122. doi: 10.1302/0301-620x.79b1.6808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.