Abstract
Purpose
Ischemia/reperfusion results in tissue damage, a rapid increase in cytokines and chemokines and inflammatory cell infiltration. Herein we investigated the ability of a selective TLR2/4 antagonist, Sparstolonin B (SsnB), to protect rat cultured left ventricular tissue (LV) slices from hypoxic injury by inhibiting the myocardial inflammatory response independent of inflammatory cell infiltration.
Methods and Results
Media Lactate dehydrogenase (LDH) levels were measured to reflect hypoxia-induced cytotoxicity and cell injury with and without SsnB. Incubation with SsnB (15 and 30 μM) significantly reduced by 20 and 40 %, respectively, the amount of LDH released from the hypoxic LV slices. TUNEL staining showed that SsnB significantly attenuated the levels of hypoxia-induced apoptotic cells from 61.5 ±4.0 to 27.0±2.1 (15 μM SsnB) and 23.5±2.2 (30 μM SsnB) cells/unit area. Similarly, the Periodic Acid-Schiff (PAS) staining of ischemic areas in untreated hypoxic LV slices was increased 17 fold from 0.26±0.09 to 4.41±0.43 %, while in hypoxic slices incubated with 15 and 30 μM of SsnB, the PAS positive ischemic areas were increased by only 6.4 fold to 1.66±0.39 % and 3.8 fold to 1.00±0.22 %, respectively. Rt-PCR confirmed that MCP1 and IL-6 expression during hypoxia was elevated by 2 and 4 fold, respectively, while their upregulation was significantly inhibited (i.e., <0.7 fold increase) by SsnB.
Conclusion
The selective TLR2/4 antagonist, Sparstolonin B, can substantially protect LV myocardium via its ability to inhibit injury resulting from hypoxic myocardial-generated inflammation. Accordingly SsnB has potential as a therapeutic agent for the attenuation of myocardial ischemia-reperfusion injury.
Keywords: Myocardial ischemia injury, LDH, Apoptosis, Necrosis, Inflammation
Introduction
Ischemia and reperfusion tissue injury contributes to morbidity and mortality over a wide range of pathologies, including myocardial infarction and ischemic stroke. An imbalance in metabolic supply and demand within the ischemic organ results in profound tissue damage and microvascular dysfunction. Subsequent reperfusion enhances the activation of innate and adaptive immune responses and cell death programs [1].
A variety of pathological processes contributes to ischemia and reperfusion associated tissue injury. For example, limited oxygen availability is associated with impaired endothelial cell barrier function due to decreases in adenylate cyclase activity and intracellular cAMP levels and a concomitant increase in vascular permeability and leakage [1]. In addition, ischemia and reperfusion leads to the activation of cell death programs, including apoptosis (nuclear fragmentation, plasma membrane blebbing, cell shrinkage and loss of mitochondrial membrane potential and integrity) [2], necrosis (progressive cell and organelle swelling, plasma membrane rupture and leakage of proteases and lysosomes into the extracellular compartment), and autophagy-associated cell death (cytoplasmic vacuolization, loss of organelles and accumulation of vacuoles with membrane whorls) [3].
In particular, the ischemic period is associated with significant alterations in the transcriptional control of gene expression. Despite the fact that ischemia and reperfusion typically occurs in a sterile environment, activation of innate and adaptive immune responses occurs and contributes to injury, including activation of pattern-recognition receptors such as Toll like receptors (TLRs) and inflammatory cell trafficking into the affected organ, as well as the activation of the complement system [4]. As these responses can have adverse consequences, targeting immune activation is an emerging therapeutic strategy for minimizing ischemia and reperfusion injury.
Herbal medicine has been widely used in traditional Chinese medicine for the treatment of myocardial infarction for hundreds of years. Recently we isolated a compound, designated Sparstolonin B (SsnB), from the Chinese herb Sparganium stoloniferum and characterized it as a selective TLR2 and TLR4 antagonist [5]. In addition, we have shown that SsnB potently inhibits macrophage inflammatory responses to multiple TLR2 and TLR4 ligands in vitro and in vivo [5], attenuates hypoxia-reoxygenation induced cardiomyocyte inflammation in vitro, and blocks lipopolysaccharide (LPS)-induced endothelial cell activation [6]. The purpose of the present study was to investigate the ability of SsnB to protect rat cultured left ventricular (LV) tissue slices against hypoxic damage and to inhibit the hypoxia-related inflammation in the absence of inflammatory cell infiltration.
Materials and Methods
The study protocol was approved by the Institution’s Animal Care and Use Committee and conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For all experiments, 8-week old male Sprague–Dawley rats were purchased from Harlan Laboratories. The animals were housed under standard environmental conditions and maintained on a normal rodent diet and tap water ad libitum. Euthanasia was accomplished by removal of the heart after the rats were deeply anesthetized with an intraperitoneal injection of pentobarbital sodium (70 mg/kg).
Rat Left Ventricular Tissue Slice Culture and Hypoxia
Under deep anesthesia, the rat chest was opened aseptically and the heart was removed and washed in cold sterile saline followed by transference to Joklik media (Sigma-Aldrich, St. Louis, USA). The LV plus septum were separated from the rest of the heart and filled with 2.5 % liquefied agarose (around 40 °C) and placed into a metal cylinder containing agarose. The LV was sliced perpendicular to the long axis (250 to 300 μm in thickness) using a Brendel/Vitron Tissue Slicer (Vitron Organ Slicing Tech., Tucson, AZ). The slices were incubated in Joklik media at room temperature for 30 min and then transferred to fresh Joklik media with 0.2 uM of calcium chloride and incubated for 30 min in a culture incubator (37 °C, 95 % O2 and 5 % CO2). Following a further addition of calcium chloride to a concentration of 0.4 uM, the LV slices were incubated for another 30 min. Subsequently, the LV slices (15 to 20) from each of the four hearts studied were randomly divided into four wells (i.e., normoxia, hypoxia, hypoxia with 15 or 30 μM of SsnB) with at least three slices per well containing fresh Waymouth medium (Sigma-Aldrich, St. Louis, USA). Tissue hypoxia was induced by placing the slices into the pre-deoxygenated, serum free Waymouth medium and incubation in 1 % O2, 94 % N2 and 5 % CO2 at 37 °C, for 45 min. Tissue normoxia culture was conducted in Waymouth medium with serum and 95 % O2, 5 % CO2 at 37 °C for 45 min. Afterwards, media from each well was collected and analyzed for Lactate dehydrogenase (LDH) and the slices were weighed. Some of the slices from each group were snap frozen and stored at −80 °C for inflammatory factor assay. The remaining slices were fixed in 10 % buffered formalin for 24 h at room temperature and embedded in paraffin and sectioned to a 5 μm thickness for subsequent staining.
SsnB Treatment
SsnB (15 or 30 μM) was added into the pre-deoxygenated serum free Waymouth media. These concentrations were shown previously to have no toxic effects on cultured H9c2 cells under normoxic or hypoxic conditions [5]. SsnB powder was dissolved in Dimethyl Sulfoxide (DMSO) as a stock solution of 50 mg/ml (186.6 mM) and then diluted into appropriate media to the desired concentration. The solubility of SsnB in most of the cell culture media is ~150 μg/ml (0.56 mM) [5].
LDH Assay
LDH is normally retained in the cytosol of viable cells. To determine the degree of cell cytotoxicity induced by hypoxia, the culture media was collected and LDH activity in the media was assayed by use of a LDH cytotoxicity detection kit (Clontech, Mountain View, CA). The relative LDH activity in the culture media was normalized by the wet tissue weight.
TUNEL Staining
TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining was used to determine programmed cell death, or apoptosis. The tissue sections in each group were deparafinized and rehydrated. TUNEL staining was accomplished by using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacture’s instruction. Nuclei were stained by DAPI (Vector Laboratories Inc., Burlingame, CA). Negative control was established by incubating the fixed and permeabilized tissue in Label solution only (without terminal transferase) instead of TUNEL reaction mixture while a positive control was established by incubating the fixed and permeabilized tissue with DNase I recombinant for 10 min at room temperature to induce DNA strand breaks, prior to the labeling procedure. From each section, 10 randomly selected fields (200× magnification) were photographed with a Laser Scanning Confocal Microscope (Leica TCS SP5, Mannheim Germany). The number of TUNEL-positive cells in each field were counted using the software Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD) and divided by the field area. The average apoptotic cell density of the 10 fields was then obtained for each experimental group.
Periodic Acid-Schiff (PAS) Staining
Periodic acid acts upon the 1,2 glycol linkage of carbohydrates in tissue sections to produce aldehyde which can be highlighted with Schiff’s reagent. In the early stage of myocardial ischemia, PAS reagent is used to depict areas of abnormal sarcolemmal permeability. Accordingly, the PAS positive areas are indicative of early necrotic damage. PAS staining was performed with the Periodic Acid-Schiff Staining System (Sigma-Aldrich, St. Louis, USA), according to the manufacture’s instruction. Briefly, the tissue sections in each group were deparafinized, rehydrated, oxidized in 0.5 % Periodic acid solution and then placed in Schiff reagent. Afterwards, the tissue sections were counterstained in Mayer’s hematoxylin. In each section, ten fields (200× magnification) were randomly selected and photographed. The PAS positive area was quantified using the software Image-Pro Plus 6.0 and divided by the field area. The average percent PAS positive area of the 10 fields was obtained for each experimental group.
Real-Time Reverse-Transcription Polymerase Chain Reaction (qPCR)
Total RNA was extracted and purified from the cultured rat LV tissue slices using TRIzol reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions before performing reverse transcription using a First-strand cDNA Synthesis Kit (Bio-Rad, Hercules, CA) on the cDNA Synthesis System (Bio-Rad). qPCR analyses were carried out using iQ SYBR Green Supermix (Bio-Rad) on an Eppendorf Realplex2 Mastercycler (Bio-Rad). The primers (Integrated DNA Technologies, Coralville, IA) used in quantitative realtime-PCR are listed as follows: rat 18S RNA (internal control), 5′-TGAGGCCATGATTAAGAGGG-3′ (forward) and 5′-AGTCGGCATCGTTTATGGTC-3′ (reverse); rat MCP1, 5′-CCAATGAGTCGGCTGGAGAACT-3′ (forward) and 5′-AGTGCTTGAGGTGGTTGTGGAA-3′ (reverse); rat IL-6, 5′-AGGAGACTTCACAGAGGATACC-3′ (forward) and 5′-TCCAGAAGACCAGAGCAGATT-3′ (reverse). Samples were amplified as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 10 s, 58 °C for 15 s, and 72 °C for 20 s, then a melting curve analysis from 65 °C to 95 °C every 0.2 °C. The abundance of each gene product was calculated by relative quantification, with values for the target genes normalized with 18S RNA.
Statistical Analysis
Results are presented as mean ± SEM. GraphPad Prism 5 software (GraphPad Software, San Diego, CA) was used to perform the following analyses. One-way analysis of variance (ANOVA) was used for multiple group comparisons. If the data followed a Gaussian distribution, then Bonferroni’s multiple comparisons were used as a post-test. Otherwise, the non-parametric KruskalWallis test and Dann’s multiple comparison post-test were used. A p value of <0.05 was considered to indicate statistical significance.
Results
SsnB Reduced LDH Release from Hypoxia Damaged Heart Tissue
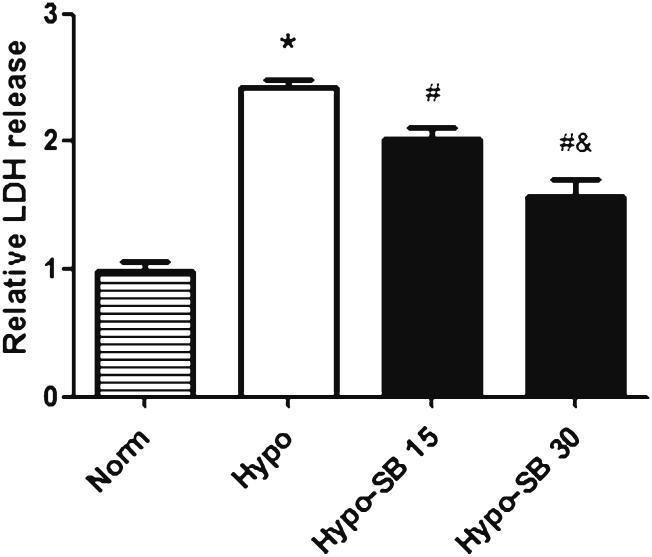
LDH activity in the culture media was assayed to determine the protective effects of SsnB against the hypoxic damage of heart tissue. As shown in Fig. 1, hypoxia for 45 min significantly increased LDH release (2.4 fold) compared to normoxia. SsnB treatment at the concentrations of 15 and 30 μM significantly attenuated hypoxia-induced LDH release by 20 and 40 %, respectively, in a dose dependent manner.
Fig. 1.
SsnB reduced hypoxia-induced LDH released from rat left ventricular tissue. LDH released from rat LV tissue slices cultured in normoxia Waymouth medium with serum (Norm) and in hypoxia serum free media without SsnB (Hypo) and with 15 and 30 μM of SsnB (Hypo-SB 15 and Hypo-SB 30) for 45 min. Data are presented as Mean ± SEM, n=4, *p<0.05 vs. normoxia, #p<0.05 vs. hypoxia, & p<0.05 vs. hypoxia with 15 μM SsnB
SsnB Decreased Heart Tissue Apoptotic Damage Induced by Hypoxia
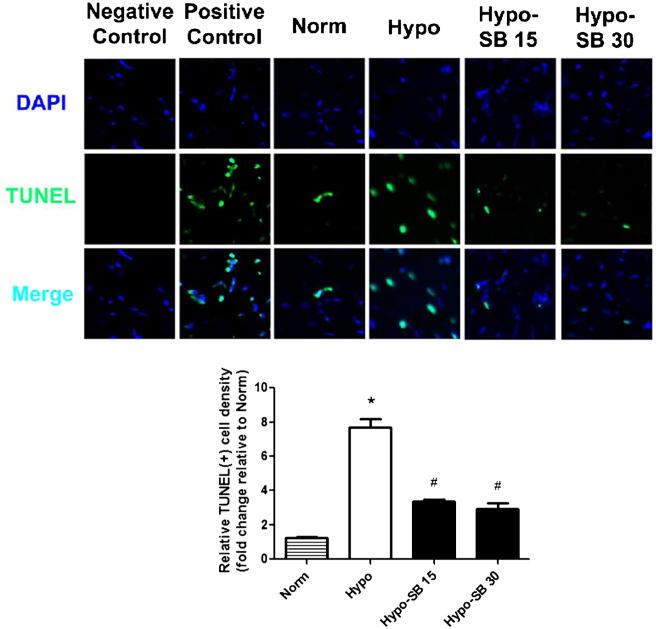
TUNEL staining results indicated that the apoptotic cell number in untreated hypoxic tissue was increased approximately 6 fold from that in normoxic tissue from 10±0.7 to 61.5±2.1 cells per unit area. Incubation with 15 and SsnB significantly inhibited the hypoxia-induced cell apoptosis by 56 % (27.0±2.1 cells per unit area) and 62 % (23.5±2.2 cells per unit area), respectively (Fig. 2); the trend to decrease further with 30 μM SsnB did not reach the level of statistical significance.
Fig. 2.
SsnB decreased hypoxia-induced apoptotic damage in rat left ventricular tissue. TUNEL staining was performed to detect apoptotic myocardial cells in rat LV tissue slices cultured in normoxia Waymouth media with serum (Norm) and in hypoxia serum free media without SsnB (Hypo) and with 15 and 30 μM of SsnB (Hypo-SB 15 and Hypo-SB 30) for 45 min. Data normalized to TUNEL results from a normoxic heart are presented as Mean ± SEM, n=4, *p<0.05 vs. Norm, #p<0.05 vs. Hypo. Representative images were obtained from the confocal microscopy. Apoptotic myocardial cells were stained by TUNEL staining (green) and nuclei were stained by DAPI (blue)
SsnB Decreased Heart Tissue Necrotic Damage Induced by Hypoxia
We also determined whether SsnB inhibits hypoxia-induced necrotic cell damage using PAS staining; the results are depicted in Fig. 3. The percent PAS positive area in untreated hypoxic heart tissue slices was increased by 17 fold from 0.26±0.09 to 4.41±0.43 %, while in hypoxic slices incubated with 15 and 30 μM of SsnB, the percent PAS positive area was increased by only 6.4 fold to 1.66±0.39 % and 3.8 fold to 1.00±0.22 %, respectively. Here, the dose dependent response was significant.
Fig. 3.
SsnB decreased the necrotic damage induced by hypoxia in rat left ventricular tissue. PAS staining was performed to delineate necrotic areas in rat LV tissue slices cultured in normoxia Waymouth medium with serum (Norm) and in hypoxia serum free media without SsnB (Hypo) and with 15 and 30 μM of SsnB (Hypo-SB 15 and Hypo-SB 30) for 45 min. PAS positive areas were stained in dark red (marked with *). Data normalized to % necrosis results from a normoxic heart are presented as Mean ± SEM, n=4, *p<0.05 vs. normoxic control group, #p<0.05 vs. hypoxic group, &p<0.05 vs. hypoxia with 15μM SsnB
SsnB Decreased the Expression of Inflammatory Molecules Induced by Hypoxia
SsnB has been shown to be a selective TLR2/4 antagonist that decreases the expression of downstream inflammatory molecules. In this study, we examined whether SsnB inhibits hypoxia-induced expression of MCP1 and IL-6 molecules in the cultured LV tissue. As shown in Fig. 4, MCP1 mRNA expression was significantly increased by nearly 2 fold as a result of 45 min hypoxia, compared to that in the normoxic group. This increase was not seen in the hypoxic tissue incubated with 15 and 30 μM of SsnB. Similarly, IL-6 mRNA expression was significantly increased 4 fold by hypoxia from that in the normoxic group, and treatment with 15 and 30 μM of SsnB prevented this increase. Taken together, these data indicate that SsnB suppresses hypoxia-induced inflammatory molecular expression in the cultured rat heart tissue.
Fig. 4.
SsnB decreased inflammatory molecular expression induced by hypoxia. mRNA levels of MCP1 and IL-6 in left ventricular (LV) tissue slices cultured in normoxia Waymouth medium with serum (Norm) and in hypoxia serum free media without SsnB (Hypo) and with 15 and 30 μM of SsnB (Hypo-SB 15 and Hypo-SB 30) for 45 min. Data are presented as Mean ± SEM, n=4, *p<0.05 vs. Normoxic group, #p<0.05 vs. Hypoxic group
Discussion
Our previous discovery of an isolated Chinese herb-derived single compound, SsnB, which functions as a selective TLR2 and TLR4 antagonist, prompted us to explore its potential therapeutic value for inflammatory diseases. Inflammation is a key detrimental component of myocardial ischemia-reperfusion injury [7] and TLRs are important components of the innate immunity response to pathogens as well as non-pathogenic components of damaged tissues. Accordingly, TLRs are involved in the pathogenesis of atherosclerosis, thrombosis, and ischemic/reperfusion injury [8-10]. Hence, TLR antagonists may be used therapeutically for a number of inflammatory conditions [11]. Since our previous studies have shown that SsnB could attenuate inflammatory responses in macrophages, endothelial cells and a cardiomyocyte cell line, we focused our efforts in the current study on whether SsnB was cardioprotective in cultured hypoxic LV tissue slices. The use of cultured tissue slices has several advantages compared to isolated cell and in vivo studies including: 1) maintaining conditions in which myocardial cells retain their 3-D structural integrity, intercellular interactions and extracellular attachments; 2) ability to perform multiple perturbations on tissue from an individual heart thereby minimizing biological variability; and 3) relatively long-term effects of a perturbation can be investigated under highly controlled conditions. Most importantly, the myocardial response to perturbations is solely intrinsic in that it would be void of factors, such as circulating cytokines, infiltrating inflammatory cells, variations in the neuro-hormonal background, and variations in preload, afterload and contractility. Clearly these advantages permit the design of clear and straightforward experiments that address the research question unambiguously.
The levels of LDH released from ischemia-induced damage to the cell membranes [9] were used to assess the efficacy of SsnB. The results indicated that incubation with 15 and 30 μM of SsnB significantly attenuated LDH release in a dose dependent manner from hypoxia-damaged LV tissue, and also reduced apoptotic and necrotic cell death and decreased inflammatory molecular expression.
The programmed cell death, apoptosis, occurs as a result of cardiac ischemia and reperfusion injury [12]. Apoptosis contributes significantly to cardiomyocyte loss during acute myocardial infarction and subsequent remodeling events [13]. However, nearly 75 % of the cells in the normal heart are fibroblasts, and endothelial cells [14] and our apoptosis assay does not distinguish the type of resident myocardial cells undergoing apoptosis during hypoxia. Therefore, we also quantified the amount of necrosis, which we assume to primarily reflect cardiomyocyte damage.
Necrosis triggers distinct changes in cell morphology that uniquely distinguishes it from apoptotic cell death [15]. A key primary event believed to distinguish necrosis from apoptosis respectively is observed permeability changes to inner mitochondrial membrane (IMM) resulting in the formation and opening of the mitochondrial permeability transition pore [16, 17]. Hence, early permeabilization changes to the outer mitochondrial membrane are features of apoptosis, while permeability changes to the IMM are more reflective of necrosis [18]. PAS reagent is used to depict areas of abnormal sarcolemmal permeability which occur during the early stage of myocardial ischemia. The results indicate the presence of PAS positive areas of necrosis in the hypoxic tissue. Statistical analysis indicated that SsnB significantly decreased the LV tissue necrotic damage induced by hypoxia in a dose dependent fashion. Given that cardiomyocytes are terminally differentiated and have little potential for division, the ability of SsnB to attenuate the apoptotic and necrotic loss of cardiomyocytes after hypoxic injury is indicative of its potential therapeutic value [19].
Inflammation plays a major role during myocardial ischemia and reperfusion injury. Typically, inflammatory responses result in myocardial damage and fibrosis, leading to progressive adverse remodeling and impairment of cardiac function post-myocardial infarction. We selected Monocyte chemo-attractant protein (MCP)-1 and Interleukin (IL)-6 as biomarkers for hypoxia-induced inflammation. MCP1, which belongs to the C-C chemokine superfamily, induces the infiltration, activation and cytokine secretion of inflammatory cells during ischemia [20]. MCP1 can be induced in numerous cell types, including vascular endothelial cells, smooth muscle cells, monocytes/macrophages and cardiomyocytes. IL-6 is a pleiotropic cytokine of the IL-6 family which is expressed in many cell types and regulates many physiological and pathophysiological processes including immune system regulation, inflammation, wound healing and cell survival [21]. In the heart, IL-6 is expressed by integral tissue cellular components such as cardiomyocytes, fibroblasts and vascular endothelial and smooth muscle cells as well as interstitial macrophages. IL-6 can be released from the cardiomyocytes in response to hypoxia [22], and it is a key player in the inflammation and damage during myocardial ischemia and reperfusion injury [23]. In our study, the results indicate that SsnB dramatically suppresses hypoxia-induced mRNA expression of MCP1 and IL-6 in the cultured LV tissue slices, which could be an important mechanism by which SsnB attenuates the hypoxic tissue damage. The present findings together with those from our previous study using H9c2 cells, which reported MCP1 mRNA levels in the cardiomyocytes were increased during hypoxia [24], suggest that the hypoxia-induced increases in MCP1 and IL-6 expression occur independently of inflammatory cells, and that these increases can be significantly suppressed by SsnB.
In summary, we have demonstrated that the Chinese herbderived compound SsnB, a novel selective TLR2 and TLR4 antagonist, can protect cultured rat LV tissue slices against hypoxia induced cell apoptosis and necrosis, and can suppress the production of inflammatory molecules. The results herein provide initial evidence that SsnB has potential to serve as a therapeutic agent for protection against myocardial ischemia-reperfusion injury. However, additional in vivo studies are required to definitively determine its therapeutic efficacy.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health (R21-HL-089483 to J.S.J. and R21AT006767 and R01HL116626 to D.F.).
Footnotes
Disclosures
None declared.
References
- 1.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feuerstein GZ, Young PR. Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res. 2000;45:560–9. doi: 10.1016/s0008-6363(99)00372-7. [DOI] [PubMed] [Google Scholar]
- 3.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med. 2013;91:183–93. doi: 10.1007/s00109-012-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang Q, Wu Q, Jiang J, Duan J, Wang C, Smith MD, et al. Characterization of sparstolonin B, a Chinese herb-derived compound, as a selective Toll-like receptor antagonist with potent anti-inflammatory properties. J Biol Chem. 2011;286:26470–9. doi: 10.1074/jbc.M111.227934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang Q, Yu F, Cui X, Duan J, Wu Q, Nagarkatti P, et al. Sparstolonin B suppresses lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Arch Pharm Res. 2013 doi: 10.1007/s12272-013-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res. 2012;110:126–44. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 8.Peri F, Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv. 2012;30:251–60. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–9. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 10.Patel H, Shaw SG, Shi-Wen X, Abraham D, Baker DM, Tsui JC. Toll-like receptors in ischaemia and its potential role in the patho-physiology of muscle damage in critical limb ischaemia. Cardiol Res Pract. 2012;2012:121237. doi: 10.1155/2012/121237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin E, Freedman JE, Beaulieu LM. Innate immunity and toll-like receptor antagonists: a potential role in the treatment of cardiovascular diseases. Cardiovasc Ther. 2009;27:117–23. doi: 10.1111/j.1755-5922.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–26. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Santini D, Abbate A, Scarpa S, Vasaturo F, Biondi-Zoccai GG, Bussani R, et al. Surviving acute myocardial infarction: survivin expression in viable cardiomyocytes after infarction. J Clin Pathol. 2004;57:1321–4. doi: 10.1136/jcp.2004.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roubille F, Barrere-Lemaire S. Apoptosis following myocardial infarction: cardiomyocytes and beyond. Eur J Clin Invest. 2013 doi: 10.1111/eci.12188. [DOI] [PubMed] [Google Scholar]
- 15.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Xi J, Zheng H, Zhang Y, Jin Y, Xu Z. Astragaloside IV inhibits oxidative stress-induced mitochondrial permeability transition pore opening by inactivating GSK-3beta via nitric oxide in H9c2 cardiac cells. Oxid Med Cell Longev. 2012;2012:935738. doi: 10.1155/2012/935738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 18.Mughal W, Dhingra R, Kirshenbaum LA. Striking a balance: autophagy, apoptosis, and necrosis in a normal and failing heart. Curr Hypertens Rep. 2012;14:540–7. doi: 10.1007/s11906-012-0304-5. [DOI] [PubMed] [Google Scholar]
- 19.Prech M, Marszalek A, Schroder J, Filas V, Lesiak M, Jemielity M, et al. Apoptosis as a mechanism for the elimination of cardiomyocytes after acute myocardial infarction. Am J Cardiol. 2010;105:1240–5. doi: 10.1016/j.amjcard.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 20.Nah DY, Rhee MY. The inflammatory response and cardiac repair after myocardial infarction. Korean Circ J. 2009;39:393–8. doi: 10.4070/kcj.2009.39.10.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal. 2013;25:898–909. doi: 10.1016/j.cellsig.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995;91:1520–4. doi: 10.1161/01.cir.91.5.1520. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya K, Farwell K, Huang M, Kempuraj D, Donelan J, Papaliodis D, et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia-reperfusion. Int J Immunopathol Pharmacol. 2007;20:69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Wang J, Liang Q, Wang D, Luo Y, Li J, et al. Sparstolonin B attenuates hypoxia-reoxygenation induced cardiomyocye inflammation. Exper Biol Med. 2014;239:376–84. doi: 10.1177/1535370213517620. [DOI] [PubMed] [Google Scholar]