Abstract
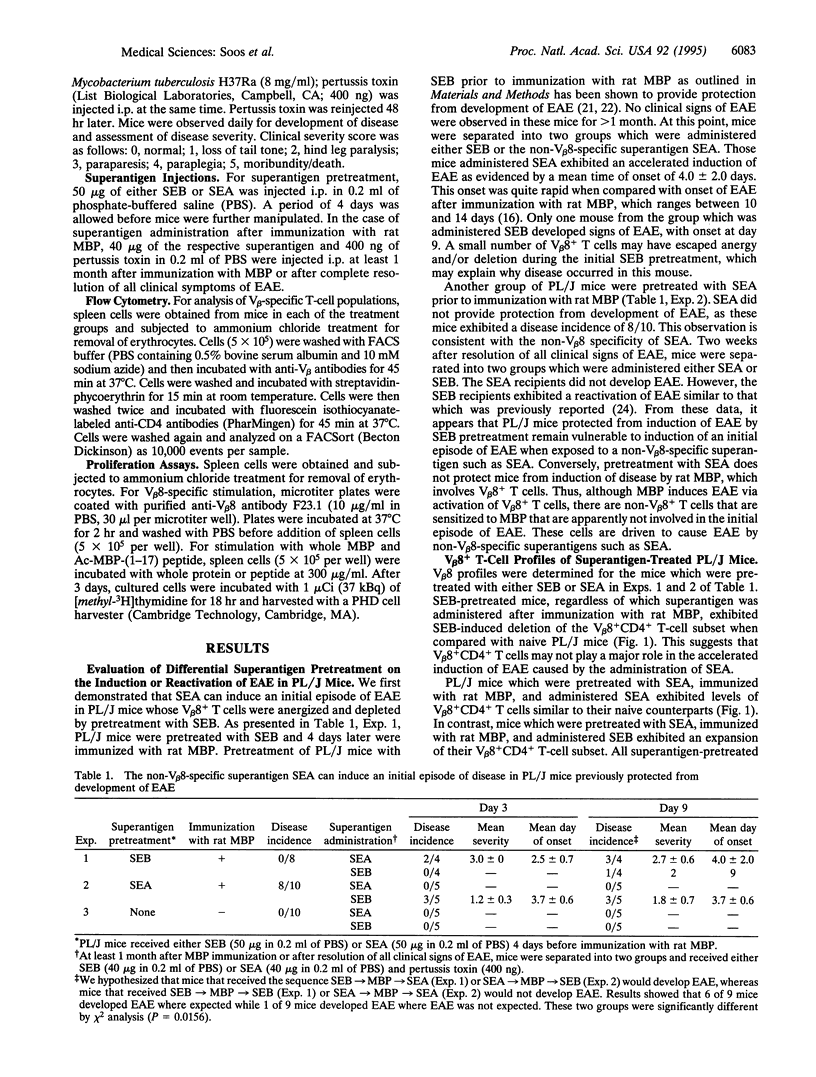
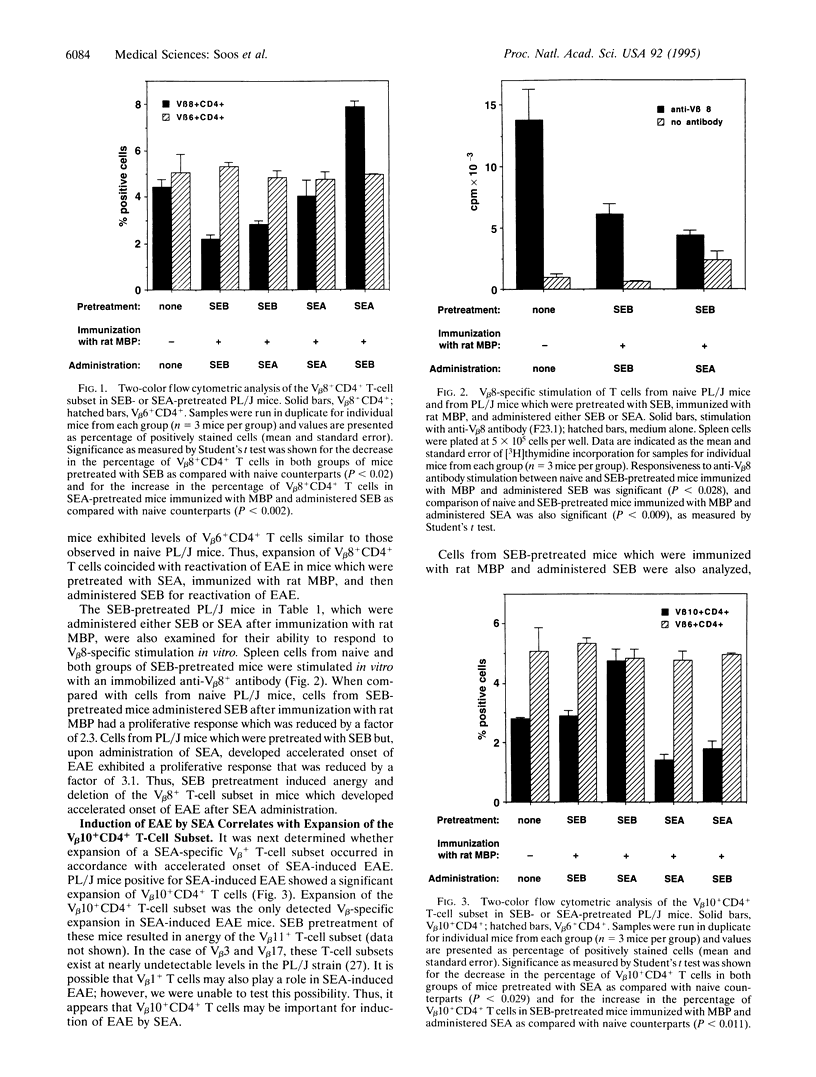
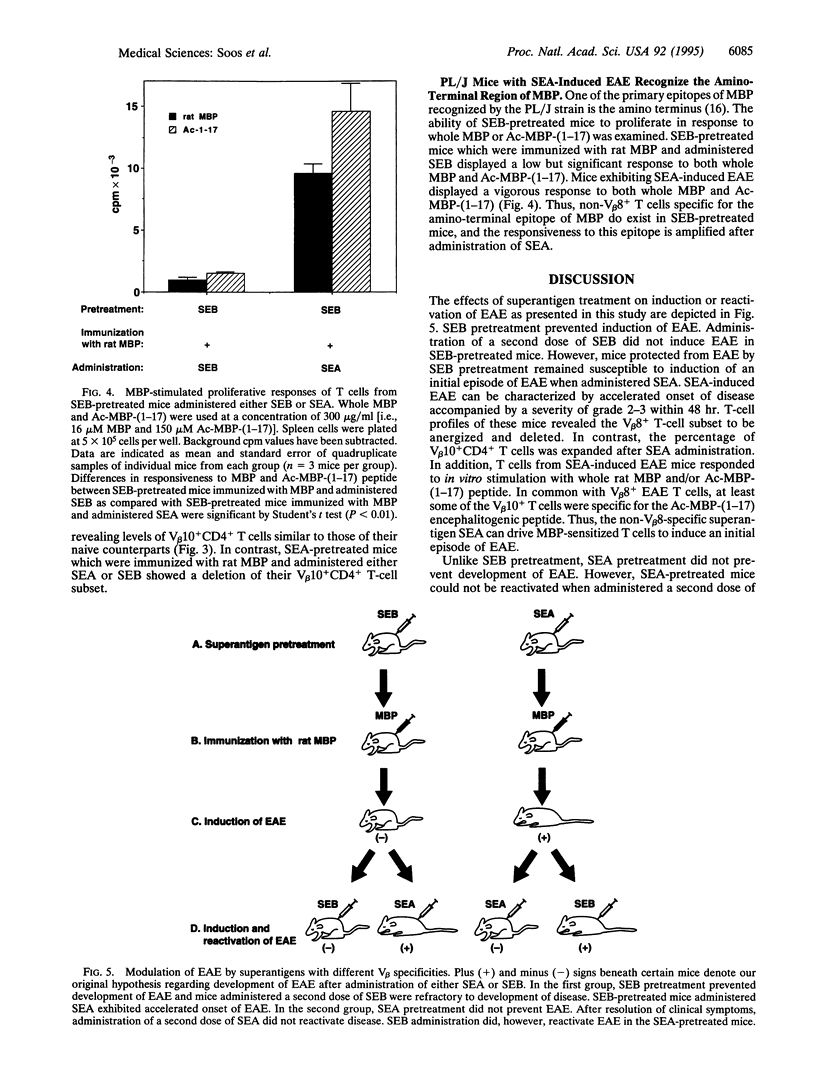
Superantigens such as the staphylococcal enterotoxins can play an important role in exacerbation of autoimmune disorders such as experimental allergic encephalomyelitis (EAE) in mice. In fact, superantigens can reactivate EAE in PL/J mice that have been sensitized to rat myelin basic protein (MBP). The T-cell subset predominantly responsible for disease in PL/J mice bears the V beta 8+ T-cell antigen receptor (TCR). The question arises as to whether T cells bearing other V beta specificities are involved in induction or reactivation of EAE with superantigen. Thus, we have investigated the ability of a non-V beta 8-specific superantigen, staphylococcal enterotoxin A (SEA) (V beta specificities 1, 3, 10, 11, and 17), to induce EAE in PL/J mice that have been previously protected from disease by anergy and deletion of V beta 8+ T cells. PL/J mice were first pretreated with the V beta 8-specific superantigen staphylococcal enterotoxin B (SEB) and then immunized with MBP. These mice exhibited V beta 8-specific anergy and depletion and did not develop EAE, even when further treated with SEB. However, administration of SEA to these same mice induced an initial episode of EAE which was characterized by severe hindleg paralysis and accelerated onset of disease. In contrast to SEB pretreatment, PL/J mice pretreated with SEA did develop EAE when immunized with MBP, and after resolution of clinical signs of disease these mice were susceptible to relapse of EAE induced by SEB but not by SEA. Thus, superantigens can activate encephalitogenic MBP-specific non-V beta 8+ T cells to cause EAE in PL/J mice. These data suggest that superantigens can play a central role in autoimmune disorders and that they introduce a profound complexity to autoimmune diseases such as EAE, akin to the complexity seen in multiple sclerosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brocke S., Gaur A., Piercy C., Gautam A., Gijbels K., Fathman C. G., Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993 Oct 14;365(6447):642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Posnett D. N., Tumang J. R., Cole B. C., Crow M. K. A potential role for microbial superantigens in the pathogenesis of systemic autoimmune disease. Arthritis Rheum. 1991 Apr;34(4):468–480. doi: 10.1002/art.1780340412. [DOI] [PubMed] [Google Scholar]
- Fritz R. B., Chou C. H., McFarlin D. E. Relapsing murine experimental allergic encephalomyelitis induced by myelin basic protein. J Immunol. 1983 Mar;130(3):1024–1026. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Russell J. K., Pontzer C. H. Staphylococcal enterotoxin microbial superantigens. FASEB J. 1991 Sep;5(12):2706–2712. doi: 10.1096/fasebj.5.12.1916093. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Russell J. K., Pontzer C. H. Superantigens in human disease. Sci Am. 1992 Apr;266(4):92-5, 98-101. doi: 10.1038/scientificamerican0492-92. [DOI] [PubMed] [Google Scholar]
- Kalman B., Lublin F. D., Lattime E., Joseph J., Knobler R. L. Effects of staphylococcal enterotoxin B on T cell receptor V beta utilization and clinical manifestations of experimental allergic encephalomyelitis. J Neuroimmunol. 1993 Jun;45(1-2):83–88. doi: 10.1016/0165-5728(93)90167-w. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990 Oct 1;172(4):1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Karuturi S., Chou Y. K., Lafferty J., Forrester J. M., Better M., Nedwin G. E., Offner H., Vandenbark A. A. Preferential T-cell receptor beta-chain variable gene use in myelin basic protein-reactive T-cell clones from patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9161–9165. doi: 10.1073/pnas.88.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Wucherpfennig K. W., Brod S. A., Benjamin D., Weiner H. L., Hafler D. A. Common T-cell receptor V beta usage in oligoclonal T lymphocytes derived from cerebrospinal fluid and blood of patients with multiple sclerosis. Ann Neurol. 1991 Jan;29(1):33–40. doi: 10.1002/ana.410290109. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Rellahan B. L., Jones L. A., Kruisbeek A. M., Fry A. M., Matis L. A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990 Oct 1;172(4):1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott O., Wekerle H., Fleischer B. Protection from experimental allergic encephalomyelitis by application of a bacterial superantigen. Int Immunol. 1992 Mar;4(3):347–353. doi: 10.1093/intimm/4.3.347. [DOI] [PubMed] [Google Scholar]
- Schiffenbauer J., Johnson H. M., Butfiloski E. J., Wegrzyn L., Soos J. M. Staphylococcal enterotoxins can reactivate experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8543–8546. doi: 10.1073/pnas.90.18.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos J. M., Schiffenbauer J., Johnson H. M. Treatment of PL/J mice with the superantigen, staphylococcal enterotoxin B, prevents development of experimental allergic encephalomyelitis. J Neuroimmunol. 1993 Mar;43(1-2):39–43. doi: 10.1016/0165-5728(93)90073-8. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Lee N. E., Moore A. C., Waldor M. K., Sakai K., Rothbard J. B., McDevitt H. O., Steinman L., Acha-Orbea H. Predominant expression of a T cell receptor V beta gene subfamily in autoimmune encephalomyelitis. J Exp Med. 1988 May 1;167(5):1586–1596. doi: 10.1084/jem.167.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Lee N. E., Moore A. C., Waldor M. K., Sakai K., Rothbard J. B., McDevitt H. O., Steinman L., Acha-Orbea H. Predominant expression of a T cell receptor V beta gene subfamily in autoimmune encephalomyelitis. J Exp Med. 1988 May 1;167(5):1586–1596. doi: 10.1084/jem.167.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Nelson P. A., Mitchell D. J., Knobler R. L., Fritz R. B., Steinman L. Encephalitogenic T cell clones specific for myelin basic protein. An unusual bias in antigen recognition. J Exp Med. 1985 Dec 1;162(6):2107–2124. doi: 10.1084/jem.162.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., al-Sabbagh A., Nelson P. A., Kaul D., Charles M. S., Mitchell D. J., Steinman L., Weiner H. L., Kuchroo V. K. 'Lupus-prone' mice are susceptible to organ-specific autoimmune disease, experimental allergic encephalomyelitis. Pathobiology. 1994;62(3):113–119. doi: 10.1159/000163887. [DOI] [PubMed] [Google Scholar]


