Abstract
Chronic exposure to alcohol produces changes in the prefrontal cortex that are thought to contribute to the development and maintenance of alcoholism. A large body of literature suggests that stress hormones play a critical role in this process. Here we review the bi-directional relationship between alcohol and stress hormones, and discuss how alcohol acutely stimulates the release of glucocorticoids and induces enduring modifications to neuroendocrine stress circuits during the transition from non-dependent drinking to alcohol dependence. We propose a pathway by which alcohol and stress hormones elicit neuroadaptive changes in prefrontal circuitry that could contribute functionally to a dampened neuroendocrine state and the increased propensity to relapse—a spiraling trajectory that could eventually lead to dependence.
Keywords: alcohol use disorders, hypothalamic pituitary adrenal axis, prefrontal cortex, animal models, dependence, glucocorticoids
Overview
Alcoholism is a neurobehavioral disorder characterized by compulsive seeking of alcohol, excessive and uncontrolled intake, and the emergence of a negative emotional state (e.g., irritability, anxiety, depression) when alcohol is unavailable (American Psychiatric Association, 1994). Preclinical studies in rodents suggest that the transition from alcohol use to abuse to dependence is due to alterations in stress-related neural pathways resulting from exposure to repeated cycles of alcohol intoxication and withdrawal (Heilig and Koob, 2007; Breese et al., 2011). Alcohol dependence is characterized by impaired functioning of the hypothalamic pituitary adrenal (HPA) axis (Adinoff et al., 1990; Wand and Dobs, 1991; Lovallo et al., 2000; Rasmussen et al., 2000; Zorrilla et al., 2001; Richardson et al., 2008). HPA dysfunction is thought to contribute to a number of symptoms, including dysphoria, alcohol craving, and enhanced propensity to relapse early in abstinence (Lovallo, 2006; Li et al., 2011; Sinha et al., 2011; Stephens and Wand, 2012).
Here we review alcohol use disorders and describe how preclinical and clinical studies together have implicated dysfunction of the HPA axis and prefrontal cortex in these disorders. We first provide an overview of some of the preclinical rodent models that have been designed to study drinking behavior at different stages of alcohol use disorders. With the focus on evidence from these drinking models, we discuss the bidirectional relationship between alcohol and stress hormones. The HPA axis undergoes adaptations from non-dependent drinking to alcohol dependence and we examine some of the mechanisms that may contribute to changes in stress hormone levels. Toward the end of the review, we pull together information from various studies that supports the following hypothesis: continued heavy use of alcohol causes glucocorticoid-mediated adaptations within the HPA axis and upstream in the prefrontal cortex that lead to neuroendocrine dysfunction and a heightened propensity to relapse. We posit that the complex interplay between alcohol, stress hormones, and the prefrontal cortex may be a critical factor in the transition from social drinking to problematic drinking and alcoholism. More research should be directed toward exploring the possibility of adaptations in the HPA dysregulation driven by alterations in the prefrontal cortex regulation over time. These studies could provide a new avenue of therapeutic intervention that may be extremely effective, as prefrontal dysfunction and HPA dysregulation are both thought to play a functional role in escalation of drinking and relapse (Stephens and Wand, 2012).
Alcohol use disorders and prefrontal cortex
The prefrontal cortex integrates information from other cortical and subcortical regions to functionally contribute to working memory, emotion regulation, and behavioral control (Wilson et al., 2010; Kesner and Churchwell, 2011). Structural, physiological, and behavioral deficits related to the prefrontal cortex have been observed in alcohol use disorder patients. These functional changes include reduced glucose metabolic rates, cortical atrophy, decreased cognitive flexibility, and memory performance (reviewed in (Fadda and Rossetti, 1998; Moselhy et al., 2001; Stephens and Duka, 2008). In addition, prefrontal deficits are tightly associated with HPA dysregulation in alcoholic men (Errico et al., 2002). Because the prefrontal cortex provides top-down control over the HPA axis, it is possible that neuroadaptive changes in this region could underlie some of the changes in stress hormones (Lovallo, 2006; Herman, 2012). Preclinical animal models can be useful tools for dissecting complex interaction between alcohol, stress hormones, and the prefrontal cortex. Below we briefly describe these models.
Animal models of alcohol use, abuse, and dependence
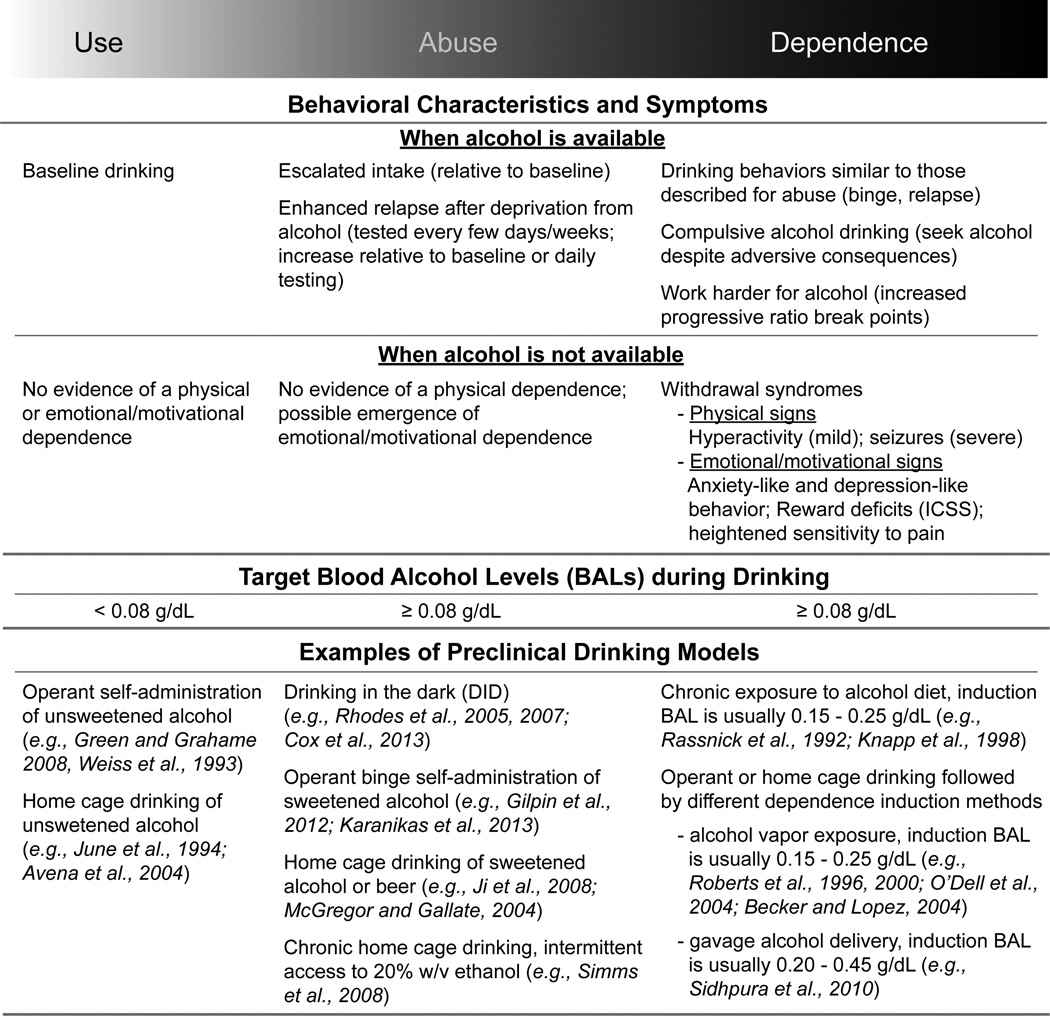
Preclinical rodent models aim to emulate as much as possible the human experience with alcohol by capturing different drinking behaviors in the early, mid, and late stages of addiction (Brown et al., 1980). Fig. 1 provides an overview of commonly used rodent models of alcohol use, abuse and dependence. For more detailed discussion of the preclinical nonhuman primate models see (Grant and Bennett, 2003; Barr and Goldman, 2006). When people consume alcohol, most of them drink low-to-moderate amounts, which is less than three drinks per day for men and less than two drinks per day for women (Eckardt et al., 1998; Boschloo et al., 2011). Similarly, rodents can be used to model this type of non-dependent drinking (Use, left column, Fig. 1). The positive reinforcing properties of the drug, such as pleasure, disinhibition and social acceptance, are thought to be the primary forces driving motivation to consume alcohol under non-dependent conditions (Eckardt et al., 1998).
Fig. 1.
An overview of preclinical rodent models capturing different drinking behaviors in the early (Use), mid (Abuse), and late (Dependence) stages of alcohol addiction. Behavioral characteristics and symptoms are described for each phase under conditions when alcohol is available versus conditions when alcohol is unavailable (withdrawal). The blood alcohol levels reached during a drinking episode differs as well, with use always remaining below the “binge” limit (0.08 g/dL) during the Use phase, but exceeding this level during the phases of Abuse and Dependence. We have provided a few examples of models used to capture drinking behavior at each phase, but it should be noted that this is not an exhaustive list. Abbreviation: ICSS, intracranial self-stimulation.
Rodent models of voluntary alcohol abuse are designed to capture more hazardous patterns of drinking (Abuse, middle column, Fig. 1). Abuse-like drinking patterns include escalations in intake, enhanced relapse after short or long withdrawal periods, stress/cue/alcohol-induced reinstatement, and episodic alcohol consumption resulting in some degree of intoxication. “Binge drinking” is an example of alcohol abuse. This is classified as the consumption of enough alcohol within a two-hour period to produce alcohol concentrations in the blood that reach an intoxication level of 0.08 g/dL or higher (~4 drinks in women, ~5 drinks in men, (NIAAA, 2004). Non-dependent alcohol use can escalate to a pattern of abuse that may be brought on by additional factors such as social pressure, age, genetic predispositions, and gender (Chassin et al., 2004; Oei and Morawska, 2004; Ceylan-Isik et al., 2010; Silveri, 2012). Many of these same factors influence drinking patterns in rodents, and these preclinical models have aided in the identification of some of the neural correlates of risky drinking (Anacker and Ryabinin, 2010; Sherrill et al., 2011; Gilpin et al., 2012; Karanikas et al., 2013; McBride et al., 2014).
A variety of strategies can be used to elicit voluntary binge drinking in animals, but a common theme in most models is intermittent access to alcohol (Mcgregor and Gallate, 2004; Rhodes et al., 2005; Simms et al., 2008; Crabbe et al., 2009; Gilpin et al., 2012; Sharko et al., 2013). If this episodic pattern of drinking persists, animals may begin to show signs of motivational and emotional—but not physical—dependence (Cox et al., 2013). Stress regulatory systems begin to undergo neuroadaptive changes and although alcohol may still have positive reinforcing properties, the negative reinforcing properties of alcohol are starting to become powerful motivators driving excessive drinking (Baker et al., 1986; Koob, 2003; Sinha et al., 2009; Koob et al., 2014; Wise and Koob, 2014).
Chronic cycling between alcohol intoxication and withdrawal can cause an individual to become dependent on alcohol (Becker, 2008) (Dependence, right column, Fig. 1). This shift from non-dependence to dependence has been described as a transition from the light side to the dark side of addiction (Schulteis and Koob, 1994; Koob and Le Moal, 2005). Laboratory rodents without a predisposition for addiction are shifted from non-dependent baseline drinking to escalated and compulsive-like drinking by combining voluntary drinking and forced alcohol exposure that induces mild to moderate physical dependence (Roberts et al., 2000; Becker and Lopez, 2004; O'Dell et al., 2004; Richardson et al., 2008; Vendruscolo et al., 2012). By incorporating voluntary drinking into the experimental design, preclinical studies have been useful for identifying biological changes specifically associated with drinking behavior at these various stages of alcohol use disorders (Roberts et al., 1996; Knapp et al., 1998; Sidhpura et al., 2010; Gilpin et al., 2012; DePoy et al., 2013).
Alcohol stimulates the release of stress hormones
When an organism experiences a physical or psychological challenge, neurons in the paraventricular nucleus of the hypothalamus (PVN) release the 41-amino acid peptide corticotropin-releasing factor (also known as corticotropin-releasing hormone) from axonal terminals in the median eminence (Vale et al., 1981). Corticotropin-releasing factor (CRF) travels through the short portal system, binds to its Type 1 G-protein coupled receptor (CRF1) (Chang et al., 1993; Chen et al., 1993; Perrin et al., 1993), and stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland (Rivier and Vale, 1983). ACTH is released into the bloodstream and within minutes this hormone reaches its target cells in the adrenal gland to stimulate the release of glucocorticoids (cortisol in primates, corticosterone in rodents, (Rivier and Vale, 1983).
The first line of evidence demonstrating that alcohol is an acute stressor that activates the HPA axis comes from studies in which alcohol-naïve animals are given a bolus dose of alcohol using “forced” delivery methods such as intragastric injection, ig (Ogilvie et al., 1997a), intubation/gavage (Pruett et al., 1998), intracerebroventricular injection (Selvage, 2012), intraperitoneal injection, ip (Rivier, 1993), and vapor inhalation (Rivier et al., 1984). This approach has been effective for identifying neural circuits that are activated by acute alcohol intoxication and exploring the molecular mechanisms by which alcohol can stimulate a stress hormone response. We briefly summarize these findings below (for a more detailed review, see Rivier, 2014).
Experimenter-administered alcohol dose dependently elicits elevations in PVN cellular activity and the release of ACTH and corticosterone in male and female rats (Ellis, 1966; Rivier, 1993; Rivier and Lee, 1996; Ogilvie et al., 1997a; Willey et al., 2012). The tight link between alcohol dose and HPA activity is further supported by correlated blood alcohol and stress hormone levels after an acute alcohol challenge (Ellis, 1966; Ogilvie et al., 1997a). These findings suggest that alcohol may directly activate HPA axis through regulating the PVN cellular activity. Indeed, in vitro application of alcohol to hypothalamic tissue or primary hypothalamic cells induces the release of CRF (Redei et al., 1988; Li et al., 2005). In addition, CRF heteronuclear RNA quickly elevates within 20 min after in vivo alcohol administration in rats (Rivier and Lee, 1996; Ogilvie et al., 1998). This transcriptional process is presumably initiated to replenish cellular stores of this peptide that were rapidly released from the nerve terminals in response to alcohol stimulation. CRF mRNA expression increases thereafter and remains elevated up to 6 hours following ethanol administration (Zoeller and Rudeen, 1992).
Alcohol is also known to activate cells outside the PVN. An intoxicating dose of alcohol administered ip or ig modulates Fos expression in the prefrontal cortex, bed nucleus of the stria terminalis, central nucleus of the amygdala, and locus coeruleus (Chang et al., 1995; Knapp et al., 2001). These targeted regions could regulate HPA reactivity through direct or indirect pathways and provide another layer of regulation in response to alcohol stimulation (Ulrich-Lai and Herman, 2009; Herman, 2012).
The acute effect of alcohol on stress hormones has been observed with voluntary drinking in humans and animals. Voluntary alcohol drinking activates the HPA axis in male rats (Richardson et al., 2008; although see Korányi et al., 1987) and in men and women (Jenkins and Connolly, 1968; Schuckit et al., 1987; Lex et al., 1991; Ekman et al., 1994; King et al., 2006). These key findings demonstrate that alcohol acts as a stressor, even if this drug is experienced through a natural route of administration. We and others postulate that the HPA axis is a biological system that is both sensitive to alcohol and may also play a functional role in the progression from non-dependent drinking to abuse and dependence (Koob and Kreek, 2007; Stephens and Wand, 2012; Vendruscolo et al., 2012; Koob et al., 2014). As mentioned earlier, binge drinking—but not moderate drinking—brings blood alcohol concentrations to a level of intoxication. Consequently, engaging in this type of hazardous drinking will activate a robust stress response, which could be costly to an individual if the pattern of abuse continues (Romero et al., 2009; Koob et al., 2014). Moreover, the effects alcohol abuse has on physiological and mental health may be more profound in individuals already sensitive to stress. Sex differences in HPA reactivity are thought to contribute to differential alcohol-related vulnerabilities in men and women (Adinoff et al., 2010; Lovallo et al., 2012; Stephens and Wand, 2012).
Chronic exposure to alcohol leads to neuroendocrine tolerance
Chronic heavy alcohol use eventually leads to dampened functioning of the neuroendocrine stress system and this dysregulated hormonal state may contribute to some of the symptoms of alcoholism (Lovallo, 2006; Li et al., 2011; Sinha et al., 2011; Stephens and Wand, 2012). Animal studies have elucidated some of the functional changes in the HPA axis that emerge after varying degrees of prolonged alcohol exposure in drinking models of addiction. Early in abstinence after chronic alcohol exposure, basal/resting levels of ACTH and corticosterone are significantly lower at the start of the inactive (light) phase of the light/dark cycle in dependent rats compared to non-dependent rats, but this difference in basal hormone levels was not measurable in the active (dark) phase (Richardson et al., 2008). Blunted basal levels of corticosterone have also been observed in both phases of the light/dark cycle in adult male rats weeks after removal from chronic alcohol liquid diet, as compared to alcohol naïve-controls (Rasmussen et al., 2000; Zorrilla et al., 2001).
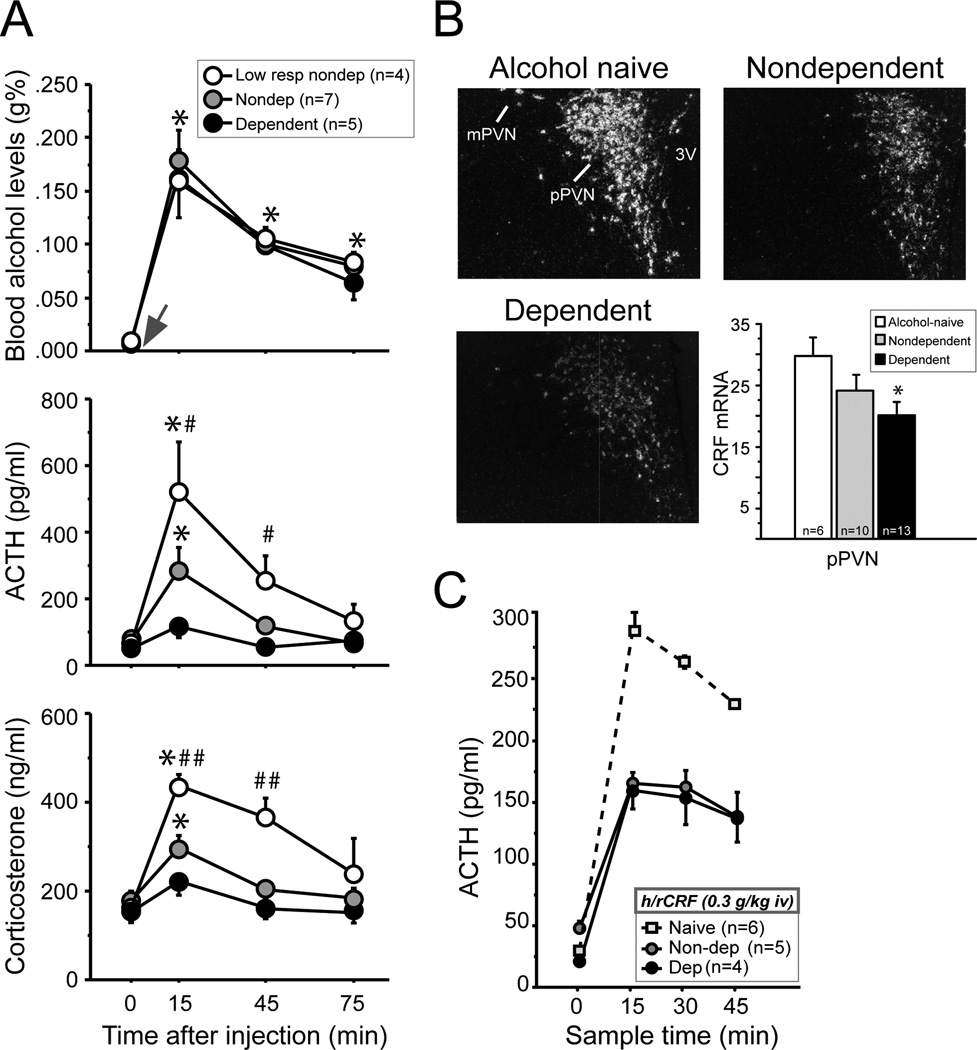
The most reliable indicator of chronic alcohol-induced changes in HPA function is a reduced response of this neuroendocrine system to an acute challenge of alcohol—also known as “neuroendocrine tolerance.” Neuroendocrine tolerance emerges after prolonged drinking and the magnitude of decrease in neuroendocrine sensitivity to alcohol appears to be dose-dependently related to the overall amount of alcohol consumed. When animals are given an alcohol challenge of 1 g/kg iv—the dose of alcohol that dependent rats voluntarily binge drink in a single 30-min session (Heyser et al., 1997; Gilpin et al., 2009; Li et al., 2011)—HPA responses differ greatly across individuals depending on their previous experience with alcohol (Richardson et al., 2008). This 1 g/kg dose elicits binge-like blood alcohol levels in all animals (Fig. 2A). However, it stimulates robust ACTH and corticosterone responses in low-drinking non-dependent rats, mid-range responses in moderate drinking non-dependent rats, and blunted responses in high drinking dependent rats (Fig. 2A).
Fig. 2.
The hypothalamic pituitary adrenal (HPA) axis is functionally different depending on an individual’s prior experience with alcohol. Data were obtained from adult male rats that were either naïve (no operant training or previous exposure to alcohol), low-drinking non-dependent (several weeks of low levels of alcohol-self administration), non-dependent (several weeks of moderate levels of alcohol-self administration), or dependent (several weeks of moderate levels of alcohol self-administration followed by chronic alcohol vapor-induced dependence). All measures were taken when dependent animals were in acute withdrawal (6–8 hours after removal from chronic alcohol vapors). (A) The level of dampened HPA activity in response to 1 g/kg iv alcohol challenge depends on animals’ alcohol responsiveness and the alcohol exposure history. (B) CRF mRNA expression is low in the hypothalamus of dependent compared to alcohol-naïve controls, and CRF mRNA expression in non-dependent animals is intermediate to these two groups. (C) A CRF challenge (0.3 µg/kg, iv) elicits a lower ACTH response in drinking rats relative to alcohol-naïve controls, but the non-dependent and dependent groups do not differ from one another. Abbreviations: iv, intravenous; pPVN, parvocellular division of paraventricular nucleus of the hypothalamus; mPVN, magnocellular division of PVN; 3V, third ventricle. [Adapted from Richardson et al., 2008 European Journal of Neuroscience.]
Adaptations have been found at multiple levels within the HPA axis, which may contribute to dampened neuroendocrine function after chronic alcohol. At the level of the hypothalamus, CRF mRNA expression is reduced in dependent animals 6–8 hours after withdrawal from chronic alcohol vapors compared to alcohol-naïve controls, and CRF mRNA expression in non-dependent animals is intermediate to these two groups (Fig. 2B). Chronic alcohol consumption appears to reduce responsiveness of pituitary corticotrophs to CRF peptide. A CRF challenge (0.3 µg/mL, iv, intravenous) elicits low ACTH responses in non-dependent and dependent drinking rats relative to the responses observed in alcohol-naïve rats (Fig. 2C). However, alterations in pituitary responsiveness does not appear to further progress with increased doses of alcohol, as non-dependent and dependent rats have comparable ACTH responses after a CRF challenge (Fig. 2C). Reduced pituitary responsiveness in drinking rats versus alcohol-naïve rats could be mediated by various mechanisms, including CRF1 receptor expression within pituitary cells and changes within the arginine vasopressin system (Ogilvie et al., 1997b; Zhou et al., 2000).
Although mechanisms downstream of the pituitary were not explored in Richardson et al. (2008), the fact that a 1 g/kg (iv) alcohol challenge in the dark cycle elicited a similar timeline of change in ACTH in the three drinking groups, but a much more prolonged corticosterone response in the low-drinking non-dependent rats suggests that even moderate drinking may alter adrenal sensitivity to ACTH (Fig. 2A). Alcohol-induced alterations in splanchnic innervation of the adrenal glands could explain such group differences (Ulrich-Lai et al., 2006). The mechanisms upstream of the hypothalamus are largely unknown, but enhanced inhibitory tone from peri-PVN GABA cells or other direct and indirect targets of the prefrontal cortex are possible candidates (Li et al., 2011; Herman, 2012). Later in this review, we discuss in detail a proposed role for the prefrontal cortex in neuroendocrine tolerance after chronic alcohol use and dependence (see Fig. 4).
Fig. 4.
A proposed model illustrating differential prefrontal regulation of the HPA axis after alcohol use (A) versus alcohol dependence (B). Dorsomedial prefrontal cortex (dmPFC) in rats modulates HPA activity by activating the inhibitory control over PVN via the BNST or periPVN (Radley et al., 2006; 2009). Chronic alcohol exposure has been proposed to increase the local de novo glucocorticoid synthesis in the PFC (Little et al., 2008). Additionally, ex vitro studies demonstrates that glucocorticoids reduce GABA inhibition of layer V pyramidal cells in the dmPFC (Hill et al., 2011). Thus, the chronic alcohol-induced overflow of glucocorticoids in the dmPFC could strengthen its output to downstream targets such as the BNST and periPVN, resulting in stronger inhibition of the PVN and neuroendocrine tolerance. Abbreviations: dmPFC, dorsal medial prefrontal cortex; vmPFC, ventral medial prefrontal cortex; BNST, bed nucleus of the stria terminalis, periPVN, peri-paraventricular nucleus of the hypothalamus, PVN, parventricular nucleus of the hypothalamus; ACTH, adrenocorticotropic hormone; CRF, corticotropin releasing factor.
Neuroendocrine tolerance may trigger relapse and heavy drinking
As described above, HPA dysregulation is a common symptom associated with chronic alcohol abuse and dependence. Reduced stress hormone levels may not only be reliable indicators of the addictive stage of an individual, but could also play a functional role in driving escalated drinking and enhanced relapse. In support of this hypothesis, blunted basal stress hormone levels in alcoholics predicts craving (Kiefer et al., 2002). There is also a strong temporal relationship between dampened HPA hormone levels and increases in heavy drinking and propensity to relapse early in abstinence in humans (Gianoulakis, 1998; Kiefer et al., 2002; Junghanns et al., 2003; Adinoff et al., 2005b; 2005c; 2005a; Sinha et al., 2011) and in rodents (Rasmussen et al., 2000; Richardson et al., 2008; Li et al., 2011). Additionally, the opiate receptor antagonist, naltrexone, stimulates the HPA axis and blocks alcohol craving and self-administration in alcohol-dependent human subjects (O'Malley et al., 2002).
Transgenic manipulations of CRF1 receptors in animals demonstrate that elimination of CRF1 receptors specifically from the central nervous system while leaving pituitary CRF1 receptor expression intact reduces relapse-like drinking (Molander et al., 2012). However, if pituitary CRF1 receptors are also eliminated—as with the CRF1 null knockout—HPA hormones are dampened and relapse-like drinking increases (Molander et al., 2012). This is consistent with the hypothesis that during a drinking session alcohol-induced stimulation of ACTH and possibly its downstream hormone corticosterone curbs alcohol drinking. Other predictions of this hypothesis have been tested using pharmacological approaches. Blocking inhibitory tone on the PVN using GABAA receptor antagonists such as picrotoxin or bicuculline is known to increase HPA activity (indexed by elevated Fos-immunoreactivity in the PVN and elevated blood corticosterone levels) in alcohol-naïve rats (Cole and Sawchenko, 2002) and alcohol-dependent rats (Li et al., 2011). This treatment also prevents relapse-like drinking in animals exposed to an intermittent drinking paradigm (Li et al., 2011).
While the findings above suggest that dampened HPA activity may stimulate relapse and heavy drinking, the interplay between low peripheral glucocorticoid levels and drinking behavior is not so clear. Acute blockade of corticosterone synthesis through metyrapone administration fails to elevate (Besheer et al., 2013)—and may even block (Fahlke et al., 1994)—alcohol drinking behavior. This suggests that low glucocorticoid levels do not cause increases in drinking, at least under non-dependent conditions. Perhaps deficits in HPA reactivity upstream of the adrenal glands are driving forces in increased drinking (Li et al., 2011). In addition, dampened glucocorticoids may acutely drive heavy drinking and relapse only after key behavioral circuits have undergone significant neuroadaptive changes associated with dependence. To the best of our knowledge, this hypothesis has not been empirically tested.
Role of glucocorticoids in the transition to dependence
Even with chronic alcohol dampening the neuroendocrine stress system, glucocorticoids still play a powerful role in the transition to dependence. Chronic exposure to alcohol drinking or to vapor-induced bouts of intoxication leads to dampen peripheral glucocorticoid levels (Richardson et al., 2008; Silva et al., 2009), yet glucocorticoid signaling is required for the development of the physical, motivational, and cognitive syndromes associated with alcohol dependence in rodents (Sze, 1977; Jacquot et al., 2008; Vendruscolo et al., 2012). This seems paradoxical, but two important factors must be considered. First, chronic alcohol exposure reduces—but does not fully diminish—the ability of alcohol exposure to acutely elevate plasma levels of corticosterone (Rivier et al., 1984; Lee and Rivier, 1997; Richardson et al., 2008). In fact, corticosterone levels remain significantly elevated for several hours during the intoxication phase of chronic vapor treatment in neuroendocrine-tolerant animals (Rivier et al., 1984; Lee and Rivier, 1997). Second, as individuals experience repeated bouts of intoxication, the brain undergoes neuroadaptive changes that eventually promote the emergence of a withdrawal syndrome (Koob, 2013). It is thought that the withdrawal syndrome worsens over time, and at this point, periods of prolonged withdrawal could be a second phase in which brain circuits are exposed to high concentrations of glucocorticoids that may be synthesized centrally (Brooks et al., 2008; Little et al., 2008). Consequently, repeated cycling between binge intoxication and periods of withdrawal would conceivably give this stress hormone ample opportunity to act on its receptors in the brain and affect transcriptional regulation of multiple genes that could promote addiction.
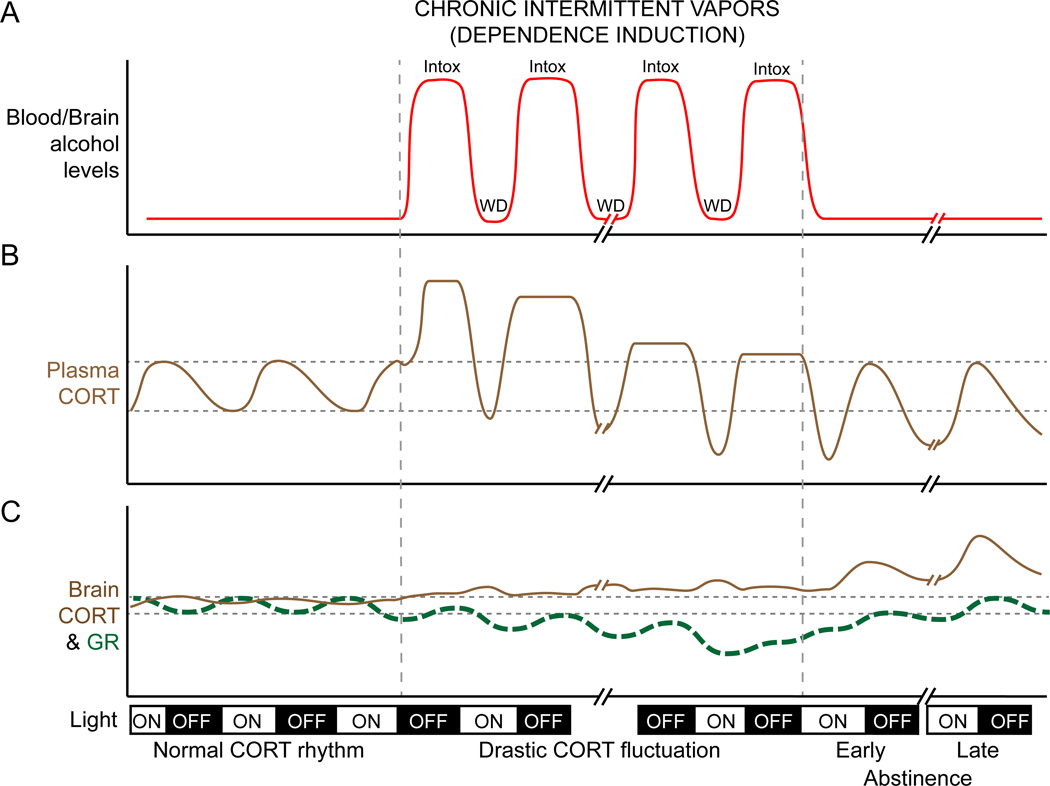
To understand how glucocorticoid signaling could promote—and glucocorticoid type II receptor (GR) antagonists could block—the transition to dependence and increase the probability of relapsing after abstinence (Vendruscolo et al., 2012), we must consider the dynamic interplay between glucocorticoid levels and their receptors. As illustrated in Fig. 3, glucocorticoid signaling in the brain is thought to be a complex process as individual’s transition from abuse to dependence. Blood levels of this stress hormone fluctuate with the pattern of alcohol exposure (Fig. 3B) and brain responsiveness to corticosterone also changes because of receptor auto-regulation (Sapolsky et al., 1984; Sapolsky and McEwen, 1985; Herman and Spencer, 1998). Accordingly, differential GR expression in the brain might give insight into which brain regions have high or low local concentrations of corticosterone during the intoxication and withdrawal phases of chronic alcohol exposure. GR expression levels differ in early versus late abstinence from chronic alcohol (Vendruscolo et al., 2012). GR mRNA expression is reduced in frontolimbic brain regions 24h into withdrawal from chronic intermittent vapors, but is normalized—or even elevated—in these same regions 3 weeks after cessation of chronic intermittent alcohol treatment (Vendruscolo et al., 2012). Down-regulated GR mRNA expression in early abstinence could reflect the recent hormonal environment in these frontolimbic regions during the 14-h intoxication phase of intermittent vapor treatment (Rivier et al., 1984).
Fig. 3.
A schematic illustrating proposed alterations in alcohol levels in the blood, corticosterone (CORT) in the blood and brain, and glucocorticoid receptors (GR) in the brain before, during and after dependence induction. (A) Blood and brain alcohol levels are strongly fluctuated during the intermittent alcohol vapor exposure. In this example, vapors are delivered for 14 h, beginning at the onset of the dark cycle. (B) Alcohol vapor stimulates CORT release to levels far exceeding the normal diurnal rhythm of plasma CORT. Neuroendocrine tolerance develops through out the induction period and eventually leads to the dampened HPA activity. (C) GR level in the brain decreased in response to the high CORT during the high alcohol period. On the other hand, increased brain CORT after dependence is hypothesized to come from de novo local synthesis or alterations in blood brain-barrier permeability or both after alcohol dependence. Abbreviations: Intox, intoxication; WD, withdrawal.
After removal from chronic alcohol treatment, peripheral corticosterone levels can remain dampened for several weeks into abstinence (Rasmussen et al., 2000; Zorrilla et al., 2001)—perhaps resulting in a compensatory elevation in GR expression within some of these brain regions important for addiction. In animals that have been exposed to high levels of alcohol for several months, abstinence is characterized by increases in prefrontal concentrations of glucocorticoids and heightened glucocorticoid/GR signaling (Brooks et al., 2008; Little et al., 2008). This could explain why repeated periods of abstinence and relapse are key elements of alcoholism (Koob and Le Moal, 2001). Fig. 3C shows a hypothetical model of how GR expression may change in the brain in response to peripheral fluctuation of glucocorticoids throughout the induction of dependence and into early and late abstinence. The complex interplay between intermittent exposure to alcohol and changes of GR responsiveness in the brain may lead to further neuronal adaptation and behavioral changes such as escalated and compulsive drinking, and increased probability of relapse after abstinence.
Glucocorticoids may target the medial prefrontal cortex (mPFC) to produce some of the neuroendocrine and behavioral changes associated with dependence
Glucocorticoids initiate non-genomic and genomic cellular events that provide both immediate and long-term effects, respectively (Kolber et al., 2008). The fluctuating levels of glucocorticoids during alcohol intoxication and after abstinence, as described above, could induce assorted adaptation processes in the brain. Although there are most likely several targets undergoing GR-mediated neuroadaptive changes following chronic alcohol, here we focus on the prefrontal cortex—a region of the brain known for this role in executive functions and regulation of emotions and behavior (Wilson et al., 2010; Kesner and Churchwell, 2011).
As shown in Fig. 4, mPFC may play a role in the long-loop negative feedback of the HPA axis (Sullivan and Gratton, 2002a). The GR has a four to five fold higher prevalence than mineralocorticoid receptor (MR) in the mPFC, which is notably different from the equal distribution of GR and MR in the hippocampus (Diorio et al., 1993; Cintra et al., 1994). Implantation of corticosterone pellets in the dorsal portion of the mPFC (dmPFC), to mimic high stress-like levels, attenuates HPA response to restraint stress (Diorio et al., 1993; Akana et al., 2001). Activation of the dmPFC dampens HPA responses, whereas lesions of the dmPFC produce exaggerated HPA responses (Diorio et al., 1993; Figueiredo et al., 2003; Radley et al., 2006; 2008; Jones et al., 2011). We hypothesize that corticosterone activates cells in the dmPFC that project to subcortical structures and inhibit HPA axis activity. In vitro studies support this hypothesis showing that corticosterone administration suppresses local GABA release in the dmPFC (prelimbic cortex)—a disinhibitory effect that would, in turn, lead to higher pyramidal cell activation and strengthen the overall dmPFC breaking effect on HPA activity (Hill et al., 2011).
In rodents, the mPFC is anatomically similar to the cingulate and premotor cortices of the frontal lobes in primates (Reep et al., 1987). The rodent mPFC also has functional similarity to the dorsolateral prefrontal cortex in primates (Kolb, 1984; Birrell and Brown, 2000; Barense et al., 2002; Seamans et al., 2008; Kesner and Churchwell, 2011). Chronic alcohol abuse and alcohol dependence can result in impaired performance on cognitive tasks associated with integrity of the mPFC (George et al., 2012; Kroener et al., 2012), suggesting that this region undergoes neuroadaptive change with prolonged exposure to moderate to high alcohol levels. We posit that repeatedly engaging in binge alcohol exposure stimulates HPA axis activity and leads to enduring GR signaling within the mPFC that produce changes in functions dependent on this region. In support of this hypothesis, mPFC GR mRNA expression is reduced and heavy, compulsive-like drinking is high early in abstinence from chronic alcohol, but prior chronic treatment with a GR antagonist prevents the development of this behavioral phenotype in rats (Vendruscolo et al., 2012). Acute treatment with a GR antagonist also reduce the mPFC-mediated memory deficit observed during acute withdrawal from chronic alcohol treatment in mice (Jacquot et al., 2008). Altogether, the findings suggest that prolonged exposure to alcohol impacts mPFC control of cognitive performance and addiction-related behaviors—at least in part—through glucocorticoid signaling.
Conclusions
We propose that acute stimulation of the HPA axis during repeated bouts of intoxication and the subsequent adaptation within this neuroendocrine axis and upstream in the prefrontal cortex are key factors in the transition from alcohol use to abuse and eventually to dependence. As individuals engage in repeated cycles of intoxication, abstinence, and relapse, a dynamic cascade of glucocorticoid signaling could trigger a series of neuroadaptive events in the prefrontal cortex that have broad implications on neural functioning and behavior. Other important factors modulating the development of alcohol use disorders are beyond the scope of the current review. However, these factors are worth noting and have been reported elsewhere: age onset of alcohol use/abuse (e.g. Dawson et al., 2008; Gilpin et al., 2012), substance co-use/abuse with alcohol (e.g. Hanson et al., 2008), genetic/epigenetic regulation (e.g. Tabakoff et al., 2009; Nieratschker et al., 2014), social components of drinking (e.g. Butler et al., 2014), sex differences (e.g. Fox et al., 2009; Wemm et al., 2013), other neurotransmitter/neuromodulator systems (e.g. Clapp et al., 2008; Gilpin, 2012) and the potential lateralized stress regulation in the prefrontal cortex (e.g. Sullivan and Gratton, 2002b). It is worth noting that the animal housing condition may be a factor that interacts with the alcohol drinking behavior and neural adaptations. The individual housing is often incorporated in the experimental during the drinking period or throughout the entire experiment. Single housing is known to induce stress (Greco et al., 1992) and increase voluntary alcohol consumption in rats (Yoshimoto et al., 2003). On the other hand, group housing may produce psychosocial stress from the hierarchy especially in male rodents (Pohorecky, 2010). Therefore, the potential stress effect from various housing conditions should be considered when interpreting alcohol effects in these studies.
Three important next steps in the field should be to (1) explore how glucocorticoid signaling changes within the prefrontal cortex during use, abuse, and dependence, (2) determine how alcohol and glucocorticoids interact to produce molecular and circuit-level neuroadaptive changes in the prefrontal cortex to impact downstream targets and alter neuroendocrine, autonomic, and behavioral functions related to stress and addiction, and (3) develop a deeper understanding and appreciation for the importance of sex, developmental status, and individual differences in this preclinical research, as most of the animal literature cited in this review was based on studies using adult male rodents. Gaining a new understanding the complex interplay among alcohol drinking, stress hormones, and the prefrontal cortex would provide further information in the development of new biomarkers to identify the progression of alcohol dependence and help guide the discovery of new promising treatments for alcohol use disorders.
Acknowledgements
We thank Jesse McClure for his critical reading of the manuscript. Our work is supported by the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism: AA021013 (HNR).
References
- Adinoff B, Best SE, Ye W, Williams MJ, Iranmenesh A. Adrenocortical and pituitary glucocorticoid feedback in abstinent alcohol-dependent women. Alcohol Clin Exp Res. 2010;34:915–924. doi: 10.1111/j.1530-0277.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. 2005a:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005b;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin Exp Res. 2005c;29:528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. Journal of Neuroendocrinology. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM IV) Washington, DC: American Psychiatric; 1994. [Google Scholar]
- Anacker AMJ, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7:473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Carrillo CA, Needham L, Leibowitz SF, Hoebel BG. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol. 2004;34:203–209. doi: 10.1016/j.alcohol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addiction Biology. 2006;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Becker HC. Alcohol Dependence, Withdrawal, and Relapse. Alcohol Res Health. 2008;31:348–361. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Lindsay TG, Cannady R. Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology. 2013;72:139–147. doi: 10.1016/j.neuropharm.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Licht CMM, Vreeburg SA, Smit JH, van den Brink W, Veltman DJ, de Geus EJC, Beekman ATF, Penninx BWJH. Heavy alcohol use, rather than alcohol dependence, is associated with dysregulation of the hypothalamic-pituitary-adrenal axis and the autonomic nervous system. Drug Alcohol Depend. 2011;116:170–176. doi: 10.1016/j.drugalcdep.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Croft AP, Norman G, Shaw SG, Little HJ. Nimodipine prior to alcohol withdrawal prevents memory deficits during the abstinence phase. Neuroscience. 2008;157:376–384. doi: 10.1016/j.neuroscience.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Brown SA, Goldman MS, Inn A, Anderson LR. Expectations of reinforcement from alcohol: their domain and relation to drinking patterns. J Consult Clin Psychol. 1980;48:419–426. doi: 10.1037//0022-006x.48.4.419. [DOI] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, Weiner JL. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front Integr Neurosci. 2014;7:102. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life Sciences. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Chassin L, Fora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: the effects of familial alcoholism and personality. J Abnorm Psychol. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Zoli M, Rosén L, Agnati LF, Okret S, Wikström AC, Gustaffsson JA, Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62:843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- Clapp P, Bhave SV, Hoffman PL. How Adaptation of the Brain to Alcohol Leads to Dependence: A Pharmacological Perspective. Alcohol Res Health. 2008;31:310–339. [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu C-H, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Patricia Chou S, June Ruan W, Grant BF. Age at First Drink and the First Incidence of Adult-Onset DSM-IV Alcohol Use Disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci USA. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ekman AC, Vakkuri O, Vuolteenaho O, Leppäluoto J. Delayed pro-opiomelanocortin activation after ethanol intake in man. Alcohol Clin Exp Res. 1994;18:1226–1229. doi: 10.1111/j.1530-0277.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, Parsons OA. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol Clin Exp Res. 2002;26:1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hård E, Thomasson R, Engel JA, Hansen S. Metyrapone-induced suppression of corticosterone synthesis reduces ethanol consumption in high-preferring rats. Pharmacol Biochem Behav. 1994;48:977–981. doi: 10.1016/0091-3057(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong K-IA, Siedlarz KM, Bergquist K, Anderson G, Kreek MJ, Sinha R. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol and Alcoholism. 2009;44:575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci USA. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Alcohol-seeking behavior: the roles of the hypothalamic-pituitary-adrenal axis and the endogenous opioid system. Alcohol Health Res World. 1998;22:202–210. [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): Effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46:329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS ONE. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Greco AM, Gambardella P, Sticchi R, D'Aponte D, de Franciscis P. Circadian rhythms of hypothalamic norepinephrine and of some circulating substances in individually housed adult rats. Physiol Behav. 1992;52:1167–1172. doi: 10.1016/0031-9384(92)90477-j. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural pathways of stress integration: relevance to alcohol abuse. Alcohol Res. 2012;34:441–447. doi: 10.35946/arcr.v34.4.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT-Y, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu Q-S, Gorzalka BB, Hillard CJ. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcohol Clin Exp Res. 2008;32:2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Jenkins JS, Connolly J. Adrenocortical response to ethanol in man. Br Med J. 1968;2:804–805. doi: 10.1136/bmj.2.5608.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural Pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104:266–271. doi: 10.1016/j.physbeh.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Murphy JM, Mellor-Burke JJ, Lumeng L, Li TK. The benzodiazepine inverse agonist RO19-4603 exerts prolonged and selective suppression of ethanol intake in alcohol-preferring (P) rats. Psychopharmacology (Berl) 1994;115:325–331. doi: 10.1007/BF02245073. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Karanikas CA, Lu Y-L, Richardson HN. Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience. 2013;249:98–105. doi: 10.1016/j.neuroscience.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, Wiedemann K. Alcohol self-administration, craving and HPA-axis activity: an intriguing relationship. Psychopharmacology (Berl) 2002;164:239–240. doi: 10.1007/s00213-002-1255-3. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcohol Clin Exp Res. 2001;25:1662–1672. [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Wieczorek L, Muglia LJ. Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress. 2008;11:321–338. doi: 10.1080/10253890701821081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Korányi L, Endröczi E, Tal E, Lévay G. The effect of acute and chronic ethanol administration on serum corticosterone concentration in rats. Acta Physiol Hung. 1987;69:123–128. [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS ONE. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci. 1997;17:8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex BW, Ellingboe JE, Teoh SK, Mendelson JH, Rhoades E. Prolactin and cortisol levels following acute alcohol challenges in women with and without a family history of alcoholism. Alcohol. 1991;8:383–387. doi: 10.1016/0741-8329(91)90618-7. [DOI] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye J-H. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addiction Biology. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37:1922–1928. doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li T-K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats - Animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgregor IS, Gallate JE. Rats on the grog: novel pharmacotherapies for alcohol craving. Addict Behav. 2004;29:1341–1357. doi: 10.1016/j.addbeh.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- NIAAA. NIAAA Council Approves Definition of Binge Drinking. 2004:1–4. [Google Scholar]
- Nieratschker V, Grosshans M, Frank J, Strohmaier J, Goltz von der C, El-Maarri O, Witt SH, Cichon S, Nöthen MM, Kiefer F, Rietschel M. Epigenetic alteration of the dopamine transporter gene in alcohol-dependent patients is associated with age. Addiction Biology. 2014;19:305–311. doi: 10.1111/j.1369-1600.2012.00459.x. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oei TPS, Morawska A. A cognitive model of binge drinking: the influence of alcohol expectancies and drinking refusal self-efficacy. Addict Behav. 2004;29:159–179. doi: 10.1016/s0306-4603(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997a;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C. Role of arginine vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Res. 1997b;744:83–95. doi: 10.1016/s0006-8993(96)01082-7. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C. Divergence in the expression of molecular markers of neuronal activation in the parvocellular paraventricular nucleus of the hypothalamus evoked by alcohol administration via different routes. J Neurosci. 1998;18:4344–4352. doi: 10.1523/JNEUROSCI.18-11-04344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Acute novel stressors modify ethanol intake of psychosocially stressed rats. Pharmacol Biochem Behav. 2010;95:390–400. doi: 10.1016/j.pbb.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Collier SD, Wu WJ. Ethanol-induced activation of the hypothalamic-pituitary-adrenal axis in a mouse model for binge drinking: role of Ro15-4513-sensitive gamma aminobutyric acid receptors, tolerance, and relevance to humans. Life Sciences. 1998;63:1137–1146. doi: 10.1016/s0024-3205(98)00375-0. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Redei E, Branch BJ, Gholami S, Lin EY, Taylor AN. Effects of ethanol on CRF release in vitro. Endocrinology. 1988;123:2736–2743. doi: 10.1210/endo-123-6-2736. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- Rivier C, Vale W. Influence of the frequency of ovine corticotropin-releasing factor administration on adrenocorticotropin and corticosterone secretion in the rat. Endocrinology. 1983;113:1422–1426. doi: 10.1210/endo-113-4-1422. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE. The Reactive Scope Model - a new model integrating homeostasis, allostasis, and stress. Horm Behav. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339:161–165. doi: 10.1016/0006-8993(85)90638-9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Koob G. Neuropharmacology. Dark side of drug dependence. Nature. 1994;371:108–109. doi: 10.1038/371108a0. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Selvage D. Roles of the locus coeruleus and adrenergic receptors in brain-mediated hypothalamic-pituitary-adrenal axis responses to intracerebroventricular alcohol. Alcohol Clin Exp Res. 2012;36:1084–1090. doi: 10.1111/j.1530-0277.2011.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcohol Clin Exp Res. 2013;37(Suppl 1):E172–E180. doi: 10.1111/j.1530-0277.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216:569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SM, Santos-Marques MJ, Madeira MD. Sexually dimorphic response of the hypothalamo-pituitary-adrenal axis to chronic alcohol consumption and withdrawal. Brain Res. 2009;1303:61–73. doi: 10.1016/j.brainres.2009.09.099. [DOI] [PubMed] [Google Scholar]
- Silveri MM. Adolescent Brain Development and Underage Drinking in the United States: Identifying Risks of Alcohol Use in College Populations. Harv Rev Psychiatry. 2012;20:189–200. doi: 10.3109/10673229.2012.714642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong K-IA, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond, B, Biol Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MAC, Wand G. Stress and the HPA Axis: Role of Glucocorticoids in Alcohol Dependence. Alcohol Res. 2012;34:468–483. doi: 10.35946/arcr.v34.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 2002a;927:69–79. doi: 10.1016/s0006-8993(01)03328-5. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002b;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Sze PY. The permissive role of glucocorticoids in the development of ethanol dependence and tolerance. Drug Alcohol Depend. 1977;2:381–396. doi: 10.1016/0376-8716(77)90040-0. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, et al. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Science. Vol. 213. New York, NY: 1981. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin; pp. 1394–1397. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wemm S, Fanean A, Baker A, Blough ER, Mewaldt S, Bardi M. Problematic drinking and physiological responses among female college students. Alcohol. 2013;47:149–157. doi: 10.1016/j.alcohol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CRE, Gaffan D, Browning PGF, Baxter MG. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends Neurosci. 2010;33:533–540. doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, Komura S, Yasuhara M. The age of drinking onset and housing condition influences rat alcohol drinking behavior. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2003;38:335–340. [PubMed] [Google Scholar]
- Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily “binge” intragastric alcohol administration. Alcohol Clin Exp Res. 2000;24:1575–1582. [PubMed] [Google Scholar]
- Zoeller RT, Rudeen PK. Ethanol blocks the cold-induced increase in thyrotropin-releasing hormone mRNA in paraventricular nuclei but not the cold-induced increase in thyrotropin. Brain Res Mol Brain Res. 1992;13:321–330. doi: 10.1016/0169-328x(92)90215-w. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]