Abstract
The process of glutamate release, activity, and reuptake involves the astrocyte, the presynaptic and postsynaptic neuron. Glutamate is released into the synapse and may occupy and activate receptors on both neurons and astrocytes. Glutamate is rapidly removed from the synapse by a family of plasma membrane excitatory amino acid transporters (EAATs), also localized to neurons and astrocytes. The purpose of the present study was to examine EAAT labeling in postmortem human cortex at the light and electron microscopic levels. Postmortem prefrontal cortex was processed for EAAT1 and EAAT2 immunohistochemistry. At the light microscopic level, EAAT1 and EAAT2 labeling was found in both grey and white matter. Most cellular labeling was in small cells which were morphologically similar to glia. In addition, EAAT1 labeled neurons were scattered throughout, some of which were pyramidal in shape. At the electron microscopic level, EAAT1 and EAAT2 labeling was found in astrocytic soma and processes surrounding capillaries. EAAT labeling was also found in small astocytic processes adjacent to axon terminals forming asymmetric (glutamatergic) synapses. While EAAT2 labeling was most prevalent in astrocytic processes, EAAT1 labeling was also present in neuronal processes including the soma, axons, and dendritic spines. Expression of EAAT1 protein on neurons may be due to the hypoxia associated with the postmortem interval, and requires further confirmation. The localization of EAATs on the astrocytic plasma membrane and adjacent to excitatory synapses is consistent with the function of facilitating glutamate reuptake and limiting glutamate spillover. Establishment that EAAT1 and EAAT2 can be measured at the EM level in human postmortem tissues will permit testing of hypotheses related to these molecules in diseases lacking analogous animal models.
Keywords: ultrastructure, electron microscopy, glutamate transporter, astrocytes, mitochondria
INTRODUCTION
Glutamate levels in the CNS are tightly regulated by a family of excitatory amino acid transporters (EAATs) localized to neurons and astroglia (for review see Danbolt, 2001). At most excitatory synapses, perisynaptic-localized EAATs bind and transport glutamate into astrocytes, where glutamate is converted to glutamine (Ottersen et al., 1992) or is utilized as a metabolic intermediate for the tricarboxylic acid cycle (Balazs et al., 1970). Glutamine is shuttled back to the presynaptic terminal via at least two distinct transport systems, and glutamine may be converted back to glutamate by the enzyme glutaminase, and repackaged for release from the presynaptic terminal (Sibson et al., 1997). The EAATs have a critical role in this cycle, as they maintain low basal levels of glutamate in the synapse, facilitating receptor-mediated responses to glutamate release (Rothstein et al., 1996; Shan et al., 2013).
To prevent non-physiologic (ie pathological) glutamate spillover, EAATs must be expressed at high levels on the plasma membrane and be localized adjacent to the synapse (Cholet et al., 2002; O'Shea, 2002). Spillover of glutamate may occur when levels of synaptic glutamate exceed the capacity of the reuptake machinery to remove glutamate from the synapse (Tsvetkov et al., 2004; Weng et al., 2007; Drew et al., 2008; Leveille et al., 2008). The extent or degree of spillover is likely a critical event, since the proximity of extrasynaptic receptors and adjacent synapses may vary (Rusakov et al., 1998). Physiologic spillover, in areas such as the hippocampus and cerebellum, facilitates activation of glutamate receptors on adjacent synapses (Kullman and Aszetly, 1998; Lozovaya et al., 2004; Marcaggi and Atwell, 2007; Tzingounis and Wadiche, 2007). However, in most brain regions, the extent of spillover of glutamate is thought to be quite low, and extrasynaptic glutamate levels are postulated to be tightly regulated, as activation of extrasynaptic glutamate receptors has potent effects (Hardingham et al., 2002; Hardingham and Bading, 2002; Bridges et al., 2012). For example, activation of extrasynaptic NMDA receptors promotes initiation of NMDA spikes, while long term depression (LTD) and long term potentiation (LTP) can be readily induced in the adult cortex by activation of extrasynaptic NR2B containing NMDA receptors (Massie et al., 2008; Chalifoux et al., 2011).
The cellular distribution of EAATs highlights their importance in synaptic functions. Five EAATs, which differ in regional and cellular distribution, have been characterized in the CNS and elsewhere in the body and are well-reviewed by Danbolt (2001). The human and rodent analogues are: 1) EAAT1 in human (Arriza et al., 1994) or GLAST in rodent (Storck et al., 1992), 2) EAAT2 in human (Arriza et al., 1994) or GLT-1 in rodent (Pines et al., 1992), 3) EAAT3 in human (Arriza et al., 1994) or EAAC1 in rodent (Kanai and Hediger, 1992), 4) EAAT4 in human (Fairman et al., 1995), and 5) EAAT5 in human (Arriza et al., 1997). GLT1 and GLAST have been localized throughout the rodent brain, where GLT is predominantly in the forebrain, while GLAST is more robust in the cerebellum (Chaudhry et al., 1995; Lehre et al., 1995). EAAT3 is highly expressed in the cortex, hippocampus, striatum, and peripheral tissues (Rothstein et al., 1994). EAAT4 is highly enriched in cerebellar Purkinje cells, and in astrocytes in the spinal cord and forebrain (Hu et al., 2003). EAAT5 is expressed predominantly in the retina (Arriza et al., 1997). Of these, EAAT2 is the most robustly expressed and is the main glutamate transporter in the brain, clearing a majority of extracellular glutamate in most brain regions (Haugeto et al., 1996; Tanaka et al., 1997).
In postmortem thalamus and cortex in schizophrenia, we have found alterations in EAAT1 and/or EAAT2 expression, as well as changes in the expression of molecules that regulate these transporters (Huerta et al., 2006; Bauer et al., 2008, Shan et al., 2013, Shan et al., 2014). In general, these changes are consistent with diminished regional expression of glial glutamate transporter expression and activity. EAAT1 and EAAT2 expression is generally found in discrete subsets of glia (Regan et al., 2007). GLAST (EAAT1) knockout mice have behavioral endophenotypes associated with schizophrenia without a decrease in EAAT2 expression (Karlsson et al., 2008). Thus, we have focused our attention on EAAT1 and EAAT2 for their potential role in schizophrenia and other severe neuropsychiatric illnesses. In the present study we have localized these transporters at the light and electron microscopic level in normal human postmortem frontal cortex. This work has been presented in preliminary form (Roberts et al., 2011).
MATERIALS AND METHODS
Human postmortem cases
Human brain tissue was obtained with the permission of the next of kin from the Maryland Brain Collection (IRB# HP-00043632) and the Alabama Brain Collection (IRB# F080306003). In addition, we have a non-human subjects protocol for tissue from the Maryland Brain Collection that is used at UAB (N110411002). The tissue was collected from nine adult control subjects (5 males, 4 females) ranging in age from 32 to 67 years, with no history of central nervous system disease or neurological disease as determined by family interviews, autopsy reports (if applicable) and gross neuropathology reports. Eight cases were used for electron microscopy, and one additional case was used for western blot analysis. Demographics are presented in Table I.
Table I.
Demographic and other information.
| ID | A/ R/ S | PMI (hrs) | pH | Cause of death | Source |
|---|---|---|---|---|---|
| 1 | 37/C/ F | 5.0 | 7.0 | ASCVD | MBC |
| 2 | 32/C/ F | 8.0 | 6.3 | Cardiac Arrest | ABC |
| 3 | 32/C/ F | 6.0 | 7.2 | Cardiac Arrhythmia | MBC |
| 4 | 36/C/M | 4.0 | 6.7 | MVA | MBC |
| 5 | 43/AA/F | 6.0 | 7.2 | ASCVD | MBC |
| 6 | 61/C/M | 4.0 | 6.5 | COPD | ABC |
| 7 | 67/C/M | 8.0 | 6.6 | Lung Cancer | ABC |
| 8 | 40/AA/M | 7.0 | 6.7 | Cardiac Arrhythmia | MBC |
| 9 | 65/C/M | 23.0 | 6.0 | Myocardial Infarction | ABC |
on the cases examined. Abbreviations: A/R/S, age/race/sex; A, African-American; C, Caucasian; M, male; F, female; PMI, postmortem interval; ASCVD, atherosclerotic cardiovascular disease; MVA, motor vehicle accident; COPD, chronic obstructive pulmonary disease; MBC, Maryland Brain Collection; ABC, Alabama Brain Collection. Cases 1-8 were used for electron microscopy. The mean ±SD for the eight EM cases for age, PMI and pH was 43.5 ±13.3 years, 6.0 ±1.60 hours and 6.78±0.34, respectively. Case #9 was used for Western Blot analysis.
Mouse
GLAST knockout and heterozygous tissues were provided by Kohichi Tanaka (mice described in Watase, et al., 1998). Brains were dissected from sacrificed animals, the frontal cortex removed, and frozen at – 80°C until processed for Western blots.
Antibodies
The EAAT 1 antibody was a polyclonal rabbit antibody purchased from Abcam (http://www.abcam.com/EAAT1-antibody-ab416.html) and directed at a synthetic peptide corresponding to 20 residues near the C-terminus of rat EAAT1. The EAAT2 antibody (http://www.millipore.com/catalogue/item/ab1783) was a polyclonal guinea pig antibody purchased from Millipore, which is directed at a synthetic peptide from the C-terminus of rat GLT1. None of the antibodies were affinity purified.
Tissue processing
Coronal blocks 1 cm thick of the dorsolateral prefrontal cortex were immersed in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (PB), pH 7.4). Tissue from cases 1-8 was cut in 10-12 series at a thickness of 40 μm with a vibratome. Adjacent series of sections were stained for EAAT1, Kluver-Barrera, or EAAT2. Tissue used for Western Blot analysis was dissected with a scalpel from frozen tissue that had been freshly frozen on dry ice and stored at −80°C.
Western Blot Analysis
Western blot analysis was performed according to our previously published techniques (Bauer et al., 2008). Briefly, homogenized human and mouse brain (fresh frozen) from frontal cortex was prepared for western blot analyses with double distilled filter purified water (dH20) and sample buffer (4.5% sodium dodecyl sulfate (SDS), 15% βmercaptoethanol, 0.018% bromophenol blue, and 36% glycerol in 170mM Tris-HCl, pH 6.8) and heated at 70C for 10 minutes. Samples were run on 4–12% gradient gels and transferred to polyvinylidene difluoride (PVDF) membranes using a semi-dry transblotter (Bio-Rad, Hercules, CA, USA). The membranes were blocked with LiCor blocking buffer (LiCor, Lincoln, NE, USA) for 1 hour at room temperature, and then probed with the primary antibodies. After three 8 minute washes in phosphate buffered saline (PBS), the membranes were then incubated with the appropriate second antibody with infared-Dye 670 or 800cw labeled in LiCor blocking buffer or 5% bovine serum albumin in PBS for 1 hour at room temperature. Washes were repeated after the secondary antibody incubation. Membranes were scanned using a LiCor Odyssey scanner, and the intensity value for each protein band was measured using the Odyssey 2.1 software.
Kluver-Barrera
Sections were prepared by rinsing in 0.1M phosphate buffer, pH7.4 (PB) (4 × 30 min), and then placed on charged slides and allowed to dry overnight. Slides were then incubated in dH2O for 5 minutes, followed by dehydration in 50% ethanol (EtOH) and 70% EtOH for 5 minutes each. Slides were then placed in 0.1% luxol fast blue solution (purchased from Electron Microscopy Science, item number 26681-01) and placed in a 65 C° water bath with slight agitation for 24 hours. Once removed, the tissue was rehydrated in 95% EtOH, 70% EtOH, 50% EtOH, and dH2O for 5 minutes each. Tissue was placed in 0.05% lithium carbonate solution, aqueous, for 12 minutes with constant agitation, followed by 70% EtOH (2 min, constant agitation), 70% EtOH (1 min, constant agitation), and 50% EtOH (1 min). Tissue was checked for differentiation under the light microscope, and then placed in dH2O (2 × 5 min). The sections were counterstained in 0.1% cresyl violet acetate solution, aqueous, for 10 minutes, followed by dehydration in 50% EtOH (2 min), 70% EtOH (2 min), 95% EtOH (2 × 2 min), 100% EtOH (2 × 2 min). The tissue was placed in xylene (2 × 2 min) and was coverslipped using Eukitt.
Immunohistochemistry
Sections were incubated in 1% sodium borohydride, 0.1 M PB solution for 15 minutes, and then placed in a pre-incubation buffer (10% normal goat serum in 0.01 M PB) for 30 minutes. Sections were then incubated for 72 hours in the EAAT1 antibody (1:2,000) or the EAAT2 antibody (1:3,000) in 3% normal goat serum in 0.01 M PB. Sections were subsequently incubated for 60 minutes in biotinylated goat anti-rabbit IgG (EAAT1) or biotinylated goat anti-guinea pig IgG (EAAT2) (1:200 dilution in 1.5% normal goat serum in 0.01M PB), and then in the avidin-biotin peroxidase complex (ABC standard kit) for 45 min (1:100 dilution in 0.01 M PBS for each solution). To visualize the reaction product, sections were then incubated in diaminobenzidine (110 mg tablet dissolved in 15 ml PBS, 12 μl 30% hydrogen peroxide), with development time varying between 2-7 min. Eliminating the primary antibody and otherwise processing the tissue in a similar fashion abolished all staining.
Electron Microscopy
Sections were processed for electron microscopic analysis using standard techniques and flat embedded. Samples were cut from representative regions, mounted on beam capsules and thin sectioned. Electron micrographs of neuropil were taken at 5,000-25,000X from serial sections.
RESULTS
Western Blots
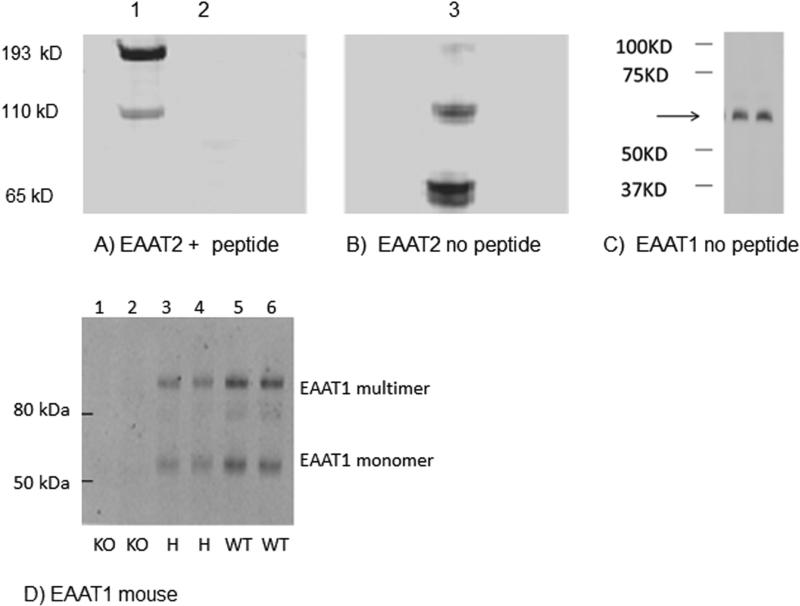
We have previously tested the specificity of EAAT1-2 with Western blot assays using varying protein concentrations of human brain homogenate and determined that our assays are in the linear range of the concentrations correlated for each protein to be assayed (Bauer, et. al., 2008, 2010). For EAAT1-2, digital images were obtained using a LiCor Odyssey scanner (LiCor, Lincoln, Nebraska, USA) as previously described (Vlachos, et al., 2009; Zhao, et al., 2009; Figure 1). EAAT1 protein appeared as a monomer at about 65kD. EAAT2 protein appeared as a monomer (65 kD) and multimer (110kD). Preadsorption with the EAAT2 immunogen peptide completely abolished staining. The same EAAT1 antibody that was used for the Western blots and immunohistochemistry did not produce any staining in Western blots using cortex from GLAST knockout mice (Figure 1). Cortex from heterozygote mice showed diminished staining compared to wild type mice (Figure 1).
Figure 1.
Western blots of human cortical homogenate (lanes 2-6, 10 ug of total protein/lane from case #9). A,B) Lane 1 shows the molecular weight standards. Blots A and B were generated from the same gel and transblot. Blot A was probed with EAAT2 antibody preincubated overnight with a blocking peptide (Lane 2, 90:1 molar equivalents peptide: antibody) identical to the peptide used to make the antibody. Blot B was probed with EAAT2 antibody (1:1000) that was not incubated with blocking peptide. Both blots were imaged together with a Licor scanner under identical conditions. EAAT2 monomer (65 kD) and multimer are present in lane 3, but not lane 2, which was blocked with the peptide. C) Blot C was probed with EAAT1 antibody (1:500). EAAT1 monomer (65 kD) is indicated by arrow; sample run in duplicate. D) Western blot analysis of EAAT1 expression in the rodent frontal cortex in GLAST knockout mice (KO, lanes 1 and 2), GLAST heterozygous mice (H, lanes 3 and 4), and wild type mice (WT, lanes 5 and 6). EAAT1 migrates as a monomer (about 60 kDa) and multimer (about 120 and 180 kDa). The two lanes for each mouse are duplicates.
Light microscopy
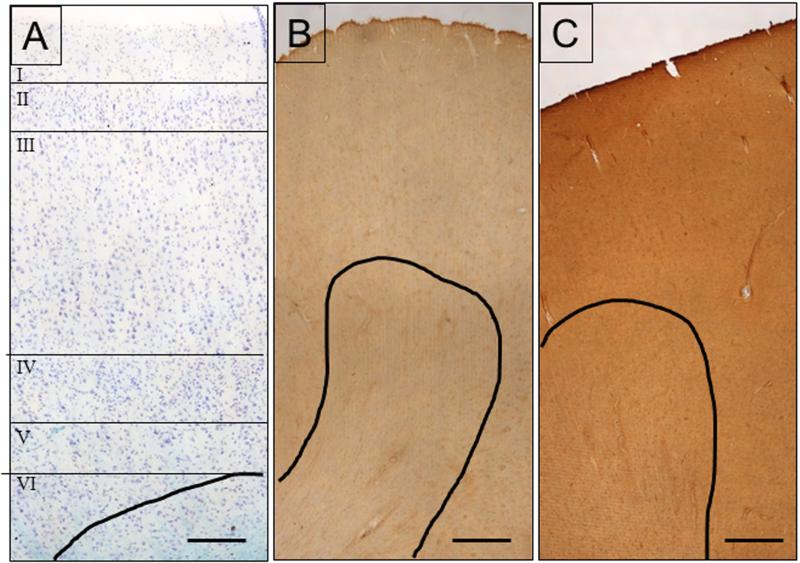
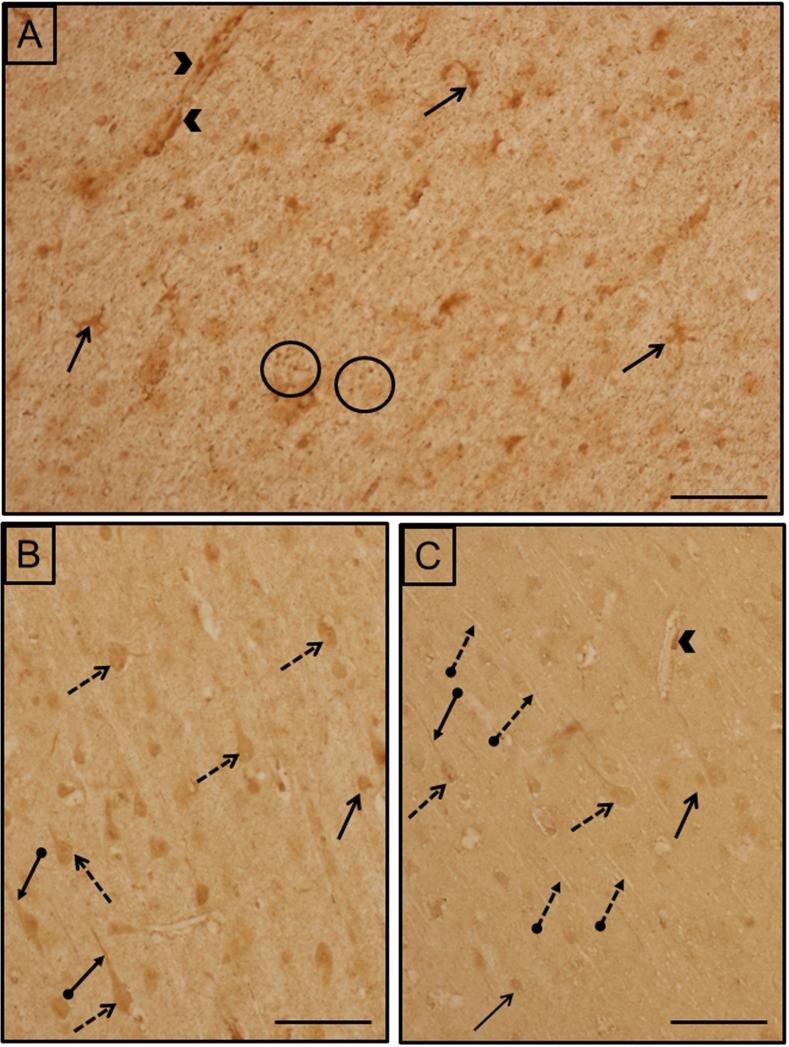
Kluver-Barrera preparations were used to assess structural normalcy and to identify cortical layers (Fig 2A). EAAT1 labeling was found in both grey and white matter, with the former being more heavily and diffusely labeled (Fig. 2B). The intensity of immunoreactivity was similar among cortical layers I-VI. Cellular labeling was in small cells which were morphologically similar to glia. Labeled processes were observed throughout the neuropil and around capillaries. In addition, labeled neurons were also scattered throughout, some of which were pyramidal in shape. Labeled neurons were more abundant in the deeper layers, than in middle or superficial layers. In the white matter, EAAT1 was present in small glial cells, their processes and punctate structures. Labeling was also present around capillaries.
Figure 2.
A) Kluver-Barrera stain used to identify cortical layers and asses cytoarchitectural integrity. The lines indicate separate cortical layers. B) EAAT1 localization. C) EAAT2 localization. The black line in all three images delineates the boundary between white matter and grey matter. All images are from case #5. Scale bars= 250 μm (A), 500 μm (B,C).
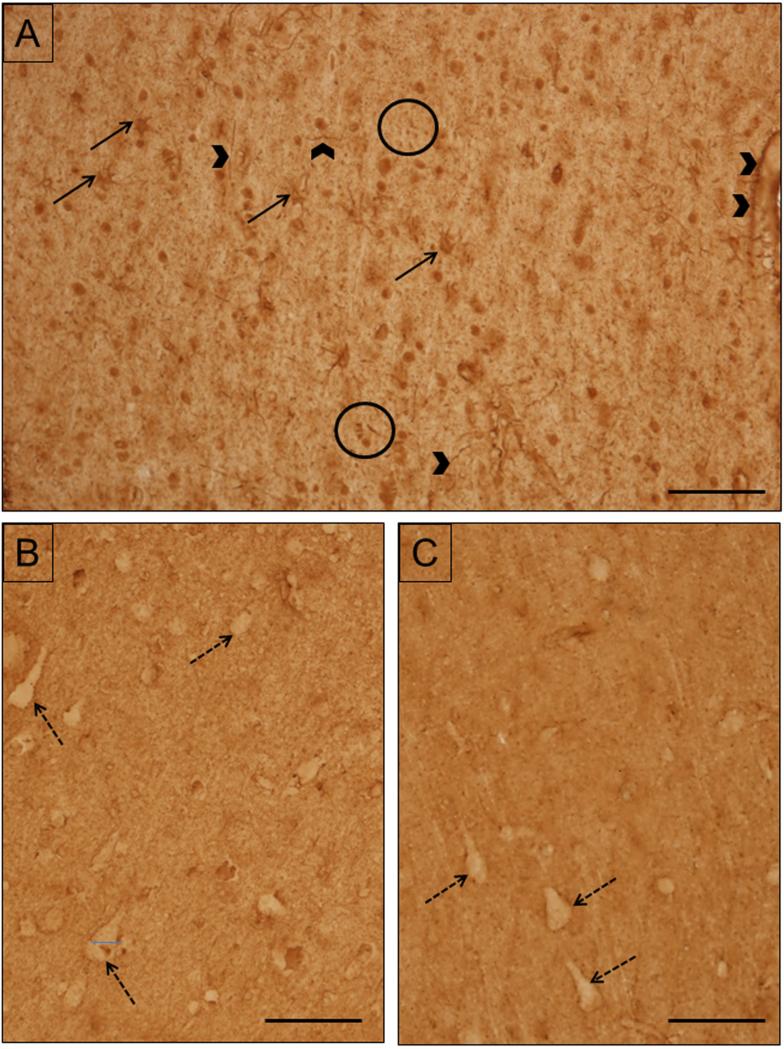
EAAT2 labeling was present throughout the grey and white matter in small cells which are most likely glial cells and their processes (Fig. 2C). The overall intensity of EAAT2 labeling was greater than that of EAAT1. EAAT2 labeling was similar in intensity throughout the cortical layers. EAAT2 labeling was homogeneously and diffusely distributed in the grey matter. Unlabeled neurons were surrounded by labeled neuropil. In the white matter, labeled glial cell bodies morphologically similar to astrocytes were abundant. Labeled thick glial processes and punctate structures were abundant.
Electron Microscopy
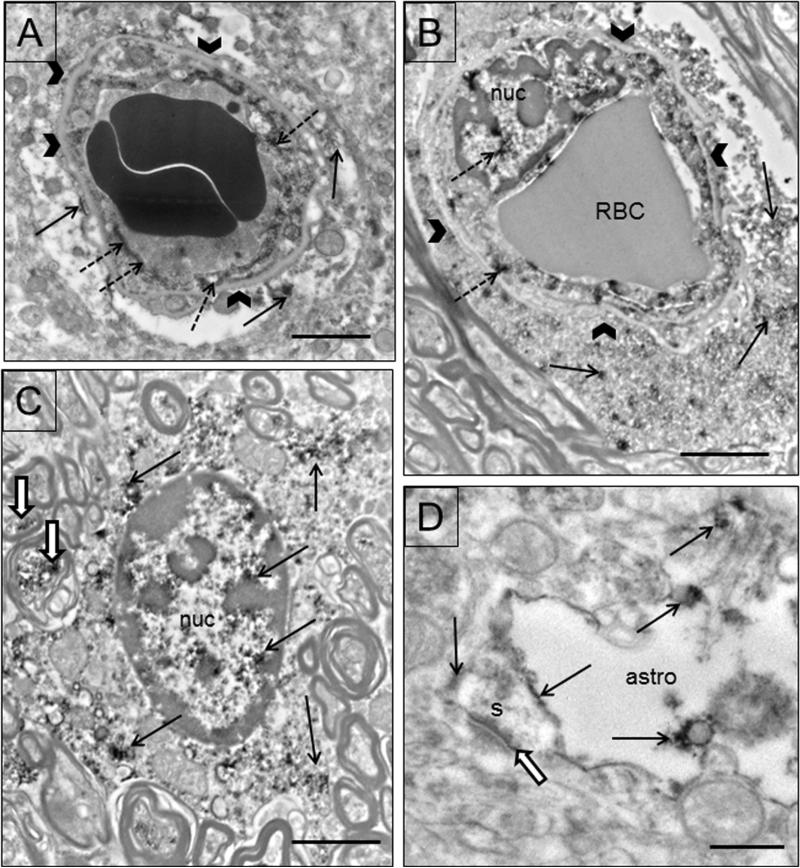
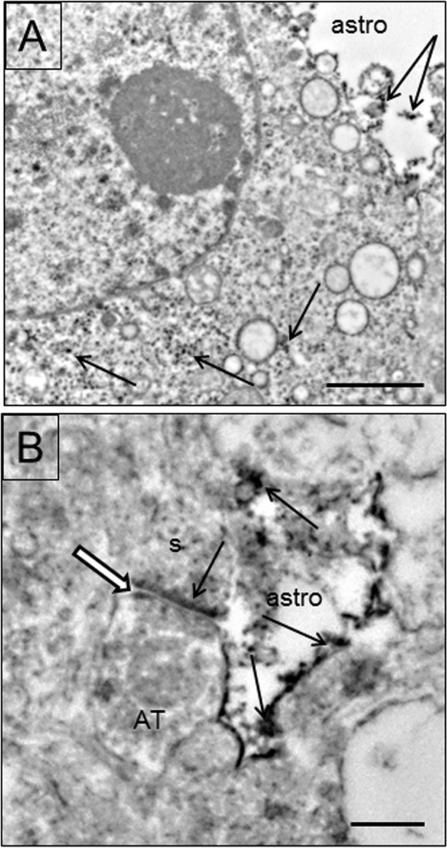
EAAT1 immunoreactivity is found in astrocytes, endothelial cells and neurons. EAAT1 immunoreactivity is located in endothelial cells and astrocytic processes surrounding capillaries (Fig. 5A,B). Both the nucleus and the cytoplasm of endothelial cells and astrocytes are immunoreactive (Fig. 5A, C). This localization was present in both grey and white matter. In grey matter, small astrocytic processes contain immunoreactivity close to asymmetric synapses (Fig. 5D). Mitochondria in glial processes sometimes display immunoreactivity.
Figure 5.
Electron micrographs of EAAT1 in glial profiles. A) A capillary in the grey matter is shown with two red blood cells in the lumen. Labeling occurs in the endothelial cell (dotted arrows), and the surrounding astrocytic endfeet (arrows). Basal lamina is indicated by arrowheads. B) A capillary in the white matter is shown with one red blood cell (RBC), a labeled endothelial cell (dotted arrows) and labeled astrocytic endfeet (arrows). Both the nucleus (nuc) and the cytoplasm of the endothelial cell are immunoreactive (dotted arrows). C) A protoplasmic astrocyte in white matter is labeled (arrows) in both the nucleus (nuc) and the cytoplasm. The cell is surrounded by myelinated axons, some of which are labeled (black and white arrows). D) A labeled (arrows) astrocytic process (astro) close to an asymmetric synapse (black and white arrow) on a spine (s). Scale bars = 2μm (A,C) and 0.5 μm in D.
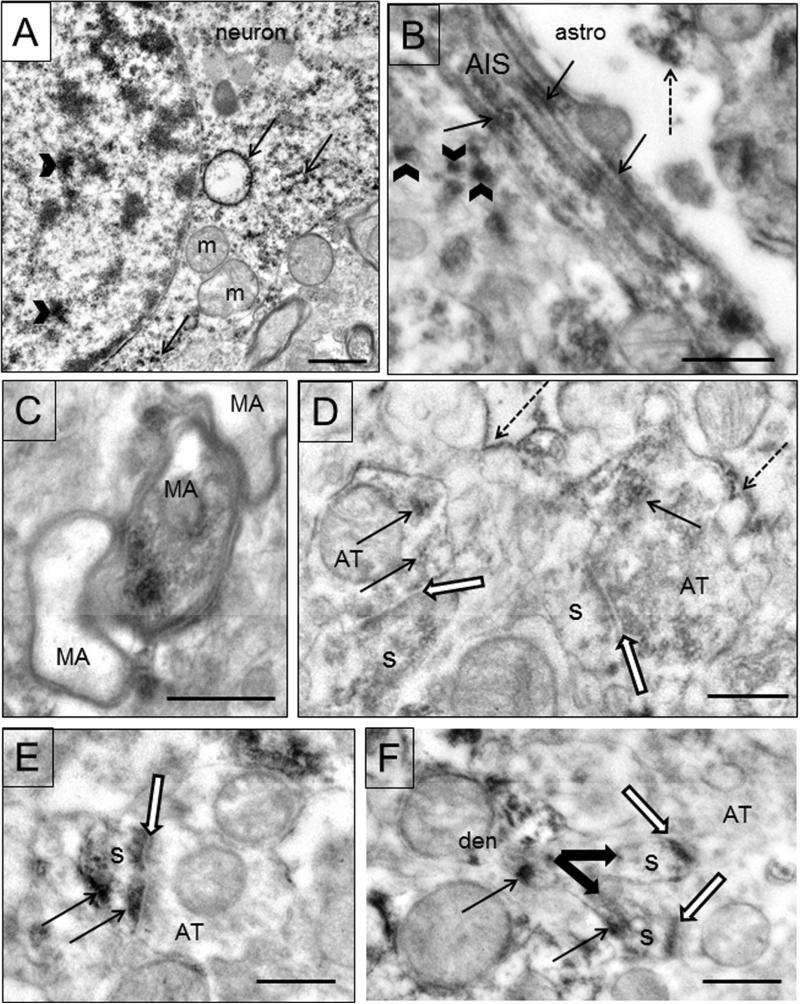
EAAT1 labeling is also present in neuronal profiles. Immunoreactivity is present in neuronal soma in both the nucleus and cytoplasm (Fig. 6A). Ribosomes and rough endoplasmic reticulum (rER) are labeled within the cytoplasm (Fig. 6A). Within axons, labeling is present in the axon initial segment, predominantly on the fasciculations of microtubules, one of their defining characteristics (Fig. 6B). EAAT1 immunoreactivity is present in some myelinated axons (Fig. 5C, 6C). In axon terminals, immunoreactivity is light and patchy (Fig. 6D). Dendrites and spines also displayed immunoreactivity (Figs. 6E,F). EAAT1 immunoreactivity is deposited at the postsynaptic density and at times elsewhere in the spine. Labeling is also found in discrete deposits in the neuropil in structures too small to be identified (Fig. 6B). Although EAAT1 labeling is present in glial cells and neurons, the mitochondria within these cells are usually not immunoreactive.
Figure 6.
Electron micrographs of EAAT1 in neuronal profiles. A) EAAT1 labeling in a neuronal soma. This neuron was identified by morphological criteria and only a portion of it is shown due to space limitations. Labeling is present in the nucleus (arrowhead) and in the cytoplasm on rER and free ribosomes (arrows). Unlabeled mitochondria (m) are present. B) An axon initial segment (AIS) has immunoreactivity deposited on the fasciculated microtubules (arrows). An adjacent astrocytic process (astro) is labeled (dotted arrow). EAAT1 immunoreactivity is also found in discrete deposits (arrowheads) in the neuropil in structures too small to be identified. C) Three myelinated axons (MA) are shown, one of which is labeled (arrow). Case #7. D) Labeling is found in axon terminals (AT) where it is light and patchy (arrows). Both terminals (AT) are forming synapses with spines (s) (black and white arrows). Immunoreactivity is also found on astrocytic membranes (dotted arrows). E) An axon terminal (AT) forms an asymmetric synapse (black and white arrow) with a labeled (arrows) spine (s). EAAT1 immunoreactivity is deposited at the postsynaptic density and elsewhere in the spine. F) Two spines (s) emerge (thick arrows) from a labeled dendrite (den). Labeling (thin arrows) is found in the dendrite, the bifurcated spines at the postsynaptic density (black and white arrows) and elsewhere in the spine. Scale bars=0.5um.
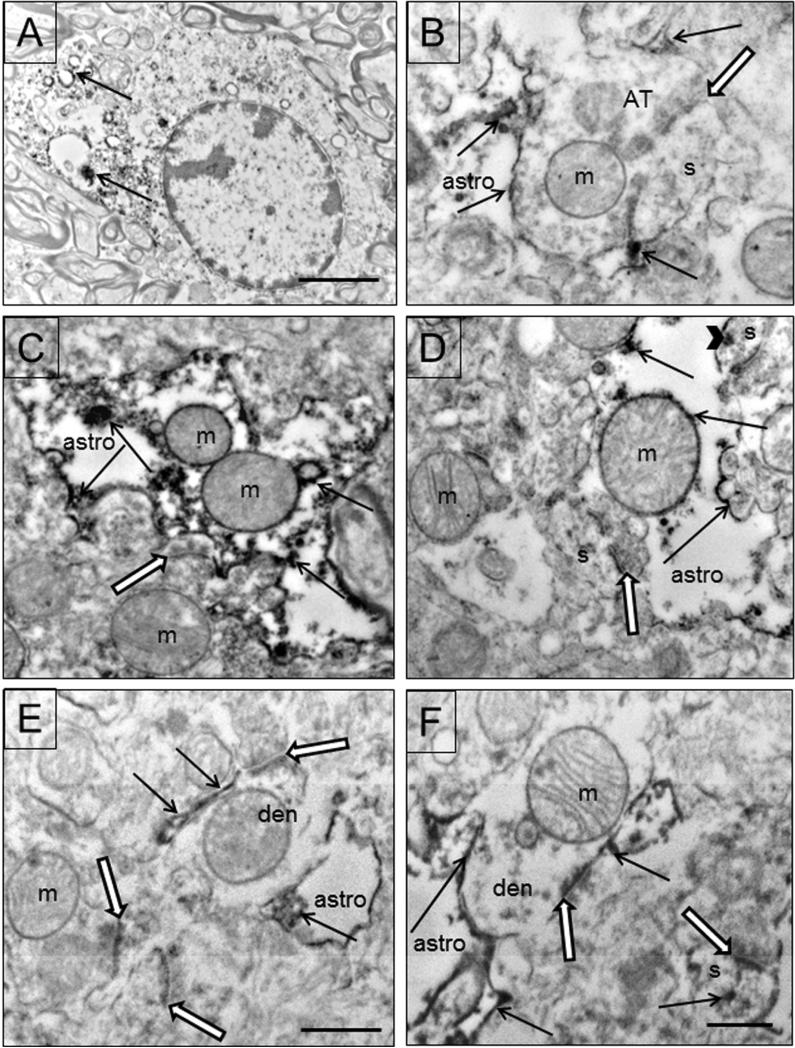
EAAT2 is prominently deposited in glial profiles. Astrocytic soma are labeled on polyribosomes and rER (Fig. 7A). Labeled astrocytic processes are abundant throughout the neuropil and are in close contact with asymmetric synapses (Figs. 7 B-E). The amount of immunoreactivity varies from light (Fig. 7B,D,E,F) to heavy (Fig. 7C). Labeling occurs throughout the astrocyte, on its cytoplasmic membrane, on the outer mitochondrial membrane, and on rER and polyribosomes. EAAT2 labeling is deposited on the astrocytic membrane that is immediately adjacent to asymmetric synapses.
Figure 7.
Electron micrographs of EAAT2 in glial profiles. A) EAAT2 labeling in an astrocyte in the white matter. Polyribosomes and rER are labeled (arrows). Case #3. B-F) Examples of labeled astrocytic processes (astro) in close contact with asymmetric synapses (black and white arrows). B) An axon terminal (AT) forms an asymmetric synapse with a spine (s). A labeled (arrows) astrocytic process is adjacent to the terminal and EAAT immunoreactivity is located right at the synapse. C) An example of a heavily labeled astrocytic process adjacent to a synapse. Immunoreactivity (arrows) is abundant on the cytoplasmic membrane, within the cytoplasm, and on the mitochondria. D) An image of a lightly labeled astrocytic (astro) process adjacent to two synapses formed on spines (s). Immunoreactivity (arrows) is present on the astrocytic cytoplasmic membrane, within the cytoplasm, and on part of the mitochondrion. The spine (s) in the upper left contains some immunoreactivity (arrowhead). Both postsynaptic densities are also labeled. E) An example of a thin labeled astrocytic process adjacent to the asymmetric synapse on a dendrite (den). There is no immunoreactivity on the PSD (black and white arrow). F) An example of a synapse (black and white arrow) on a dendrite (den) where the PSD is labeled. Labeled astrocytic (astro) processes are nearby and adjacent to the synapse. A labeled spine (s) is nearby, with a labeled PSD (black and white arrow) as well as labeleing (arrow) elsewhere in the spine. Mitochondria (m) are identified throughout the figures. Scale bars = 2μm (A), 0.5 μm (B-E).
EAAT2 labeling is occasionally observed in neuronal profiles (Fig 7F, 8A,B). The somata of neurons are lightly labeled on ribosomes and rER (Fig. 8A). Dendritic spines contain immunoreactivity on the postsynaptic density (Figs. 7E, 8B) and at times elsewhere in the spine (Fig. 7F). The most predominant neuronal labeling is on the PSD of asymmetric synapses. Labeled astrocytic processes are the predominant and most robustly labeled elements throughout the neuropil.
Figure 8.
Electron micrographs of EAAT2 in neuronal profiles. A) The soma of a neuron showing immunoreactivity on polyribosomes (arrows). A perineuronal glial process (astro) contains immunoreactivity as well (arrows). (Case #8) B) An axon terminal (AT) forms an asymmetric synapse (black and white arrow) with a spine (s). The PSD is labeled (arrow). An adjacent astroglial (astro) process is also labeled (arrows). (Case #5) Scale bars= 2 μm (A), 0.5 μm (B).
DISCUSSION
The results of the present study describe EAAT1 and EAAT2 labeling in prefrontal cortex in human postmortem control brains at the light and electron microscopic level. To our knowledge these results are the first to describe the electron microscopic localization of EAAT1 and EAAT2 in normal postmortem human brain, though there is one report of EAAT2 localization in surgical specimens of cortex adjacent to tumors (Melone et al., 2011) and one report from hippocampal resections (Bjornsen et al., 2007). EAAT1 labeling was located in astrocytes, neurons, and endothelial cells. EAAT1 labeling was located on the plasma membrane of astrocytes, in the soma and nucleus. In neurons, labeling was present in the soma, all parts of the axon, dendritic spines and the post synaptic density (PSD). EAAT2 labeled astrocytic processes were the predominant and most robustly labeled elements throughout the neuropil. Within astrocytes, the plasma membrane and mitochondria were the most heavily labeled. EAAT2 immunoreactivity was also present in neuronal profiles, the most predominant location being the postsynaptic density of asymmetric synapses.
While most of the work on EAAT1 and EAAT2 localization in the CNS has been done in human and rodent, EAATs have been localized in a variety of other species such as sheep (Northington et al., 1999), rabbits, cats, pigs, monkeys (Reye et al., 2002a; Williams et al., 2005) and even caterpillars (Gardiner et al., 2002)(Summarized in Tables II-IV). There have been quite a few disagreements as to where the EAATs are localized, with the literature continually evolving. For example, EAAT1 and EAAT2 were originally thought to be confined to astrocytes (Chaudhry et al., 1995; Lehre et al., 1995; Milton et al., 1997), but later studies began to identify them in neuronal processes as well (Brooks-Kayal et al., 1998; Chen et al., 2002, 2004; Meloni et al., 2009, 2011). Interestingly, the cellular localization of EAAT1 and EAAT2, as well as some of the other EAATs, varies across developmental stages (Bar-Peled, et al., 1997; Northington et al., 1999; DeSilva et al., 2007, 2012), with disease processes (Rothstein et al., 1995; Proper et al., 2002; Maragakis et al., 2004) and experimental manipulation (Xu et al., 2003, Sullivan et al., 2007). Thus, inconsistencies in localization may be due in part to the examination of different splice variants (Holmsmeth et al., 2009), different species, normal vs. diseased tissue, varying brain regions, different stages of development and/or variations in methodologies (such as using antibodies directed to different amino acid sequences of the transporters). The results of the present study for EAAT1 show neuronal localization, which is a departure from most of the current literature, while our results on EAAT2 are consistent with most studies. Therefore, we will discuss various technical issues as well as how our data are supported by the literature.
Table II.
Comparison of EAAT1 and GLAST localization in human and rodent
| Name species | region | Cell type | Intracellular location | method | Antibody informa | Amino acid sequence | reference |
|---|---|---|---|---|---|---|---|
| Human EAAT1 | cortex , BG, hippocampus cerebellum | cDNA cloning, NB | AA 1-542 | Arriza '94 | |||
| cortex, hippocampus, caudate, spinal cord | astrocytes | WB, IHC | rabbit polyclonal C-terminus | AA 504-518 | Rothstein '95 | ||
| fetal cortex, BG, hippocampus, adult cortex, caudate, thalamus | CA1, dentate gyrus neurons | WB, IHC | rabbit polyclonal N-terminus | As in Rothstein '94 | Bar-Peled '97 | ||
| fetal cell culture, adult white matter | oligodendrocytes | soma, processes | ISH, WB IHC | rabbit polyclonal N-terminus Alpha Diagnostics | AA 1-15 | Pitt '03 | |
| ↑cerebellum, also in hippocampus & cortex | astrocytes patchy in some cortical areas | WB, IHC | rabbit polyclonal N-terminus | As in Pow and Barnett '99 | Williams '05 | ||
| optic nerve in MS and controls | oligodendrocytes | QPCR, WB, IHC | rabbit polyclonal C-terminus | Vallejo-Illarramendi '06 | |||
| hippocampus | only astrocytes | soma, processes mitochondria (Fig 5a) | WB, IHC, EM | rabbit polyclonal C-terminus | AA 522-541 | Bjornsen '07 | |
| cortex | glia, some neurons | soma, processes, axons, spines, PSD | WB, IHC, EM | rabbit polyclonal C-terminus Abcam | Present paper | ||
| Rodent GLAST | ↑cerebellum | cerebellum | Bergmann glia | cDNA cloning NB, ISH | AA 1-543 | Storck '92 | |
| ↑cerebellum | mainly glia | ISH | Torp '94 | ||||
| throughout brain | astrocytes everywhere also in striatum and cortex some neurons | astrocytic soma and processes. Golgi in neurons | WB, IHC, EM | rabbit polyclonal C-terminus | NRDVEMGNSVI.....EENE | Rothstein '94 | |
| brain | Just in and in all astrocytes. | Varies with location of astrocytic process. | IHC, EM | rabbit polyclonal C-terminus | AA 522-541 | Chaudhry '95 | |
| ↑cerebellum, hippocampus, corpus callosum, spinal cord | astrocytes | Not in terminals; between neurons and around axosomatic synapses | IHC, EM | rabbit polyclonal C-terminus | AA 522-541 | Lehre '95 | |
| hippocampus | Gltl&GLAST in same astrocytes. | plasma membrane around synapses | WB, IHC, EM | rabbit polyclonal C-terminus | AA 522-541 | Haugeto '96 | |
| cultured cortical astrocytes | C terminus interacts with PSD and NHERF1,2 | Proteomic WB, IP | guinea pig polyclonal C-terminus rabbit polyclonal N-terminus | AA 1-50 | Ritter '11 | ||
| hippocampus | astrocytes | astrocytes | ISH | Chen '04 | |||
| ↑cerebellum, also in hippocampus, cortex | homogeneous cortical staining of astrocytes | WB, IHC | rabbit polyclonal N-terminus | Williams '05 |
The table gives details of brain region, cell type, intracellular localization, methods, antibody information including amino acid (AA) sequence (either numbered or named) and reference. Empty parts of Table indicate information was not relevant or not provided. Abbreviations: BG, basal ganglia. MS, multiple sclerosis. PSD, postsynaptic density. NHERF1,2, Na+/H+ exchanger regulatory factors 1 and 2. NB, northern blot. WB, western blot. IP, immunoprecipitation. ISH, in situ hybridization. QPCR, quantitative polymerase chain reaction. IHC, immunohistochemistry. EM, electron microscopy.
Table IV.
Comparison of EAAT3,4,5 and EAAC1 localization in human and rodent
| EAAT3 human | ↑ cortex | cDNA cloning, NB | Arriza '94 | ||
| fetal cortex, BG, hippocampus adult cortex, GP caudate, thalamus | CA1 & dentate | probably neurons in pyramidal & granular cell layers | WB, IHC | Bar-Peled '97 | |
| cerebellum | Purkinje cells | WB, IHC | Furuta '97 | ||
| EAAC1 rodent | cortex, cerebellum, hippocampus, gut and other organs | in brain, probably neurons | cDNA cloning, NB, ISH | Kanai & Hediger '92 | |
| cortex, cerebellum, hippocampus, caudate | neurons not glia | soma, axon terminals | WB, IHC, EM | Rothstein '94 | |
| cortex, cerebellum | astrocytes, neurons | spines, dendrites, PSD | WB, IHC, EM | Furuta '97 | |
| cortex | neurons, astrocytes | dendrites, spines, small astrocytic processes | IHC, EM | Conti '98 | |
| throughout brain | neurons, not glia | mostly somata and dendrites, and a little in spines | ISH, WB, IHC, EM | Holmseth'12 | |
| EAAT4 human | cerebellum | cDNA cloning, NB | Fairman '95 | ||
| fetal cortex, hippocampus cerebellar cortex; adult cerebellum | CA1 & dentate pyramidal & granular layers | probably neurons | WB, IHC | Bar-Peled '97 | |
| ↑ adult cerebellum, lighter in hippocampus, cortex | Purkinje cells & other neurons | soma, dendrites | WB, IHC | Furuta '97 | |
| developing cerebellum | Purkinje cells | soma, processes, spines | IHC | Itoh '97; Inage '98 | |
| EAAT4 rodent | ↑ cerebellum lighter in hippocampus, cortex | neurons, astrocytes | spines, dendrites, PSD, some small astrocytic processes | WB, IHC, EM | Furuta '97 |
| ↑ cerebellum | Purkinje cells | soma, dendrites, spines | WB, IHC, EM | Dehnes '98 | |
| ↑ cerebellum | Purkinje cells | soma, dendrites | cDNA cloning, NB, ISH, IHC | Lin '98 | |
| forebrain, spinal cord | astrocytes | Rt-PCR, WB, IHC | Hu '03 | ||
| EAAT5 human | retina | photoreceptors bipolar neurons | cDNA cloning, NB | Arriza '97 | |
| EAAT5 rodent | retina, non-neural tissue (gut, kidney, heart, lung) | photoreceptors bipolar neurons | photoreceptor terminals | Rt-PCR, WB, IHC | Lee '13 |
The table gives details of brain region, cell type, intracellular localization, methods, and reference. Empty parts of Table indicate information was not relevant or not provided. Abbreviations: BG, basal ganglia. GP, globus pallidus. PSD, postsynaptic density. NB, northern blot. WB, western blot. IP, immunoprecipitation. ISH, in situ hybridization. Rt-PCR, real time-polymerase chain reaction. IHC, immunohistochemistry. EM, electron microscopy.
Technical issues
Diaminobenzidine (DAB) has been criticized as capable of diffusing to areas where the antigen is not actually located and thus creating nonspecific labeling at the ultrastructural level (Danbolt, 2001). However, eliminating the primary antibody as a control abolished any DAB staining. There is an immense literature using DAB at the ultrastructural level to visualize various antibodies, showing unique staining patterns for each antibody; this would not be possible if DAB labeling was nonspecific. Importantly, our EAAT2 data are highly consistent with other reports using immunogold labeling in human cortical biopsies (Meloni et al., 2011).
Postmortem Interval: The effect of postmortem interval (PMI) on the localization of EAATs in mice has shown that the localization diffuses after 12 hours raising concern over the effect of PMl in human studies (Li et al., 2012). Our postmortem tissue used for electron microscopy was fixed in eight hours or under, so there should be minimal diffusion due to PMI. Also, the effect of PMI on a small mouse brain is likely to be much more dramatic than in the much larger human brain, meaning that the PMI could probably be much longer in humans without deleterious effects. Several studies using postmortem human tissue with PMIs equivalent to or greater than ours show no correlation between PMI and EAAT protein levels and/or show a similar distribution to that of the present study, suggesting that EAAT protein is not degraded in tissues with PMIs < 20. (Ikematsu et al., 2001; Bauer et al, 2008; Shan et al., 2013, 2014). There were no qualitative differences in staining pattern between the brains used in the present study, where the PMIs ranged from four to eight hours. Finally, the ultrastructural data in human hippocampal resections (Bjornsen et al., 2007) and cortical biopsies (Meloni et al., (2011), which have no postmortem interval, are largely in agreement with our data in postmortem tissue.
Antibodies
The antibodies we used are well characterized and have been used by us previously (Bauer et al. 2008; Shan et al., 2013, 2014). In the present study EAAT1 showed no reactivity in the GLAST knockout brain tissue and EAAT2 labeling was abolished with a blocking peptide. These antibodies were directed against the C-terminus, typical of most antibodies to EAAT1 and EAAT2 (see Tables II and III). Antibody cross reactivity with other EAATs is not likely to be a confound for two reasons. First, the antibodies we used are at the C-terminus end and there is no sequence homology between EAATs in that region in rodent or human (Arriza et al., 1994). Secondly, preadsorption of individual EAAT antibodies with peptides at the C-terminal domains of other EAATs does not abolish any immunoreactivity (Rothstein et al., 1994).
Table III.
Comparison of EAAT2 and GLT localization in human and rodent
| Name species |
region | Cell type | Intracellular location | method | Antibody informatic | Amino acid sequence |
reference |
|---|---|---|---|---|---|---|---|
| EAAT2 Human | ↑cortex, BG hippocampus | cDNA cloning, NB | AA 1-574 | Arriza '94 | |||
| fetal and adult cortex, BG, thalamus, cerebellum | CA1, dentate gyrus neurons | WB, IHC | rabbit polyclonal C-terminus | AA 559-573 | Bar-Peled '97 | ||
| cortex, striatum, hippocampus | diffuse in grey matter | WB, IHC | polyclonal C-terminus | AA 559-573 | Ikematsu '01 | ||
| fetal cell culture adult white matter | oligodendrocytes | soma, processes | ISH, WB, IHC | rabbit polyclonal C-terminus | AA 525-573 | Pitt '03 | |
| throughout brain | astrocytes | patchy in temporal but not motor cortex | WB, IHC | rat, rabbit polyclonal C-terminus | PFPFLDIETCI AA 558-573 | Williams '05 | |
| cortex, hippocampus, caudate, spinal cord | astrocytes | WB, IHC | rabbit polyclonal C-terminus | AA 560-574 | Rothstein '95 | ||
| optic nerve in MS and controls | astrocytes | QPCR, WB, IHC | rabbit polyclonal C-terminus | Vallejo-Illarramendi '06 | |||
| hippocampus | only astrocytes | soma, processes | WB, IHC, EM | rabbit polyclonal C-terminus | AA 563-573 | Bjornsen '07 | |
| biopsied cortex | neurons astrocytes | axons, terminals dendrites plasma membrane | IHC, EM | polyclonal EAAT2a C-terminus | AA 559-573 | Melone '11 | |
| developing cortex | neurons, astrocytes | ISH, WB, IHC | N-terminus | AA 1-15 | DeSilva '07; '12 | ||
| postmortem human cortex | mostly astrocytes, some neurons | glial processes, some terminals, PSD, mitochondria | WB, IHC, EM | guinea pig polyclonal C-terminus Millipore | Present paper | ||
| GLT1 Rodent | rat brain | cDNA cloning, NB | rabbit polyclonal | AA 1-573 | Pines '92; Danbolt '92 | ||
| cerebellum | Golgi epithelial cells, astrocytes | processes | WB, IHC, EM | mouse monoclonal | Hees '92 | ||
| hippocampus | astrocytes | processes | WB, ELISA, IHC, EM | mouse monoclonal | AA 518-536 | Levy '93 | |
| ↑hippocampus, cortex & striatum | Glia, neurons | ISH | Torp '94 | ||||
| throughout brain | astrocytes | soma, processes | WB, IHC, EM | rabbit polyclonal C-terminus | AA 559-573 | Rothstein '94 | |
| ↑hippo, cortex, striatum, corpus callosum | astrocytes only | around synapses; plasma membranes | IHC, EM | rabbit polyclonal N-terminus | AA 12-26 | Lehre '95 | |
| GLT1 Rodent | cerebellum, hippocampus | astrocytes | IHC, EM | C-terminus N-terminus | AA 493-508 AA 12-26 | Chaudry '95 | |
| hippocampus | GLAST and GLT in same astrocytes | Glt1&GLAST in same plasma membranes | WB, IHC, EM | rabbit polyclonal C-terminus N-terminus | AA 493-508 AA 12-26 | Haugeto '96 |
| hippocampus | CA3 neurons | ISH | Schmitt '96; Torp '97; Berger & Hediger '98 | ||||||
| hippocampal slices | neurons and some astrocytes | dendrites of excitatory neurons | WB, IHC | rabbit polyclonal C-terminus N-terminus | AA 559-573 AA 12-26 |
Mennerick '98 | |||
| cortex, hippocampus, striatum, white matter | astrocytes and neurons | Neuronal soma w/ISH astrocytes ISH, IHC | ISH, WB, IHC | rabbit polyclonal C-terminus | AA 544-573 | Schmitt '02 | |||
| hippocampus: cell culture & morphine treated rats | astrocytes, neurons | ↑axon terminals & dendrites w/morphine | ISH, WB, IHC, EM | goat polyclonal N-terminus | AA 1-19 | Xu '03 | |||
| hippocampal slices | 80% glial processes; 20% axons and axon terminals | WB, IHC, EM | rabbit polyclonal C-terminus N-terminus | AA 493-508 518-536, 563-573 AA 12-26 |
Furness '08 | ||||
| slice culture/In vivo | hippocampus/cortex | interacts with mitochondria and glycolytic enzymes | cDNA cloning IHC, mass spectroscopy | mouse monoclonal rabbit polyclonal | AA 559-573 (from Rothstein) | Genda '11 | |||
| GLT1a | hippocampus, cortex, striatum, external capsule, pituitary | astrocytes | not patchy in cortex | IHC | rat, rabbit polyclonal C-terminus | GLT1b | C-terminus | AA 558-573 | Reye '02c |
| astrocytes | WB, IHC | rat, rabbit polyclonal C-terminus | GLT1b | C-terminus | AA 558-573 | Williams '05 | |||
| hippocampus | astrocytes, neurons | 14-29% of AT forming AS, and PSD | ISH, IHC, EM | GLT1a N-terminus GLT1a C-terminus GLT1b C-terminus |
AA 1-15 AA 559-573 AA 548-562 |
Chen '02, '04 | |||
| GLT1b | hippocampus | astrocytes, neurons | PSD, AT, spines, soma | ISH, IHC, EM | |||||
| cortex, hippocampus, striatum | astocytes | patchy in cortex | IHC | rat, rabbit polyclonal C-terminus | GLT1b | C-terminus | PFPFLDIETCI | Reye '02c | |
| ↑cerebellum, also in cortex & hippocampus | astrocytes | WB, IHC | rat, rabbit polyclonal C-terminus | GLT1b | C-terminus | PFPFLDIETCI | Williams '05 | ||
| cell culture | neurons | PSD, spines | IP, IHC | rabbit polyclonal GLTlb C-terminus | Gonzalez-Gonzalez '08 | ||||
| astrocytes | small processes around synapses | WB, IHC, EM | rabbit polyclonal N-terminus | AA 12-26 | Holmseth '09 | ||||
| GLT1c | retina | photoreceptors | QPCR, ISH, WB, IHC | rabbit polyclonal GLT1c C-terminus | Rauen '04 | ||||
| brain | astrocytes | ||||||||
| GLT1v | retina, various brain regions | neurons, all glial cell types | neuropil, granular label in cytoplasm | ISH, IHC | rabbit polyclonal GLT1v C-terminus | AA 550-562 | Schmitt '02 | ||
| GLT1aGLT1b | retina | various cell types depending on species | IHC | rat, rabbit polyclonal GLT1a C-terminus GLT1b C-terminus | Reye '02a,b |
The table gives details of brain region, cell type, intracellular localization, methods, antibody information including amino acid (AA) sequence (either numbered or named) and reference. Empty parts of Table indicate information was not relevant or not provided. Abbreviations: BG, basal ganglia. MS, multiple sclerosis. PSD, postsynaptic density. AT, axon terminal. AS, asymmetric axospinous synapse. NB, northern blot. WB, western blot. IP, immunoprecipitation. ISH, in situ hybridization. QPCR, quantitative polymerase chain reaction. IHC, immunohistochemistry. EM, electron microscopy.
Thus, any localization differences we report herein, from that observed previously, are probably not due to artifacts of DAB, diffusion or degradation during the PMl, antibody specificity or cross reactivity with other EAATs, such as those found predominantly in neurons. However, hypoxia associated with the process of dying, as well as during the postmortem interval, may impact transporter expression in neurons. In a porcine model of hypoxia, pigs were exposed to lower oxygen levels for 45 minutes and allowed to recover for 72 hours (Sullivan et.al. 2007). In this study, protein for a splice variant of EAAT1, lacking exon 9, was expressed in neurons in brain tissues from hypoxic animals. This EAAT1 variant was detected with C-terminal antibodies, but not N-terminal antibodies. Although brains from subjects with prolonged agonal status were excluded from our study, expression of EAAT1 in neurons in postmortem brain could be due to mild to moderate hypoxia associated with dying and the human brain collection process. Taken together, our data and the hypoxia findings suggest that EAAT1 may be expressed in neurons.
Interestingly, one study found EAAT1 expression in neurons using C-terminal antibodies (Rothstein et al, 1994). Subsequent confirmation studies using N-terminal antibodies did not detect EAAT1 (Lehre et al., 1995), and it was concluded that the studies with the C-terminal antibodies must have been an artifact (Ginsburg et al., 1995). We used a C-terminal antibody and the isoform of EAAT1 expressed in neurons was only detectable with a C-terminal antibody, providing a parsimonious explanation for the all of the previous divergent findings for this protein.
At the light microscopic level, EAAT1 was distributed throughout the neuropil in small processes and punctate structures, in glial cells and in selected neurons. We found EAAT2 localized most heavily in astrocytic processes and their cell bodies, in both grey and white matter in agreement with previous studies (Danbolt et al., 1992; Torp et al., 1994; Lehre et al., 1995). Previous results of EAAT2 labeling in cortex have described two patterns, regular and irregular. We found the EAAT2 labeling to be homogeneously distributed in the form of small punctate structures throughout the neuropil and in the soma and proximal processes of cells with astrocytic morphologies. Thus, our results are consistent with what has been described as the regular pattern (Melone et al., 2011) and observed by several investigators (Rothstein et al., 1995; Bar-Peled et al., 1997; Ikematsu et al., 2001; Bjornsen et al., 2007). Notably, long PMIs (>20 hours)have been associated with the irregular pattern of staining (Milton et al., 1997; Proper et al., 2002), and our postmortem intervals were very short (<10 hours).
EAAT1 and EAAT2 labeled astrocytes were most often observed around asymmetric synapses, characteristic of glutamatergic synapses. However, EAAT labeled astrocytic processes were not exclusively around excitatory synapses. EAAT1 and EAAT2 labeled astrocytic processes were adjacent to many types of neuropil profiles, including symmetric synapses, which are characteristic of inhibitory transmission. Our data are consistent with other results showing EAAT2 or GLT1 labeling located both adjacent to and far from synapses (Chaudhry et al., 1995; Bjornsen et al., 2007; Minelli et al., 2001). The EAATs function to remove glutamate from the synaptic cleft, begging the question of why are EAATs localized in regions where there are no synapses, such as the white matter, and throughout the grey matter neuropil far from excitatory synapses? Glutamate may enter the extracellular space in several ways: 1) spillover following release at the synapse; 2) non vesicular release, sometimes called leaking; 3) exocytosis from astrocytes; and 4) release by the cystine / glutamate antiporter (Montana et al., 2004; Hayden and Carmigoto, 2006; Baker et al., 2008; Parpura et al., 2011; Bridges et al., 2012). Glutamate has been measured in the white matter in humans by in vivo imaging (Ota et al., 2012; for review see Marsman et al., 2013) and plays an important role therein (for review see Matute and Ransom, 2012). Although the EAATs certainly regulate glutamate at excitatory synapses, their role in glutamate regulation may not be restricted to the synapse.
One possible explanation for the expression of EAATs far from synapses is that they regulate and partition glutamate levels in distinct extracellular pools or microdomains. Evidence for extracellular (and extrasynaptic) glutamate microdomains includes a large body of work that has described localization of functional glutamate receptors outside of synapses, as well as the observation that glial cells may form electrically-independent morphological structures of unknown function that ensheath neuronal structures (Grosche et al., 1999, 2002; Szabadkai and Rizzuto, 2007; Spat et al., 2009; Genda et al., 2011). It may be that glutamate microdomains are formed by specialized protein clusters on the membranes of astrocytic processes opposed to extrasynaptic glutamate receptors expressed on specialized regions of neuronal membranes (Grosche et al., 1999; Genda et al., 2011; Shan et. al., 2012). Diffusion of glutamate between domains or domains and synapses could be limited by the dense expression of glutamate transporters between these specialized structures (Danbolt et al., 1998; Shan et al., 2012).
Many previous studies of EAAT1 have reported localization only in astrocytes, including processes in perivascular locations (Chaudhry et al., 1995; Lehre et al., 1995; Haugeto et al., 1996; Williams et al., 2005; Bjornsen et al., 2007). However, some studies localized EAAT1 to endothelial cells as well (O'Kane et al., 1999; Hawkins et al., 2006; Helms et al., 2012), as we show in the present study at the ultrastructural level. Our results, that EAAT1 is localized in astrocytic endfeet and endothelial cells, supports a role for EAAT1 in maintaining glutamate concentrations across the blood brain barrier, as previously posited (O'Kane et al., 1999; Hawkins et al., 2006; Helms et al., 2012). The observation that EAAT1 is present in two cell types associated with the blood brain barrier, whereas EAAT2 is only present in astrocytic end feet, might suggest a more prominent role for EAAT1 than EAAT2 in maintaining glutamate concentrations at this site.
Our finding of EAAT1 protein expression in neurons was somewhat unexpected because EAAT1 has been considered by many to be only a glial transporter (see Table II). That said, several studies have supported a neuronal localization of EAAT1 (Rothstein et al., 1994; Bar-Peled et al., 1997; Sullivan et.al., 2007; Ritter et al., 2011) and EAAT2 (Melone et al., 2009, 2011). In fact, it has been suggested (Conti et al., 1998) that the division of EAATs into neuronal and glial transporters is no longer warranted. In the present study, EAAT1 was frequently observed in axons and axon terminals, and EAAT2 was observed occasionally in axon terminals. Excitatory amino acids are taken up into presynaptic terminals (Divac et al., 1977; Storm-Mathisen and Iversen, 1979; Storm-Mathisen and Wold, 1981; Taxt and Storm-Mathisen, 1984; Gundersen et al., 1993, 1996). It has been suggested (Danbolt, 2001) that the molecular basis of this uptake cannot be accounted for solely by the expression of the neuronal glutamate transporter EAAT3/EAAC1. The location of EAAT1 in axon terminals, the postsynaptic density and in perisynaptic astrocytes suggests that it may have important and possibly multiple roles for regulating synaptic glutamate. Taken together, EAAT1 and EAAT2 might to have a role in presynaptic glutamate regulation.
Our data show EAAT1 and EAAT2 on the postsynaptic density, and elsewhere in the spine. Using immunoprecipitation studies, Ritter et al. (2011) found that the C-terminus portion of GLAST (EAAT1) robustly co-immunoprecipitated with the post synaptic density domains of two scaffolding proteins (Na+/H+ exchanger regulatory factors 1 and 2, NHERF-1 and NHERF-2). The interaction of EAAT1 with proteins in the postsynaptic density supports our data localizing EAAT1 in the post synaptic density. EAAT2 has not always been found in dendritic spines (Table III), but Chen et al, (2004) have localized GLT1a labeling on the postsynaptic density in spines receiving asymmetric synapses. In other studies, GLT1b was found to interact with proteins located in the postsynaptic density, PSD-95 (Gonzalez-Gonzalez et al., 2008) and the NMDA receptor NR1 (Bassan et al., 2008), placing GLT1b at the postsynaptic density. These data suggest that postsynaptic glutamate reuptake may not be limited to EAAT3, whose postsynaptic localization is well-characterized (Levenson et al., 2002).
Our observations of EAAT2 labeled mitochondria, especially in astrocytes, is supported by Genda et al., (2011) whose data suggests that GLT-1 exists in a multiprotein complex in close association with mitochondria. These authors demonstrated co-localization of mitochondria and GLT-1 in astrocytic processes of organotypic hippocampal slices and in vivo. It is unclear why electron microscopic studies have not discussed GLT1/EAAT2 labeling on mitochondria (Chaudry et al., 1995; Lehre et al., 1995; Haugeto et al., 1996; Chen et al., 2004). However, labeled mitochondria are illustrated, but not discussed in both rodent (Lehre et al., 1995; see Fig. 12a), and human (Melone et al., 2011; see fig. 5c). The functional significance of EAAT2 labeled mitochondria is unclear. Distinct subtypes of glutamate transporters (non-EAAT) are expressed on mitochondria (Fiermonte et al., 2002; Berkich et al., 2007; Palmieri, 2004). The calcium-binding mitochondrial carrier protein Aralar1, and the mitochondrial glutamate carriers 1 and 2 (GC1 and GC2) move glutamate across the mitochondrial membrane from the cytosol. Our data suggest that some mitochondria in astrocytic processes also have EAAT2 in the mitochondrial outer membrane. Another possibility is that one or more of these other transporters may have an epitope detectable by our EAAT2 antibody. However, there is no overlap of the sequences of these proteins (based on Protein BLAST, unpublished observation) for the EAAT2 epitope used to generate the EAAT2 antibody. Finally, it may be that EAAT2 is trafficked to the mitochondria where mitochondrial proteins interact and join a so-called glutamate transport protein complex before insertion in the plasma membrane (Genda et al., 2011; Shan et al., 2014). Regardless of the functional role or the orientation in the mitochondrial membrane, further study of the selective localization of EAAT2 to mitochondria is warranted.
Summary
EAAT1 localization has not been previously studied in human cortex at the electron microscopic level. We found that EAAT1 was distributed in more varied cell populations and intracellular locations than EAAT2, suggesting more diverse functions. Our finding of EAAT1 protein expression in neurons could be secondary to the brain collection process, and needs to be confirmed with follow up studies. The predominant localization of EAAT2 on the astrocytic plasma membranes adjacent to excitatory synapses is consistent with the function of facilitating glutamate reuptake and limiting glutamate spillover. Neuronal and extrasynaptic localization suggests additional functions, especially for EAAT1, which has a more diverse distribution than EAAT2. Finally, establishing that EAAT1 and EAAT2 may be studied at the ultrastructural level in postmortem brain extends the tools available to study neuropsychiatric diseases which do not have animals models that fully recapitulate the illness.
Highlights.
EAAT1-2 protein expression may be assessed by electron microscopy in postmortem brain
EAAT2 expression in postmortem brain is very similar to findings in other species
EAAT1 protein was found in many cell types, including astrocytes and neurons
EAAT1 expression in neurons may be secondary to hypoxia
Figure 3.
Light micrographs of EAAT1. A) Labeling in white matter. EAAT1 is present in glial cell bodies (arrows), processes (arrowheads) and punctate structures (encircled). Note the staining around a capillary (upper left). B) EAAT1 is present throughout the neuropil and in scattered neurons (dashed arrows) and glial cells (black arrows) in layer III. Apical dendrites are labeled, at least in proximal portions (solid arrows with balls). C) In layer V fewer labeled neuronal somata (dashed arrows) are present, and unlabeled large dendrites (dashed arrows with balls) are seen coursing through the labeled neuropil. Glial profiles (arrows and arrowhead). All images are from case #4. Scale bars =100 μm.
Figure 4.
Light micrographs of EAAT2. A) Labeling in white matter is very heavy. EAAT2 is present in glial cell bodies (black arrows), processes (arrowheads) and punctate structures (encircled). Note the staining around the capillary (right edge). Case #5. B) In layer III, EAAT2 is present throughout the neuropil, where it avoids neurons (black dashed arrows). Case #7. C) Layer V is similar in appearance to layer III. Case #5. Scale bars =100 μm.
Acknowledgements
The authors would like to thank the Alabama and Maryland Brain Collections for providing tissue. We also thank Dr. Kohichi Tanaka for providing GLAST knockout and heterozygote mice brains. This work was supported by MH087752 (REM, RCR), MH074016 (REM), MH094445 (REM) and Doris Duke Clinical Scientist Award (REM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arriza JL, Furman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology. 2008;33(7):1760–1772. doi: 10.1038/sj.npp.1301532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs R, Machiyama Y, Hammond BJ, Julian T, Richter D. The operation of the gamma-aminobutyrate bypath of the tricarboxylicacid cycle in brain tissue in vitro. Biochem. 1970;J116:445–461. doi: 10.1042/bj1160445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. J Neurochem. 1997;69(6):2571–80. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- Bassan M, Liu H, Madsen KL, Armsen W, Zhou J, Desilva T, Chen W, Paradise A, Brasch MA, Staudinger J, Gether U, Irwin N, Rosenberg PA. Interaction between the glutamate transporter GLT1b and the synaptic PDZ domain protein PICK1. Eur J Neurosci. 2008;(1):66–82. doi: 10.1111/j.1460-9568.2007.05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104:108–20. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117(1):92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol (Berl) 1998;198(1):13–30. doi: 10.1007/s004290050161. [DOI] [PubMed] [Google Scholar]
- Berkich DA, Ola MS, Cole J, Sweatt AJ, Hutson SM, LaNoue KF. Mitochondrial transport proteins of the brain. J Neurosci Res. 2007;85(15):3367–77. doi: 10.1002/jnr.21500. [DOI] [PubMed] [Google Scholar]
- Bjørnsen LP, Eid T, Holmseth S, Danbolt NC, Spencer DD, de Lanerolle NC. Changes in glial glutamate transporters in human epileptogenic hippocampus: inadequate explanation for high extracellular glutamate during seizures. Neurobiol Dis. 2007;25(2):319–30. doi: 10.1016/j.nbd.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker AM. Thinking outside the cleft to understand synaptic activity: Contribution of the cystine-glutamate antiporter (system xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev. 2012;2012;64(3) doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Munir M, Jin H, Robinson MB. The glutamate transporter, GLT-1, is expressed in cultured hippocampal neurons. Neurochem Int. 1998;33:95–100. doi: 10.1016/s0197-0186(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. Glutamate spillover promotes the generation of NMDA spikes. J Neurosci. 2011;31:16435–16446. doi: 10.1523/JNEUROSCI.2777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Lebre KP, van Lookeren Campagne M, Ottersen OP, Danholt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002;22(6):2142–52. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12(5):515–25. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate transporter, is localized to astrocytes and GABAergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8:108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Chaudhry FA, Dehnes Y, Lehre KP, Levy LM, Ullensvang K, Storm-Mathisen J. Properties and localization of glutamate transporters. Prog Brain Res. 1998;116:23–43. doi: 10.1016/s0079-6123(08)60428-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Kanner BI. An [Na.+K.]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience. 1992;51:295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18(10):3606–19. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva TM, Borenstein NS, Volpe JJ, Kinney HC, Rosenberg PA. Expression of EAAT2 in neurons and protoplasmic astrocytes during human cortical development. J Comp Neurol. 2012;520(17):3912–3932. doi: 10.1002/cne.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilva TM, Kinney HC, Borenstein NS, Trachtenberg FL, Irwin N, Volpe JJ, Rosenberg PA. The glutamate transporter EAAT2 is transiently expressed in developing human cerebral white matter. J Comp Neurol. 2007;501:879–890. doi: 10.1002/cne.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divak I, Fonnum F, Storm-Mathisen J. High affinity uptake of glutamte in terminals of corticostriatal axons. Nature. 1997;266:377–378. doi: 10.1038/266377a0. [DOI] [PubMed] [Google Scholar]
- Drew GM, Mitchell VA, Vaughan CW. Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. J Neurosci. 2008;28:808–15. doi: 10.1523/JNEUROSCI.4876-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fiermonte G, Palmieri L, Todisco S, Agrimi G, Palmieri F, Walker JE. Identification of the mitochondrial glutamate transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J Biol Chem. 2002;277(22):19289–94. doi: 10.1074/jbc.M201572200. [DOI] [PubMed] [Google Scholar]
- Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K, Wojewodzic M, Zhou Y, Attwell D, Danbolt NC. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience. 2008;157(1):80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin CLG, Dykes-Hoberg M, Rothstein JD. Cellular and synaptic localization of the neuronal glutamate transporters excitatory amino acid transporter 3 and 4. Neuroscience. 1997;81:1031–1042. doi: 10.1016/s0306-4522(97)00252-2. [DOI] [PubMed] [Google Scholar]
- Gardiner RB, Ullensvang K, Danbolt NC, Caveney S, Donly BC. Cellular distribution of a high-affinity glutamate transporter in the nervous system of the cabbage looper Trichoplusia ni. J Exp Biol. 2002;205:2605–2613. doi: 10.1242/jeb.205.17.2605. [DOI] [PubMed] [Google Scholar]
- Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O'Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neuroscience. 2011;31(50):18275–18288. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Martin LJ, Rothstein JD. Regional Deafferentiation Down-Regulates Subtypes of Glutamate Transporter Protein. J. Neurochem. 1995;65:2800–03. doi: 10.1046/j.1471-4159.1995.65062800.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Garcia-Tardon N, Cubelos B, Gimenez C, Zafra F. The glutamate transporter GLT1b interacts with the scaffold protein PSD-95. J Neurochem. 2008;105:1834–1848. doi: 10.1111/j.1471-4159.2008.05281.x. [DOI] [PubMed] [Google Scholar]
- Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res. 2002;68(2):138–49. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2(2):139–43. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Gundersen V, Danbolt NC, Ottersen OP, Storm-Mathisen J. Demonstration of glutamate/aspartate uptake activity in nerve endings by use of antibodies recognizing exogenous D-aspartate. Neuroscience. 1993;57:97–111. doi: 10.1016/0306-4522(93)90114-u. [DOI] [PubMed] [Google Scholar]
- Gundersen V, Ottersen OP, Storm-Mathisen J. Selective excitatory amino acid uptake in glutamatergic nerve terminals and in glia in the rat striatum: quantitative electron microscopic immunocytochemistry of exogenous (D)-aspartate and endogenous glutamate and GABA. Eur J Neurosci. 1996;8(4):758–65. doi: 10.1111/j.1460-9568.1996.tb01261.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–153. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Haugeto Ø , Ullensvang K, Levy LM, Chaudhry FA, Honoré T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological reviews. 2006;86(3):1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, O'Kane RL, Simpson IA, Viña JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. 2006;136(1 Suppl):218S–26S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- Hees B, Danbolt NC, Kanner BI, Haase W, Heitmann K, Koepsell H. A monoclonal antibody against a Na(+)-L-glutamate cotransporter from rat brain. J Biol Chem. 1992;267(32):23275–23281. [PubMed] [Google Scholar]
- Helms HC, Madelung R, Waagepetersen HS, Nielsen CU, Brodin B. In vitro evidence for the brain glutamate efflux hypothesis: brain endothelial cells cocultured with astrocytes display a polarized brain-to-blood transport of glutamate. Glia. 2012;60(6):882–93. doi: 10.1002/glia.22321. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci 25. 2012;32(17):6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162(4):1055–71. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Hu WH, Walters WM, Xia XM, Karmally SA, Bethea JR. Neuronal glutamate transporter EAAT4 is expressed in astrocytes. Glia. 2003;44(1):13–25. doi: 10.1002/glia.10268. [DOI] [PubMed] [Google Scholar]
- Huerta I, McCullumsmith RE, Haroutunian V, Giménez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59:394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- Ikematsu K, Tsuda R, Orihara Y, Nakasono I. The expression of excitatory amino acid transporter 2 (EAAT2) in forensic autopsy cases. Forensic Sci Int. 2001;118:49–55. doi: 10.1016/s0379-0738(00)00378-9. [DOI] [PubMed] [Google Scholar]
- Inage YW, Itoh M, Wada K, Takashima S. Expression of two glutamate transporters, GLAST and EAAT4, in the human cerebellum: their correlation in development and neonatal hypoxic-ischemic damage. J Neuropathol Exp Neurol. 1998;57:554–562. doi: 10.1097/00005072-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Itoh M, Watanabe Y, Watanabe M, Tanaka K, Wada K, Takashima S. Expression of a glutamate transporter subtype, EAAT4, in the developing human cerebellum. Brain Res. 1997;767:265–271. doi: 10.1016/s0006-8993(97)00572-6. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Heilig M, Holmes A. Loss of Glial Glutamate and Aspartate transporter (Excitatory Amino Acid Transporter 1) Causes Locomotor Hyperactivity and Exaggerated responses to Psychotomimetics: Rescue by Haloperidol and Metabotropic Glutamate 2/3 Agonist. Biol Psychiatry. 2008;64(9):810–14. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Lee A, Anderson AR, Stevens M, Beasley S, Barnett NL, Pow DV. Excitatory Amino Acid Transporter 5 is widely expressed in peripheral tissues. Eur J Histochem. 2013;57(1):e11. doi: 10.4081/ejh.2013.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, El-Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. Faseb J. 2008;12:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nature Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Levy LM, Lehre KP, Rolstad B, Danbolt NC. A monoclonal antibody raised against an [Na.+K.] coupled L-glutamate transporter purified from rat brain confirms glial localization. Fed Eur Biochem Soc Lett. 1993;317:79–84. doi: 10.1016/0014-5793(93)81495-l. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou Y, Danbolt NC. The rates of postmortem proteolysis of glutamate transporters differ dramatically between cells and between transporter subtypes. J Histochem Cytochem. 2012;60(11):811–21. doi: 10.1369/0022155412458589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Tzingounis AV, Jin L, Furuta A, Kavanaugh MP, Rothstein JD. Molecular cloning and expression of the rat EAAT4 glutamate transporter subtype. Brain Res Mol Brain Res. 1998;63(1):174–9. doi: 10.1016/s0169-328x(98)00256-3. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape 'superslow' afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Short- and long-term depression of rat cerebellar parallel fibre synaptic transmission mediated by synaptic crosstalk. J Physiol. 2007;578:545–550. doi: 10.1113/jphysiol.2006.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013;1:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, Arckens L, Michotte Y. High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J Comp Neurol. 2008;511(2):155–72. doi: 10.1002/cne.21823. [DOI] [PubMed] [Google Scholar]
- Matute C, Ransom BR. Roles of white matter in central nervous system pathophysiologies. ASN Neuro. 2012;4(2):89–101. doi: 10.1042/AN20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57(1):108–17. doi: 10.1002/glia.20744. [DOI] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Ducati A, Lacoangeli M, Conti F. Cellular and Synaptic Localization of EAAT2a in Human Cerebral Cortex. Front Neuroanat. 2011;144:151. doi: 10.3389/fnana.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Dhond RP, Benz A, Xu W, Rothstein JD, Dandolt NC, Isenberg KE, Zorumski CF. Neuronal expression of the glutamate transporter GLT-1 in hippocampal microcultures. J Neurosci. 1998;18:4490–4499. doi: 10.1523/JNEUROSCI.18-12-04490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton ID, Banner SJ, Ince PG, Piggott NH, Fray AE, Thatcher N, Horne CH, Shaw PJ. Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study. Brain Res Mol Brain Res. 1997;52(1):17–31. doi: 10.1016/s0169-328x(97)00233-7. [DOI] [PubMed] [Google Scholar]
- Minelli A, Barbaresi P, Reimer RJ, Edwards RH, Conti F. The glial glutamate transporter GLT-1is localized both in the vicinity of and at distance from axon terminals in the rat cerebral cortex. Neuroscience. 2001;108:151–159. doi: 10.1016/s0306-4522(01)00375-x. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24(11):2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Traystman RJ, Koehler RC, Martin LJ. GLT1, glial glutamate transporter, is transiently expressed in neurons and develops astrocyte specificity only after midgestation in the ovine fetal brain. J Neurobiol 15. 1999;39(4):515–526. [PubMed] [Google Scholar]
- O'Kane RL, Martinez-Lopez I, DeJoseph MR, Jr, Hawkins RA. Na(1)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood–brain barrier. A mechanism for glutamate removal. J Biol Chem. 1999;274:31891–5. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- O'Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exper Pharmacol Physiol. 2002;29:1018–1023. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46(3):519–34. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Ota M, Ishikawa M, Sato N, Hori H, Sasayama D, Hattori K, Teraishi T, Nakata Y, Kunugi H. Glutamatergic changes in the cerebral white matter associated with schizophrenic exacerbation. Acta Psychiatr Scand. 2012;126(1):72–78. doi: 10.1111/j.1600-0447.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447(5):689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- Parpura V, Grubiši V, Verkhratsky A. Ca(2+) sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta. 2011;1813(5):984–91. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seebert E, Kanner B. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61(8):1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Changing patterns of spatial buffering of glutamate in developing rat retinae are mediated by the Müller cell glutamate transporter GLAST. Cell Tissue Res. 1999;1:57–66. doi: 10.1007/s004410051333. [DOI] [PubMed] [Google Scholar]
- Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PN. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125(Pt 1):32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochem Int. 2004;5(7):1095–1106. doi: 10.1016/j.neuint.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Fletcher EL, Pow DV. Distribution of two splice variants of the glutamate transporter GLT1 in the retinas of humans, monkeys, rabbits, rats, cats and chickens. J Comp Neurol. 2002a;445:1–12. doi: 10.1002/cne.10095. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Pow DV. Distribution of two splice variants of the glutamate transporter GLT-1 in the developing rat retina. J Comp Neurol. 2002b;447(4):323–330. doi: 10.1002/cne.10218. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Scott H, Pow DV. Distribution of two splice variants of the glutamate transporter GLT-1 in rat brain and pituitary. Glia. 2002c;38(3):246–255. doi: 10.1002/glia.10059. [DOI] [PubMed] [Google Scholar]
- Ritter SL, Asay MJ, Paquet M, Paavola KJ, Reiff RE, Yun CC, Hall RA. GLAST Stability and Activity Are Enhanced by Interaction with the PDZ Scaffold NHERF-2. Neurosci Lett. 2011;487:3–7. doi: 10.1016/j.neulet.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, McCullumsmith R. The localization of the excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: A light and electron microscopic study. Program No. 899.02/HH16. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, van Kammen M, Levey AI, Martin LJ, Kunel RW. Selective loss of glial glutamate transporter GLT-l in amyotrophic lateral sclerosis. Ann Neural. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18(9):3158–70. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt TE, Eaton SA, Turner JP. Characterization of the metabotropic glutamate receptors (mGluRs) which modulate GABA-mediated inhibition in the ventrobasal thalamus. Neurochem Internatl. 1996;29(3):317–322. doi: 10.1016/0197-0186(95)00146-8. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Jons T, Kugler P. Expression of the glutamate transporter GLTI in neuronal cells of the rat central nervous system: non-radioactive in situ hybridization and comparative immunocytochemistry. Neuroscience. 1996;71:989–1004. doi: 10.1016/0306-4522(95)00477-7. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Lesch KP, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience. 2002;109:45–61. doi: 10.1016/s0306-4522(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res. 2013;144(1-3):1–8. doi: 10.1016/j.schres.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan D, Mount D, Moore S, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr Res. 2014 Feb 20; doi: 10.1016/j.schres.2014.01.028. pii: S0920-9964(14)00051-6. doi: 10.1016/j.schres.2014.01.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan D, Yates S, Roberts RC, McCullumsmith RE. Update on the Neurobiology of Schizophrenia: a Role for Extracellular Microdomains. MINERVA PSICHIATA. 2012;53:233–49. [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA. 1997;94(6):2699–704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spat A, Fulop L, Koncz P, Szanda G. When is high-Ca+ microdomain required for mitochondrial Ca+ uptake? Acta Physiologica. 2009;195(1):139–47. doi: 10.1111/j.1748-1716.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na -dependent glutamate/ aspartate transporter from rat brain. Proc NatI Acad Sci USA. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm-Mathisen J, Iversen LL. Uptake of [3H] glutamic acid in excitatory nerve endings: light and electron microscopic observations in the hippocampal formation of the rat. Neuroscience. 1979;4:1237–1253. doi: 10.1016/0306-4522(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Wold JE. In vivo high-affinity uptake and axonal transport of D-[2,3-3H]aspartate in excitatory neurons. Brain Res. 1981;230(1-2):427–33. doi: 10.1016/0006-8993(81)90428-5. [DOI] [PubMed] [Google Scholar]
- Sullivan SM, Macnab ST, Bjorkman ST, Coldizt PB, Pow DV. GLAST1b, the exon-9 skipping form of the glutamate-aspartate transporter EAAT1 is a sensitive marker of neuronal dysfunction in the hypoxic brain. Neuroscience. 2007;149:434–45. doi: 10.1016/j.neuroscience.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Rizzuto R. Chaperones as parts of organelle networks. Adv Exp Med Biol. 2007;594:64–77. doi: 10.1007/978-0-387-39975-1_7. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Taxt T, Storm-Mathisen J. Uptake of D-aspartate and L-glutamate in excitatory axon terminals in hippocampus: autoradiographic and biochemical comparison with gamma-aminobutyrate and other amino acids in normal rats and in rats with lesions. Neuroscience. 1984;11:79–100. doi: 10.1016/0306-4522(84)90215-x. [DOI] [PubMed] [Google Scholar]
- Torp R, Danbolt NC, Babaie E, Bjoras M, Seeberg E, Storm-Mathisen J, Ottersen OP. Differential expression of two glial glutamate transporters in the rat brain: an in situ hybridization study. Eur J Neurosci. 1994;6:936–942. doi: 10.1111/j.1460-9568.1994.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Torp R, Hoover F, Danbolt NC, Storm-Mathisen J, Ottersen OP. Differential distribution of the glutamate transporters GLT1 and EAAC1 in rat cerebral cortex and thalamus: an in situ hybridization analysis. Anat Embryol (Berl) 1997;195(4):317–326. doi: 10.1007/s004290050051. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Shin RM, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41:139–51. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8(12):935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Utsunomiya-Tate N, Endou H, Kanai Y. Tissue specific variants of glutamate transporter GLT-1. FEBS Lett. 1997;416:312–316. doi: 10.1016/s0014-5793(97)01232-5. [DOI] [PubMed] [Google Scholar]
- Velaz-Faircloth M, McGraw TS, Malandro MS, Fremeau RT, Jr, Kilberg MS, Anderson K. Characterization and distribution of the neuronal glutamate transporter EAACI in rat brain. Am J Physiol. 1996;270:C67–C75. doi: 10.1152/ajpcell.1996.270.1.C67. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Pérez-Cerdá F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol Dis. 2006;21:154–64. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10(3):976–88. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Pan ZZ, Nie H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience. 2007;149:898–907. doi: 10.1016/j.neuroscience.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, Lingwood BE, Dodd PR, Pow DV. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49(4):520–541. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci. 2003;23:4775–4784. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]