Abstract
Backgrounds
It has been extensively proved that the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) is superior to that of cytotoxic chemotherapy in advanced non-small cell lung cancer (NSCLC) patients harboring sensitive EGFR mutations. However, the question of whether the efficacy of EGFR-TKIs differs between exon 19 deletion and exon 21 L858R mutation has not been yet statistically answered.
Methods
Subgroup data on hazard ratio (HR) for progression-free survival (PFS) of correlative studies were extracted and synthesized based on random-effect model. Comparison of outcomes between specific mutations was estimated through indirect and direct methods, respectively.
Results
A total of 13 studies of advanced NSCLC patients with either 19 or 21 exon alteration receiving first-line EGFR-TKIs were included. Based on the data from six clinical trials for indirect meta-analysis, the pooled HRTKI/chemotherapy for PFS were 0.28 (95% CI 0.20–0.38, P<0.001) in patients with 19 exon deletion and 0.47 (95% CI 0.35–0.64, P<0.001) in those with exon 21 L858R mutation. Indirect comparison revealed that the patients with exon 19 deletion had longer PFS than those with exon 21 L858R mutation (HR19 exon deletion/exon 21 L858R mutation = 0.59, 95% CI 0.38–0.92; P = 0.019). Additionally, direct meta-analysis showed similar result (HR19 exon deletion/exon 21 L858R mutation = 0.75, 95% CI 0.65 to 0.85; P<0.001) by incorporating another seven studies.
Conclusions
For advanced NSCLC patients, exon 19 deletion might be associated with longer PFS compared to L858 mutation at exon 21 after first-line EGFR-TKIs.
Introduction
Lung cancer, mainly non-small-cell lung cancer (NSCLC), remains to be the leading cause of cancer-related mortality worldwide [1]. Unfortunately, few treatment options are available for the majority of patients with advanced or metastatic disease [2]. In spite of marginal improvement in survival, most advanced patients require systemic therapy [3]. Recent advances in genetic discoveries have proved that EGFR-dependent pathway is activated in more than half of the patients with NSCLC and it plays an important role in the development and the progression of epithelial cells [4]. Small-molecule tyrosine kinase inhibitors (TKIs), including gefitinib and erlotinib, which specifically block the EGFR-dependent pathway, were the first targeted drugs to enter the clinical use for the treatment of lung cancer [5]. It has been extensively proved that NSCLC patients harboring sensitive EGFR mutations, which mainly refer to exon 19 deletions or L858R substitution in exon 21, usually benefit more from EGFR-TKIs than wild-type patients [6], [7]. However, whether the efficacy of EGFR-TKIs varies among different sensitive EGFR mutations is still controversial. Several studies have reported that advanced NSCLC patients with EGFR exon 19 deletion had a longer overall survival (OS) and/or progression-free survival (PFS) following treatment with gefitinib or erlotinib compared with those with the L858R mutation [8], [9], [10], but this result has not been shown in all reports [12], [13], [14], [15], [16].
Based on these scattered data, it is not convincing to conclude that a specific EGFR mutation may affects the response to EGFR-TKIs. Therefore, we sought to perform a meta-analysis by incorporating relevant studies to evaluate whether the clinical outcome differs between exon 19 deletion and exon 21 L858R mutation in advanced NSCLC patients treated with first-line EGFR-TKIs.
Methods
Literature search
All relevant articles were retrieved by searching through PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library, using a combination of the terms “EGFR”, “epidermal growth factor receptor”, “tyrosine kinase inhibitors”, “TKI”, “exon”, “mutation”, “non-small-cell lung cancer” and “NSCLC”. An additional search through Google Scholar and a manual search through reference lists of pertinent reviews and included studies were performed. Two authors (FW and KS) carried out the search independently. No language or date restrictions were set in the search.
Inclusion and exclusion criteria
Eligible studies should meet the following criteria: (i)clinical trials or retrospective studies which investigated the local advanced or metastatic (IIIB or IV) stage NSCLC with first-line monotherapy of EGFR-TKIs (e.g. gefitinib, erlotinib or afatinib) (ii) clinical trials or retrospective studies with a subset of NSCLC patients with specific sensitive EGFR mutation (exon 19 deletion or exon 21 L858R mutation); (iii) EGFR mutation analysis was performed on available tumor tissue samples instead of circulating free DNA in serum; (iv) prior neoadjuvant or adjuvant chemotherapy in patients with recurrence after surgery was permitted if it had elapsed from last administration to relapse at least 6-month; (v) hazard ratios (HRs) of EGFR-TKIs compared to conventional chemotherapy for progression-free survival (PFS) and HRs of exon 19 deletion compared to exon 21 L858R mutation for PFS in terms of EGFR-TKIs were available. Studies failing to meet the inclusion criteria will be excluded.
Outcomes measures, data extraction and quality assessment
The clinical outcome for this meta-analysis was PFS. The data collection and assessment of methodological quality followed the QUORUM and the Cochrane Collaboration guidelines (http://www.cochrane.de). Data of PFS were extracted as HR and its 95% confidence interval (CI) from subgroup analysis by two investigators (YY and FW) independently. We used median PFS and the P-value to calculate the HR and its 95% CI then reviewed them with Review Manager (version 5.1 for Windows; the Cochrane Collaboration, Oxford, UK) if they were not displayed directly. The following main information was also extracted from included studies: author, year, trial name, details of therapeutic regimens, and number of participants with either 19 or 21 exon alterations. Three reviewers (YY, KS and FW) used the Jadad scale to assess the quality of included random control trials (RCTs) and a modified Newcastle-Ottawa scale to assess other studies. Discrepancies were discussed by all investigators to reach a consensus.
Statistical analysis
Pooled HRs for PFS with 95% CI were calculated. Heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). The random-effects model was employed in case of potential heterogeneity and to avoid underestimation of standard errors of pooled estimates in direct meta-analyses as well as subsequent indirect comparison. All calculations were performed using STATA 11.0 (StataA Corp, College Station, TX). Based on the assumption that no significant difference of chemotherapy efficacy existed between exon 19 deletions and L858R mutations, we calculated the adjusted indirect comparison using the following formulas as previously described [17]. The log hazard ratio (log HR) of the adjusted indirect comparison for arm A versus arm B was estimated by 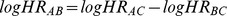 , and its standard error for the log HR was
, and its standard error for the log HR was 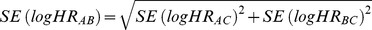 , in which log HRAC was the log HR for the direct comparison of patients with exon 19 deletion who received EGFR-TKIs versus those who received chemotherapy and log HRBC were the log HR for the direct comparison of patients with exon 21 mutation who received EGFR-TKIs versus those who received chemotherapy. SE (log HR) was the standard error of the log HR for the direct comparisons. A HR value of less than 1 standed for patients receiving EGFR-TKIs with exon 19 deletion demonstrated longer PFS than those with exon 21 mutation. All CIs had two-sided probability coverage of 95%. A statistical test with p value less than 0.05 was considered as significant.
, in which log HRAC was the log HR for the direct comparison of patients with exon 19 deletion who received EGFR-TKIs versus those who received chemotherapy and log HRBC were the log HR for the direct comparison of patients with exon 21 mutation who received EGFR-TKIs versus those who received chemotherapy. SE (log HR) was the standard error of the log HR for the direct comparisons. A HR value of less than 1 standed for patients receiving EGFR-TKIs with exon 19 deletion demonstrated longer PFS than those with exon 21 mutation. All CIs had two-sided probability coverage of 95%. A statistical test with p value less than 0.05 was considered as significant.
Publication bias
An extensive search strategy was made to minimize the potential of publication bias. Graphical funnel plots were generated to visually assess a publication bias. The statistical methods used to detect funnel plot asymmetry were the rank correlation test of Begg and Mazumdar and the regression asymmetry test of Egger [18], [19].
Results
Eligible studies
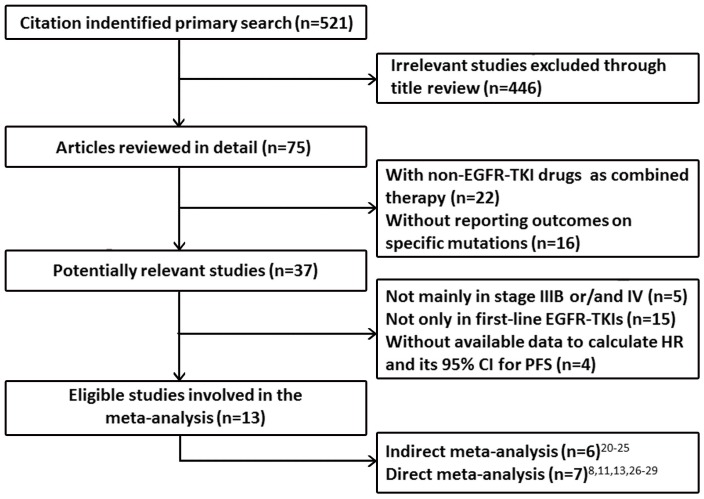
We identified 521 records according to the search strategy and focused on 13 studies which reported advanced NSCLC patients with either 19 or 21 exon alteration who received first-line monotherapy of EGFR-TKIs. Six phase III RCTs (IPASS [20], WJTOG3405 [21], OPTIMAL [22], EUTRAC [23], LUXLUNG3 [24], and LUXLUNG6 [25]), involving 1382 advanced NSCLC chemo-naïve patients, investigated the efficacy of EGFR-TKIs (gefitinib, erlotinib or afatinib) and conventional platinum-based chemotherapy. PFS and subgroup analyses of different sensitive EGFR mutations were reported. Therefore, these studies were selected for indirect meta-analysis. Another seven studies (clinical trials or retrospective studies) [8], [11], [13], [26], [27], [28], [29] involving 549 advanced NSCLC patients receiving first-line EGFR-TKIs (gefitinib or erlotinib) presented direct comparison of exon 19 deletion and L858R mutation for PFS. We incorporated these 7 studies for direct meta-analysis. Figure 1 summarizes the flow chart. Table 1 and Table 2 summarize the characteristics of involved studies for indirect meta-analysis and direct meta-analysis, respectively.
Figure 1. Profile summarizing the trial flow.
CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
Table 1. Characteristics of included studies for indirect meta-analysis.
| Lead author (y) | Trial name (phase) | Therapeutic regimen of TKI | Therapeutic regimen of Chemo | Exon of EGFR mutationa | Sample size (TKI/Chemo) | HRTKI/chemotherapy for PFS (95% CI) |
| Mok TS (2009) | IPASS (III) | Gefitinib 250 mg/d, po | Paclitaxel 200 mg/m2, d1, iv, q3w + carboplatin | 19 | 140 (66/74) | 0.38 (0.26–0.56) |
| (AUC = 5–6) d1, iv, q3w×6 cycles | 21 | 111 (64/47) | 0.55 (0.35–0.87) | |||
| Mitsudomi T (2010) | WJTOG3405 (III) | Gefitinib 250 mg/d, po | Docetaxel 60 mg/m2, d1, iv, q3w + cisplatin 80 | 19 | 87 (50/37) | 0.453 (0.268–0.768) |
| mg/m2, d1, iv, q3w×3–6 cycles | 21 | 85 (36/49) | 0.514 (0.294–0.899) | |||
| Zhou CC (2011) | OPTIMAL (III) | Erlotinib 150 mg/d, po | Gemcitabine 1000 mg/m2, d1,8, iv, q3w + | 19 | 82 (43/39) | 0.13 (0.07–0.25) |
| carboplatin (AUC = 5) d1, iv, q3w×4 cycles | 21 | 72 (39/33) | 0.26 (0.14–0.49) | |||
| Rosell R (2012) | EUTRAC (III) | Erlotinib 150 mg/d, po | Docetaxel 75 mg/m2, d1 or gemcitabine | 19 | 115 (57/58) | 0.30 (0.18–0.50) |
| 1000–1250 mg/m2, d1,8, iv, q3w + cisplatin 75 | ||||||
| mg/m2, d1 or carboplatin (AUC = 5–6) d1, iv, | 21 | 58 (29/29) | 0.55 (0.29–1.02) | |||
| q3w×4 cycles | ||||||
| Sequist LV (2013) | LUXLUNG3 (III) | Afatinib 40 mg/d, po | Pemetrexed 500 mg/m2, d1, iv, q3w + cisplatin | 19 | 170 (113/57) | 0.28 (0.18–0.44) |
| 75 mg/m2, d1, iv, q3w×≤6 cycles | 21 | 138 (91/47) | 0.73 (0.46–1.17) | |||
| Wu YL (2014) | LUXLUNG6 (III) | Afatinib 40 mg/d, po | Gemcitabine 1000 mg/m2, d1,8, iv, q3w + | 19 | 186 (124/62) | 0.20 (0.13–0.33) |
| cisplatin 75 mg/m2, d1, iv, q3w×≤6 cycles | 21 | 138 (92/46) | 0.32 (0.19–0.52) |
Exon of EGFR mutation means either exon 19 deletion or exon 21 L858R mutation.
Abbreviations: AUC = area under the concentration time curve; Chemo = chemotherapy; CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
Table 2. Characteristics of included studies for direct meta-analysis.
| Lead author (y) | Study (phase) | Therapeutic regimen of TKI | Exon of EGFR mutationa | Sample size | Median PFS (m) | P-valueof PFS | HR19/21 of TKIb for PFS (95% CI) |
| Maemondo M (2010) | NEJ002 (III) | Gefitinib 250 mg/d, po | 19 | 58 | 11.5 | 0.90 | 0.939 (0.3518–2.5061) |
| 21 | 49 | 10.8 | |||||
| Asahina Hc (2006) | Prospective (II) | Gefitinib 250 mg/day, po | 19 | 13 | 8.3 | 0.678 | 1.410 (0.2785–7.1388) |
| 21 | 3 | 11.7 | |||||
| Jackman DMc (2006) | Retrospective | Gefitinib 250 mg/day, po or erlotinib 150 mg/day, po | 19 | 22 | 24 | 0.04 | 0.417 (0.1810–0.9608) |
| 21 | 10 | 10 | |||||
| Li JJ (2012) | Retrospective | Gefitinib 250 mg/day, po or erlotinib 150 mg/day, po | 19 | 33 | 9.0 | 0.002 | 0.778 (0.6635–0.9123) |
| 21 | 21 | 7.0 | |||||
| Lee VHF (2013) | Retrospective | Gefitinib 250 mg/day, po or erlotinib 150 mg/day, po | 19 | 64 | 12.8 | 0.040 | 0.649 (0.416–0.983) |
| 21 | 80 | 11.4 | |||||
| Lu RL (2014) | Retrospective | Gefitinib or erlotinib | 19 | 42 | 14.2 | <0.05d | 0.676 (0.4570–1.0000) |
| 21 | 34 | 9.6 | |||||
| Choi CM (2014) | Retrospective | Gefitinib 250 mg/day, po or erlotinib 150 mg/day, po | 19 | 77 | NA | NA | 0.846 (0.2815–2.5437) |
| 21 | 43 | NA |
Exon of EGFR mutation means either exon 19 deletion or exon 21 L858R mutation.
HR19/21 of TKI represents HR19 exon deletion/exon 21 L858R mutation in TKI therapy cohort.
We considered time to progression (TTP) as PFS in studies of Asahina H and Jackman DM.
We considered P-value as 0.05 in Lu RL's study to calculate the HR19/21 of TKI for PFS and its 95% CI.
Abbreviations: CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; NA = not available; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
Indirect meta-analysis of EGFR exon 19 deletions vs. exon 21 L858R mutations under TKI therapy for PFS
For advanced NSCLC patients with EGFR exon 19 deletion, the pooled HR of EGFR-TKIs compared to conventional chemotherapy were 0.28 (95% CI, 0.20–0.38, P<0.001). For another sensitive EGFR mutation type, L858R mutation in exon 21, the pooled HR were 0.47 (95% CI 0.35 to 0.64, P<0.001). In addition, subgroup analyses revealed similar results that both exon 19 deletion (HRgefitinib/chemotherapy = 0.40, 95% CI 0.30 to 0.55, P<0.001; HRerlotinib/chemotherapy = 0.20, 95% CI 0.09 to 0.46, P<0.001; HRafatinib/chemotherapy = 0.24, 95% CI 0.17 to 0.33, P<0.001) and exon 21 L858R mutation (HRgefitinib/chemotherapy = 0.54, 95% CI 0.38 to 0.76, P = 0.001; HRerlotinib/chemotherapy = 0.38, 95% CI 0.18 to 0.79, P = 0.009; HRafatinib/chemotherapy = 0.49, 95% CI 0.22 to 1.09, P = 0.080) resulted in reduced progression risk through different types of TKIs ( Figure 2 and Figure 3 ). Figure 4 shows the relationship of the indirect comparisons. Present evidence suggested that there was little difference in the efficacy of platinum-based doublet for patients with exon 19 deletion and those with exon 21 L858R mutation. Indirect comparison revealed that patients with exon 19 deletion had more favorable outcome for PFS than those with exon 21 L858R mutation (HR19/21 = 0.59, 95% CI 0.38 to 0.92; P = 0.019) under TKIs therapy. Besides, we found similar results through subgroup analyses stratified by TKI types (Gefitinib: HR19/21 = 0.76, 95% CI 0.47 to 1.21, P = 0.244; Erlotinib: HR19/21 = 0.53, 95% CI 0.18 to 1.61, P = 0.264; Afatinib: HR19/21 = 0.49, 95% CI 0.21 to 1.17, P = 0.108) ( Table 3 ).
Figure 2. Direct comparison of TKI versus chemotherapy in EGFR exon 19 deletions cohort in terms of HR for PFS. CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
Figure 3. Direct comparison of TKI versus chemotherapy in EGFR exon 21 L858R mutations cohort in terms of HR for PFS. CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
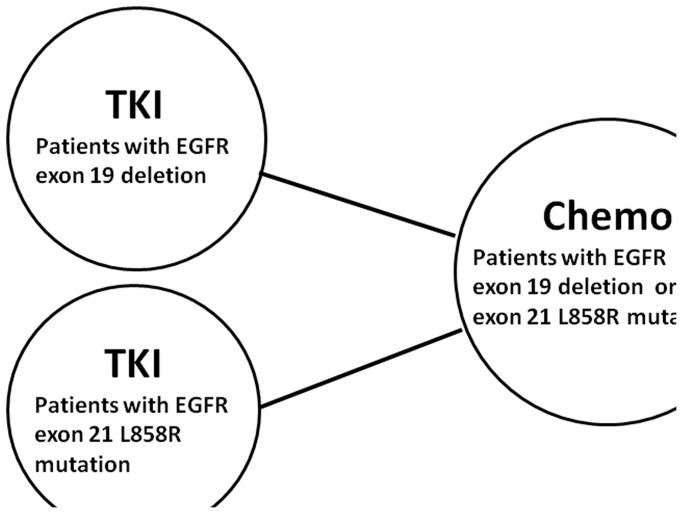
Figure 4. Geometric distribution of indirect comparisons.
Solid lines between regimens represented the existence of direct comparisons. Chemo = chemotherapy; EGFR = epidermal growth factor receptor; TKI = tyrosine kinase inhibitor.
Table 3. Indirect comparison of EGFR exon 19 deletion versus EGFR exon 21 L858R mutation in TKI therapy cohort in terms of HR for PFS.
| TKI | HR19/21 of TKIa for PFS (95% CI) | P-value |
| Gefitinib | 0.76 (0.47–1.21) | 0.244 |
| Erlotinib | 0.53 (0.18–1.61) | 0.264 |
| Afatinib | 0.49 (0.21–1.17) | 0.108 |
| Overall | 0.59 (0.38–0.92) | 0.019 |
HR19/21 of TKI represents HR19 exon deletion/exon 21 L858R mutation in TKI therapy cohort.
Abbreviations: CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
Direct meta-analysis of EGFR exon 19 deletions vs. exon 21 L858R mutations under TKI therapy for PFS
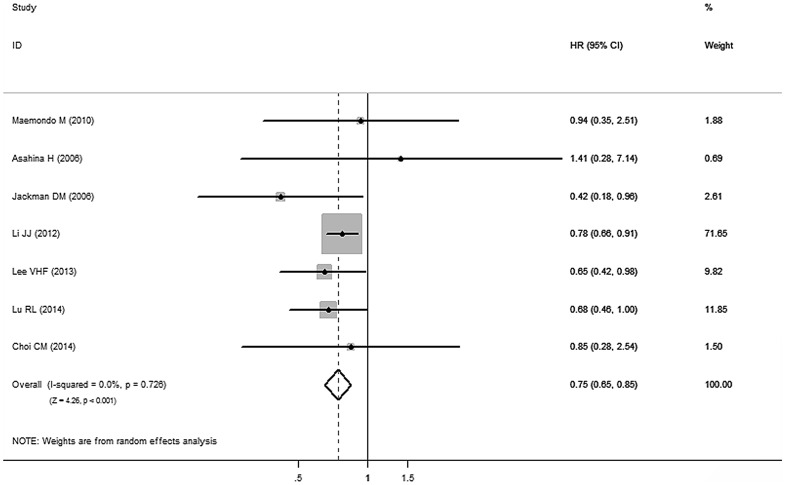
Based on the data from the above seven studies [8], [11], [13], [26], [27], [28], [29], advanced NSCLC patients with exon 19 deletion benefited from longer PFS than those with exon 21 L858R mutation (HR19/21 = 0.75, 95% CI 0.65 to 0.85; P<0.001) ( Figure 5 ). There was no publication bias for outcome measures, as the funnel plot analysis showed a symmetrical appearance and all p values were greater than 0.05 in Begg's test and Egger's test.
Figure 5. Direct comparison of EGFR exon 19 deletions versus EGFR exon 21 L858R mutations in TKI therapy cohort in terms of HR for PFS. CI = confidence interval; EGFR = epidermal growth factor receptor; HR = Hazard ratio; PFS = progression-free survival; TKI = tyrosine kinase inhibitor.
Discussion
For patients with advanced NSCLC, the association of specific EGFR mutation genotype and the efficacy or prognosis of first-line EGFR-TKIs therapy remains to be unclear. A meta-analysis incorporating all available data from correlative studies was a good way to address this question. We conducted this study and found that patients with exon 19 deletion had significantly reduced disease progression risk than those with exon 21 L858R mutation after front-line TKIs. Additionally, similar trends of favorable outcome of PFS in exon 19 deletion among different first-line EGFR-targeted agents (gefitinib, erlotinib and afatinib), were presented in our work, but the statistical significances were not approached.
Our results derived three interpretations. Firstly, exon 19 deletions might be more efficiently inhibited by EGFR-TKIs than exon 21 L858R mutations[8].Mutant forms of exon 19 deletion and L858R mutation had a reduced affinity for its natural substrate, ATP, but equivalent or increased affinity for reversible EGFR-TKIs, which explained why tumors with these EGFR mutations had higher sensitivity to TKIs [30], [31]. If exon 19 deletion results in EGFR structural alterations which bind TKIs more tightly than L858R mutation, it could support our hypothesis. However, an in vitro study showed that the growth of NSCLC cell lines harboring exon 19 deletion or L858R mutation were almost equally inhibited by equivalent concentrations of gefitinib, and the degree of EGFR phosphorylation [32].
Secondly, T790M mutation, which was associated with acquired resistance to reversible EGFR-TKIs [33], might occur more frequently in L858R mutation. However, a prospective rebiopsy protocol among different EGFR mutated patients with acquired resistance to EGFR-TKIs showed that no difference between exon 19 deletions (63%) and L858R mutations (61%) existed in terms of the presence of T790M mutation [34]. Another recent phase II trials found similar results [35].
Thirdly, exon 21 L858R mutation co-existing with other uncommon mutations might affect the hypersensitivity of L858R to EGFR-TKIs. A retrospective study which investigated the frequency of complex mutations in 783 NSCLC patients found the majority was G719S plus L858R mutation (n = 8), the result also suggested poor response to gefitinib in patients with G719S plus L858R mutation [36]. Besides, previous studies revealed that patients with exon 18 (G719S) plus L858R mutation showed poor outcomes with gefitinib [37], [38], [39].
Based on the above hypotheses, the mechanism of more favorable efficacy in exon 19 deletion compared with L858R mutation is still controversial. Nonetheless, regardless of what the true causes might be, this comprehensive analysis statistically confirmed that patients with exon 19 deletion were associated with longer PFS compared to those with L858 mutation at exon 21. The result leads to some important hints. Firstly, we suggest that investigators should consider the proportion of type of sensitive EGFR mutation as a stratification factor in designing or reviewing clinical studies involving target therapy. In addition, the mechanism of EGFR-TKIs acting on different genotypes of EGFR mutants is more complicated than we imagined before. Therefore, more efforts should be made to investigate the pharmacological essence inside.
One research reported that patients with EGFR-mutant after front-line TKIs might develop as acquired resistance to TKI [20]. Besides, some previous investigations found inferior response to EGFR-TKIs following treatment of chemotherapy [40], [41]. According to previous evidence, getting second-line or third-line EGFR-TKIs involved will tangle the discussion. Therefore, in order to minimize the crossover effects, we only focused on first-line EGFR-TKIs in our analyses.
This is the first study to comprehensively answer the impact of different EGFR mutation types on TKIs. However, there existed several limitations. First, our meta-analysis was based on subgroup data of included articles, which might compromise the evidence level. In addition, the small number of included studies negated statistically significances in subgroup analyses. Finally, our work was conducted based on the assumption that there were no significant differences in the efficacy of platinum-based chemotherapy between exon 19 deletion and L858R mutation for advanced NSCLC patients. Few studies focused on the prognostic value of different EGFR mutation in patients with advanced NSCLC with chemotherapy [42], and as a consequence, our hypothesis is in need of confirmation by more convincing evidence.
In conclusion, for patients with advanced NSCLC, EGFR exon 19 deletion might be associated with longer PFS than those harboring L858 mutation at exon 21 after first-line EGFR-TKIs. Sensitive EGFR mutation type should be considered an essential factor in studies regarding EGFR-targeted agents.
Clinical Practice Points
The association of specific EGFR mutation genotype with the prognosis in patients with advanced NSCLC after first-line EGFR-TKIs was controversial based on previous reports. This comprehensive analysis statistically confirmed exon 19 deletions were associated with longer PFS compared with L858 mutations at exon 21. Additionally, the superior benefits of PFS in former genotype of EGFR mutation showed similar trends among different EGFR-targeted agents (gefitinib, erlotinib and afatinib), although the significances were not approached statistically. The result gave us some important hints. Firstly, we strongly suggested that investigators should consider the proportion of type of sensitive EGFR mutation as a stratification factor in designing or reviewing clinical studies regarding target therapy. In addition, the mechanism of EGFR-TKIs acting on different genotypes of EGFR mutants was more complicated than what we acknowledged. Therefore, more efforts should be made to investigate pharmacological essence inside.
Supporting Information
PRISMA Checklist.
(DOC)
Acknowledgments
We thank Mr. Avira Som (undergraduate student from Massachusetts Institute of Technology) and Dr. Aiham Qdaisat (visiting scientist from MD Anderson Cancer Center) for help revising this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National High Technology Research and Development Program of China, Grant No. 2012AA02A502; Innovative drug R&D center based on real-time high-throughput cell-based screening platform and large capacity compound library, Grant No. 2013ZX09401003-002; National Natural Science Funds of China, Grant No. 81372502; Wu Jieping Medical Foundation Project, Grant No. 320.6750.131. All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Wakelee H, Belani CP (2005) Optimizing first-line treatment options for patients with advanced NSCLC. Oncologist 10 Suppl 3 1–10. [DOI] [PubMed] [Google Scholar]
- 3. Ramalingam S, Belani C (2008) Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 13 Suppl 1 5–13. [DOI] [PubMed] [Google Scholar]
- 4. Herbst RS, Heymach JV, Lippman SM (2008) Lung cancer. N Engl J Med 359: 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cataldo VD, Gibbons DL, Perez-Soler R, Quintas-Cardama A (2011) Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med 364: 947–955. [DOI] [PubMed] [Google Scholar]
- 6. Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, et al. (2013) Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 105: 595–605. [DOI] [PubMed] [Google Scholar]
- 7. Paz-Ares L, Soulieres D, Melezinek I, Moecks J, Keil L, et al. (2010) Clinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med 14: 51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, et al. (2006) Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 12: 3908–3914. [DOI] [PubMed] [Google Scholar]
- 9. Riely GJ, Pao W, Pham D, Li AR, Rizvi N, et al. (2006) Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 12: 839–844. [DOI] [PubMed] [Google Scholar]
- 10. Goto K, Nishio M, Yamamoto N, Chikamori K, Hida T, et al. (2013) A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 82: 109–114. [DOI] [PubMed] [Google Scholar]
- 11. Lu RL, Hu CP, Yang HP, Li YY, Gu QH, et al. (2014) Biological Characteristics and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors Efficacy of EGFR Mutation and its Subtypes in Lung Adenocarcinoma. Pathol Oncol Res 20: 445–451. [DOI] [PubMed] [Google Scholar]
- 12. Yang CH, Yu CJ, Shih JY, Chang YC, Hu FC, et al. (2008) Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 26: 2745–2753. [DOI] [PubMed] [Google Scholar]
- 13. Asahina H, Yamazaki K, Kinoshita I, Sukoh N, Harada M, et al. (2006) A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer 95: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue A, Suzuki T, Fukuhara T, Maemondo M, Kimura Y, et al. (2006) Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 24: 3340–3346. [DOI] [PubMed] [Google Scholar]
- 15. Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T (2009) Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol 4: 22–29. [DOI] [PubMed] [Google Scholar]
- 16. Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, et al. (2008) First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 26: 2442–2449. [DOI] [PubMed] [Google Scholar]
- 17. Yang Q, Wei Y, Chen YX, Zhou SW, Jiang ZM, et al. (2013) Indirect comparison showed survival benefit from adjuvant chemoradiotherapy in completely resected gastric cancer with d2 lymphadenectomy. Gastroenterol Res Pract 2013: 634929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 20. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 21. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 22. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 23. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 24. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, et al. (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 25. Wu YL, Zhou C, Hu CP, Feng J, Lu S, et al. (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15: 213–222. [DOI] [PubMed] [Google Scholar]
- 26. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 27. Choi CM, Kim MY, Lee JC, Kim HJ (2014) Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings after tyrosine kinase inhibitor therapy. Radiology 270: 574–582. [DOI] [PubMed] [Google Scholar]
- 28. Lee VH, Tin VP, Choy TS, Lam KO, Choi CW, et al. (2013) Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic non-small-cell lung cancer. J Thorac Oncol 8: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 29. Li J, Qu L, Wei X, Gao H, Wang W, et al. (2012) [Clinical observation of EGFR-TKI as a first-line therapy on advanced non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi 15: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, et al. (2006) Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res 66: 8163–8171. [DOI] [PubMed] [Google Scholar]
- 31. Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, et al. (2007) Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, et al. (2005) Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst 97: 1185–1194. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, et al. (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352: 786–792. [DOI] [PubMed] [Google Scholar]
- 34. Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, et al. (2011) Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 17: 1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katakami N, Atagi S, Goto K, Hida T, Horai T, et al. (2013) LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 31: 3335–3341. [DOI] [PubMed] [Google Scholar]
- 36. Hata A, Yoshioka H, Fujita S, Kunimasa K, Kaji R, et al. (2010) Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol 5: 1524–1528. [DOI] [PubMed] [Google Scholar]
- 37. Chou TY, Chiu CH, Li LH, Hsiao CY, Tzen CY, et al. (2005) Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res 11: 3750–3757. [DOI] [PubMed] [Google Scholar]
- 38. Mitsudomi T, Yatabe Y (2007) Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 98: 1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu JY, Wu SG, Yang CH, Gow CH, Chang YL, et al. (2008) Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res 14: 4877–4882. [DOI] [PubMed] [Google Scholar]
- 40. Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, et al. (2012) First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 30: 3002–3011. [DOI] [PubMed] [Google Scholar]
- 41. Chin TM, Quinlan MP, Singh A, Sequist LV, Lynch TJ, et al. (2008) Reduced Erlotinib sensitivity of epidermal growth factor receptor-mutant non-small cell lung cancer following cisplatin exposure: a cell culture model of second-line erlotinib treatment. Clin Cancer Res 14: 6867–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamashita F, Azuma K, Yoshida T, Yamada K, Kawahara A, et al. (2013) Prognostic value of EGFR mutation and ERCC1 in patients with non-small cell lung cancer undergoing platinum-based chemotherapy. PLoS One 8: e71356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.