Abstract
The renal vasculature, like all vessels, is lined by simple squamous epithelium, called an endothelium. These endothelial-lined vessels can be subdivided into four major compartments: arteries, veins, capillaries and lymphatics. The renal vasculature is a highly integrated network that forms through the active processes of angiogenesis and vasculogenesis. The precise contribution of these two processes and the molecular signaling that governs the differentiation, specification and maturation of these critical cell populations is an actively evolving field. Though much of the focus has concentrated on the origin of the glomerular capillaries, this review extends the investigation to the origins of the endothelial cells throughout the entire kidney and the signaling events that cause their distinct functional and molecular profiles. A thorough understanding of endothelial cell biology may play a critical role in better understanding renal vascular diseases.
Keywords: Endothelium, kidney, development, vasculature, angiogenesis, vasculogenesis
Introduction
The development of the adult kidney involves the intricate molecular and cellular interactions of the metanephric mesenchyme (including the nephron progenitor and the renal stroma) and the ureteric bud. Less focus has been given to the formation of the vasculature and the role that the renal vasculature plays in patterning the developing kidney. The formation of an appropriately patterned vasculature is critical as the kidneys receive between 20–25% of the total cardiac output. This is of particular significance when one considers the relatively small size of the kidneys in relation to total body mass (approximately 0.5%). Recent studies have shifted the primary focus from glomerular capillaries to an expanded interrogation of the more numerous and encompassing vascular components that join together to form the post-glomerular renal vasculature. This manuscript will provide an up-to-date review of the literature related to the many and various interactions of the renal endothelium and its role in patterning the formation of the kidney.
Angiogenesis vs vasculogenesis
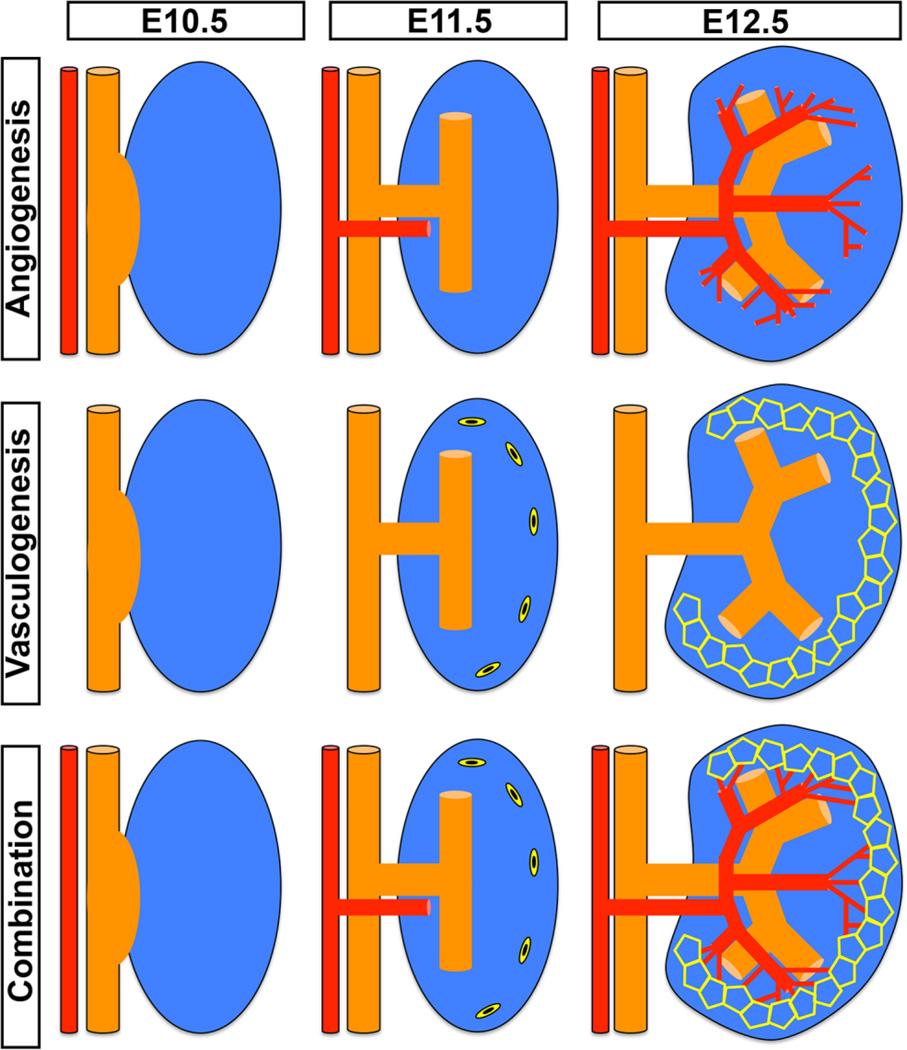
The formation of the developing vasculature throughout organogenesis and as the kidney develops is the combination of two biological processes; the first being angiogenesis, which is defined by the outgrowth (sprouting) of vessels from an established central vessel [1–3]. In the renal vasculature there are two primary angiogenic sites: vessels that sprout off the renal artery and the formation of the vasa recta, which branches off the efferent arterioles. The second is termed vasculogenesis, this process is the formation of vascular networks from inherent endothelial progenitors [4]. At the initiation of kidney development the angiogenic vessels track with the invading ureteric bud, concurrently the vasculogenic vessels are forming a primitive vascular network (Figure 1). Angiogenic vessels then connect with the vasculogenic network and produce the functional and highly integrated kidney vasculature. These two critical vascular events happen in a coordinated manner in the developing kidney to integrate into the glomeruli and attach to the major efferent renal vessels.
Figure 1. The contribution of angiogenesis and vasculogenesis in the developing kidney.
This schematic represents the interactions of angiogenesis and vasculogenesis throughout the early stages of kidney development. In the top panel the angiogenic vessels are seen to branch from the major renal vessels once the ureteric bud has invaginated into the metanephric mesenchyme. In a series of coordinated steps the angiogenic vessels continue to grow and branch closely tracking with the ureteric epithelial branching. As early as E11.5 sporadic cells are seen in the periphery of the kidney that express endothelial markers and these are the endothelial progenitors. These cells form a primitive vascular plexus that is representative of the vasculogenic vessels. Angioblasts have been found to populate the glomerulus and contribute to the vasculogenic vessels. The angiogenic and the vasculogenic vessels then join forming the functional renal vasculature.
The developmental origin and specification of endothelial progenitors in the kidney
The early intermediated mesoderm that is fated to become metanephric mesenchyme initially expresses the transcription factor Odd-Skipped-Related 1 (OSR1) and it is this cell lineage that gives rise to all the cell types of the metanephric mesenchyme. In mice this OSR1 positive mesenchyme also gives rise to a subset of endothelial precursors that are fated to become Flk1 (Vascular endothelial growth factor receptor 2) positive [5]. The endothelium of the kidney is then subdivided into the various subcompartments including the arteries, veins, capillaries and lymphatics [6]. The specification of the endothelium into its various four distinct types is a dynamic process that involves critical cross talk between VEGF, Ephrin, Notch and Sox signaling pathways (as reviewed in [6]).
Glomerular endothelium develops from inherent angioblasts via vasculogenesis
The human kidney contains between 200,000 and 2 million glomeruli [7]. One of the critical structures that reside within the glomerulus are the capillary loops. These capillary loops consist of fenestrated endothelial cells that act in the initial filtration of the blood, and podocytes (Figure 2). Unlike other fenestrated endothelial vessels, the glomerular capillaries are not spanned by diaphragms, which allows for free exchange of fluid, however they are not wide enough to allow red blood cells to pass. The glomerular basement membrane, synthesized by the glomerular endothelial cells and the podocytes, forms an integral part of the filtration system of the glomerulus. Previously, in mice it has been determined that the glomerular endothelium likely develops from hemangioblasts [8–10]. These cells are thought to circulate into the kidney through the angiogenic vessels and take up residency in the lower limb of the developing S-shaped body, and are closely associated and reliant on the mesangial cells and the podocytes for normal formation and function. This process was confirmed using a murine model which lineage tagged endothelial cells in combination with kidney explants either under the renal capsule or into the anterior chamber of the eye. Here it was found that explanted kidneys, even as early as embryonic day 12 (E12), contained the necessary cells to form the renal microvasculature including the endothelium of the glomerulus [8].
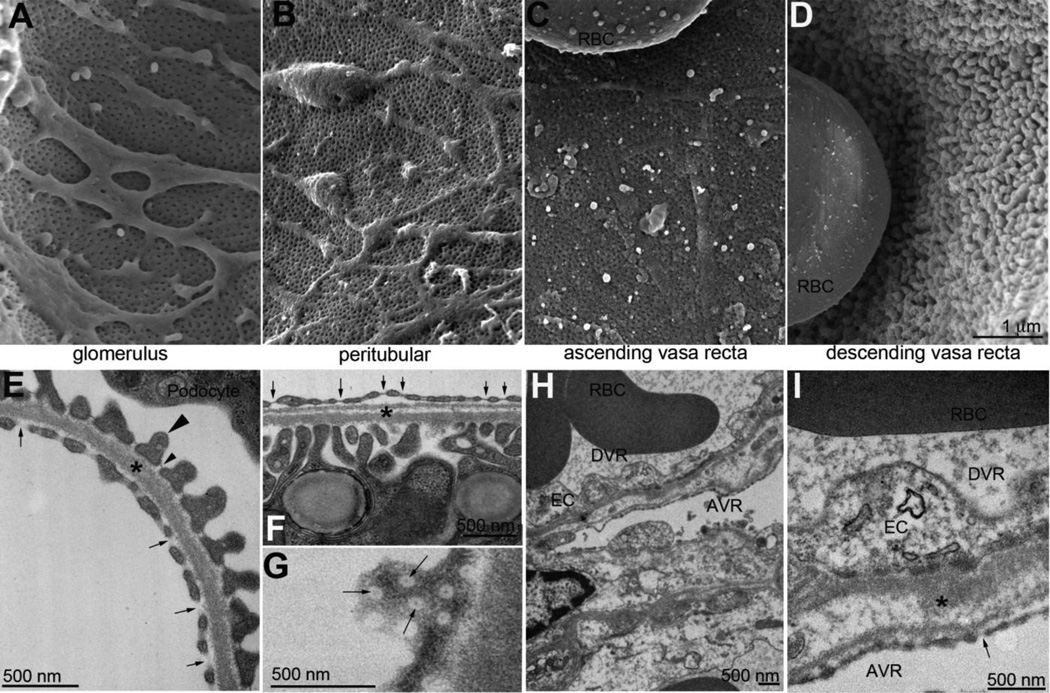
Figure 2. Ultrastructural examination of renal microvasculature.
Scanning electron micrographs (A–D) and transmission electron micrographs (E-I) of the major microvascular beds of the mouse kidney. Ultrastructure of the glomerular capillaries (A,E) showing fenestrated endothelium without proteinaceous diaphragms (E, arrows). Glomerular endothelial cells share a basement membrane (*) with podocyte foot processes (example, large arrowhead). Slit diaphragms (small arrowhead), span between individual foot processes. Peritubular endothelium (B) are also fenestrated, but the fenestrations are covered with diaphragms (F,G, arrows). I thick basement membrane (*) separates the peritubular vasculature from the tubular cells. Ascending vasa recta (AVR) are fenestrated endothelium with diaphragms (C,H,I, arrow) but descending vasa recta (DVR) endothelium are not fenestrated, but thick and continuous (D,H,I). In H, a thick basement membrane (*) separates the two microvascular components. Size bar A–D = 1 micron. RBC: red blood cell, EC: endothelial cell.
Vasculogenic vessels give rise to many of the peritubular capillaries
Each glomerulus is connected to the collecting duct system by a series of tubular segments. A rich vascular network known as the peritubular capillaries surround these tubular segments. The peritubular capillaries are critical for electrolyte balance by modulating reabsorption and secretion between the blood and filtrate. The origin of the peritubular capillaries is much less studied than those invested in the glomeruli. However, it has recently been shown by our lab and others in mice, that the peritubular capillary network arises from both resident endothelial progenitors as well the invading angiogenic vessels [4,11,12]. Studies from our laboratory have shown that the renal stroma gives rise to a subset of endothelial progenitors as early as E12.5 that are present in the peritubular capillaries, but not in glomerular endothelium. We utilized renal stroma that was permanently labeled (using Foxd1cre mice bred with a mouse that contains RFP driven under the Rosa locus) and tracked these cells through to adulthood, revealing that the renal stroma gave rise to mature peritubular endothelium. Furthermore, we determined that the renal stroma, when isolated and cultured in vitro, had the ability to differentiate into endothelial cells given the appropriate environmental cues [11]. Studies have determined that the metanephric mesenchyme contains a c-kit positive fraction of cells that also have the ability to differentiate into endothelial cells [13].
The origin of the renal endothelium has been shown to arise from angiogenesis and vasculogenesis, however, these vessels have a heterogeneous expression profile [14]. This suggests that the various vascular compartments in the kidney have unique expression profiles that may allude to their differing functional roles. Interestingly, the endothelium that forms in the kidney contains several histological types including fenestrated and fenestrated with diaphragms, as well as continuous capillaries. Fenestrated endothelia that do not have diaphragms are seen in the glomerular capillaries, and this is important for selective sieving of molecules from the blood into the filtrate (Figure 2 A,E). Fenestrated diaphragmed endothelia are found in the peritubular area (Figure 2 B,F,G) and ascending vasa recta capillaries (Figure 2 C,H,I). Continuous endothelial cells are seen lining the large vessels such as arterioles and venules as well as the descending vasa recta capillaries (Figure 2 D,H,I). Figure 3 shows that the density and patterning of the vasculature is unique between the cortex and medullary compartments.
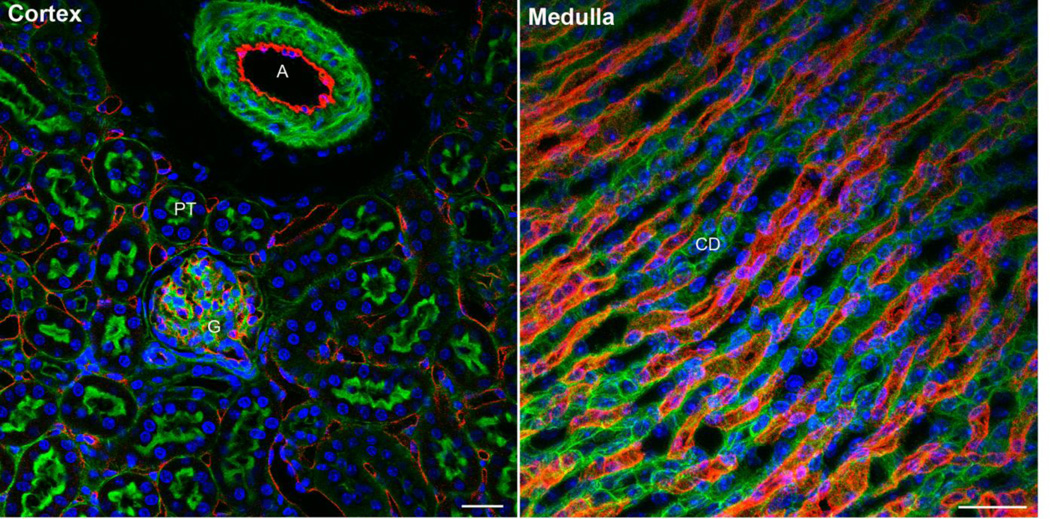
Figure 3. Vascularity in the kidney.
Frozen sections of mouse kidney were labeled to accentuate the endothelium (CD31, red) and counterstained to show F-actin (Phalloidin, green microvilli) and nuclei (Hoechst’s dye, blue). In cortex, the vasculature is located within the glomerulus (G) and surrounding the tubules (PT, proximal tubules). Occasional large arteries (A, like the arcuate or interlobular) are also visible. In the inner medulla, the vasa recta are packed very densely surrounding the collecting ducts and loops of Henle. Size bar = 50 microns.
Several years ago, a molecular profile was determined for mouse endothelial cells in the kidney [15]. In this study it was determined that the developing renal endothelium contained markers of both an endothelial and hematopoetic nature suggesting that there are multiple sources contributing to the endothelial cells within the kidney. The heterogenic expression pattern of the kidney endothelium allows unique therapeutic opportunities. If one can specifically target the various endothelial compartments of the kidney to either stimulate, when vascular formation is perturbed or insufficient, or inhibit vessel formation, to treat renal cancers, this could be highly beneficial to patients with kidney diseases.
Critical role of vascular endothelial growth factor signaling in patterning the kidney
Perhaps the most critical of all of the signaling pathways that regulate endothelial cells is the vascular endothelial growth factor (VEGF) pathway. This signaling pathway comprises 5 major ligands VEGF-A,B and C,D and placenta growth factor (PIGF). These ligands bind to 1 or more receptor tyrosine kinases known as VEGFR1,2 and 3 [16,17]. Knockout experiments in mice have shown that the binding of VEGF-A to VEGFR2 is critical for vascular development. Homozygous VEGF-A knockouts die at E8–9, while even a loss of a single allele leads to early embryonic lethality [18,19]. While both VEGFR1 [20] and VEGFR2 [21] mice die at a similar time, their phenotypes differ from one another. VEGFR2 mice have a lack of differentiated endothelial cells and also do not contain organized blood vessels. Conversely, VEGFR1 mice do possess mature endothelial cells but the vessels are large disorganized, suggesting alternate and non-overlapping roles of these two receptors. Furthermore, lymphatic endothelium is highly dependent upon VEGF-C binding to VEGFR3. The VEGF ligands and receptors are expressed at high levels in the developing and adult kidney. At the initiation of nephrogenesis, VEGF-A has been found in the E11 metanephric mesenchyme, while the expression of endothelial progenitor markers was also observed. At this early developmental stage there were no patent capillaries. However, it was postulated that these endothelial progenitors were able to respond to VEGF-A signals and form patent glomerular capillaries [22]. VEGF ligands are widely expressed in the kidney, typically in the podocytes, distal tubules and collecting ducts and at lower levels in the proximal tubules [23,24], while the receptors have been found expressed on endothelial cells of glomeruli and peritubular capillaries. It has also been found that VEGFR2 is expressed on the apical surface of the ureteric epithelium [25,26]. Further, in vitro experiments have shown a critical role for VEGF in mediating ureteric branching morphogenesis [25,27]. VEGF activity is regulated on many levels. It has recently been determined that the Semaphorin 3a gene product competes with VEGF-A; subsequently when Semaphorin 3a is knocked down, VEGF-A is able to elicit more of an effect on the ureteric epithelium leading to an increase in ureteric branching. Conversely when recombinant Semaphorin 3a is added to organ culture a knockdown in the activity of VEGF-A is seen followed by a reduction in ureteric branching [28]. These findings taken together suggest that the effect on the ureteric epithelium is direct, as VEGF-A could directly signal to VEGFR2 on the ureteric epithelium. As well as being a potent inhibitor of ureteric branching Semaphorin 3a has also been shown to play an important role in regulating kidney vasculature patterning. Global deletion of Semaphorin 3a leads to vascular patterning defects including glomerular malformation characterized by an overabundance of endothelial cells in the glomerulus and effaced podocytes [29].
VEGF-A is produced by the developing nephrons and can act as a chemoattractant for the invading vascular cells that populate the glomerulus [30]. Conditional deletion of VEGF in mice within the glomerular podocytes leads to perinatal lethality [31]. This highlights one of the critical roles of VEGF-A signaling which is a major component of crosstalk existing between podocytes and endothelial cells to produce functional glomeruli. It has also been elucidated that blocking of VEGF-A signaling during the early post-natal period, when most of the other developing organs have already formed, therefore circumventing the early lethality, leads to a reduction in the number of vessels in the renal cortex and glomeruli that are avascular, suggesting that the VEGF-A programming of the endothelial cells is critical through all developmental time points of kidney development [32]. It has been determined that renal development is dependent on appropriate VEGF signaling. In humans, it has been shown that splicing of Exon 8 of VEGF-A leads to two classes of factors; angiogenic (VEGF165)and anti-angiogenic (VEGF165b) [33,34]. During kidney development VEGF165b is expressed in the developing glomerulus through all developing stages and also in the immature podocytes. However, expression of this isoform is down-regulated in the mature glomerulus when angiogenesis is critical for formation of the glomerular capillary tuft. Subsequently, it is the role of anti-angiogenic factors to mediate the actions of the VEGF isoforms in normal kidney vasculature [35].
Role of angiogenic factors during kidney development
Formation of the vascular networks of the kidney requires highly specialized molecular signaling. There is a critical role for angiogenic factors in patterning the developing vasculature and kidney. The hypoxia inducible factors (HIFs) are a potent group of transcription factors that control the expression of angiogenic genes. As their names suggest, these molecules are activated under hypoxic conditions. When oxygen levels are low, as is the case during kidney development, HIF genes are seen to be expressed in the mouse at E14 and also in the early postnatal period [36], HIF subunits are able to translocate to the nucleus and avoid interaction with Von Hippel-Lindau protein (VHL), an E3 ubiquitin ligase, that leads to HIF degradation. However, under levels of high oxygenation that occur later in kidney development, kidney development ends at P4–5 [37], HIF subunits are subjected to oxygen-dependent propyl hydroxylation which leads to their interaction with VHL and their subsequent degradation [38]. These pathways are critical in mediating vascular signaling in the developing kidney. HIF subunits are largely found in the nephrogenic zone and in the developing collecting ducts during embryogenesis, which are regions that are known to be hypoxic. The major HIF-induced genes during kidney development are VEGF, Flt1 and Flk1 and these genes are paramount to normal renal microvascular development [36,39]. Both VEGF and Flt1 (VEGFR1) contain the conserved hypoxia responsive elements, and these two factors have been shown to be responsive to hypoxia both in vitro and in vivo [40–42]. Furthermore, VEGFR2 has also been shown to be responsive under hypoxic conditions [43]. It was previously determined that when embryonic kidneys are cultured under normoxic conditions they do not contain endothelial cells. However, when these cultures are maintained in hypoxic conditions, the kidneys are able to develop endothelial cells [44].
Notch genes are one of the critical signaling components that drive angiogenic vessels. These genes play an essential role in mediating the expression of VEGFR2 in tip cells, while down-regulating VEGFR2 in the adjacent stalk cell. This intricate balance determines the correct location of the branching vessels [45]. Further, it has previously been determined that the Notch genes are important factors for formation of the glomerular vasculature. When mice are hypomorphic for the Notch2 gene (which is normally expressed in the glomerular epithelial cells) they are unable to signal appropriately to its ligand Jagged (expressed by the endothelium), which leads to glomeruli that are lacking both endothelial cells and mesangial cells [46,47].
Angiopoietins are a family of growth factors that have been shown to regulate vascular development in the developing kidney. Angiopoietin 1 (Angpt-1) is expressed in the metanephric mesenchyme, maturing tubules and mature podocytes and binds to its endothelial receptor Tie-2 leading to endothelial survival, cell-cell stabilization and maturation of the vessel [48]. One of the major roles of Angpt-1 is to drive endothelial cell proliferation; this was confirmed in vitro via the addition of Angpt-1 to kidney organ cultures resulting in an increased proportion of vascularized glomeruli. Global deletion of Angpt-1 leads to early developmental lethality. To circumvent this problem, conditional knockout approaches have been utilized and have shown that Angpt-1 is critical for regulating vessel diameter and number. Global deletion of Angpt-1 causes glomerular capillary defects; it was observed that the endothelial cells did not attach appropriately to the basement membrane leading to dilated capillary loops [49]. Furthermore, null deletion of the Angpt-1 receptor Tie-2 has similar vascular abnormalities to Angpt-1 global mutants and early embryonic lethality [50]. Furthermore, the Angpt-1/Tie-2 transduction pathway is further modulated by VEGF-A signaling [51]. Signaling can be down-regulated by Angpt-2 (expressed in renal smooth muscle and pericytes)/Tie-1 (endothelial cells) interactions. This is particularly evident as deletion of Angpt-2 leads to alterations in kidney vascular patterning. This is driven by an increase in Tie-2 expression leading to peritubular capillary dysmorphogenesis [52]. Taken together, these findings suggest that there is a delicate balance between Angpt-1/Angpt-2 signaling and alterations in this balance lead to vessel instability and inappropriate vascular patterning.
The renin angiotensin system (RAS) is a complex developmental pathway that has previously been shown to regulate vascular formation of the kidney (as reviewed in [53]). In the developing kidney, renin expressing cells have been found to mediate branching of the renal arterioles suggesting a role in angiogenesis [54]. Pharmacological blockage of any individual component of the RAS pathway (including angiotensin, angiotensinogen or angiotensin converting enzyme) has been shown to cause morphological changes including renal vascular development that is severely stunted [55,56]. However, inactivation of single receptor subunits has not recapitulated this phenotype suggesting a functional redundancy in these receptor subunits [57–60]. Angiotenisin II has subsequently been shown to promote development of the renal microvasculature through AT1 receptors. This was shown by treating rats with the angiotensin II type 1 receptor antagonist candesartan in the perinatal period, which led to a decrease in cortical and medullary capillaries as well as inhibiting the organization of the vasa recta bundles. Similar findings were observed when the AT1 receptors were deleted in mice [61].
Renal lymphatics developmental origins to disease progression
In the kidney, the least characterized of all the endothelial cells is the lymphatic endothelium. The lymphatic vessels are important for the drainage of plasma and interstitial fluids. Lymphatic vessels are responsive to signaling from VEGF-C via VEGFR3. Recent studies have focused on characterizing the lymphatic endothelium throughout kidney development. Lymphatic vessels have been shown to branch off veins and in the kidney it has been found that the LYVE-1 positive lymphatic vessels are closely associated with the interlobular and arcuate veins. Interestingly there is also a subset of LYVE-1 positive cells that also express the macrophage marker F4/80 [62]. It is known that macrophages can contribute to lymphangiogenesis via the release of VEGF-C, so it may be postulated that their role in the kidney is to guide the ingrowing lymphatic vessels into the appropriate locations. It has recently been determined in a mouse model that there is a relationship between the pro-fibrotic marker TGF-β1 and the pro-lymphatic marker VEGF-C. Both in vitro and in vivo it was found that TGF-β1 stimulated VEGF-C and subsequently increased the amount of fibrosis. Furthermore, by blocking TGF-β signaling a reduction in the appearance of lymphatic vessels was observed [63].
Vascular related malformations
The formation of the renal vessels is a process that is highly variable and alterations in the anatomical position and number of arteries and veins that enter the kidney is seen in as many as 30 percent of all patients [64]. The precise pathological consequences of these malformations is unknown, however it is hypothesized that these alterations may be critical factors in mediating renal hypertension. Renal vascular shunting is a less uncommon malformation which occurs when there is shunting directly between the arteries and the veins that bypass the capillary beds [65]. These abnormalities display symptoms that range from hypertension to development of renal masses.
Targeting microvasculature for a therapy to renal disease
In a recent review it was described how targeting of the renal microvasculature may prove critical in tackling chronic kidney disease [66]. During chronic disease, it is suggested that the kidneys are in an anti-angiogenic condition and this leads to renal vascular loss. Angiogenesis has been shown to be stimulated using many of the angiogenic factors mentioned above. In rat [67] and pig [68] models VEGF-A administration has been shown to lead to a reduced endothelial dropout and a reduction in the amount of fibrosis. Furthermore, supplementation with Angpt-1 leads to reduced peritubular capillary dropout in a mouse model of unilateral ureteral obstruction [69]. It has also been suggested that adoptive transfer of endothelial progenitors could be utilized to stimulate vasculogenesis. Transplantation of endothelial progenitor cells at the early stages of ischemia reperfusion injury in mice has been shown to regenerate tubules as well as preserving peritubular capillaries via elevation of VEGF-A [70]. This technique has also been shown to play a functional role in chronic kidney disease. Injection of endothelial progenitor cells after microvascular disease was established in mice led to an increase in renal blood flow, enhanced capillary proliferation and attenuation of fibrosis [71].
Future directions
Recent advances in understanding the kidney vasculature have included identification and manipulation of key molecules characterized via transgenics, systemic and conditional knockouts and clinical trials. However, several critical questions arise in relation to the future directions of this field of research including: What is the precise origin and contribution of angiogenesis and vasculogenesis to kidney vasculature? – this is a hotly debated topic and the answers will dictate the treatment of many kidney vascular disorders; Would the early detection of vascular malformation lead to a better renal prognosis or predispose as a risk factor for future disease progression? - determining whether a patient is susceptible to future illnesses is difficult at this stage as it is likely that even subtle variations may significantly alter blood flow, however this is an area that requires significant study.
Conclusion
This article describes the shift in focus regarding renal endothelial cell biology, from the glomerulus as the primary site of vascular importance, to the entire renal vascular tree as an integrated system critical for maintaining electrolyte balance and proper renal function. An intricate relationship of the vasculature in patterning both the vessels and the parenchyma is clearly apparent. Furthermore, interrogation of the molecular signaling that maintains these endothelial cells and regulates their spatial-temporal function may be important in tackling congenital vascular-related renal defects as well as offering insight into the vascular component relevant to pathological conditions.
Acknowledgements
SSL is supported by an NIDDK Mentored Research Scientist Development Award (DK096996).
References
- 1.Abrahamson DR, Robert B, Hyink DP, St John PL, Daniel TO. Origins and formation of microvasculature in the developing kidney. Kidney Int. 1998;(Suppl 67):S7–S11. doi: 10.1046/j.1523-1755.1998.06702.x. [DOI] [PubMed] [Google Scholar]
- 2.Robert B, St John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol. 1998;275:F164–F172. doi: 10.1152/ajprenal.1998.275.1.F164. [DOI] [PubMed] [Google Scholar]
- 3.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancrin C, Sroczynska P, Serrano AG, Gandillet A, Ferreras C, Kouskoff V, Lacaud G. Blood cell generation from the hemangioblast. J Mol Med (Berl) 2010;88:167–172. doi: 10.1007/s00109-009-0554-0. [DOI] [PubMed] [Google Scholar]
- 5.Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kume T. Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol. 2010;25:637–646. doi: 10.14670/hh-25.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 8.Hyink DP, Tucker DC, St John PL, Leardkamolkarn V, Accavitti MA, Abrass CK, Abrahamson DR. Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol. 1996;270:F886–F899. doi: 10.1152/ajprenal.1996.270.5.F886. [DOI] [PubMed] [Google Scholar]
- 9.Ballermann BJ. Glomerular endothelial cell differentiation. Kidney Int. 2005;67:1668–1671. doi: 10.1111/j.1523-1755.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development. 2005;132:5437–5449. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- 11.Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, Gittes G, Bates CM. Endothelial Progenitors Exist within the Kidney and Lung Mesenchyme. PLoS One. 2013;8:e65993. doi: 10.1371/journal.pone.0065993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Valerius MT, Duah M, Staser K, Hansard JK, Guo JJ, McMahon J, Vaughan J, Faria D, Georgas K, Rumballe B, Ren Q, Krautzberger AM, Junker JP, Thiagarajan RD, Machanick P, Gray PA, van Oudenaarden A, Rowitch DH, Stiles CD, Ma Q, Grimmond SM, Bailey TL, Little MH, McMahon AP. Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development. 2012;139:1863–1873. doi: 10.1242/dev.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunskill EW, Potter SS. Gene expression programs of mouse endothelial cells in kidney development and disease. PLoS One. 2010;5:e12034. doi: 10.1371/journal.pone.0012034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 20.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 21.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 22.Loughna S, Hardman P, Landels E, Jussila L, Alitalo K, Woolf AS. A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis. 1997;1:84–101. doi: 10.1023/A:1018357116559. [DOI] [PubMed] [Google Scholar]
- 23.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 24.Simon M, Rockl W, Hornig C, Grone EF, Theis H, Weich HA, Fuchs E, Yayon A, Grone HJ. Receptors of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and [125I]VEGF binding sites. J Am Soc Nephrol. 1998;9:1032–1044. doi: 10.1681/ASN.V961032. [DOI] [PubMed] [Google Scholar]
- 25.Marlier A, Schmidt-Ott KM, Gallagher AR, Barasch J, Karihaloo A. Vegf as an epithelial cell morphogen modulates branching morphogenesis of embryonic kidney by directly acting on the ureteric bud. Mech Dev. 2009;126:91–98. doi: 10.1016/j.mod.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Robert B, St John PL, Hyink DP, Abrahamson DR. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol. 1996;271:F744–F753. doi: 10.1152/ajprenal.1996.271.3.F744. [DOI] [PubMed] [Google Scholar]
- 27.Karihaloo A, Karumanchi SA, Cantley WL, Venkatesha S, Cantley LG, Kale S. Vascular endothelial growth factor induces branching morphogenesis/tubulogenesis in renal epithelial cells in a neuropilin-dependent fashion. Mol Cell Biol. 2005;25:7441–7448. doi: 10.1128/MCB.25.17.7441-7448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tufro A, Teichman J, Woda C, Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev. 2008;125:558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, Thomas D, Tufro A. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufro A. VEGF spatially directs angiogenesis during metanephric development in vitro. Dev Biol. 2000;227:558–566. doi: 10.1006/dbio.2000.9845. [DOI] [PubMed] [Google Scholar]
- 31.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamoto Y, Tokunaga H, Tomita K. Vascular endothelial growth factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis. J Clin Invest. 1997;99:2351–2357. doi: 10.1172/JCI119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 34.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 35.Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, Nishikawa A, Satchell SC, Harper SJ, Gittenberger-de Groot AC, Bates DO. The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol. 2008;110:p57–p67. doi: 10.1159/000177614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeburg PB, Robert B, St John PL, Abrahamson DR. Podocyte expression of hypoxia-inducible factor (HIF)-1 and HIF-2 during glomerular development. J Am Soc Nephrol. 2003;14:927–938. doi: 10.1097/01.asn.0000059308.82322.4f. [DOI] [PubMed] [Google Scholar]
- 37.Sims-Lucas S, Caruana G, Dowling J, Kett MM, Bertram JF. Augmented and accelerated nephrogenesis in TGF-beta2 heterozygous mutant mice. Pediatr Res. 2008;63:607–612. doi: 10.1203/PDR.0b013e31816d9130. [DOI] [PubMed] [Google Scholar]
- 38.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 39.Steenhard BM, Freeburg PB, Isom K, Stroganova L, Borza DB, Hudson BG, St John PL, Zelenchuk A, Abrahamson DR. Kidney development and gene expression in the HIF2alpha knockout mouse. Dev Dyn. 2007;236:1115–1125. doi: 10.1002/dvdy.21106. [DOI] [PubMed] [Google Scholar]
- 40.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 41.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5' enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 43.Kappel A, Ronicke V, Damert A, Flamme I, Risau W, Breier G. Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood. 1999;93:4284–4292. [PubMed] [Google Scholar]
- 44.Loughna S, Yuan HT, Woolf AS. Effects of oxygen on vascular patterning in Tie1/LacZ metanephric kidneys in vitro. Biochem Biophys Res Commun. 1998;247:361–366. doi: 10.1006/bbrc.1998.8768. [DOI] [PubMed] [Google Scholar]
- 45.Tung JJ, Tattersall IW, Kitajewski J. Tips, stalks, tubes: notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb Perspect Med. 2012;2:a006601. doi: 10.1101/cshperspect.a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCright B. Notch signaling in kidney development. Curr Opin Nephrol Hypertens. 2003;12:5–10. doi: 10.1097/00041552-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 47.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 48.Kolatsi-Joannou M, Li XZ, Suda T, Yuan HT, Woolf AS. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev Dyn. 2001;222:120–126. doi: 10.1002/dvdy.1170. [DOI] [PubMed] [Google Scholar]
- 49.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tachibana K, Jones N, Dumont DJ, Puri MC, Bernstein A. Selective role of a distinct tyrosine residue on Tie2 in heart development and early hematopoiesis. Mol Cell Biol. 2005;25:4693–4702. doi: 10.1128/MCB.25.11.4693-4702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolf AS, Gnudi L, Long DA. Roles of angiopoietins in kidney development and disease. J Am Soc Nephrol. 2009;20:239–244. doi: 10.1681/ASN.2008020243. [DOI] [PubMed] [Google Scholar]
- 52.Pitera JE, Woolf AS, Gale NW, Yancopoulos GD, Yuan HT. Dysmorphogenesis of kidney cortical peritubular capillaries in angiopoietin-2-deficient mice. Am J Pathol. 2004;165:1895–1906. doi: 10.1016/S0002-9440(10)63242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez RA. Role of angiotensin in renal vascular development. Kidney Int. 1998;(Suppl 67):S12–S16. doi: 10.1046/j.1523-1755.1998.06703.x. [DOI] [PubMed] [Google Scholar]
- 54.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol. 1998;9:63–71. doi: 10.1681/ASN.V9163. [DOI] [PubMed] [Google Scholar]
- 55.Friberg P, Sundelin B, Bohman SO, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA. Renin-angiotensin system in neonatal rats: induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int. 1994;45:485–492. doi: 10.1038/ki.1994.63. [DOI] [PubMed] [Google Scholar]
- 56.Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol. 1995;269:F110–F115. doi: 10.1152/ajprenal.1995.269.1.F110. [DOI] [PubMed] [Google Scholar]
- 57.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 58.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 59.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugaya T, Nishimatsu S, Tanimoto K, Takimoto E, Yamagishi T, Imamura K, Goto S, Imaizumi K, Hisada Y, Otsuka A, Uchida H, Sugiura M, Fukuta K, Fukamizu A, Murakami K. Angiotensin II type 1a receptor-deficient mice with hypotension and hyperreninemia. J Biol Chem. 1995;270:18719–18722. doi: 10.1074/jbc.270.32.18719. [DOI] [PubMed] [Google Scholar]
- 61.Madsen K, Marcussen N, Pedersen M, Kjaersgaard G, Facemire C, Coffman TM, Jensen BL. Angiotensin II promotes development of the renal microcirculation through AT1 receptors. J Am Soc Nephrol. 2010;21:448–459. doi: 10.1681/ASN.2009010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee HW, Qin YX, Kim YM, Park EY, Hwang JS, Huo GH, Yang CW, Kim WY, Kim J. Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell Tissue Res. 2011;343:429–444. doi: 10.1007/s00441-010-1098-x. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-beta induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int. 2012;81:865–879. doi: 10.1038/ki.2011.464. [DOI] [PubMed] [Google Scholar]
- 64.Kumar S, Neyaz Z, Gupta A. The utility of 64 channel multidetector CT angiography for evaluating the renal vascular anatomy and possible variations: a pictorial essay. Korean J Radiol. 2010;11:346–354. doi: 10.3348/kjr.2010.11.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cura M, Elmerhi F, Suri R, Bugnone A, Dalsaso T. Vascular malformations and arteriovenous fistulas of the kidney. Acta Radiol. 2010;51:144–149. doi: 10.3109/02841850903463646. [DOI] [PubMed] [Google Scholar]
- 66.Long DA, Norman JT, Fine LG. Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- 67.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 68.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant. 2010;25:1079–1087. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim W, Moon SO, Lee SY, Jang KY, Cho CH, Koh GY, Choi KS, Yoon KH, Sung MJ, Kim DH, Lee S, Kang KP, Park SK. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006;17:2474–2483. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Park HC, Addabbo F, Ni J, Pelger E, Li H, Plotkin M, Goligorsky MS. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]