Abstract
Ketamine is a dissociative anaesthetic, analgesic drug as well as an N-methyl-d-aspartate receptor antagonist and has been reported to influence otoacoustic emission amplitudes. In the present study, we assess the effect of ketamine–xylazine on high-frequency distortion-product otoacoustic emissions (DPOAE) in the bat species Carollia perspicillata, which serves as model for sensitive high-frequency hearing. Cubic DPOAE provide information about the nonlinear gain of the cochlear amplifier, whereas quadratic DPOAE are used to assess the symmetry of cochlear amplification and potential efferent influence on the operating state of the cochlear amplifier. During anaesthesia, maximum cubic DPOAE levels can increase by up to 35 dB within a medium stimulus level range from 35 to 60 dB SPL. Close to the -10 dB SPL threshold, at stimulus levels below about 20-30 dB SPL, anaesthesia reduces cubic DPOAE amplitudes and raises cubic DPOAE thresholds. This makes DPOAE growth functions steeper. Additionally, ketamine increases the optimum stimulus frequency ratio which is indicative of a reduction of cochlear tuning sharpness. The effect of ketamine on cubic DPOAE thresholds becomes stronger at higher stimulus frequencies and is highly significant for f2 frequencies above 40 kHz. Quadratic DPOAE levels are increased by up to 25 dB by ketamine at medium stimulus levels. In contrast to cubic DPOAEs, quadratic DPOAE threshold changes are variable and there is no significant loss of sensitivity during anaesthesia. We discuss that ketamine effects could be caused by modulation of middle ear function or a release from ipsilateral efferent modulation that mainly affects the gain of cochlear amplification.
Keywords: DPOAE, otoacoustic emissions, bat, anaesthesia, cochlear amplifier, high-frequency hearing
INTRODUCTION
Distortion-product otoacoustic emissions (DPOAE) are a by-product of nonlinear ear mechanics and are used to obtain a noninvasive measure of sensitivity and tuning of the cochlear amplifier (e.g. Brown and Kemp 1984; Gaskill and Brown 1990; Probst et al. 1991; Brown et al. 1992; Janssen et al. 2000; Shera et al. 2002; Boege and Janssen 2002). They provide a fast and efficient assessment of mechanical threshold sensitivity in different vertebrates and even in insects (e.g. Brown 1987; Manley et al. 1993; Taschenberger and Manley 1998; Kössl et al. 1999; Mills and Shepherd 2001; van Dijk et al. 2002; Kössl et al. 2008). The cubic distortion tones (CDTs), most prominently the 2f1-f2 DPOAE, depend on the gain of the cochlear amplifier (e.g. Mills and Rubel 1996). Since their amplitudes usually are largest among DPOAEs, they are most frequently used to evaluate the state of cochlear amplification and of outer hair cells in humans and animal models. Quadratic distortion tones (QDTs) like the f2–f1 difference tone depend on the symmetry of cochlear nonlinearity and their amplitude is minimal when the operation point is at a symmetrical position along the transducer function (see, e.g. Frank and Kössl 1996; Lukashkin and Russell 1998, 1999; Drexl et al. 2012) There is evidence that quadratic distortions provide information about a frequency specific modulation of the operating point of the cochlear amplification (Abel et al. 2009; Wittekindt et al. 2009; Althen et al. 2012). In addition, dynamic compression of the transducer gain also can change the amplitude of the f2–f1 DPOAE (Bian 2004).
A difficulty when measuring DPOAE in animal models is the sensitivity of DPOAE to anaesthetics (e.g. Cederholm et al. 2012). Hence, the reliability of DPOAE data obtained at low stimulus levels is an issue to be considered (Valero and Ratnam 2011). Ketamine is a widely used anaesthetic and analgesic which also acts as an N-methyl-d-aspartate receptor antagonist (NMDA antagonist). In animal research, it is often used in combination with xylazine, an alpha-2-adrenergic agonist with sedative properties. When compared to isoflurane, ketamine–xylazine anaesthesia appears to have less effect on auditory sensitivity in terms of OAE or auditory brainstem responses (Ruebhausen et al. 2012; Harel et al. 1997). According to Harel et al. (1997) ketamine–xylazine anaesthesia can even increase the amplitudes of transiently evoked OAE (TEOAE) and DPOAE. This could be caused by blocking possible efferent suppression of inner ear mechanics due to ketamine–xylazine as discussed by Harel et al. (1997). The DPOAE adaptation that is caused by efferent activity (e.g. Liberman et al. 1996) is also reduced by ketamine (Boyev et al. 2002). In addition, DPOAE also could be altered by changing middle ear mechanics as suggested by Hatzopoulos et al. (2002).
High-frequency hearing and corresponding DPOAE recordings are particularly sensitive to factors that interfere with outer hair cell structure or function and the cochlear amplifier: furosemide or aminoglycosids lead to auditory threshold and DPOAE deterioration that increases with sound frequency (e.g. Puel et al. 1987; Kössl and Vater 2000; Schmiedt et al. 2002; Mills and Schmiedt 2004) resembling sensory presbyacusis (Schmiedt et al. 2002). If ketamine anaesthesia would affect the cochlear amplifier directly, e.g. by changing metabolic conditions in the cochlea or indirectly via the efferents, effects on DPOAE should also be frequency specific and grow towards higher frequencies. To evaluate high-frequency effects of Ketamine, we chose a bat animal model (Carollia perspicillata) that is quite sensitive in the ultrasonic range (Sterbing et al. 1994; Koay et al. 2002) and, in addition, has prominent cubic DPOAE (Kössl 1992). It is important to note that C. perspicillata is not specialised on perception of specific echolocation frequencies like, e.g. horseshoe bats, and therefore can serve as a general model for good high-frequency hearing capabilities that can be studied using otoacoustic emission techniques.
MATERIAL AND METHODS
Animals and Anaesthesia
A total of 29 adult specimens of C. perspicillata of both sexes (14 females and 15 males, body weight 19–24 g), taken from our breeding colony at the Faculty of Biosciences, University of Frankfurt, Germany, were used in this study. The DPOAE data initially were obtained from 12 anaesthetised bats and from another group of 12 awake animals. Five additional bats were investigated both awake and while they were anaesthetised. In these individuals, the awake versus anaesthetised recording sessions were separated by at least 3 days. The anaesthetic was delivered by subcutaneous injection using a mixture of 0.005 mg/g body weight ketamine (Ketavet©, Pfitzer GmbH, Berlin, Germany) and 0.035 mg/g body weight xylazine-hydrochloride (Rompun© 2 % solution, Bayer HealthCare AG, Mohnheim, Germany). Effects of the anaesthesia (e.g. loss of the pedal withdrawal reflex) were apparent 1 min after the injection, and anaesthesia lasted for as long as 120 min.
Experimental System and Procedures
The experiments took place in a sound-attenuated chamber. The body temperature of the bats was kept constant at 37 °C. To ensure that bats were not able to move during the measurement, their heads were fixed with a custom-made dental acrylic mouth holder. To measure DPOAE, an acoustic coupler was placed in the outer ear canal in about 0.3–1.0 mm distance to the tympanum under visual control (Zeiss OPMI 1-FR binocular, Carl Zeiss AG, Jena, Germany). The coupler consisted of three acoustic channels that converged in the coupler’s tip. Two of the coupler channels were connected to reversely driven condenser microphones used as loudspeakers (1/2″, MTG MK202, Microtech Gefell GmbH, Gefell, Germany) and the third channel contained a sensitive microphone (1/4″, B&K 4939, Brüel & Kjær, Nærum, Denmark) for recording DPOAE. To generate the two pure tone stimuli and to record DPOAE, a soundcard (RME fireface UC, RME Audio AG, Haimhausen, Germany; sampling rate, 192 kHz) was used. Data acquisition and analysis software was written in MATLAB (MATLAB 2007b, MathWorks Inc., Ismaning, Germany). The sound system was calibrated in situ using white noise. Two tone stimuli (f1 and f2) were used with f2 frequencies between 15 and 90 kHz (5 kHz step size) within a f2 level range from −10 to 60 dB SPL. At L2 levels above 74.1–89.9 dB SPL (2f1–f2) and 70.4–89.2 dB SPL (f2–f1), setup distortions reached a level of −10 dB SPL within used f2 frequency range. The amplitude of DPOAE depends on the level and frequency parameter of the two stimuli. In the present study, a difference of 10 dB between the levels of the primary stimuli L1 and L2 (i.e. L1 = L2 + 10 dB) was used that has been proven to produce maximal 2f1–f2 DPOAE levels at medium stimulus levels in C. perspicillata (Kössl 1992) and other mammals (e.g. Brown and Kemp 1984; Probst et al. 1991; Mills 1997). For each f2 frequency, the optimum frequency ratio (f1/f2) for both the 2f1–f2 DPOAE and the f2-f1 DPOAE was determined to optimise DPOAE generation in the following measurements. After adjusting f1 to the optimum frequency ratio, DPOAE level growth functions were measured for each f2 frequency and for each of the two DPOAE types. The recorded waveforms (42.7 ms duration) were averaged hundred times and a fast Fourier transformation (FFT) was calculated. The average noise level of the recording system within the used frequency range was between −15.0 ± 4.4 dB SPL (f2: 15 kHz) and −20.0 ± 5.7 dB SPL (f2: 90 kHz). From the DPOAE growth functions, the −10 dB SPL threshold was determined for each tested frequency by calculating the level of f2 sufficient to evoke a distortion level of −10 dB SPL. For all average curves, the data were given with error bars of one standard deviation. For more detailed description of the experimental procedures, see Eckrich et al. (2008) and Althen et al. (2012).
Statistical analysis was done using JMP (Version 7.0; SAS Institute Inc., Cary, NC). Both, ratio and threshold curves were tested in two-way ANOVAs for influences of sound frequency (16 values) and the condition (awake vs. anesthetised) followed by a post hoc analysis (independent contrasts). We also tested for interactions between these two parameters. P values were adjusted for multiple comparisons according to the Bonferroni–Holm correction. Statistical analysis of QDT data was restricted to the frequency range 35–90 kHz as the data for low frequencies were incomplete (close to noise level). After this correction, all data were examined under a criterion of global p < 0.005.
RESULTS
Optimum Frequency Ratio
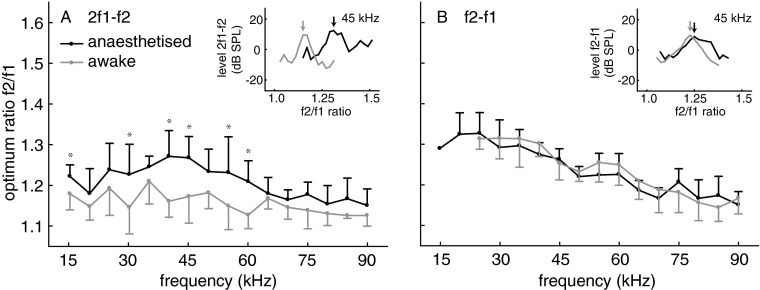
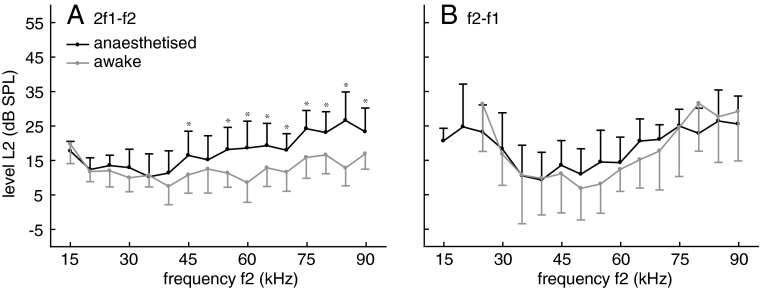
To maximise DPOAE amplitudes at low levels of the two stimuli, we determined the optimum f2/f1 frequency ratio for each tested f2 stimulus frequency in each individual bat and for each of the two investigated DPOAE (2f1–f2, f2–f1). For this purpose, f2 was kept constant and f1 varied. The L2 stimulus level which was used to determine the optimum ratio was 30 dB SPL and the level of the evoked DPOAE was between about −10 and 18 dB SPL. The optimum frequency ratio can provide information about the sharpness of cochlear frequency tuning in bats (Kössl 1994). The statistical analysis of ratio data revealed different influences of f2 frequency and wake state on the CDT and QDT optimum ratios. CDT ratios were significantly lower for the awake compared to the anesthetised condition (F1,512 = 209.7; p < 0.0001) and depended on f2 frequency (F15,512 = 12.0; p < 0.0001). The influence of anaesthesia was most pronounced at f2 frequencies in the range of 40 to 60 kHz (two-way interaction state × frequency: F15,512 = 3.1; p < 0.0001; see Fig. 1 for the results from post-hoc analysis). QDT ratios significantly depend on f2 frequency (F11,384 = 44.5; p < 0.0001) but a dependency on the awake versus anesthetised condition was not found (F1,384 = 1.5; p < n.s.). In C. perspicillata, there was a general trend towards smaller optimum ratios with increasing f2 for both types of DPOAE (Fig. 1). Such a frequency-dependent decrease of optimum ration is also found in certain other mammals (e.g. Eckrich et al. 2008). The decrease of the optimum ratio with f2 was more pronounced for QDTs than for CDTs, both in the anaesthetised and in the awake bats. The optimum CDT ratio in anaesthetised animals reached a maximum of close to 1.28 at f2 frequencies between 40 and 45 kHz.
FIG. 1.
Optimum frequency ratio f2/f1 for awake (grey) and anaesthetised (black) bats at different f2 frequencies (n = 17). Error bars: absolute value of SD, shown for graphical reasons only as upward (anesthetised) or downward bar (awake). Inlays: dependence of DPOAE level on f2/f1 ratio at 45 kHz for L1/L2: 40/30 dB SPL. Arrows: DPOAE amplitude at the optimum ratio. A: 2f1–f2 DPOAE: the f2/f1 ratio is smaller for awake than for anaesthetised bats over the entire frequency range. B: f2-f1 DPOAE: the optimum f2/f1 ratio is not significantly influenced by anaesthesia. Statistical analysis: two-way ANOVA with p values adjusted for multiple comparisons according to the Bonferroni–Holm correction.
DPOAE Growth Functions
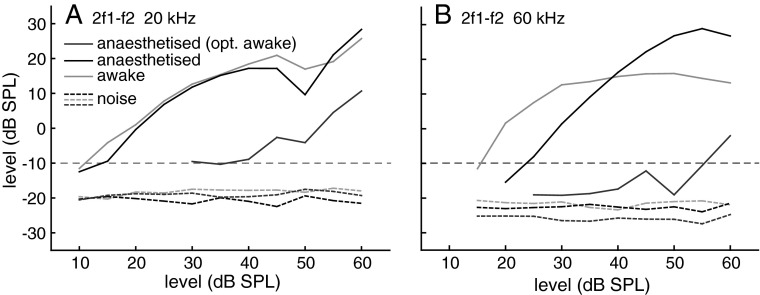
After the optimum ratio f2/f1 had been determined, the frequency of f1 was adjusted accordingly and DPOAE level growth functions were recorded for each f2 frequency. Both stimulus levels were increased in 5 dB steps. It is important to note that an adjustment of the frequency ratio to its optimum value for each growth function measurement results in slightly different f1 frequencies for the awake versus anaesthetised condition, in particular for the 2f1-f2 measurements where the optimum ratios are significantly different between both conditions. We use this procedure since it has proven of large benefit when determining threshold sensitivity in different bat species (e.g. Kössl 1992, Kössl et al. 1999; Kössl and Vater 2000; Wittekindt et al. 2005; Macias et al. 2006) and also in other mammalian species (e.g. Drexl et al. 2003; Eckrich et al. 2008). In Figure 2, two example growth functions are shown that illustrate the advantage of this procedure. Growth functions for the anaesthetised state that did not take into account a changed optimum ratio, but were obtained with the ‘awake’ optimum ratio, are quite insensitive due to the use of an inadequate f1 frequency and give the incorrect impression of largely changed thresholds.
FIG. 2.
Example data from an individual bat for 2f1–f2 DPOAE growth functions at 20 and 60 kHz. Growth functions measured with the optimal ratio for the awake condition (grey) and during anaesthesia (black) are compared to a growth function obtained during anaesthesia measured with the optimal ratio for awake condition (dark grey). Dotted lines: mean noise levels + 1 standard deviation (SD) for both conditions. Horizontal line: −10 dB SPL DPOAE threshold criterion.
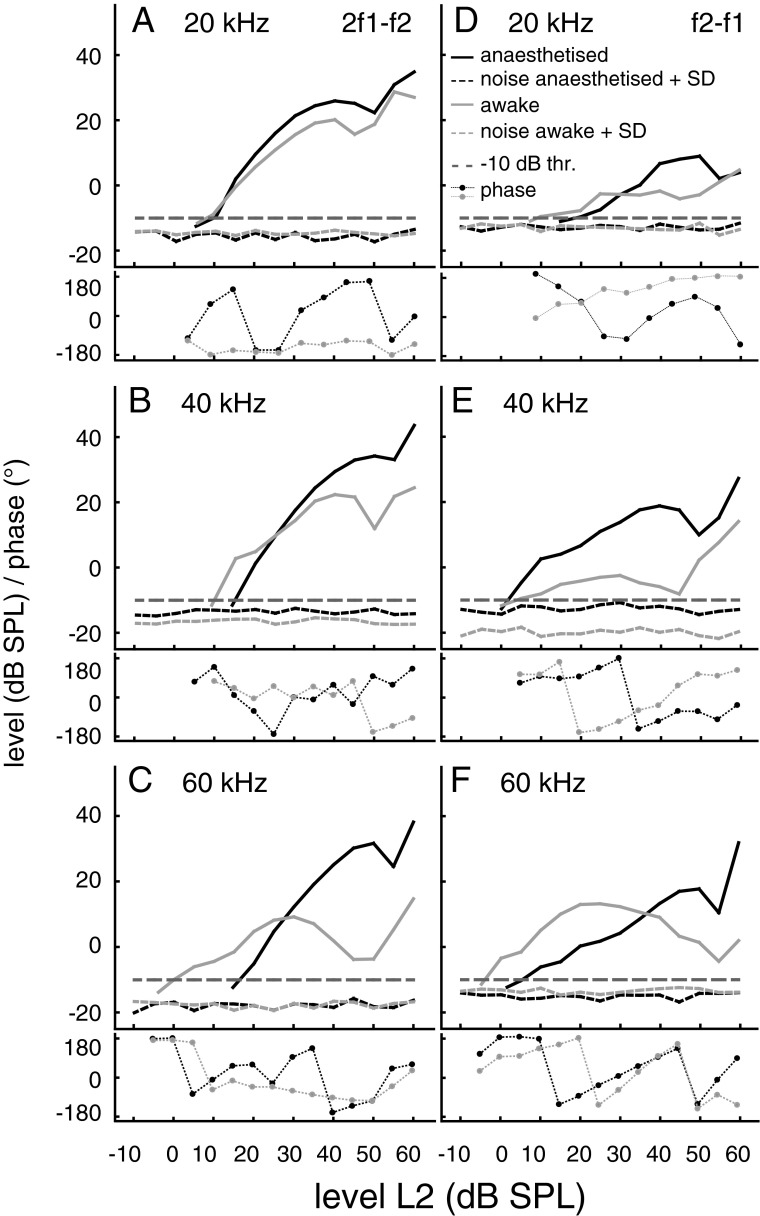
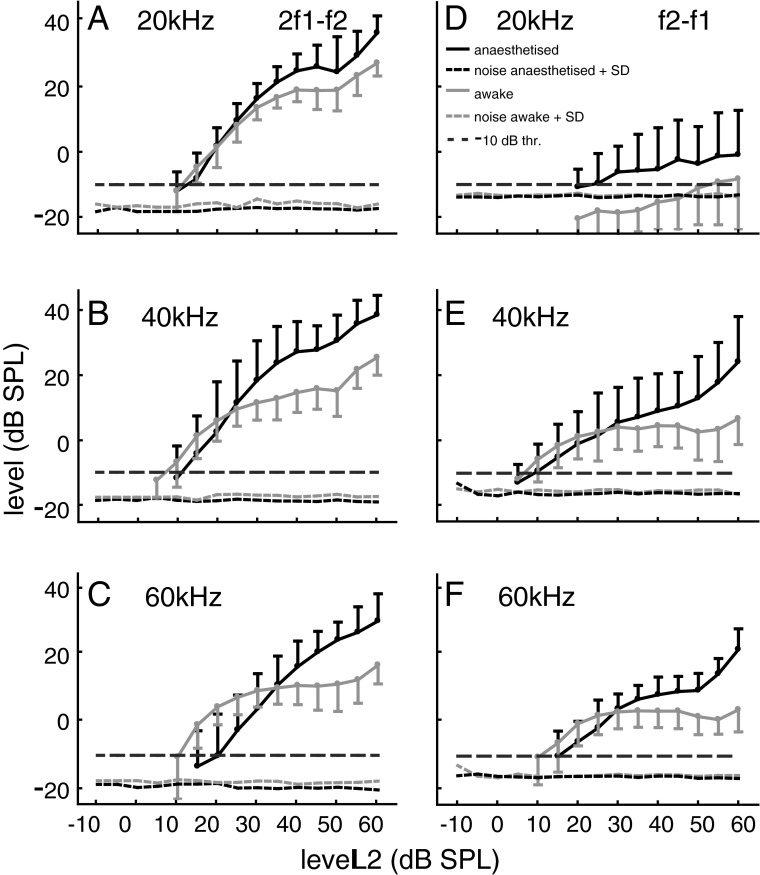
Examples for such optimised growth functions for both emission types obtained from an individual bat that was recorded while awake and during anaesthesia are shown in Figure 3. Averaged data (n = 17) for growth functions of both emission types are given in Figure 4. At f2 frequencies of 40 and 60 kHz, the threshold level of the 2f1–f2 emission in awake bats was more sensitive than in anaesthetised bats. The sensitivity at 20 kHz was nearly the same for both conditions. However, the maximum 2f1–f2 levels within a f2 level range of 35–60 dB SPL were much higher in the anaesthetised condition. In the given examples, the maximum 2f1–f2 levels at the three different f2 frequencies (Fig. 3A–C) were between 11.6 and 35.3 dB higher during anaesthesia compared with the awake state. Averaged across all 17 data sets, the differences between the awake and the anaesthetised state were between 9.1 to 15.8 dB for the maximum 2f1–f2 levels at f2 frequencies of 20, 40 and 60 kHz. The increase of 2f1–f2 amplitude at a medium stimulation level produced a pronounced change of the initial slope of the growth function, which was much steeper in the anaesthetised animal. The initial slopes were determined within a 10-dB window starting at the first data point above the −10 dB threshold. In the example data set, for f2 = 20, 40 and 60 kHz, the initial slopes for the 2f1–f2 emission were between 0.8 and 1.5 dB/dB (awake) and between 1.6 and 1.9 dB/dB (anaesthesia). The slopes of the average growth functions obtained at f2 frequencies of 20, 40 and 60 kHz were between 0.8 and 1.5 dB/dB in the awake versus 1.3 and 1.7 dB/dB in the anaesthetised condition. For the f2-f1 DPOAE, the differences between awake and anaesthetised condition were more variable and threshold deterioration during anaesthesia was less obvious. However, a large increase of the f2-f1 DPOAE level at medium stimulus levels is obvious at all f2 frequencies both for the example data (Fig. 3D–F: increase up to 25.7 dB) and the averaged data set (f2 = 20, 40 and 60 kHz: increase of 3.5 to 21.9 dB, n = 17). In addition, the initial slopes of the average f2–f1 growth functions (f2 = 40 and 60 kHz) slightly increased during anaesthesia and were between 0.6 and 0.9 dB/dB (awake) and between 0.8 and 1.0 dB/dB (anaesthetised). The phase of 2f1–f2 and f2–f1 DPOAE (lower graphs in Fig. 3) changed in a level-dependent manner, often the phase angle increased with increasing stimulus level (e.g. Fig. 3C, E, F). However, phase data were variable and there was no conspicuous or consistent difference between the awake and anaesthetised state.
FIG. 3.
DPOAE growth functions of 2f1–f2 and f2–f1 DPOAE at 20, 40 and 60 kHz f2 frequency (grey awake, black anaesthetised). All example data are from the same individual bat. The used ratio for both conditions (awake/anaesthetised) was in A 1.15/1.23, B 1.21/1.25, C 1.13/1.25, D 1.29/1.29, E 1.33/1.29 and F 1.25/1.21. Dotted lines: mean noise levels + 1SD for both conditions. Horizontal line: −10 dB SPL DPOAE threshold criterion. Lower graphs: corresponding phase data (grey awake, black anaesthetised).
FIG. 4.
Average DPOAE growth functions (n = 17 anaesthetised and 17 awake animals) of 2f1–f2 and f2–f1 DPOAE at 20, 40 and 60 kHz f2 frequency (Error bars give absolute value of SD, shown for graphical reasons only as upward (anesthetised) or downward bar (awake)). See legend Figure 3 for details.
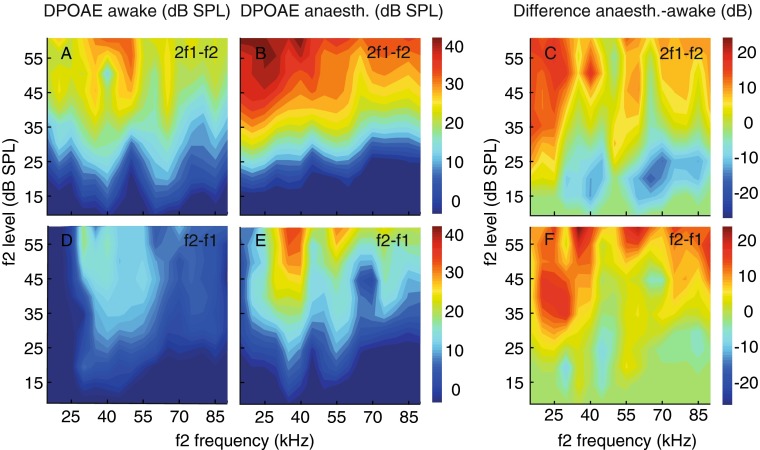
To illustrate the profound changes of the levels of both types of DPOAE, a more detailed analysis, covering all used f2 stimulus frequencies, is given for a single individual that was recorded both in the awake state and during anaesthesia (Fig. 5). In this individual bat, largest anaesthesia-related DPOAE level increase was apparent at low f2 frequencies between 15 and 40 kHz. Anaesthesia appeared to smooth the 2f1–f2 DPOAE iso-level contours (compare Fig. 5A, B) but increased contour sharpness for the f2–f1 DPOAE (compare Fig. 5D, E). During anaesthesia, there was a clear reduction of 2f1–f2 levels for low stimulus levels between 15 and 30 dB SPL and for stimulus frequencies above 30 kHz.
FIG. 5.
Dependence of DPOAE level on f2 frequency and level sampled at a resolution of 5 kHz in an individual bat. 2f1–f2 and f2–f1 DPOAE levels increased during anaesthesia (compare A, D with B, E), largest differences at low f2 frequencies (C, F). At low stimulus levels, high-frequency CDTs decreased in amplitude. Colour-coded bars: DPOAE level (A, B, D, E) or the level difference between the anaesthetised and awake condition (C, F).
DPOAE Thresholds
The growth functions were used to calculate DPOAE threshold curves for a threshold criterion of −10 dB SPL. For this purpose, the f2 stimulus level which was sufficient to produce a DPOAE of −10 dB SPL was interpolated from the growth function. In some cases, in particular for low f2 frequencies when noise level + 1 SD > −10 dB SPL, the threshold value was extrapolated from the first two data points of the growth function that were above the noise level.
The distortion threshold curve is plotted as a function of f2, since the cochlear location of primary distortion generation is thought to be close to the f2 frequency location (e.g. Brown and Kemp 1984). The data of the threshold curve are averaged from 17 individual threshold data sets of each condition (Fig. 6). The statistical analysis of threshold data revealed different influences of frequency and wake state for CDTs and QDTs, comparable to the frequency ratio data (see above). Likewise the comparison of the awake and the anesthetised condition revealed a significant difference (F1,512 = 107.3; p < 0.0001) which depended on f2 frequency (F15,512 = 14.0; p < 0.0001). The influence of anaesthesia was especially pronounced at frequencies above of 55 kHz (two-way interaction state × frequency: F15,512 = 4.4; p < 0.0001; see Fig. 6 for the results from post hoc analysis). For the QDT thresholds, there was no significant difference between the awake and anesthetised condition (F1,384 = 0.8; p < n.s.).
FIG. 6.
DPOAE threshold for 2f1–f2 (A) and f2–f1 (B). Awake (grey), anaesthetised (black). Error bars: absolute value of SD, shown for graphical reasons only as upward (anesthetised) or downward bar (awake). A: In awake bats, 2f1–f2 DPOAE threshold was between 8.7 and 17.5 dB SPL for f2 between 20–90 kHz. Under anaesthesia, the threshold was more insensitive for frequencies above 40 kHz. B For the f2-f1 DPOAE, minimum thresholds were about 6 dB SPL at 50–55 kHz. During anaesthesia, the threshold minimum shifted to 40 kHz. Statistical analysis: two-way ANOVA with p values adjusted for multiple comparisons according to the Bonferroni–Holm correction.
The 2f1–f2 threshold curve obtained in awake animals is rather flat for frequencies between 20 and 90 kHz and thresholds are between 8.6 and 20.2 dB SPL (Fig. 6a). During anaesthesia, significant threshold increases were found for all f2 frequencies ≥55 kHz and there was a progressive threshold rise with increasing f2 frequency. Maximum threshold increase of 14.4 dB was observed at 85 kHz. Quite remarkably are the minimal average and individual threshold values for the f2-f1 DPOAE between 35 and 55 kHz in C. perspicillata (Fig. 6b) which were slightly more sensitive than even the lowest 2f1–f2 thresholds. In 12 of the awake bats, the −10 dB threshold was lower than 5 dB SPL at one or more frequencies. The lowest f2-f1 threshold of −4.1 dB SPL could be observed at 35 kHz and was about 7 dB more sensitive than the lowest 2f1–f2 threshold at the same frequency. The fact that there are substantial f2–f1 distortions at very low SPLs can indicate pronounced asymmetry of cochlear amplification at hearing threshold. The f2-f1 threshold curve of awake bats is characterised by a broad threshold minimum of about 6 dB SPL between 50 and 55 kHz that shifts to 40 kHz during anaesthesia.
DISCUSSION
General Properties of CDTs and QDTs in C. perspicillata
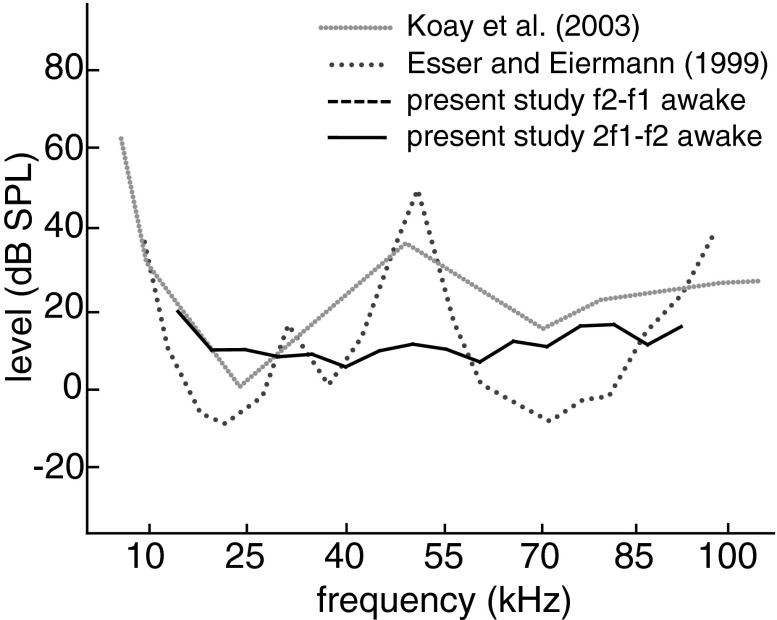
Large 2f1-f2 DPOAE amplitudes and low thresholds over the whole tested frequency range up to 90 kHz demonstrate that cochlear mechanics in this bat species is adapted for sensitive high-frequency hearing. C. perspicillata can be viewed as a broadband hearing generalist whose hearing range is shifted towards ultrasonic frequencies to allow high-resolution echolocation. For most frequencies, the 2f1–f2 threshold sensitivity is in the range of behavioural threshold data of this species (Koay et al. 2002) but less sensitive than neuronal thresholds (Sterbing et al. 1994; Esser and Eiermann 1999; see Fig. 7).
FIG. 7.
Hearing threshold data from C. perspicillata. Behavioural threshold (Koay et al. 2002) and auditory cortex neurophysiological threshold (Esser and Eiermann 1999) were both obtained under free field conditions. DPOAE threshold (green lines: 2f1–f2 (solid), f2-f1 (dashed)) were measured with the in-ear acoustic coupler which prevented modification of threshold sensitivity by the outer ear. The free field curves are characterised by an insensitivity between 40 and 55 kHz which is absent in the DPOAE data.
The f2-f1 DPOAE is quite prominent in C. perspicillata and can be evoked by surprisingly low stimulus levels in the mid frequency range. In this respect, f2–f1 DPOAE are more sensitive than 2f1–f2 emissions. This is exceptional since in other mammals studied, the generation of the f2-f1 emission usually requires higher stimulus levels (e.g. Kujawa et al. 1995; Frank and Kössl 1996; Lukashkin et al. 2002; Wittekindt et al. 2009). The fact that in the present study the stimulus frequency ratio was optimised for the f2-f1 distortion before the respective measurements may have contributed to this sensitivity. If prevalence of f2-f1 is taken as indicator of an asymmetric position of the operating point of cochlear transduction (Frank and Kössl 1996; Bian 2004; Lukashkin and Russell 2005; Abel et al. 2009; Drexl et al. 2012), forward or reverse transduction should be largely asymmetric at the threshold level for a wide range of high frequencies in C. perspicillata. The f2-f1 threshold curve is most sensitive in a frequency range of about 40–55 kHz. In this respect, the f2-f1 as well as the 2f1–f2 threshold curves do not resemble the behavioural or neurophysiological thresholds that have local threshold maxima in this frequency range (Fig. 7). This discrepancy is most likely due to the exclusion of outer ear filtering during DPOAE measurement. This is in accordance with Koay et al. (2002) who could influence the 50-kHz behavioural threshold maximum by changing the direction of sound incidence. Comparable data on outer ear related threshold discrepancies are also found in other bat species (Wittekindt et al. 2005; Macias et al. 2006).
The Effect of Anaesthesia on High-Frequency DPOAE
Ketamine–xylazine anaesthesia reduces cubic DPOAE threshold sensitivity preferentially at high frequencies and broadens mechanical cochlear tuning assessed by optimum stimulus frequency ratios. A quite unexpected result of the present study was the pronounced anaesthesia related amplitude increase of both quadratic and cubic DPOAE at medium to high stimulus levels. To assess these effects, we discuss possible ketamine–xylazine effects on cochlear physiology and the cochlear amplifier, on middle ear mechanics and on efferent neuronal feedback.
General Impact on Cochlear Physiology
Ketamine leads to moderate cardiac stimulation and increase of blood pressure combined with respiratory depression while xylazine has stronger depressive effects on respiration and reduces heart rate (Sanford and Colby 1980). This could affect cochlear sound transduction, if reduction of metabolic energy supply would change the endocochlear potential. Endocochlear potential decrease is expected to induce a strong decrease of cubic DPOAE at levels close to hearing threshold (Mills et al. 1993; Rübsamen et al. 1995). Endocochlear potential related changes of quadratic DPOAE are not directly related to the change in cubic DPOAE level (Mills et al. 1993). Endocochlear potential change affects cochlear thresholds more strongly at higher sound frequencies than at lower frequencies, which presumably is related to blockage of the cochlear amplifier whose gain may increase with increasing sound frequency towards the cochlear base (Schmiedt et al. 2002). The fact that in our study cubic DPOAE thresholds were elevated during anaesthesia in a frequency-specific manner, while the quadratic DPOAE threshold is not changed significantly, could be a consequence of a metabolic influence on the cochlear amplifier. The optimum frequency ratio during measurements of cubic DPOAE is indicative of mechanical cochlear tuning sharpness (e.g. Kössl 1994). The significant increase of optimum frequency ratios during anaesthesia could be caused by endocochlear potential related deterioration of mechanical cochlear frequency tuning (Ruggero and Rich 1991) as a consequence of reduction of cochlear amplifier action.
The fact that the slopes of cubic DPOAE growth functions increased strongly during anaesthesia is comparable to a steepening of DPOAE growth functions in patients with cochlear hearing loss or tinnitus (Janssen et al. 1998, 2000, 2006). Indeed, the slopes can be used as indicator of changes in cochlear function (Neely et al. 2003; Müller and Janssen 2004). Since the quadratic DPOAE function slopes also changed during anaesthesia, a compromising level-dependent effect on the operating point of the mechanisms that generate and/or transmit DPOAE is likely.
If anaesthesia would reduce the gain of the cochlear amplifier, a reduction of level-dependent phase change or a shift of amplitude notches and phase jumps along the level growth function of 2f1–f2 DPOAE could be produced as it can be observed during furosemide application (e.g. Lukashkin et al. 2002; Mills and Rubel 1994). However, in our case, phase data were quite variable and did not allow a consistent interpretation. This could be caused by optimization of the level growth functions using individual optimal ratios. It also could be a consequence of a combination of different anaesthesia effects on cochlear amplifier and efferent activity (see below).
Middle Ear as Possible Target of Ketamine Action
The most striking effect of anaesthesia in the present study is a large increase of DPOAE level of up to 35 dB for 2f1–f2 and 25 dB for f2-f1. Ketamine related closure of the Eustachian tubes and hence a change in middle ear pressure may contribute to this effect. Hatzopulos et al. (2002) demonstrated in the rat that ketamine–xylazine causes a reduction of 2f1–f2 level of up to about 10 dB within a time window of up to an hour after injection of the anaesthetics. This level reduction could be prevented by making a small hole in the tympanum or by adding atropine to the injection narcotics. Atropine is known to reduce mucus production and closure of Eustachian tubes. The data strongly indicate that middle ear pressure was changed during ketamine anaesthesia in the rat. In the present study, we found the opposite effect in bats, i.e. a large increase in DPOAE amplitude during anaesthesia. Middle ear pressure variations may also work to increase DPOAE amplitudes at the ear drum. The size of the shown effect surprises since this would suggest that middle ear pressure adjustment at medium to high levels in the awake bat prevents optimal sound propagation through the middle ear. Aside from ketamine-induced pressure change in the middle ear, ketamine could also block the middle ear muscle reflex. A study by Smith et al. (2008) investigating rats showed that most of the effects of contralateral acoustic stimulation on DPOAE were abolished when the middle ear muscles were cut. The middle ear muscle-related DPOAE changes were already seen at low ipsilateral DPOAE stimulation levels of about 50 dB SPL. But in C. perspicillata, there are no data available on the threshold of the middle ear muscle reflex. In Pteronotus parnellii, an insectivorous bat that uses narrow band echolocation signals, the most sensitive middle ear muscle reflex threshold is at about 63 dB SPL (Suga et al. 1974). This threshold value would not explain an amplitude reduction based on a middle ear muscle contraction in awake bats at stimulus levels between about 20 and 60 dB SPL as shown in the present study. In addition, a contribution of the middle ear to the pronounced change of slope of DPOAE growth as observed in the present study under anaesthesia seems unlikely. In an attempt to distinguish middle ear dysfunction from cochlear hearing loss on the basis of DPOAE growth functions in humans, Gehr et al. (2004) showed that middle ear changes did not influence the slope of DPOAE growth, whereas cochlear hearing loss was related to changes to the slope.
Possible Release from Efferent Cochlear Modulation
In addition to changes in middle ear function, a suppression of efferent activity could also play a role. Ketamine acts as a noncompetitive NMDA antagonist (Harrison and Simmonds 1985) which raises the possibility that it could block efferent neurons or their input systems. Efferent deactivation can lead to a deterioration of behavioural measures of frequency discrimination (Hienz et al. 1998). A change of mechanical tuning sharpness and optimum DPOAE frequency ratios due to blocking of medial olivocochlear ipsilateral efferent fibres to the outer hair cells seems to be possible but is hard to prove in the present experimental setup, without invasive manipulation of efferent fibres. In addition, electrical stimulation and hence activation of the medial olivocochlear system lead to a reduction of the gain of cochlear amplification and of mechanical tuning sharpness in the cochlea (Murugasu and Russell 1996; Dolan et al. 1997; Guinan and Cooper 2003) which contradicts an increase of optimum frequency ratios due to efferent deactivation by ketamine. It also has to be pointed out that NMDA antagonists could directly influence the cochlea since NMDA receptors are present in the cochlea and are involved in generating tinnitus and excitotoxicity (Puel et al. 1994; Guitton et al. 2003). Harel et al. (1997) showed that ketamine can increase DPOAE levels slightly in the chinchilla and suggested that efferents could be involved in this effect since it was independent of middle ear muscle deactivation. Accordingly, a release from efferent suppression of cochlear amplification or from a reduction of MOC-mediated DPOAE adaptation (see Boyev et al. 2002) could contribute to the massive increase of the level of both types of DPOAE evoked at medium stimulus levels in the bat during anaesthesia. In addition, ketamine produces a differential effect on 2f1–f2 versus f2-f1 DPOAE, i.e. f2-f1 is less affected at its threshold, which could imply that a potential ketamine-generated efferent release influences more the gain than the operating point of cochlear amplification. This would be in contrast to studies in humans and gerbils where at low stimulus levels, the f2-f1 DPOAE is more sensitive to contralateral acoustic stimulation than the 2f1–f2 DPOAE (Wittekindt et al. 2009; Abel et al. 2009; Althen et al. 2012). In our data, the f2–f1 threshold did not significantly change during anaesthesia. This would argue against a possible spontaneous or tonic efferent influence that could adjust the operating point of cochlear amplification at low levels. Hence, the apparent cochlear desensitization during anaesthesia, reflected by increasing 2f1–f2 thresholds, most likely is not due to efferent action but could be caused by a general impact of the anaesthetic on cochlear physiology (see above).
If the middle ear muscle reflex does not play a major role, then ketamine blockage of efferent cochlear modulation remains the most likely candidate to explain the increase of DPOAE levels for medium to high stimulation levels in the present study. In this case, efferent action would induce a compression of cochlear mechanical responses at higher sound levels. The prominent effects found in this study indicate that C. perspicillata is an excellent model to study efferent modulations in hearing processes. While our present data cannot unambiguously resolve if the observed effects are due to middle ear or efferent mechanisms or both, a strong attenuation at medium to high sound levels during the normal awake state could be of behavioural value for the bat as it should primarily affect the emitted sonar signals and less the faint returning echoes and thus enable the bat to focus on low-level echo signals.
Acknowledgments
We would like to thank PD Dr. Bernhard Gaese for the help with the statistics. This work was supported by the DFG.
Contributor Information
D. Schlenther, Phone: 0049 69/798-42065, Email: d.schlenther@stud.uni-frankfurt.de
C. Voss, Phone: 0049 69/798-42062, Email: voss@bio.uni-frankfurt.de
M. Kössl, Phone: 0049 69/798-42052, Email: koessl@bio.uni-frankfurt.de
References
- Abel C, Wittekind A, Kössl M. Contralateral acoustic stimulation modulates low-frequency biasing of DPOAE: efferent influence on cochlear amplifier operating state? J Neurophysiol. 2009;101:2362–2371. doi: 10.1152/jn.00026.2009. [DOI] [PubMed] [Google Scholar]
- Althen H, Wittekindt A, Gaese B, Kössl M, Abel C. Effect of contralateral pure tone stimulation on distortion emissions suggests a frequency-specific functioning of the efferent cochlear control. J Neurophysiol. 2012;107(7):1962–1969. doi: 10.1152/jn.00418.2011. [DOI] [PubMed] [Google Scholar]
- Bian L. Cochlear compression: effects of low-frequency biasing on quadratic distortion product otoacoustic emission. J Acoust Soc Am. 2004;116(6):3559–3571. doi: 10.1121/1.1819501. [DOI] [PubMed] [Google Scholar]
- Boege P, Janssen T. Pure-tone threshold estimation from extrapolated distortion product otoacoustic emission I/O-functions in normal and cochlear hearing loss ears. J Acoust Soc Am. 2002;111(4):1810–1818. doi: 10.1121/1.1460923. [DOI] [PubMed] [Google Scholar]
- Boyev KP, Lieberman MC, Brown MC. Effects of anaesthesia an efferent-mediated adaptation of the DPOAE. JARO. 2002;3:362–373. doi: 10.1007/s101620020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM. Acoustic distortion from rodent ears: a comparison of responses from rats, guinea pigs and gerbils. Hear Res. 1987;31:25–38. doi: 10.1016/0378-5955(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Brown AM, Kemp DT. Suppressibility of the 2f1–f2 stimulated acoustic emissions in gerbil and man. Hear Res. 1984;13(1):29–37. doi: 10.1016/0378-5955(84)90092-3. [DOI] [PubMed] [Google Scholar]
- Brown AM, Gaskill SA, Williams DM. Mechanical filtering of sound in the inner ear. Proc R Soc Lond B. 1992;250:29–34. doi: 10.1098/rspb.1992.0126. [DOI] [PubMed] [Google Scholar]
- Cederholm JME, Froud KE, Wong ACY, Ko M, Ryan AF, Housley GD. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hear Res. 2012;292:71–79. doi: 10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan DF, Guo MH, Nuttall AL. Frequency dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation. J Acoust Soc Am. 1997;102:3587–3596. doi: 10.1121/1.421008. [DOI] [PubMed] [Google Scholar]
- Drexl M, Faulstich M, von Stebut B, Radtke-Schuller S, Kössl M. Distortion-product otoacoustic emissions and auditory evoked potentials in the hedgehog tenrec Echinops telfairi. JARO. 2003;4:555–564. doi: 10.1007/s10162-002-3043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexl M, Gürkov R, Krause E. Low-frequency modulated quadratic and cubic distortion product otoacoustic emissions in humans. Hear Res. 2012;287:91–101. doi: 10.1016/j.heares.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Eckrich T, Foeller E, Stuermer IW, Gaese BH, Kössl M. Strain-dependence of age-related cochlear hearing loss in wild and domesticated Mongolian gerbils. Hear Res. 2008;235:72–79. doi: 10.1016/j.heares.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Esser KH, Eiermann A. Tonotopic organization and parcellation of auditory cortex in the FM-bat Carollia perspicillata. Eur J Neurosci. 1999;11:3669–3682. doi: 10.1046/j.1460-9568.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- Frank G, Kössl M. The acoustic two-tone distortions 2f1–f2 and f2-f1 and their possible relation to changes in the operating point of the cochlear amplifier. Hear Res. 1996;98:104–115. doi: 10.1016/0378-5955(96)00083-4. [DOI] [PubMed] [Google Scholar]
- Gaskill SA, Brown AM. The behavior of the acoustic distortion product, 2f1–f2, from the human ear and its relation to auditory sensitivity. J Acoust Soc Am. 1990;88:821–839. doi: 10.1121/1.399732. [DOI] [PubMed] [Google Scholar]
- Gehr DD, Janssen T, Michaelis CE, Deingruber K, Lamm K. Middle ear and cochlear disorders result in different DPOAE growth behaviour: implications for the differentiation of sound conductive and cochlear hearing loss. Hear Res. 2004;193:9–19. doi: 10.1016/j.heares.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Cooper NP. Fast effects of efferent stimulation on basilar membrane motion. In: Gummer AW, editor. The biophysics of the cochlea: molecules to models. Singapore: World Scientific; 2003. pp. 245–251. [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23(9):3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel N, Kakigi A, Hirakawa H, Mount RJ, Harrison RV. The effects of anesthesia on otoacoustic emissions. Hear Res. 1997;110:25–33. doi: 10.1016/S0378-5955(97)00061-0. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl d-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985;84(2):381–91. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzopoulos S, Petruccelli J, Laurell G, Finesso M, Martini A. Evaluation of anesthesia effects in a rat animal model using otoacoustic emission protocols. Hear Res. 2002;170:12–21. doi: 10.1016/S0378-5955(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Hienz RD, Stiles P, May BJ. Effects of bilateral olivocochlear lesions on vowel formant discrimination in cats. Hear Res. 1998;116:10–20. doi: 10.1016/S0378-5955(97)00197-4. [DOI] [PubMed] [Google Scholar]
- Janssen T, Kummer P, Arnold W. Growth behavior of the 2 f12f2 distortion product otoacoustic emission in tinnitus. J Acoust Soc Am. 1998;103(6):3418–3430. doi: 10.1121/1.423053. [DOI] [PubMed] [Google Scholar]
- Janssen T, Boege P, Oestreicher E, Arnold W. Tinnitus and 2f1–f2 distortion product otoacoustic emissions following salicylate overdose. J Acoust Soc Am. 2000;107(3):1790–1792. doi: 10.1121/1.428578. [DOI] [PubMed] [Google Scholar]
- Janssen T, Niedermeyer HP, Arnold W. Diagnostics of the cochlear amplifier by means of distortion product otoacoustic emissions. ORL. 2006;68:334–339. doi: 10.1159/000095275. [DOI] [PubMed] [Google Scholar]
- Koay G, Bitter KS, Heffner HE, Heffner RS. Hearing in American leaf-nosed bats. I: Phyllostomus hastatus. Hear Res. 2002;171:96–102. doi: 10.1016/S0378-5955(02)00458-6. [DOI] [PubMed] [Google Scholar]
- Kössl M. High frequency distortion products from the ears of two bat species, Megaderma lyra and Carollia perspicillata. Hear Res. 1992;60(2):156–164. doi: 10.1016/0378-5955(92)90018-I. [DOI] [PubMed] [Google Scholar]
- Kössl M. Otoacoustic emissions from the cochlea of the ‘constant frequency’ bats, Pteronotus parnellii and Rhinolophus rouxi. Hear Res. 1994;72:59–72. doi: 10.1016/0378-5955(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Kössl M, Vater M. Consequences of outer hair cell damage for otoacoustic emissions and audio-vocal feedback in the mustached bat. JARO. 2000;1:300–314. doi: 10.1007/s101620010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kössl M, Mayer F, Frank G, Faulstich M, Russell IJ. Evolutionary adaptations of cochlear function in Jamaican mormoopid bats. J Comp Physiol A. 1999;185:217–228. doi: 10.1007/s003590050381. [DOI] [PubMed] [Google Scholar]
- Kössl M, Möckel D, Weber M, Seyfarth EA. Otoacoustic emissions from insect ears: evidence of active hearing? J Comp Physiol A. 2008;194:597–609. doi: 10.1007/s00359-008-0344-0. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Fallon M, Bobbin RP. Time-varying alterations in the f2-f1 DPOAE response to continuous primary stimulation I: Response characterization and contribution of the olivocochlear efferents. Hear Res. 1995;85:142–154. doi: 10.1016/0378-5955(95)00041-2. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Puria S, Guinan JJ. The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1–f2 distortion product otoacoustic emission. J Acoust Soc Am. 1996;99:3572–3584. doi: 10.1121/1.414956. [DOI] [PubMed] [Google Scholar]
- Lukashkin AN, Russell IJ. Dependence of the DPOAE amplitude pattern on acoustical biasing of the cochlear partition. Hear Res. 1998;203:45–53. doi: 10.1016/j.heares.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Lukashkin AN, Russell IJ. Analysis of the f2–f1 and 2 f1–f2 distortion components generated by the hair cell mechanoelectrical transducer: dependence on the amplitudes of the primaries and feedback gain. J. Acoust. Soc. Am. 1999;106:2661. doi: 10.1121/1.428096. [DOI] [Google Scholar]
- Lukashkin AN, Lukashkina VA, Russell IJ. One source for distortion product otoacoustic emissions generated by low- and high-level primaries. J Acoust Soc Am. 2002;111(6):2740–2748. doi: 10.1121/1.1479151. [DOI] [PubMed] [Google Scholar]
- Macias S, Mora EC, Garcia A. Acoustic identification of Mormoopid Bats: a survey during the evening exodus. J Mammal. 2006;87:324–330. doi: 10.1644/05-MAMM-A-124R1.1. [DOI] [Google Scholar]
- Manley GA, Köppl C, Johnstone BM. Distortion‐product otoacoustic emissions in the bobtail lizard. I: General characteristics. J Acoust Soc Am. 1993;93:2820–2833. [Google Scholar]
- Mills DM. Interpretation of distortion product otoacoustic emission measurements. I. Two stimulus tones. J Acoust Soc Am. 1997;102:413–429. doi: 10.1121/1.419763. [DOI] [PubMed] [Google Scholar]
- Mills DM, Rubel EW. Variation of distortion product otoacoustic emissions with furosemide injection. Hear Res. 1994;77:183–199. doi: 10.1016/0378-5955(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Mills DM, Rubel EW. Development of the cochlear amplifier. J Acoust Soc Am. 1996;100(1):428–441. doi: 10.1121/1.415857. [DOI] [PubMed] [Google Scholar]
- Mills DM, Schmiedt RA. Metabolic presbycusis: differential changes in auditory brainstem and otoacoustic emission responses with chronic furosemide application in the gerbil. JARO. 2004;5:1–10. doi: 10.1007/s10162-003-4004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DM, Shepherd RK. Distortion product otoacoustic emission and auditory brainstem responses in the echidna (Tachyglossus aculeatus) JARO. 2001;2:130–146. doi: 10.1007/s101620010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DM, Norton SJ, Rubel EW. Vulnerability and adaptation of distortion product otoacoustic emissions to endocochlear potential variation. J Acoust Soc Am. 1993;94:2108–2122. doi: 10.1121/1.407483. [DOI] [PubMed] [Google Scholar]
- Müller J, Janssen T. Similarity in loudness and distortion product otoacoustic emission input/output functions: implications for an objective hearing aid adjustment. J Acoust Soc Am. 2004;115(6):3081–3091. doi: 10.1121/1.1736292. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely ST, Gorga MP, Dorn PA. Cochlear compression estimates from measurements of distortion-product otoacoustic emissions. J Acoust Soc Am. 2003;114(3):1499–1507. doi: 10.1121/1.1604122. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am. 1991;89(5):2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Puel JL, Lenoir M, Uziel A. Dose-dependent changes in the rat cochlea following aminoglycoside intoxication. I. Physiological study. Hear Res. 1987;26:191–197. doi: 10.1016/0378-5955(87)90111-0. [DOI] [PubMed] [Google Scholar]
- Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341:241–256. doi: 10.1002/cne.903410209. [DOI] [PubMed] [Google Scholar]
- Rübsamen R, Mills DM, Rubel EW. Effects of furosemide on distortion product otoacoustic emissions and on neuronal responses in the anteroventral cochlear nucleus. J Neurophysiol. 1995;74(4):1628–1638. doi: 10.1152/jn.1995.74.4.1628. [DOI] [PubMed] [Google Scholar]
- Ruebhausen MR, Brozoski TJ, Bauer CA. A comparison of the effects of isoflurane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hear Res. 2012;287:25–29. doi: 10.1016/j.heares.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters organ of Corti mechanics: evidence for feedback of outer hair cells upon basilar membrane. J Neurosci. 1991;11:1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford TD, Colby ED. Effect of xylazine and ketamine on blood pressure, heart rate and respiratory rate in rabbits. Lab Anim Sci. 1980;30(3):519–523. [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura H, Schulte BA. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Jr, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. PNAS. 2002;99(5):3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Sterns AR, Prieve BA, Woods CI. Effects of anesthesia on DPOAE level and phase in rats. Hear Res. 2008;235:47–59. doi: 10.1016/j.heares.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Sterbing SJ, Schmidt U, Rübsamen R. The postnatal development of frequency-place code and tuning characteristics in the auditory midbrain of the phyllostomid bat, Carollia perspicillata. Hear Res. 1994;76:133–146. doi: 10.1016/0378-5955(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Suga N, Simmons JA, Shimozawa T. Neurophsiological studies on echolocation systems in awake bats producing CF-FM orientation sounds. J Exp Biol. 1974;61:379–399. doi: 10.1242/jeb.61.2.379. [DOI] [PubMed] [Google Scholar]
- Taschenberger G, Manley GA. General characteristics and suppression tuning properties of the distortion-product otoacoustic emission 2f1–f2 in the barn owl. Hear Res. 1998;123:183–200. doi: 10.1016/S0378-5955(98)00120-8. [DOI] [PubMed] [Google Scholar]
- Valero MD, Ratnam R. Reliability of distortion-product otoacoustic emissions in the common marmoset (Callithrix jacchus) Hear Res. 2011;282:265–271. doi: 10.1016/j.heares.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk P, Mason MJ, Narins PM. Distortion product otoacoustic emissions in frogs: correlation with middle and inner ear properties. Hear Res. 2002;173:100–108. doi: 10.1016/S0378-5955(02)00605-6. [DOI] [PubMed] [Google Scholar]
- Wittekindt A, Drexel M, Kössl M. Cochlear sensitivity in the lesser spear-nosed bat, Phyllostomus discolor. J comp Physiol. 2005;191:31–36. doi: 10.1007/s00359-004-0564-x. [DOI] [PubMed] [Google Scholar]
- Wittekindt A, Gaese BH, Kössl M. Influence of contralateral acoustic stimulation on the quadratic distortion product f2–f1 in humans. Hear Res. 2009;247:27–33. doi: 10.1016/j.heares.2008.09.011. [DOI] [PubMed] [Google Scholar]