ABSTRACT
Literature often refers to a 300 pps limit for cochlear implant (CI) electrical stimulation, above which pulse rate discrimination deteriorates or above which rate pitch is not perceived to increase. The present study investigated the effect on pulse rate difference limens (PRDLs) when using compound stimuli in which identical pulse trains were applied to multiple electrodes across the length of the electrode array and compared the results to those of single-electrode stimuli. PRDLs of seven CI users were determined in two stimulus pulse phase conditions, one in which the phase delays between pulses on different electrodes were minimised (burst mode) and a second in which they were maximised (spread mode). PRDLs were measured at base rates of 100 to 600 pps in 100 pps intervals, using compound stimuli on one, two, five, nine and 18 electrodes. As smaller PRDLs were expected to reflect improved rate pitch perception, 18-electrode spread mode stimuli were also included in a pitch ranking task. PRDLs improved markedly when multi-electrode compound stimuli were used, with average spread mode PRDLs across listeners between 6 and 8 % of the base rate in the whole range tested (i.e. up to 600 pps). PRDLs continued to improve as more electrodes were included, up to at least nine electrodes in the compound stimulus. Stimulus pulse phase had a significant influence on the results, with PRDLs being smaller in spread mode. Results indicate that pulse rate discrimination may be manipulated with stimulus parameter choice so that previously observed deterioration of PRDLs at 300 pps probably does not reflect a fundamental limitation to rate discrimination. However, rate pitch perception did not improve in the conditions that resulted in smaller PRDLs. This may indicate that listeners used cues other than pitch to perform the rate discrimination task or may reflect limitations in the electrically evoked neural excitation patterns presented to a rate pitch extraction mechanism.
Keywords: cochlear implants, rate pitch, rate discrimination thresholds, multi-electrode stimuli, across-channel integration
INTRODUCTION
It has been known for a long time that users of cochlear implants (CIs) can perceive pitch based on either place cues or rate cues (Tong et al. 1982, 1983; Townshend et al. 1987), and it has been shown that these cues are independent (Tong et al. 1983; McKay et al. 2000). Several factors limit the effectiveness of place pitch coding in CI. Pitch-place mismatches often arise, as filter bands associated with electrodes are typically not matched to the cochlear locations of the electrodes (Nardo et al. 2008). Also, the limited number of physical electrodes (Eisen and Franck 2005) and the effect of current spread (Boex et al. 2003; Xi et al. 2009) both limit place pitch resolution.
Rate coding of pitch, on the other hand, may provide more precise control of pitch. Action potentials are entrained to stimulation pulses at low stimulation pulse rates, resulting in these occurring at the stimulation pulse rate. Most CI users have good pulse rate discrimination at low rates. It has been shown that some CI users can identify melodies when pulse rate is varied on a single electrode channel (Pijl and Schwarz 1995b) and can also identify musical intervals (Pijl and Schwarz 1995a; McDermott and McKay 1997; Pijl 1995, 1997). Pulse rate discrimination varies greatly between users. Discrimination thresholds at a base rate of 200 pps have been measured to be as small as 10 pps (McDermott and McKay 1997) for some users and up to 100 pps (Townshend et al. 1987) for others. A repeated observation in the CI literature has been that CI users have a steeply decreasing sensitivity to changes in the rate of a pulse train applied to a single channel of a CI above around 300 pps (Shannon 1983; Tong et al. 1987; Townshend et al. 1987; McDermott and McKay 1997; McKay et al. 2000; Zeng 2002). This has often been referred to as the "300-Hz limit" to rate discrimination. This limit may restrict the usable range of rate encoding of pitch in CIs (see, for example, Carlyon et al. 2010a).
In contrast, normal hearing (NH) listeners presented with filtered pulse trains can detect rate changes at significantly higher base rates than CI users discriminating electric pulse rates on a single electrode (Carlyon and Deeks 2002). NH listeners may use phase-locking cues to estimate pure tone frequencies of up to 2 kHz (Micheyl et al. 1998) or higher. While phase-locking cues in mammals are weak above 4 to 5 kHz (Johnson 1980), the ability to estimate the pitch of pure tones may still depend on temporal mechanisms up to 8 kHz (Moore and Ernst 2012). This poses the question: is it possible for cochlear implantees to achieve smaller pulse rate discrimination thresholds (or pulse rate difference limens, PRDLs) than has been demonstrated?
A number of studies have endeavoured to answer this question. Different approaches have been followed, ranging from varying stimulus parameters and the place of stimulation to adding more electrodes. Carlyon et al. (2010a) found that neither increased stimulus duration nor addition of an amplitude ramp to the stimulus had any significant influence on the upper limit of rate pitch. The rationale was that stimulus manipulations that are expected to markedly influence auditory nerve (AN) spike train patterns may influence discrimination thresholds that rely on temporal aspects of spike trains. They also established that a more stochastic pattern of neural firing, obtained by the addition of 5,000-pps background pulses, had no influence on the upper limit of rate pitch. One study tested rate discrimination thresholds with deeply inserted electrodes using the MED-EL COMBI 40+ implant (Baumann and Nobbe 2004), as it was thought that rate coding might be more effective closer to the apical end of the cochlea because of its tuning to lower characteristic frequencies. Applying stimulation pulses to a more apical site (in this case, about 25.5 mm from the round window) in the cochlea, however, did not result in improved PRDLs. Some recent studies, though, did show that stimulation of more apical neural populations resulted in improved phase locking in cats (Middlebrooks and Snyder 2010) and indeed Macherey et al. (2011) found that the upper limit of temporal pitch could be extended somewhat when asymmetric pulses were used on apical electrodes. Asymmetric pulses can potentially direct stimulation more towards the cochlear apex (Macherey and Carlyon 2012). Carlyon et al. (2008) showed that the deterioration of rate discrimination thresholds at higher pulse rates could not be alleviated by introducing interaural timing cues in bilateral implantees. Kong et al. (2009) found that users of the Med-El C40+ implant performed significantly better at a rate discrimination task with pulse rates of 300 to 500 pps than a group of Cochlear CI24 users. These improvements at higher rates were later confirmed in a second study with the same group of participants (Kong and Carlyon 2010). These two studies suggest that CI design may influence rate discrimination abilities of CI users to some extent, but the high inter-subject variability of rate discrimination experiments and the limited number of participants preclude clear conclusions from these results.
The effects of using multiple electrodes for the coding of rate pitch have not been investigated until relatively recently. The data of Macherey and Carlyon (2010) show some differences between single-channel and dual-channel stimuli. Dual-channel stimuli were applied to neighbouring electrode pairs, using narrow bipolar stimulation (BP+1 or BP+2 modes). While single-channel pitch ranking asymptoted at around 300 pps for all participants, dual-channel pitch continued to increase beyond this point for three of the six participants, up to the highest rate tested (516 pps). However, when applying pulse trains to seven neighbouring electrodes (using MP1+2 monopolar stimulation mode), Carlyon et al. (2010a) found no improvement over single-electrode stimuli in the upper limit of rate pitch.
The present study endeavoured to answer the question: will stimulation pulse trains applied over a wide area of the cochlea (as wide as the electrode array allows) yield better PRDLs than on a single electrode or a limited number of adjacent electrodes? The effect of two main stimulus manipulations on PRDL was tested wherein the stimulus phase was varied, as explained in the next section. As the intention of stimulus manipulations was to find ways to extend the range of rates over which CI listeners had good pulse rate discrimination ability and as the underlying assumption was that the latter would directly influence rate pitch and could potentially expand the rate pitch range, pitch ranking was also tested with the stimuli that were found to have the largest influence on PRDLs.
Three main experiments were carried out with CI users as participants. The objective of the first experiment was to determine whether smaller PRDLs than those reported previously for single-electrode stimuli were possible in an all-electrode condition. As smaller PRDLs were indeed observed, the second experiment considered the influence of the number of electrodes in a compound stimulus. The third experiment considered whether observed smaller PRDLs would reflect in improved rate pitch perception. An additional experiment tested whether loudness cues may have influenced measured PRDLs.
METHODS
Participants and Equipment
Ethics clearance was obtained for this study, which has been carried out in accordance with the Declaration of Helsinki. Seven postlingually deafened CI users (Table 1) participated in experiments 1 and 3 (measurement of PRDLs with 18-electrode stimuli and pitch ranking), of which a subset also participated in the second experiment (measurement of PRDLs with fewer electrodes in a set). All participants used Cochlear's Nucleus devices. All experiments involved the real-time generation of pulse trains on a personal computer using the Matlab Nucleus Toolbox version 4.03 provided by Cochlear. These pulse sequences were transferred to the implants using an L34 speech processor provided by Cochlear.
TABLE 1.
Participant information in this study
| Subject | Gender | Age at testing (years) | Onset of deafness (age in years) | Years of implant use | Implant | Ear |
|---|---|---|---|---|---|---|
| S3 | F | 62 | 21 | 7 | Nucleus 24R | Left |
| S5 | F | 44 | 32 | 12 | Nucleus 24M | Right |
| S8 | M | 61 | 45 | 16 | Nucleus 24RE | Left |
| S15 | F | 23 | 2 | 19 | Nucleus 24R | Right |
| S18 | M | 68 | 50 | 8 | Nucleus 24RE | Left |
| S21 | F | 41 | 5 | 4 | Nucleus 24RE | Left |
| S24 | F | 21 | 12 | 5 | Nucleus 24RE | Right |
Stimuli
All electrodes were stimulated with biphasic pulses in monopolar mode with both return electrodes activated (MP1+2). The minimum available phase durations of 25 μs per phase and an inter-phase gap of 8 μs were used for all stimuli. Similar to commercial multi-electrode processing strategies, multi-electrode stimuli used in this study always involved the stimulation of electrodes from the basal towards the apical end of the cochlea (the natural direction of the cochlear travelling wave).
Stimuli consisted of pulse trains presented on either single or multiple electrodes. Multi-electrode stimuli were created by applying pulse trains with identical pulse rates to two or more electrodes in the array. As explained below, hardware limitations placed restrictions on the highest pulse rates that could be achieved when using larger numbers of electrodes. The intention was to test up to base rates of at least 600 pps, which is double that which was previously observed rate discrimination limit so that with the pulse widths used, a set containing 18 electrodes was the largest set possible. Consecutive pulses can be applied with a minimum pulse period of 70 μs for implants using Cochlear CIC3 (Cochlear Implant Chip, third generation) implant devices. The maximum pulse rate that can be achieved on each electrode is 793.65 pps with an 18-electrode compound stimulus within each signal period. Consequently, the highest base rate used was 600 pps and a limit of 750 pps was imposed on the probe (at 600 pps, a maximum allowed PRDL of 150 pps).
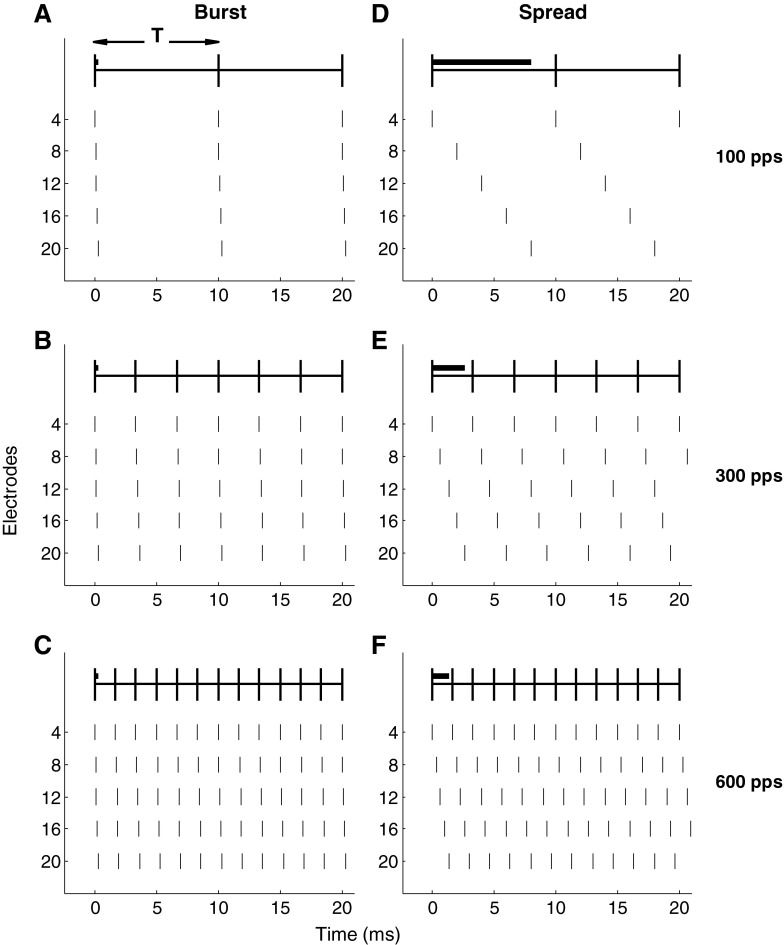
The complete set of 18 electrodes was included in the stimuli of experiment 1, while the number of electrodes was varied in experiment 2. Two types of multi-electrode stimuli were generated (Fig. 1). The first minimised the phase delay between the onsets of pulses on successive electrodes. This burst of stimulus pulses was applied at the start of each signal period ("signal period" is defined in Fig. 1). This minimum-phase delay condition is referred to as "burst mode". The second maximised the phase difference between pulses on electrodes by applying pulses on subsequent electrodes at equally spaced intervals within the signal period so that the duration of one sweep across the set of electrodes was one signal period. This maximum-phase delay condition is referred to as "spread mode".
FIG. 1.
Exemplar electrode stimulation patterns for burst (left column) and spread (right column) mode stimuli are shown for a compound stimulus with five electrodes. Each vertical line represents a biphasic stimulation pulse. The three rows show examples of 100, 300 and 600 pps stimuli, respectively, with 100, 300 and 600 pps signals represented by these five-electrode stimuli appearing at the top of the panels. T defines one signal period, which is 10 ms in the pulse trains in the top row. Burst mode stimuli occur at the beginning of a signal period, while spread mode stimuli are spread across a signal period. The bold horizontal bars indicate the duration of a stimulus sweep relative to one period of the signal.
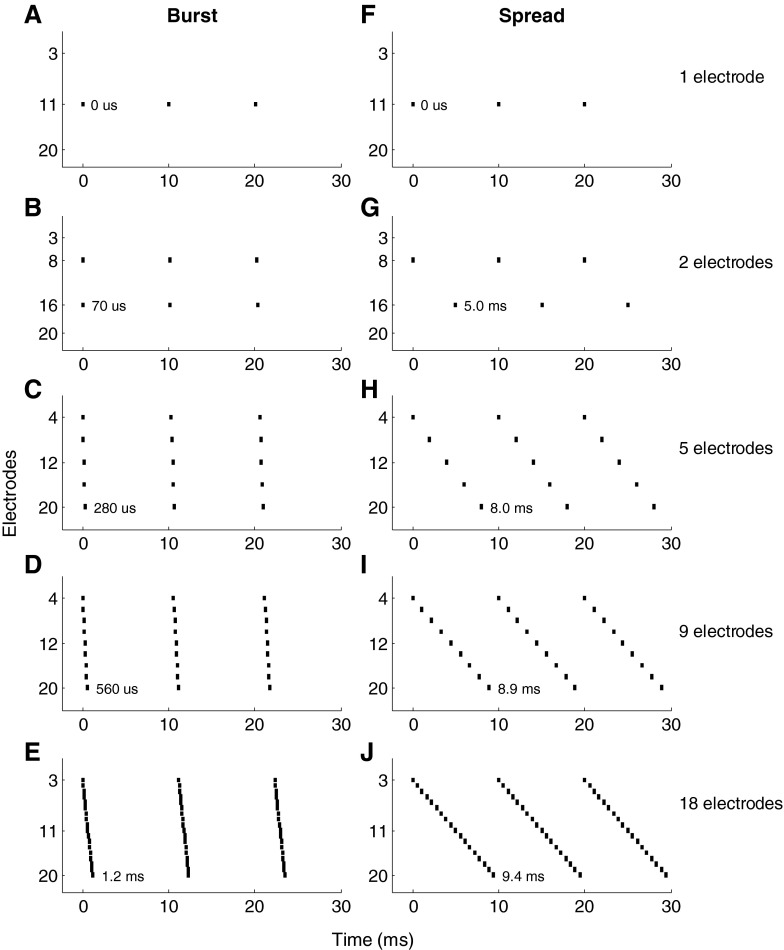
Figure 2 shows representations of stimuli over three periods of a stimulus as used in the experiments for different numbers of electrodes. The example shown is for a signal period of 10 ms (100 pps stimulus rate). Note that the unit pulses per second used in the remainder of this text refers to the per-channel pulse rate.
FIG. 2.
Three periods of the stimulation patterns used in the present study for different numbers of electrodes. Each vertical line represents a biphasic stimulation pulse. The stimuli shown are for a channel rate of 100 pps/channel. The two columns illustrate burst and spread mode stimuli, respectively. Three modes of stimulation are shown: single-electrode stimuli in panels (A) and (F), burst mode stimuli that included two, five, nine, and 18 electrodes, respectively, in panels (B)–(E) and the same stimuli for spread mode in panels (G)–(J). Panels (A) and (F) are the same but were included in both columns to simplify comparison with the burst and spread mode stimuli in the two columns. The duration of each stimulus sweep is given next to the last stimulation pulse of the first sweep.
For the 18-electrode stimuli, electrodes 3 to 20 were used. Stimuli involving fewer electrodes were generated by spreading the activated electrode set across a similarly wide range across the cochlea. For the nine-electrode stimuli, every second electrode of the 18-electrode stimuli was omitted, resulting in stimuli consisting of all even numbered electrodes between 3 and 20. Likewise, five-electrode stimuli included electrodes 4, 8, 12, 16 and 20, while two-electrode stimuli included only electrodes 8 and 16. The single-electrode stimuli were applied in the middle of the array on electrode 11. PRDLs were measured at base rates of 100 to 600 pps at intervals of 100 pps for each of these stimulus conditions, explained below.
The 18-electrode and nine-electrode spread mode stimuli of experiment 1 were used in experiment 3 (pitch ranking) and an additional 700-pps stimulus was included. The seven stimuli (with rates 100 to 700 pps in steps of 100 pps and duration 500 ms) were carefully loudness balanced before commencement of the pitch ranking task, as described in the next section. Stimuli were presented at 85 % of dynamic range.
Loudness Balancing
Before experiments 1 and 2 commenced, loudness balancing was carried out to ensure that all base rates were presented at the same loudness level. The reference loudnesses of all stimulus configurations (conditions with different numbers of electrodes, stimulated in spread and burst modes, i.e. a total of nine stimulus configurations as shown in Fig. 2) at all six base rates were carefully balanced to be the same. This was to ensure that presentation level or loudness could not influence measured PRDLs at different base rates or with different stimulus configurations.
The reference sound was a compound stimulus produced by stimulation of electrodes 5, 11 and 18 in spread mode at a base rate of 200 pps. The dynamic ranges of each electrode (difference between a current level at a loud but comfortable loudness level and the current level at threshold) from the clinical maps of the participants were used to obtain starting values of current levels. Participants first had to adjust the reference sound to a comfortable loudness level which had to be between 50 and 75 % of the dynamic range for the compound stimulus. Each electrode in the compound stimulus was stimulated at the same percentage of dynamic range of the particular electrode. When adjusting the loudness, each electrode in the set was adjusted by the same percentage of dynamic range. This was based on a recent observation that the adjustment of pulse rate had an insignificant effect on the tilt and curvature of threshold and comfort level profiles (Wesarg et al. 2010). After setting a comfortable level, participants had to adjust the stimuli at all six base rates of all the other stimulus configurations (burst and spread mode with different numbers of electrodes, a total of 54 conditions) to this reference loudness level.
The loudness balancing procedure consisted of the presentation of two intervals (500 ms each, with a 300-ms quiet gap between these) of which the first contained the reference stimulus and the second contained one of the probe stimuli. The probe stimulus could be adjusted to obtain a loudness equal to that of the reference sound. To adjust probe stimulus loudness, all electrodes included in the compound stimulus were adjusted in level by an equal percentage of the dynamic ranges of the respective electrodes.
In the experiments that followed (experiments 1 and 2), the base and probe stimuli were always presented at the current levels determined in the loudness balancing procedure with the pulse rate of the probe stimulus the only variable. Although all the reference stimuli (stimuli presented at a base rate) were loudness balanced, the probe stimuli were not, i.e. stimuli presented at rates that vary from the base rate during the staircase procedure used to determine the PRDL (explained below) were not loudness balanced. The number of probe stimuli used during the staircase procedure made loudness balancing impractical.
It is conceivable that small differences in rate (as the probe rate varies from the base rate during the staircase procedure) may have resulted in small differences in loudness. Level roving was used in some studies to encourage listeners to attend to pitch differences, rather than to remaining loudness cues (e.g. Baumann and Nobbe 2004; Chatterjee and Oberzut 2011). This approach is, however, not universal and some authors preferred not to rove level (e.g. Kong et al. 2009; Carlyon et al. 2008; Zeng 2002). The reason for this is that, although important to eliminate possible loudness cues, level roving may be a confounding factor that could influence the data. Carlyon et al. (2010b) showed that small level differences could influence pitch judgements so that roving may adversely influence a pitch perception experiment. Roving generally degrades overall performance in pitch perception tasks (see, e.g. Cousineau et al. 2009). For example, Baumann and Nobbe (2004) showed that PRDLs more than doubled when level roving was applied. The strength of the deleterious effect of roving varied across listeners, and in their work, Baumann and Nobbe decided on a level of 5 % roving as an adequate compromise. The influence on rate discrimination thresholds when presentation level varied by up to 5 % was seen as acceptably small.
The approach in the present study was different. To avoid the deleterious effect of roving in the present work, loudness was carefully balanced for all reference stimuli, but no level roving was used. Rather, an additional experiment was carried out to determine whether loudness differences may potentially have influenced the measured rate discrimination thresholds. Participants had to loudness balance a stimulus presented at the base rate against a stimulus presented at a higher rate reflecting their measured PRDL in a particular condition. This is explained below (experiment 4).
Loudness balancing of stimuli for the pitch ranking experiment closely followed the procedure used to loudness balance stimuli of experiments 1 and 2. The seven base rates (100 to 700 pps) of each of the three electrode-number conditions (single, 9 and 18) were loudness balanced to a reference stimulus in three separate loudness balancing tasks.
Procedure of Experiments 1 and 2: Measurement of PRDLs
PRDLs were measured using an adaptive four-interval two-alternative forced choice (4I2AFC) procedure. Two intervals were presented with a silent gap of 500 ms between these. Each interval contained two 500-ms sound bursts separated by a silent gap of 300 ms. The first sound burst in each interval was presented at the base rate, while the second was presented at the same rate in one interval and at a rate differing from the reference in the other interval. This probe burst had a higher rate than the reference and randomly appeared in either of the two intervals. Participants were asked to identify the interval where the two sound bursts differed in pitch. No feedback was provided to indicate correct or incorrect responses.
For the base rates of 100, 200, 300, 400, 500 and 600 pps, the starting probe rates were 200, 300, 400, 500, 650 and 750 pps, respectively. A two-down, one-up protocol was used, converging on the point where the listener could discriminate the base rate from the probe rate 71 % of the time (Levitt 1971). Two successive correct responses were required before the probe rate was adjusted to be closer to the base rate, while one error resulted in a larger difference between probe and base rates. The difference between the base and probe rates was initially adjusted by a factor of 1.6, and this factor was adjusted after two and four reversals to values of 1.4 and 1.2, respectively. The adaptive procedure terminated after ten reversals. The PRDL values were estimated by taking the geometric average between the rates of the last six reversals.
With the expectation that single-electrode PRDLs would deteriorate steeply from a base rate of around 300 pps, PRDLs were determined at base rates from 100 up to 600 pps at intervals of 100 pps. Six repetitions were completed for each condition. Conditions were presented in a different random order for each participant, and participants had no training. Each participant completed around 42 h of experiments, divided into sessions of typically 3 h each. Participants were compensated for their time.
The maximum allowable probe rate was 750 pps, as explained earlier. The experiment was aborted when the probe stimulus exceeded this limit. Also, it was assumed that the procedure did not converge at a specific base rate when the difference between reference and probe rates exceeded 500 pps. No PRDL value was then recorded.
The analysis of variance (ANOVA) analyses that are reported in the "RESULTS" section were carried out with SPSS version 21. For these analyses, the arithmetic means of the six repetitions of PRDL measurements in each condition were used. Zhao’s z statistic for comparing curves (2011) was used (using the mean and variance of the six PRDL measurements in each condition) to determine if differences between PRDL curves were statistically significant. These analyses were carried out with custom code developed in Matlab version 7.11.
Procedure of Experiment 3: Pitch Ranking
As the task in experiment 1 was to discriminate between rates by listening for pitch differences, it was hypothesised that smaller PRDLs should also reflect improved rate pitch perception. Experiment 3 characterised this by measuring the ability of listeners to pitch rank stimuli of different rates. Since the smallest PRDLs were obtained in 18-electrode and nine-electrode spread mode (see "RESULTS" section), differences in pitch ranking ability between multi-electrode and single-electrode conditions were expected to be most prominent for these conditions.
Pitch ranking was carried out using the midpoint comparison procedure of Long et al. (2005) separately for single-electrode, nine-electrode and 18-electrode spread conditions. This pitch ranking procedure was repeated 15 times for each participant. Each rate was then assigned a pitch rank position, from 1 to 7.
Procedure of Experiment 4: Testing for Loudness Cues
To test whether loudness cues existed that may have influenced PRDL data, the loudness of a stimulus at a reference rate (the base rate) was balanced to the loudness of a probe stimulus presented at a rate that was just noticeably different from this, i.e. the probe rate was the base rate plus the PRDL as determined in experiment 1. These measurements were repeated for each of the six base rates in 18-electrode spread mode.
First, the reference (interval 1) was presented at its loudness balanced level, and the participant’s task was to adjust the probe (interval 2) to be equal in loudness to the reference. The amount of adjustment expressed as percentage of dynamic range was recorded. This was repeated with the probe presented at a fixed level so that the reference had to be adjusted. These two adjustments were repeated four times to obtain eight loudness adjustments at each base rate. The same was repeated in a control condition where both stimuli (reference and probe) were presented at the base rate, alternating the interval in which loudness had to be adjusted and repeating to obtain eight loudness adjustments at each base rate.
Zhao’s method for testing for differences between curves (Zhao 2011) was then used to determine whether any loudness differences between the test and control conditions were significant. Zhao’s method was used to obtain a t statistic for comparing the test and control condition curves. Equivalence testing (Streiner 2003), in which the null hypothesis was that the two conditions differed by δ% or more of the dynamic range, was then carried out. Equivalence intervals of δ = 5 and 3 % were tested. The latter was chosen to be smaller than 5 %, a value at which Baumann and Nobbe (2004) observed acceptably small influence of level variation on PRDLs. Failure to reject the null hypothesis would reflect the availability of salient loudness cues so that participants could have used loudness differences (rather than pitch differences) to perform the discrimination task. Rejection of the null hypothesis would mean that differences in loudness between the test condition (where rates of reference and probe differed) and the control condition were smaller than 3 % of dynamic range so that any loudness differences would be expected to have little influence on the discrimination of pitch differences.
RESULTS
Experiment 1: PRDLs Using 18-Electrode Stimuli
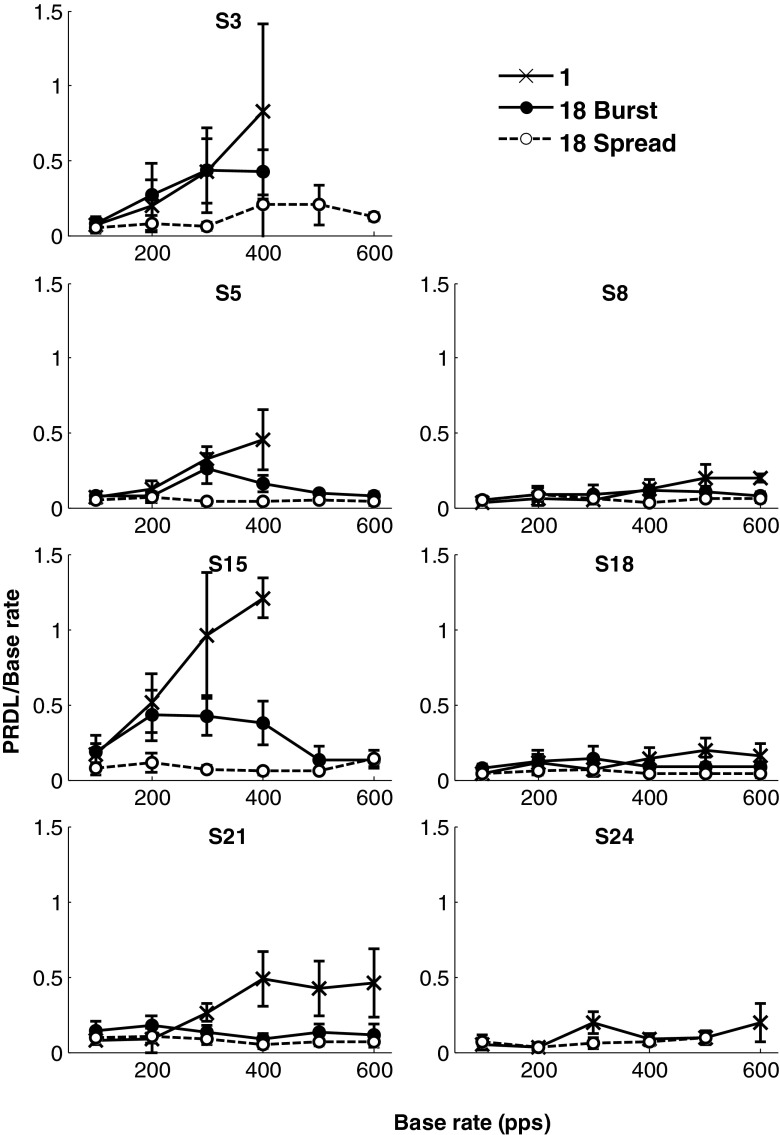
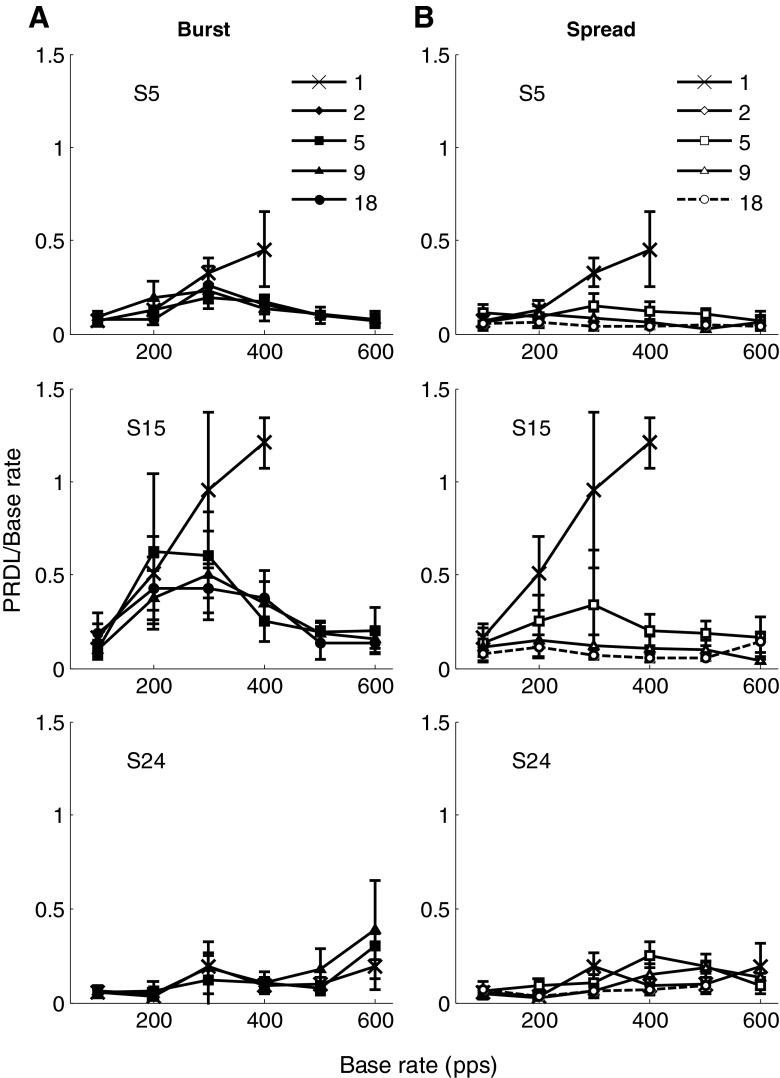
Figure 3 contains the results for each of the participants for single-electrode stimuli and for 18-electrode compound stimuli (both burst and spread mode). The error bars indicate one standard deviation at each of the measurement points. Participant S24 was not available for measurements in the burst mode. Figure 4 shows the same PRDL values averaged over all the participants. PRDLs in these figures are expressed as a relative threshold (or Weber fraction), i.e. PRDL/base rate.
FIG. 3.
Results of experiment 1. Weber fractions (PRDL/base rate) at different base rates for the single-electrode condition (X markers), 18-electrode burst condition (solid markers) and 18-electrode spread condition (open markers) are shown. The seven panels show individual relative PRDLs for seven participants measured at base rates of 100, 200, 300, 400, 500 and 600 pps with error bars indicating one standard deviation. Eighteen-electrode burst mode was not measured for S24, and all other missing data points were rates for which the PRDL could not be measured.
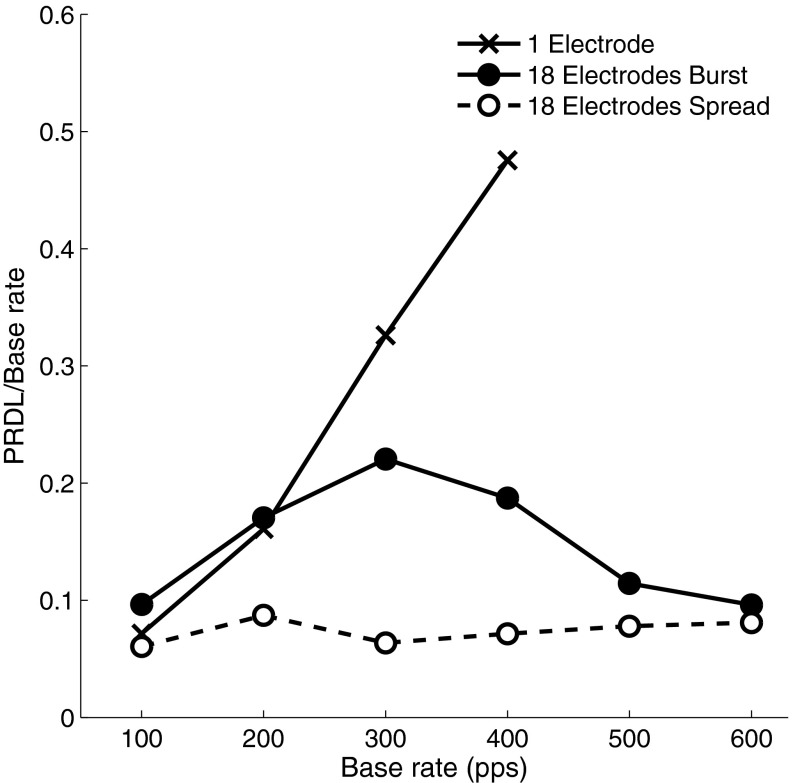
FIG. 4.
Mean values over participants for each of the conditions are shown in Figure 3.
Trends in the rate discrimination thresholds for single-electrode stimuli were as expected. These are expressed as relative thresholds (or relative PRDLs) below, with absolute values of the PRDL given in pulses per second as well. Relative thresholds for the different participants were between 0.03 and 0.16, (i.e. PRDLs between 3 and 16 pps) at a base rate of 100 pps and increased towards higher base rates. The previously documented increase in growth rate of PRDLs above 300 pps is reflected in the data of some participants, but not all, with a change in slope at around 300 pps. The general trend was for single-electrode discrimination thresholds to increase steeply from a base rate of 300 pps. Average relative thresholds across participants increased by 0.16 (PRDL of 32 pps), as base rate increased from 100 to 200 pps, and by 0.33 (98 pps) and 0.48 (190 pps) as base rate increased to 300 and 400 pps, respectively. Relative threshold differences between participants also increased as base rates increased (ranges of 0.13, 0.47, 0.9 and 1.02 at base rates 100, 200, 300 and 400 pps, respectively). For three of the participants, threshold values for single-electrode stimuli did not converge (i.e. were larger than the set PRDL limits) at base rates of 500 and 600 pps.
While relative thresholds for single-electrode stimuli increased linearly, those of 18-electrode spread mode (Fig. 4) remained almost constant with increasing base rates, and on average (across participants), this was always between 6 and 8 % of the base rate. Comparing 18-electrode spread mode thresholds with single-electrode thresholds, Zhao’s z statistic indicated a statistically significant difference (p < 0.05) between the relative PRDL curves of these two conditions for six out of seven participants (S3: p = 0.012, z = 2.245; S5: p < 0.0001, z = 4.371; S8: p = 0.012, z = 2.240; S15, p < 0.0001, z = 8.929; S18: p = 0.005, z = 2.568; S21: p = 0.0001, z = 3.646) and approaching significance for participant S24 (p = 0.063, z = 1.530).
Relative thresholds for burst mode were between those for single-electrode stimuli and those for spread mode. A comparison of relative thresholds in 18-electrode burst mode with single-electrode thresholds using Zhao’s z statistic indicated a statistically significant difference (p < 0.05) between the curves of these two conditions for three of the participants (S5: p < 0.001, z = 3.039; S15: p < 0.0001, z = 6.187; S21: p = 0.002, z = 2.834), while the remaining participants did not show a significant improvement (S3: p = 0.161, z = 0.992; S8: p = 0.155, z = 1.017; S18: p = 0.218, z = 0.780). Considering Figure 4, relative thresholds in 18-electrode burst mode tracked the single-electrode thresholds at the low pulse rate end of the range tested (100 and 200 pps) but tracked the spread mode thresholds at the highest pulse rates tested (500 and 600 pps). The average relative rate discrimination threshold for burst mode was 0.1 and 0.19 at base rates of 100 and 200 pps, respectively, around the same as for the single-electrode condition. Average thresholds decreased at rates above 300 pps and were lower than those for the single-electrode condition from this pulse rate onwards. The inter-participant differences were considerably smaller for this condition than for the single-electrode stimuli.
Figure 4 shows that relative PRDLs of the maximum-phase (spread) and minimum-phase (burst) modes approach each other as the base rate increases. This may be expected, since the inter-pulse delays between different electrodes in the two modes will be the same at approximately 790 Hz, where the maximum-phase shift is equal to the minimum achievable (hardware limit) time difference between pulses.
Experiment 2: PRDLs Using Two, Five and Nine Electrodes
Participants S5, S15 and S24 repeated the experiments with two-, five- and nine-electrode compound stimuli in both the burst (Fig. 2B–D) and spread (Fig. 2G–I) modes of stimulation. The results are shown in Figure 5 along with those of the single-electrode and 18-electrode stimuli of experiment 1.
FIG. 5.
Results of experiment 2: PRDL/base rate at different base rates for single-, two-, five-, nine- and 18-electrode conditions for burst mode (solid symbols in left hand column) and spread mode (open symbols in right hand column). Error bars indicate one standard deviation. Data for single-electrode and 18-electrode conditions are repeated from Figure 3.
Zhao’s z statistic for comparing curves (2011) was used to determine if observed differences between relative PRDL curves were statistically significant. As curves were individually compared across conditions, hypotheses tests were conducted using alpha levels that were Bonferroni corrected (alpha = 0.0125). In spread mode (right column of Fig. 5), using nine instead of 18 electrodes made no statistically significant difference in relative PRDLs for all participants tested (S5: z = 1.18, p = 0.12; S15: z = 0.45, p = 0.33; S24: z = 1.79, p = 0.037). In all cases, however, using nine electrodes rather than 18 resulted in increased variance in measurements. With five-electrode stimuli, measured relative PRDLs were significantly larger than for 18 electrodes in two participants (S5: z = 2.38, p = 0.009; S15: z = 1.95, p = 0.026; S24: z = 3.14, p = 0.001). When two-electrode stimuli were used, relative PRDLs were significantly higher than for the five-electrode condition in participant S15 (z = 2.78, p = 0.003) but not in S5 (z = 1.95, p = 0.025) or S24 (z = 0.056, p = 0.48). There was no significant difference between single- and dual-electrode stimuli in two of the participants (S5: z = 1.89, p = 0.029; S24: z = 0.39, p = 0.35), while these conditions differed significantly in S15 (z = 3.22, p = 0.001). Also, there was no significant difference between any of the other conditions for participant S24. This was also the participant that had the lowest thresholds for single-electrode stimuli.
There was no significant difference between the average relative PRDLs measured for two, five or nine electrodes for any of the three participants in burst mode (left column of Fig. 5), although the variance increased when fewer electrodes were used. Although relative PRDLs in burst mode appear to be higher for two-electrode stimuli than for five-, nine- and 18-electrode compound stimuli in participants S5 and S15, these differences were not statistically significant (five-electrode, S5: z = 1.09, p = 0.14; S15: z = 67, p = 0.25; nine-electrode, S5: z = 0.66, p = 0.25; S15: z = 1.38, p = 0.083; 18-electrode, S5: z = 0.99, p = 0.16; S15: z = 1.58, p = 0.057).
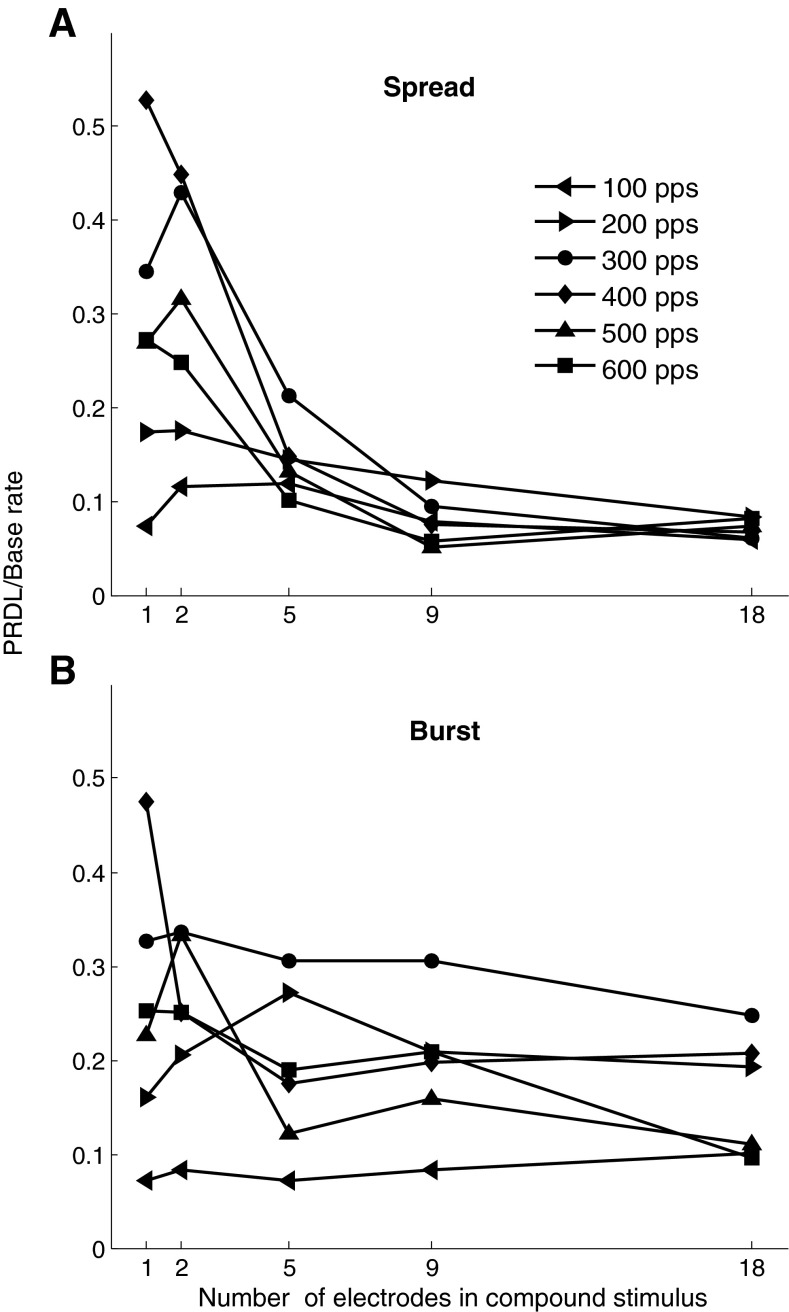
The average relative PRDLs (across listeners) are replotted in Figure 6 as a function of the number of electrodes in the compound stimulus. Figure 6A, B shows the spread and burst mode data, respectively. Different curves are for the different base rates. Here, it can be seen more clearly that the number of electrodes used in a compound stimulus has considerable influence. The data were analysed in a repeated-measures ANOVA with phase mode of stimulation (burst or spread), number of electrodes and base rate as the treatment factors. The effect of phase mode of stimulation was not significant [F(1, 2) = 9.355, p = 0.092]. Overall, the effect of the number of electrodes was not significant [F(4,8) = 2.25, p = 0.27], but a significant interaction between number of electrodes and phase mode [F(4,8) = 4.265, p = 0.039] and an almost significant interaction between number of electrodes and base rate [F(8,20) = 1.709, p = 0.074] show that the influence of the number of electrodes on thresholds was dependent on both the base rate and the phase mode. This can be seen in Figure 6. A strong dependence of relative PRDL on the number of electrodes is observed for the higher base rates in spread mode (Fig. 6A) but not for the lower base rates. A similar but weaker dependence of relative PRDL on number of electrodes is seen for burst mode (Fig. 6B). The dependence of the influence of the number of electrodes on relative PRDL is further reflected in interaction between base rate, mode and number of electrodes that approached significance [F(20,40) = 1.819, p = 0.053], while the interaction between phase mode and base rate was not significant [F(5,10) = 1.905, p = 0.18].
FIG. 6.
The data of Figure 5 replotted in a different format. Mean PRDL/base rate across listeners are shown for different numbers of electrodes in a compound stimulus for spread mode (A) and burst mode (B). The different curves are for different base rates.
Experiment 3: Pitch Ranking
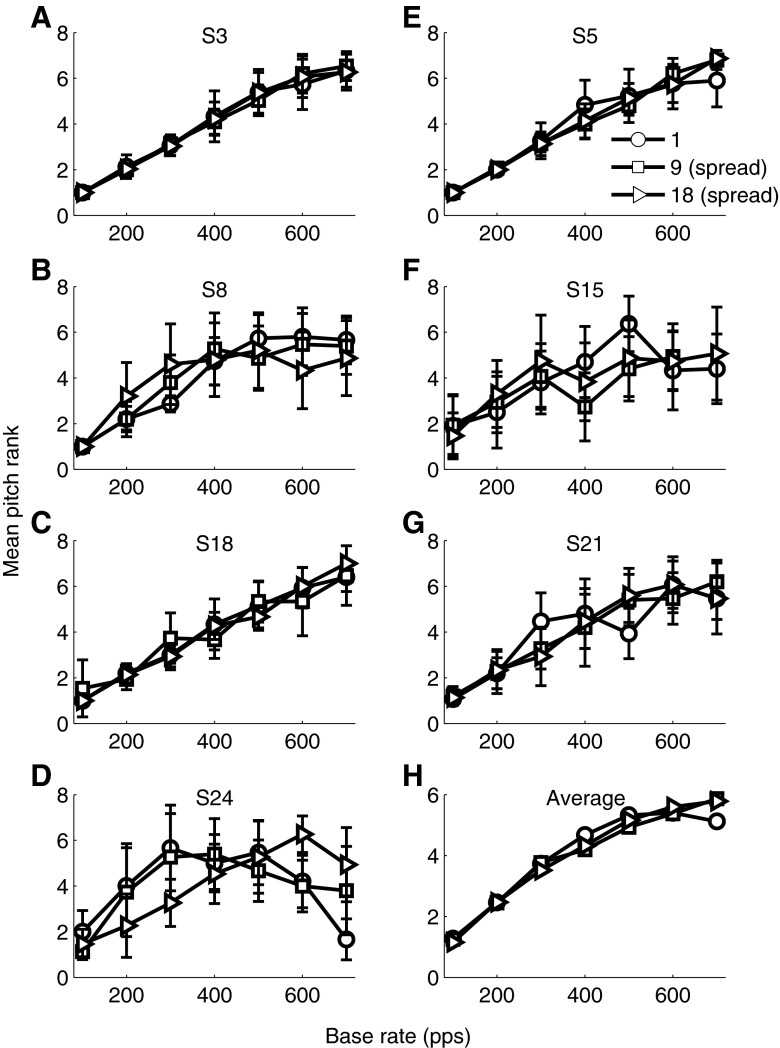
Pitch ranking data obtained for all the participants of the first experiment are plotted in Figure 7.
FIG. 7.
Data for pitch ranking (experiment 3). Individual data are plotted on pitch rank axes in panels (A) to (G), with error bars indicating one standard deviation, while panel (H) shows pitch ranking data averaged over the seven participants.
While some participants had multi-electrode rate pitch that continued to increase up to the highest rates tested (S3, S5, S18 and S21), this was not true for all participants. Rate pitch of S24 also continued to increase up to 600 pps for 18-electrode stimuli but not for nine-electrode stimuli. Single-electrode and nine-electrode stimuli asymptoted at 300 pps for this participant. S15 did not show rate pitch sensitivity above 300 pps for multi-electrode stimuli, while single-electrode stimuli increased in pitch up to 500 pps. All three variations of electrode numbers asymptoted at around 400 pps for S8.
The data were analysed in a repeated-measures ANOVA with number of electrodes and base rate as factors. This indicates that rate influenced pitch significantly [F(6, 30) = 24.44, p = 0.003]. The ANOVA analysis indicated that number of electrodes in a stimulus set does not significantly influence pitch [F(2,10) = 1.00, p = 0.363]. Also, the interaction between number of electrodes and rate was not significant [F(12,60) = 1.026, p = 0.398] so that the observation that relative PRDLs are influenced by the number of electrodes, depending the base rate, was not reflected in the pitch ranking data.
In general, there was no clear relationship between pitch ranking and relative PRDL data. While a shallower slope of relative PRDLs across rate (18-electrode spread) sometimes corresponded to the expected steeper slope in the pitch ranking curve (S3, S5 above 400 Hz, S18), the opposite was also observed. Whereas the slopes for single-electrode and 18-electrode spread condition relative thresholds did not differ for S24, the slopes of the pitch ranking data differed markedly in these two conditions. The opposite trend is seen in the data of S15 and S21, where small thresholds in the 18-electrode spread condition and large thresholds in the single-electrode condition did not reflect in the ability to pitch rank the different rates.
Experiment 4: Loudness Cues
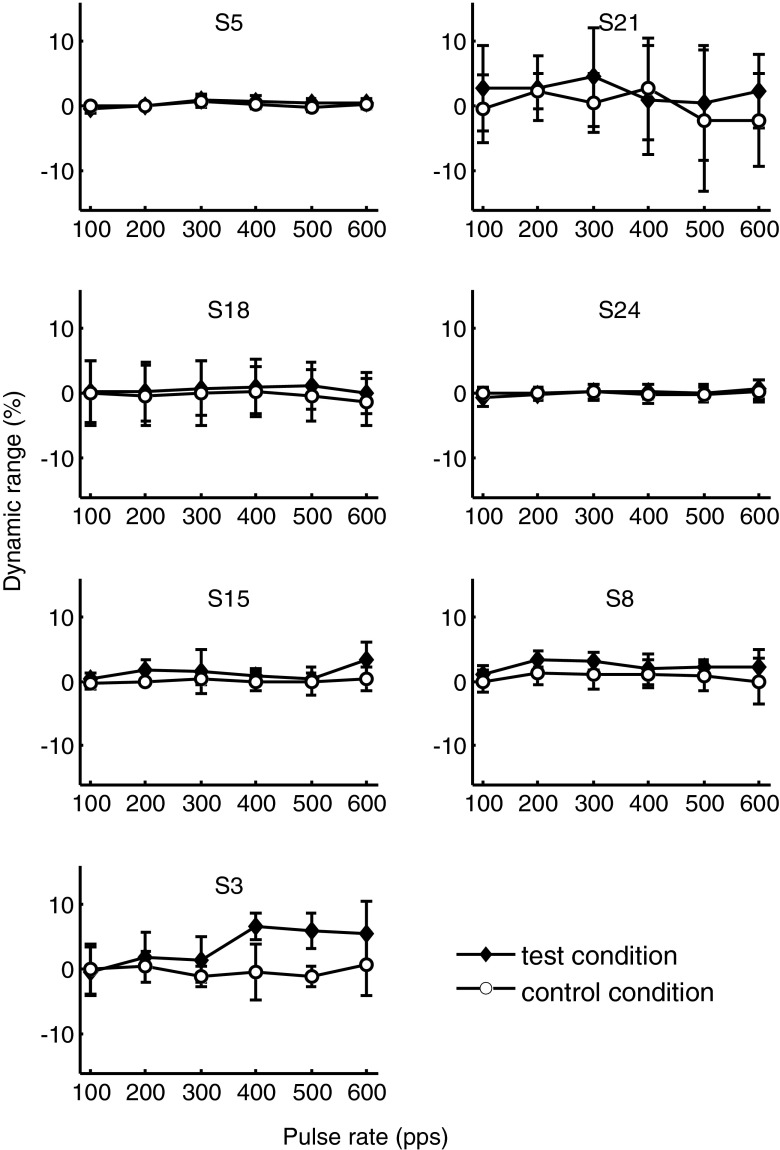
Results from experiment 4 are shown in Figure 8.
FIG. 8.
Loudness balancing data of experiment 4. Solid diamond markers indicate the average amount (arithmetic mean of eight loudness adjustments) by which loudness had to be adjusted when the reference and probe were presented at different pulse rates, and open circles show the loudness adjustments in the control condition (no difference in pulse rate between reference and probe). Error bars indicate the standard deviation of the eight repetitions.
The null hypothesis (that the test and control conditions did not differ by more than δ% of dynamic range) was rejected for six of the seven participants at δ = 5 % and for five participants when a value of δ = 3 % was tested. The statistical power of the test is determined by the choice of δ and by the variance in individual data. Statistical power is above 0.8 for all participants when δ is above 5 % and above 0.8 for five participants for δ = 3 %. The exceptions are S3 (0.55) and S21 (0.07) for whom low power at this choice of δ results from large variances in the loudness adjustment task. Two-tailed t tests with 14 degrees of freedom indicated that the probabilities that loudness adjustments in the test condition (curve T) were larger than loudness adjustments in the control condition (curve C) by more than 3 % of dynamic range were smaller than p = 0.05 for five participants (S3: t = 1.59, p = 1.86; S5: t = 18.99, p < 0.0001; S8: t = 3.29, p = 0.005; S15: t = 4.96, p < 0.0001; S18: t = 2.35, p = 0.03; S21: t = 0.76, p = 0.46; S24: t = 11.71, p < 0.0001). The null hypothesis may also be rejected for S3 at δ = 5.45 % (t = 2.15, p = 0.049) and for S21 at δ = 5.15 % (t = 2.15, p = 0.049). At these values of δ, statistical power is above 0.8. This means that the two loudness adjustment curves (curves T and C) differed by less than 5.5 % of dynamic range for all participants and by less than 3 % for five out of seven participants. Based on the conclusions of Baumann and Nobbe (2004), these loudness differences are not expected to have a marked influence on PRDL values. This is supported by published data that suggest that rate does not have a strong influence on loudness at comfortably loud levels. Data of McKay and McDermott (1998), shown in their Figure 5, indicate that loudness increased with a shallow slope with increasing pulse rate. Current level had to be reduced by around 0.65 dB on average for their four subjects to maintain equal loudness as rate increased from 100 to 600 pps, i.e. for a rate increase of, e.g. 50 pps in an adaptive procedure, current decrease would have to be 0.065 dB to maintain equal loudness. For a small DR, e.g. 5 dB, this is 1.3 % of DR. Differences this small are not expected to provide prominent loudness cues to rate changes, although weak loudness cues may have been available. Loudness cues may have influenced PRDL determination at specific rates in the present data. Loudness adjustment variances were large for S21, reaching almost 10 % of DR, and this uncertainty about the loudness of stimuli may have influenced pitch discrimination, as is reflected in larger variances in her relative PRDLs at 400 to 600 pps. However, loudness uncertainty across all rates tested also indicates that loudness could not provide a consistent cue. The largest loudness adjustments in experiment 4 were for S3 at 400 and 500 pps at values smaller than 7 % of dynamic range. Notably, the relative PRDLs of S3 (Fig. 3) exhibited relatively large variances at 400 and 500 pps. This uncertainty in the pitch discrimination task may have resulted from loudness differences between the reference and probe so that PRDL data at these two base rates may have been contaminated by loudness differences.
DISCUSSION
Summary of Results
The main observations from the data are the following:
PRDLs of CI users improved when pulses were applied to multiple electrodes across the array instead of on a single electrode. This was found up to the highest rate tested (600 pps). Variation in the pattern of phase delays between pulses on multiple electrodes had a significant influence on the PRDLs. The maximum-phase delay pattern (spread mode) resulted in the smallest PRDLs, and in this condition, PRDLs systematically decreased when more electrodes were stimulated.
This suggests that the 300-pps limit to rate discrimination observed for single electrodes does not reflect some fundamental limit but rather that stimulus parameters may influence the upper limit of rate discrimination.
As PRDLs were significantly influenced by the number of electrodes in the maximum-phase condition, these improvements were expected to reflect improved rate pitch perception. Therefore, rate pitch perception was tested in the condition resulting in the largest improvement in PRDL (18-electrode spread). However, rate pitch did not reflect the significant effect of number of electrodes on PRDLs. Neither nine-electrode nor 18-electrode spread conditions resulted in statistically significant improvement in pitch ranking above the single-electrode condition.
Comparison to Previous Rate Discrimination Data
Single-Electrode PRDLs
Rate dependence of single-electrode PRDLs followed the trends of data in the literature. Similar to previously published data (McDermott and McKay 1997; McKay et al. 2000; Shannon 1983; Tong et al. 1987; Townshend et al. 1987; Zeng 2002), single-electrode PRDLs generally deteriorated steeply at base rates greater than 300 pps, although this was not true in all participants. S24 had unusually good relative PRDLs of 9, 10 and 19 % at base rates of 400, 500 and 600 pps, respectively, for the single-electrode condition. This is uncommon but not unprecedented. Although published data indicate that many CI listeners have poor single-electrode pulse rate discrimination above 300 pps, exceptions have been reported. For example, participant 5 in a study by McKay et al. (1994) had relative PRDLs of around 8 and 9 % for base rates of 400 and 500 pps. More recently, a group of CI users of the Med-El COMBI 40+ implants were reported as being able to distinguish a 35 % difference in single-electrode reference pulse rates of 400 and 500 pps with an accuracy between 70 and 80 % (Kong et al. 2009).
Multi-Electrode PRDLs
Some conditions in the present study resembled those used in previous studies, and these resulted in similar findings to those of earlier studies, as explained below. However, some of the conditions differed distinctly from those of earlier studies. First, previous multi-electrode rate discrimination studies used fewer electrodes, and no other studies have considered rate discrimination thresholds with a number as large as nine or 18 electrodes. Bahmer and Baumann (2013) measured PRDLs of single-electrode and three-electrode stimuli, while the study of Chen et al. (2005) included conditions with two and four electrodes. Experiment 3 of Carlyon et al. (2010a) pitch ranked seven-electrode stimuli. Second, while previous studies mostly used adjacent or closely spaced electrodes in multi-electrode stimuli, the conditions in the present study generally maximised the distance between electrodes in a multi-electrode stimulus. The stimuli of Bahmer and Baumann (2013) were applied on three adjacent electrodes and those of Carlyon et al. (2010a) on seven adjacent electrodes (8–14). Third, the multi-electrode phase conditions used vary across studies. The maximum-phase condition (spread) of the present study resulted in a systematic effect of rate. Six out of seven participants showed significant improvements in PRDLs in the 18-electrode spread mode condition as compared to their single- or dual-electrode results. This systematic decrease in rate discrimination thresholds was not observed for the minimum-phase condition (burst). The same is true for other studies that used minimum-phase conditions. Bahmer and Baumann (2013), using simultaneous stimulation, did not find significant differences in PRDLs between single- and multi-electrode stimuli. They noted, however, that a subgroup of their participants had smaller PRDLs at higher rates for the three-electrode condition. Chen et al. (2005), using minimum-phase stimuli similar to the burst condition, also concluded that multi-electrode stimulation did not significantly influence rate pitch discrimination thresholds at the rates tested (up to 1,000 pps). In contrast, Carlyon et al. (2010a), using stimulation pulses that were spaced evenly over the signal period (i.e. spread mode) on the different electrodes, did observe improved rate discrimination with multi-electrode stimuli. PRDLs were not measured, however.
Considering the present data together with these previous data, pulse rate discrimination appears to be dependent on the number of electrodes in a stimulus for some but not all stimulus configurations. Specifically, PRDLs are clearly dependent on stimulus phase. The systematic decrease in PRDLs with more electrodes was observed for spread mode stimuli but not for burst mode stimuli. The data therefore suggest that there is no fundamental limit to pulse rate discrimination at 300 pps but that the upper limit of rate discrimination is dependent on stimulus parameters.
Multi-Electrode Rate Pitch
Improvements in PRDLs were expected to reflect in improved rate pitch perception. However, this was not borne out in the data. Likewise, Carlyon et al. (2010a) performed pitch ranking experiments with seven-electrode stimuli that shared similarity with those of the present study and observed no significant differences in pitch ranking between single-electrode and multi-electrode stimuli across a wide range of rates (112.5 to 1,800 pps). Potential explanations for the apparent disagreement between PRDL and pitch ranking data are considered below.
Possible Explanations of the PRDL Data
Spatial and Temporal Integration in Overlapping Neural Populations
PRDLs are dependent on pulse timing (or stimulus phase) and on the number of electrodes in a stimulus. The latter covaries with distance between the electrodes used in the stimulus. All of these stimulus parameters are expected to influence neural firing patterns. Two main effects in neural excitation patterns are expected for the different stimulus variations. First, adjacent electrodes may stimulate an overlapping neural population as a result of current spread from electrodes, with the amount of overlap depending on the distance between electrodes. The resulting interleaving of pulses in this overlapping population is expected to increase the effective pulse rate in this population. Second, the specific pattern of interleaving would depend on stimulus phase or pulse timing, which remained the same for burst mode stimuli (Fig. 1) as stimulus pulse rate increased, while it depended on pulse rate in spread mode. Both the amount of overlap and the pattern of interleaving could influence rate discrimination thresholds in at least two ways.
First, observed differences in PRDLs in conditions with different numbers of electrodes and different stimulus phase patterns may have been influenced by loudness differences. A temporal integration model of loudness in cochlear electrical stimulation (McKay and McDermott 1998) indicates that the number of pulses in an integration window may have an influence on loudness. For multi-electrode stimuli, where pulses of adjacent or nearby electrodes may activate overlapping neural populations, pulses separated by short delays may lead to charge accumulation, and therefore residual polarisation (De Balthasar et al. 2003) on neural membranes of these nerve fibres. This may result in higher neural spike rates when multiple electrodes are stimulated (as opposed to the spike rates for single electrodes), potentially resulting in increased loudness. Because of decay of charge on neural membranes, the effect on loudness from this temporal integration mechanism is expected to be weaker for longer delays between pulses applied to adjacent electrodes. McKay and McDermott (1998) estimated that pulses separated by delays shorter than around 400 μs may have an accumulative effect on loudness, but this effect is unlikely to be important for longer delays. For the present stimuli, the effect in spread mode would be smaller than in burst mode (the latter having no pulse delays between adjacent electrodes of the stimulus set) but may still exist in some of the spread mode conditions. Pulse delays between adjacent electrodes varied between 522 μs at 100 pps and 70 μs at 600 pps for 18-electrode stimuli in spread mode and were longer for multi-electrode stimuli that incorporated fewer electrodes. The intention of the loudness balancing procedure carried out across all conditions was to compensate for these potential loudness cues.
However, loudness differences may still have had an influence on PRDL measurements within a given condition, as the increased-rate stimuli (relative to the base rate) used during the staircase procedure were not loudness balanced to the base rate, as explained before. Multi-electrode stimuli may be expected to have steeper slopes of the loudness vs. rate function as a result of more overlap in stimulated neural populations so that the steepest slopes are expected in electrode sets with the largest number of electrodes. Experiment 4 confirmed that these anticipated loudness differences were small in all participants. Furthermore, any effect of loudness cues on PRDLs should be larger in burst mode than in spread mode. The data, however (Fig. 3), show that burst mode had larger relative PRDLs than spread mode, except at low pulse rates, where the temporal integration model of loudness would predict a small or non-existing effect of rate on loudness. Thus, loudness variation with pulse rate does not provide a consistent explanation for the observed PRDLs.
A second explanation for the data to consider is adaptation. Litvak et al. (2001) showed that higher pulse rates can lead to more adaptation. From this and because of interleaving of pulses, it is expected that stimuli with more electrodes would lead to more adaptation. This may potentially be used as a cue to discriminate between different rates. Carlyon et al. (2010a) argued that differences in adaptation for stimuli with different numbers of electrodes may in this way allow rate changes to become discriminable but would not necessarily lead to pitch changes. In addition, it is conceivable that burst mode would lead to more adaptation than spread mode at the same rate of stimulation so that adaptation may partially explain differences in PRDLs between burst and spread modes. More adaptation would lead to lower spike rates, and a mechanism that needs to discriminate between two different spike rates may fare worse if the spike rates were lower. Thus, adaptation may provide a feasible explanation for the differences between the PRDLs of burst and spread modes and may explain why significant differences in PRDLs do not lead to significant changes in perceived pitch.
Combining of Temporal Information Across Channels
Burst mode is more likely than spread mode to activate overlapping neural populations when stimulation electrodes are spaced closely (e.g. in the 18-electrode condition) due to residual polarisation (De Balthasar et al. 2003) so that smaller PRDLs may be expected than in burst mode. However, smaller PRDLs were measured in spread mode so that PRDLs do not appear to reflect residual polarisation. Furthermore, Figure 3 shows clear differences between spread and burst modes, pointing towards timing-sensitive differences in the processing of these. It is known that information from different cochlear areas of electrical stimulation can be combined with preservation of phase information: Carlyon et al. (2000) observed that CI users could detect phase differences between two widely separated electrodes, even in the presence of masker pulses between the two electrodes under test. Thus, given differences in the PRDLs of spread and burst modes, it is possible that observed PRDLs reflect across-channel integration. However, this does not explain why pitch perception did not improve with multi-electrode stimulation. This may mean that the data do not reflect an across-channel integration mechanism, or that cues for rate pitch extraction were suboptimal (discussed below) or that non-pitch cues were used to perform the rate discrimination task.
Best Electrode Explanation
It is possible that discrimination thresholds were smaller for multi-electrode stimuli simply because these included a "best electrode", i.e. it may be that a participant had better discrimination ability on one particular electrode than on others. If this were true, the probability that these better electrodes were included would increase with a larger number of electrodes in a set. This best electrode hypothesis may be ruled out for several reasons, however. Burst and spread modes delivered the same number of pulses per stimulus period to the same set of electrodes, and these multi-electrode stimuli were presented at equal loudness. The same best electrode would therefore be included. But, as shown in Figure 6, relative PRDLs in these two modes were significantly different, which cannot be explained by participant performance being in accordance with their best discrimination ability on a particular electrode. Also, the data in Figure 6 show that the decrease in relative PRDL in spread mode was generally monotonic as the number of electrodes in a set increased, although the specific electrodes included in a multi-electrode set varied with the number of electrodes included (see Fig. 2) so that a best electrode included in some conditions may not have been included in others. Finally, large differences were observed between relative PRDLs for different pulse rates in burst mode but not in spread mode (e.g., compare panels A and B of Fig. 6, five-electrode and nine-electrode stimuli), pointing to a stimulus phase-sensitive mechanism.
Possible Explanations of the Pitch Ranking Data
Despite significant improvements in rate discrimination ability, 18-electrode and nine-electrode spread conditions did not result in significant improvement in rate pitch. Explanations for this are considered below.
Non-Pitch Cues
While the PRDL data are not consistent with the use of loudness as a main cue, as argued above, loudness cannot be entirely precluded as a potential cue. As remarked by Carlyon et al. (2010a), listeners will use any available cue in a forced-choice procedure, and even small loudness differences may (perhaps in combination with other cues) allow rates that differ slightly to be discriminated. Also, adaptation (considered above) may provide a reasonable explanation for improvement in rate discrimination that does not result in pitch differences.
Discrimination of Pitch Differences and Direction of Pitch Change
Studies that considered the differences in sensitivity to detection of frequency or pitch changes and the identification of the direction of change show that these two tasks may be served by separate neural processes. Psychoacoustic data of patients that had undergone selective surgical removal of temporal lobe brain tissue led Johnsrude et al. (2000) to this conclusion, while Demany and Ramos (2005) interpreted their data as providing evidence of specialised frequency-shift detectors in the auditory pathway. So, although multi-electrode stimuli create action potential trains from which stimulation rate differences can be detected more easily than from single-electrode stimuli, it is conceivable that the signal available to a separate mechanism specialised for extraction of rate pitch is no more salient than for single-electrode stimuli.
Suboptimality of Cues for Rate Pitch Extraction
Even though rate discriminability improves with multi-electrode stimuli, the possibility exists that the multi-electrode stimuli that were used are suboptimal for pitch extraction. Cedolin and Delgutte (2010) demonstrated that the pitch of harmonic complexes may be extracted from the relative timings of action potentials from consecutive cochlear locations, i.e. locations stimulated sequentially by the cochlear travelling wave. Consecutive pulses in a multi-electrode stimulus create action potential patterns that to some extent emulate the basal-to-apical cochlear delays of the cochlear travelling wave. Octopus cells of the cochlear nucleus (CN) appear to be specialised to compensate for cochlear delays by resynchronising neural activity from the consecutive cochlear regions activated by the cochlear travelling wave (McGinley et al. 2012). Any divergence from the natural relative timing of action potentials originating from consecutive cochlear places that arrive at the CN may result in degrading of resynchronisation. As such, the reliability of any mechanism deeper into the auditory pathway that relies on synchronised neural activity to extract rate pitch may be compromised. For example, pitch-sensitive neurons that have been identified in the low-frequency region of the auditory cortex (Bendor et al. 2012) are sensitive to temporal envelope regularity.
CONCLUSION
It is not clear yet why stimuli that resulted in systematic and significant improvements in pulse rate discrimination at higher rates do not have a marked effect on rate pitch perception. There does not appear to be a fundamental limit to pulse rate discrimination at 300 pps. Rather, the correct choice of stimulus parameters of multi-electrode stimuli allowed smaller PRDLs at higher pulse rates than those that are typically observed for single-electrode stimuli. The failure of these stimuli to enhance rate pitch perception at high rates, however, may indicate that listeners used cues other than pitch to perform the rate discrimination task or may reflect limitations in the electrically evoked neural excitation patterns presented to a rate pitch extraction mechanism deeper into the auditory pathway.
Acknowledgments
The authors wish to thank all the participants for their contribution. Also, we would like to thank the reviewers and associate editor for their many constructive comments. Financial assistance by the National Research Foundation (South Africa) is acknowledged.
Contributor Information
Pieter J. Venter, Email: pjventermail@gmail.com
Johan J. Hanekom, Phone: +27 12 4202461, Email: johan.hanekom@up.ac.za
References
- Bahmer A, Baumann U. New parallel stimulation strategies revisited: effect of synchronous multi electrode stimulation on rate discrimination in cochlear implant users. Cochlear Implants Int. 2013;14:142–149. doi: 10.1179/1754762812Y.0000000011. [DOI] [PubMed] [Google Scholar]
- Baumann U, Nobbe A. Pulse rate discrimination with deeply inserted electrode arrays. Hear Res. 2004;196:49–57. doi: 10.1016/j.heares.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bendor D, Osmanski MS, Wang X. Dual-pitch processing mechanisms in primate auditory cortex. J Neurosci. 2012;32:16149–16161. doi: 10.1523/JNEUROSCI.2563-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boex C, De Balthasar C, Kos MI, Pelizzone M. Electrical field interactions in different cochlear implant systems. J Acoust Soc Am. 2003;114:2049–2057. doi: 10.1121/1.1610451. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Deeks JM. Limitations on rate discrimination. J Acoust Soc Am. 2002;112:1009–1025. doi: 10.1121/1.1496766. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Geurts L, Wouters J. Detection of small across-channel timing differences by cochlear implantees. Hear Res. 2000;141:140–154. doi: 10.1016/S0378-5955(99)00215-4. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Long CJ, Deeks JM. Pulse-rate discrimination by cochlear-implant and normal-hearing listeners with and without binaural cues. J Acoust Soc Am. 2008;123:2276–2286. doi: 10.1121/1.2874796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon RP, Deeks JM, McKay CM. The upper limit of temporal pitch for cochlear-implant listeners: stimulus duration, conditioner pulses, and the number of electrodes stimulated. J Acoust Soc Am. 2010;127:1469–1478. doi: 10.1121/1.3291981. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Lynch C, Deeks JM. Effect of stimulus level and place of stimulation on temporal pitch perception by cochlear implant users. J Acoust Soc Am. 2010;127:2997–3008. doi: 10.1121/1.3372711. [DOI] [PubMed] [Google Scholar]
- Cedolin L, Delgutte B. Spatiotemporal representation of the pitch of harmonic complex tones in the auditory nerve. J Neurosci. 2010;30:12712–12724. doi: 10.1523/JNEUROSCI.6365-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Oberzut C. Detection and rate discrimination of amplitude modulation in electrical hearing. J Acoust Soc Am. 2011;130:1567–1580. doi: 10.1121/1.3621445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ishihara YC, Zeng FG. Pitch discrimination of patterned electric stimulation. J Acoust Soc Am. 2005;118:338–345. doi: 10.1121/1.1937228. [DOI] [PubMed] [Google Scholar]
- Cousineau M, Demany L, Pressnitzer D. What makes a melody: the perceptual singularity of pitch sequences. J Acoust Soc Am. 2009;126:3179–3187. doi: 10.1121/1.3257206. [DOI] [PubMed] [Google Scholar]
- De Balthasar C, Boëx C, Cosendai G, Valentini G, Sigrist A, Pelizzone M. Channel interactions with high-rate biphasic electrical stimulation in cochlear implant subjects. Hear Res. 2003;182:77–87. doi: 10.1016/S0378-5955(03)00174-6. [DOI] [PubMed] [Google Scholar]
- Demany L, Ramos C. On the binding of successive sounds: perceiving shifts in nonperceived pitches. J Acoust Soc Am. 2005;117(2):833–841. doi: 10.1121/1.1850209. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrode interaction in pediatric cochlear implant subjects. J Assoc Res Otolaryngol. 2005;6:160–170. doi: 10.1007/s10162-005-5057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DH. The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am. 1980;68:1115–1122. doi: 10.1121/1.384982. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123:155–163. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- Kong YY, Carlyon RP. Temporal pitch perception at high rates in cochlear implants. J Acoust Soc Am. 2010;127:3114–3123. doi: 10.1121/1.3372713. [DOI] [PubMed] [Google Scholar]
- Kong YY, Deeks JM, Axon PR, Carlyon RP. Limits of temporal pitch in cochlear implants. J Acoust Soc Am. 2009;125:1649–1657. doi: 10.1121/1.3068457. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Litvak L, Delgutte B, Eddington D. Auditory nerve fibre responses to electric stimulation: modulated and unmodulated pulse trains. J Acoust Soc Am. 2001;110:368–379. doi: 10.1121/1.1375140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Nimmo-Smith I, Baguley DM, O'Driscoll M, Ramsden R, Otto SR, Axon PR, Carlyon RP. Optimizing the clinical fit of auditory brain stem implants. Ear Hear. 2005;26:251–262. doi: 10.1097/00003446-200506000-00002. [DOI] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP. Temporal pitch percepts elicited by dual-channel stimulation of a cochlear implant. J Acoust Soc Am. 2010;127(1):339–349. doi: 10.1121/1.3269042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP. Place-pitch manipulations with cochlear implants. J Acoust Soc Am. 2012;131:2225–2236. doi: 10.1121/1.3677260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Deeks JM, Carlyon RP. Extending the limits of place and temporal pitch perception in cochlear implant users. J Assoc Res Otolaryngol. 2011;12:233–251. doi: 10.1007/s10162-010-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott HJ, McKay CM. Musical pitch perception with electrical stimulation of the cochlea. J Acoust Soc Am. 1997;101:1622–1631. doi: 10.1121/1.418177. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, Charles Liberman M, Bal RM, Oertel D. Generating synchrony from the asynchronous: compensation for cochlear traveling wave delays by the dendrites of individual brainstem neurons. J Neurosci. 2012;32:9301–9311. doi: 10.1523/JNEUROSCI.0272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ. Loudness perception with pulsatile electrical stimulation: the effect of interpulse intervals. J Acoust Soc Am. 1998;104:1061–1074. doi: 10.1121/1.423316. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ, Clark GM. Pitch percepts associated with amplitude-modulated current pulse trains in cochlear implantees. J Acoust Soc Am. 1994;96:2664–2673. doi: 10.1121/1.411377. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ, Carlyon RP. Place and temporal cues in pitch perception: are they truly independent? Acoust Res Lett Online. 2000;1:25–30. doi: 10.1121/1.1318742. [DOI] [Google Scholar]
- Micheyl C, Moore BCJ, Carlyon RP. The role of excitation-pattern cues and temporal cues in the frequency and modulation-rate discrimination of amplitude-modulated tones. J Acoust Soc Am. 1998;104:1039–1050. doi: 10.1121/1.423322. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Snyder RL. Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci. 2010;30:1937–1946. doi: 10.1523/JNEUROSCI.4949-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Ernst SMA. Frequency difference limens at high frequencies: evidence for a transition from a temporal to a place code. J Acoust Soc Am. 2012;132:1542–1547. doi: 10.1121/1.4739444. [DOI] [PubMed] [Google Scholar]
- Nardo WD, Cantore I, Marchese MR, Cianfrone F, Scorpecci A, Giannantonio S, Paludetti G. Electric to acoustic pitch matching: a possible way to improve individual cochlear implant fitting. Eur Arch Otorhinolaryngol. 2008;265:1321–1328. doi: 10.1007/s00405-008-0655-3. [DOI] [PubMed] [Google Scholar]
- Pijl S. Musical pitch perception with pulsatile stimulation of single electrodes in patients implanted with the Nucleus cochlear implant. Ann Otol Rhinol Laryngol. 1995;166:224–227. [PubMed] [Google Scholar]
- Pijl S. Labeling of musical interval size by cochlear implant patients and normally hearing subjects. Ear Hear. 1997;18:364–372. doi: 10.1097/00003446-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Pijl S, Schwarz DWF. Intonation of musical intervals by musical intervals by deaf subjects stimulated with single bipolar cochlear implant electrodes. Hear Res. 1995;89:203–211. doi: 10.1016/0378-5955(95)00138-9. [DOI] [PubMed] [Google Scholar]
- Pijl S, Schwarz DWF. Melody recognition and musical interval perception by deaf subjects stimulated with electrical pulse trains through single cochlear implant electrodes. J Acoust Soc Am. 1995;98:886–895. doi: 10.1121/1.413514. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics. Hear Res. 1983;11:157–189. doi: 10.1016/0378-5955(83)90077-1. [DOI] [PubMed] [Google Scholar]
- Streiner DL. Unicorns do exist: a tutorial on "proving" the null hypothesis. Can J Psychiatr. 2003;48:756–761. doi: 10.1177/070674370304801108. [DOI] [PubMed] [Google Scholar]
- Tong YC, Clark GM, Blamey PJ, Busby PA, Dowell RC. Psychophysical studies for two multiple-channel cochlear implant patients. J Acoust Soc Am. 1982;71:153–160. doi: 10.1121/1.387342. [DOI] [PubMed] [Google Scholar]
- Tong YC, Blamey PJ, Dowell RC, Clark GM. Psychophysical studies evaluating the feasibility of a speech processing strategy for a multiple-channel cochlear implant. J Acoust Soc Am. 1983;74:73–80. doi: 10.1121/1.389620. [DOI] [PubMed] [Google Scholar]
- Tong YC, Clark GM, Lim HH. Estimation of the effective spread of neural excitation produced by a bipolar pair of scala tympani electrodes. Ann Otol Rhinol Laryngol. 1987;96:37–38. [Google Scholar]
- Townshend B, Cotter N, Van Compernolle D, White RL. Pitch perception by cochlear implant subjects. J Acoust Soc Am. 1987;82:106–115. doi: 10.1121/1.395554. [DOI] [PubMed] [Google Scholar]
- Wesarg T, Battmer RD, Garrido LC, Dillier N, Garcia-Ibez L, Hey M, Macias AR, Irujo AH, Morsnowski A, Offeciers EF, Zarowski A, Pesch J, Rypkema G, Smoorenburg GF. Effect of changing pulse rate on profile parameters of perceptual thresholds and loudness comfort levels and relation to ECAP thresholds in recipients of the Nucleus CI24RE device. Int J Audiol. 2010;49:775–787. doi: 10.3109/14992027.2010.492401. [DOI] [PubMed] [Google Scholar]
- Xi X, Ji F, Han D, Hong M, Chen A. Electrode interaction in cochlear implant recipients: comparison of straight and contour electrode arrays. J Oto-Rhino-Laryngol. 2009;71:228–237. doi: 10.1159/000229303. [DOI] [PubMed] [Google Scholar]
- Zeng FG. Temporal pitch in electric hearing. Hear Res. 2002;174:101–106. doi: 10.1016/S0378-5955(02)00644-5. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Power of tests for comparing trend curves with application to national immunization survey (NIS) Stat Med. 2011;30:531–540. doi: 10.1002/sim.4295. [DOI] [PubMed] [Google Scholar]