Abstract
Encapsulating peritoneal sclerosis (EPS) is a fatal complication that can occur in patients undergoing long-term peritoneal dialysis. It is characterized by bowel obstruction and marked sclerotic thickening of the peritoneal membrane. Although the mechanisms underlying the development of EPS are complex, angiogenesis, inflammation, and peritoneal fibrosis are known to be essential factors. Now, several animal models that exhibit EPS have pathophysiology similar to that of human EPS and have been proposed for use in research to provide insights into it. Recent histochemical methods also help us to understand the pathophysiology of EPS. Advances in basic research based on the findings in those animal models have enabled the development of several strategies for the prevention and treatment of EPS. We describe here interventional studies in some animal models for peritoneal fibrosis, one of the histological disorders findings characteristic to EPS, and we highlight the need for a sophisticated animal model that closely resembles human conditions.
Keywords: peritoneal dialysis, encapsulating peritoneal sclerosis, angiogenesis, inflammation, peritoneal fibrosis
I. Introduction
Peritoneal dialysis (PD) is an effective self-care therapy that improves the quality of life of patients with end-stage renal disease (ESRD). Encapsulating peritoneal sclerosis (EPS) is a serious complication that can occur during long-term PD, although only a small percentage of PD patients develop EPS. The clinical characteristics of EPS are adherent intestinal loops, thickened visceral peritoneum, and recurrent adhesive bowel obstruction, followed by cocoon formation [27]. The histological hallmarks of EPS are fibrin deposition, fibroblast swelling, capillary angiogenesis, mononuclear cell infiltration, and a thickened submesothelial compact zone [27]. Although the precise mechanism underlying EPS remains unclear, key factors known to be involved are inflammation, angiogenesis, and fibrosis of the peritoneum [17]. Infiltrating inflammatory cells, especially macrophages, produce cytokines and chemokines and cause membrane deterioration. Angiogenesis is consistent with fibrin deposition and aids in the infiltration of inflammatory cells. Finally, proinflammatory cytokines increased by inflammation and angiogenesis cause peritoneal fibrosis, which is seen in EPS. In the development of peritoneal fibrosis, heat shock protein (HSP) 47, a collagen-specific molecular chaperone, is known to play a key role.
Clinical research has identified several strategies for the treatment of EPS. Total parenteral nutrition, surgical treatment, glucocorticoids, and tamoxifen all have beneficial effects [14, 28–30, 34, 42], but the optimal management regime for EPS is yet to be established, and EPS remains life threatening.
With a view to determining its pathogenesis and establishing treatment options, many studies on EPS relied on in vivo animal models or in vitro cell-based systems. The ideal EPS animal model should be clinically relevant, with clinical characteristics and a disease progression similar to those of EPS patients. Our group has investigated EPS and focused on peritoneal fibrosis, a main lesion of EPS, on both human and animal models. Then we realized that histochemical methods, such as immunohistochemical staining, in situ hybridization, and southwestern histochemistry, were very powerful to analyze them.
This review summarizes the findings from some of the reported animal models for peritoneal fibrosis, a representative histological marker of EPS.
II. Mechanisms of EPS
Although the main cause of EPS is obscure, it is most likely multifactorial [23]. Exposure to dialysis fluids, uremia, and other risk factors (such as genetic predisposition, kidney transplantation, discontinuation of PD therapy, long-term PD therapy, peritonitis, younger age, and medication) can lead to peritoneal changes [35, 57]. Continued exposure to these risk factors may eventually lead to EPS, but the extent of the contribution of each of these factors to EPS development is not known. It is known that advanced glycation end products (AGEs), derived from the glucose and glucose degradation products (GDPs) contained in the PD dialysate, bind to receptors for AGEs (RAGE), and activate numerous signaling cascades that play important roles in EPS [15, 31]. Notably, RAGE activation stimulates the upregulation of nuclear factor (NF)-κB and leads to increased levels of vascular endothelial growth factor (VEGF), monocyte chemotactant protein-1 (MCP-1), and proinflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α [8, 9]. Therefore, angiogenesis and inflammation, followed by the upregulation of NF-κB are essential factors of peritoneal fibrosis or peritoneal loss of function [4, 13].
With respect to angiogenesis, the density of blood vessels per unit length of peritoneum was found to be significantly high in patients with membrane, and it correlated with the degree of fibrosis [61]. In addition, blood vessel permeability is increased in neovessels, allowing fibrin to elute from these vessels; extravascular fibrin deposition correlates to the number of sites of inflammation and the degree of tissue adhesion [4].
The increase in vascularity increases the infiltration of inflammatory cells and induces inflammation [62]. In humans and animal models, myofibroblasts, macrophages, neutrophils, T lymphocytes, and B lymphocytes are observed in the fibrotic peritoneum [5, 47]. These cells are principal sources of proinflammatory cytokines and fibrotic mediators, such as connective tissue growth factor (CTGF), transforming growth factor (TGF)-β, and VEGF. Peritoneal macrophage infiltration correlates to the baseline peritoneal solute transport rate in peritoneal dialysis patients [54], and intraperitoneal IL-6 serves as a marker for predicting EPS [51].
RAGE activation also mediates the generation of reactive oxygen species (ROS) and activates TGF-β-Smad signaling [1, 16]. TGF-β is one of the most potent regulators of extracellular matrix (ECM) production; it stimulates fibroblasts to produce ECM [7], and it decreases the production of enzymes that degrade ECM, including collagenase, heparinase, and stromelysin. Furthermore, TGF-β increases the production of proteins such as plasminogen-activator inhibitor type-1 and tissue inhibitor of metalloprotease, which inhibit the degradation of ECM [5]. During fibrogenesis, ECM-integrin signaling induces the expression of MCP-1 and leads to macrophage infiltration of the inflammatory sites. Subsequently, the infiltrated macrophages secrete several cytokines, such as TGF-β, and they may amplify the fibrotic process. In addition, TGF-β and inflammatory cytokines induce a complete transition of mesothelial cells. The epithelial-to-mesenchymal transition (EMT) of mesothelial cells in peritoneal fibrosis has been the subject of many studies [3, 19, 38].
The biosynthesis and the secretion of procollagen are essential to the progression of peritoneal fibrosis. In this process, HSP47, a collagen-specific molecular chaperone, plays a key role [41]. We previously reported that HSP47 expression correlates to the degree of peritoneal fibrosis in PD patients [57].
The above findings make it clear that the mechanisms underlying EPS are complex; it has been suggested that inflammation and angiogenesis are both interconnected and connected to other elements that participate in the process that leads to peritoneal fibrosis [4]. Animal models can be extremely useful to clarify the mechanism of EPS and its development, and to test therapeutic interventions. Animal models that reproduce the same pathological feature of EPS, such as the formation of a cocoon, peritoneal fibrosis, and inflammation have been developed.
III. Animal Models
The generation and production of animal models used to validate clinical applications should be based on the clinically relevant risk factors found in actual EPS.
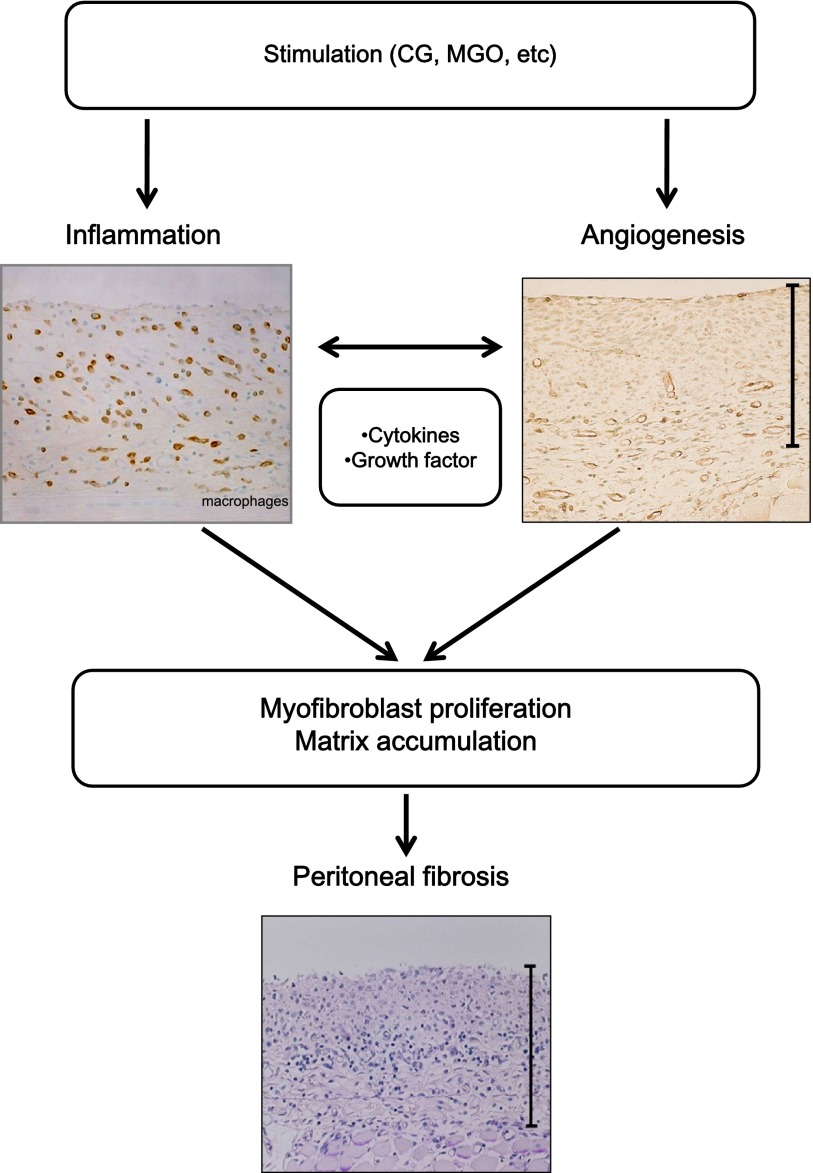
Until now, because it has been difficult to reproduce the pathology of EPS completely, several different reagents have been used to alter the peritoneal tissue mimicking the tissue histology of peritoneal fibrosis. An outline of the proposed pathogenesis of peritoneal fibrosis from animal models is shown in Fig. 1. The reagents that have been used include chlorhexidine gluconate (CG), acidic (pH 3.8) glucose solution, GDPs (e.g., methylglyoxal (MGO) and 3,4-deoxyglucoson-3-ene), and such chemical irritants as silica or household bleach [49].
Fig. 1. .
Mechanisms for the progression of peritoneal fibrosis in animal models. A variety of factors or stimuli, such as chlorhexidine gluconate (CG) or methylglyoxal (MGO), are thought to be involved in peritoneal fibrosis. They lead to inflammation and angiogenesis in the peritoneum. The subsequent myofibroblast proliferation by several cytokines and growth factors, and the accompanying accumulation of extracellular matrix in the peritoneum, are the key events that lead to peritoneal fibrosis.
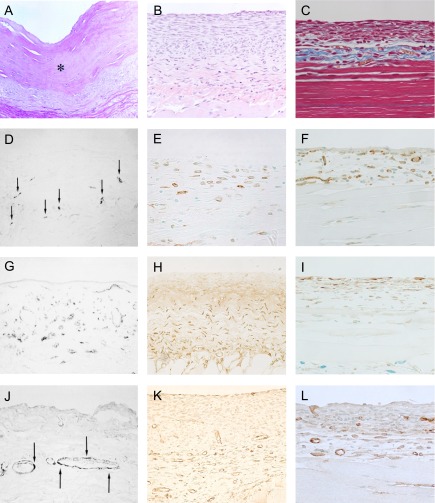
Tissue specimens from the animal models were compared to the peritoneum from long-term peritoneal dialysis patients, as shown in Fig. 2A, D, G, J. As can be seen, most of the mesothelial cells were detached and the peritoneal tissues were markedly thickened by the irregular proliferation of collagen fibers. Immunohistochemical methods also showed CD68-positive macrophages and α-SMA-positive myofibroblasts in the markedly thickened area, with a proliferation of collagen fibers [57].
Fig. 2. .
Peritonea from long-term peritoneal dialysis patients (A), (D), (G), and (J). Hematoxylin and eosin staining of peritoneal tissue from a patient presenting ultrafiltration loss. Mesothelial cells were mostly detached, and peritoneal tissue was markedly thickened, by the irregular proliferation of collagen fibers (×100). * Indicates hyalinous changes of collagen fibers (A). Many cells positive for CD68, a macrophage marker (arrow) were present in the area thickened with the collagen fibers (×100) (D). The α-smooth muscle actin (SMA) was abundantly expressed in the thickened submesothelial compact zone (×400) (G). Blood vessels (arrow) were observed in the lower area, in the submesothelial compact zone (×100) (J). These figures are extracted from Shioshita, et al. 2000 [57] with permission. The peritoneal tissues from the mouse CG model are shown in (B), (E), (H), and (K). The injection of CG induced a significant thickening of the peritoneum within 3 weeks (×100) (B). F4/80-positive macrophages were found in the submesothelial compact zone (×400) (E). Note the presence of a large number of α-SMA-positive cells in the submesothelial compact zone (×200) (H). Numerous vessels were positively stained for CD31 (×200) (K). These figures are extracted from Arai et al. 2011 [2] and Nakazawa et al. 2013 [44] with permission. The peritoneal tissues obtained from the MGO-induced peritoneal fibrosis model (C), (F), (I), and (L). As shown in masson-trichrome staining, the injection of MGO induced a significant thickening of the peritoneum (×200) (C). F4/80-positive cells in the markedly thickened peritoneal tissues (×200) (F). Note the presence of numerous α-SMA-expressing cells in the thickened submesothelial compact zone (×200) (I). Numerous vessels that stained positive for CD31 appeared in the lower submesothelial compact zone (×200) (L). These figures are extracted from Kitamura et al. 2012 [32] with permission.
The CG model
Among the several experimental rodent models of EPS, the most common, due to its ease of use and adaptability, is the CG model, prepared with 0.1% CG and 15% ethanol [2, 22, 44, 46, 47, 62]. Suga et al. were the first to report a rat peritoneal fibrosis model made with CG [58], and this model, as well as derivatives of it, has been used in many studies. CG is a chemical irritant; repeated injections cause mesothelial cell degeneration and inflammatory responses that lead to excessive fibrosis. Inflammation cells, such as macrophages, α-SMA-positive myofibroblasts, and newborn blood vessels were observed in the submesothelial compact zone; these findings resemble those of peritoneal dialysis patients. The figures shown here are from our previous reports (Fig. 2B, E, H, K) [2, 44]. Although the CG injection leads to significant peritoneal thickening, deposition of fibrin was relatively weak for the thickened peritoneum [32]. Because the mechanisms to create peritoneal fibrosis in the CG animal model differ from those that operate in human EPS patients, more refined animal models have been needed.
The MGO model
As mentioned previously, GDPs and AGEs play an important role in peritoneal fibrosis, and GDPs have been used to develop animal models. MGO is an extremely toxic GDP in PD fluid and a potent promoter of AGE formation. AGEs induce inflammation and angiogenesis, as described above. Compared to the CG model, virtually the same histological features, such as thickening of the submesothelial compact zone, the presence of inflammatory cells, and neovessels, could be observed in the MGO-induced peritoneal fibrosis model (Fig. 2C, F, I, L) [32]. In addition, the fibrin deposition in the MGO model was greater than that in the CG model. In the MGO-induced model, the AGEs confirmed in peritoneal tissue with immunohistochemistry were similar to those of EPS patients [20, 55]. Hirahara et al. reported that mesenchymal-like mesothelial cells, which are typically found in EPS, were observed in the MGO model, but not in the CG model [21]. Therefore, the MGO model more closely resembles the mechanism of actual EPS than does the CG model.
Limitations of animal models
Although the experimental models that are challenged with the infusion of a chronic chemical irritant such as CG show progression to the abdominal cocoon and encapsulation, appropriate animal models, which exactly reflect the pathogenesis of EPS, notably angiogenesis and fibrosis, should be used to establish suitable treatments for EPS. However, it is difficult to make an animal model that exhibits peritoneal fibrosis and sclerosis after long-term exposure to an unphysilogical PD fluid that is low pH and high osmolality. One factor is the time span: while it takes a long time for a human subject to develop EPS, the peritoneal degeneration induced in animal models by some chemical compounds develops in a short time. Furthermore, the reagents are generally administered into the peritoneal cavity, and these injections give mechanical damage to the peritoneal tissue.
The choice of species and strain when using animal models is essential. Among rodent models, the advantages of the rat are its size and valid response to reagent exposure. On the other hand, mice as knock-out or knock-in models are more amenable to study than rats. However, heterogeneity in TGF-β responses among mouse strains has been reported in a TGF-β overexpression model [6]. Therefore differences in animal species and strain may affect the outcome of in vivo studies, requiring careful assessment of the peritoneum in animal models.
IV. Therapeutic Strategies in Animal Models
Some reagents that were effective in the treatment of other disorders accompanying both inflammation and angiogenesis have been investigated as therapeutic candidates for peritoneal fibrosis. In Table 1, we summarized the representative strategies for preventing peritoneal fibrosis, based on the previous literature.
Table 1. .
Targeted strategies for the prevention of peritoneal fibrosis
| Angiogenesis | TNP-470 [ref. 62], sulodexide [ref. 50], sunitinib [ref. 11], anti-VEGF neutralizing antibody [ref. 25], thalidomide [ref. 2] |
| Inflammation | macrophage depletion [ref. 36], glucocorticoid [ref. 12], azathioprine [ref. 12], cyclosporine [ref. 12], mycophenol mophetil [ref. 24], mizoribine [ref. 59] |
| Collagen synthesis | HSP47 inhibition by antisense oligonucleotides [ref. 46] or siRNA [ref. 48] |
| Oxidative stress | sodium sulfite [ref. 43], N-acetyl cysteine [ref. 10], epigallocatechin gallate [ref. 32] |
| Others | angiotensin converting enzyme inhibitors [ref. 18, 53], angiotensin II receptor blockers [ref. 18], tamoxifen [ref. 37], analog of vitamin D [ref. 22], erythropoietin [ref. 40], rosiglitazone [ref. 52], suberoylanilide hydroxamic acid [ref. 26] |
VEGF, vascular endothelial growth factor; HSP47, heat shock protein 47; siRNA, small interfering ribonucleic acid.
Strategies for inhibiting angiogenesis
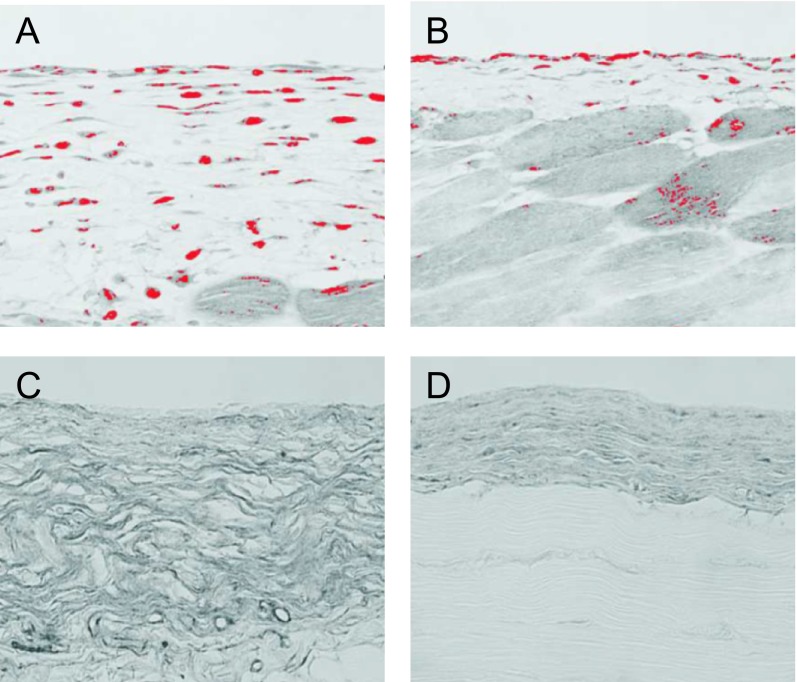
The most consistent change observed in long-term PD patients is an increase in the thickening of the submesothelial compact zone, along with peritoneal fibrosis and angiogenesis. Therefore, at therapeutic strategy in treating peritoneal fibrosis includes the inhibition of angiogenesis. TNP-470 is a well-known angiogenesis inhibitor that can also reduce peritoneal fibrosis, accompanied by the suppression of myofibroblasts and VEGF [62]. In fact, the administration of TNP-470 prevented angiogenesis by inhibiting endothelial cell proliferation (Fig. 3A–C). Immunohistochemistry analysis for collagen type III showed that treating CG-induced, peritoneal fibrosis mice with TNP-470 suppressed the peritoneal fibrosis that was observed in the untreated mice (Fig. 3D, E).
Fig. 3. .
Note the abundant expression of proliferating cell nuclear antigen (PCNA)-positive cells among the vascular endothelial cells in the CG-induced peritoneal fibrosis model (arrowheads) (×400) (A). Administration of TNP-470 to the CG-induced peritoneal fibrosis model decreased the number of PCNA-positive cells in the submesothelial area (B). Note that CD31-positive cells were also positive for PCNA (×800). Double staining for CD31 (brown) and PCNA (blue) in the same section (C). Immunohistochemistry for collagen type III showed that the administration of TNP-470 suppressed significantly the progression of peritoneal thickening (E) compared to that of the untreated mouse (×200) (D). These figures are extracted from Yoshio et al. 2004 [62] with permission.
Endostatin also produces antiangiogenic effects and has been shown to have anti-fibrotic effects in a mouse CG model [60]. Other agents, such as sulodexide and sunitinib, manifest antiangiogenic effects and attenuated peritoneal fibrosis via a similar mechanism [11, 50]. Similarly, in a rat CG model, VEGF blockade with a VEGF neutralizing antibody inhibited angiogenesis and suppressed the progression of peritoneal fibrosis [25]. Thalidomide, also known to have antiangiogenic effects, has been used clinically as a therapeutic agent in the treatment of multiple myeloma. Our group also reported that thalidomide, by inhibiting angiogenesis, attenuated peritoneal fibrosis in a mouse CG model [2].
Strategies for reducing inflammation
Since inflammation is one of the most important factors contributing to EPS, targeting infiltrating macrophages can be a potential therapeutic candidate. When liposome-encapsulated clodronate was used in a rat CG model to deplete macrophages, the peritoneal fibrosis was attenuated significantly [36]. As the upregulation of cytokines and the infiltration of macrophages are unavoidable in developing peritoneal fibrosis, immunosuppressants should exert robust effects on the inhibition of the fibrosis. In fact, glucocorticoid, azathioprine, and cyclosporine all prevented peritoneal fibrosis in animal models [12]. Other immunosuppressants, such as mycophenolate mofetil and mizoribine, have some inhibitory effects on peritoneal fibrosis similary [24, 28, 59]. Inhibition of angiogenesis also prevents inflammatory cells from infiltrating and results in anti-inflammatory effects. For example, TNP-470 suppressed angiogenesis effectively and also suppressed infiltrating inflammatory cells [62].
Strategies for inhibiting collagen synthesis
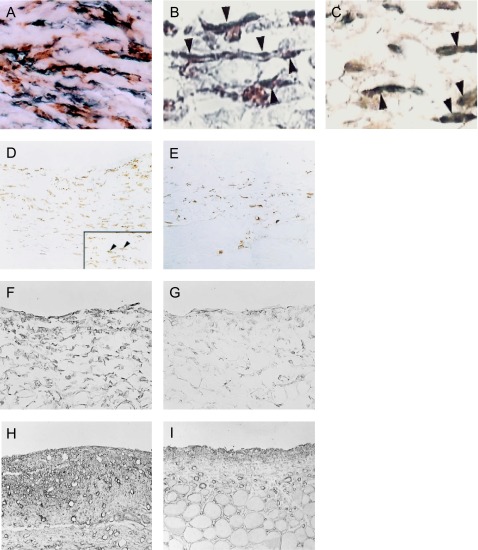
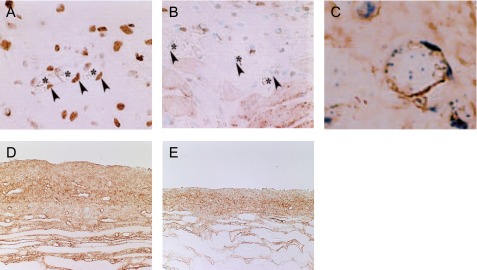
Collagen deposition is concurrent with peritoneal fibrosis and plays a role in EPS. Since HSP47 is a collagen-specific molecular chaperone with a role in procollagen synthesis and secretion, and its expression is associated with the progression of peritoneal fibrosis in humans [56], targeting HSP47 in EPS models seems to be reasonable. In both human and animal models, immunohistochemical analysis revealed that HSP47 was co-expressed in α-SMA-positive myofibroblasts and collagen type III [39, 56]. The double staining results were shown in Fig. 4A–C. These findings suggested that the suppression of HSP47 prevented peritoneal fibrosis by suppressing the activity of the myofibroblasts. Moreover, our group has found that antisense oligonucleotides against HSP47 suppressed peritoneal fibrosis in a rat CG model [46]. Antisense oligonucleotides were absorbed into α-SMA-positive myofibroblasts, effectively decreasing the expression of HSP47 in animal models (Fig. 4D, E). The results of in situ hybridization also proved that the expression of HSP47 mRNA is diminished by antisense oligonucleotide (Fig. 4F, G). Consequently, antisense oligonucleotide decreased collagen type III deposition in the submesothelial compact zone (Fig. 4H, I). In addition, HSP47-small interfering RNA conjugated with cationized gelatin microspheres also suppressed the development of peritoneal fibrosis induced by CG in mice [48].
Fig. 4. .
Heat shock protein (HSP) 47 co-localized with α-SMA-positive myofibroblasts. Note that α-SMA-positive cells in the peritoneal tissue were stained with anti-HSP47 antibody as well as α-SMA. HSP47 (brown) and α-SMA (blue) (×400) (A). Double staining for HSP47 (blue) and α-SMA (brown) in rat CG peritoneal tissue was shown (arrowheads) (×800) (B). HSP47-positive cells were also positive for collagen type III (arrowheads) (×800) (C). The administration of the antisense oligonucleotide of HSP47 suppressed the formation of peritoneal fibrosis compared to the sense oligonucleotide of HSP47. Spindle-shaped cells were positive for HSP47 in CG-induced peritoneal fibrosis in the untreated group (arrowheads) (×200) (D). Antisense oligonucleotides diminished the expression of HSP47 (×200) (E). Analysis of in situ hybridization showed that antisense oligonucleotide significantly suppressed HSP47mRNA (G) compared to the untreated CG-induced peritoneal fibrosis model (F) (×400). The untreated CG-induced peritoneal fibrosis model showed a marked accumulation of collagen type III (H), while antisense oligonucleotide decreased the accumulation of collagen type III (I). These figures are extracted from Mishima et al. 2003 [39], Nishino et al. 2003 [46], and Shioshita et al. 2000 [57] with permission.
Strategies for reducing oxidative stress
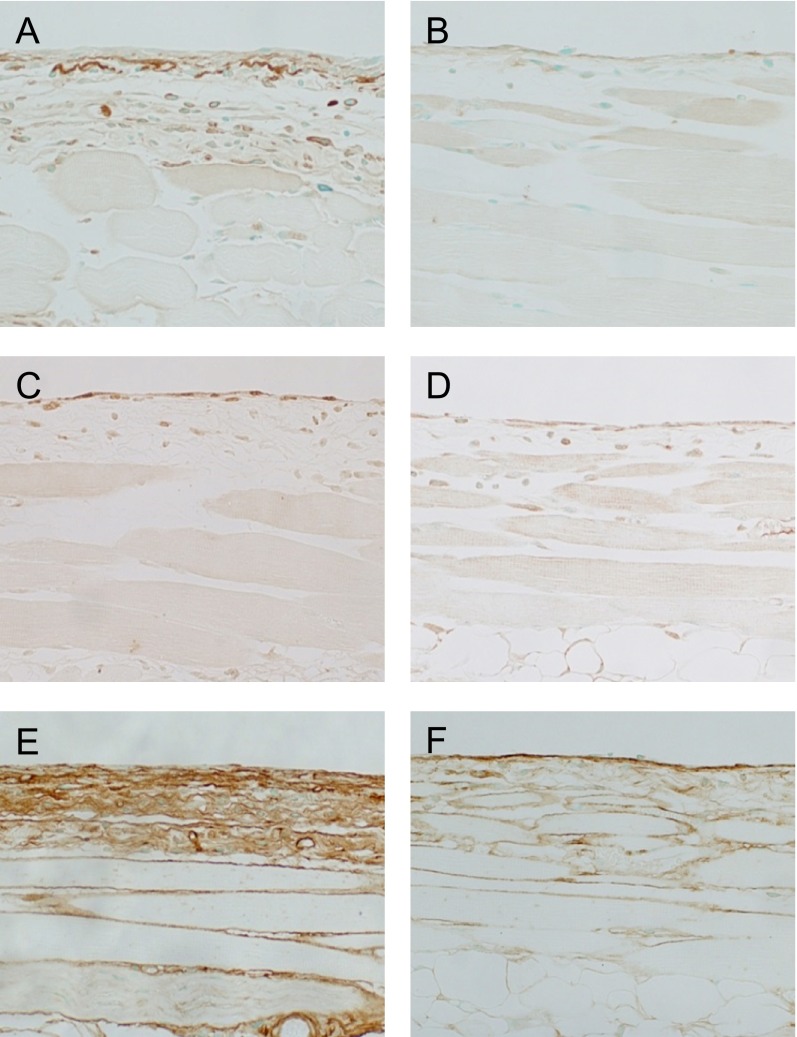
There is a consensus that PD patients suffer increased oxidative stress. Based on the findings in animal models, an increase in oxidative stress has been suggested to play an important role in peritoneal fibrosis, either through upregulating the renin-angiotensin system or by increasing AGEs [15, 18]. Sodium sulfite, a food additive and antioxidant, suppressed hyper-vascular changes and AGEs production, and attenuated peritoneal fibrosis in a rat MGO model [43]. N-acetyl cysteine, an expectorant with antioxidant effects, decreased inflammation in a rat CG model [10]. Our group has reported that epigallocatechin gallate (EGCG), a tea polyphenol with strong antioxidative effects, suppressed peritoneal fibrosis in a mouse MGO model [32]. The deposition of AGEs in the peritoneum were diminished in the group administrated EGCG, and the number of cells expressing 8-hydroxydeoxyguanosine (8-OHdG), an oxidative stress marker, were also diminished (Fig. 5A–D). In addition, the positive areas of collagen type III in the mice administered EGCG significantly diminished compared to that of mice administered with only the vehicle (Fig. 5E, F). From these results, the addition of antioxidant agents in PD fluid would seem to be effective to prevent EPS.
Fig. 5. .
Carboxy methyl lysine (CML), a well-known AGE, deposited on peritoneal tissue in an MGO-induced peritoneal fibrosis model (×200) (A). In contrast, the deposition of CML was suppressed by epigallocatechin gallate (EGCG) in the MGO fibrosis model (×200) (B). Cells positive for 8-hydroxydeoxyguanosine (8-OHdG) were observed on the surface of the peritoneum in an MGO-induced peritoneal fibrosis model (×200) (C). The administration of EGCG decreased the number of 8-OHdG-positive cells (×200) (D). The positive area of collagen type III in the peritoneum was significantly inferior in mice that received EGCG (F) compared to those that received only vehicle (E) (×200). These figures are extracted from Kitamura et al. 2012 [32] with permission.
Other strategies
Since the components of the renin-angiotensin-aldosterone system are constitutively expressed in peritoneal mesothelial cells and are upregulated in the presence of acute inflammation and high glucose PD fluid [45], angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have been used to attenuate peritoneal fibrosis [18, 53]. The synthetic estrogen tamoxifen has been used successfully to treat retroperitoneal fibrosis, a disease featuring the proliferation of fibrous tissue in the retroperitoneum, and EPS [34]. Loureiro et al. investigated the mechanism of action of tamoxifen in an EPS model and showed that, in the highly concentrated glucose PD model, it blocked mesothelial to mesenchymal transition [37]. As a previous in vitro study reported that vitamin D suppressed the activation of renal interstitial myofibroblasts and collagen expression [23], the vitamin D analog, 22-oxacalcitriol, was tested on the mouse CG model [22]. Southwestern histochemistry analysis using a haptenized double-stranded DNA has been used to find the transcription regulatory factors that bind to specific sequences of DNA and regulate the transcriptional activity of the genes [33, 56]. Southwestern histochemistry analysis revealed that 22-oxacalcitriol suppressed the activation of NF-κB in the cells of the submesothelial compact zone (Fig. 6A, B) and inhibited angiogenesis and inflammation of the peritoneum. The accumulation of collagen type III was also significantly suppressed in the group administered 22-oxacalcitriol, compared to the group treated with the vehicle (Fig. 6C, D) [22]. Erythropoietin, a potent stimulator of erythroid progenitor cells, is used worldwide to treat renal anemia; its administration reduced the degree of peritoneal fibrosis generation [40]. Rosiglitazone, an anti-diabetic drug that has an insulin-sensitizing effect, protected against peritoneal dialysis fluid-induced damage by reducing the accumulation of AGEs [52]. Recently, we reported that the histone deacetylase inhibitor, suberoylanilide hydroxamic acid, suppressed the progression of peritoneal fibrosis [26].
Fig. 6. .
Administration of 22-oxacarcitol to CG peritoneal fibrosis model significantly suppressed the formation of nuclear factor (NF)-κB-activated cells (B) compared to those administered the vehicle alone (A) (×200). The southwestern histochemistry panels for activated NF-κB were subjected to an image analyzer. The red color was assigned to positive cells. From the immunohistochemistry for collagen type III, 22-oxacarcitol was found to suppress the peritoneal fibrosis in CG-induced animals (D) compared to models administered only vehicle (C) (×200). These figures are extracted from Hirose et al. 2013 [22] with permission.
V. Conclusion
EPS development occurs due to multifactorial mechanisms. Several immunohistochemical investigations in animal models of peritoneal fibrosis have shed light on the pathogenesis of EPS. In this review, we have focused on the involvement of angiogenesis and inflammation and have identified several therapeutic strategies for the prevention and treatment of peritoneal fibrosis. We hope that further advances in the understanding of the pathogenesis of EPS will lead to the development of an effective therapy.
VI. Conflict of Interest
The authors have no conflict of interest to disclose.
VII. References
- 1.Alhamdani, M. S., Al-Kassir, A. H., Abbas, F. K., Jaleel, N. A. and Al-Taee, M. F. (2007) Antiglycation and antioxidant effect of carnosine against glucose degradation products in peritoneal mesothelial cells. Nephron Clin. Pract. 107; 26–34 [DOI] [PubMed] [Google Scholar]
- 2.Arai, H., Furusu, A., Nishino, T., Obata, Y., Nakazawa, Y., Nakazawa, M., Hirose, M., Abe, K., Koji, T. and Kohno, S. (2011) Thalidomide prevents the progression of peritoneal fibrosis in mice. Acta Histochem. Cytochem. 44; 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aroeira, L. S., Aguilera, A., Sánchez-Tomero, J. A., Bajo, M. A., del Peso, G., Jiménez-Heffernan, J. A., Selgas, R. and López-Cabrera, M. (2007) Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J. Am. Soc. Nephrol. 18; 2004–2013 [DOI] [PubMed] [Google Scholar]
- 4.Augustine, T., Brown, P. W., Davies, S. D., Summers, A. M. and Wilkie, M. E. (2009) Encapsulating peritoneal sclerosis: clinical significance and implications. Nephron Clin. Pract. 111; 149–154 [DOI] [PubMed] [Google Scholar]
- 5.Bertoli, S. V., Barone, M. T., Vago, L., Bonetto, S., De Vecchi, A., Scalamogna, A. and Barbiano di Belgiojoso, G. (1999) Changes in peritoneal membrane after continuous ambulatory peritoneal dialysis—a histopathological study. Adv. Perit. Dial. 15; 28–31 [PubMed] [Google Scholar]
- 6.Bodenham, T., Topley, N. and Fraser, D. (2013) Peritoneal fibrosis is mouse strain dependent. Nephrol. Dial. Transplant. 28; 1966–1969 [DOI] [PubMed] [Google Scholar]
- 7.Border, W. and Noble, N. (1994) Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 331; 1286–1292 [DOI] [PubMed] [Google Scholar]
- 8.Boulanger, E., Wautier, M. P., Wautier, J. L., Boval, B., Panis, Y., Wernert, N., Danze, P. M. and Dequiedt, P. (2002) AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 61; 148–156 [DOI] [PubMed] [Google Scholar]
- 9.Boulanger, E., Grossin, N., Wautier, M. P., Taamma, R. and Wautier, J. L. (2007) Mesothelial RAGE activation by AGEs enhances VEGF release and potentiates capillary tube formation. Kidney Int. 71; 126–133 [DOI] [PubMed] [Google Scholar]
- 10.Bozkurt, D., Hur, E., Ulkuden, B., Sezak, M., Nar, H., Purclutepe, O., Sen, S. and Duman, S. (2009) Can N-acetylcysteine preserve peritoneal function and morphology in encapsulating peritoneal sclerosis? Perit. Dial. Int. 29; 202–205 [PubMed] [Google Scholar]
- 11.Bozkurt, D., Sarsik, B., Hur, E., Ertilav, M., Karaca, B., Timur, O., Bicak, S., Akcicek, F. and Duman, S. (2011) A novel angiogenesis inhibitor, sunitinib malate, in encapsulating peritoneal sclerosis. J. Nephrol. 24; 359–365 [DOI] [PubMed] [Google Scholar]
- 12.Bozkurt, D., Sipahi, S., Cetin, P., Hur, E., Ozdemir, O., Ertilav, M., Sen, S. and Duman, S. (2009) Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit. Dial. Int. 29; 206–210 [PubMed] [Google Scholar]
- 13.Braun, N., Alscher, M. D., Kimmel, M., Amann, K. and Büttner, M. (2011) Encapsulating peritoneal sclerosis—an overview. Nephrol. Ther. 7; 162–171 [DOI] [PubMed] [Google Scholar]
- 14.Chin, A. I. and Yeun, J. Y. (2006) Encapsulating peritoneal sclerosis: an unpredictable and devastating complication of peritoneal dialysis. Am. J. Kidney Dis. 47; 697–712 [DOI] [PubMed] [Google Scholar]
- 15.De Vriese, A. S., Flyvbjerg, A., Mortier, S., Tilton, R. G. and Lameire, N. H. (2003) Inhibition of the interaction of AGE-RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. J. Am. Soc. Nephrol. 14; 2109–2118 [DOI] [PubMed] [Google Scholar]
- 16.De Vriese, A. S. (2005) The John F. Maher Recipient Lecture 2004: RAGE in the peritoneum. Perit. Dial. Int. 25; 8–11 [PubMed] [Google Scholar]
- 17.Devuyst, O., Margetts, P. J. and Topley, N. (2010) The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 21; 1077–1085 [DOI] [PubMed] [Google Scholar]
- 18.Duman, S., Sen, S., Duman, C. and Oreopoulos, D. G. (2005) Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int. J. Artif. Organs 28; 156–163 [DOI] [PubMed] [Google Scholar]
- 19.Fang, C. C., Huang, J. W., Shyu, R. S., Yen, C. J., Shiao, C. H., Chiang, C. K., Hu, R. H. and Tsai, T. J. (2012) Fibrin-induced epithelial-to-mesenchymal transition of peritoneal mesothelial cells as a mechanism of peritoneal fibrosis: effects of pentoxifylline. PLoS One 7; e44765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirahara, I., Kusano, E., Yanagiba, S., Miyata, Y., Ando, Y., Muto, S. and Asano, Y. (2006) Peritoneal injury by methylglyoxal in peritoneal dialysis. Perit. Dial. Int. 26; 380–392 [PubMed] [Google Scholar]
- 21.Hirahara, I., Ishibashi, Y., Kaname, S., Kusano, E. and Fujita, T. (2009) Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol. Dial. Transplant. 24; 437–447 [DOI] [PubMed] [Google Scholar]
- 22.Hirose, M., Nishino, T., Obata, Y., Nakazawa, M., Nakazawa, Y., Furusu, A., Abe, K., Miyazaki, M., Koji, T. and Kohno, S. (2013) 22-Oxacalcitriol prevents progression of peritoneal fibrosis in a mouse model. Perit. Dial. Int. 33; 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoff, C. M. (2005) Experimental animal models of encapsulating peritoneal sclerosis. Perit. Dial. Int. 25; 57–66 [PubMed] [Google Scholar]
- 24.Hur, E., Bozkurt, D., Timur, O., Bicak, S., Sarsik, B., Akcicek, F. and Duman, S. (2012) The effects of mycophenolate mofetil on encapsulated peritoneal sclerosis model in rats. Clin. Nephrol. 77; 1–7 [DOI] [PubMed] [Google Scholar]
- 25.Io, H., Hamada, C., Ro, Y., Ito, Y., Hirahara, I. and Tomino, Y. (2004) Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int. 65; 1927–1936 [DOI] [PubMed] [Google Scholar]
- 26.Io, K., Nishino, T., Obata, Y., Kitamura, M., Koji, T. and Kohno, S. (2014) SAHA prevents the progression of peritoneal fibrosis in mice. Perit. Dial. Int. (Epub ahed of print). [DOI] [PMC free article] [PubMed]
- 27.Kawaguchi, Y. and Tranaeus, A. (2005) A historical review of encapsulating peritoneal sclerosis. Perit. Dial Int. 25; 7–13 [PubMed] [Google Scholar]
- 28.Kawaguchi, Y., Saito, A., Kawanishi, H., Nakayama, M., Miyazaki, M., Nakamoto, H. and Tranaeus, A. (2005) Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit. Dial. Int. 25; 83–95 [PubMed] [Google Scholar]
- 29.Kawanishi, H., Kawaguchi, Y., Fukui, H., Hara, S., Imada, A., Kubo, H., Kin, M., Nakamoto, M., Ohira, S. and Shoji, T. (2004) Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am. J. Kidney Dis. 44; 729–737 [PubMed] [Google Scholar]
- 30.Kawanishi, H., Watanabe, H., Moriishi, M. and Tsuchiya, S. (2005) Successful surgical management of encapsulating peritoneal sclerosis. Perit. Dial. Int. 25; 39–47 [PubMed] [Google Scholar]
- 31.Kihm, L. P., Wibisono, D., Müller-Krebs, S., Pfisterer, F., Morath, C., Gross, M. L., Morcos, M., Seregin, Y., Bierhaus, A., Nawroth, P. P., Zeier, M. and Schwenger, V. (2008) RAGE expression in the human peritoneal membrane. Nephrol. Dial. Transplant. 23; 3302–3306 [DOI] [PubMed] [Google Scholar]
- 32.Kitamura, M., Nishino, T., Obata, Y., Furusu, A., Hishikawa, Y., Koji, T. and Kohno, S. (2012) Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chem. Biol. Interact. 195; 95–104 [DOI] [PubMed] [Google Scholar]
- 33.Koji, T., Komuta, K., Nozawa, M., Yamada, S. and Nakane, P. K. (1994) Localization of cyclic adenosine 3',5'-monophosphate-responsive element (CRE)-binding proteins by southwestern histochemistry. J. Histochem. Cytochem. 42; 1399–1405 [DOI] [PubMed] [Google Scholar]
- 34.Korte, M. R., Fieren, M. W., Sampimon, D. E., Lingsma, H. F., Weimar, W., Betjes, M. G. and investigators of multicenter of the Dutch Multicentre EPS Study (2011) Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol. Dial. Transplant. 26; 691–697 [DOI] [PubMed] [Google Scholar]
- 35.Korte, M. R., Sampimon, D. E., Betjes, M. G. and Krediet, R. T. (2011) Encapsulating peritoneal sclerosis: the state of affairs. Nat. Rev. Nephrol. 7; 528–538 [DOI] [PubMed] [Google Scholar]
- 36.Kushiyama, T., Oda, T., Yamada, M., Higashi, K., Yamamoto, K., Oshima, N., Sakurai, Y., Miura, S. and Kumagai, H. (2011) Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrol. Dial. Transplant. 26; 3143–3154 [DOI] [PubMed] [Google Scholar]
- 37.Loureiro, J., Sandoval, P., del Peso, G., Gónzalez-Mateo, G., Fernández-Millara, V., Santamaria, B., Bajo, M. A., Sánchez-Tomero, J. A., Guerra-Azcona, G., Selgas, R., López-Cabrera, M. and Aguilera, A. I. (2013) Tamoxifen ameliorates peritoneal membrane damage by blocking mesothelial to mesenchymal transition in peritoneal dialysis. PLoS One 8; e61165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLoughlin, R. M. and Topley, N. (2011) Switching on EMT in the peritoneal membrane: considering the evidence. Nephrol. Dial. Transplant. 26; 12–15 [DOI] [PubMed] [Google Scholar]
- 39.Mishima, Y., Miyazaki, M., Abe, K., Ozono, Y., Shioshita, K., Xia, Z., Harada, T., Taguchi, T., Koji, T. and Kohno, S. (2003) Enhanced expression of heat shock protein 47 in rat model of peritoneal fibrosis. Perit. Dial. Int. 23; 14–22 [PubMed] [Google Scholar]
- 40.Mondello, S., Mazzon, E., Di Paola, R., Crisafulli, C., Italiano, D., Buemi, M., Aloisi, C. and Cuzzocrea, S. (2009) Erythropoietin suppresses peritoneal fibrosis in rat experimental model. Eur. J. Pharmacol. 604; 138–149 [DOI] [PubMed] [Google Scholar]
- 41.Nagata, K. (2003) Therapeutic strategy for fibrotic diseases by regulating the expression of collagen-specific molecular chaperone HSP47. Nihon Yakurigaku Zasshi 121; 4–14 [DOI] [PubMed] [Google Scholar]
- 42.Nakamoto, H., Kawaguchi, Y. and Suzuki, H. (2002) Encapsulating peritoneal sclerosis in patients undergoing continuous ambulatory peritoneal dialysis in Japan. Adv. Perit. Dial. 18; 119–123 [PubMed] [Google Scholar]
- 43.Nakayama, M., Sakai, A., Numata, M. and Hosoya, T. (2003) Hyper-vascular change and formation of advanced glycation endproducts in the peritoneum caused by methylglyoxal and the effect of an anti-oxidant, sodium sulfite. Am. J. Nephrol. 23; 390–394 [DOI] [PubMed] [Google Scholar]
- 44.Nakazawa, M., Obata, Y., Nishino, T., Abe, S., Nakazawa, Y., Abe, K., Furusu, A., Miyazaki, M., Koji, T. and Kohno, S. (2013) Involvement of leptin in the progression of experimentally induced peritoneal fibrosis in mice. Acta Histochem. Cytochem. 46; 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nessim, S. J., Perl, J. and Bargman, J. M. (2010) The renin-angiotensin-aldosterone system in peritoneal dialysis: is what is good for the kidney also good for the peritoneum? Kidney Int. 78; 23–28 [DOI] [PubMed] [Google Scholar]
- 46.Nishino, T., Miyazaki, M., Abe, K., Furusu, A., Mishima, Y., Harada, T., Ozono, Y., Koji, T. and Kohno, S. (2003) Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int. 64; 887–896 [DOI] [PubMed] [Google Scholar]
- 47.Nishino, T., Ashida, R., Obata, Y., Furusu, A., Abe, K., Miyazaki, M., Koji, T. and Kohno, S. (2012) Involvement of lymphocyte infiltration in the progression of mouse peritoneal fibrosis model. Ren. Fail. 34; 760–766 [DOI] [PubMed] [Google Scholar]
- 48.Obata, Y., Nishino, T., Kushibiki, T., Tomoshige, R., Xia, Z., Miyazaki, M., Abe, K., Koji, T., Tabata, Y. and Kohno, S. (2012) HSP47 siRNA conjugated with cationized gelatin microspheres suppresses peritoneal fibrosis in mice. Acta Biomater. 8; 2688–2696 [DOI] [PubMed] [Google Scholar]
- 49.Park, S. H., Kim, Y. L. and Lindholm, B. (2008) Experimental encapsulating peritoneal sclerosis models: pathogenesis and treatment. Perit. Dial. Int. 28; S21–28 [PubMed] [Google Scholar]
- 50.Pletinck, A., Van Landschoot, M., Steppan, S., Laukens, D., Passlick-Deetjen, J., Vanholder, R. and Van Biesen, W. (2012) Oral supplementation with sulodexide inhibits neo-angiogenesis in a rat model of peritoneal perfusion. Nephrol. Dial. Transplant. 27; 548–556 [DOI] [PubMed] [Google Scholar]
- 51.Sampimon, D. E., Korte, M. R., Barreto, D. L., Vlijm, A., de Waart, R., Struijk, D. G. and Krediet, R. T. (2010) Early diagnostic markers for encapsulating peritoneal sclerosis: a case-control study. Perit. Dial. Int. 30; 163–169 [DOI] [PubMed] [Google Scholar]
- 52.Sandoval, P., Loureiro, J., González-Mateo, G., Pérez-Lozano, M. L., Maldonado-Rodríguez, A., Sánchez-Tomero, J. A., Mendoza, L., Santamaría, B., Ortiz, A., Ruíz-Ortega, M., Selgas, R., Martín, P., Sánchez-Madrid, F., Aguilera, A. and López-Cabrera, M. (2010) PPAR-γ agonist rosiglitazone protects peritoneal membrane from dialysis fluid-induced damage. Lab. Invest. 90; 1517–1532 [DOI] [PubMed] [Google Scholar]
- 53.Sawada, T., Ishii, Y., Tojimbara, T., Nakajima, I., Fuchinoue, S. and Teraoka, S. (2002) The ACE inhibitor, quinapril, ameliorates peritoneal fibrosis in an encapsulating peritoneal sclerosis model in mice. Pharmacol. Res. 46; 505–510 [DOI] [PubMed] [Google Scholar]
- 54.Sawai, A., Ito, Y., Mizuno, M., Suzuki, Y., Toda, S., Ito, I., Hattori, R., Matsukawa, Y., Gotoh, M., Takei, Y., Yuzawa, Y. and Matsuo, S. (2011) Peritoneal macrophage infiltration is correlated with baseline peritoneal solute transport rate in peritoneal dialysis patients. Nephrol. Dial. Transplant. 26; 2322–2332 [DOI] [PubMed] [Google Scholar]
- 55.Schwenger, V. (2006) GDP and AGE receptors: mechanisms of peritoneal damage. Contrib. Nephrol. 150; 77–83 [DOI] [PubMed] [Google Scholar]
- 56.Shin, M., Hishikawa, Y., Izumi, S. and Koji, T. (2002) Southwestern histochemistry as a molecular histochemical tool for analysis of expression of transcription factors: application to paraffin-embedded tissue sections. Med. Electron. Microsc. 35; 217–224 [DOI] [PubMed] [Google Scholar]
- 57.Shioshita, K., Miyazaki, M., Ozono, Y., Abe, K., Taura, K., Harada, T., Koji, T., Taguchi, T. and Kohno, S. (2000) Expression of heat shock proteins 47 and 70 in the peritoneum of patients on continuous ambulatory peritoneal dialysis. Kidney Int. 57; 619–631 [DOI] [PubMed] [Google Scholar]
- 58.Suga, H., Teraoka, S., Ota, K., Komemushi, S., Furutani, S., Yamauchi, S. and Margolin, S. (1995) Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp. Toxicol. Pathol. 47; 287–291 [DOI] [PubMed] [Google Scholar]
- 59.Takahashi, S., Taniguchi, Y., Nakashima, A., Arakawa, T., Kawai, T., Doi, S., Ito, T., Masaki, T., Kohno, N. and Yorioka, N. (2009) Mizoribine suppresses the progression of experimental peritoneal fibrosis in a rat model. Nephron Exp. Nephrol. 112; 59–69 [DOI] [PubMed] [Google Scholar]
- 60.Tanabe, K., Maeshima, Y., Ichinose, K., Kitayama, H., Takazawa, Y., Hirokoshi, K., Kinomura, M., Sugiyama, H. and Makino, H. (2007) Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int. 71; 227–238 [DOI] [PubMed] [Google Scholar]
- 61.Williams, J. D., Craig, K. J., Topley, N., Von Ruhland, C., Fallon, M., Newman, G. R., Mackenzie, R. K., Williams, G. T. and Group, P. B. S. (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 13; 470–479 [DOI] [PubMed] [Google Scholar]
- 62.Yoshio, Y., Miyazaki, M., Abe, K., Nishino, T., Furusu, A., Mizuta, Y., Harada, T., Ozono, Y., Koji, T. and Kohno, S. (2004) TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int. 66; 1677–1685. [DOI] [PubMed]