Abstract
The epidemiology, genetics, and clinical manifestations of Crohn's disease (CD) vary considerably among geographic areas and ethnic groups. Thus, identifying the characteristics of Korean CD is important for establishing management strategies appropriate for Korean patients. Since the mid-2000s, many studies have investigated the characteristic features of Korean CD. The incidence and prevalence rates of CD have been increasing rapidly in Korea, especially among the younger population. Unlike Western data, Korean CD shows a male predominance and a lower proportion of isolated colonic disease. Perianal lesions are more prevalent than in Western countries. Genome-wide association studies have confirmed that genetic variants in TNFSF15, IL-23R, and IRGM, but not ATG16L1, are associated with CD susceptibility in the Korean population. Studies of the associations between genetic mutations and the clinical course of CD are underway. Although it has been generally accepted that the clinical course of Korean CD is milder than that in Western countries, recent studies have shown a comparable rate of intestinal resection in Korean and Western CD patients. An ongoing nationwide, hospital-based cohort study is anticipated to provide valuable information on the natural history and prognosis of Korean CD in the near future.
Keywords: Crohn disease, Epidemiology, Genetics, Prognosis, Korea
INTRODUCTION
Crohn's disease (CD) is a chronic relapsing inflammatory disease that can involve any part of the gastrointestinal tract. The etiopathogenesis of CD remains unclear. Patients with CD go through repeated periods of aggravation and improvement during the course of the disease and, many eventually require operations due to stricturing or penetrating complications [1,2].
Although higher incidences and prevalences of CD have been reported in Western countries, recent studies have shown gradually increasing incidences and prevalences in Asian countries, including Korea [3]. It is widely accepted that notable differences exist in terms of the epidemiology, genetics, and clinical characteristics of CD between Western countries and Eastern Asia [4,5]. However, most current guidelines for the diagnosis and treatment of CD were established based on Western data.
Thus, there is an ongoing need for research on the natural history, genetic susceptibility, and clinical characteristics of Korean CD patients to establish more suitable management strategies. Since the 1960s, when the first CD case series were reported [6], to the early 2000s, most data on Korean CD have come from small, single-center studies. In the mid-2000s, however, Korean guidelines for the diagnosis and treatment of CD were prepared at the urging of the Korean Association for the Study of Intestinal Diseases (KASID), and many large-scale, multi-center studies have provided valuable information regarding the characteristic features of Korean CD [3,7,8,9,10,11]. Recently, a nationwide hospital-based CD cohort was launched. It is anticipated that this will facilitate understanding of the natural history and prognosis of Korean CD in the near future [12]. In this review, we describe the current status and clinical characteristics of Korean CD based on recent domestic studies.
EPIDEMIOLOGY
Incidence and prevalence
Although there are regional differences, the incidence and prevalence rates of CD are increasing generally around the globe, with the highest rates in Europe and North America [13,14,15,16]. Interestingly, within the European continent, the West shows twice the annual incidence of the East [17].
According to a population-based epidemiological study carried out in the Songpa-Kangdong district of Seoul, the mean annual incidence rate of CD increased from 0.05 per 100,000 in 1986 to 1990 to 1.34 per 100,000 in 2001 to 2005 [3]. The adjusted prevalence rate of CD per 100,000 inhabitants was 11.24 (95% confidence interval, 9.29 to 13.18). The incidence rate of CD in this study was one-third that of ulcerative colitis (UC), but their ratio has decreased from 6.8 in 1986 to 1990 to 2.3 in 2001 to 2005, suggesting that the incidence of CD is increasing considerably more rapidly than that of UC. In an interesting study of the incidence of inflammatory bowel disease (IBD) among young males due for conscription in 2003 to 2008, the mean annual incidence of CD increased from 1.8 per 100,000 persons in 2003 to 2004, to 2.7 per 100,000 persons in 2005 to 2006, and to 5.1 per 100,000 persons in 2007 to 2008 [18]. This indicates that the incidence of CD is increasing rapidly in young Korean males.
Age at diagnosis
In a population-based study, the median age at diagnosis of Korean CD was 21.5 years (range, 9 to 64) [3]. A similar result was seen in a nationwide cohort study of 465 CD patients, in which the median age at diagnosis was 22.4 years (range, 10 to 65) [19]. As the median age at diagnosis of CD for Westerners is 31 to 39 years [15,16,20], the age at diagnosis of Korean CD seems to be lower. According to a recent inception cohort study by the European Crohn's and Colitis Organisation Epidemiology Committee (ECCO-EpiCom), the median age at diagnosis of adult CD in Western European centers was 34 years (range, 10 to 85), and 32 years (range, 15 to 89) in Eastern European centers [17]. However, the age at diagnosis of CD in Japan was reported to be 22 years [21], and 25 years in China [22]. Compared with UC, the age at diagnosis for CD is about 10 years earlier in both the West and the East.
Male-to-female ratio
Korean CD studies show a male predominance, as in other East Asian studies [22,23,24]. According to a population-based study, the incidence ratio between males and females was 2.8:1, and a more recent multicenter study and nationwide cohort studies reported ratios of 2.5:1 and 2.8:1, respectively [3,10,19]. This differs from Western findings, which show a general female predominance [20,25,26]. However, some studies from Western countries have shown a male predominance, especially in pediatric CD [15,16].
Time from symptoms to diagnosis
If proper treatment for CD is delayed due to late diagnosis, the risk of complications increases due to its progressive nature. In a Danish population-based study, the median time from symptom onset to diagnosis was 8.3 months (range, 0 months to 48 years) [20]. A Japanese study reported that the median time to diagnosis was 29.9 months and tended to be longer in ileitis-type than colitis- or ileocolitis-type disease [21]. According to the recent ECCO-EpiCom inception cohort study, the median time from symptoms to diagnosis was 3.4 months in Eastern European centers and 4.6 months in Western European centers, significantly shorter than reported previously [17]. This outcome is likely attributable to the development of diagnostic technology and rising awareness of the disease generally. In Korea, the median interval from symptoms to diagnosis was reported to be 12 months (range, 0.5 to 198) in a population-based study and 5 months (range, 1 to 126) in a more recent nationwide cohort study [3,19]. The trend is easily observed from a study that categorized CD patients chronologically into cohorts according to the year of diagnosis. The cohort in which the diagnosis was made in 1981 to 2000 showed a median time to diagnosis of 24 months (range, 0 to 287), whereas the cohort in which the diagnosis was made in 2006 to 2012 showed a median time of 14 months (range, 0 to 270), significantly shorter than the earlier cohort [27].
Family history
A positive family history is known to be a strong risk factor for the development of IBD. It has been reported that more than 10% of Western CD patients have a family history of IBD [15,20]. Previous Korean studies reported the frequency of family history in CD patients to be 1.4% to 2.9%, lower than Western levels [3,10,19,28].
ETIOLOGY
Although the cause of CD is largely unknown, it is assumed to involve the gut flora, together with numerous environmental factors interacting to induce a dysregulated immune response of the intestinal mucosa in genetically susceptible individuals [29,30]. Since NOD2 was identified as the first CD susceptibility gene in 2001, many studies have aimed to find susceptibility genes related to CD. To date, more than 140 CD susceptibility genes or loci have been identified through genome-wide association studies (GWAS) in Caucasians [31]. As with phenotypes, there seem to be considerable differences in genetic susceptibility to CD between Asians and Caucasians [4,32,33,34].
According to a genetic polymorphism study involving a large number of Northern European and Korean patients with CD and normal controls, the three disease-associated single-nucleotide polymorphisms (SNPs) of the CARD15 (NOD2) gene that are found frequently among European CD patients were not found in Korean CD patients. Subsequently, several other studies confirmed the absence of NOD2 gene mutations in Korean CD populations [33,35]. Many replicate studies have investigated the associations of genes found to be susceptibility loci for CD in Caucasians through GWAS in Korean CD patients. TNFSF15, IL-23R, and IRGM genetic variants, but not ATG16L1, have been confirmed to be associated with Korean CD [34,36,37,38]. A recent GWAS in a Korean population also found three novel risk loci for CD, including ATG16L2 [39]. Additionally, genetic variants of tumor necrosis factor (TNF) α, IL-12β, and IL-27 were reported to be associated with Korean CD [40,41,42]. It is important to investigate the effects of these susceptibility genes on the clinical course of CD because they may be helpful in predicting the clinical course and treatment responses of patients with certain genotypes and establishing treatment plans. In a study including 380 CD patients, SNP rs1004819 and rs1495965 of IL-23R were shown to have associations with stricturing and penetrating behaviors [34]. In another study, the SNP rs6478108 CC genotype, among five SNPs of TNFSF15, had an association with stricture and nonperianal penetrating complications and the SNP rs4574921 CC genotype was found to be related to perianal fistula [43]. Further studies that aim to identify additional susceptibility genes or loci and determine their effects on the clinical course of Korean CD are needed.
CLINICAL FEATURES
Presenting symptoms
Common symptoms at the time of diagnosis were abdominal pain (84.9%), weight loss (69.8%), and diarrhea (49.3%) from a study analyzing 278 CD patients [44]. According to a nationwide cohort study, similar symptoms were reported; abdominal pain (64.5%), diarrhea (60.9%), and weight loss (32.7%). Hematochezia and febrile sensations were also commonly reported symptoms, and 35% to 43% of patients had perianal symptoms, such as fistula or abscess, at the time of diagnosis.
Disease location and behavior
CD shows different clinical manifestations and responses to therapy according to its location and behavior type. The Montreal classification defines L1 to have lesions in the terminal ileum, L2 in the colon, and L3 in the ileocolon [45]. Regarding disease behavior, B1 indicates nonstricturing and nonpenetrating, B2 indicates stricturing, and B3 indicates penetrating behaviors. In Korean CD, L3 is the most common (53% to 71%), followed by L1 (21% to 32%) and L2 (7% to 14%) [3,10,19]. A recent Chinese study showed a similar result (L1, 24%; L2, 6%; L3, 71%) [24]. In contrast, L2 (30% to 40%) seems to be the most common form in Western studies, supporting the view that Western and the Eastern Asian diseases occur in different anatomical locations [15,17,20]. According to disease behavior, 60% to 77% of Korean CD is categorized as B1, 10% to 25% as B2, and 12% to 30% as B3 at the time of diagnosis [10,19,27,44].
The risk of CD complications, such as stricture and fistula, increases gradually as the disease progresses. In a study of 2,043 CD patients, 77.9% were categorized as B1, 10.1% as B2, and 12% as B3 at the time of diagnosis. After 5 years, the percentages were 54.4% as B1, 17.4% as B2, and 28.2% as B3; and 20 years later, 13.4% as B1, 15.4% as B2, and 71.2% as B3. Thus the percentages of the B2 and B3 phenotypes increased markedly [27]. In contrast, change in disease location was uncommon; during follow-up, 31 patients changed from L1 to L3, and 36 patients from L2 to L3.
The cumulative frequency of perianal fistula was reported to be 40.7% after 1 year, 46.1% after 5 years, 49.7% after 10 years, and 54.3% after 5 years when including perianal fistula that resolved before diagnosis of CD [44]. This differs from Western data, in which the cumulative frequency of perianal fistula is 13% to 38% [46].
Disease activity
The most widely used index to evaluate disease activity is the Crohn's disease activity index (CDAI), which was developed by the National Cooperative Crohn's Disease Study in 1976. In a large single-center study, 11.5% of patients were in remission at diagnosis (CDAI < 150), 21.6% had mild (150 to 220), 58.6% moderate (220 to 450), and 8.3% severe activity (≥ 450) [44].
DIAGNOSIS
Generally, CD is diagnosed from combined clinical, endoscopic, laboratory, radiological, pathological, and/or operative findings [7]. In some cases, however, it is difficult to differentiate CD from acute infectious enterocolitis, irritable bowel syndrome, UC, intestinal tuberculosis (ITB), or intestinal Behcet's disease (BD). The prevalence of ITB and intestinal BD in Korea are considerably higher than in Western countries, so the differential diagnosis is important. Endoscopy is now the fundamental tool for differentiation, along with several serological markers.
Differential diagnosis of CD and UC
Although CD and UC have distinct clinical, endoscopic, radiological, and pathological findings, there are occasions in which differentiating the two is difficult. In a nationwide cohort study of 342 CD patients, 20 patients (4.3%) were previously misdiagnosed as UC [19].
ASCA and perinuclear antineutrophil cytoplasmic antibody
Anti-Saccharomyces cerevisiae antibody (ASCA) and perinuclear antineutrophil cytoplasmic antibody (pANCA) are helpful serological markers that can be used adjunctively in differentiating CD and UC. It has been reported that 35% to 60% of CD patients are positive for ASCA, whereas only 1% to 11% of UC patients are positive [47,48]. However, pANCA is commonly positive in UC patients. Korean studies have reported that 38.3% to 49.4% of CD patients are positive for ASCA and 44.2% of UC patients are positive for pANCA [49,50]. For CD, the sensitivity and specificity of diagnosis were 48% and 87% for the combination of positive ASCA and negative pANCA. For UC, the sensitivity and specificity were 36.4% and 97.6% for the combination of positive pANCA and negative ASCA [49]. Furthermore, ASCA is associated with the clinical course of CD. Compared with patients negative for ASCA, patients positive for ASCA tend to show B2/B3 disease behavior, have higher Harvey-Bradshaw index scores, use more steroids and immunosuppressive drugs, and are admitted more frequently [50].
Differential diagnosis of CD and ITB
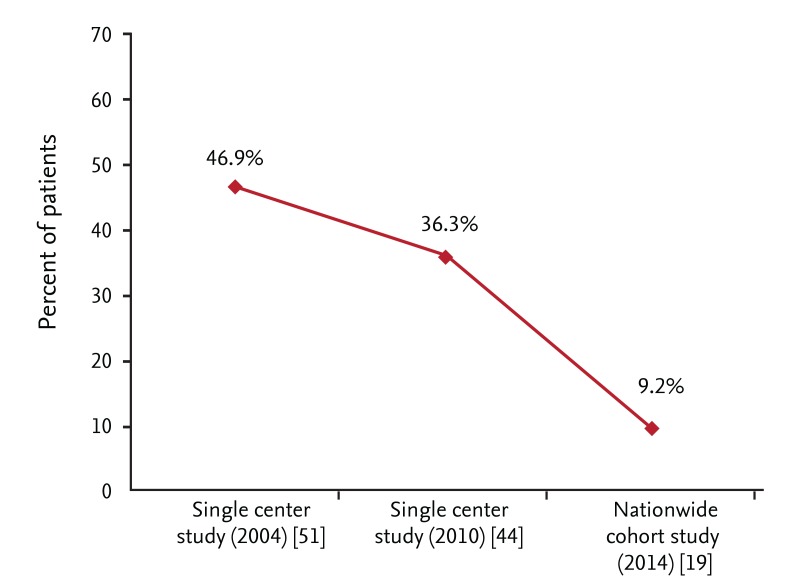
The differential diagnosis between ITB and CD is challenging in Korea. According to a single-center study, almost 50% of patients received anti-TB medication prior to a CD diagnosis [51]. However, the proportion has decreased markedly during last decade, probably due to greater clinical experience among physicians (Fig. 1) [19,44].
Figure 1.
Change in proportion of patients who received anti-tuberculosis (anti-TB) medication prior to Crohn's disease (CD) diagnosis. Proportion of patients who received anti-TB medication prior to CD diagnosis has decreased markedly during last decade.
Endoscopy
The characteristic endoscopic findings of CD and ITB are well known. For example, longitudinal ulcers are usually present in CD, whereas transverse ulcers are commonly present in ITB. However, the differential diagnosis becomes obscure in many clinical situations. An interesting study assessed the diagnostic value of various endoscopic findings in differentiating the two diseases [52]. Four parameters (anorectal lesions, longitudinal ulcers, aphthous ulcers, and cobblestone appearance) were prevalent in CD. In ITB, four other parameters (involvement of fewer than four segments, a patulous ileocecal valve, transverse ulcers, and scars or pseudopolyps) were prevalent. A score system was devised in which +1 point was assigned for an endoscopic finding characteristic of CD and -1 for an endoscopic finding indicative of ITB. If the sum is a positive value, the diagnosis was CD and if the sum is negative, it was ITB. Using this system, 87.5% of cases were correctly diagnosed, 8% misdiagnosed, and 4.5% remained unclear. However, this scoring system (Yang's criteria) has not yet been validated.
Interferon-γ releasing assay and ASCA
In addition to endoscopy, an interferon-γ assay (QuantiFERON-TB gold test [QFT], Cellestis Limited, Carnegie, Australia) and ASCA are helpful in the differential diagnosis of CD and ITB. In multi-center studies, ASCA was positive in 44.4% of CD patients, 13.3% of ITB patients, and 15% of healthy controls. Additionally, QFT was positive in 9% of CD patients and 66.6% of ITB patients. In cases that were ASCA-positive and QFT-negative, the sensitivity, specificity, positive predictive value, and negative predictive value for a diagnosis of CD were 44.4%, 96.0%, 91.4%, and 64.3%, respectively [53]. In another KASID multi-center study, QFT showed a sensitivity of 67%, a specificity of 90%, a positive predictive value of 87%, and a negative predictive value of 73% for ITB [54].
Differential diagnosis of CD and intestinal BD
A Korean study that used a classification and regression tree model based on endoscopic parameters (such as ulcer shape, distribution, number, margin, and border contour, the presence of aphthous ulcer, cobblestone, perianal, and strictured lesion) reported a sensitivity of 94.3%, a specificity of 90%, a positive predictive value of 94.7%, and a negative predictive value of 89.2% [55]. In this study, round ulcers or irregular/geographic-shaped ulcers with focal distributions were suggestive of intestinal BD.
Small bowel evaluation of CD
Evaluating small bowel involvement in CD in the clinical situation is challenging. Currently, "small bowel follow-through" (SBFT) is the primary diagnostic tool for diagnosing small-bowel CD. However, the efficacy of computed tomography enterography (CTE) or magnetic resonance enterography (MRE) is comparable to that of SBFT, so increased use of CTE and/or MRE is anticipated in the near future. A prospective Korean study reported that SBFT, CTE, and MRE appear to be equally accurate in identifying active inflammation in the small bowel [56].
Despite the efficacy of SBFT and CTE, the risk of radiation exposure is a major concern for patients who undergo these tests repeatedly. According to a multi-center study on radiation exposure (cumulative effective dose > 50 mSv) in IBD patients, MRE was reported as a radiation-free alternative and an effective exam for those who are young and require examinations on a repeated basis [57].
TREATMENT
The conventional treatment strategy for CD is a step-up approach. After the administration of first-line drugs, which are less effective but safer, more potent drugs are added, if unresponsive to first-line drugs. However, many patients during the course of disease progression suffer complications even with proper treatments, and eventually, undergo surgical treatment [1,2]. Systemic corticosteroids are effective in short-term use but show disappointing long-term results; only one-third of patients show prolonged responses after 1 year, 28% show steroid dependency, and 38% undergo surgery [58]. Additionally, although the use of immunomodulators has increased, the percentage of patients who undergo colectomies has not changed markedly over the past 25 years [59]. Thus, an accelerated step-up approach, in which immunomodulators or biologic agents are introduced at an early stage of treatment, is the current accepted practice. Furthermore, the concept of tailored therapy is emerging, in which patients with high risk factors and who are expected to have a poor prognosis are screened and treated more intensively at an early stage [60,61,62].
Medical management
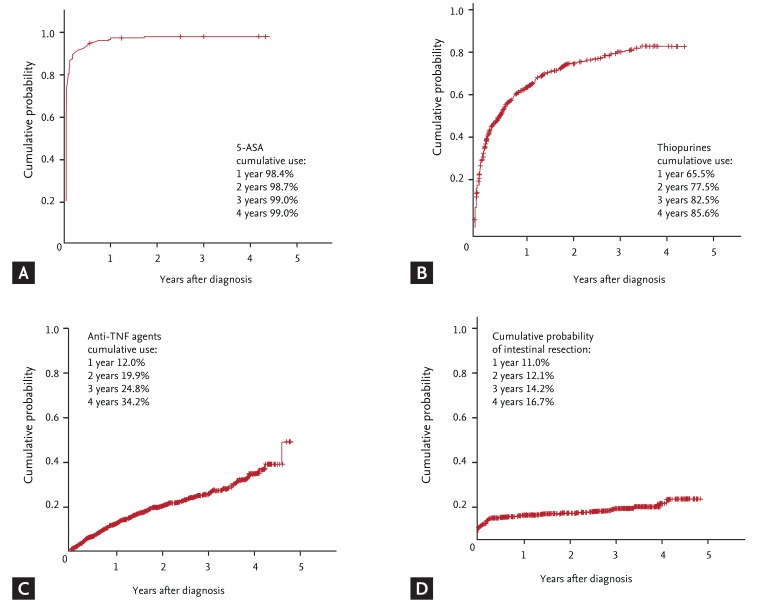
Several large-scale studies have been conducted to assess the medical management of Korean CD. The KASID multicenter study of 728 CD patients reported that 98.1% of patients were prescribed 5-aminosalicylate (5-ASA), and 59.5% and 18.0% were prescribed oral or intravenous corticosteroids, respectively. Thiopurine drugs were used in 65.0%, and infliximab was used in 26.9% of patients [10]. Another large single-center study showed that systemic corticosteroids were administered to 61.2% of patients with CD at diagnosis and/or during follow-up. The cumulative probabilities of corticosteroid treatment at 1, 5, 10, 20, and 25 years after diagnosis were 44.2%, 58.7%, 69.8%, 80.3%, and 82.3%, respectively [27]. A nationwide cohort study showed that the cumulative use rates of thiopurines and anti-TNF agents at 1, 2, and 3 years after diagnosis were 65.5%, 77.5%, and 82.5%, and 12.0%, 19.9%, and 24.8%, respectively (Fig. 2) [19]. Overall, Korean physicians prescribe 5-ASA to most of their patients regardless of questions as to its efficacy. Approximately 80% of patients have experienced use of corticosteroids or immunomodulators, and 25% of patients received anti-TNF therapy.
Figure 2.
Cumulative probability of medication use and surgery in Korean patients with Crohn disease. (A) 5-aminosalicylates (5-ASA), (B) thiopurines, (C) anti-tumor necrosis factor (anti-TNF) agents, and (D) intestinal resection. Adapted from Ye et al. [19] 9th Congress of European Crohn's and Colitis Organization.
Corticosteroids are a first-line therapy for active CD. In a single-center study of 96 CD patients who had received oral corticosteroids as induction therapy, 89.5% of patients showed responses (complete remission 38.5%, partial remission 51.0%) to therapy. At 4 months and 1 year after treatment, 69.5% and 56.6% of patients showed prolonged responses, 23.2% and 24.1% steroid dependency, and 7.4% and 19.3% were steroid-refractory, respectively [63]. That is, although the short-term response rate to oral corticosteroid therapy in Korean CD was very high, as seen in Western studies, the responsiveness decreased with time. In this study, the B1 (inflammatory) phenotype and lower CDAI were predictors of a response.
It has been reported that immunomodulators, such as azathiopurine (AZA) and 6-mercaptopurine (6-MP), are effective for maintenance therapy of CD, and current treatment strategies recommend adopting immunomodulators at an earlier stage. A retrospective single-center study of 168 CD patients showed that clinical remission and corticosteroid-free remission rates were significantly higher in the early immunomodulator group, in which immunomodulators were administered within 6 months of diagnosis, compared with a 'conventional' therapy group. However, there was no difference in the disease relapse rate between the two groups [64]. Also, drug-related adverse events were more frequent in the early immunomodulator group. Thus, it is important to be aware of adverse events when immunomodulators are to be administered.
Myelotoxicity induced by treatment with thiopurines, such as AZA or 6-MP, has been reported to be more frequent in Koreans than Westerners [65]. Gene mutations in thiopurinemethyltransferase (TPMT) are known to be related to such myelotoxicity. From a Korean multicenter study, 2.4% of patients were detected with the *1/*3C mutation and the patients with heterozygous *3C-type TPMT had a higher probability of leukopenia than those with wild-type TPMT. Additionally, patients with intermediate TPMT activity had a lower probability of leukopenia than those with lower activity [66]. In clinical practice, however, TPMT genotyping or measuring TPMT activity are not commonly carried out due to the low frequency of the TPMT mutation and nonavailability of the test in Korea.
The adoption of anti-TNF agents since the late 1990s for steroid- and/or thiopurine-refractory CD has altered the CD treatment paradigm. Currently, two anti-TNF agents, infliximab and adalimumab, are available in clinical practice. A study of 40 refractory luminal and fistulizing CD patients revealed that the clinical response rate at 10 weeks after starting infliximab was 85%. The response rate was higher in luminal CD than fistulizing CD (96.2% vs. 72.4%) [67]. In another study of 80 CD patients who were observed for a mean follow-up period of 33.7 months, the clinical response rate after 10 weeks of infliximab was 96%, and there was no marked difference in response rates between luminal and fistulizing CD. Among them, 76.6% of the patients showed sustained clinical responses, until the end of the follow-up period [68]. In both studies, ~20% reported adverse events; most were minor side-effects, such as infusion reactions; 5% of patients experienced severe adverse events, such as liver abscess, herpes zoster, and pulmonary TB. A multi-center study by KASID from 29 referral centers with 317 CD patients demonstrated that the clinical response and remission rates after 2 weeks of infliximab in luminal CD were 71.8% and 29.2% and the rates after 14 weeks of infliximab in fistulizing CD were 85% and 56.2%, respectively. The response was maintained even at week 30, until week 54, in patients who showed responses to induction therapy [69]. Although 12.3% of the patients experienced adverse events, only 6% experienced severe adverse events. Although each study was designed differently in terms of the time at which the treatment response was evaluated or length of follow-up period, infliximab has been shown to be effective and safe for induction and maintenance therapy of refractory Korean CD in real-life settings.
Because the use of anti-TNF agents for various immune-mediated inflammatory diseases (IMIDs) is generally increasing, the reactivation of latent TB infection (LTBI) has become an important issue in Korea, where TB is endemic. In a single-center study of 101 IMID patients (including 55 CD patients) who have a history of anti-TNF therapy in the past due to TB infection, active TB was reported in one patient, 6 years after the therapy, with a median follow-up period of 31.5 months. TB was not reported in patients who received LTBI treatment prior to anti-TNF therapy [70]. According to a recent KASID multi-center study, among 873 IBD patients (CD 643, UC 230) who received anti-TNF therapy, TB occurred in 25 (2.9%). This result showed that anti-TNF therapy increased the risk of TB by 41.7-fold compared with the general Korean population [71]. Thus, meticulous care, such as LTBI screening and TB prophylaxis, must be considered prior to administration of anti-TNF therapy.
Surgical management
Although it is not curative for CD, surgery should be an option to improve the patient's quality of life, the ultimate goal of treatment [72,73]. Surgery should not be considered as a final resort or a failure of medical treatment. Indications for surgery include intestinal perforation, uncontrolled bleeding, recurrent stricture, dysplasia or tumor, and inadequate response to therapy due to refractoriness to, or side effects of, drugs [59,74]. To date, few Korean studies of the effects of surgical treatment of CD have been conducted.
For active CD with intraabdominal abscess, surgical treatment has become the conventional therapeutic modality [75,76]. Percutaneous drainage currently acts as a bridging therapy or alternative to surgical measures. In a single-center retrospective study, there was no significant difference in overall treatment response rates (100% vs. 87.9%) or recurrence rates (27.8% vs. 30.8%) between a surgical treatment group and an antibiotic group (with or without percutaneous drainage) for CD-related intraabdominal abscesses [77]. In another study, the overall success rate of nonsurgical treatment was reported to be 66.7% [78]. Thus, nonsurgical treatment represents an initial treatment for CD-related intraabdominal abscesses.
Treatment of fistulizing CD
An asymptomatic and simple perianal fistula does not require special treatment; however, if symptomatic, a noncutting seton or fistulectomy may be an option. Antibiotics are used at first as a medical therapeutic option, thiopurines as the second option, and infliximab as the third. In a complex perianal fistula, antibiotics and thiopurines together with surgical measures are considered the first choice for therapy and anti-TNF therapy as a second-line treatment [8,59].
In a clinical study of 94 CD patients with perianal lesions, the remission rate with specific medical treatments, such as 5-ASA, thiopurine, and metronidazole, was 77%, higher than that of nonspecific treatments and surgery [79]. It was suggested that perianal lesions should be managed initially with medical treatments. Anti-TNF agents are effective in inducing remission and the maintenance of fistulizing CD that is refractory to conventional medical treatments [80,81,82]. These agents reduce rates of hospitalization and operations. In various Korean clinical studies, infliximab has been shown to be a safe and effective agent for fistulizing CD [63,64,65].
Despite their efficacy, half of all patients treated with anti-TNF agents experience nonresponsiveness or recurrence of the fistula. Recently, an open-label, phase II, multicenter study demonstrated the efficacy of autologous adipose tissue-derived stem cells (ASCs) treatment of CD fistulas [83]. In this study, 82% of 33 patients showed complete fistula healing after 8 weeks of treatment. Among them, 88% showed sustained complete closure at 1 year. ASCs treatment is expected to become a safe, effective remedy for CD fistula.
PROGNOSIS AND PREDICTORS OF OUTCOMES
In a single-center study of 113 newly diagnosed CD patients, the cumulative remission rate was 75.5% at 1 year, 80.7% at 3 years, and 85.5% at 5 years after diagnosis [51]. The remission rate was higher in cases without diarrhea and older patients (≥ 30 years). In this study, the cumulative relapse rate was 30.7% at 1 year, 53.0% at 3 years, and 73.4% at 5 years after remission. The recurrence rate was higher in patients group with higher CDAI scores at the time of diagnosis and when remission was achieved by medical as opposed to surgical treatment.
In a study of 2,043 CD patients, at the same institution, 35.5% of patients underwent intestinal resection during the median follow-up of 80 months. The cumulative probabilities of intestinal resection at 5, 10, 20, and 30 years after diagnosis were 28.9%, 43.5%, 70.0%, and 76.1%, respectively. Early use of thiopurines, stricturing/penetrating disease behaviors, and ileal involvement were significantly associated with delayed need for intestinal resection [27]. The reported surgery rate was similar to that of Western data. However, caution is required in interpreting these results because they were derived from a referral center-based cohort that may include more severe patients. From the KASID multicenter study, the cumulative operation rates at 5, 7, and 10 years after diagnosis were 15.0%, 20.0%, and 35.3%, respectively, conspicuously lower than in the above-mentioned single-center study [10]. In this study, smoking habits and penetrating disease behavior were independent predictors of surgery. However, in a nationwide cohort study, the cumulative probabilities of intestinal resection at 1, 2, 3, and 4 years after diagnosis were 11.0%, 12.1%, 14.2%, and 16.7%, respectively [18]. In the near future, a better understanding of the surgery rate in Korean CD will be possible when longer-term follow-up data become available.
Because surgery is not a curative treatment for CD, postoperative recurrence is common and many patients undergo reoperations. It has been reported that 18.3% of the patients who undergo a first intestinal resection needed a second resection, and the cumulative probabilities of reoperation were 2.9% after 1 year, 19.9% after 5 years, and 30.8% after 10 years [44]. In a multi-ecenter study of 708 patients who had undergone abdominal surgery, young age (< 16 years), stricturing behavior, intra-abdominal abscesses, and history of emergency surgery were found to be possible risk factors for repeated abdominal surgery [84]. In CD, ~70% of patients undergo postoperative endoscopic recurrence (PER) after 1 year of intestinal resection. PER is also a known risk factor for subsequent clinical recurrence. Thus, colonoscopic monitoring and proper prophylactic therapy are recommended to prevent PER [85,86]. According to a recent retrospective study, the cumulative PER rates at 6, 12, and 24 months were 33.3%, 42.9%, and 66.1%, respectively, comparable with Western results [87].
CONCLUSIONS
During recent decades, the incidence and prevalence rates of CD have increased dramatically in Korea. There are some differences in the clinical and genetic characteristics of CD between Western countries and Korea. Unlike Westerners, Korean patients with CD show a male predominance and a lower proportion of isolated colonic disease. Perianal lesions are more prevalent than in Western countries. A GWAS confirmed that genetic variants in TNFSF15, IL-23R, and IRGM, but not ATG16L1, are associated with CD susceptibility in Korean populations. Previously, the clinical course of CD in Korea was thought to be milder than that in Western countries; however, recent studies have shown comparable rates of intestinal resection in Korean CD patients to those of Westerners. A nationwide, hospital-based cohort study was recently launched by KASID. We anticipate that much information regarding the typical natural history and genetic causes of Korean CD will be gleaned from this study.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 4.Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:1164–1176. doi: 10.1002/ibd.21845. [DOI] [PubMed] [Google Scholar]
- 5.Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010;4:1–14. doi: 10.5009/gnl.2010.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KY, Suh SH, Chang MW, Min KS. Crohn's disease: case report and article review. J Korean Surg Soc. 1964;6:121–131. [Google Scholar]
- 7.Ye BD, Jang BI, Jeen YT, et al. Diagnostic guideline of Crohn's disease. Korean J Gastroenterol. 2009;53:161–176. [PubMed] [Google Scholar]
- 8.Ye BD, Yang SK, Shin SJ, et al. Guidelines for the management of Crohn's disease. Intest Res. 2012;10:26–66. [Google Scholar]
- 9.Lee CK, Kim HS, Ye BD, et al. Patients with Crohn's disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis. 2014;8:384–391. doi: 10.1016/j.crohns.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Moon CM, Park DI, Kim ER, et al. Clinical features and predictors of clinical outcomes in Korean patients with Crohn's disease: a Korean association for the study of intestinal diseases multicenter study. J Gastroenterol Hepatol. 2014;29:74–82. doi: 10.1111/jgh.12369. [DOI] [PubMed] [Google Scholar]
- 11.Bae JH, Park J, Yang KM, Kim TO, Yi JM IBD Study Group of Korean Association for Study of Intestinal Diseases (KASID) Detection of DNA hypermethylation in sera of patients with Crohn's disease. Mol Med Rep. 2014;9:725–729. doi: 10.3892/mmr.2013.1840. [DOI] [PubMed] [Google Scholar]
- 12.Cheon JH, Kim YS, Ye BD, et al. Crohn's Disease Clinical Network and Cohort (CONNECT) study: the first step toward nationwide multicenter research of Crohn's disease in Korea. Intest Res. 2014;12:173–175. doi: 10.5217/ir.2014.12.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakatos L, Kiss LS, David G, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011;17:2558–2565. doi: 10.1002/ibd.21607. [DOI] [PubMed] [Google Scholar]
- 16.Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn's disease in Quebec, Canada. Inflamm Bowel Dis. 2009;15:429–435. doi: 10.1002/ibd.20756. [DOI] [PubMed] [Google Scholar]
- 17.Burisch J, Pedersen N, Cukovic-Cavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588–597. doi: 10.1136/gutjnl-2013-304636. [DOI] [PubMed] [Google Scholar]
- 18.Shin DH, Sinn DH, Kim YH, et al. Increasing incidence of inflammatory bowel disease among young men in Korea between 2003 and 2008. Dig Dis Sci. 2011;56:1154–1159. doi: 10.1007/s10620-010-1403-2. [DOI] [PubMed] [Google Scholar]
- 19.Ye BD, Han DS, Youn EJ, et al. The clinical features and prognosis of Crohn's disease: a Korean multicenter nationwide cohort study; 9th Congress of European Crohn's and Colitis Organization; 2014 Feb 20-22; Copenhagen. Vienna: ECCO; 2014. p. 591. [Google Scholar]
- 20.Vind I, Riis L, Jess T, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 21.Oriuchi T, Hiwatashi N, Kinouchi Y, et al. Clinical course and longterm prognosis of Japanese patients with Crohn's disease: predictive factors, rates of operation, and mortality. J Gastroenterol. 2003;38:942–953. doi: 10.1007/s00535-003-1177-9. [DOI] [PubMed] [Google Scholar]
- 22.Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn's disease in Japan. J Gastroenterol. 2009;44:659–665. doi: 10.1007/s00535-009-0057-3. [DOI] [PubMed] [Google Scholar]
- 23.Chuang CH, Lin SH, Chen CY, Sheu BS, Kao AW, Wang JD. Increasing incidence and lifetime risk of inflammatory bowel disease in Taiwan: a nationwide study in a low-endemic area 1998-2010. Inflamm Bowel Dis. 2013;19:2815–2819. doi: 10.1097/01.MIB.0000435436.99612.27. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28:1148–1153. doi: 10.1111/jgh.12164. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 26.Gearry RB, Richardson A, Frampton CM, et al. High incidence of Crohn's disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm Bowel Dis. 2006;12:936–943. doi: 10.1097/01.mib.0000231572.88806.b9. [DOI] [PubMed] [Google Scholar]
- 27.Park SH, Yang SK, Park SK, et al. Long-term prognosis of Crohn's disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014;20:488–494. doi: 10.1097/01.MIB.0000441203.56196.46. [DOI] [PubMed] [Google Scholar]
- 28.Park JB, Yang SK, Byeon JS, et al. Familial occurrence of inflammatory bowel disease in Korea. Inflamm Bowel Dis. 2006;12:1146–1151. doi: 10.1097/01.mib.0000235094.01608.59. [DOI] [PubMed] [Google Scholar]
- 29.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 30.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JZ, Anderson CA. Genetic studies of Crohn's disease: past, present and future. Best Pract Res Clin Gastroenterol. 2014;28:373–386. doi: 10.1016/j.bpg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croucher PJ, Mascheretti S, Hampe J, et al. Haplotype structure and association to Crohn's disease of CARD15 mutations in two ethnically divergent populations. Eur J Hum Genet. 2003;11:6–16. doi: 10.1038/sj.ejhg.5200897. [DOI] [PubMed] [Google Scholar]
- 33.Lee GH, Kim CG, Kim JS, Jung HC, Song IS. Frequency analysis of NOD2 gene mutations in Korean patients with Crohn's disease. Korean J Gastroenterol. 2005;45:162–168. [PubMed] [Google Scholar]
- 34.Yang SK, Park M, Lim J, et al. Contribution of IL23R but not ATG16L1 to Crohn's disease susceptibility in Koreans. Inflamm Bowel Dis. 2009;15:1385–1390. doi: 10.1002/ibd.20921. [DOI] [PubMed] [Google Scholar]
- 35.Jang JY, Song SM, Kim KM, Oh SH, Lee YJ, Rhee KW. Lack of common NOD2 mutations in Korean pediatric patients with inflammatory bowel disease. Pediatr Int. 2010;52:888–889. doi: 10.1111/j.1442-200X.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang SK, Lim J, Chang HS, et al. Association of TNFSF15 with Crohn's disease in Koreans. Am J Gastroenterol. 2008;103:1437–1442. doi: 10.1111/j.1572-0241.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim SW, Kim ES, Moon CM, et al. Genetic polymorphisms of IL-23R and IL-17A and novel insights into their associations with inflammatory bowel disease. Gut. 2011;60:1527–1536. doi: 10.1136/gut.2011.238477. [DOI] [PubMed] [Google Scholar]
- 38.Moon CM, Shin DJ, Kim SW, et al. Associations between genetic variants in the IRGM gene and inflammatory bowel diseases in the Korean population. Inflamm Bowel Dis. 2013;19:106–114. doi: 10.1002/ibd.22972. [DOI] [PubMed] [Google Scholar]
- 39.Yang SK, Hong M, Zhao W, et al. Genome-wide association study of Crohn's disease in Koreans revealed three new susceptibility loci and common attributes of genetic susceptibility across ethnic populations. Gut. 2014;63:80–87. doi: 10.1136/gutjnl-2013-305193. [DOI] [PubMed] [Google Scholar]
- 40.Kim TH, Kim BG, Shin HD, et al. Tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in Korean patients with inflammatory bowel disease. Korean J Gastroenterol. 2003;42:377–386. [PubMed] [Google Scholar]
- 41.Yang SK, Lee SG, Cho YK, Lim J, Lee I, Song K. Association of TNF-alpha/LTA polymorphisms with Crohn's disease in Koreans. Cytokine. 2006;35:13–20. doi: 10.1016/j.cyto.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Li CS, Zhang Q, Lee KJ, et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol. 2009;24:1692–1696. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang DH, Yang SK, Song K, et al. TNFSF15 is an independent predictor for the development of Crohn's disease-related complications in Koreans. J Crohns Colitis. 2014 May 13; doi: 10.1016/j.crohns.2014.04.002. [Epub]. http://dx.doi.org/10.1016/j.crohns.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Ye BD, Yang SK, Cho YK, et al. Clinical features and long-term prognosis of Crohn's disease in Korea. Scand J Gastroenterol. 2010;45:1178–1185. doi: 10.3109/00365521.2010.497936. [DOI] [PubMed] [Google Scholar]
- 45.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Gastroenterological Association Clinical Practice Committee. American Gastroenterological Association medical position statement: perianal Crohn's disease. Gastroenterology. 2003;125:1503–1507. doi: 10.1016/j.gastro.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 47.Canani RB, Romano MT, Greco L, et al. Effects of disease activity on anti-Saccharomyces cerevisiae antibodies: implications for diagnosis and follow-up of children with Crohn's disease. Inflamm Bowel Dis. 2004;10:234–239. doi: 10.1097/00054725-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Kim BG, Kim YS, Kim JS, Jung HC, Song IS. Diagnostic role of anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic antibodies in patients with inflammatory bowel disease. Dis Colon Rectum. 2002;45:1062–1069. doi: 10.1007/s10350-004-6361-3. [DOI] [PubMed] [Google Scholar]
- 50.Kim BC, Park S, Han J, Kim JH, Kim TI, Kim WH. Clinical significance of anti-Saccharomyces cerevisiae antibody (ASCA) in Korean patients with Crohn's disease and its relationship to the disease clinical course. Dig Liver Dis. 2007;39:610–616. doi: 10.1016/j.dld.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Park JB, Yang SK, Myung SJ, et al. Clinical characteristics at diagnosis and course of Korean patients with Crohn's disease. Korean J Gastroenterol. 2004;43:8–17. [PubMed] [Google Scholar]
- 52.Lee YJ, Yang SK, Byeon JS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn's disease. Endoscopy. 2006;38:592–597. doi: 10.1055/s-2006-924996. [DOI] [PubMed] [Google Scholar]
- 53.Kim YS, Kim YH, Kim WH, et al. Diagnostic utility of anti-Saccharomyces cerevisiae antibody (ASCA) and interferon-gamma assay in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Clin Chim Acta. 2011;412:1527–1532. doi: 10.1016/j.cca.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 54.Kim BJ, Choi YS, Jang BI, et al. Prospective evaluation of the clinical utility of interferon-gamma assay in the differential diagnosis of intestinal tuberculosis and Crohn's disease. Inflamm Bowel Dis. 2011;17:1308–1313. doi: 10.1002/ibd.21490. [DOI] [PubMed] [Google Scholar]
- 55.Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behcet's disease and Crohn's disease by colonoscopic findings. Endoscopy. 2009;41:9–16. doi: 10.1055/s-0028-1103481. [DOI] [PubMed] [Google Scholar]
- 56.Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 57.Jung YS, Park DI, Kim ER, et al. Quantifying exposure to diagnostic radiation and factors associated with exposure to high levels of radiation in Korean patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1852–1857. doi: 10.1097/MIB.0b013e31828c844f. [DOI] [PubMed] [Google Scholar]
- 58.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 59.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panaccione R, Rutgeerts P, Sandborn WJ, Feagan B, Schreiber S, Ghosh S. Review article: treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:674–688. doi: 10.1111/j.1365-2036.2008.03753.x. [DOI] [PubMed] [Google Scholar]
- 61.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Antunes O, Filippi J, Hebuterne X, Peyrin-Biroulet L. Treatment algorithms in Crohn's: up, down or something else? Best Pract Res Clin Gastroenterol. 2014;28:473–483. doi: 10.1016/j.bpg.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Kim DH, Cheon JH, Park JJ, et al. Clinical outcomes and predictive factors for response after the first course of corticosteroid therapy in patients with Crohn's disease. Gut Liver. 2013;7:58–65. doi: 10.5009/gnl.2013.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwak MS, Kim DH, Park SJ, et al. Efficacy of early immunomodulator therapy on the outcomes of Crohn's disease. BMC Gastroenterol. 2014;14:85. doi: 10.1186/1471-230X-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Cheon JH, Kim WH. The frequency and the course of the adverse effects of azathioprine/6-mercaptopurine treatment in patients with inflammatory bowel disease. Korean J Gastroenterol. 2008;51:291–297. [PubMed] [Google Scholar]
- 66.Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010;44:e242–e248. doi: 10.1097/MCG.0b013e3181d6baf5. [DOI] [PubMed] [Google Scholar]
- 67.Kim SH, Yang S, Kim KJ, et al. Efficacy of infliximab in the treatment of Korean patients with Crohn's disease. Korean J Gastroenterol. 2009;54:108–116. doi: 10.4166/kjg.2009.54.2.108. [DOI] [PubMed] [Google Scholar]
- 68.Kim YJ, Kim JW, Lee CK, et al. Clinical outcome of treatment with infliximab in Crohn's disease: a single-center experience. Korean J Gastroenterol. 2013;61:270–278. doi: 10.4166/kjg.2013.61.5.270. [DOI] [PubMed] [Google Scholar]
- 69.Choi CH, Song ID, Cha BK, Chang SK, Kim YH, Kim WH. The efficacy and safety of infliximab therapy in Korean patients with Crohn's diseases: a retrospective, multicenter study. Gastroenterology. 2012;142(5 Suppl 1):S353. [Google Scholar]
- 70.Jo KW, Hong Y, Jung YJ, et al. Incidence of tuberculosis among anti-tumor necrosis factor users in patients with a previous history of tuberculosis. Respir Med. 2013;107:1797–1802. doi: 10.1016/j.rmed.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Byun JM, Lee CK, Rhee SY, et al. Risk of tuberculosis in inflammatory bowel disease patients treated with tumor necrosis factor-a blockers: a multicenter study in Korea; Oral presented at: The 2nd Annual Meeting of Asian Organization for Crohn's & Colitis; 2014 Jun 19-21; Seoul, Korea. [Google Scholar]
- 72.McLeod RS. Surgery for inflammatory bowel diseases. Dig Dis. 2003;21:168–179. doi: 10.1159/000073248. [DOI] [PubMed] [Google Scholar]
- 73.Thirlby RC, Land JC, Fenster LF, Lonborg R. Effect of surgery on health-related quality of life in patients with inflammatory bowel disease: a prospective study. Arch Surg. 1998;133:826–832. doi: 10.1001/archsurg.133.8.826. [DOI] [PubMed] [Google Scholar]
- 74.Lichtenstein GR, Hanauer SB, Sandborn WJ Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 75.Garcia JC, Persky SE, Bonis PA, Topazian M. Abscesses in Crohn's disease: outcome of medical versus surgical treatment. J Clin Gastroenterol. 2001;32:409–412. doi: 10.1097/00004836-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Gervais DA, Hahn PF, O'Neill MJ, Mueller PR. Percutaneous abscess drainage in Crohn disease: technical success and short- and long-term outcomes during 14 years. Radiology. 2002;222:645–651. doi: 10.1148/radiol.2223010554. [DOI] [PubMed] [Google Scholar]
- 77.Kim DH, Cheon JH, Moon CM, et al. Clinical efficacy of nonsurgical treatment of Crohn's disease-related intraabdominal abscess. Korean J Gastroenterol. 2009;53:29–35. [PubMed] [Google Scholar]
- 78.Lee H, Kim YH, Kim JH, et al. Nonsurgical treatment of abdominal or pelvic abscess in consecutive patients with Crohn's disease. Dig Liver Dis. 2006;38:659–664. doi: 10.1016/j.dld.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Kim HD, Kim CG, Kim JW, et al. Clinical features and therapeutic responses of perianal lesions in Crohn's disease. Korean J Gastroenterol. 2003;42:128–133. [PubMed] [Google Scholar]
- 80.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 81.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 82.Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128:862–869. doi: 10.1053/j.gastro.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 83.Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013;31:2575–2581. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 84.Lee KY, Yu CS, Lee KY, et al. Risk factors for repeat abdominal surgery in Korean patients with Crohn's disease: a multi-center study of a Korean inflammatory bowel disease study group. J Korean Soc Coloproctol. 2012;28:188–194. doi: 10.3393/jksc.2012.28.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 86.De Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn's disease: a systematic review. Inflamm Bowel Dis. 2012;18:758–777. doi: 10.1002/ibd.21825. [DOI] [PubMed] [Google Scholar]
- 87.Lee YW, Lee KM, Chung WC, Paik CN, Sung HJ, Oh YS. Clinical and endoscopic recurrence after surgical resection in patients with Crohn's disease. Intest Res. 2014;12:117–123. doi: 10.5217/ir.2014.12.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]