Abstract
Background/Aims
Most current knowledge regarding amiodarone toxicity derives from clinical trials. This study was performed to investigate the incidence and risk factors of overall adverse effects of amiodarone in real-world practice using a large sample size.
Methods
Between January 1, 2000 and March 10, 2012, a total of 930 consecutive patients who had been treated with amiodarone for arrhythmia were reviewed retrospectively. An amiodarone-associated adverse event was considered in cases of discontinuation or drug dose reduction due to an unexpected clinical response.
Results
The mean daily dose of amiodarone was 227 ± 126 mg, and the mean duration was 490 ± 812 days. During the mean follow-up duration of 982 ± 1,137 days, a total of 154 patients (16.6%) experienced adverse effects related to amiodarone, the most common being bradycardia or conduction disturbance (9.5%). Major organ toxicities in the thyroid (2.5%), liver (2.2%), eyes (0.6%), and lungs (0.3%) were rare. All patients recovered fully without complications after amiodarone discontinuation or dose reduction. The only independent predictor of adverse effects was the duration of amiodarone treatment (odds ratio, 1.21; 95% confidence interval, 1.03 to 1.41; p = 0.016, per year).
Conclusions
Low-dose amiodarone is well tolerated in a real-world clinical population. Further studies with a prospective design are needed to confirm this finding.
Keywords: Adverse effects, Amiodarone, Incidence, Risk factors
INTRODUCTION
Amiodarone is one of the most commonly used antiarrhythmia drugs. It is used to control a wide spectrum of cardiac tachyarrhythmias ranging from premature ventricular and atrial contraction to sustained tachyarrhythmias, such as ventricular tachycardia and atrial fibrillation. However, the use of amiodarone is limited clinically because of the relatively high incidence of adverse reactions. Amiodarone toxicity involves the heart, lungs, thyroid gland, liver, eyes, skin, and nerves [1,2]. Many studies have investigated the adverse effects of amiodarone; however, most were performed decades ago using a small sample size. Most of these studies were case reports or case series and focused on the toxicity of amiodarone toward specific organ systems [3,4,5,6]. In addition, the overall adverse events associated with amiodarone were not assessed in those studies. Although recent well-controlled clinical trials and meta-analyses provided information regarding the adverse effects of amiodarone [7,8,9,10,11,12,13], it is difficult to apply the results from these studies to daily practice in patients because of disparities between patient characteristics and the way in which the treatment was applied [14]. Given these limitations, there is a need to estimate the overall adverse effects of amiodarone among patients in a real-world population using a larger sample size.
The purpose of this study was to investigate the incidence of and risk factors for the overall adverse effects of amiodarone in real-world clinical practice.
METHODS
Study subjects and clinical data collection
This single center study was performed at Boramae Medical Center (Seoul, Korea). Between January 1, 2000 and March 10, 2012, 961 consecutive patients who had been treated with amiodarone for arrhythmia were reviewed retrospectively. Their medications were identified using the drug identification number in the hospital prescription database. Patients without an amiodarone prescription were assumed to not have taken amiodarone during the time period under review. Thirty-one patients were excluded from the 961 patients based on the following criteria: 1) aged < 18 years (n = 4); 2) insufficient data regarding the amiodarone dose and duration (n = 13); 3) no clinical follow-up (n = 11); and 4) lack of baseline clinical parameters (n = 3). Therefore, 930 patients were included in the study. During the medical record review, we collected relevant clinical data, including age, gender, body mass index, and underlying medical and heart disease, as well as the type of arrhythmia at the time of prescribing amiodarone. Individual medical histories were noted for diabetes, hypertension, stroke, and lung, thyroid, and liver diseases. In addition, any cases of underlying heart disease were reviewed for the presence of coronary artery disease and heart failure. Arrhythmias were classified as atrial fibrillation, supraventricular tachycardia, atrial premature contraction, atrial tachycardia, ventricular premature beats, ventricular tachycardia, or ventricular fibrillation. The duration of amiodarone treatment was calculated from the start date of the first prescription to the end date of the last filled prescription plus 60 days or the end of follow-up, whichever came first [15]. The mean daily dose of amiodarone was categorized as < 200, 200 to 399, or ≥ 400 mg/day. The follow-up of each patient was censored after either the first report of an adverse effect from amiodarone, discontinuation of amiodarone without adverse events, death, or the end of follow-up, whichever came first. Clinical follow-up was performed every 3 to 6 months in stable patients. The last day of follow-up was February 28, 2013. The Institutional Review Board of Boramae Medical Center approved the study protocol, and the need for informed consent was waived due to the retrospective study design.
Adverse effects of amiodarone
A patient was considered to have experienced amiodarone-associated adverse events if the medical record review revealed discontinuation of or a dose reduction in amiodarone due to an unexpected clinical response, as judged by the treating physician. The conclusion of amiodarone toxicity was based on patient symptoms, physical examinations, laboratory findings, radiographic images, and electrocardiograms. Several specific organ toxicities were defined as follows [7]. Bradycardia or conduction disturbance was defined as an asymptomatic resting heart rate < 50 beats per minute or the development of a second- or third-degree atrioventricular block. QT prolongation was defined as a prolongation of the corrected QT interval beyond 500 msec. In cases of QT prolongation accompanied by bradycardia, the patient was defined as having bradycardia, rather than QT prolongation. Thyroid toxicity was defined as clinical hypothyroidism or hyperthyroidism or changes in thyroid function (thyroid-stimulating hormone, thyroxine, and tri-iodothyroxine) requiring medical therapy. Eye toxicity was defined as the presence of visual complaints or corneal microdeposits on slit-lamp examination. Pulmonary toxicity was defined as respiratory symptoms with new-onset chest X-ray or computed tomography findings. Hepatic toxicity was defined by a > 2- to 3-fold elevation in alanine transaminase or aspartate transaminase. All toxicity definitions were based on the premise that there were no other reasons for these findings.
Statistical analysis
Continuous variables were presented as mean ± SD, and categorical variables were expressed as percentages. Continuous variables were compared using Student t tests, and categorical variables were compared using chi-square tests. Multivariable logistic regression analysis was performed to assess independent risk factors for amiodarone-associated adverse effects. Age, gender, body mass index, underlying illness, and amiodarone dose and duration were adjusted in the model. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline characteristics of the study patients
The baseline characteristics of the study patients are summarized in Table 1. The mean age at the initiation of amiodarone therapy was 65.5 ± 12.7 years, and 57.4% of the patients were male. Histories of diabetes, hypertension, coronary artery disease, congestive heart failure, and stroke were identified in 24.8%, 54.5%, 17.2%, 9.7%, and 12.9% of patients, respectively. At the time of amiodarone initiation, ischemic heart disease, acute decompensated heart failure, and cardiac arrest were present in 2.4%, 2.6%, and 8.7% of patients, respectively. The most frequent indication for amiodarone was atrial fibrillation (69.5%), followed by ventricular tachycardia (16.8%), and paroxysmal supraventricular tachycardia (10.0%). The mean daily dose of amiodarone was 227 ± 126 mg, and the mean duration was 490 ± 812 days.
Table 1.
Baseline clinical characteristics of the study patients
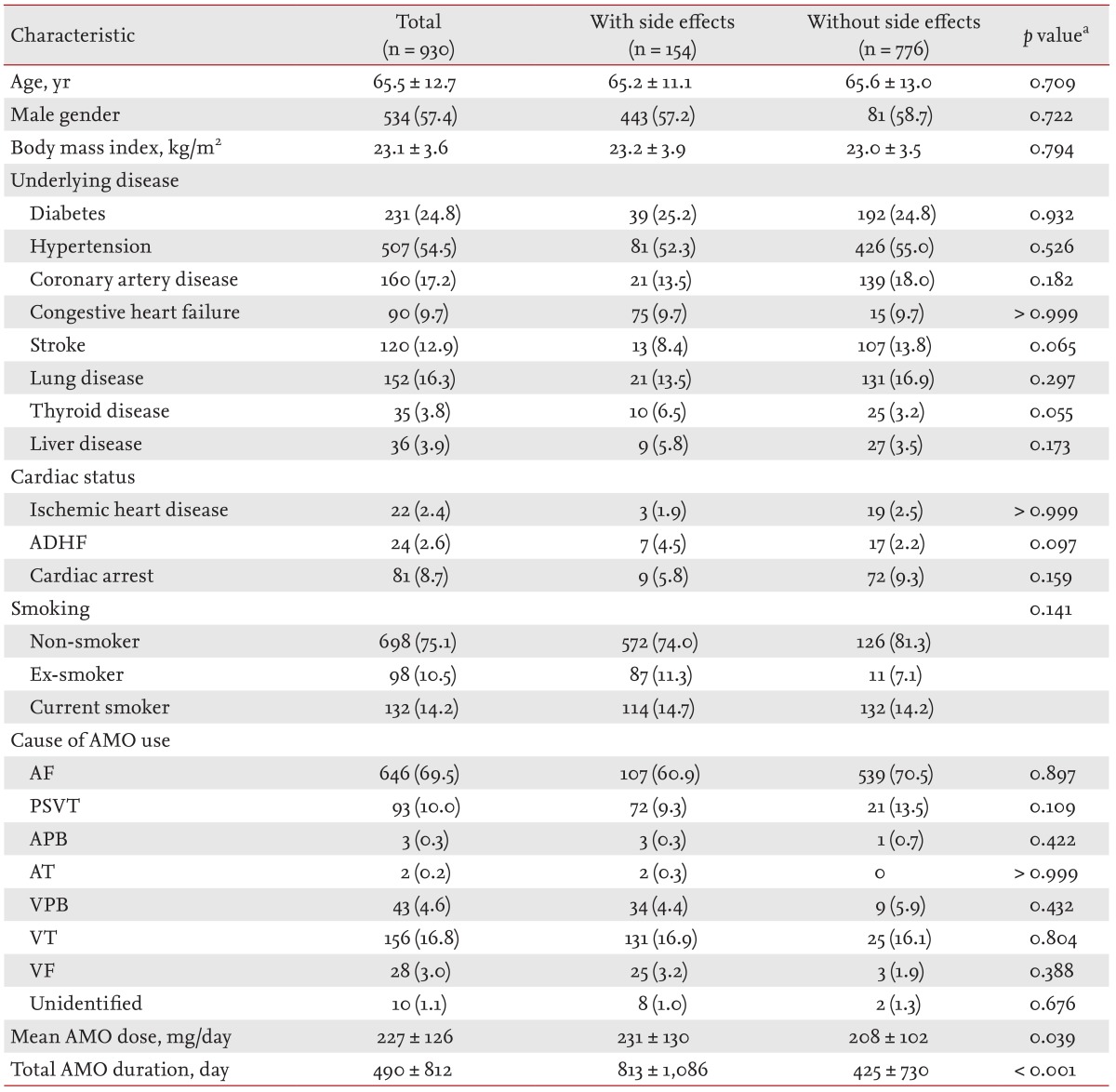
Values are presented as number (%) or mean ± SD.
ADHF, acute decompensated heart failure; AMO, amiodarone; AF, atrial fibrillation; PSVT, paroxysmal supraventricular tachycardia; APB, atrial premature beat; AT, atrial tachycardia; VPB, ventricular premature beat; VT, ventricular tachycardia; VF, ventricular fibrillation.
aDifferences between groups with and without side effects.
Clinical parameters associated with amiodarone-associated adverse effects
During the mean follow-up period of 982 ± 1,137 days, a total of 154 patients (16.6%) experienced adverse effects related to amiodarone. A comparison of the clinical characteristics of patient groups with and without adverse effects is shown in Table 1. There were no significant differences between the two groups, except for the mean dose and total duration of amiodarone treatment. The mean daily dose of amiodarone was higher in patients with adverse events than in those without adverse events (231 ± 130 mg vs. 208 ± 102 mg, p = 0.039). The duration of amiodarone treatment was longer in patients with side effects than in those without (813 ± 1,086 days vs. 425 ± 730 days, p < 0.001).
Incidence of adverse effects
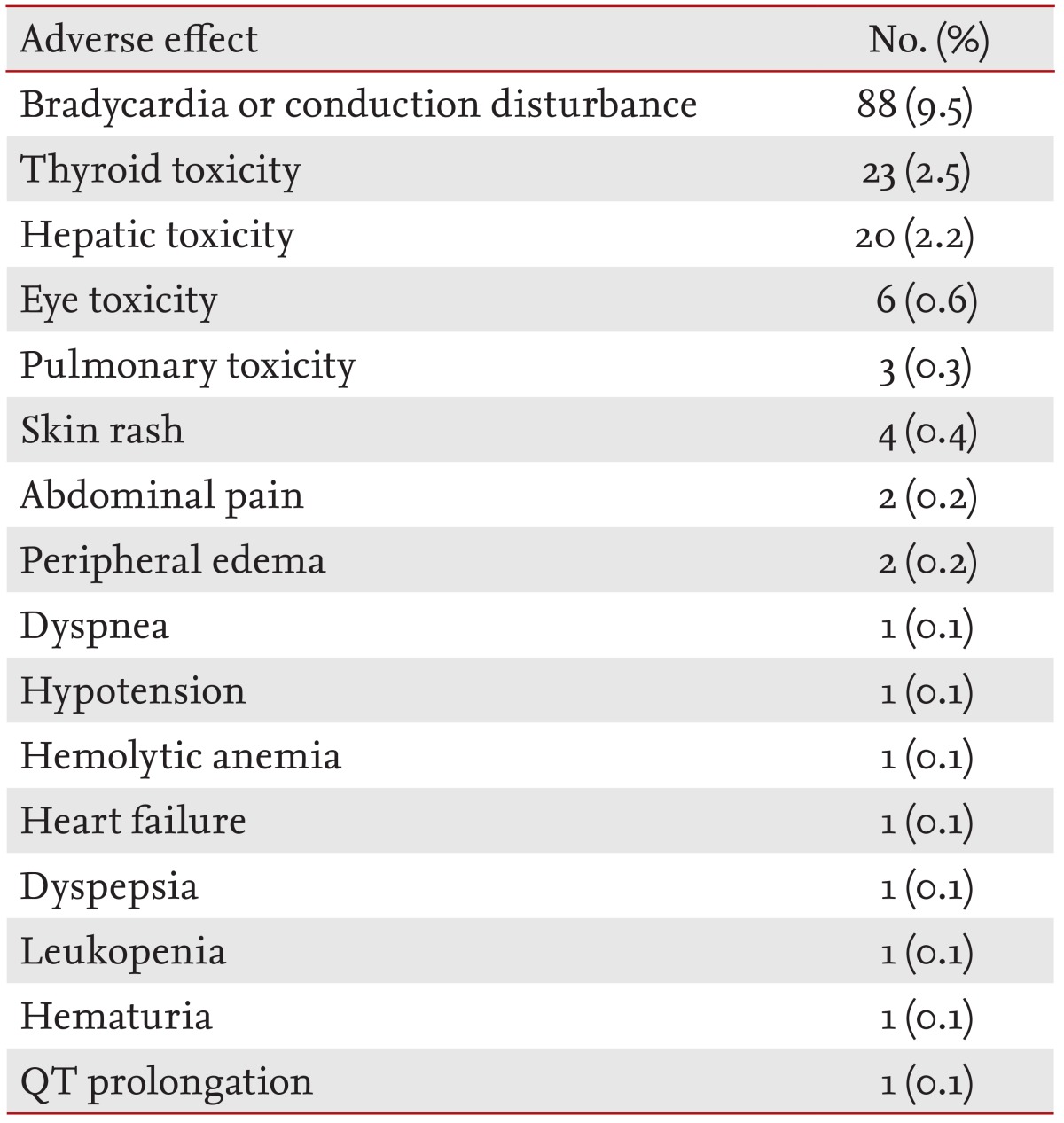
All amiodarone-related adverse effects are summarized in Table 2. The most frequent adverse effect was bradycardia or conduction disturbance, which occurred in 88 patients (9.5%). Among these, 34 patients (38.6%) took concomitant medications related to bradycardia, including β-blockers (n = 18, 20.4%), nondihydropyridine calcium channel blockers (n = 6, 6.8%), and digoxin (n = 2, 2.2%). Thyroid and hepatic toxicities occurred in 23 patients (2.5%) and 20 patients (2.2%), respectively. Six patients (0.6%) complained of visual disturbance, four of whom had undergone an eye examination by an ophthalmologist, all revealing amiodarone-associated corneal deposition. Pulmonary toxicity occurred in four patients (0.4%). Two adverse effects occurred simultaneously in three patients: one experienced bradycardia with thyroid toxicity, and the remaining two patients experienced both thyroid and hepatic toxicities. Amiodarone treatment was stopped or the dose was reduced in 140 patients (15.1%) or 14 patients (1.5%), respectively, after the identification of adverse effects. All the patients who experienced adverse effects recovered fully after discontinuation or dose reduction.
Table 2.
Adverse effects of amiodarone requiring drug discontinuation or reduction (n = 930)
Independent predictors of overall adverse effects
To identify the independent risk factors for amiodarone-related adverse effects, multiple logistic regression analyses were performed. After controlling for confounders, the duration of amiodarone treatment was the only independent risk factor for adverse events (odds ratio, 1.21; 95% confidence interval, 1.03 to 1.41; p = 0.016, per year). Underlying illness and daily amiodarone dose were not associated with adverse events (Table 3).
Table 3.
Independent predictors for adverse effects of amiodarone
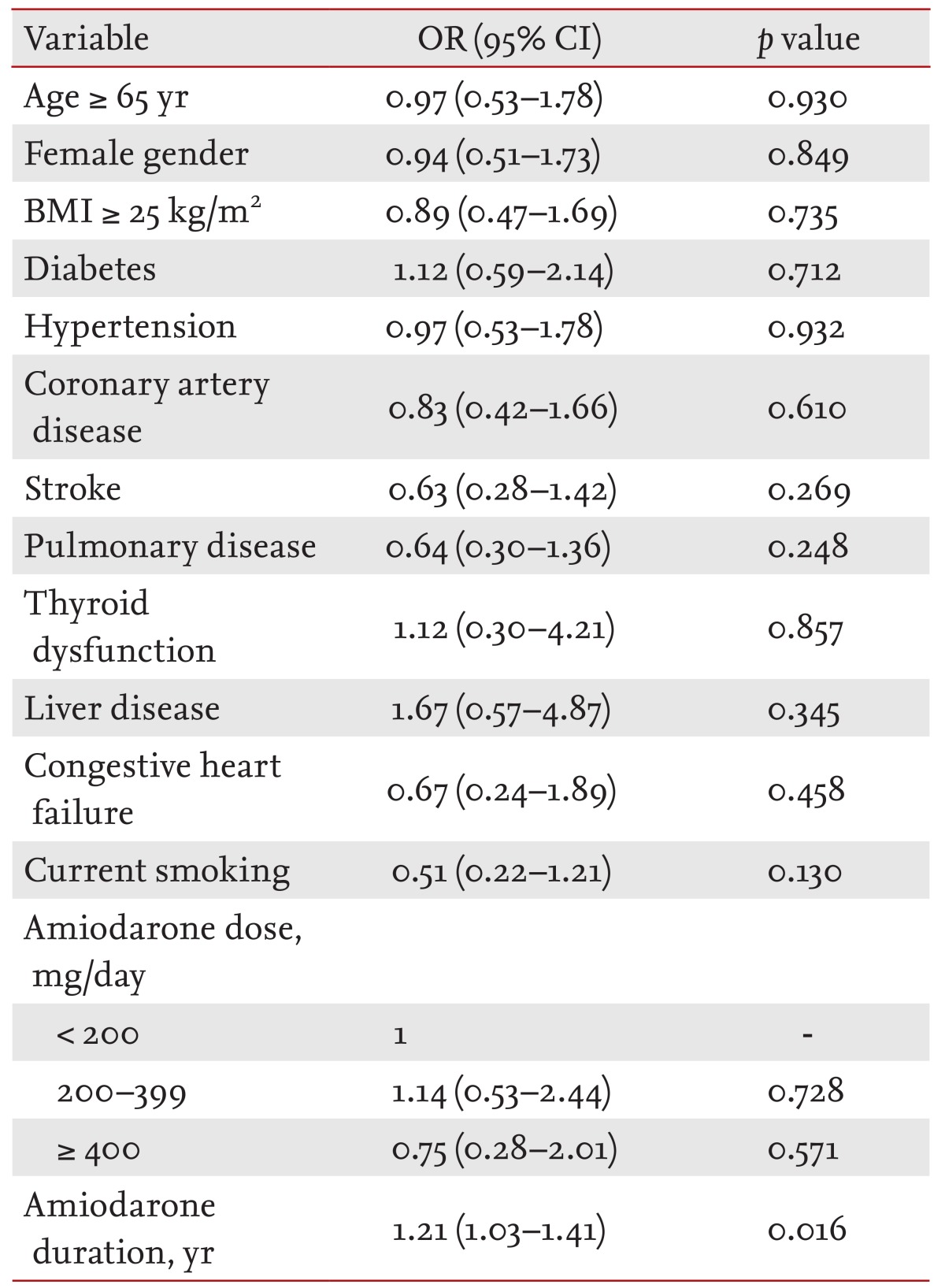
OR, odds ratio; CI, confidence interval; BMI, body mass index.
DISCUSSION
The present study, performed in real-world clinical practice, revealed that 16.6% of patients taking amiodarone at relatively low maintenance doses (227 mg) for a mean duration of 32 months suffered from adverse effects that required drug discontinuation or dose reduction. The most common adverse event was bradycardia or conduction disturbance (9.5%). The incidence of major organ toxicities for thyroid, liver, eyes, and lungs were rare. All patients recovered fully without complications after the discontinuation of or dose reduction in amiodarone. The only independent predictor for adverse events was the duration of amiodarone treatment.
Study rationale
The best way to assess an adverse reaction to a drug is to compare the rate of adverse events in patients who were exposed to the drug with those who were exposed to the placebo; randomized double-blinded and placebo-controlled trials are best suited for such comparisons. Moreover, meta-analysis of individual clinical trials increases the statistical power of likelihood estimates for adverse effects. Many clinical trials and meta-analyses have investigated the efficacy of amiodarone and provided information that has been applied to clinical practice. However, there is a large disparity between clinical trials and real-world performance regarding patient characteristics and the way in which patients receive treatment. As such, there are concerns over how well the information obtained from clinical trials can be generalized to different clinical settings and populations with broader patient characteristics compared with those in clinical trials [14]. Therefore, clinicians can sometimes obtain vital information from data gathered in real clinical settings. However, little real-world clinical data specifically addressing the adverse effects of amiodarone are available. As such, the results of the current study are valuable and informative to physicians.
The overall incidence of adverse effects with amiodarone
The present study revealed that the overall incidence of adverse events requiring discontinuation of amiodarone was 15.1% in patients receiving a mean daily dose of 227 mg and a mean follow-up duration of 32 months. Despite the relatively longer follow-up period in the current study, the incidence of adverse effects was lower than that found in previous studies. In a randomized controlled trial by Roy et al. [8], 18% of patients who had received 200 mg/day amiodarone for the maintenance of sinus rhythm discontinued taking the drug because of adverse events during a 16-month follow-up. In another trial with a similar study design, the incidence of adverse effects requiring discontinuation was 12.3% during the first year [12]. The guidelines published by the North American Society of Pacing and Electrophysiology in 2000 estimated that the incidence of adverse effects of amiodarone was 15% during the first year and as high as 50% with long-term therapy [16]. In a placebo-controlled trial investigating amiodarone use in a large population of myocardial infarction survivors with a left ventricular ejection fraction ≤ 40%, Julian et al. [10] demonstrated that 38.5% of patients in the amiodarone group (200 mg/day) discontinued medication, compared with 21.4% of those in the placebo group, during the 21-month study period. Bardy et al. [11] demonstrated study drug discontinuation rates of 32% in the amiodarone group and 22% in the placebo group among patients with congestive heart failure. Their study used a median dose of 300-mg/day amiodarone and a median study follow-up period of 45.5 months [11]. In meta-analyses of trials assessing long-term amiodarone treatment (mean dose of 152-400 mg and mean follow-up of 12 months), there was a significantly higher rate of drug discontinuation with amiodarone compared with the placebo (22.9% to 41.0% vs. 15.4% to 27.0%) [7,9,13].
The incidence of adverse events of amiodarone in this study is lower than that observed in previous studies. Although the underlying mechanisms behind this difference remain unknown, we can suggest several hypotheses. First, the mean dose of amiodarone was lower in our studies than in previous reports. Second, most of the previous studies were well-controlled trials, and the enrolled patients were monitored intensively and questioned frequently regarding adverse effects. As such, even subtle changes related to amiodarone might be reported in these patients. In contrast, the current study was performed using patients from a real-world clinical population. Our patients were monitored less frequently; therefore, some minor side effects might not have been identified. In addition, due to the retrospective study design, it is possible that certain amiodarone adverse effects might not have been detected, particularly among the 15.2% of patients who were lost during the study period. Some of this missing information could lead to nonresponse bias in the data. Therefore, the incidence of adverse events might have been underestimated in the current study. The different ethnicities of the patient groups might also have influenced the differences in adverse reactions to amiodarone, although there is no current evidence to support this. Nevertheless, additional studies are needed to confirm our hypothesis.
Bradycardia and atrioventricular block are common adverse effects of amiodarone owing to its calcium channel blocking activity. The chronic use of low dose amiodarone was associated with an increased risk of bradycardia compared with placebo (3.3% vs. 1.4%) in a previous meta-analysis [7]. An additional study reported that the overall incidence of bradycardia was 5% [16]. Santangeli et al. [17] summarized the results of several randomized controlled trials using amiodarone for the long-term maintenance of sinus rhythm in patients with atrial fibrillation, and demonstrated that the rate of symptomatic bradycardia leading to drug discontinuation was 1.22%. In the present study, bradycardia or heart block occurred in 9.5% of patients, which is somewhat higher than the findings of previous studies. Differences in the study protocols might explain this discrepancy. Many patients who had bradycardia < 50 beats per minutes were required to discontinue the drug, and each of these instances was counted as an adverse effect even though the patients were asymptomatic in the current study. This definition of bradycardia differed from that in previous studies, in which only symptomatic bradycardia or advanced heart block requiring discontinuation of the drug were considered to be adverse reactions to amiodarone [7].
Thyroid, hepatic, eye, and pulmonary toxicities were relatively rare in our study compared with previous reports. Amiodarone is very rich in iodine, and a 100-mg tablet contains 250-fold more than the recommended daily iodine requirement. This high iodine content and the direct toxic effects of amiodarone on the thyroid parenchyma alter thyroid function [18], and the incidence of amiodarone-induced thyroid dysfunction in previous studies was 14% to 18% [19]. However, in the current study only 2.5% of patients experienced thyroid dysfunction that required discontinuation or dose reduction. Due to its high lipophilicity, amiodarone can easily accumulate in organs with high-adipose contents; this lipophilic character is presumed to cause liver injury [20]. Hepatotoxicity due to amiodarone was 2.2% in the current study, which is considerably lower than the previously reported incidences of 14% to 82% [20], among which 20% to 40% of patients required drug discontinuation because of hepatotoxicity [21]. A meta-analysis performed by Lewis et al. [22] estimated that asymptomatic elevations of serum tansaminases to 4-fold the normal range developed in 23% of 1,306 patients. Ocular changes caused by amiodarone have also been reported. The incidence of visual disturbance in patients receiving amiodarone ranges from 1.4% to 40%. Corneal epithelial deposits are extremely common and are found in 70% to 100% of patients receiving amiodarone [23]. In the present study, only six patients (0.6%) complained of ocular symptoms during the study period. Of the four that underwent eye examinations, all had corneal deposits. Amiodarone can induce pulmonary toxicity via direct toxic effects, through an immune-mediated mechanism, and via activation of the angiotensin system. It is a rare complication but can be life-threatening [24]. Recent studies reported that the incidence of amiodarone-induced pulmonary toxicity (AIPT) was 5% to 13% [25]. Mortality due to AIPT was reported to range from 10% to 75% [15,26]. In the current study, three patients (0.3%) experienced AIPT but without mortality.
Independent predictors of adverse effects of amiodarone
Multivariate analysis revealed that the duration of amiodarone treatment was the only independent risk factor for amiodarone toxicity, which is consistent with previous studies showing that the cumulative administered dose of amiodarone was a major risk factor for amiodarone-induced organ toxicity [1,2,16,17]. During long-term treatment, amiodarone accumulates easily in the liver, lungs, myocardium, fat, skin, and muscle. However, the elimination half-life of amiodarone is very long and variable. Therefore, tissue accumulation plays a major role in the development of adverse events [17]. Age, gender, and underlying disease have also been suggested to be predictors of specific organ toxicities [15,24,27,28]; however, these factors were not associated with the overall adverse effects of amiodarone in the current study. We also performed multivariate analyses to identify independent predictors for specific organ toxicities, but the data were limited because of the small number of adverse events.
Study limitations
In addition to the retrospective design, this study has several inherent limitations. First, it is possible that adverse effects of amiodarone might have gone unnoticed in some patients, and that the incidence of adverse events might have been underestimated. Second, the follow-up schedules and laboratory tests varied among physicians, even though current guidelines recommend routine follow-up intervals and laboratory tests for patients receiving amiodarone [16]. Third, the definition of adverse events based on discontinuation or dose reduction was not an objective parameter, because the decisions were made by the attending physician in charge of each patient. Fourth, it is possible that bradycardia or conduction disturbance might be caused by other concomitant medications such as β-blockers, nondihydropyridine calcium channel blockers, and digoxin. Finally, the mean dose of amiodarone used in the current study was 227 mg. The incidence and risk factors for adverse events might differ in patients receiving different doses of amiodarone.
Conclusion
The incidence of overall adverse effects of low-dose amiodarone was relatively low in real-world clinical practice. Furthermore, major organ toxicity associated with the use of low-dose amiodarone, including thyroid, hepatic, eye, and lung toxicities, were quite rare, and no serious complications or mortality associated with amiodarone occurred during the mean follow-up period of 32 months. In addition, all patients recovered fully from these adverse effects after discontinuation or dose reduction. Our results suggest that low-dose amiodarone can be used safely in most patients with tachyarrhythmia. However, the duration of amiodarone treatment was identified as an independent predictor of adverse effects. Therefore, physicians should monitor patients taking amiodarone continuously and make every effort to minimize the treatment duration. These findings will be informative for physicians who treat patients with amiodarone in real clinical settings. However, our data should be interpreted with caution in view of their retrospective nature, and the fact that low-maintenance doses (227 mg) for a mean duration of 32 months of amiodarone were used. Therefore, it is difficult to generalize our results to other patients prescribed different doses and durations of amiodarone. These results also do not imply that amiodarone is safe and recommended as a first-line therapy. Further studies using a prospective design are needed to confirm our findings.
KEY MESSAGE
Low-dose amiodarone is well tolerated in real-world practice: most adverse effects of low-dose amiodarone were not serious, and all patients with adverse effects recovered fully after drug discontinuation or dose reduction.
The duration of amiodarone use was an independent predictor for adverse effects, emphasizing the need for continuous monitoring and efforts to minimize the duration of amiodarone use.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Haffajee CI, Love JC, Alpert JS, Asdourian GK, Sloan KC. Efficacy and safety of long-term amiodarone in treatment of cardiac arrhythmias: dosage experience. Am Heart J. 1983;106(4 Pt 2):935–943. doi: 10.1016/0002-8703(83)90019-4. [DOI] [PubMed] [Google Scholar]
- 2.Nademanee K, Singh BN, Hendrickson J, et al. Amiodarone in refractory life-threatening ventricular arrhythmias. Ann Intern Med. 1983;98(5 Pt 1):577–584. doi: 10.7326/0003-4819-98-5-577. [DOI] [PubMed] [Google Scholar]
- 3.Parra O, Ruiz J, Ojanguren I, Navas JJ, Morera J. Amiodarone toxicity: recurrence of interstitial pneumonitis after withdrawal of the drug. Eur Respir J. 1989;2:905–907. [PubMed] [Google Scholar]
- 4.Kang HM, Kang YS, Kim SH, et al. Amiodarone-induced hepatitis and polyneuropathy. Korean J Intern Med. 2007;22:225–229. doi: 10.3904/kjim.2007.22.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavares AB, Paula SK, Vaisman M, Teixeira Pde F. Amiodarone and thyrotoxicosis: case reports. Arq Bras Cardiol. 2010;95:e122–e124. doi: 10.1590/s0066-782x2010001500022. [DOI] [PubMed] [Google Scholar]
- 6.Kwon KH, Jeong SW, Uh S, et al. A case of amiodarone-associated pulmonary toxicity. Korean J Intern Med. 1995;10:155–159. doi: 10.3904/kjim.1995.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30:791–798. doi: 10.1016/s0735-1097(97)00220-9. [DOI] [PubMed] [Google Scholar]
- 8.Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation: Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 9.Piccini JP, Berger JS, O'Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J. 2009;30:1245–1253. doi: 10.1093/eurheartj/ehp100. [DOI] [PubMed] [Google Scholar]
- 10.Julian DG, Camm AJ, Frangin G, et al. European Myocardial Infarct Amiodarone Trial Investigators. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. Lancet. 1997;349:667–674. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 11.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 12.AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20–29. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 13.Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350:1417–1424. [PubMed] [Google Scholar]
- 14.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 15.Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705–710. doi: 10.1016/j.amjcard.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B. Practical guidelines for clinicians who treat patients with amiodarone: Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med. 2000;160:1741–1748. doi: 10.1001/archinte.160.12.1741. [DOI] [PubMed] [Google Scholar]
- 17.Santangeli P, Di Biase L, Burkhardt JD, et al. Examining the safety of amiodarone. Expert Opin Drug Saf. 2012;11:191–214. doi: 10.1517/14740338.2012.660915. [DOI] [PubMed] [Google Scholar]
- 18.Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118:706–714. doi: 10.1016/j.amjmed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22:240–254. doi: 10.1210/edrv.22.2.0427. [DOI] [PubMed] [Google Scholar]
- 20.Babatin M, Lee SS, Pollak PT. Amiodarone hepatotoxicity. Curr Vasc Pharmacol. 2008;6:228–236. doi: 10.2174/157016108784912019. [DOI] [PubMed] [Google Scholar]
- 21.Podrid PJ. Amiodarone: reevaluation of an old drug. Ann Intern Med. 1995;122:689–700. doi: 10.7326/0003-4819-122-9-199505010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JH, Ranard RC, Caruso A, et al. Amiodarone hepatotoxicity: prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9:679–685. doi: 10.1002/hep.1840090504. [DOI] [PubMed] [Google Scholar]
- 23.Mantyjarvi M, Tuppurainen K, Ikaheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998;42:360–366. doi: 10.1016/s0039-6257(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 24.Camus P, Martin WJ, 2nd, Rosenow EC., 3rd Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25:65–75. doi: 10.1016/S0272-5231(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 25.Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66:82–87. doi: 10.1111/j.1365-2125.2008.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyama N, Oyama N, Yokoshiki H, et al. Detection of amiodarone-induced pulmonary toxicity in supine and prone positions: high-resolution computed tomography study. Circ J. 2005;69:466–470. doi: 10.1253/circj.69.466. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg BA, Miles WM, Klein LS, et al. Five-year follow-up of 589 patients treated with amiodarone. Am Heart J. 1993;125:109–120. doi: 10.1016/0002-8703(93)90063-f. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y, Shiga T, Matsuda N, Hagiwara N, Kasanuki H. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71:1610–1616. doi: 10.1253/circj.71.1610. [DOI] [PubMed] [Google Scholar]


