Abstract
Background/Aims
The clinical outcomes of some patients with pleural infection may be favorable with medical treatment alone, but in others, the disease progresses and requires additional surgical treatment. However, little is known about the factors affecting this difference. The aim of this study was to investigate the factors predictive of failure of medical treatment in patients with pleural infection.
Methods
A cohort of 127 consecutive patients who were admitted to the hospital with pleural infection was studied. Clinical manifestations and laboratory findings in patients in whom medical treatment succeeded or failed were reviewed.
Results
In univariate analysis, the significant factors associated with medical treatment outcome were age, smoking history, duration of chief complaint, serum albumin level, and pleural fluid glucose and lactate dehydrogenase levels (p < 0.05). Multivariate logistic regression analysis identified age and duration of chief complaint as independent predictive factors for failure of medical treatment, with odds ratios of 0.871 (p = 0.013) and 0.797 (p = 0.026), respectively. Receiver operating characteristic curve analysis determined cutoff values of 50.5 years for age and 4.5 days for duration of chief complaint.
Conclusions
We demonstrated that a younger age < 50.5 years and shorter duration of chief complaint < 4.5 days were independent predictive factors for the failure of medical treatment in patients with pleural infection. This suggests their role as evaluative criteria in setting indications for the optimal treatment in patients with pleural infection. A larger, prospective study is required to confirm these findings.
Keywords: Empyema, pleural; Drainage; Fibrinolytic agents; Treatment outcome
INTRODUCTION
Approximately 40% of patients admitted to the hospital with pneumonia have parapneumonic effusion. The overall mortality increases threefold in cases of unilateral and sevenfold in cases of bilateral parapneumonic effusion. An estimated 5% to 10% of these patients progress to pleural infection, defined as complicated parapneumonic effusion or empyema, and require additional interventional procedures along with antibiotics [1,2].
There are two treatment options for pleural infection, medical or surgical [1,3,4]. Medical treatment includes adequate intravenous antibiotics and percutaneous catheter drainage of pleural effusion with or without intrapleural fibrinolysis. Surgical procedures include video-assisted thoracoscopic surgery (VATS) and open thoracotomy. Although there are some guidelines regarding primary treatment choice, there is no consensus regarding the optimal treatment.
Although medical measures are often administered first in the treatment of pleural infection, large case-control prospective studies and meta-analyses have shown that medical treatment does not affect overall mortality [5,6,7]. Among the surgical procedures, decortication with open thoracotomy is not selected as a first-line treatment because it is invasive and results in considerable postoperative pain. The less-invasive VATS technique has been reported to be equally effective in the treatment of pleural infection [8,9]. Therefore, when initial medical treatment fails, surgical treatment with VATS is the next option. This sequential algorithm results in multiple procedures, prolonged hospitalization, hospitalization-related complications and economic burden [10]. Delay in the drainage of infected pleural fluid may also cause aggravation of the disease, development of other complications, or increased mortality. Subsequent surgical treatment for these patients is limited and associated with increased surgery-related mortality, reduced treatment success rates, or increased overall mortality. Therefore, surgery has recently been suggested as the preferred initial method of treatment in some patients with pleural infection [8,9]. However, there is no consistent indication for surgical treatment.
The purpose of this study was to identify the clinical, laboratory, and/or radiological factors predictive of the need for surgical treatment in patients with pleural infection. The underlying aim was to determine predictive factors for the failure of pleural fluid drainage using minimally invasive techniques during pleural infection that could be used to identify the patients who would benefit from early surgical interventions.
METHODS
Subjects
The study consisted of 127 consecutive patients with pleural infection who were admitted from February 2002 to June 2011 at St. Vincent's Hospital, College of Medicine, The Catholic University of Korea (Suwon, Korea). This study was approved by our hospital's Institutional Review Board (No. VC10RISI0193). Because this was a retrospective analysis of stored medical records, the need for informed consent was waived. Empyema was, by definition, pus in the pleural space. Complicated parapneumonic effusion was defined as parapneumonic effusion for which an invasive procedure, such as tube thoracostomy, was necessary for its resolution, or parapneumonic effusion with positive bacterial culture results [1,2,11]. The indication for pleural drainage was left to the clinical judgment of the managing physician. Criteria contributing to this decision included macroscopically purulent pleural fluid, pleural fluid acidosis (pH < 7.2), glucose < 40 mg/dL, lactate dehydrogenase (LDH) > 1,000 IU/L (Light's criteria) [11], and/or the presence of loculations/septations on radiological imaging in association with persistent systemic sepsis. Success of minimally invasive procedures was defined as the condition responding to medical treatment alone and the patient being discharged without any complication, while failure was defined as the requirement for surgery. There were no patients with previous pleural disease.
All patients were initially treated with empirical antibiotic therapy and chest catheter drainage with or without the use of intrapleural fibrinolytics. Antibiotics consisted of intravenous third-generation cephalosporin and metronidazole. When cultures were positive, the antibiotics were changed to those appropriate for the organism. All chest catheters were inserted into the most dependent area of the effusion or into the largest locule under ultrasonography (US) guidance by an interventional radiologist. Immediately after catheter insertion, on-site follow-up US was performed to confirm the catheter position on a routine basis. The sizes of the chest catheters inserted were 10-, 12-, 14-, or 16-Fr. All catheters were connected to underwater seal drainage with -20 cmH2O suction and flushed with 20- to 30-mL saline every 12 hours. Simple chest radiographs (posterior-anterior and lateral view) and drainage measurement were obtained daily. If radiological response and drainage were not satisfactory, patients received intrapleural urokinase (UK) to enhance drainage. UK (100,000 international units [IU]) was dissolved in 10-mL 0.9% saline and retained in the pleural space for 4 hours after each administration. Intrapleural UK was administered once daily until daily drainage was < 100 mL. When pleural effusion remained, despite defervescence and normal white blood cell (WBC) counts and C-reactive protein (CRP) with these managements, the catheter was replaced with one of a larger size or an additional catheter was placed in a different location under US guidance.
Failure of medical treatment was determined by the presence of persistent sepsis syndrome (fever and/or persistent leukocytosis and/or elevated CRP) in association with a residual pleural fluid collection. These patients were referred to surgical treatment with VATS adhesiolysis and debridement. In those patients who responded to medical treatment, the chest catheter was removed when drainage fell to below 50 to 100 mL daily for 2 consecutive days.
Data collection
Epidemiological and clinical data were collected by thorough review of the medical records of each patient. The following data were gathered for each patient: gender, age, alcohol and smoking history, comorbidity, chief complaint, duration of chief complaint, body temperature, mean arterial blood pressure, simplified acute physiologic score (SAPS) II, time from admission to first antibiotics (time to antibiotics), time from admission to first catheter insertion (time to catheter), catheter size, the number of catheter changes or addition and intrapleural UK instillation, partial pressure of oxygen in arterial blood (PaO2), WBC counts, neutrophil percentage of WBC, total protein level, albumin level, and CRP. Pleural fluid analysis, including WBC count, neutrophil count, protein, glucose, and LDH level, gross finding, and bacterial cultures, was also performed. Radiological analysis was performed by chest X-ray and computed tomography (CT). The size of pleural effusion and the presence of fluid shifting was determined using plain film images. The size of the pleural effusion was defined as follows: (1) small when the hemothorax was < one-third; (2) moderate when the hemothorax was one-third to two-thirds; and (3) large when the hemothorax was > two-thirds. CT images were used to visualize the presence of loculation and pleural thickening and the number (none, single, and multiple) of loculations was recorded. Three patients were excluded from the analysis of CT results due to unavailability of pretreatment images. Data were classified into clinical, laboratory (blood test and pleural fluid analysis), and radiological parameters.
Statistical analysis
Means and standard deviations (SD) were determined for continuous variables, and discrete variables were expressed as the counts of observed events. For comparison of continuous variables, Student t test was used when they followed a normal distribution; otherwise, the Mann-Whitney U test was used; the chi-square test or Fisher exact test was used for discrete variables. To identify independent factors predictive of failure of medical treatment, multivariate logistic regression analysis with variables found to be significantly different between the two groups by univariate analysis was used. Receiver operating characteristic (ROC) curve analysis was used to identify cutoff values for variables in predicting the failure of medical treatment and calculating the sensitivity and specificity. The maximum sum of sensitivity and specificity was defined as the cutoff value. The data analysis was performed using the SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics of patients
Clinical characteristics of patients are summarized in Table 1. A total of 127 patients with pleural infection were analyzed, which comprised 86 males and 41 females. There were 89 patients in the medical treatment success (MTS) group and 38 in the medical treatment failure (MTF) group. No significant difference in gender was noted between these two groups. The mean age of the study population was 58.42 ± 12.68 years, and the MTF group was on average younger than the MTS group (p < 0.001). There was no significant difference in alcohol consumption history between the two groups. The MTF group, however, had a significantly higher percentage of patients with a history of smoking than the MTS group (p = 0.032). There was no significant difference in comorbidity. The chief complaints were pleuritic chest pain (45.7%), dyspnea (23.6%), fever (12.6%), cough (9.4%), and other constitutional symptoms such as malaise, anorexia, or fatigue (8.7%). There was no significant difference in frequency of chief complaint between the two groups. The mean duration of the chief complaint was 11.52 ± 12.35 days, and was significantly shorter in the MTF group than the MTS group (p = 0.001). There were no significant differences in body temperature, SAPS II, time to antibiotics, time to catheter, catheter size, duration with catheter, the number of catheter changes or additions, and intrapleural UK instillation.
Table 1.
Patients' clinical characteristics
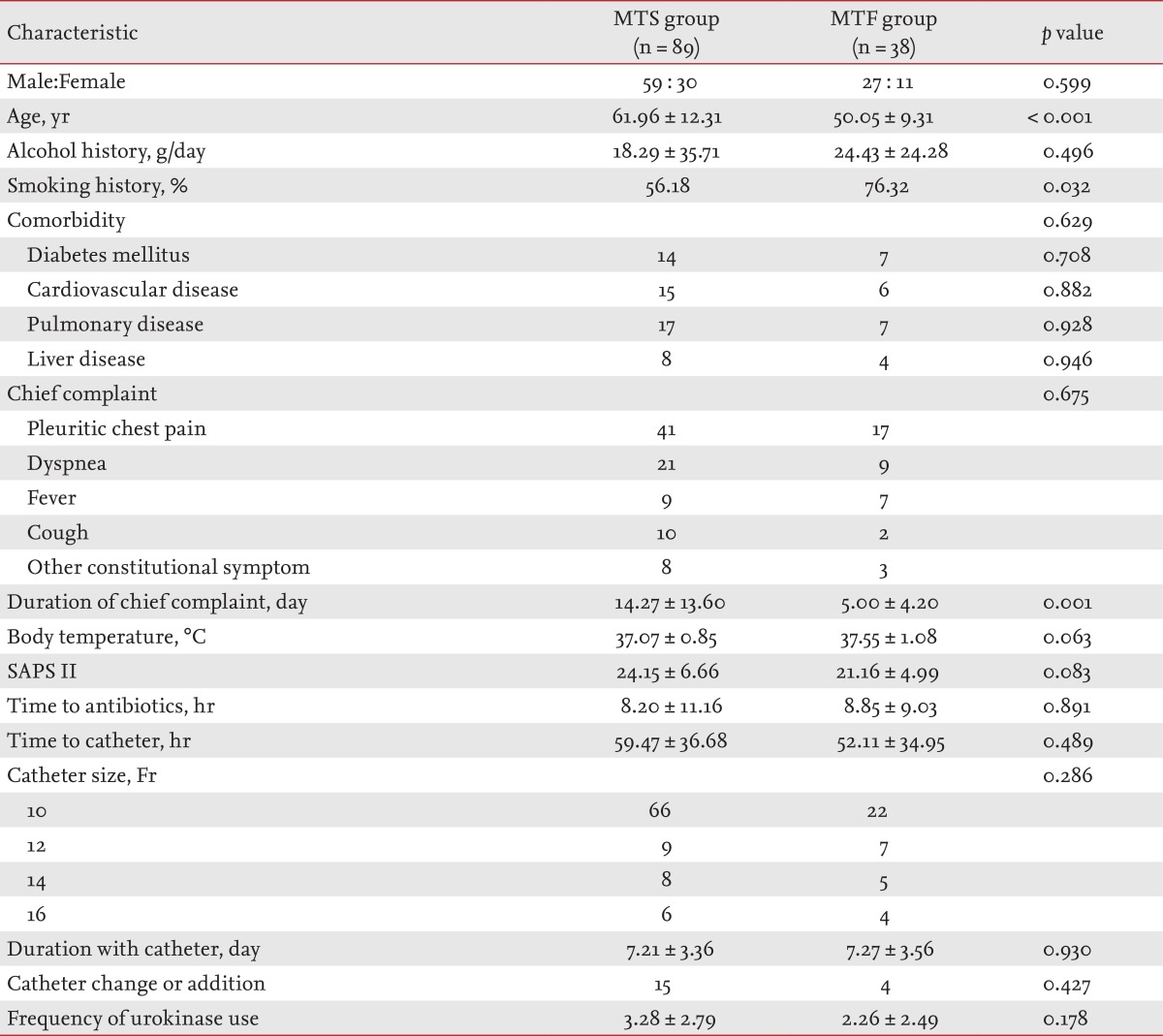
Values are presented as mean ± SD or number.
MTS, medical treatment success; MTF, medical treatment failure; SAPS, simplified acute physiologic score.
Blood tests
Serum albumin was higher in the MTF group than in the MTS group (p = 0.008) (Table 2). There was no significant difference in PaO2, blood WBC count, neutrophil content of WBC, total protein, or CRP between the two groups.
Table 2.
Blood test results
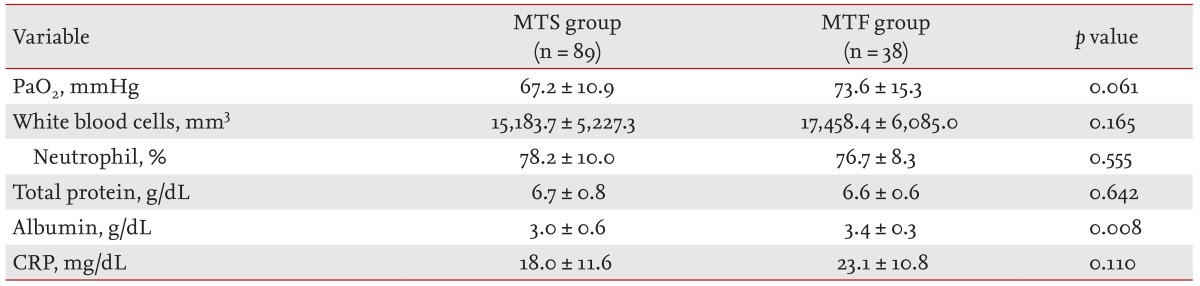
Values are presented as means ± SD.
MTS, medical treatment success; MTF, medical treatment failure; PaO2, partial pressure of oxygen; CRP, C-reactive protein.
Pleural fluid analysis
The pleural glucose level was significantly lower (p = 0.025) and the pleural LDH level was significantly higher (p = 0.027) in the MTF group than in the MTS group (Table 3). Pleural WBC and neutrophil count, protein level, gross findings, and bacterial culture of pleural fluid in both groups showed no significant difference between the two groups.
Table 3.
Pleural fluid analysis
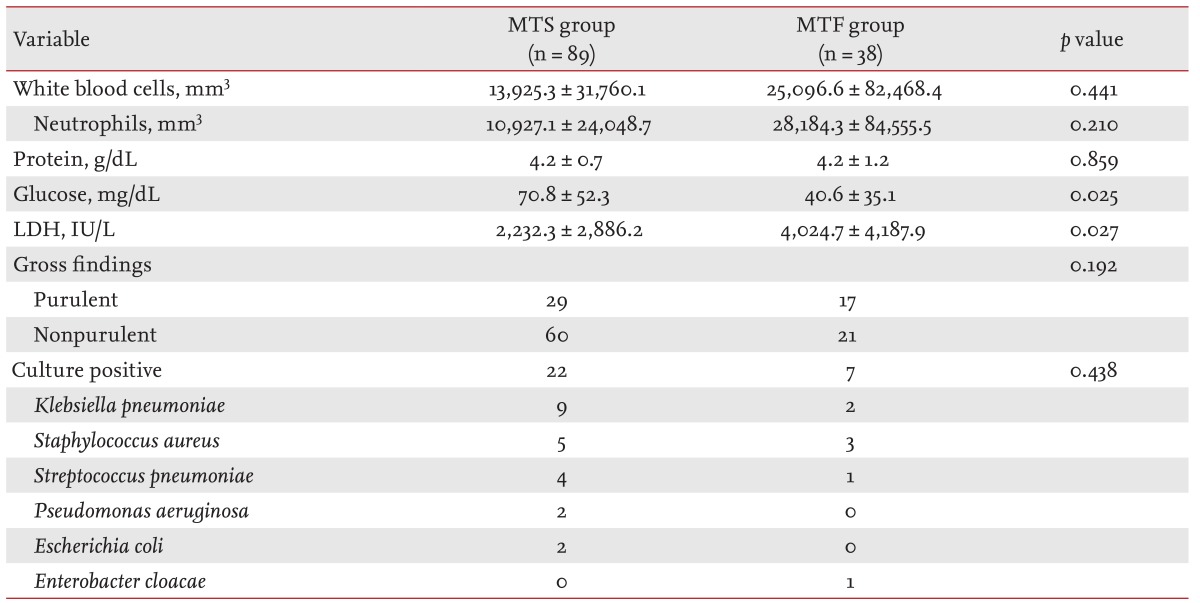
Values are presented as mean ± SD or number.
MTS, medical treatment success; MTF, medical treatment failure; LDH, lactate dehydrogenase.
Radiological findings
There was no significant difference in the amount of pleural effusion, the presence of fluid shifting, the presence and number of loculations, and the presence of pleural thickening between the two groups (Table 4).
Table 4.
Radiological characteristics
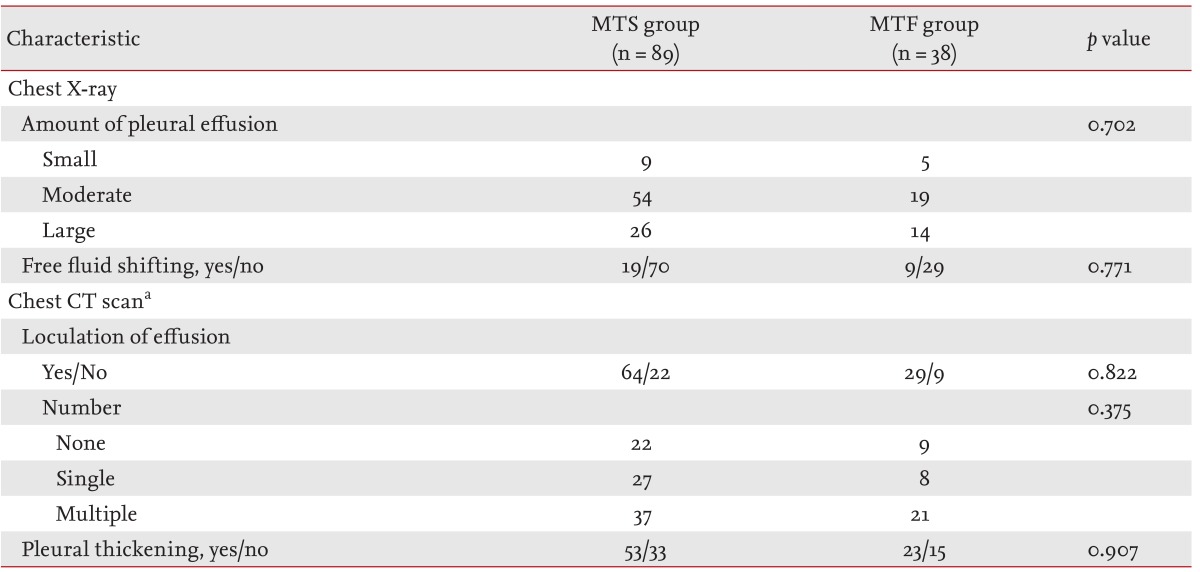
MTS, medical treatment success; MTF, medical treatment failure; CT, computed tomography.
aThree patients who had a CT scan before treatment were excluded from the analysis of CT scan results.
Analysis of predictive factors for medical treatment failure
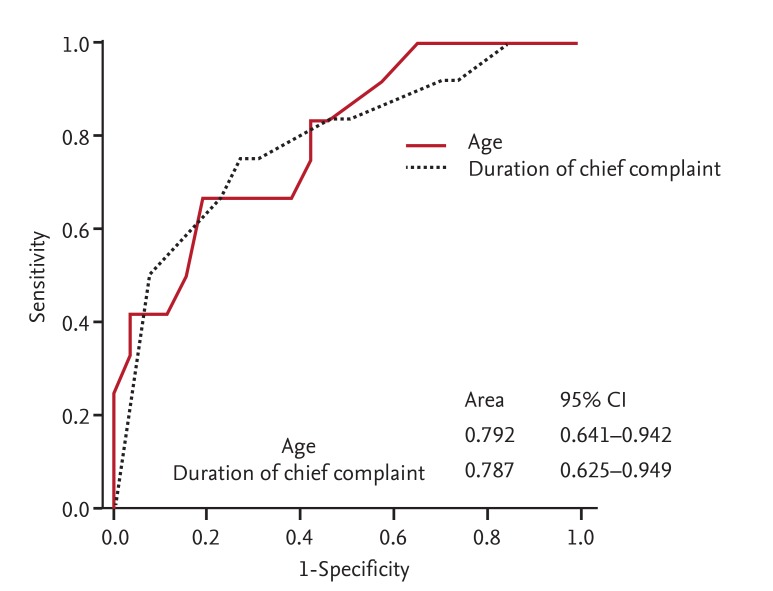
We performed multivariate logistic regression analyses comparing age, smoking history, duration of chief complaint, serum albumin, and pleural glucose and LDH levels to identify predictive factors for medical treatment failure. Age and duration of chief complaint were identified as significant independent factors predictive of failure of medical treatment, with odds ratios of 0.871 (p = 0.013) and 0.797 (p = 0.026), respectively (Table 5). ROC curve analysis was used to assess optimal cutoff values for age and duration of chief complaint. The areas under the curve for age and duration of chief complaint were 0.792 (95% confidence interval [CI], 0.641 to 0.942) and 0.787 (95% CI, 0.625 to 0.949), respectively (Fig. 1). The maximum sum of sensitivity and specificity was at 50.5 years for age and 4.5 days for duration of chief complaint. In patients with pleural infection, independent factors predictive of failure of medical treatment were age < 50.5 years (sensitivity 76.3%, specificity 84.3%, positive predictive value 67.4%, negative predictive value 89.3%) and duration of chief complaint < 4.5 days (sensitivity 79.0%, specificity 83.2%, positive predictive value 66.7%, negative predictive value 90.2%) (Table 6).
Table 5.
Multivariate logistic regression analysis of factors predicting failure of medical treatment
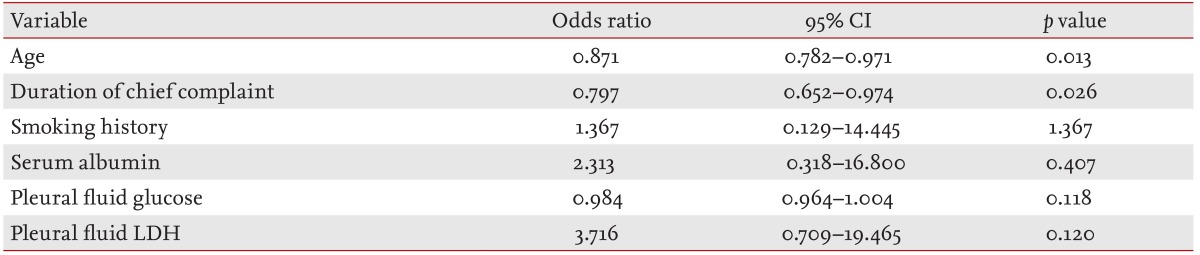
CI, confidence interval; LDH, lactate dehydrogenase.
Figure 1.
Receiver operating characteristic (ROC) curve for age (< 50.5 years) and duration of chief complaint (< 4.5 days) as factors predictive of failure of medical treatment. CI, confidence interval.
Table 6.
Accuracy of age and duration of chief complaint as predictive factors for failure of medical treatment

PPV, positive predictive value; NPV, negative predictive value.
Association of pleural LDH and glucose levels with age and duration of chief complaint
When the patients were divided into two groups according to age (≤ 50 and ≥ 51 years) and duration of chief complaint (≤ 4 and ≥ 5 days), the pleural LDH level was significantly higher in the group with younger age (p = 0.049) and shorter duration of chief complaint (p = 0.038). Although the pleural glucose level was lower in the group with a younger age and shorter duration of chief complaint, the difference was not significant (Table 7).
Table 7.
Association of p-LDH and glucose levels with age and duration of chief complaint
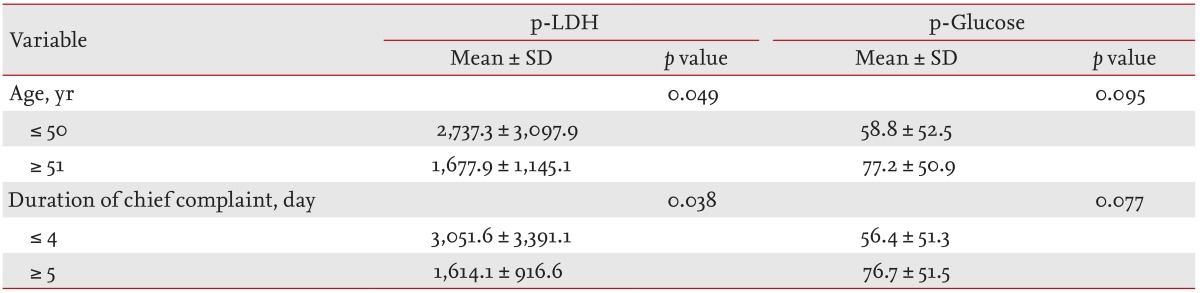
Values are presented as mean ± SD.
p-LDH, pleural lactate dehydrogenase level; p-glucose, pleural glucose level.
DISCUSSION
In this study, we showed that age, duration of chief complaint, smoking history, serum albumin level, and pleural glucose and LDH level of the MTS and MTF groups were significantly different. Further, we found that younger age (< 50.5 years) and shorter duration of chief complaint (< 4.5 days) were independent factors predictive of failure of medical treatment in patients with pleural infection. We recognize that these results are in contrast to the typical course of patients with pleural infection. It is logical that patients who are older fare worse than younger patients. In addition, untreated pleural infection tends to have a worse outcome over time, compared to in patients treated early with antibiotics. However, we focused on the pathogenesis of pleural infection and normal host defense mechanisms.
In general, parapneumonic effusions can be divided into three stages: 1) an exudative stage with a small amount of WBC; 2) a fibrinopurulent stage characterized by bacterial invasion, numerous WBCs, and deposition of fibrin; and 3) an organization stage involving formation of inelastic peels caused by attachment of pleura and fibroblast ingrown into the pleural fluid with higher viscosity [1,4]. Pleural infection occurs during the fibrinopurulent stage. In this stage, bacteria invade the pleural space, and polymorphonuclear leukocytes increase significantly in number. Various inflammatory mediators, including tumor necrosis factor (TNF)-α and interleukin (IL)-8, which are reported to play a role in the pathophysiology of infectious pleural effusion are recruited into the pleural space. This process enhances the focal inflammatory reaction, and the pleural fluid becomes more viscous and purulent [12,13]. Fibrin balance in the pleural space is disturbed, inhibiting fibrin degradation, and loculations finally begin to form. Therefore, in severe focal inflammatory reactions, prompt drainage is bound to fail because of the formation of viscous and purulent fluid and loculation in the pleural space, despite it being an essential treatment.
The focal inflammatory reaction is associated with the normal host defense mechanism against pathogens in the pleural space, and occurs as an innate immune response [14,15], which is closely related to age [16]. According to the literature, younger people have more rapid and active immune responses [16]. The younger the patient, the more active the immune response against pathogens, causing a stronger and more rapid inflammatory reaction. Therefore, the outcome of medical treatment in pleural infection was closely associated with the degree of focal inflammatory reaction in the pleural cavity. Because younger patients with pleural infection have more active and rapid focal inflammatory reactions, the duration of symptoms was shortened. A young age and short duration of symptoms were associated with an excessive focal inflammatory reaction, which may in turn lead to failure of medical treatment. This would explain our finding that younger age (< 50.5 years) and shorter duration of chief complaint (< 4.5 days) are independent factors predictive of MTF in patients with pleural infection.
Our data showed a significantly higher pleural LDH level in the patients with a younger age (≤ 50 years) and short duration of chief complaint (≤ 4 days). Although the pleural glucose level failed to reach statistical significance, it showed a lower trend in such patients. The LDH and glucose levels of pleural fluid are indicators of the degree of inflammation in pleural fluid [1,2,3,4]. In other words, patients with a younger age and shorter duration of chief complaint tended to have more severe pleural infection. Although a larger number of patients is required to reach statistical significance, this supports our suggestion that younger age (< 50.5 years) and shorter duration of chief complaint (< 4.5 days) are independent factors predictive of the failure of medical treatment in patients with pleural infection. The LDH and glucose levels of pleural fluid were not predictors of MTF according to our multivariate analysis. Poe et al. [17] implied that Light's criteria (including evidence of frank purulence, glucose level < 40 mg/dL, pH < 7.0, and/or LDH > 1,000 IU/L), known indicators for tube thoracostomy, had limited usefulness in terms of predicting the need for medical or surgical treatment.
In our study, a univariate analysis determined that smoking history and serum albumin level were significantly different between the two groups. Smoking causes imbalances in inflammatory cytokine levels by participating in inflammatory reactions [18,19]. In particular, smoking increases the level of proinflammatory cytokines, including TNF-α, IL-1, or IL-6, and inhibits the release of tissue-plasminogen activator and fibrinolytic activity by inducing vascular endothelial dysfunction and hyperactivity of plasminogen activator inhibitor-I [19,20]. Smoking in patients with pleural infection plays a role in the failure of medical treatment by aggravating the inflammatory response in the pleural cavity by promotion of the release of proinflammatory cytokines and inducing loculations via inhibition of fibrinolytic activity.
Our data showed that the serum albumin level was significantly higher in the MTF group than in the MTS group. This could be because the serum albumin level, in general, is higher in younger than older individuals [21], and younger age was an independent factor associated with MTF in our study. However, further studies are required to reach conclusions regarding the relationship between serum albumin level and the outcome of medical treatment.
We investigated the factors related to the outcome of medical treatment among those associated with the decision to treat by the primary physician. There was no significant difference in time to antibiotic treatment or catheterization between the two groups. However, because these two factors are closely associated with morbidity and mortality in patients with pleural infection [10,22,23], early use of appropriate antibiotics and prompt drainage in medical treatment of pleural infection should not be overlooked. Catheter size, catheter changes or additions, and the number of intrapleural UK instillations had no significant relationship with the outcome of medical treatment. This suggests that additional medical treatment, including change to a larger catheter, additional catheterization, or additional instillation of UK, in patients that have not responded to medical treatment would not result in an improvement.
Our study had several limitations. First, this was a non-randomized retrospective study with a small sample size. Therefore, any conclusions are limited in their implications, and we cannot rule out the presence of other confounding factors, such as selection bias or missing data, that might have affected the results. In addition, as our findings are from a single institution, they cannot be generalized. Second, our results showed that the MTF group was significantly younger than the MTS group. According to our protocol, the MTF group received surgical treatment; surgeons may be more willing to operate on younger patients, which could have been a confounding variable. However, our data demonstrated that the degree of pleural infection tended to be more severe in younger patients. Thus the degree of pleural infection might have been so severe in the younger patients that they underwent surgical treatment more frequently than older patients. Third, our study did not examine long-term follow-up results. At times, treatment of pleural infection may leave long-term pulmonary sequelae, such as pleural thickening or fibrothorax, which are diagnosed several months later. These complications can occasionally impede lung function; long-term follow-up data and simple chest radiographs or pulmonary function tests would be needed to evaluate such outcomes.
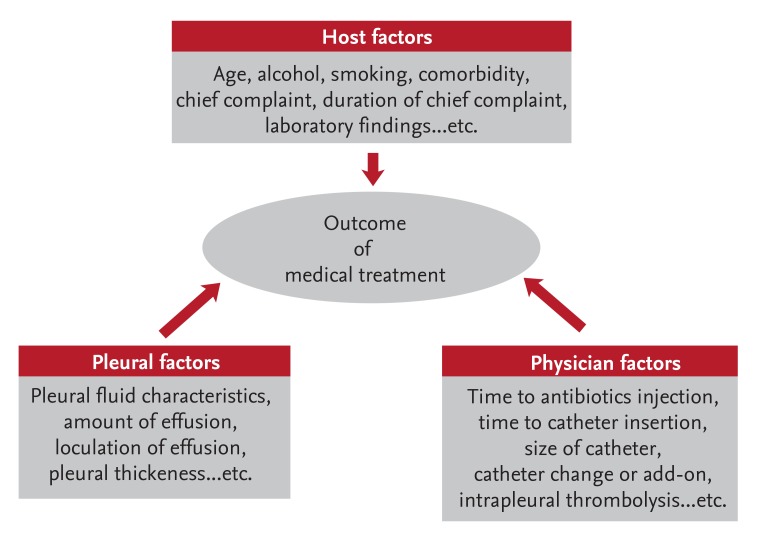
Although two similar studies investigated the factors associated with medical treatment outcomes in patients with pleural infection [24,25], they failed to show consistent results. However, based on these studies, the prognostic factors in patients with pleural infection may be divided into host, pleural, and physician factors (Fig. 2). Although all of these factors can affect the outcome of medical treatment to some degree, the identification of the most important independent factor would improve the prognosis and mortality rate of patients with pleural infection.
Figure 2.
Schematic model of factors influencing the outcome of medical treatment.
In conclusion, we herein characterized the independent factors predictive of the failure of medical treatment in patients with pleural infection. These were included age < 50.5 years and duration of chief complaint < 4.5 days, but not laboratory or radiological findings, which reiterates the findings of previous studies. Based on our results, pleural infection patients of relatively younger age and with a short duration of chief complaint should undergo surgical treatments to reduce the use of time-consuming medical management, or should undergo early surgical interventions without additional medical treatment after the failure of initial medical treatment. Although our study was small and retrospective, we cautiously suggest new criteria for selection of the optimal treatment in patients with pleural infection. Further large prospective studies are required.
KEY MESSAGE
A younger age < 50.5 years and shorter duration of chief complaint < 4.5 days were independent predictive factors for the medical treatment failure in patients with pleural infection.
Pleural infection patients of relatively younger age and with a short duration of chief complaint should undergo surgical treatments to reduce the use of time-consuming medical management, or should undergo early surgical interventions without additional medical treatment after the failure of initial medical treatment.
This could be used as a new criteria for selection of the optimal treatment in patients with pleural infection.
Acknowledgments
We thank Seok Hui Kang and Mi Na Oh for assistance with data collection and processing. We thank Young Joo Park for linguistic advice.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3:75–80. doi: 10.1513/pats.200510-113JH. [DOI] [PubMed] [Google Scholar]
- 2.Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980;69:507–512. doi: 10.1016/0002-9343(80)90460-x. [DOI] [PubMed] [Google Scholar]
- 3.Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;118:1158–1171. doi: 10.1378/chest.118.4.1158. [DOI] [PubMed] [Google Scholar]
- 4.Davies HE, Davies RJ, Davies CW BTS Pleural Disease Guideline Group. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–ii53. doi: 10.1136/thx.2010.137000. [DOI] [PubMed] [Google Scholar]
- 5.Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev. 2008;(2):CD002312. doi: 10.1002/14651858.CD002312.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352:865–874. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 7.Tokuda Y, Matsushima D, Stein GH, Miyagi S. Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: a meta-analysis. Chest. 2006;129:783–790. doi: 10.1378/chest.129.3.783. [DOI] [PubMed] [Google Scholar]
- 8.Luh SP, Chou MC, Wang LS, Chen JY, Tsai TP. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest. 2005;127:1427–1432. doi: 10.1378/chest.127.4.1427. [DOI] [PubMed] [Google Scholar]
- 9.Petrakis IE, Kogerakis NE, Drositis IE, Lasithiotakis KG, Bouros D, Chalkiadakis GE. Video-assisted thoracoscopic surgery for thoracic empyema: primarily, or after fibrinolytic therapy failure? Am J Surg. 2004;187:471–474. doi: 10.1016/j.amjsurg.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 10.LeMense GP, Strange C, Sahn SA. Empyema thoracis: therapeutic management and outcome. Chest. 1995;107:1532–1537. doi: 10.1378/chest.107.6.1532. [DOI] [PubMed] [Google Scholar]
- 11.Light RW. A new classification of parapneumonic effusions and empyema. Chest. 1995;108:299–301. doi: 10.1378/chest.108.2.299. [DOI] [PubMed] [Google Scholar]
- 12.Aleman C, Alegre J, Monasterio J, et al. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin Sci (Lond) 2003;105:601–607. doi: 10.1042/CS20030115. [DOI] [PubMed] [Google Scholar]
- 13.Porcel JM, Vives M, Esquerda A. Tumor necrosis factor-alpha in pleural fluid: a marker of complicated parapneumonic effusions. Chest. 2004;125:160–164. doi: 10.1378/chest.125.1.160. [DOI] [PubMed] [Google Scholar]
- 14.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 16.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poe RH, Marin MG, Israel RH, Kallay MC. Utility of pleural fluid analysis in predicting tube thoracostomy/decortication in parapneumonic effusions. Chest. 1991;100:963–967. doi: 10.1378/chest.100.4.963. [DOI] [PubMed] [Google Scholar]
- 18.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83:148–156. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 19.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 20.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Gom I, Fukushima H, Shiraki M, et al. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J Nutr Sci Vitaminol (Tokyo) 2007;53:37–42. doi: 10.3177/jnsv.53.37. [DOI] [PubMed] [Google Scholar]
- 22.Ashbaugh DG. Empyema thoracis: factors influencing morbidity and mortality. Chest. 1991;99:1162–1165. doi: 10.1378/chest.99.5.1162. [DOI] [PubMed] [Google Scholar]
- 23.Cham CW, Haq SM, Rahamim J. Empyema thoracis: a problem with late referral? Thorax. 1993;48:925–927. doi: 10.1136/thx.48.9.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies CW, Kearney SE, Gleeson FV, Davies RJ. Predictors of outcome and long-term survival in patients with pleural infection. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1682–1687. doi: 10.1164/ajrccm.160.5.9903002. [DOI] [PubMed] [Google Scholar]
- 25.Huang HC, Chang HY, Chen CW, Lee CH, Hsiue TR. Predicting factors for outcome of tube thoracostomy in complicated parapneumonic effusion for empyema. Chest. 1999;115:751–756. doi: 10.1378/chest.115.3.751. [DOI] [PubMed] [Google Scholar]