Abstract
Purpose
To compare the risk of hospitalization between patients with early-stage breast cancer who received different chemotherapy regimens.
Patient and Methods
We identified 3,567 patients older than age 65 years from the SEER/Texas Cancer Registry-Medicare database and 9,327 patients younger than age 65 years from the MarketScan database who were diagnosed with early-stage breast cancer between 2003 and 2007. The selection was nonrandomized and nonprospectively collected. We categorized patients according to the regimens they received: docetaxel (T) and cyclophosphamide (C), doxorubicin (A) and C, TAC, AC + T, dose-dense AC + paclitaxel (P) or AC + weekly P. We compared the rates of chemotherapy-related hospitalizations that occurred within 6 months of chemotherapy initiation and used multivariable logistic regression analysis to identify the factors associated with these hospitalizations.
Results
Among patients younger than age 65 years, the hospitalization rates ranged from 6.2% (dose-dense AC + P) to 10.0% (TAC), and those who received TAC and AC + T had significantly higher rates of hospitalization than did patients who received TC. Among patients older than age 65 years, these rates ranged from 12.7% (TC) to 24.2% (TAC) and the rates of hospitalization of patients who received TAC, AC + T, AC, or AC + weekly P were higher than those of patients who received TC.
Conclusion
TAC and AC + T were associated with the highest risk of hospitalization in patients younger than age 65 years. Among patients older than age 65 years, all regimens (aside from dose-dense AC + P) were associated with a higher risk of hospitalization than TC. Results may be affected by selection biases where less aggressive regimens are offered to frailer patients.
INTRODUCTION
In patients with early-stage breast cancer, chemotherapy reduces recurrence and mortality rates and improves survival rates.1 Anthracyclines are effective drugs, but they infrequently cause congestive heart failure (approximately 2% of patients).2–4 Anthracyclines have also been associated with secondary leukemia.5–8 Therefore, equally effective nonanthracycline-based regimens have been sought. In the United States, the use of anthracyclines has decreased, whereas the use of taxane-based regimens without an anthracycline has increased.9 This shift was likely influenced by studies initially presented in December of 2005 that suggested that taxane-based regimens without anthracycline might provide equivalent or superior results to anthracycline-based regimens.10,11
There is no evidence that taxane-based regimens without an anthracycline are superior to third-generation regimens that combine an anthracycline with a taxane. A phase III randomized clinical trial showed that the disease-free survival and overall survival of patients who received four cycles of docetaxel and cyclophosphamide was higher than that of patients who received four cycles of doxorubicin and cyclophosphamide, a first-generation anthracycline-based regimen.12,13 A phase III clinical trial comparing docetaxel and cyclophosphamide with a third-generation anthracycline/taxane regimen is ongoing.14 Thus, the evidence to recommend routinely replacing anthracyclines with taxanes in the adjuvant treatment of breast cancer is insufficient, and the optimal chemotherapy regimen in this setting remains unknown.15,16
Several chemotherapy regimens17 are available to patients diagnosed with early-stage breast cancer. Characterizing subsets of patients who are at the greatest risk for developing toxicities may help guide clinicians in the choice of a regimen. Given the uncertainty surrounding the optimal chemotherapy regimen for this disease, the toxicities of each regimen become more relevant when selecting therapy. No population-based studies have compared the toxicities of specific chemotherapy regimens.
In the present study, we used claims-based data to compare the risk of chemotherapy-related hospitalization between patients with early-stage breast cancer identified in two databases who received commonly used chemotherapy regimens.
PATIENTS AND METHODS
Data Sources
We identified patients older than age 65 years from the SEER/Texas Cancer Registry (TCR) –Medicare–linked database18,19 and patients younger than age 65 years from the MarketScan database.20
The TCR,21 which was legislatively mandated in 1979 as a component of the Texas Department of State Health Services, is the fourth largest state population-based registry. Annually, it receives approximately 250,000 reports of cancer cases from diverse clinical sources and other state registries. Vital status and cause of death information is obtained through data linkages with Texas vital statistics and mortality data, the National Death Index, and the Social Security Death Index. The TCR meets the high-quality data standards of the Centers for Disease Control and Prevention's National Program of Cancer Registries and has a Gold Standard for Registry Certification from the North American Association of Central Cancer Registries. The TCR collects data according to standardized registry rules and has core data items similar to those collected by the SEER registries.
The SEER registries provide information on approximately 25% of the nation's incident cancers and have been linked to Medicare claims.18,19 The details of the SEER-Medicare linked database have been previously published.22 The National Cancer Institute has also linked TCR data to Medicare claims, using the same standards and algorithms used to create the SEER-Medicare linkage. For the analyses in the present study, the TCR-Medicare and SEER-Medicare data sets were combined.
The MarketScan20 database consists of proprietary data sets that meet Health Insurance Portability and Accountability Act requirements for confidentiality, and undergoes quality validity checks. Files are released once 100% of the claims have been paid. In the present study, we used the Commercial Claims and Encounters data set, a large nationwide employment-based database that contains information about medical claims and outpatient prescription claims paid on behalf of employees and their dependents. The data set is considered a convenience sample that includes a younger population, covers a comprehensive geographic area, and contains demographic and comorbidity data from nationwide private medical insurance claims.
Study Population
From the SEER/TCR-Medicare linked database, we identified patients older than age 65 years who received a first diagnosis of stage I, II, or III breast cancer (identified using the International Classification of Diseases, ninth revision, and Current Procedural Terminology codes in Appendix Table A1; online only) between 2003 and 2007 and underwent lumpectomy, mastectomy, or axillary lymph node dissection. To ensure data completeness, we included only patients who had full medical insurance coverage provided by Medicare Part A and Part B during the 12 months before and after diagnosis and had not been Health Maintenance Organization members. Patients with a prior diagnosis of any other cancer were excluded. Of the 52,838 patients we identified, 10,921 had received chemotherapy; of these patients, 3,567 had received 1 of the 6 regimens described below.
From the MarketScan database, using an algorithm9 adapted from previously described algorithms,23–26 we identified patients age less than 65 years who received a first diagnosis of breast cancer between 2003 and 2007, and underwent lumpectomy, mastectomy, or axillary lymph node dissection. We included patients who had continuous medical insurance coverage during the 3 months before and 12 months after diagnosis. Of the 47,050 patients we identified, 21,833 received chemotherapy; of these patients, 9,327 had received 1 of the 6 regimens described below. The regimens received by the patients identified from either database who were excluded from the present study are given in Appendix Table A2 (online only).
Study Variables
Chemotherapy regimens.
We used Healthcare Common Procedure Coding System codes for chemotherapy drugs (“J” codes)27–30 to identify patient's chemotherapy regimens (Appendix Table A3, online only). We selected only patients who received chemotherapy within 3 months before and 12 months after the date of diagnosis (SEER/TCR-Medicare) or the date of surgery (MarketScan). We then categorized patients into 1 of 6 groups according to the specific chemotherapy regimen they received. Patients had to have received at least 2 consecutive cycles of each regimen to be included. If the time interval between claims was greater than 18 days, the regimen was considered to have been cycled every 3 weeks; if the interval was 12 to 18 days, it was considered cycled every 2 weeks, and if it was less than 12 days, it was considered a weekly dose. We selected regimens that were commonly used during the time period of the study. The selected regimens were docetaxel and cyclophosphamide administered once every 3 weeks (TC)12,13; doxorubicin and cyclophosphamide administered once every 3 weeks (AC)31,32; docetaxel, doxorubicin, and cyclophosphamide administered once every 3 weeks (TAC)33; doxorubicin and cyclophosphamide administered once every 3 weeks followed or preceded by docetaxel once every 3 weeks (AC + T; patients who received fluorouracil with this regimen were also included)34; dose-dense doxorubicin and cyclophosphamide administered every 2 weeks followed or preceded by paclitaxel once every 2 weeks (ddAC + P)35; and doxorubicin and cyclophosphamide administered once every 3 weeks followed or preceded by paclitaxel once per week (AC + wP; patients who received fluorouracil with this regimen were also included).34
Patient demographics, tumor characteristics, and treatments.
From the MarketScan database, we obtained the ages, geographic regions, diagnosis years, surgery types (lumpectomy or mastectomy), hormone therapy (identified using the National Drug Code numbers in Appendix Table A4, online only), and primary prophylaxis (PP) use of granulocyte colony-stimulating factor (G-CSF). We defined G-CSF PP use in those patients who had claims for G-CSF that occurred within 5 days after the first cycle of chemotherapy.36–38 We also identified nonsurgery-related hospitalization claims that occurred 90 days before the beginning of chemotherapy, which served as surrogate indicators of comorbidity. From the SEER/TCR-Medicare linked database, we obtained the ages, races, geographic regions, census variables (urban/rural, education, poverty level), diagnosis years, stages (localized or regional), hormone receptor status (estrogen receptor and progesterone receptor), surgery types, and G-CSF PP use. We used the Klabunde adaptation of the Charlson comorbidity index to identify these patients' comorbidities.39,40
Hospitalization claims.
To assess chemotherapy-related toxicities, we followed a published algorithm41 and evaluated the rates of the first hospitalization that occurred within 6 months of chemotherapy initiation. Using Common Procedural Terminology codes, we identified eight reasons for hospitalization: neutropenia, infection, fever, thrombocytopenia, anemia, adverse effects of chemotherapy, dehydration, and delirium. We also conducted two sensitivity analyses: we calculated the chemotherapy-related hospitalization rates of the patients who received chemotherapy but were excluded from the present study (Appendix Table A2); we calculated the nonchemotherapy-related hospitalization rates resulting from nonsurgical reasons (which were not part of the eight chemotherapy-related reasons).
Statistical Analysis
We used χ2 analysis to compare the proportions of patients who were hospitalized within 6 months of the initiation of each regimen. We used logistic regression models adjusted for patients' demographics, comorbidities, and tumor characteristics to compare the odds of hospitalization between patient groups. We used the Hosmer and Lemeshow test to check the goodness-of-fit of the models. P values less than .05 were considered statistically significant. The SAS software program version 9.2 (SAS Institute, Cary, NC) was used to perform all data management and statistical analyses.
RESULTS
Our study population consisted of 9,327 patients identified from the MarketScan database and 3,567 patients identified from the SEER/TCR-Medicare linked database. Each cohort's use of each regimen per year is shown in Figure 1. In both cohorts, the percentage of patients treated with TC increased over time, and the percentage of patients treated with AC and AC + T decreased over time.
Fig 1.
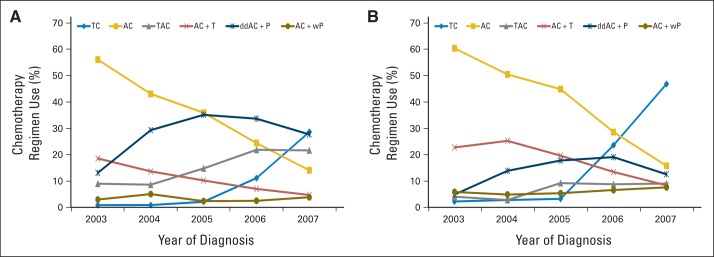
Chemotherapy regimen use according to year of diagnosis of patients with early-stage breast cancer (A) younger than age 65 years and (B) older than age 65 years. A, doxorubicin; C, cyclophosphamide; dd, dose dense; P, paclitaxel; T, docetaxel; w, weekly.
The characteristics of the patients younger than age 65 years are given in Table 1. The distribution of geographic regions varied among patients who received different regimens. The difference in hormone therapy likely reflects the rates of hormone receptor–positive disease. Among the 4,701 patients who received G-CSF PP, 96% received one dose of pegfilgrastim. The proportions of patients who received G-CSF PP were higher among those who received TAC and ddAC + P (both 79%) compared with patients who received other regimens (AC, 21%; TC, 49%). The rates of hospitalization before the initiation of chemotherapy (used as surrogate indicators of comorbidity) were consistently low (4.2%) and did not differ significantly between regimens.
Table 1.
Characteristics of Patients With Early-Stage Breast Cancer Younger Than Age 65 Years According to Chemotherapy Regimen
| Variable | Total (N = 9,327) |
TC (n = 1,060) |
AC (n = 2,889) |
TAC (n = 1,516) |
AC + T (n = 894) |
ddAC + P (n = 2,657) |
AC + wP (n = 311) |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Age, years | < .001 | ||||||||||||||
| Range | 23-64 | 26-64 | 24-64 | 27-64 | 27-64 | 23-64 | 30-64 | ||||||||
| Mean | 51 ± 8 | 52 ± 7 | 52 ± 8 | 50 ± 8 | 51 ± 8 | 50 ± 8 | 51 ± 8 | ||||||||
| Median | 52 | 52 | 52 | 51 | 52 | 51 | 52 | ||||||||
| < 35 | 271 | 2.9 | 18 | 1.7 | 65 | 2.2 | 57 | 3.8 | 18 | 2.0 | 102 | 3.8 | 11 | 3.5 | |
| 35-44 | 1,734 | 18.6 | 176 | 16.6 | 451 | 15.6 | 310 | 20.4 | 169 | 18.9 | 561 | 21.1 | 67 | 21.5 | |
| 45-54 | 3,929 | 42.1 | 436 | 41.1 | 1,240 | 42.9 | 673 | 44.4 | 360 | 40.3 | 1,111 | 41.8 | 109 | 35.0 | |
| 55-64 | 3,393 | 36.4 | 430 | 40.6 | 1,133 | 39.2 | 476 | 31.4 | 347 | 38.8 | 883 | 33.2 | 124 | 39.9 | |
| Region* | < .001 | ||||||||||||||
| Northeast | 694 | 7.4 | 84 | 7.9 | 200 | 6.9 | 89 | 5.9 | 32 | 3.6 | 275 | 10.4 | 14 | 4.5 | |
| North Central | 2,454 | 26.3 | 234 | 22.1 | 796 | 27.6 | 309 | 20.4 | 193 | 21.6 | 829 | 31.2 | 93 | 29.9 | |
| South | 4,689 | 50.3 | 578 | 54.5 | 1,412 | 48.9 | 884 | 58.3 | 516 | 57.7 | 1,130 | 42.5 | 169 | 54.3 | |
| West | 1,448 | 15.5 | 163 | 15.4 | 470 | 16.3 | 229 | 15.1 | 148 | 16.6 | 406 | 15.3 | 32 | 10.3 | |
| Surgery | < .001 | ||||||||||||||
| Lumpectomy | 4,701 | 50.4 | 579 | 54.6 | 1,754 | 60.7 | 631 | 41.6 | 369 | 41.3 | 1,234 | 46.4 | 134 | 43.1 | |
| Mastectomy | 4,529 | 48.6 | 470 | 44.3 | 1,099 | 38.0 | 871 | 57.5 | 515 | 57.6 | 1,397 | 52.6 | 177 | 56.9 | |
| Axillary LND | 97 | 1.0 | 11 | 1.0 | 36 | 1.2 | 14 | 0.9 | ≤ 10 | 26 | 1.0 | ≤ 10 | |||
| Hormone therapy† | 5,018 | 53.8 | 607 | 57.3 | 1,592 | 55.1 | 815 | 53.8 | 432 | 48.3 | 1,431 | 53.9 | 141 | 45.3 | < .001 |
| G-CSF primary prophylaxis | 4,701 | 50.4 | 518 | 48.9 | 597 | 20.7 | 1,204 | 79.4 | 220 | 24.6 | ,2089 | 78.6 | 73 | 23.5 | < .001 |
| Hospitalization in previous 90 days‡ | 394 | 4.2 | 49 | 4.6 | 105 | 3.6 | 76 | 5.0 | 46 | 5.1 | 107 | 4.0 | 11 | 3.5 | .172 |
Abbreviations: AC, doxorubicin and cyclophosphamide; AC + T, doxorubicin and cyclophosphamide followed or preceded by docetaxel; AC + wP, doxorubicin and cyclophosphamide followed or preceded by weekly paclitaxel; ddAC + P, dose-dense doxorubicin and cyclophosphamide followed or preceded by paclitaxel; G-CSF, granulocyte colony-stimulating factor; LND, lymph node dissection; TAC, docetaxel, doxorubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide.
Unknown in 42 patients.
Received tamoxifen or an aromatase inhibitor.
Occurring within 90 days before initiation of chemotherapy.
The characteristics of the patients younger than age 65 years are given in Table 2. The distributions of geographic regions and race/ethnicity varied among patients who received different regimens. Among the 2,501 patients who received G-CSF PP, 83% received one dose of pegfilgrastim. The proportions of patients who received G-CSF PP by regimen ranged from 56% (AC) to 94% (ddAC + P).
Table 2.
Characteristics of Patients With Early-Stage Breast Cancer Older Than Age 65 Years According to Chemotherapy Regimen
| Variable | Total (N = 3,567) |
TC (n = 597) |
AC (n = 1,407) |
TAC (n = 240) |
AC + T (n = 629) |
ddAC + P (n = 477) |
AC + wP (n = 217) |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Age, years | < .001 | ||||||||||||||
| Range | 66-91 | 66-91 | 66-88 | 66-82 | 66-88 | 66-85 | 66-85 | ||||||||
| Mean | 71 ± 4 | 72 ± 4 | 72 ± 4 | 70 ± 4 | 71 ± 3 | 70 ± 3 | 71 ± 4 | ||||||||
| Median | 70 | 70 | 71 | 70 | 70 | 70 | 70 | ||||||||
| 66-70 | 1,872 | 52.5 | 278 | 46.6 | 714 | 50.7 | 164 | 68.3 | 321 | 51.0 | 299 | 62.7 | 96 | 44.2 | |
| 71-75 | 1,129 | 31.7 | 199 | 33.3 | 458 | 32.6 | 61 | 25.4 | 205 | 32.6 | 137 | 28.7 | 69 | 31.8 | |
| > 75* | 566 | 15.9 | 120 | 20.1 | 235 | 16.7 | 15 | 6.3 | 103 | 16.4 | 41 | 8.6 | 52 | 24.0 | |
| Region | < .001 | ||||||||||||||
| CA + HI | 823 | 23.1 | 168 | 28.1 | 339 | 24.1 | 55 | 22.9 | 115 | 18.3 | 112 | 23.5 | 34 | 15.7 | |
| NJ | 421 | 11.8 | 80 | 13.4 | 169 | 12.0 | 19 | 7.9 | 53 | 8.4 | 84 | 17.6 | 16 | 7.4 | |
| TX | 1,114 | 31.2 | 178 | 29.8 | 383 | 27.2 | 83 | 34.6 | 277 | 44.0 | 89 | 18.7 | 104 | 47.9 | |
| Other† | 1,209 | 33.9 | 171 | 28.6 | 516 | 36.7 | 83 | 34.6 | 184 | 29.3 | 192 | 40.3 | 63 | 29.0 | |
| Race/ethnicity | < .001 | ||||||||||||||
| White | 2,858 | 80.1 | 483 | 80.9 | 1,127 | 80.1 | 208 | 86.7 | 477 | 75.8 | 400 | 83.9 | 163 | 75.1 | |
| Black | 276 | 7.7 | 49 | 8.2 | 103 | 7.3 | < 25 | 64 | 10.2 | 30 | 6.3 | < 25 | |||
| Hispanic | 308 | 8.6 | 45 | 7.5 | 118 | 8.4 | 12 | 5.0 | 75 | 11.9 | 32 | 6.7 | 26 | 12.0 | |
| Other | 125 | 3.5 | 20 | 3.4 | 59 | 4.2 | < 25 | 13 | 2.1 | 15 | 3.1 | < 25 | |||
| Stage | < .001 | ||||||||||||||
| Localized | 1,390 | 39.0 | 287 | 48.1 | 817 | 58.1 | 56 | 23.3 | 112 | 17.8 | 77 | 16.1 | 41 | 18.9 | |
| Regional | 2,177 | 61.0 | 310 | 51.9 | 590 | 41.9 | 184 | 76.7 | 517 | 82.2 | 400 | 83.9 | 176 | 81.1 | |
| ER/PR status | < .001 | ||||||||||||||
| Negative | 680 | 19.1 | 127 | 21.3 | 294 | 20.9 | 30 | 12.5 | 90 | 14.3 | 110 | 23.1 | 29 | 13.4 | |
| Positive | 1,618 | 45.4 | 273 | 45.7 | 660 | 46.9 | 114 | 47.5 | 236 | 37.5 | 257 | 53.9 | 78 | 35.9 | |
| Unknown | 1,269 | 35.6 | 197 | 33.0 | 453 | 32.2 | 96 | 40.0 | 303 | 48.2 | 110 | 23.1 | 110 | 50.7 | |
| Surgery | < .001 | ||||||||||||||
| Lumpectomy | 1,599 | 44.8 | 297 | 49.7 | 715 | 50.8 | 91 | 37.9 | 222 | 35.3 | 193 | 40.5 | 81 | 37.3 | |
| Mastectomy | 1,968 | 55.2 | 300 | 50.3 | 692 | 49.2 | 149 | 62.1 | 407 | 64.7 | 284 | 59.5 | 136 | 62.7 | |
| G-CSF primary prophylaxis | 2,501 | 70.1 | 458 | 76.7 | 788 | 56.0 | 214 | 89.2 | 450 | 71.5 | 449 | 94.1 | 142 | 65.4 | < .001 |
| Charlson comorbidity index | .0167 | ||||||||||||||
| 0 | 2,578 | 72.3 | 409 | 68.5 | 1,049 | 74.6 | 174 | 72.5 | 440 | 70.0 | 364 | 76.3 | 142 | 65.4 | |
| 1 | 709 | 19.9 | 127 | 21.3 | 262 | 18.6 | 49 | 20.4 | 137 | 21.8 | 78 | 16.4 | 56 | 25.8 | |
| ≥ 2 | 280 | 7.8 | 61 | 10.2 | 96 | 6.8 | 17 | 7.1 | 52 | 8.3 | 35 | 7.3 | 19 | 8.8 | |
NOTE. Variables with small numbers were suppressed for confidentiality reasons.
Abbreviations: AC, doxorubicin and cyclophosphamide; AC + T, doxorubicin and cyclophosphamide followed or preceded by docetaxel; AC + wP, doxorubicin and cyclophosphamide followed or preceded by weekly paclitaxel; ddAC + P, dose-dense doxorubicin and cyclophosphamide followed or preceded by paclitaxel; ER, estrogen receptor; G-CSF, granulocyte colony-stimulating factor; PR, progesterone receptor; TAC, docetaxel, doxorubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide.
The age categories 76-80 years and > 80 years were combined as a result of small numbers.
Regions combined as a result of small numbers: Atlanta and rural GA; CT; Detroit, MI; IA; KY; LA; NM; Seattle, WA; and UT.
The proportions of patients who were admitted to the hospital for chemotherapy-related reasons within 6 months of the initiation of chemotherapy are shown in Figure 2A. Among patients younger than age 65 years, the proportions ranged from 6.2% (ddAC + P) to 10% (TAC). Only 1.2% of patients younger than age 65 years were hospitalized more than once, whereas 6.0% were hospitalized for neutropenia, fever, or infection. The hospitalization rates resulting from nonchemotherapy-related reasons ranged from 3.1% (TAC) to 6.7% (AC + wP). Among patients older than age 65 years, the proportions of those who were admitted to the hospital ranged from 12.7% (TC) to 24.2% (TAC); less than 5% of patients were hospitalized more than once, and 12.4% were hospitalized for neutropenia, fever, or infection. The overall hospitalization rate among patients older than age 65 years for nonchemotherapy-related reasons was 4.5%, whereas those treated with AC + wP had the lowest rate, and those treated with AC + T had the highest rate (5.1%). Figure 2B shows the hospitalization rates for each regimen adjusted by G-CSF PP use.
Fig 2.
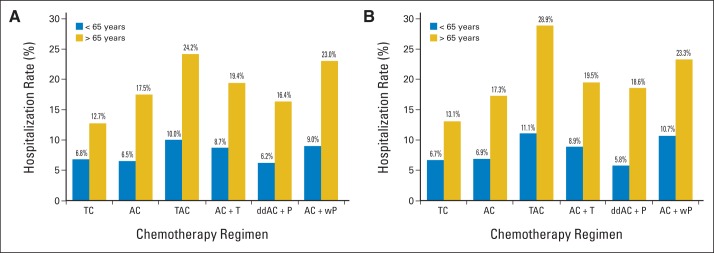
(A) Proportions of patients with early-stage breast cancer younger than age 65 years (blue) and older than age 65 years (gold) hospitalized for chemotherapy-related reasons (neutropenia, fever, infection, thrombocytopenia, anemia, adverse effects of chemotherapy, dehydration, delirium) per chemotherapy regimen. (B) Hospitalization rates adjusted for granulocyte colony-stimulating factor primary prophylaxis. A, doxorubicin; C, cyclophosphamide; dd, dose dense; P, paclitaxel; T, docetaxel; w, weekly.
The results of the multivariable logistic regression analysis are given in Tables 3 and 4. Patients younger than age 65 years who received TAC (odds ratio [OR], 1.74; 95% CI, 1.28 to 2.37) or AC + T (OR, 1.50; 95% CI, 1.05 to 2.15) had increased odds of hospitalization for chemotherapy-related toxicities compared with those who received TC. The odds of hospitalization of patients who received other regimens did not differ significantly from those patients treated with TC. Additionally, patients who had been hospitalized within 90 days before beginning chemotherapy were 85% more likely to be hospitalized for chemotherapy-related toxicities (OR, 1.85; 95% CI, 1.35 to 2.52). In patients older than age 65 years, the regimens TAC, AC + T, AC, and AC + wP had odds of hospitalization for chemotherapy-related toxicities that were significantly higher than for those treated with TC. The odds of hospitalization of the patients who received ddAC + P did not differ significantly from patients who received TC. Older age was also associated with higher odds of hospitalization. The Charlson comorbidity score was independently associated with hospitalization; compared with patients with a score of 0, those with a score of 1 had an OR of 1.48 (95% CI, 1.19 to 1.84), and patients with a score of ≥ 2 had an OR of 2.70 (95% CI, 2.03 to 3.59).
Table 3.
Logistic Regression Analysis for the Odds of Chemotherapy-Related Hospitalizations Within 6 Months of Therapy Initiation Among Patients With Early-Stage Breast Cancer Age Younger Than 65 Years
| Variable | OR* | 95% CI |
|---|---|---|
| Chemotherapy regimen | ||
| TC | Reference | |
| AC | 1.14 | 0.83 to 1.55 |
| TAC | 1.74 | 1.28 to 2.37 |
| AC + T | 1.50 | 1.05 to 2.15 |
| ddAC + P | 1.06 | 0.78 to 1.44 |
| AC + wP | 1.46 | 0.91 to 2.32 |
| Age at diagnosis, years | ||
| < 35 | Reference | |
| 35-44 | 0.75 | 0.47 to 1.21 |
| 45-54 | 0.82 | 0.52 to 1.28 |
| 55-64 | 1.02 | 0.65 to 1.60 |
| Year of diagnosis | ||
| 2003 | Reference | |
| 2004 | 0.96 | 0.72 to 1.29 |
| 2005 | 0.99 | 0.72 to 1.35 |
| 2006 | 0.93 | 0.69 to 1.26 |
| 2007 | 1.34 | 1.01 to 1.79 |
| Surgery | ||
| Mastectomy | Reference | |
| Lumpectomy/axillary | 0.87 | 0.74 to 1.02 |
| Hormonal therapy | 0.88 | 0.75 to 1.03 |
| G-CSF primary prophylaxis | 0.94 | 0.77 to 1.14 |
| Hospitalization in previous 90 days† | 1.85 | 1.35 to 2.52 |
NOTE. Bold font indicates statistical significance.
Abbreviations: AC, doxorubicin and cyclophosphamide; AC + T, doxorubicin and cyclophosphamide followed or preceded by docetaxel; AC + wP, doxorubicin and cyclophosphamide followed or preceded by weekly paclitaxel; ddAC + P, dose-dense doxorubicin and cyclophosphamide followed or preceded by paclitaxel; G-CSF, granulocyte colony-stimulating factor; OR, odds ratio; TAC, docetaxel, doxorubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide.
Odds ratios are adjusted for the variables listed in Table 1 in addition to the variable region.
Occurring within 90 days before initiation of chemotherapy.
Table 4.
Logistic Regression Analysis for the Odds of Chemotherapy-Related Hospitalizations Within 6 Months of Therapy Initiation Among Patients With Early-Stage Breast Cancer Older Than Age 65 Years
| Variable | OR* | 95% CI |
|---|---|---|
| Chemotherapy regimen | ||
| TC | Reference | |
| AC | 1.50 | 1.08 to 2.08 |
| TAC | 2.43 | 1.61 to 3.68 |
| AC + T | 1.49 | 1.04 to 2.14 |
| ddAC + P | 1.45 | 0.99 to 2.13 |
| AC + wP | 1.80 | 1.18 to 2.76 |
| Age at diagnosis, years | ||
| 66-70 | Reference | |
| 71-75 | 1.39 | 1.13 to 1.70 |
| 76-80 | 1.39 | 1.06 to 1.83 |
| > 80 | 2.72 | 1.75 to 4.23 |
| Year of diagnosis | ||
| 2003 | Reference | |
| 2004 | 0.92 | 0.69 to 1.22 |
| 2005 | 0.93 | 0.69 to 1.25 |
| 2006 | 1.07 | 0.79 to 1.45 |
| 2007 | 0.92 | 0.67 to 1.25 |
| Race/ethnicity | ||
| White | Reference | |
| African American | 1.04 | 0.74 to 1.45 |
| Hispanic | 1.05 | 0.74 to 1.48 |
| Other | 1.91 | 1.21 to 3.04 |
| Stage | ||
| Localized | Reference | |
| Regional | 1.22 | 0.99 to 1.51 |
| ER/PR | ||
| Negative | Reference | |
| Positive | 0.94 | 0.74 to 1.21 |
| Unknown | 0.61 | 0.36 to 1.04 |
| Surgery | ||
| Mastectomy | Reference | |
| Lumpectomy | 0.92 | 0.76 to 1.11 |
| Use of G-CSF primary prophylaxis | 0.85 | 0.69 to 1.04 |
| Charlson comorbidity index | ||
| 0 | Reference | |
| 1 | 1.48 | 1.19 to 1.84 |
| ≥ 2 | 2.70 | 2.03 to 3.59 |
NOTE. Bold font indicates statistical significance.
Abbreviations: AC, doxorubicin and cyclophosphamide; AC + T, doxorubicin and cyclophosphamide followed or preceded by docetaxel; AC + wP, doxorubicin and cyclophosphamide followed or preceded by weekly paclitaxel; ddAC + P, dose-dense doxorubicin and cyclophosphamide followed or preceded by paclitaxel; ER, estrogen receptor; G-CSF, granulocyte colony-stimulating factor; OR, odds ratio; PR, progesterone receptor; TAC, docetaxel, doxorubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide.
Adjusted for the variables listed in Table 1 in addition to SEER region and Census variables
DISCUSSION
We used medical insurance claims to compare the chemotherapy-related hospitalization rates of patients with early-stage breast cancer who received specific chemotherapy regimens. In both cohorts, the variations in the risk of hospitalization among patients who received different regimens were significant. Overall, patients older than age 65 years had a high rate of chemotherapy-related hospitalization compared with patients younger than age 65 years. Comorbid conditions were also associated with higher rates of hospitalization in both cohorts. These results suggest that, for all age groups, the regimens TAC and AC + T may be associated with a higher risk of chemotherapy-related hospitalizations.
The durations of the chemotherapy regimens are characteristically different. For example, the TC regimen is usually given over 12 weeks, whereas the AC + wP regimen is usually given over 24 weeks. These inherent differences may affect the results, given that patients who have a longer exposure to chemotherapy may have an increased likelihood of chemotherapy-related toxicities compared with patients who receive regimens of a shorter duration.
By using hospitalization claims in the SEER-Medicare linked database, Du et al41 reported that (compared with cyclophosphamide, methotrexate, and fluorouracil) anthracycline-containing agents were associated with greater odds of chemotherapy-related toxicities in older women with breast cancer. However, taxane-based regimens were not considered in this study, and the study was not limited to adjuvant therapy. Rajan et al36–38 have reported that G-CSF PP significantly reduces hospitalization rates in SEER-Medicare patients and improves the ability of elderly patients to complete all cycles of chemotherapy. Using the MarketScan database, Hassett et al42 suggested that chemotherapy-related adverse events among younger patients with breast cancer (< age 64 years) may be more common than those reported by clinical trials.
A systematic review by Kuderer et al43 of 17 randomized controlled trials that had enrolled patients with solid tumor and malignant lymphoma (age range, 15-90 years) showed that G-CSF PP reduced the risk of febrile neutropenia in patients, independently of tumor type, age, or type of G-CSF used. Using a prospective cohort of 3,760 patients with solid tumor and malignant lymphoma (39% with breast cancer), Lyman et al44 developed a risk prediction model for neutropenic complications. Interestingly, the initial clinical trials reported that febrile neutropenia occurred in only 4% to 8% of patients who received the regimen TC.12,13 However, febrile neutropenia has been recently reported to occur in 24% to 30% of patients treated with TC,45,46 placing this regimen in a high-risk category for febrile neutropenia. Because of differences in reporting toxicities, we could not directly compare the findings of our study with the adverse events described in clinical trials, which typically report toxicities according to the National Cancer Institute Common Toxicity Criteria. Noticeably, the use of G-CSF PP was not significantly protective in the multivariable regression models, which could be explained by a potential bias characteristic of observational data, in which patients more vulnerable to chemotherapy-related toxicities may be more likely to receive G-CSF PP.36,38
The potential limitations of the present study are inherent to retrospective claims-based research,47 which is at best hypothesis-generating. Administrative data do not sufficiently describe individual patients' treatment elements and outcomes and do not permit full adjustment for all key clinical confounders for hospitalization such as severity of comorbid conditions, patient's performance status, frailty, and laboratory abnormalities, which may also influence the choice of the chemotherapy regimen. Those who could not continue chemotherapy after the first cycle as a result of toxicities would be excluded from our study. Given that the intended treatment plan for each patient is unknown, it is impossible to measure the rates of early stopping of treatment for each regimen. In MarketScan, those who suffered severe toxicities that affected their employment status and possible loss of medical insurance may also have been excluded. The MarketScan database does not include tumor stage information, and patients younger than age 65 years may have been misclassified as having early-stage disease. Hospitalization data underrepresents chemotherapy-induced toxicities, because mild adverse events are usually managed in the outpatient setting. Long-term chemotherapy-related effects, such as cardiac toxicities associated with anthracyclines, or debilitating neurological toxicities were beyond the scope of the present study, and we recognize that these toxicities may factor into a clinician's choice of regimen. Errors in Medicare billing and errors in capturing claims may have also occurred. Misclassification errors usually generate an underestimation of the strength of association. The eligibility criteria of the present study were designed to reduce the frequency of misclassification errors, which tend to even out over larger groups of patients. These results are from nonrandomized and nonprospective collected administrative data sets, and the toxicity results are not adjusted for the selection bias in which physicians tend to select sicker patients for less aggressive regimens; however, this bias would make these results less pronounced.
A substantial number of patients who received chemotherapy were excluded because of our selection criteria. The sensitivity analysis showed that, of the 12,506 patients younger than age 65 years who were excluded, 9.0% had been hospitalized; of the 7,354 patients older than age 65 years who were excluded, 20.8% had been hospitalized (Appendix Table A2). Overall, these rates are similar to those of the patients included in the present study. In addition, death information was available only for patients older than age 65 years; the percentage of these patients who died within 6 months after starting chemotherapy was less than 1% (the absolute numbers are not reported owing to confidentiality agreements).
In conclusion, these findings suggest that the TAC and AC + T chemotherapy regimens may be associated with the highest risk of chemotherapy-related hospitalization in patients with early-stage breast cancer who are younger than age 65 years. In patients older than age 65 years, all regimens included in this study (aside from ddAC + P) may also be associated with a higher risk of hospitalization compared with the TC regimen.
Acknowledgment
This study used the SEER-Medicare linked database. We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in creating the SEER-Medicare linked database.
Appendix
Table A1.
ICD-9 and CPT Codes Used to Identify Diagnosis or Procedures
| Diagnosis or Procedure | ICD-9 Code | CPT Code |
|---|---|---|
| Breast cancer | 174.x | |
| Lumpectomy | 85.20-85.23, 85.25 | 19110, 19120, 19125, 19126, 19160, 19162, 19301, 19302 |
| Mastectomy | 85.41-85.48 | 19180, 19182, 19200, 19220, 19240, 19303-19307 |
| Axillary lymph node dissection | 40.3 | 38740, 38745, 38525 |
Abbreviations: CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, ninth revision.
Table A2.
Chemotherapy Regimens Received by Excluded Patients With Early-Stage Breast Cancer and Proportion of Patients Hospitalized for Chemotherapy-Related Reasons (neutropenia, infection, fever, thrombocytopenia, anemia, adverse effects of chemotherapy, dehydration and delirium)
| Chemotherapy Regimen | Patients Hospitalized |
|||
|---|---|---|---|---|
| ≥ Age 65 Years (n = 7,354) |
≤ Age 65 Years (n = 12,506) |
|||
| No. | % | No. | % | |
| Trastuzumab containing | 1,737 | 19.8 | 3,688 | 7.9 |
| Other anthracycline and taxane containing | 2,283 | 23.5 | 4,552 | 10.7 |
| Anthracycline without a taxane | 812 | 24.9 | 1,939 | 9.2 |
| Cyclophosphamide, methotrexate, and fluorouracil | 1,456 | 15.2 | 661 | 4.7 |
| Other | 1,066 | 24.3 | 1,666 | 8.3 |
Table A3.
Health Care Common Procedure Coding System J Codes Used to Identify Chemotherapy Agents
| Agent | J Code |
|---|---|
| Doxorubicin | J9000 |
| Docetaxel | J9170 |
| Paclitaxel | J9265 |
| Cyclophosphamide | J9070, J9080, J9090, J9091, J9092, J9093, J9094, J9095, J9096, J9097 |
| Granulocyte colony-stimulating factor | J1440, J1441, J2505, J2820 |
Table A4.
National Drug Codes Used to Identify Hormone Products
| Hormone Product | National Drug Code |
|---|---|
| Anastrozole | 00054016413, 00093753656, 00378603405, 00378603477, 00781535631, 00904619546,16571042103, 16729003510, 38779227403, 38779227404, 38779227406, 42043018003, 51079032301, 51079032306, 51991062010, 51991062033, 54569619800, 55111064730, 60258086603, 63323012930, 63275993001, 63275993002, 63275993003, 63275993004, 66435041530, 68084044821, 68382020906, 68382020910 |
| Arimidex | 00310020130, 00310020137, 12280034630, 35356027030, 54569573100, 54868500000, 55175550503 |
| Aromasin | 00009766304, 49999098630, 54569573200, 54868526100, 63629126201 |
| Femara | 00078024915, 35356040930, 54569571400, 54868415100 |
| Nolvadex | 00310060018, 00310060025, 00310060060, 00310060075, 00310060412, 00310060430, 00310060490, 00403150571, 54569038200, 54569038202, 54569853100, 55175550006, 55289058530, 57866661501, 57866661801, 58016065760, 60346004832, 66105083201, 66105083203, 66105083206, 66105083209, 66105083210 |
| Soltamox | 13632012301 |
| Tamoxifen citrate | 00054483121, 00054483126, 00054483413, 00054483422, 00054883125, 00054883425, 00093078201, 00093078205, 00093078210, 00093078256, 00093078405, 00093078406, 00093078410, 00093078486, 00172565649, 00172565658, 00172565670, 00172565680, 00172565746, 00172565760, 00172565770, 00172565780, 00310073060, 00310073130, 00378014405, 00378014491, 00378027401, 00378027493, 00555044603, 00555044605, 00555044609, 00555044663, 00555090401, 00555090405, 00555090414, 00591223218, 00591223260, 00591223319, 00591223330, 54569376500, 54569376501, 54569571600, 54569585700, 54569860200, 54868300401, 54868300402, 54868300403, 54868300404, 54868300405, 54868428700, 54868428701, 54868428702, 54868428703, 54868428704, 60346090060, 63739026910, 63739026915 |
Footnotes
Supported in part by Grant No. RP101207 from the Center for Comparative Effectiveness Research on Cancer in Texas, a multiuniversity consortium funded by the Cancer Prevention and Research Institute of Texas; Grant No. 2P30 CA016672 from the National Institutes of Health through MD Anderson's Cancer Center Support; and the Dickson Fund for Breast Cancer Research and the Duncan Family Institute. S.H.G. is supported by Grant No. RSG-09-149-01-CPHPS from the American Cancer Society. The collection of cancer incidence data used in this study was supported by the Texas Department of State Health Services and Cancer Prevention Research Institute of Texas as part of the statewide cancer reporting program and the Centers for Disease Control and Prevention National Program of Cancer Registries Cooperative Agreement 5U58/DP000824-05.
Presented in poster format at the Cancer Prevention Research Annual Conference, Austin, TX, October 24-26, 2012.
The contents of this study are solely the responsibility of its authors and do not necessarily represent the official views of the Texas Department of State Health Services, Cancer Prevention Research Institute of Texas, or Centers for Disease Control and Prevention.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Linda S. Elting, Helsinn; Benjamin D. Smith, Varian Medical Systems Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Carlos H. Barcenas, Jiangong Niu, Ning Zhang, Thomas A. Buchholz, Benjamin D. Smith, Sharon H. Giordano
Administrative support: Gabriel N. Hortobagyi
Collection and assembly of data: Carlos H. Barcenas, Jiangong Niu, Ning Zhang, Yufeng Zhang, Benjamin D. Smith, Sharon H. Giordano
Data analysis and interpretation: Carlos H. Barcenas, Jiangong Niu, Ning Zhang, Yufeng Zhang, Linda S. Elting, Gabriel N. Hortobagyi, Sharon H. Giordano
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal SK, Childs BH, Pegram M. Emergence of nonanthracycline regimens in the adjuvant treatment of breast cancer. Breast Cancer Res Treat. 2010;119:25–32. doi: 10.1007/s10549-009-0567-y. [DOI] [PubMed] [Google Scholar]
- 4.Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Curtis RE, Boice JD, Jr, Stovall M, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 6.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 7.Campone M, Roché H, Kerbrat P, et al. Secondary leukemia after epirubicin-based adjuvant chemotherapy in operable breast cancer patients: 16 years experience of the French Adjuvant Study Group. Ann Oncol. 2005;16:1343–1351. doi: 10.1093/annonc/mdi251. [DOI] [PubMed] [Google Scholar]
- 8.Patt DA, Duan Z, Fang S, et al. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: Understanding risk. J Clin Oncol. 2007;25:3871–3876. doi: 10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 9.Giordano SH, Lin YL, Kuo YF, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones SE, Savin MA, Holmes FA, et al. Final analysis: TC (docetaxel/cyclophosphamide, 4 cycles) has a superior disease-free survival compared to standard AC (doxorubicin/cyclophosphamide) in 1016 women with early-stage breast cancer. Breast Cancer Res Treat. 2005;(suppl 1):94. abstr 40. [Google Scholar]
- 11.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2neu positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat. 2005;94(suppl 1) abstr 1. [Google Scholar]
- 12.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov. TAC Versus TC for Adjuvant Breast Cancer: NCT00493870. http://clinicaltrials.gov/show/NCT00493870.
- 15.Gianni L, Norton L, Wolmark N, et al. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27:4798–4808. doi: 10.1200/JCO.2008.21.4791. [DOI] [PubMed] [Google Scholar]
- 16.Henderson IC. Can we abandon anthracyclines for early breast cancer patients? Oncology (Williston Park) 2011;25:115–124. 127. [PubMed] [Google Scholar]
- 17.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 18.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Hansen LG, Chang S. White Paper: Health Research Data for the Real World: The Thomson Reuters MarketScan Databases. Thomson Reuters. 2011:32. [Google Scholar]
- 21.Risser DR, Bowcock CL, Miller EA, et al. Austin, TX: Texas Department of State Health Services; Cancer Prevention Research Institute of Texas; 2011. Cancer in Texas, Texas Cancer Registry 2011; p. 25. [Google Scholar]
- 22.Barcenas CH, Zhang N, Zhao H, et al. Anthracycline regimen adherence in older patients with early breast cancer. Oncologist. 2012;17:303–311. doi: 10.1634/theoncologist.2011-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman JL, Zhang D, Freeman DH, et al. An approach to identifying incident breast cancer cases using Medicare claims data. J Clin Epidemiol. 2000;53:605–614. doi: 10.1016/s0895-4356(99)00173-0. [DOI] [PubMed] [Google Scholar]
- 24.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold HT, Do HT. Evaluation of three algorithms to identify incident breast cancer in Medicare claims data. Health Serv Res. 2007;42:2056–2069. doi: 10.1111/j.1475-6773.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldi I, Vicari P, Di Cuonzo D, et al. A high positive predictive value algorithm using hospital administrative data identified incident cancer cases. J Clin Epidemiol. 2008;61:373–379. doi: 10.1016/j.jclinepi.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: Findings from Medicare claims data. J Clin Oncol. 2001;19:1455–1461. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 28.Du X, Goodwin JS. Increase of chemotherapy use in older women with breast carcinoma from 1991 to 1996. Cancer. 2001;92:730–737. doi: 10.1002/1097-0142(20010815)92:4<730::aid-cncr1376>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(suppl 8):IV-55-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 30.Lamont EB, Herndon JE, 2nd, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. J Natl Cancer Inst. 2005;97:1080–1083. doi: 10.1093/jnci/dji189. [DOI] [PubMed] [Google Scholar]
- 31.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 32.Fisher B, Anderson S, Tan-Chiu E, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001;19:931–942. doi: 10.1200/JCO.2001.19.4.931. [DOI] [PubMed] [Google Scholar]
- 33.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 34.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 36.Rajan SS, Stearns SC, Lyman GH, et al. Effect of primary prophylactic G-CSF use on systemic therapy administration for elderly breast cancer patients. Breast Cancer Res Treat. 2011;130:255–266. doi: 10.1007/s10549-011-1553-8. [DOI] [PubMed] [Google Scholar]
- 37.Rajan SS, Lyman GH, Carpenter WR, et al. Chemotherapy characteristics are important predictors of primary prophylactic CSF administration in older patients with breast cancer. Breast Cancer Res Treat. 2011;127:511–520. doi: 10.1007/s10549-010-1216-1. [DOI] [PubMed] [Google Scholar]
- 38.Rajan SS, Lyman GH, Stearns SC, et al. Effect of primary prophylactic granulocyte-colony stimulating factor use on incidence of neutropenia hospitalizations for elderly early-stage breast cancer patients receiving chemotherapy. Med Care. 2011;49:649–657. doi: 10.1097/MLR.0b013e318215c42e. [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081-1090. [DOI] [PubMed] [Google Scholar]
- 41.Du XL, Osborne C, Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol. 2002;20:4636–4642. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassett MJ, O'Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 43.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 44.Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117:1917–1927. doi: 10.1002/cncr.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton EP, Topping EL, Peppercorn JM, et al. Clinical impact of febrile neutropenia (FN) increase among patients receiving adjuvant docetaxel/cyclophosphamide (TC) chemotherapy compared to TC plus pegfilgrastim for breast cancer. J Clin Oncol. 2013;31(suppl):67s. abstr 1076. [Google Scholar]
- 46.Lakhanpal R, Stuart-Harris R, Chan A, et al. A multicenter audit of the incidence of febrile neutropenia and neutropenia associated with docetaxel and cyclophosphamide (TC) chemotherapy for early breast cancer. J Clin Oncol. 2013;31(suppl):68s. abstr 1079. [Google Scholar]
- 47.Giordano SH, Kuo YF, Duan Z, et al. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]


