Abstract
Purpose
Most research regarding fertility in young women with breast cancer has focused on long-term survivors. Little is known about how fertility concerns affect treatment decisions or fertility preservation strategies at the time of initial cancer diagnosis.
Patients and Methods
As part of an ongoing prospective multicenter cohort study, we surveyed women with newly diagnosed early-stage breast cancer at age ≤ 40 years. The baseline survey included sociodemographic, medical, and treatment data as well as a modified Fertility Issues Survey, including fertility concern and preservation items. Univariable and multivariable modeling were used to investigate predictors of greater fertility concern.
Results
Among the first 620 eligible respondents included in this analysis, median age was 37 years (range, 17 to 40 years); 425 women (68%) discussed fertility issues with their physicians before starting therapy, and 319 (51%) were concerned about becoming infertile after treatment. Because of concerns about fertility, four women (1%) chose not to receive chemotherapy, 12 (2%) chose one chemotherapy regimen over another, six (1%) considered not receiving endocrine therapy, 19 (3%) decided not to receive endocrine therapy, and 71 (11%) considered receiving endocrine therapy for < 5 years; 65 (10%) used fertility preservation strategies. Greater concern about fertility was associated with younger age, nonwhite race, not having children, and receipt of chemotherapy.
Conclusion
Many young women with newly diagnosed breast cancer have concerns about fertility, and for some, these substantially affect their treatment decisions. Only a minority of women currently pursue available fertility preservation strategies in this setting.
INTRODUCTION
Breast cancer is the most commonly diagnosed malignancy in women of reproductive age.1 For premenopausal patients with breast cancer, oncologic treatments may impair fertility either by direct gonadotoxicity (eg, resulting from chemotherapy) or by delays to conception that allow natural ovarian aging (eg, resulting from endocrine therapy). A woman's ovaries contain a finite number of primordial follicles (termed ovarian reserve) that declines over time and at a higher rate when exposed to gonadotoxic chemotherapy. Potential infertility resulting from breast cancer treatments may be concerning to many young women and may contribute to the higher levels of distress found in younger patients.2
Adjuvant or neoadjuvant chemotherapy for early-stage breast cancer can cause immediate or early menopause, and even in those who continue to menstruate, fertility may be impaired.3–6 Surgery and radiation therapy do not generally affect fertility, but adjuvant endocrine therapy (eg, tamoxifen), which has traditionally been recommended for 5 years (and may be recommended for 10 years going forward)7 for endocrine-responsive cancers, necessitates postponing child bearing because of potential teratogenicity. Most young women with breast cancer receive both chemotherapy and tamoxifen.8
Prior studies have suggested that many patients with cancer are interested in future fertility at diagnosis and do not receive the information they need at that time about risks to fertility and fertility preservation options.9–12 The American Society of Clinical Oncology recommends that oncologists ask patients with newly diagnosed cancer about interest in future fertility as early as possible and that interested patients be immediately referred to specialists who can offer fertility preservation techniques when appropriate.13,14 However, it is not clear how often these guidelines are followed. A recent Swedish study showed that only 48% of young female survivors of a variety of cancers recalled being informed about risks to fertility, only 14% recalled being informed about fertility preservation techniques, and only 2% used one of these techniques.11
We sought to better understand the burden of concern about fertility, how fertility concerns affect treatment decisions, and fertility preservation strategies used by women in a large cohort of young women with newly diagnosed breast cancer.
PATIENTS AND METHODS
Between November 2006 and December 13, 2012, we invited eligible women identified through pathology record review from 11 sites in Massachusetts and one site in Colorado to participate in an ongoing prospective cohort study: Helping Ourselves, Helping Others: The Young Women's Breast Cancer Study. Eligibility requirements included age ≤ 40 years and diagnosis with stage 0 to IV breast cancer < 6 months before enrollment. The initial accrual goal was 600 based on assessment of fertility concerns as the primary end point. Surveys were mailed to all enrollees, and only those who returned the questionnaire within 9 months of diagnosis were included. Time since diagnosis was calculated as the number of days between first pathology report revealing cancer and the date the baseline survey was returned (or the date survey data were entered in the database plus 30 days if no date of survey return was available). Medical record review was used to gather data on tumor stage, grade, estrogen (ER) and progesterone receptor expression, and human epidermal growth factor receptor 2/neu overexpression. Surveys included items about sociodemographics and medical history and the Fertility Issues and Outcomes Scale (FIS), modified from a previously studied survey that included items regarding fertility concerns, preferences, communications, and decision making.9,15 Concern about fertility at the time of decision making regarding treatment was evaluated using a 4-point Likert scale ranging from a lot of concern to no concern. The Hospital Anxiety and Depression Scale (HADS) was used to identify anxious and depressed patients, using a threshold of > 10 on the subscales. Patients with stage IV disease were excluded from this analysis.
Univariable logistic regression assessed the association between fertility concern (dichotomized as very or somewhat concerned versus a little or not at all concerned) and sociodemographic factors, tumor characteristics and treatments, psychological state, and prior fertility-related issues. Variables associated with univariable P ≤ .20 were evaluated in a multivariable logistic regression model using stepwise selection, and variables achieving significance at P < .05 were included in the final model.
The protocol for this research was approved by participating site institutional review boards. Participants provided signed informed consent before enrollment.
RESULTS
As of December 13, 2012, we had invited 1,511 and enrolled 919 patients. Of these, 724 had returned the baseline survey that included the FIS questions. We excluded patients from the analysis for the following reasons: they did not answer the FIS questions (n = 8), they did not have disease stage information (n = 56), and they had stage IV disease at the time of diagnosis (n = 40). Thus, 620 women were included in this analysis (flow diagram provided in Fig 1).
Fig 1.
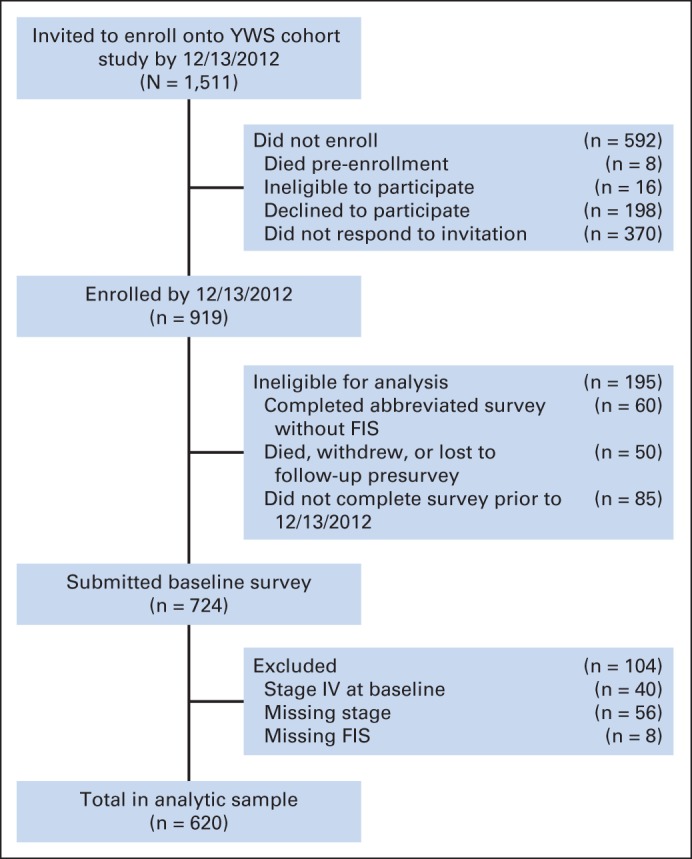
Flow diagram of participants. FIS, Fertility Issues and Outcomes Scale; YWS, The Young Women's Breast Cancer Study.
Median age at diagnosis was 37 years (range, 17 to 40 years). Median time between diagnosis and return of baseline survey was 141.5 days (range, 44 to 496 days). Eighty-eight percent of women were white, 76% were married, and 66% had children already at the time they were diagnosed with cancer. Table 1 lists patient and disease characteristics.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age < 35 years | 229 | 37 |
| Race | ||
| White | 547 | 88 |
| Black | 16 | 3 |
| Asian | 42 | 7 |
| American Indian/Alaskan | 2 | 0.3 |
| Did not answer race question | 13 | 2 |
| College educated | 518 | 84 |
| Married | 474 | 76 |
| Employed full time | 267 | 43 |
| Financially comfortable | 309 | 50 |
| Medically insured | 619 | 99.9 |
| Current drinker | 407 | 66 |
| Current or past smoker | 217 | 35 |
| First-degree relative with breast or ovarian cancer | 89 | 14 |
| ≥ 1 comorbidity | 27 | 4 |
| Received/receiving chemotherapy | 426 | 69 |
| Already receiving endocrine therapy | 116 | 19 |
| Underwent mastectomy | 333 | 54 |
| Anxious by HADS (anxiety score > 10) | 144 | 24 |
| Depressed by HADS (depression score > 10) | 48 | 8 |
| Pregnant at time of survey | 13 | 2 |
| Pregnant at diagnosis | 25 | 4 |
| Had children before cancer diagnosis | 409 | 66 |
| Never pregnant | 158 | 25 |
| History of miscarriages/stillbirths | 149 | 24 |
| History of therapeutic abortions | 109 | 18 |
| History of infertility treatments before diagnosis | 65 | 10 |
| History of difficulty becoming pregnant | 98 | 16 |
| No longer menstruating at time of survey | 40 | 6 |
| Stage | ||
| 0 | 48 | 8 |
| I | 223 | 36 |
| II | 267 | 43 |
| III | 82 | 13 |
| Grade | ||
| 1 | 44 | 7 |
| 2 | 207 | 33 |
| 3 | 362 | 58 |
| Missing | 7 | 1 |
| ER | ||
| Positive | 435 | 70 |
| Negative | 182 | 29 |
| Missing | 3 | 0.5 |
| PR | ||
| Positive | 392 | 63 |
| Negative | 224 | 36 |
| Missing | 4 | 0.6 |
| HER2 | ||
| Positive | 185 | 30 |
| Negative | 405 | 65 |
| Missing | 30 | 5 |
Abbreviations: ER, estrogen receptor; HADS, Hospital Anxiety and Depression Scale; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Fertility Concerns
Two hundred thirty women (37%) reported that before their breast cancer diagnosis, they had wished to have future biologic children or additional biologic children; 160 women (26%) reported that they wished to have biologic children in the future at the time of the survey. Table 2 lists fertility concerns and steps taken to prevent infertility. Four hundred twenty-four (68%) reported that they had discussed fertility issues with their physicians before starting therapy, and there was no obvious increasing or decreasing trend in likelihood of these discussions over time between 2006 and 2012 (Table 3). Three hundred one (49%) reported being not at all concerned about becoming infertile, 83 (13%) were a little concerned, 88 (14%) were somewhat concerned, and 148 (24%) were very concerned. Concerns about fertility affected treatment decisions a little in 55 (9%), somewhat in 53 (9%), a lot in 52 (8%), and not at all in 456 (74%).
Table 2.
Fertility Concerns, Decision Making, and Strategies
| Concern, Decision, or Strategy | No. | % |
|---|---|---|
| Before breast cancer diagnosis, wished to have biologic children in future | 230 | 37 |
| At time of survey, wished to have biologic children in future | 160 | 26 |
| Felt pressured by partner to have children, somewhat or a lot | 31 | 5 |
| Felt pressured by family to have children, somewhat or a lot | 33 | 5 |
| If wanted more children, concerned about: | ||
| Caring for them if cancer recurred | 27 | 4 |
| Children having increased risk of developing cancer | 70 | 11 |
| If did not want more children, concerned about: | ||
| Caring for them if cancer recurred | 18 | 3 |
| Children having increased risk of developing cancer | 33 | 5 |
| Pregnancy would increase risk of recurrence | 59 | 9 |
| At time of decision making about treatment, concerned about fertility | ||
| Not at all | 301 | 49 |
| A little | 83 | 13 |
| Somewhat | 88 | 14 |
| Very | 148 | 24 |
| Concerns about fertility affected treatment decisions: | ||
| Not at all | 456 | 74 |
| A little | 55 | 9 |
| Somewhat | 53 | 9 |
| A lot | 52 | 8 |
| Fertility concerns led patient to choose not to receive chemotherapy | 4 | 1 |
| Fertility concerns led patient to choose one chemotherapy over another | 12 | 2 |
| Fertility concerns led patient to consider not receiving endocrine therapy | 6 | 1 |
| Fertility concerns led patient to not receive endocrine therapy | 19 | 3 |
| Fertility concerns led patient to consider receiving endocrine therapy for < 5 years | 71 | 11 |
| Fertility concerns led patient to undergo mastectomy | 5 | 1 |
| Took special steps to lessen chance of infertility (some patients indicated > 1) | 65 | 10 |
| Embryo cryopreservation | 46 | 7 |
| Oocyte cryopreservation | 7 | 1 |
| GnRH agonist | 19 | 3 |
| Acupuncture | 1 | 0.2 |
| Discussed fertility issues with physician before starting therapy | 424 | 68 |
Abbreviation: GnRH, gonadotropin-releasing hormone.
Table 3.
Fertility Concerns, Discussions, and Preservation Techniques Over Time
| Diagnosis Year | Somewhat or Very Concerned About Becoming Infertile (%) | Recalled Discussion About Fertility (%) | Took Special Steps to Lessen Chance of Infertility (%) |
|---|---|---|---|
| 2006 | 43 | 78 | 5 |
| 2007 | 43 | 67 | 9 |
| 2008 | 30 | 62 | 12 |
| 2009 | 39 | 71 | 8 |
| 2010 | 41 | 67 | 12 |
| 2011 | 38 | 74 | 12 |
| 2012 | 37 | 71 | 15 |
When women's concerns were dichotomized as more concerned (somewhat or very) or less concerned (not at all or a little), 38% of women were characterized as being more concerned. There was no obvious change over time in concern about fertility; 30% to 43% of participants diagnosed in each year (2006 to 2012) expressed at least moderate concern about fertility. Table 3 lists women's fertility-related data by year of diagnosis.
In the multivariable model (Table 4), receipt of chemotherapy, age < 35 years, nonwhite race, and not already having children were associated with greater likelihood of fertility concern. Education, marital status, HADS anxiety, stage, finances, tobacco use, comorbidities, breast surgery, having been pregnant at diagnosis, never having been pregnant, and history of miscarriage, stillbirth, or infertility were associated (P < .20) with more fertility concern in univariable models but were not statistically significant in multivariable models. Factors that were not associated with fertility concern in univariable models (P > .20) included employment status, history of alcohol use, family history of breast cancer, receipt of endocrine therapy, HADS depression, pregnancy at time of survey, history of abortion, difficulty becoming pregnant, and tumor biology (grade, ER, progesterone receptor, and human epidermal growth factor receptor 2 expression).
Table 4.
Logistic Regression Models Evaluating Associations Between Patient/Disease Characteristics and Fertility Concerns
| Variable | Univariable Models |
Final Multivariable Model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age ≥ 35 v < 35 years | 0.16 | 0.11 to 0.23 | < .001 | 0.26 | 0.18 to 0.40 | < .001 |
| White v not | 0.31 | 0.18 to 0.54 | < .001 | 0.38 | 0.20 to 0.72 | .003 |
| College educated v not | 1.35 | 0.85 to 2.15 | .20 | |||
| Married v not | 0.26 | 0.17 to 0.38 | < .001 | |||
| Employed full time v not | 1.19 | 0.86 to 1.65 | .30 | |||
| Financially comfortable v not | 0.76 | 0.55 to 1.06 | .11 | |||
| Current alcohol use v none | 0.88 | 0.63 to 1.25 | .48 | |||
| Current or past smoker v not | 0.76 | 0.54 to 1.07 | .11 | |||
| First-degree relative with breast or ovarian cancer v none | 1.06 | 0.67 to 1.69 | .79 | |||
| ≥ 1 comorbidity v none | 0.56 | 0.23 to 1.34 | .19 | |||
| Received chemotherapy v none | 1.66 | 1.15 to 2.39 | .007 | 1.61 | 1.04 to 2.50 | .03 |
| Receiving endocrine therapy v not | 1.19 | 0.79 to 1.79 | .42 | |||
| Mastectomy v none | 0.52 | 0.37 to 0.72 | < .001 | |||
| Anxious by HADS v not | 1.59 | 1.09 to 2.32 | .02 | |||
| Depressed by HADS v not | 1.44 | 0.80 to 2.61 | .23 | |||
| Currently pregnant v not | 1.90 | 0.63 to 5.72 | .25 | |||
| Pregnant at diagnosis v not | 2.56 | 1.13 to 5.80 | .02 | |||
| Has children v does not | 0.12 | 0.08 to 0.18 | < .001 | 0.17 | 0.11 to 0.26 | < .001 |
| Never pregnant v history of pregnancy | 5.26 | 3.56 to 7.79 | < .001 | |||
| History of miscarriage or stillbirth v none | 0.55 | 0.37 to 0.83 | .004 | |||
| History of abortion v none | 0.88 | 0.57 to 1.35 | .55 | |||
| History of infertility treatments v none | 1.82 | 1.02 to 3.25 | .04 | |||
| History of difficulty becoming pregnant v none | 1.36 | 0.83 to 2.21 | .22 | |||
| Stage (stage III, referent group) | ||||||
| 0 | 0.49 | 0.22 to 1.10 | .08 | |||
| I or II | 1.08 | 0.67 to 1.75 | .76 | |||
| Grade 3 v 1 or 2 | 1.15 | 0.82 to 1.60 | .42 | |||
| ER positive v negative | 1.12 | 0.78 to 1.60 | .55 | |||
| PR positive v negative | 1.17 | 0.83 to 1.64 | .38 | |||
| HER2 positive v not | 0.90 | 0.63 to 1.29 | .57 | |||
Abbreviations: ER, estrogen receptor; HADS, Hospital Anxiety and Depression Scale; HER2, human epidermal growth factor receptor 2; OR, odds ratio; PR, progesterone receptor.
Fertility Concerns and Treatment Decisions
In the 160 women (26%) who reported that concerns about fertility affected their treatment decisions, 90 provided specific details about how their decisions were affected. In the 419 who either reported that concerns about fertility did not affect their treatment decisions or did not respond to this item, 41 still went on to provide specific details about decisions that were affected. Some reported that > one treatment decision was affected. Overall, four (1%) chose not to receive chemotherapy, 12 (2%) chose one chemotherapy regimen over another, six (1%) considered not receiving endocrine therapy, 19 (3%) decided not to receive endocrine therapy, and 71 (11%) considered receiving endocrine therapy for < 5 years. Five reported that they underwent mastectomies because of their fertility concerns.
Fertility Preservation Strategies
Sixty-five women (10%), including 27% of the nonwhite women and 9% of the white women, took special steps to lessen the chance of infertility; 46 (7%) underwent embryo cryopreservation, seven (1%) underwent oocyte cryopreservation, and 19 (3%) received gonadotropin-releasing hormone agonist (GnRH-a). Table 3 shows that the proportion who pursued fertility preservation strategies seemed smaller earlier on (5% in 2006, 9% in 2007) than in later years (12% in 2011, 15% in 2012).
DISCUSSION
Our current results confirm and further elucidate several of the critical findings of earlier work. In our prior Web-based study, 72% of women reported discussing fertility concerns with their physicians before starting therapy, 57% reported that they had been somewhat or very concerned about fertility at the time of diagnosis, and 29% reported that these concerns influenced their treatment decisions.15 This similarity in the proportion of women who recalled fertility discussions and in the proportion whose fertility concerns affected treatment decision making supports the accuracy of these results and documents limited change over the past decade. The 657 participants in that Web-based study were younger at diagnosis (mean age, 33 years), suggesting that fewer may have been finished with their desired childbearing at the time of diagnosis. Because it was, on average, 3 years after their diagnosis at the time of the survey, recall bias may have affected those results more than in the present study. The somewhat lower rates of fertility concern reported by participants in our current study are likely more generalizable because the earlier Web-based study sample may have been biased toward women who were more concerned about fertility given that that study was explicitly focused on fertility rather than more broadly on breast cancer in young women.
In the current large prospective multicenter cohort study of young women with newly diagnosed stage 0 to III breast cancer, more concern about fertility was found to be associated with receipt of chemotherapy, younger age, nonwhite race, and not having children already. It is not unexpected that women who did not already have children were more concerned about fertility, nor that chemotherapy increased concern—the latter likely reflects an accurate understanding of the gonadotoxicity of these drugs. Older patients may have been less concerned because many of them had completed their planned childbearing or decided before their cancer diagnosis that they would not be having children. The association between nonwhite race and fertility concern is more surprising and warrants additional study. The fact that more nonwhite women took special steps to maintain fertility (eg, embryo cryopreservation) suggests that their higher level of concern was not caused by inadequate access to fertility preservation techniques, at least in this sample.
Despite the fact that endocrine therapy allows ovarian reserve to decline naturally over time, we did not identify any link between fertility concerns and either endocrine therapy or ER positivity (the primary indication for eventual endocrine therapy). This might reflect inadequate education regarding the role that delayed conception can play in infertility. It is important that future work not only increases the proportion of patients with breast cancer who receive information about fertility in a timely fashion, but also assures that this information is comprehensive and accurate. One recent study found that young patients with breast cancer expressed a desire for written materials to inform their choices about fertility preservation,16 and another found that young women who are concerned about fertility may benefit from access to a fertility-focused decision aid.17 An online survey of medical oncologists, surgical oncologists, and clinical nurse specialists in the United Kingdom revealed that many health care providers are uncertain about fertility preservation options, potentially leading to misinformation.18
In our study, nearly three quarters of those concerned about fertility at diagnosis did not make use of fertility preservation techniques. This may reflect apprehension about the safety or efficacy of existing techniques, or it may have been the result of inadequate awareness of or access to these techniques. New ovarian stimulation regimens using tamoxifen and aromatase inhibitors attenuate the transient elevated estrogen levels normally associated with ovarian stimulation techniques but still allow collection of multiple oocytes (for freezing or fertilization followed by freezing) after only a few weeks for most women.19–21 Nonetheless, some physicians and patients may worry about a theoretic risk that cancer recurrence could be encouraged by hormonal surges during ovarian stimulation or subsequent pregnancy or by delays in systemic cancer therapy. However, most evidence to date suggests that women who become pregnant after breast cancer may actually have a better prognosis.22 This may be because of the so-called healthy mother effect (ie, healthy breast cancer survivors are more likely than unhealthy survivors to conceive and carry a term pregnancy).23
The majority of those who did use fertility preservation techniques reported that they underwent embryo cryopreservation, the most efficacious widely available fertility preservation option. Oocyte cryopreservation, used by only seven women in this cohort, is a newer technique that is generally offered to women who do not have a male partner and do not wish to use donor sperm; in experienced centers, this technique seems to be nearly as successful as embryo cryopreservation.24 Oocyte cryopreservation will likely increase in popularity now that it is no longer classified as experimental; as of late 2012, it is now considered a standard fertility preservation option by the American Society of Reproductive Medicine, although most sites are still more experienced with embryo cryopreservation.24 Perhaps because of improving access to fertility preservation techniques, the proportion of patients who took special steps to reduce the chance of infertility seemed to increase over time from 5% in 2006 to 15% in 2012 (although we did not perform formal statistical testing of this trend).
Although GnRH-a administration during chemotherapy has not been proven to protect ovarian function,25 this technique was used by more than twice the number of women who used oocyte cryopreservation. This may be because GnRH-a injections are less invasive and carry no hypothetic risk of increasing tumor growth via heightened levels of estrogen during ovarian stimulation. Alternatively or in addition, insurance coverage issues may play a role. This finding suggests that young women with breast cancer need more widespread access to techniques that have been well established to protect fertility (eg, oocyte and embryo cryopreservation).
Important factors in fertility-related decision making and prompt referral to an experienced reproductive endocrinologist include insurance coverage and financial resources. Kim et al26 recently reported that fertility preservation techniques were more often pursued by older and wealthier patients, as well as by those whose cancers were lower stage and who did not receive neoadjuvant chemotherapy. Although the participants in our cohort reported being relatively financially comfortable, young women may be discouraged from pursuing fertility preservation options by a lack of financial resources or by limited medical insurance coverage for these procedures.
Our study represents one of the largest prospective evaluations of fertility issues in young women with recently diagnosed breast cancer. Its strengths include multicenter recruitment using pathology record review and ongoing longitudinal follow-up. Nevertheless, these findings should be considered in the context of the study limitations, including the potential for sample bias, unmeasured confounders, and some recall bias (surveys were sent ≤ 6 months after diagnosis, and some patients did not complete and return them for months after that). Still, these results are important in the care of young women with breast cancer. They support the need to address concerns about fertility and explore the connection between such fears and choices made about treatment. Nearly one third of the patients in our study did not recall discussing the impact of oncologic therapies on fertility before initiating treatment, suggesting that it is crucial that we continue to improve communication about fertility risks and options for fertility preservation, as well as to provide emotional support as young women come to terms with the impact of cancer on their hopes for a normal future. Given that many participants were enrolled at sites that focus on care of young women, fertility discussions and related support may be rarer and less substantive elsewhere. To assist current and future patients, we need to understand the predictors of subsequent pregnancies in this setting, the consequences of delaying or limiting therapy (eg, taking a break from endocrine therapy to attempt conception), and how new data supporting longer courses of endocrine therapy may affect fertility concerns among young breast cancer survivors.
Footnotes
Supported by Susan G. Komen for the Cure Komen Scholars Grant No. SAC100008 (A.H.P.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shari I. Gelber, Elizabeth S. Ginsburg, Ann H. Partridge
Financial support: Ann H. Partridge
Administrative support: Meghan E. Meyer
Provision of study materials or patients: Kathryn J. Ruddy, Lidia Schapira, Steven E. Come, Virginia F. Borges, Ann H. Partridge
Collection and assembly of data: Kathryn J. Ruddy, Virginia F. Borges, Meghan E. Meyer, Ann H. Partridge
Data analysis and interpretation: Kathryn J. Ruddy, Shari I. Gelber, Rulla M. Tamimi, Lidia Schapira, Steven E. Come, Virginia F. Borges, Ann H. Partridge
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Howard-Anderson J, Ganz PA, Bower JE, et al. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 5.Abusief ME, Missmer SA, Ginsburg ES, et al. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116:791–798. doi: 10.1002/cncr.24835. [DOI] [PubMed] [Google Scholar]
- 6.Oktay K, Oktem O, Reh A, et al. Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol. 2006;24:4044–4046. doi: 10.1200/JCO.2006.06.9823. [DOI] [PubMed] [Google Scholar]
- 7.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pronzato P, Mustacchi G, De Matteis A, et al. Biological characteristics and medical treatment of breast cancer in young women: A featured population—Results from the NORA study. Int J Breast Cancer. doi: 10.4061/2011/534256. [epub ahead of print on October 4, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruddy KJ, Gelber S, Ginsburg ES, et al. Menopausal symptoms and fertility concerns in premenopausal breast cancer survivors: A comparison to age- and gravidity-matched controls. Menopause. 2011;18:105–108. doi: 10.1097/gme.0b013e3181ef39f8. [DOI] [PubMed] [Google Scholar]
- 10.Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23:766–773. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 11.Armuand GM, Rodriguez-Wallberg KA, Wettergren L, et al. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30:2147–2153. doi: 10.1200/JCO.2011.40.6470. [DOI] [PubMed] [Google Scholar]
- 12.Gorman JR, Bailey S, Pierce JP, et al. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv. 2012;6:200–209. doi: 10.1007/s11764-011-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 14.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 16.Hill KA, Nadler T, Mandel R, et al. Experience of young women diagnosed with breast cancer who undergo fertility preservation consultation. Clin Breast Cancer. 2012;12:127–132. doi: 10.1016/j.clbc.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Peate M, Meiser B, Cheah BC, et al. Making hard choices easier: A prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br J Cancer. 2012;106:1053–1061. doi: 10.1038/bjc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King JW, Davies MC, Roche N, et al. Fertility preservation in women undergoing treatment for breast cancer in the UK: A questionnaire study. Oncologist. 2012;17:910–916. doi: 10.1634/theoncologist.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: A prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 20.Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–3859. doi: 10.1200/JCO.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 22.Azim HA, Jr, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: A multicenter retrospective study. J Clin Oncol. 2013;31:73–79. doi: 10.1200/JCO.2012.44.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sankila R, Heinävaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: “Healthy mother effect.”. Am J Obstet Gynecol. 1994;170:818–823. doi: 10.1016/s0002-9378(94)70290-x. [DOI] [PubMed] [Google Scholar]
- 24.Mature oocyte cryopreservation. A guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Turner NH, Partridge A, Sanna G, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: The benefit remains uncertain. Ann Oncol. 2013;24:2224–2235. doi: 10.1093/annonc/mdt196. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Oktay K, Gracia C, et al. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil Steril. 2012;97:671–676. doi: 10.1016/j.fertnstert.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


