Abstract
Background
A number of potentially modifiable risk factors are known to be associated with poor pregnancy outcomes. These include smoking, drinking excess alcohol, and poor nutrition. Routine health promotion (encompassing education, advice and general health assessment) in the pre‐pregnancy period has been proposed for improving pregnancy outcomes by encouraging behavioural change, or allowing early identification of risk factors. While results from observational studies have been encouraging, this review examines evidence from randomised controlled trials of preconception health promotion.
Objectives
To assess the effectiveness of routine pre‐pregnancy health promotion for improving pregnancy outcomes when compared with no pre‐pregnancy care or usual care.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (February 2009).
Selection criteria
Randomised and quasi‐randomised trials examining health promotion interventions which aim to identify and modify risk factors before pregnancy. The review focuses on all women of childbearing age rather than those in high‐risk groups. We have excluded trials where interventions are aimed specifically at women with established medical, obstetric or genetic risks or already receiving treatment as part of programmes for high‐risk groups.
Data collection and analysis
Two review authors independently assessed eligibility and carried out data extraction.
Main results
Four trials (2300 women) are included. The interventions ranged from brief advice through to education on health and lifestyle over several sessions. For most outcomes, data were only available from individual studies. Only one study followed up through pregnancy and there was no strong evidence of a difference between groups for preterm birth, congenital anomalies or weight for gestational age; only one finding (mean birthweight) reached statistical significance (mean difference ‐97.00, 95% confidence interval (CI) ‐168.05 to ‐25.95). This finding needs to be interpreted with caution as pregnancy outcome data were available for only half of the women randomised. There was some evidence that health promotion interventions were associated with positive maternal behavioural change including lower rates of binge drinking (risk ratio 1.24, 95% CI 1.06 to 1.44). Overall, there has been little research in this area and there is a lack of evidence on the effects of pre‐pregnancy health promotion on pregnancy outcomes.
Authors' conclusions
There is little evidence on the effects of pre‐pregnancy health promotion and much more research is needed in this area. There is currently insufficient evidence to recommend the widespread implementation of routine pre‐pregnancy health promotion for women of childbearing age, either in the general population or between pregnancies.
Keywords: Adult, Female, Humans, Pregnancy, Young Adult, Pregnancy Outcome, Health Behavior, Health Education, Health Education/methods, Health Promotion, Health Promotion/methods, Maternal Behavior, Preconception Care, Preconception Care/methods, Randomized Controlled Trials as Topic, Risk Factors
Plain language summary
Health promotion before pregnancy to improve outcomes for mothers and babies
Smoking, drinking excess alcohol, poor nutrition and other lifestyle factors can lead to poor outcomes for mothers and babies. The provision of routine health promotion (including advice and education and sometimes screening tests) before conception may encourage changes to improve health, and may be an opportunity to identify risk factors such as infection that can be treated before pregnancy begins. The review looks at randomised controlled trials examining routine health promotion before pregnancy to see whether it changes behaviour and leads to improved health for mothers and babies. Four trials with 2300 women provided information for the review. The health promotion offered to women in these studies ranged from very brief advice on a specific topic through to more general advice and education on health and lifestyle over several sessions. In only one study were women followed up through pregnancy and there was little evidence of any differences between groups, although the babies of women who had received the health promotion intervention had slightly lower birthweights. There was some evidence that health promotion interventions encourage women to have more healthy lifestyles, such as lower rates of binge drinking. Overall, there was little evidence on the effects of pre‐pregnancy health promotion on the health of mothers and babies, and more evidence is needed before its widespread implementation can be recommended.
Background
Introduction
A number of potentially modifiable risk factors are known to be associated with poor pregnancy outcomes. These include maternal lifestyle and behavioural factors such as smoking, drinking excess alcohol, drug abuse, exposure to occupational and environmental hazards, and poor nutrition (Czeizel 1999).
Description of the intervention
Routine health promotion (encompassing education, advice and general health assessment) in the pre‐pregnancy period has been proposed as an intervention for improving pregnancy outcomes by encouraging behavioural change, or by allowing early identification of risk factors. Pre‐pregnancy advice and assessment for women with medical conditions such as diabetes or epilepsy, or who are known to be at high risk, are provided in many countries. Such care has been offered much less frequently to women without identified risk factors, but where it has been provided, results from observational studies have been encouraging. A large study carried out in Hungary suggested that infertile couples were identified and treated sooner, and women attending for pre‐pregnancy care had early access to treatment for genito‐urinary infections and genetic counselling. In addition, positive maternal behavioural modification (smoking cessation) was associated with increased infant birthweights, and a reduction in congenital abnormalities (Czeizel 1999). Such observational studies may be subject to bias, and it was noted in this study that women receiving care tended to be from more affluent social groups.
Despite the fact that routine pre‐pregnancy health promotion is generally not available, surveys of women of childbearing age have demonstrated that its introduction would be welcomed and would potentially be of benefit. An American study reported that 60% of mothers surveyed had planned their pregnancies and more than half of these had at least one indication for pre‐pregnancy advice (Adams 1993). A study of women of childbearing age in the Netherlands revealed that 70% would be interested in attending for pre‐pregnancy care (de Jong‐Potjer 2003).
Surveys also suggest that there is consensus amongst healthcare professionals that pre‐pregnancy health promotion may be a worthwhile addition to routine maternal health care (Heyes 2004; Wallace 1998). There is, similarly, agreement on the broad aims of such care: i.e. to promote optimum maternal health at the beginning of pregnancy, and to identify, and attempt to modify, risk factors associated with maternal and infant morbidity. However, there is less consensus on what type of care should be offered; when it should be offered; how care should be funded; who should provide care; and how vulnerable groups might best be targeted (Adams 1993; Wallace 1998). In spite of this lack of agreement, a number of policy recommendations relating to pre‐pregnancy health promotion have emerged from professional and government bodies. With respect to the timing of care, recommendations are vague. It has been estimated that 92% of women actively attempting conception will become pregnant within a year (Evers 2002; Gnoth 2003) and the National Committee on Perinatal Health in the US recommends that women have a pre‐pregnancy visit in the "months" before conception, whilst the UK Royal College of Obstetricians and Gynaecologists gives no guidance on the timing of multi‐agency pre‐pregnancy advice (Johnson 2006; RCOG 2008). Recommendations have also been made without there being any clear health service mechanisms for implementation. For example, the UK Department of Health recommends that women of childbearing age should be made aware of the benefits of prenatal and early antenatal folate supplementation, and should be provided with appropriately timed nutritional advice and folate supplements, without there being any established means of targeting women who might be planning pregnancy or liable to become pregnant (Clark 1994).
Why it is important to do this review
While results from observational studies have been encouraging, such studies are subject to bias and difficult to interpret. There has been no review of evidence from randomised controlled trials in this area. Evidence from trials may provide information on whether or not routine health promotion before pregnancy is associated with improved pregnancy outcomes. Despite enthusiasm amongst women and health professionals for routine pre‐pregnancy care, evidence on effectiveness is needed before widespread implementation is advocated.The review may also offer insight into the sort of health service arrangements that have been set up to provide pre‐pregnancy health promotion, along with information on service costs.
Objectives
To assess the effectiveness of routine pre‐pregnancy health promotion for improving pregnancy outcomes when compared with no pre‐pregnancy care or usual care.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials and quasi‐randomised trials.
Types of participants
Women of childbearing age. The review focuses on care for all women rather than those identified as being in high‐risk groups. We have excluded trials where interventions are aimed specifically at women with established medical, obstetric or genetic risks (e.g. women with epilepsy or previous pregnancy loss attending specialist clinics). Pre‐conception care for women with diabetes will be considered in a related Cochrane review (Tieu 2009). We also exclude trials where interventions have been aimed specifically at women already receiving care as part of programmes for high‐risk groups (e.g. women identified as having serious alcohol or substance abuse problems). We include interventions which target all women of childbearing age, but which happen to include women from high‐risk groups.
Types of interventions
Pre‐pregnancy health promotion as defined by the trial authors. Interventions focus on health promotion which includes advice, basic health assessment (such as measuring weight and checking rubella immune status), or both, and which aims to identify and modify risk factors before pregnancy. We planned to include studies which examined interventions aimed at couples. We have included a range of interventions from single, brief advice sessions through to intensive one‐to‐one advice and health assessment taking place over multiple sessions. The locations where interventions are delivered has been defined by trial authors and include both community and healthcare settings.
Types of outcome measures
Primary outcomes
Perinatal death.
Small‐for‐gestational age.
Extremely preterm birth (defined as birth < 28 weeks' gestation).
Maternal death.
Secondary outcomes
Pregnancy outcomes
Reported maternal behavioural change: smoking, diet, alcohol or drug use.
Development of antenatal complications.
Preterm birth (defined as birth < 37 weeks' gestation).
Spontaneous miscarriage.
Therapeutic abortion.
Pregnancy within one year of intervention.
Mode of birth.
Infant outcomes
Parameters of birth asphyxia.
Neonatal intensive care unit admission.
Birthweight < 2500 g.
Respiratory distress syndrome.
Congenital anomaly.
Measures of maternal satisfaction and anxiety
Woman not satisfied with care.
Women's preferences for care.
Maternal anxiety (measured on validated scales or visual analogue scales).
Costs
Costs associated with pre‐pregnancy health promotion versus standard care (including follow‐up visits and tests).
Number of antenatal visits.
Number of antenatal admissions to hospital.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (February 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We have not applied any language restrictions.
Data collection and analysis
Selection of studies
Both review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person with expertise in the topic area.
Data extraction and management
We designed and piloted a form to extract data. Both review authors extracted data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2008) and checked them for accuracy
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the methods used to generate the allocation sequence.
We assessed the methods as:
adequate (any truly random method, e.g. random number table; computer random‐number generator);
inadequate (alternation; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during, recruitment, or could be changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We have noted for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. With this type of intervention, blinding participants and clinical staff is usually not possible, but it may be feasible to have partial blinding. We have noted where there has been partial blinding (e.g. in situations where participants are not blind to the intervention but where outcome assessors may be).
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analyses at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and any re‐inclusions in analyses which we have undertaken.
We assessed the methods as:
adequate (e.g. where there are no missing data or where there are low levels and the reasons for missing data are balanced across groups);
inadequate (e.g. where missing data are not balanced across groups, or where levels of missing data are so high as to introduce serious risk of bias);
unclear (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to be made).
(5) Selective reporting bias
We have described for each included study how we examined the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We have described for each included study any important concerns we have about other possible sources of bias. For example, we have noted any potential source of bias related to a specific study design or where there was extreme baseline imbalance.
We have assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We have made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above, we have assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
As interventions, participants, or the way outcomes were measured were different in different studies, we have not combined results from trials; rather we have presented results separately for individual studies. We have provided descriptions of the interventions and participants in the Characteristics of included studies tables. We have carried out statistical analysis using Review Manager software (RevMan 2008). Although we have not pooled results from trials in this review in updates, as more data become available, we plan to use fixed‐effect meta‐analysis.
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data we have used the mean difference. If in updates of the review we combine data from trials we will use the mean difference if outcomes are measured in the same way between trials and plan to use the standardised mean difference to combine trials that measure the same outcome, but use different methods (e.g. anxiety measured using different scales).
Unit of analysis issues
Cluster‐randomised trials
In updates of the review, if we identify any cluster‐randomised trials, we plan to include such trials in the analyses along with individually randomised trials. To take account of the design effect, we will adjust standard errors from cluster‐randomised trials using the methods described in Higgins 2008 and carry out meta‐analysis using the generic inverse‐variance method. We will use an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC (Gates 2005). If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis. Therefore, we will perform the meta‐analysis in two parts as well.
Dealing with missing data
For included studies, we have noted levels of attrition. We are aware that with an intervention such as pre‐pregnancy care, women may be followed up over several years and levels of attrition may be relatively high compared to interventions with outcomes assessed soon after the intervention. This needs to be taken into account in the interpretation of findings.
Intention‐to‐treat analysis
We had intended to carry out outcome analyses, as far as possible, on an intention‐to‐treat basis; i.e. by attempting to include all participants randomised to each group in the analyses, with the denominator for each outcome in each trial being the number randomised minus any participants known to be missing. For one trial included in the review (Lumley 2006), outcome data were reported only for that subset of the sample that became pregnant and were not otherwise lost to follow up during the study period (pregnancy outcome data were available for only half of the sample randomised). For pregnancy outcomes, we have presented results for this subset that remained available to follow up and that became pregnant (and were therefore eligible for pregnancy outcomes), but recognise that this is a potentially serious source of bias. We have also included a sensitivity analysis where women that remained available to follow up, but who did not become pregnant are restored to the randomisation group denominators.
We had also planned to analyse data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we restored them to the correct group. If there was not sufficient information in the trial reports, we tried to contact the study authors for more information. We contacted several authors during the process of conducting the review.
Assessment of heterogeneity
We planned to examine heterogeneity between trials by visual inspection and by considering the I² statistic. If we identified heterogeneity among the trials, we planned to explore it by pre‐specified subgroup analyses and by performing sensitivity analyses. In this review, we have not pooled data from studies, and we were not able to carry out planned subgroup analyses. In updates of the review as more data become available, if any heterogeneity is not explained by further analyses, we will draw attention to this when we present overall results, indicating that there was high unexplained heterogeneity so caution is needed in the interpretation of results.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we contacted study authors asking them to provide missing outcome data. Where missing data were thought to introduce serious bias, we have noted this.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001.
1. Primiparous versus multiparous women.
Sensitivity analysis
If, in updates of this review, we combine more data from trials in meta‐analysis, we will carry out sensitivity analyses to explore the effect of trial quality for important outcomes in the review. Where there is risk of bias associated with a particular aspect of study quality (e.g. inadequate allocation concealment), we will explore this by sensitivity analyses.
Results
Description of studies
Results of the search
The search strategy identified 11 reports describing seven trials examining preconception health promotion interventions.
Included studies
Using prespecified inclusion and exclusion criteria, we identified six studies eligible for inclusion. However, in this review we have only been able to include results from four trials (Floyd 2007; Lumley 2006; Robbins 2005; Velott 2008). Two studies did not report findings for the whole sample according to group allocation, but rather for non‐random subsets. Results according to randomisation group were not available and therefore, we have not included data from these trials (Elsinga 2006; Jack 1998). We have provided information about these studies in the Characteristics of included studies tables.
Studies contributing data to the review were carried out in different settings, recruited different groups of participants (although all included women capable of becoming pregnant) examined different types of interventions, and collected information on different outcomes.
The study by Floyd 2007 was carried out in the USA between 2002 and 2004 and specifically focused on changing behaviour with regard to alcohol consumption and contraception. At the time of baseline assessment, women recruited to the trial were engaged in what was described as "risky" drinking (i.e. consuming more than seven alcoholic drinks per week or more than five drinks on any one occasion). The intervention involved four counselling sessions on alcohol use and one on contraception. Outcomes relating to alcohol consumption were reported at three, six and nine months following the intervention.
In the study described by Lumley 2006, women recruited to the trial were attending government funded clinics in Australia after the birth of their first child. The intervention was delivered by a specially trained pre‐pregnancy midwife and involved a home visit where risks were identified, with hospital referral for any risk factors identified, and advice on lifestyle factors related to poor pregnancy outcomes (e.g. smoking). The main outcomes in this review were infant birthweight, preterm delivery, birth defects and perinatal deaths.
Robbins 2005 describes an intervention aimed at women of childbearing age to encourage the use of folate supplements. Women recruited to the intervention group received brief advice on the benefits of folic acid and a free bottle of folate supplements.
A study in Pennsylvania (USA) recruited women in 15 low‐income rural areas (Velott 2008). The intervention was delivered over six two‐hour sessions and involved education to encourage behavioural change to improve health in women of child‐bearing age. All women were assessed at baseline and were referred to healthcare providers if measurements (e.g. blood pressure or cholesterol) were outside normal limits. The aim of the intervention was to improve women's self‐efficacy and reduce risk factors associated with poor pregnancy outcomes.
Excluded studies
One study was excluded as it looked at an intervention for women with multiple sclerosis to help them decide whether or not to become pregnant (Prunty 2008).
Risk of bias in included studies
Results from two trials reporting results for only subsets of the main sample are at high risk of bias and results have not been included. Therefore, in the sections below we focus on those four studies contributing data to the review (Floyd 2007; Lumley 2006; Robbins 2005; Velott 2008).
Allocation
In the study by Floyd 2007, the randomisation sequence was computer generated and allocation concealment was assessed as adequate. The study report states that there was block randomisation in some of the centres recruiting women to the trial, but no details were provided on block size or whether this was random. Similarly, the study by Lumley 2006 used a balanced block design for sequence generation, with allocation according to a trial "log book" held in the participating centres. In the study by Robbins 2005, there was block randomisation (block size specified) but methods to conceal allocation were not described. In the study by Velott 2008, methods to generate the randomisation sequence and to conceal group allocation were unclear.
Blinding
With interventions involving education, it is generally not feasible to blind clinical staff and participants to group allocation. While blinding outcome assessors is possible, participants may disclose group allocation or case notes may reveal it, so blinding may be difficult to achieve in practice. In three of the studies it was not stated whether there was any attempt to blind outcome assessors (Floyd 2007; Lumley 2006; Robbins 2005). In the Pennsylvania study (Velott 2008), it was reported that assessors carrying out physical assessments were blind to group allocation. With outcomes such as perinatal death or congenital anomaly, the lack of blinding is unlikely to have an impact on outcome. However, lack of blinding may well have an effect on other outcomes such as reported behaviour. Outcomes such as alcohol consumption, smoking or folic acid consumption (where information depends on maternal self‐report) may well be affected by the lack of blinding.
Incomplete outcome data
All of the studies had relatively high levels of attrition (between 13% and 48%), hence results may be at risk of bias and should be interpreted with caution. In the study by Floyd 2007, overall attrition was approximately 29%, although it was possible for us to re‐include women lost to follow up in an intention‐to‐treat analysis. In the study by Lumley 2006, women who did not become pregnant in the follow‐up period were not included in the analyses and there were further losses to follow up for other reasons (34.2% of the sample randomised were lost to follow up and we do not know how many of these women did or did not become pregnant; further, of those women available at follow up 18% did not become pregnant and were not eligible to experience pregnancy outcomes). Overall, half of the women randomised were not followed up. Although missing data were balanced across groups, this level of attrition makes interpretation of results very difficult. In the Pennsylvania study, 48% of the randomised sample failed to complete post‐test assessments (Velott 2008). In the Robbins 2005 trial, there was approximately 13% attrition.
Selective reporting
Selective reporting is difficult to identify. The intervention in the Lumley 2006 study included advice on lifestyle and referral for identified health problems. It is not clear whether information on outcomes such as reduced alcohol consumption was collected but not reported, and process information (e.g. on the numbers of women referred for rubella immunisation) was not provided.
At the time the review was conducted, Velott 2008 was in the writing‐up stage, and further results may become available in the future.
As previously mentioned, several of the outcomes relied on self‐reported information on behaviour. With a socially stigmatised behaviour such as excessive alcohol consumption, outcomes not validated by biochemical tests may be susceptible to reporting bias.
Other potential sources of bias
A source of bias in the Lumley 2006 trial was that data included in the analyses were for those women who became pregnant in the study period (786 women of 1579 randomised); it is possible that women who become pregnant are different in a number of respects from those that do not, and that the intervention may have had a different effect on those women that did or did not become pregnant. Restricting the analyses to pregnant women and to pregnancy outcomes may have led to the size of the treatment effect being either over or under‐estimated. (No data were provided on the characteristics of women that did not become pregnant or on any outcomes for this group.) In this review denominators in the main analyses (comparison one) are for those women who became pregnant, and these results need to be interpreted with caution. We carried out a sensitivity analysis where we have attempted to include all women who remained available to follow up; in the sensitivity analysis, the denominators for dichotomous outcomes include women who did (n = 786) plus those who did not (n = 253) become pregnant.
Effects of interventions
Pre‐pregnancy interventions versus routine or alternative care: four studies with 2300 women
As the four studies contributing data to the review focused on different outcomes, we were unable to pool data, so findings are from individual studies. Only one of the included studies followed women through pregnancy and reported on pregnancy outcome (Lumley 2006).
In the Velott 2008 study, information was collected at baseline as well as following the intervention. In the analysis below, we have included data collected at follow up and have indicated whether there was clear evidence of differences between groups at baseline. In Appendix 1 we have included both baseline and follow‐up data (unpublished data provided by the study authors).
Primary outcomes
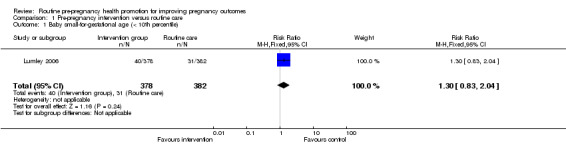
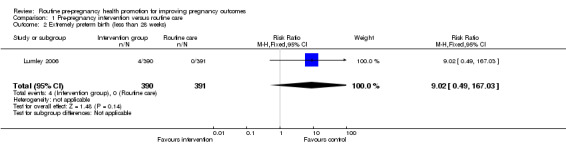
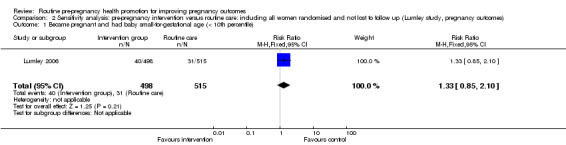
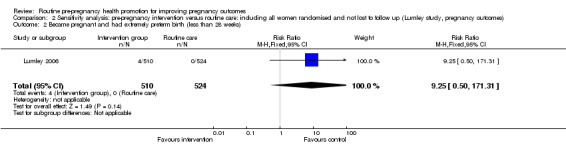
Data for two of the review's primary outcomes (extremely preterm birth and baby small‐for‐gestational age) were collected in one study (Lumley 2006). Births where babies were small‐for‐gestational age (< 10th percentile) were not significantly different between groups (risk ratio (RR) 1.30, 95% confidence interval (CI) 0.83 to 2.04). There were four extremely preterm births (babies born at less than 28 weeks' gestation) in the intervention group compared with none in the control group, but the difference between groups was not statistically significance (RR 9.02, 95% CI 0.49 to 167.03). No data were available by randomisation group for the primary outcomes of perinatal or maternal death.
Secondary outcomes
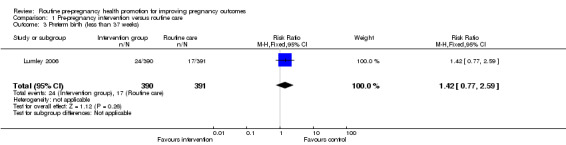
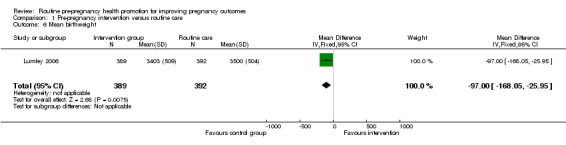
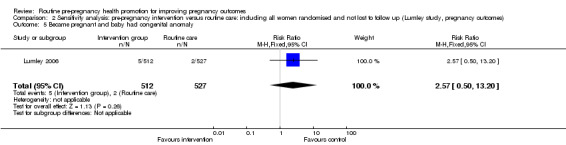
Data on a number of secondary outcomes pertaining to the neonate were collected in the Australian study (Lumley 2006). The rate of preterm births (less than 37 weeks) was lower in the control than in the intervention group, but results were not significant (RR 1.42, 95% CI 0.77 to 2.59). There were no significant differences in rates of congenital anomalies or birthweight less than 2500 g (Analysis 1.4; Analysis 1.5). Babies in the intervention group were, on average, 97 g lighter than those in the control group and this difference was significant (mean difference ‐97.00, 95% CI ‐168.05 to ‐25.95), but may be partly explained by the non‐significant increase in preterm births in the intervention group.
1.4. Analysis.
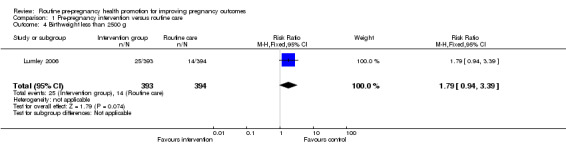
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 4 Birthweight less than 2500 g.
1.5. Analysis.
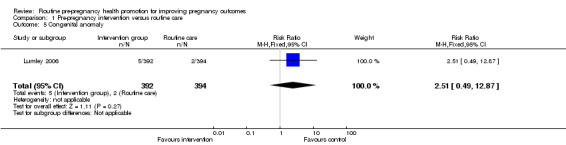
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 5 Congenital anomaly.
Data on maternal behavioural changes were reported in three studies; Robbins 2005 described the use of folic acid supplements, Floyd 2007 decribed changes in alcohol consumption and Velott 2008 described findings for a range of outcomes including use of folic acid, smoking, alcohol use and physical activity.
Two studies reported on the use of folic acid supplements following interventions. In view of differences in the interventions (very brief advice on folic acid (Robbins 2005) versus group education sessions taking place over several weeks (Velott 2008)), we have not pooled data from these two studies but have provided sub‐totals only (Analysis 1.7). The very brief intervention appeared to have virtually no effect (RR 1.09, 95% CI 0.80 to 1.47) whereas more intensive group education appeared to have a positive effect on consumption of folic acid supplements (RR 1.92, 95% CI 1.49 to 2.46).
1.7. Analysis.
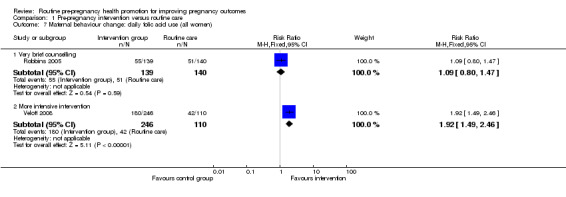
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 7 Maternal behaviour change: daily folic acid use (all women).
In the Robbins 2005 study, the use of folic acid supplements was reported for the small subset of the sample who were planning to become pregnant within a year of the intervention; the difference between groups was not significant (RR 1.06, 95% CI 0.68 to 1.66).
There was some evidence that alcohol consumption was reduced following the motivational interviewing intervention described by Floyd 2007. For women followed up at nine months, those in the intervention group were more likely to be drinking less than eight drinks per week (RR 1.25, 95% CI 1.06 to 1.47) and/or more likely to be avoiding binge drinking compared with controls (RR 1.24, 95% CI 1.06 to 1.44). When we repeated the analysis, including all women randomised, and assumed that all women lost to follow up continued to drink at baseline levels, the differences between groups remained, but failed to reach statistical significance (Analysis 1.10; Analysis 1.12). There were no differences in alcohol consumption between groups at follow up in the Velott 2008 study; the numbers of women consuming alcohol, and the numbers reporting drinking on 10 or more days in the previous month were not significantly different (Analysis 1.13; Analysis 1.14).
1.10. Analysis.
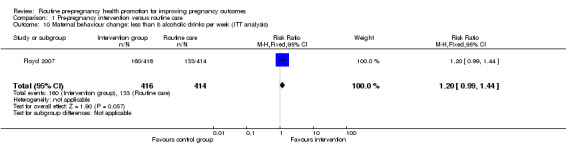
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 10 Maternal behaviour change: less than 8 alcoholic drinks per week (ITT analysis).
1.12. Analysis.
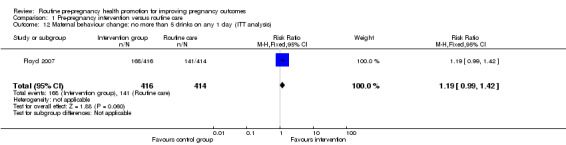
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 12 Maternal behaviour change: no more than 5 drinks on any 1 day (ITT analysis).
1.13. Analysis.
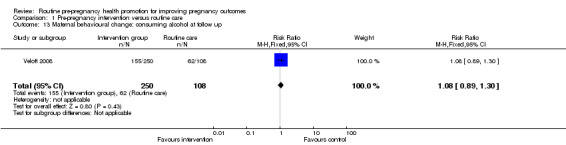
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 13 Maternal behavioural change: consuming alcohol at follow up.
1.14. Analysis.

Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 14 Maternal behaviour change: consumed alcohol on 10 or more days in the last month.
Data on smoking behaviour between baseline and follow up were reported for the Velott 2008 study. There were more smokers in the control group at baseline (33% versus 27% in the intervention group). At follow up at three months, there was very little change from baseline in either group, and there was no significant evidence that the intervention achieved a positive effect (Analysis 1.15).
1.15. Analysis.
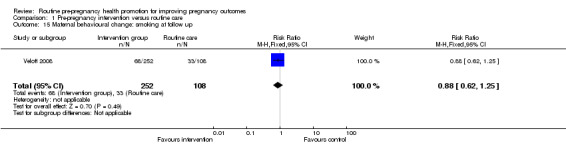
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 15 Maternal behavioural change: smoking at follow up.
Information on the costs of interventions and the impact of attending for preconception care on health service utilisation was not provided in these studies.
Non pre‐specified outcomes
The interval between births was measured in the Lumley 2006 study. While there was no significant difference between groups in the mean interval between births (Analysis 1.16), the authors reported that there was unequal variance, with women in the intervention group having greater variance in birth intervals.
1.16. Analysis.
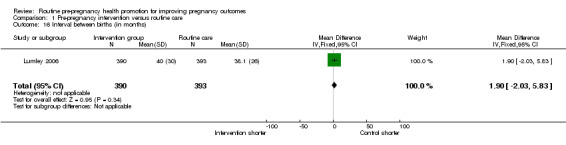
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 16 Interval between births (in months).
Information on a range of lifestyle and behavioural factors was collected in the Velott 2008 study, including women's consumption of fruit and vegetables. Findings at baseline and follow up are set out in Appendix 1.
Sensitivity analysis
We carried out a sensitivity for the dichotomous pregnancy outcomes reported in the Lumley 2006 study (comparison two). We included all women that were available to follow up in the study denominators so that both women that did and did not become pregnant were included. Findings were very similar to those in the analysis, which included only those women who became pregnant.
Discussion
Summary of main results
We have included results from only four trials and for most review outcomes there were no significant differences between intervention and control groups.
In the Lumley 2006 trial, most findings were not significant; there was no strong evidence of a difference between groups for preterm birth, congenital anomalies or small for dates; only one finding (mean birthweight) reached statistical significance (mean difference ‐97.00, 95% CI ‐168.05 to ‐25.95). Results from this trial need to be interpreted with caution as pregnancy outcome data were available for only half of the women randomised.
In the Floyd 2007 study, there was evidence that women in the intervention group reduced their alcohol consumption to less risky levels following a relatively intensive intervention. The use of folic acid was increased in the Pennsylvania study (Velott 2008), again following a relatively intensive intervention. A very brief intervention encouraging the use of folic acid supplements had little effect (Robbins 2005).
It is not clear why the intervention seemed to be associated with negative outcomes in the Australian study (Lumley 2006). The authors propose a number of possible explanations: the intervention may have increased stress in mothers which led to increased preterm birth, or the intervention meant that more babies with anomalies or with poor placentation were sustained longer in utero, leading to fewer miscarriages but more very preterm births in the intervention arm (although data on spontaneous miscarriages before 20 weeks were not reported). On the other hand, it is possible that the differences in outcomes between groups relating to prematurity and birthweight (which are likely to be related) occurred by chance or were due to some other explanation not considered by the authors.
Overall completeness and applicability of evidence
Only four studies contributed data to the review, and for most outcomes, results were derived from individual studies. Therefore, data are limited and the results of the review should be interpreted with caution. No data were available for many of the review's prespecified outcomes (e.g. the development of pregnancy complications, miscarriage, therapeutic abortion, neonatal condition at birth, women's preferences for care and costs) although these outcomes are undoubtedly important for mothers, babies and health service providers. It is surprising that so little data are available in this important topic area. Where information is available, it is not clear how easy it would be to transfer interventions to other settings, or to generalise the results to other population groups. While the interventions in the Floyd 2007, Lumley 2006 and Velott 2008 studies were well described, replication in another setting may not be easily accomplished; interventions focusing on lifestyle factors are likely to be highly culturally dependent, and the quality of an intervention will depend on the particular expertise of those delivering it, as well as on the particular content and intensity of interventions. In the Lumley 2006 study the intervention arose out of a new service development; innovative service developments often attract more motivated clinical staff and replicating interventions as part of normal practice may not achieve the same results.
We were unable to include findings from two studies, as results were not provided for the whole sample. Rather, results were for those women who had actually attended for pre‐pregnancy care. In the study by Elsinga 2006, only a very small proportion of women of childbearing age invited to take part attended for preconception care. For example, in one intervention year only 20% of pregnancies subsequently occurred in women who had attended for care. Women who attended for care tended to be more educated and were planning pregnancy. Women that did attend for care were found to be more knowledgeable compared with matched controls, but these findings may not be generalisable.
Two of the included studies involved in‐depth assessment of both intervention and comparison group women at baseline (Floyd 2007; Lumley 2006). Such assessments may in themselves prompt behavioural change (or indeed prompt anxiety) and may thereby dilute the intervention effect.
Quality of the evidence
The evidence from the four trials included in the review is likely to be at some risk of bias. While the trialists made efforts to reduce bias by aspects of study design such as sequence generation and allocation concealment, risk associated with lack of blinding could not easily be addressed. In addition, three of the studies relied on maternal self‐report for information on behavioural outcomes (Floyd 2007; Robbins 2005; Velott 2008), evidence which may not be reliable.
Missing data are a particular problem in studies where women are followed up over time, and where only a relatively small proportion of the women randomised become eligible to experience possible outcomes results are very difficult to interpret. These problems apply to studies included in this review. Findings for those women available to follow up at all data collection points may not be applicable to those women with missing data, and women that become pregnant may not be be representative of all the women randomised.
One of the trials which did not contribute outcome data to the review illustrate some of the difficulties of conducting trials in this area (Elsinga 2006). The subset of the randomised group that did attend for preconception care was not representative of the wider population, and may have been at lower risk of adverse outcomes compared with those that did not attend.
Potential biases in the review process
There were a number of potential biases in the review process. We attempted to minimise bias in several ways: two review authors independently assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. However, carrying out reviews is not an exact science and may require a number of subjective judgements; it is possible that a different review team may have reached different decisions regarding assessments of eligibility and risk of bias. We would encourage readers to examine the characteristics of included studies tables to assist in the interpretation of results.
Agreements and disagreements with other studies or reviews
The limited data presented in this review have not demonstrated that pre‐pregnancy health promotion results in improved outcomes for the neonate; an earlier observational study had reported increased infant birthweights, and reduced congenital abnormalities (Czeizel 1999). There is some evidence in this review that a relatively intensive intervention encourages women who consume alcohol to cut down; while it is known that once women know they are pregnant they do tend to reduce their drinking, achieving reductions before pregnancy or before pregnancy has been recognised may result in improved outcomes for mothers and babies. There is some evidence that brief interventions promoting abstention from alcohol or reduced drinking during pregnancy do encourage behavioural change; but trials have not had sufficient power to demonstrate that this leads to improved outcomes for babies (Stade 2009).
Authors' conclusions
Implications for practice.
There is little evidence from trials about the effects of general advice and education in the pre‐pregnancy period on outcomes for mothers and babies. It is possible that advice on specific risk factors (such as encouraging pregnancy planning and alcohol reduction) can achieve behavioural change, but it is not known whether this translates into improved health for mothers and their babies. It is possible that such interventions may be worthwhile if directed at women at increased risk. It is not clear that any behavioural changes recorded following interventions are sustained in the long term and in particular amongst those women that subsequently become pregnant. There is currently insufficient evidence to recommend the widespread implementation of pre‐pregnancy counselling for women of childbearing age, either in the general population or between pregnancies.
Implications for research.
Very little research has been carried out in the area of pre‐pregnancy health promotion, perhaps because most pregnancies are not planned and only a relatively small proportion of women respond to invitations to attend for pre‐pregnancy care. Perhaps researchers need to adopt a wider public health format when planning studies on pre‐pregnancy care and encompass all women of child‐bearing age rather than women planning a pregnancy.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Maternal behavioural change: unpublished data (Velott et al 2008 study)
|
Intervention (n = 252) |
Control (n = 110) |
|||
| Baseline | Follow up | Baseline | Follow up | |
| Smoking | 67/251 | 68/252 | 36/109 | 33/108 |
| Number of cigarettes smoked per day in past month* | 2.74 (5.66) 0 (0, 0) |
2.72 (5.65) 0 (0, 1) |
3.53 (6.24) 0 (0, 5) |
3.44 (6.03) 0 (0, 5.5) |
| Number of cigarettes smoked per day in past month (among smokers)* | 9.53 (6.83) 10 (5, 15) |
9.36 (6.92) 10 (5, 13) |
10.41 (6.59) 10 (5, 15) |
10.05 (6.29) 10 (5, 15) |
| Alcohol consumption | 172/251 | 155/250 | 64/109 | 62/108 |
| Number of drinks per day in past month* | 1.70 (1.67) 1 (0, 3) |
1.58 (1.64) 1 (0, 3) |
1.30 (1.48) 1 (0, 2) |
1.34 (1.44) 1 (0, 2) |
| Number of drinks per day in past month (among drinkers)* | 2.45 (1.48) 2 (1, 4) |
2.17 (1.55) 2 (1, 3) |
2.08 (1.37) 2 (1, 3) |
2.04 (1.31) 2 (1, 3) |
| Number of days had at least one drink in past month | ||||
| 0 days | 110/251 | 122/250 | 55/109 | 54/108 |
| 1‐2 days | 74/251 | 63/250 | 24/109 | 23/108 |
| 3‐5 days | 33/251 | 27/250 | 21/109 | 17/108 |
| 6‐9 days | 19/251 | 19/250 | 5/109 | 6/108 |
| 10+ days | 15/251 | 19/250 | 4/109 | 8/108 |
| Number of days had at least one drink in past month (among drinkers) | ||||
| 0 days | 42/183 | 54/182 | 18/72 | 17/71 |
| 1‐2 days | 74/183 | 63/182 | 24/72 | 23/71 |
| 3‐5 days | 33/183 | 27/182 | 21/72 | 17/71 |
| 6‐9 days | 19/183 | 19/182 | 5/72 | 6/71 |
| 10+ days | 15/183 | 19/182 | 4/72 | 8/71 |
| Take daily vitamin with folic acid | 88/250 | 180/246 | 34/109 | 42/110 |
| Meet PA guidelines | 82/250 | 102/250 | 34/108 | 33/110 |
| Daily intake of fruits | 62/252 | 64/251 | 21/110 | 29/110 |
| Daily intake of vegetables | 58/252 | 71/252 | 27/110 | 19/110 |
| Read food labels (all or most of the time) | 72/252 | 82/252 | 33/110 | 29/110 |
* mean (standard deviation) and median (1st quartile, 3rd quartile)
Data and analyses
Comparison 1. Pre‐pregnancy intervention versus routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Baby small‐for‐gestational age (< 10th percentile) | 1 | 760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.83, 2.04] |
| 2 Extremely preterm birth (less than 28 weeks) | 1 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.02 [0.49, 167.03] |
| 3 Preterm birth (less than 37 weeks) | 1 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.77, 2.59] |
| 4 Birthweight less than 2500 g | 1 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.94, 3.39] |
| 5 Congenital anomaly | 1 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.49, 12.87] |
| 6 Mean birthweight | 1 | 781 | Mean Difference (IV, Fixed, 95% CI) | ‐97.0 [‐168.05, ‐25.95] |
| 7 Maternal behaviour change: daily folic acid use (all women) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Very brief counselling | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.47] |
| 7.2 More intensive intervention | 1 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.49, 2.46] |
| 8 Maternal behaviour change: daily folic acid use (women planning pregnancy in next year) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| 9 Maternal behaviour change: less than 8 alcoholic drinks per week at 9 months follow up | 1 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.47] |
| 10 Maternal behaviour change: less than 8 alcoholic drinks per week (ITT analysis) | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.99, 1.44] |
| 11 Maternal behaviour change: no more than 5 drinks on any 1 day (9 months follow up) | 1 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.44] |
| 12 Maternal behaviour change: no more than 5 drinks on any 1 day (ITT analysis) | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.99, 1.42] |
| 13 Maternal behavioural change: consuming alcohol at follow up | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.30] |
| 14 Maternal behaviour change: consumed alcohol on 10 or more days in the last month | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.27] |
| 15 Maternal behavioural change: smoking at follow up | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.25] |
| 16 Interval between births (in months) | 1 | 783 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐2.03, 5.83] |
1.1. Analysis.
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 1 Baby small‐for‐gestational age (< 10th percentile).
1.2. Analysis.
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 2 Extremely preterm birth (less than 28 weeks).
1.3. Analysis.
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 3 Preterm birth (less than 37 weeks).
1.6. Analysis.
Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 6 Mean birthweight.
1.8. Analysis.

Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 8 Maternal behaviour change: daily folic acid use (women planning pregnancy in next year).
1.9. Analysis.

Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 9 Maternal behaviour change: less than 8 alcoholic drinks per week at 9 months follow up.
1.11. Analysis.

Comparison 1 Pre‐pregnancy intervention versus routine care, Outcome 11 Maternal behaviour change: no more than 5 drinks on any 1 day (9 months follow up).
Comparison 2. Sensitivity analysis: pre‐pregnancy intervention versus routine care: including all women randomised and not lost to follow up (Lumley study, pregnancy outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Became pregnant and had baby small‐for‐gestational age (< 10th percentile) | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.85, 2.10] |
| 2 Became pregnant and had extremely preterm birth (less than 28 weeks) | 1 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.25 [0.50, 171.31] |
| 3 Became pregnant and had preterm birth (less than 37 weeks) | 1 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.79, 2.67] |
| 4 Became pregnant and birthweight less than 2500 g | 1 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.97, 3.50] |
| 5 Became pregnant and baby had congenital anomaly | 1 | 1039 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.50, 13.20] |
2.1. Analysis.
Comparison 2 Sensitivity analysis: pre‐pregnancy intervention versus routine care: including all women randomised and not lost to follow up (Lumley study, pregnancy outcomes), Outcome 1 Became pregnant and had baby small‐for‐gestational age (< 10th percentile).
2.2. Analysis.
Comparison 2 Sensitivity analysis: pre‐pregnancy intervention versus routine care: including all women randomised and not lost to follow up (Lumley study, pregnancy outcomes), Outcome 2 Became pregnant and had extremely preterm birth (less than 28 weeks).
2.3. Analysis.

Comparison 2 Sensitivity analysis: pre‐pregnancy intervention versus routine care: including all women randomised and not lost to follow up (Lumley study, pregnancy outcomes), Outcome 3 Became pregnant and had preterm birth (less than 37 weeks).
2.4. Analysis.

Comparison 2 Sensitivity analysis: pre‐pregnancy intervention versus routine care: including all women randomised and not lost to follow up (Lumley study, pregnancy outcomes), Outcome 4 Became pregnant and birthweight less than 2500 g.
2.5. Analysis.
Comparison 2 Sensitivity analysis: pre‐pregnancy intervention versus routine care: including all women randomised and not lost to follow up (Lumley study, pregnancy outcomes), Outcome 5 Became pregnant and baby had congenital anomaly.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Elsinga 2006.
| Methods | Cluster‐randomised trial carried out in the Netherlands (unit of randomisation was GP practices). | |
| Participants | Women aged 18 to 40 years attending for care with 67 GPs (in 54 GP practices). There were a total of 27,226 women aged between 18 and 40 years attending the 30 intervention practices during the 3‐year study period (it was not clear what the figure was for control practices; information on the 37 control practices and women attending these practices was not provided in the study publications). In the intervention practices, GPs excluded 12,306 (45%) of women on grounds of completed family, infertility or sterility, inability to understand Dutch, or adverse family circumstances. Of 14,915 women offered preconception care, 45% responded (6782). Of these 1820 (27%) intended to get pregnant within the next 5 years. 348 women attended for preconception counselling over the 3‐year study period (representing 1.3% of the women in the relevant age group eligible for the study intervention). Results reported in study publications relate to those women attending for care. |
|
| Interventions | Intervention group: women were asked to express an interest in pre‐conception care. Those expressing interest were sent a risk assessment questionnaire and invited to attend with their partner for pre‐conception care provided by their GP. Advice addressed both general risk factors and specific advice on risks identified from the risk assessment questionnaire. Control group: women received routine care (no routine invitation to attend for pre‐conception care). |
|
| Outcomes | Knowledge, anxiety, pregnancy rates, changes in lifestyle and behaviour to reduce pregnancy risk factors (folic acid use, alcohol, smoking, infection, exposure to hazardous substances). | |
| Notes | Thanks to Dr Elsinga for providing helpful additional information about the study. The analyses for this trial were not carried out by randomisation group. Women in the intervention group who attended for pre‐conception care (a small proportion of the eligible age group) were matched with women attending for routine care. Information is not available on the whole randomised sample. We have been unable to include the data from the trial in the analyses in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Cluster randomisation. Randomisation was stratified. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? Clinical staff | High risk | It is not feasible to blind clinical staff to this type of intervention. |
| Blinding? Participants | High risk | Educational intervention. |
| Blinding? Outcome assessors | Unclear risk | Most analyses were not according to randomisation group. It may have been possible to blind outcome assessors to group allocation for some outcomes. |
| Incomplete outcome data addressed? All outcomes | High risk | Large numbers of women were excluded from the intervention group by their GP both before and after the intervention. Response rates were low and attendance for care was low. |
| Free of selective reporting? | High risk | Due to low attendance for care, an ITT analysis was not performed. Rather, women in the intervention group who had attended for care were matched with women from the comparison group. Figures on outcomes were not available for all women randomised who became pregnant during the study period. |
Floyd 2007.
| Methods | RCT. | |
| Participants | 830 women aged 18‐44 capable of becoming pregnant and who had had sexual intercourse in the previous 3 months without contraception, who were not pregnant or planning pregnancy at the time of the intervention and who were "risky" drinkers (defined as have had 8 drinks per week or more than 5 drinks on 1 occasion). Setting: community‐based settings including primary care practices, hospital clinics, prisons and drug and alcohol treatment centres in the USA. Recruitment between 2002‐4. (Project CHOICES). |
|
| Interventions | The aim of the intervention was to reduce the number of alcohol‐exposed pregnancies by encouraging women to avoid risky drinking or to use contraception, or both. Intervention: motivational interviews by trained counsellors. Women attended 4 sessions (approximately 1 hour each) and were given advice on reducing alcohol, with personalised feedback and goal setting. Women also received an appointment with a healthcare provider to discuss contraception. 63% of the sample attended all 4 counselling sessions. Comparison group: written information on alcohol risks and women's health. Both groups were assessed at baseline and at 3, 6 and 9 months following the intervention. Women were reimbursed for the time taken to complete assessments or to attend counselling sessions and for travel expenses. |
|
| Outcomes | Alcohol use (< 7 drinks per week or < 5 drinks on any 1 occasion). Risk of alcohol exposed pregnancies (risky drinking and no contraception). |
|
| Notes | A number of women in the sample were recruited from alcohol treatment centres. We had intended to exclude studies recruiting women actively participating in alcohol treatment programmes. In the context of the study, such women formed part of a larger sample mainly recruited from other community‐based settings and we decided to include this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated (some block randomisation in some recruitment centres). |
| Allocation concealment? | Low risk | Sealed, opaque, sequentially numbered envelopes. |
| Blinding? Clinical staff | High risk | Not feasible. |
| Blinding? Participants | High risk | Not feasible. |
| Blinding? Outcome assessors | Unclear risk | Follow up was conducted by staff unaware of group allocation. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 830 women were randomised. 237 were lost to follow up (29% attrition). There was further loss at longer‐term follow up. It was possible to include all women randomised in an ITT analysis by assuming that behaviour did not change in women lost to follow up. |
| Free of selective reporting? | Unclear risk | Not apparent. |
| Free of other bias? | Low risk | No baseline imbalance was apparent. Analysis was conducted to take account of attrition and recruitment setting. |
Jack 1998.
| Methods | RCT. | |
| Participants | 170 women recruited and assessed (it was not clear how many women were randomised to intervention and control groups). Inclusion criteria: women with negative pregnancy test results enrolled by a nurse experienced in prenatal risk assessment. Exclusion criteria: women who had had a pregnancy test as part of a specific medical assessment (e.g. for abdominal pain). |
|
| Interventions | All women completed a preconception risk survey to identify medical, psychosocial and behavioural risk factors. Intervention group: women and clinical staff caring for them were notified about risk factors identified; women received a booklet on reducing risks before pregnancy and were offered an appointment with their primary care doctor to discuss them. Primary care doctors received a letter describing the findings of the assessment and asked to initiate interventions or treatment to address risks and to document actions. Routine care: no feedback on the assessment was provided. |
|
| Outcomes | Risk factors (e.g. alcohol use, infection, nutrition) identified and addressed by interventions. | |
| Notes | Thanks to Dr Jack for providing the review team with helpful additional information about this study. Analysis in this study was carried out for a subgroup of women who had attended for care in the year following the initial assessment (100 women). Findings were not available for all women randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | A coin was tossed by the enrolling nurse (it was not clear at what stage in the recruitment process the coin was tossed). |
| Allocation concealment? | High risk | It would be possible for the enrolling nurse to change group allocation. |
| Blinding? Clinical staff | High risk | Primary care doctors were informed of risk assessment. |
| Blinding? Participants | High risk | Education and advice intervention. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Data on most outcomes were only available for those women attending for care in the year following assessment. |
| Free of selective reporting? | Unclear risk | Most of the analyses was for a subgroup of women who attended for care. |
Lumley 2006.
| Methods | RCT. | |
| Participants | Setting: study in Melbourne, Australia in a newly established pre‐pregnancy walk‐in service. 1579 women randomised (786 included in analysis). The aim was to recruit women at higher risk of poor pregnancy outcomes including recent migrants, lone parents and women with low incomes. An interpreter was available. Inclusion criteria: women attending government funded maternal and child health centres after the birth of their first child living in designated areas. Exclusion criteria: women attending for specialist care (for example, after the death of their first child). |
|
| Interventions | Intervention group: home visit by pre‐pregnancy midwife to discuss first pregnancy, labour, etc and to answer questions. In addition, midwife counselling including identification of risk factors (genetic, social, health or lifestyle) and timing and preparation for next pregnancy. Offer of referral for problems identified (e.g. to dietician or genetic services). Arrangements for rubella immunisation, advice on smoking and medication. Comparison group: home visit by pre‐pregnancy midwife to discuss first pregnancy and to answer questions. No active counselling intervention. |
|
| Outcomes | Primary outcomes: low birthweight, gestational age at birth, preterm birth, perinatal deaths and birth defects. Interval between pregnancies (not pre‐specified). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number tables, balanced block randomisation. |
| Allocation concealment? | Unclear risk | Not described. Allocation occurred before the midwife delivering the intervention met the women recruited. |
| Blinding? Clinical staff | Unclear risk | State that usual caregivers were blind to group allocation. |
| Blinding? Participants | High risk | Not feasible. |
| Blinding? Outcome assessors | High risk | |
| Incomplete outcome data addressed? All outcomes | High risk | 1579 women randomised. 176 became ineligible before the start of the trial. Of the remaining 1403 women there was further attrition (44%). 364 (26%) women were lost to follow up and 253 (18%) did not become pregnant during the study period. For the 786 women included in analyses there were low levels of missing data. (As part of this review we have carried out a sensitivity analysis where we attempted to include all women potentially eligible for that outcome, so we have added those women failing to become pregnant (but not lost to follow up) to the denominators of the study groups.) |
| Free of other bias? | Low risk | Not apparent. |
Robbins 2005.
| Methods | RCT. | |
| Participants | 322 women randomised aged 18‐45 years, capable of becoming pregnant, attending routine gynaecology clinics. Approximately 12% of the sample were planning pregnancy within the next year and 60% at some stage in the future. Exclusion criteria: women who were already pregnant or specifically attending for preconception or non‐routine care. Women who could not speak English or who had had hysterectomy, tubal ligation or a previous NTD pregnancy. |
|
| Interventions | Intervention group: brief counselling (approximately 1 minute) specifically about folic acid use by gynaecologist, pamphlet on benefits of folic acid and starter bottle of folic acid tablets. Follow‐up phone call reinforcing the message 1‐2 weeks later. (160 women.) Comparison group: routine brief counselling on health issues (e.g. breast examination, seat belt and sunscreen use. The advice could include information on folic acid depending on the usual practice of the clinician) and voucher for folic acid (which could be redeemed by posting off the voucher) (162 women). Groups were followed up after 2 months. |
|
| Outcomes | Weekly and daily folic acid use. | |
| Notes | The author kindly provided additional unpublished data for inclusion in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as permuted block method. Block size specified ‐ 10. |
| Allocation concealment? | Unclear risk | Described as "assigned randomly". |
| Blinding? Clinical staff | High risk | Not feasible. |
| Blinding? Participants | High risk | Not feasible. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Data available for 279 of 322 randomised (attrition 13%). |
Velott 2008.
| Methods | RCT (individual randomisation). | |
| Participants | Setting: 15 low‐income rural communities in Pennsylvania (USA) (The Central Pennsylvania Women's Health Study). Women in the area reported high levels of risk for obesity, poor nutrition, depression, alcohol use, smoking, infection and psycho‐social stress. Inclusion criteria: non‐pregnant pre‐ and inter‐conceptional women aged 18‐35 who were capable of becoming pregnant. Exclusion criteria: women who were known to be infertile (e.g. following hysterectomy or sterilisation). |
|
| Interventions | 362 women followed up (692 randomised, 2:1 intervention: control ratio). All women completed baseline risk assessment and women with blood pressure, cholesterol or other bio‐physical markers outside the normal range were referred to healthcare providers. Intervention: group education addressing pregnancy and conception, managing stress, improving health through lifestyle changes and preventing infection. Women attended 6 2‐hour sessions over a 12‐week period, with "buddy" mutual support and home work. Comparison group: no active intervention. Women received gift voucher incentives to attend intervention sessions and assessments. Women were followed up 14 weeks after the baseline assessment. |
|
| Outcomes | Attitudes, self‐reported behaviour (e.g. physical activity, use of multi‐vitamins and folic acid, alcohol use and smoking), cholesterol and blood pressure and other physical measurements. | |
| Notes | There was some baseline imbalance between intervention and control groups at baseline and at follow up. The authors provided additional, helpful, unpublished information on the study to the review team. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not clear. |
| Allocation concealment? | Unclear risk | Not clear. |
| Blinding? Clinical staff | High risk | Not feasible, educational intervention. |
| Blinding? Participants | High risk | |
| Blinding? Outcome assessors | Unclear risk | Those carrying out physical assessments were described as "blind" to the treatment condition. |
| Incomplete outcome data addressed? All outcomes | High risk | There were high levels of attrition in both the intervention and control groups (48%) at follow up. Post‐test data were not available for women lost to follow up. |
| Free of selective reporting? | Unclear risk | None apparent. |
| Free of other bias? | Unclear risk | Different methods were used in the 15 participating communities to recruit women. It was not clear whether this affected follow up. |
GP: general practitioner ITT: intention to treat NTD: neural tube defect RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Prunty 2008 | This study focuses on women with multiple sclerosis and examines decision‐aid materials to assist women in deciding whether to become pregnant. There was no health promotion intervention to improve pregnancy outcomes. |
Contributions of authors
Both authors were involved in drafting and revising the review.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
-
National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews: CPGS02
Declarations of interest
None known.
New
References
References to studies included in this review
Elsinga 2006 {published and unpublished data}
- Elsinga J, Pal‐de Bruin K, Cessie S, Jong‐Potjer L, Verloove‐Vanhorick S, Assendelft W. Preconception counselling initiated by general practitioners in the Netherlands: reaching couples contemplating pregnancy [ISRCTN53942912]. BMC Family Practice 2006;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong‐Potjer LC, Elsinga J, Cessie S, Pal‐de Bruin KM, Neven A Knuistingh, Buitendijk SE, et al. GP‐initiated preconception counselling in a randomised controlled trial does not induce anxiety. BMC Family Practice 2006;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Floyd 2007 {published data only}
- Ceperich S, Ingersoll K, Kareakashian M, Penberthy JK, Farrell L, Fabbri S, et al. Early outcomes of a 1‐session intervention to prevent alcohol‐exposed pregnancy in preconception women. Proceedings of the 70th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2008 June 14‐19; San Juan, Puerto Rico. 2008:32.
- Floyd RL, Sobell M, Velasquez MM, Ingersoll K, Nettleman M, Sobell L, et al. Preventing alcohol‐exposed pregnancies: a randomized controlled trial. American Journal of Preventive Medicine 2007;32(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jack 1998 {published and unpublished data}
- Jack BW, Culpepper L, Babcock J, Kogan MD, Weismiller D. Addressing preconception risks identified at the time of a negative pregnancy test. A randomized trial. Journal of Family Practice 1998;47(1):33‐8. [PubMed] [Google Scholar]
Lumley 2006 {published and unpublished data}
- Lumley J. A randomized trial of pre‐pregnancy counselling in inner city Melbourne. Personal communication 1993.
- Lumley J, Donohue L. Aiming to increase birth weight: a randomised trial of pre‐pregnancy information, advice and counselling in inner‐urban Melbourne. BMC Public Health 2006;6:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robbins 2005 {published and unpublished data}
- Robbins JM, Cleves MA, Collins HB, Andrews N, Smith LN, Hobbs CA. Randomized trial of a physician‐based intervention to increase the use of folic acid supplements among women. American Journal of Obstetrics & Gynecology 2005;192(4):1126‐32. [DOI] [PubMed] [Google Scholar]
Velott 2008 {published and unpublished data}
- Downs DS, Feinberg M, Hillemeier MM, Weisman CS, Chase GA, Chuang CH, et al. Design of the Central Pennsylvania Women's Health Study (CePAWHS) Strong Healthy Women Intervention: Improving Preconceptional Health. Maternal and Child Health Journal 2009;13(1):18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velott DL, Baker SA, Hillemeier MM, Weisman CS. Participant recruitment to a randomized trial of a community‐based behavioral intervention for pre‐ and interconceptional women findings from the Central Pennsylvania Women's Health Study. Womens Health Issues 2008;18(3):217‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Prunty 2008 {published data only}
- Prunty MC, Sharpe L, Butow P, Fulcher G. The motherhood choice: a decision aid for women with multiple sclerosis. Patient Education and Counseling 2008;71(1):108‐15. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 1993
- Adams MM, Bruce FC, Shulman HB, Kendrick JS, Brogan DJ. Pregnancy planning and pre‐conception counseling. The PRAMS Working Group. Obstetrics & Gynecology 1993;82(6):955‐9. [PubMed] [Google Scholar]
Clark 1994
- Clark NA, Fisk NM. Minimal compliance with the Department of Health recommendation for routine folate prophylaxis to prevent fetal neural tube defects. British Journal of Obstetrics and Gynaecology 1994;101(8):709‐10. [DOI] [PubMed] [Google Scholar]
Czeizel 1999
- Czeizel AE. Ten years of experience in periconceptional care. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;84(1):43‐9. [DOI] [PubMed] [Google Scholar]
de Jong‐Potjer 2003
- Jong‐Potjer LC, Bock GH, Zaadstra BM, Jong ORW, Verloove‐Vanhorick SP, Springer MP. Women's interest in GP‐initiated pre‐conception counselling in The Netherlands. Family Practice 2003;20(2):142‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Evers 2002
- Evers JL. Female subfertility. Lancet 2002;360:151‐9. [DOI] [PubMed] [Google Scholar]
Gates 2005
- Gates S. Methodological Guidelines. In: The Editorial Team. Pregnancy and Childbirth Group. About the Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005, Issue 2 .
Gnoth 2003
- Gnoth C, Frank‐Herrmann P, Freundl G, Godehardt D, Godehardt E. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Human Reproduction 2003;18:1959‐66. [DOI] [PubMed] [Google Scholar]
Heyes 2004
- Heyes T, Long S, Mathers N. Preconception care: practice and beliefs of primary care workers. Family Practice 2004;21(1):22‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Johnson 2006
- Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care‐‐United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. Morbidity and Mortality Weekly Reports 2006;55(RR‐6):1‐23. [PubMed] [Google Scholar]
RCOG 2008
- RCOG, RCM, RCA, RCPCH. Standards for maternity care. Report of a Working Party. London: RCOG Press, 2008. [Google Scholar]
RevMan 2008 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Stade 2009
- Stade BC, Bailey C, Dzendoletas D, Sgro M. Dowswell T. Psychological and/or educational interventions for reducing prenatal alcohol consumption in pregnant women and women planning pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004228] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tieu 2009
- Tieu J, Middleton P, Crowther CA. Preconception care for diabetic women to improve maternal and infant health. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007776] [DOI] [PubMed] [Google Scholar]
Wallace 1998
- Wallace M, Hurwitz B. Preconception care: who needs it, who wants it, and how should it be provided?. British Journal of General Practice 1998;48(427):963‐6. [PMC free article] [PubMed] [Google Scholar]


