Abstract
7-Ketocholesterol (7-KC) is found at an elevated level in patients with cancer and chronic liver disease. The up-regulation of an efflux pump, P-glycoprotein (P-gp) leads to drug resistance. To elucidate the effect of 7-KC on P-gp, P-gp function and expression were investigated in hepatoma cell lines Huh-7 and HepG2 and in primary hepatocyte-derived HuS-E/2 cells. At a subtoxic concentration, 48-h exposure to 7-KC reduced the intracellular accumulation and cytotoxicity of P-gp substrate doxorubicin in hepatoma cells, but not in HuS-E/2 cells. In Huh-7 cells, 7-KC elevated efflux function through the activation of phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway. 7-KC activated the downstream protein synthesis initiation factor 4E-BP1 and induced P-gp expression post-transcriptionally. The stimulation of efflux was reversible and could not be prevented by N-acetyl cysteine. Total cellular ATP content remained the same, whereas the lactate production was increased and fluorescence lifetime of protein-bound NADH was shortened. These changes suggested a metabolic shift to glycolysis, but glycolytic inhibitors did not eliminate 7-KC-mediated P-gp induction. These results demonstrate that 7-KC induces P-gp through PI3K/mTOR signaling and decreased the cell-killing efficacy of doxorubicin in hepatoma cells.
Keywords: 7-ketocholesterol, P-glycoprotein, PI3K/mTOR, glycolysis, hepatoma
1. Introduction
The multidrug resistance (MDR)1 (ABCB1) gene-encoded P-glycoprotein (P-gp) pumps out a variety of xenobiotics and endogenous substances from inside cells to the extracellular region [1]. Its induction contributes to intrinsic resistance to chemotherapeutic agents before the drugs are taken and to acquired resistance after repeated cycles of chemotherapy. Hepatocellular carcinoma (HCC) usually has an exceptionally poor response to systemic treatment with chemotherapeutic agents [2]. Doxorubicin is commonly used as a chemotherapeutic agent in the treatment of hepatoma. The concentration of doxorubicin required for a 50% decrease of cell proliferation in P-gp (+) Hep3B and HepG2 cells was approximately 4-fold higher than that in P-gp(−) SK-HEP-1 cells [3], indicating that the level of functional P-gp represented one of the key factors affecting the cell-killing efficacy of doxorubicin. In a study of HCC patients, patients with a detectable level of P-gp protein in tumor sections had a lower disease-free interval and survival time [4]. However, P-gp expression levels did not exhibit a significant correlation with cell proliferation, mitotic counts, and the existence of immunodetected p53. In a study of liver samples of HCC patients, the fractions of patients carrying immunodetectable P-gp in groups that had (27 patients) and had not (16 patients) undergone previous chemotherapy were similar [5]. In addition, P-gp protein levels in tumor tissues tend to be higher than the levels in the surrounding normal tissues in HCC patients with or without cirrhosis [6,7]. Thus, we postulated that microenvironmental factor(s) in tumor tissues might up-regulate the expression of P-gp.
Recent evidence has emphasized the importance of the tumor microenvironment (e.g., alterations in the membrane lipid composition) in intrinsic resistance to chemotherapy [8]. Hypercholesterolemia appears to be a key paraneoplastic syndrome in HCC patients [9]. Oxysterols, including 7-ketocholesterol (7-KC), are generated by the oxidation of cholesterol either by autooxidation or by enzymes such as cytochrome P450s [10]. 7-KC is one of the main oxysterols found in healthy human plasma and is abundant in retina and atherosclerotic plaques. Exposure of cells to 7-KC elicited a variety of defense responses, including inflammation, apoptosis, and the stimulation of vascular endothelial growth factor [10]. The blood concentration of 7-KC was about 7–20 ng/ml (17 – 50 nM) [11,12] in healthy controls. In patients with chronic hepatitis, lung cancer, and rectal cancer, blood 7-KC concentrations were 2- to 4-fold higher than those in healthy controls [11,12]. The mean hepatic contents of 7-KC in different groups were in a wide range of 0.24 – 36.85 µg/g liver [11,13]. According to the density of liver (1.051g/ml) [14], the mean hepatic contents of 7-KC were 0.57 – 91.98 µM, which was higher than its blood concentration. However, the hepatic content or blood concentration of 7-KC in hepatoma patients has not been reported. In hepatoma-bearing rats with hyperlipidemia, the mRNA and protein levels of cholesterol efflux pumps ABCA1 and ABCG1 in tumor tissues were significantly higher than their respective levels in host or control livers [15]. In patients with familial combined hyperlipidemia, the cholesterol lowering agent atorvastatin efficiently decreased plasma 7-KC levels to those of normolipidemic controls [16]. In hypercholesterolemia patients, the ABCB1 mRNA level in peripheral blood mononuclear cells (PBMC) was significantly reduced after taking a daily dose of 10 mg atorvastatin for 4 weeks [17]. There was a significant correlation between the reduction of total cholesterol and the mRNA level of ABCB1 in PBMC. In hepatoma cell line HepG2, 24-h exposure to atorvastatin decreased the expression level and efflux function of P-gp [17]. However, this decrease was not affected by mevalonic acid lactone, suggesting that the intermediates produced during cholesterol synthesis might not be involved. However, the effects of cholesterol oxidation products on P-gp were not reported.
P-gp-mediated efflux requires an energy supply from ATP, which is primarily provided by mitochondrial oxidative phosphorylation and cytosolic glycolysis. Reduced nicotinamide adenine dinucleotide (NADH) is a key cofactor in these sources and it acts as a principal electron and proton donor in mitochondria [18]. Although it exists in both oxidized (NAD+) and a reduced (NADH) forms, only NADH is intrinsically fluorescent. This auto-fluorescence of NADH allows time-resolved fluorescence decay studies to be performed, thereby providing a noninvasive method to analyze energy metabolism in viable cells [18]. The fluorescence lifetime and the ratio of free (mainly localized in the cytoplasm) to protein-bound (mainly localized in the mitochondria) NADH are likely related to the NADH/NAD+ ratio [19]. Among oxysterols, 7-KC was first studied due to its marked pathological and toxicological effects [10] and elevated concentrations in patients with chronic hepatitis [11]. To examine the effect of 7-KC on P-gp, the efflux function and expression of P-gp were investigated in hepatoma cell lines Huh-7 and HepG2 and in the immortalized primary hepatocyte cell line, HuS-E/2 [20]. The signaling participated in the P-gp up-regulation and the changes in energetic machinery were illustrated.
2. Materials and methods
2.1. Chemicals, siRNA, and antibodies
Akt kinase inhibitor (AKI), bromopyruvate, cholesterol, doxorubicin, 17β-estradiol, 7-KC, N-acetyl cysteine, LY294002, 2-(N-morpholino)-ethanesulfonic acid (MES), 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT), oligomycin, rapamycin, rhodamine 123 (Rh123), sodium oxamate, and verapamil were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, NaCl, sucrose, and Triton X-100 were purchased from Merck KGaA (Darmstadt, Germany). Dihydroethidium and 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolylcarbocyanine iodide (JC-1) were purchased from Invitrogen (Carlsbad, CA, USA). ON-TARGETplus™ (SMARTpool) mTOR and scramble siRNAs were purchased from Thermo Scientific-Dharmacon RNAi Technologies (Suwanee, GA, USA). Antibodies against phosphorylated and total Akt, the mammalian target of rapamycin (mTOR), p70 ribosomal protein S6 kinase (S6K), and eukaryotic translation initiation factor (eIF) 4E-binding protein 1 (4E-BP1) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal antibody Mdr (H-241): sc-8313 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fluorescein isothiocyanate (FITC)-labeled monoclonal anti-P-gp was purchased from Abcam (Cambridge, MA, USA). Rabbit polyclonal anti-caveolin-1 was purchased from BD Biosciences Pharmingen (Franklin Lakes, NJ, USA). Antibodies against α-tubulin and β-actin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cell culture and treatments
HuS-E/2 cells (HPV18/E6E7-immortalized primary human hepatocytes) were generously provided by Dr. K. Shimotohno (Kyoto University, Japan) and were cultured following the method established by Aly et al. [20]. The doxorubicin-resistant breast cancer cell line MCF-7/ADR was cultured as described previously [21]. Huh-7 and HepG2 hepatoma cells were cultured using complete Dulbecco’s modified Eagle’s medium (DMEM), pH 7.2, containing 25 mM sodium bicarbonate, 1% non-essential amino acid, 1% glutamine, 10% fetal bovine serum (FBS, purchased from Biological Industries, Kibbutz Beit HaeMek, Israel), and penicillin/streptomycin. The serum-free medium contained 100 nM sodium selenite (Na2SeO3), 0.1% bovine serum albumin, and penicillin/streptomycin [22]. Cells were seeded and cultured for 24 h prior to drug treatment. 7-KC and cholesterol were dissolved in ethanol, and the final concentration of ethanol in medium was less than 0.1%. During cell treatment, 7-KC- and cholesterol-containing media were changed every 24 h.
2.3. Cell viability determination
Cell viability was monitored using MTT reduction [23], trypan blue exclusion, and lactate dehydrogenase (LDH) release analyses. In the trypan blue exclusion assay, cells were trypsinized and resuspended in a complete medium containing 0.04% trypan blue and the viable cells were enumerated using a hemocytometer. The activity of LDH released from cells to the medium was determined using a Cytotox 96 non-radioactive cytotoxicity assay kit (Promega, Madison, WI, USA). The IC50 value of cell growth inhibition was calculated by curve fitting using Grafit software (Erithacus Software, Surrey, UK).
2.4. Cellular accumulation of doxorubicin and efflux function of P-gp
To determine the accumulation of doxorubicin, 80% confluent cells were exposed to 10 µM (Huh-7 and HuS-E/2) and 50 µM (HepG2) doxorubicin for 1 h. The mean fluorescence of doxorubicin retained in 2 × 104 cells was measured using a flow FACSCalibur cytometer (Ex: 480 nm; Em: 564–660 nm; BD Biosciences, San Jose, CA, USA). The Rh123 efflux function of P-gp was determined using a method modified from Pan et al. [23]. Primarily, cells were seeded on a 12-well plate (1 × 105/ well) for 24 h and then incubated with 5 µM Rh123 for 1 h. After three washes with ice-cold phosphate buffered saline (PBS) in the dark, cells were incubated with Rh123-free medium in the absence and presence of 100 µM verapamil (a P-gp inhibitor) for 3 h. The fluorescence of Rh123 retained in the cells was measured (Ex: 485 nm; Em: 538 nm) and the percentage of Rh123 exported from the cells (E) was determined as the decrease of cellular Rh123. The efflux function of P-gp was monitored in terms of the decrease of export of Rh123 in the presence of verapamil (Etotal − E+verapamil, where Etotal is the value in the absence of verapamil).
2.5. Immunoblotting analyses
Cell lysate was prepared using 38 mM Tris·HCl buffer, pH 7.5, containing 0.15 M NaCl, 0.25% sodium deoxycholate, and 0.01% Triton X-100. Protein concentration was determined using Bradford reagent with bovine serum albumin as the standard (Bio-Rad, Hercules, CA, USA). Electrophoresis and the following electrotransfer of proteins (25 µg/well of cell lysate and 3.5 µg/well of sucrose gradient fractions were loaded) from the slab gel (7.5% or 10% polyacrylamide gel) to a nitrocellulose membrane were performed as described previously [23]. Immunoreacted proteins were visualized using a chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Relative band intensity was analyzed by densitometry using the Image Master software (Pharmacia Biotech, Uppsala, Sweden) and normalized by the band density of internal controls (β-actin, glyceraldehydes-3-phosphate dehydrogenase (GAPDH), or α-tubulin). To determine the expression level of cell surface P-gp, cells were collected, immunostained with FITC-labeled anti-P-gp, and analyzed using the flow cytometric determination. Fluorescent isotype control immunoglobulin G, FITC-IgG2a (Abcam, Cambrige, MA, USA) was used for immunostaining as the internal control. Mean fluorescence intensity was determined using FACS/Cell Quest software (BD Biosciences, San Jose, CA, USA).
2.6. Isolation of lipid rafts and quantification of 7-KC and cholesterol
Lipid rafts were isolated from Huh-7 cells using a method modified from the report of Royer et al. [24]. After 48-h exposure, 5×107 cells were washed with ice-cold PBS, treated with trypsin/EDTA (Biological Industries, Kibbutz Beit HaeMek, Israel), neutralized with medium, and centrifuged at 200×g for 5 min at room temperature. The cell pellet was washed with PBS and then treated with 1 ml of 25 mM MES buffer (pH6.5) containing 150 mM NaCl, 1% (w/v) Triton X-100, and a mixture of protease inhibitors (Roche Applied Science, Indianapolis, IN, USA). After 30 min at 4°C, the cells were homogenized on ice using a Teflon pestle-glass homogenizer for 10 strokes. Cell lysate was mixed with 1 ml MES and 2 ml 80% (w/v) sucrose in MES and layered on the bottom of centrifugation tubes. 4.5 ml of 30% and 3 ml of 5% sucrose in MES buffer were sequentially overlaid on the top of the mixture of cell lysate and sucrose. After 24-h centrifugation at 36,000 rpm in a SW41 rotor (Beckman Coulter, Inc., Fullerton, CA, USA), each fraction of 1.1 ml were collected from the top to the bottom of tubes, mixed, and stored at −20°C. An aliquot (1 ml) of each fraction was extracted with 2 volumes of dichloromethane and mixed. After centrifugation, 1 ml of the bottom layer was dried under N2 gas in the chemical hood. Samples were dissolved in a acetonitrile/methanol mixture and subjected to the liquid chromagraphy (LC)-mass spectrometry (MS, with atmospheric pressure chemical ionization (APCI)) analysis as described before [25]. Protein concentration of each fraction was determined as described above.
2.6. Real-time reverse transcription (RT)–polymerase chain reaction (PCR)
Cellular total RNA was prepared from cells using the TRIzol reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Total RNA (20 µg) was subjected to the RT reaction using RevertAid™ reverse transcriptase (ThermoFischer Scientific, Waltham, MA, USA). The cDNA products were further subjected to PCR amplification with SYBR Green using the LightCycler® 480 Real-Time PCR System (Roche Molecular Systems, Branchburg, NJ, USA). The primer sets used were as follows: MDR1, forward: CAGCTATTCGAAGAGTGGGC; reverse: CCTGACTCACCACACCAATG; and β-actin, forward: GAGCCACATCGCTCAGACAC; reverse: CATGTAGTTGAGGTCAATGAAGG. An initial enzyme activation step of 94°C for 4 min was followed by 40 cycles each of 55°C for 45 s and 72°C for 1 min. The threshold cycle (Ct) number was determined. The relative mRNA expression levels were normalized to the β-actin expression level, which allowed target cDNA calculation as 2−(Ct MDR1 − Ct β-actin).
2.7. Determination of superoxide radical, mitochondrial membrane potential, ATP content, and lactate production
To determine the superoxide radical, Huh-7 cells were incubated with 5 µM dihydroethidium in a complete medium for 1 h in the dark [26]. The fluorescence intensity of cells resuspended in PBS was measured using the flow cytometric determination. Mitochondrial membrane potential was monitored using a detection kit with the cationic JC-1 dye (Invitrogen, Eugene, OR, USA) following the manufacturer’s instructions. The ratio of the fluorescence intensities of red (Ex: 550 nm; Em: 600 nm) to green (Ex: 485 nm; Em: 535 nm) fluorescence was determined. Total cellular ATP content was quantitatively determined using ATP bioluminescence assay kit HS II (Roche Diagnostics, Mannheim, Germany). Lactate concentration of the medium was determined using an L-lactate assay kit purchased from Eton Bioscience (San Diego, CA, USA). The amount of lactate was calculated by interpolation from the standard curve established following the manufacturer’s instructions.
2.8. Transfection of mTOR siRNA
Huh-7 cells were transfected with mTOR siRNA (25 nM) and scramble siRNA (25 nM) using DharmaFECT transfection reagents (Thermo Scientific-Dharmacon RNAi Technologies, Suwanee, GA, USA) following the instruction manual and then cells were incubated in a CO2 incubator for 48 h. The knockdown of mTOR expression was examined by immunoblotting as described above. Transfected cells were exposed to 7-KC for 48 h and the Rh123 efflux function was determined as described above.
2.9. Time-domain fluorescence lifetime imaging (FLIM)
Time-domain FLIM was performed following the method of Ghukasyan et al. [27] to determine the fluorescence lifetime (τ) and the fractional contribution ratio of free to protein-bound forms of NADH (a1/a2). An oil immersion objective lens (40 ×, 1.3 N.A., Olympus), average power between 3 to 5 mW, and a bandpass filter of 450 ± 40 nm (Edmund Optics, Barrington, NJ, USA) were used. Images (256 × 256-pixel) of grouped cells were taken from 3 – 5 different locations per dish with minimum spacing of cells. The full width at half maximum of instrument response function (IRF) was measured to be about 200 ps. Fluorescence lifetime and fractional contribution (relative amplitude, a) were analyzed using the SymPhoTime software package (PicoQuant, Berlin, Germany) [19]. Curve fitting of the experimental data to a two-exponential decay model was performed using the equation F(t) = a1e(−t/τ1) + a2e(−t/τ2), where F(t) is the fluorescence intensity at time t. The a and τ values of the free and protein-bound NADH are indicated by 1 and 2, respectively. A time-optimized procedure was used to determine the quality of fitting with a minimum reduced χ2 value [27].
2.10. Statistical analyses
All data are given as the mean ± SD. The statistical significance of the differences between the control and treated groups was evaluated using Student’s t test. The results from cells treated with increasing concentrations of 7-KC were analyzed using one-way analysis of variance followed by Dunnett’s test (GraphPad Prism 3.02, GraphPad Software, La Jolla, CA, USA). A p value of less than 0.05 was considered statistically significant.
3. RESULTS
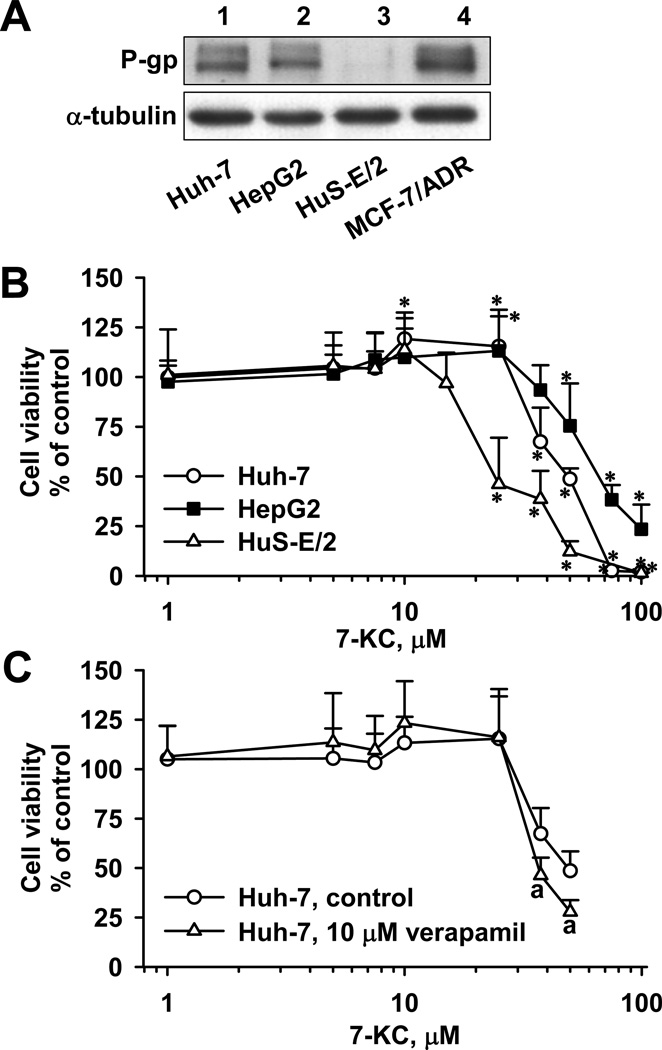
3.1. Basal P-gp levels and effects of 7-KC on cell viability in Huh-7, HepG2, and HuS-E/2 cells
In Huh-7 and HepG2 cells, an immune-reacted protein band was detected with the same electrophoretic mobility of the band detected in a P-gp-overexpressed MCF-7/ADR cell line. In HuS-E/2 cells, the P-gp level was relatively low (Fig. 1A). MTT assay was performed to monitor the number of viable cells with functional mitochondria. In Huh-7 cells, cell viability was slightly elevated by 10 and 25 µM 7-KC, but viability declined when the concentration was greater than 37.5 µM (IC50 for growth inhibition: 46.4 ± 2.5 µM) (Fig. 1B). To ensure a concentration without cell growth inhibition (defined as “sub-toxic”) of 7-KC in Huh-7 cells in later experiments, cell viability was further examined using trypan blue exclusion assays and LDH release to monitor the plasma membrane integrity. Cell viability was unchanged for 7-KC concentrations up to 10 µM (data not shown). The influence of 7-KC on HepG2 cells was similar to that on Huh-7 cells. The growth of HepG2 cells was stimulated at 25 µM and then started to decline at higher concentrations (IC50: 68.3 ± 2.2 µM), whereas there was no 7-KC-mediated growth stimulation in HuS-E/2 cells. Compared to Huh-7 and HepG2 cells, HuS-E/2 cells were more susceptible to 7-KC-induced cell death (IC50: 27.4 ± 2.5 µM). Consequently, cells were treated with 7-KC at sub-toxic concentrations (Huh-7, ≤ 10 µM; HepG2, ≤ 37.5µM; HuS, ≤ 10 µM) in the following P-gp studies. To examine the contribution of the higher P-gp levels found in hepatoma cells to their increased resistance to 7-KC-induced toxicity, the effect of verapamil (a P-gp inhibitor) on cell growth was studied in Huh-7 cells. Verapamil slightly enhanced the cytotoxicity of 7-KC at the concentrations higher than 37.5 µM (Fig. 1C). Because the toxicity enhancement only occurred when cells were exposed to a cytotoxic concentration of 7-KC, the enhancement by verapamil could not support the contribution of P-gp to the greater resistance to 7-KC toxicity in hepatoma cells.
Fig. 1.
Differential basal P-gp protein levels and susceptibilities to 7-KC-induced toxicity in Huh-7, HepG2, and HuS-E/2 cells. (A) Representative blots of immuno-detected P-gp protein in untreated cells. As a positive control of P-gp expression, cell lysate of drug-resistant breast cancer cell line MCF-7/ADR was loaded in lane 4. (B) Effects of 7-KC on cell viability monitored by MTT reduction activities. Cells were exposed to a range of concentrations of 7-KC for 48 h. (*Values significantly different from the vehicle-treated control cells, p < 0.05.) (C) Effects of verapamil on the cell viability in Huh-7 cells. MTT reduction activities was determined in Huh-7 cells with single or co-exposure to 7-KC and verapamil for 48 h. (aValues significantly different from the respective cells treated with the same concentration of 7-KC but without verapamil, p < 0.05.) Data are the results of 3 independent experiments with 4–8 determinations.
3.2. Effects of 7-KC on the accumulation of doxorubicin in Huh-7, HepG2, and HuS-E/2 cells
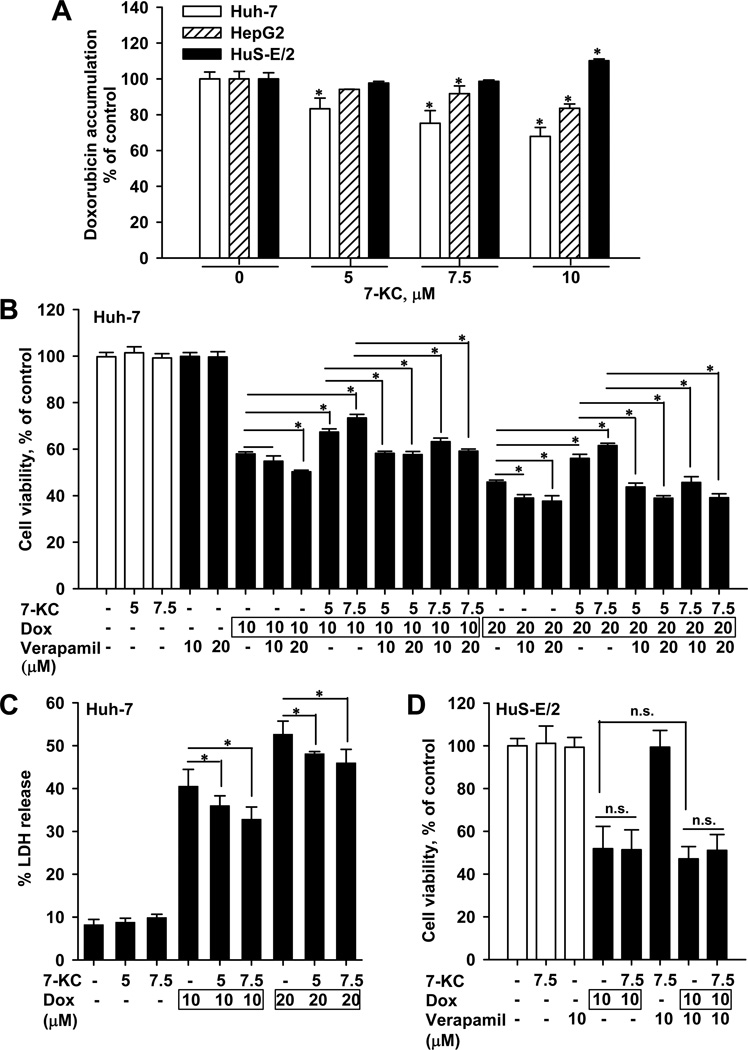
Cellular clearance of doxorubicin was found to be approximately half by diffusion and half by P-gp-mediated efflux [28]. The P-gp substrate doxorubicin predominately accumulated in the nucleus of control and 7-KC-treated Huh-7 cells (fluorescence microscopy images not shown). After 1-h exposure to doxorubicin in the accumulation assay, cell morphology and MTT values were not affected in cells (data not shown). Pre-exposure to 7-KC for 48 h caused a concentration-dependent decrease of the doxorubicin accumulation in Huh-7 cells and this decrease was greater than that in HepG2 cells under the same exposure concentration (Fig. 2A). In contrast, doxorubicin accumulation in HuS-E/2 cells was not reduced by 7-KC at concentrations up to 7.5 µM. On increasing the concentration of 7-KC to 10 µM, cellular doxorubicin accumulation increased slightly in HuS-E/2 cells (Fig. 2A), which was the opposite of the responses in Huh-7 and HepG2 hepatoma cells. The following determinations of doxorubicin toxicity and the P-gp inductive mechanism in hepatoma cells were performed using Huh-7, not HepG2, because 7-KC had a stronger effect on Huh-7.
Fig. 2.
The influence of 7-KC (48 h) on cellular accumulation and cytotoxicity of doxorubicin. (A) The differential effects of 7-KC on doxorubicin accumulation in Huh-7, HepG2, and HuS-E/2 cells. The 1-h accumulation of doxorubicin was determined as described in Materials and Methods. Values are the results of 3 independent experiments with 2 – 4 determinations (*p < 0.05). (B) Effect of 7-KC pre-exposure and verapamil co-exposure on doxorubicin-induced cytotoxicity determined by MTT assay in Huh-7 cells. Following pre-exposure to 7-KC, cells were exposed to doxorubicin (Dox) in the presence or absence of verapamil for 24 h. (C) Effect of 7-KC on doxorubicin-induced cytotoxicity in Huh-7 cells as assessed by LDH release. Following the 7-KC pre-exposure, cells were exposed to doxorubicin for 24 h and then the activity of LDH released to the medium was determined. (D) Effect of 7-KC on doxorubicin-induced cytotoxicity determined by MTT assay in HuS-E/2 cells. Values are the results of 3 independent experiments with 4 – 8 determinations. (*p < 0.05, n.s.: no significant difference in values between the two groups of cells).
3.3. Effects of 7-KC on the cytotoxicity of doxorubicin in Huh-7 and HuS-E/2 cells
To examine the impact of 7-KC on the cell-killing efficacy of doxorubicin, a doxorubicin-treatment causing cytotoxicity was designed and the cytotoxicity was assessed by MTT assay and LDH release. Huh-7 cells were pre-exposed to 7.5 µM 7-KC for 48 h and then to doxorubicin for a further 24 h. Compared with doxorubicin treatment alone, results of MTT assay showed that pre-exposure to 7-KC significantly diminished the cytotoxicity of doxorubicin (Fig. 2B). Treatment of cells with verapamil alone did not affect cell viability, whereas the presence of verapamil enhanced the cytotoxicity of doxorubicin in 7-KC-pre-exposed cells, indicating that P-gp played a crucial role in the 7-KC-mediated increase of resistance to doxorubicin in hepatoma cells. Consistent with the results of MTT assay, 7-KC pre-exposure reduced the LDH release induced by doxorubicin in Huh-7 cells (Fig. 2C). In contrast, in HuS-E/2 cells, 7-KC pre-exposure (7.5 µM, 48 h) did not affect the cytotoxicity of doxorubicin (Fig. 2D). Neither did verapamil enhance the cytotoxicity of doxorubicin in HuS-E/2 cells. The failure of verapamil and 7-KC to affect doxorubicin cytotoxicity in HuS-E/2 cells is consistent with their low P-gp expression level (Fig. 1A) and the absence of changes in doxorubicin accumulation on treatment with 7.5 µM 7-KC (Fig. 2A).
3.4. Effects of 7-KC on the efflux function of P-gp in Huh-7 and HepG2 cells
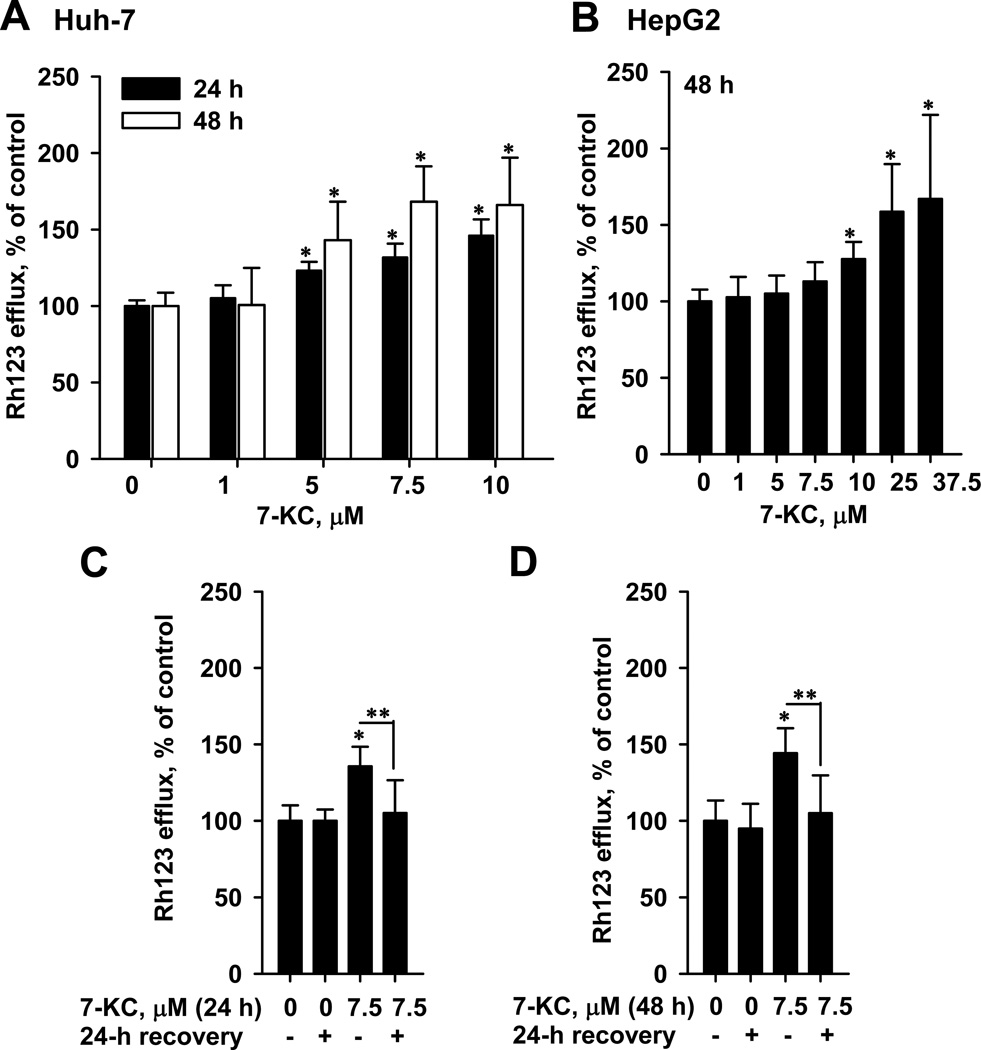
To determine the efflux function of P-gp, Rh123 was used as a substrate. The basal export of Rh123 in Huh-7 and HepG2 cells were 21.6 ± 1.5% and 19.4 ± 5.9% (mean ± SD), respectively. However, the efflux function of HuS-E/2 cells was below the detection limit. To evaluate the competitive potential of 7-KC against Rh123, Huh-7 cells were co-exposed to 7-KC (7.5 µM) and Rh123 for 1h. The co-exposure could not interfere with the basal export of Rh123 (data not shown), suggesting that 7-KC did not compete with Rh123 for export. However, the P-gp efflux function was elevated by 43–68% after 24-h and 48-h pre-exposure to 7-KC at concentrations of 5–10 µM (Fig. 3A). Because the increase of efflux function after 48-h exposure to 7-KC was greater than that after 24-h exposure, P-gp induction was further studied after 48-h exposure. In HepG2, 48-h exposure to 10–37.5 µM 7-KC also stimulated Rh123 efflux by up to 67% (Fig. 3B). To examine the reversibility of this efflux stimulation, Huh-7 cells pre-exposed to 7.5 µM 7-KC (24 h (Fig. 3C) or 48 h (Fig. 3D)) were cultured in a complete medium without 7-KC for a further 24 h. After recovery in 7-KC-free complete medium, the increased efflux function returned to the basal level, indicating that P-gp induction by 7-KC is reversible.
Fig. 3.
Effects of 7-KC on the Rh123 efflux function of P-gp in Huh-7 (A) and HepG2 (B) hepatoma cells and the recovery of efflux induction by removal of 7-KC in Huh-7 cells (C and D). In the study of recovery effect, Huh-7 cells were pre-exposed to 7-KC for 24 h (C) or 48 h (D). After pre-exposure, cells were washed and cultured in 7-KC-free medium for 24 h and then the efflux function was determined. Values are the results of 3 independent experiments with 2–3 determinations (*,**p < 0.05).
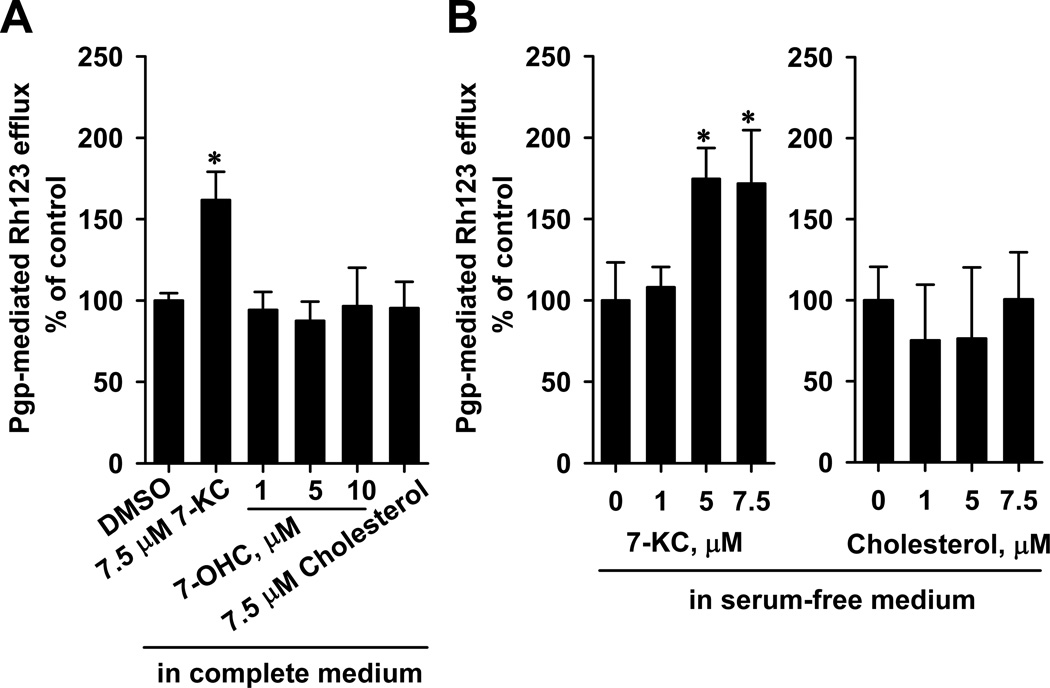
3.5. Differential effects of 7-KC, 7α-hydroxycholesterol, and cholesterol on the efflux function of P-gp in Huh-7 cells
Research has shown that serum has a high concentration of cholesterol (828 – 931 µM in FBS, data provided by Biological Industries, Kibbutz Beit HaeMek, Israel) and possibly exhibited trace amounts of oxysterols. Therefore, the effects of 7-KC and cholesterol on efflux function were determined in Huh-7 cells cultured in a serum-free medium as described in Materials Methods. It was found that 7-KC (7.5 µM), but not cholesterol (7.5 µM), elevated the efflux function of P-gp after 48-h culture in both a serum-containing medium (Fig. 4A) and a serum-free medium (Fig. 4B).7α-Hydroxycholesterol at 1–10 µM did not exhibit cytotoxicity (data not shown) and could not affect the Rh123 efflux function of P-gp in Huh-7 cells cultured in a serum-containing medium (Fig. 4A).
Fig. 4.
Effects of 48-h exposure to 7-KC, 7-hydroxycholesterol (7-OHC), and cholesterol on Rh123 efflux function in Huh-7 cells. Cells were cultured in complete (10% serum-fortified) (A) and serum-free media (B). Data are from 3 independent experiments with 3 determinations (*p < 0.05).
3.6. Differential effects of 7-KC on the expression of P-gp in Huh-7 and HuS-E/2 cells
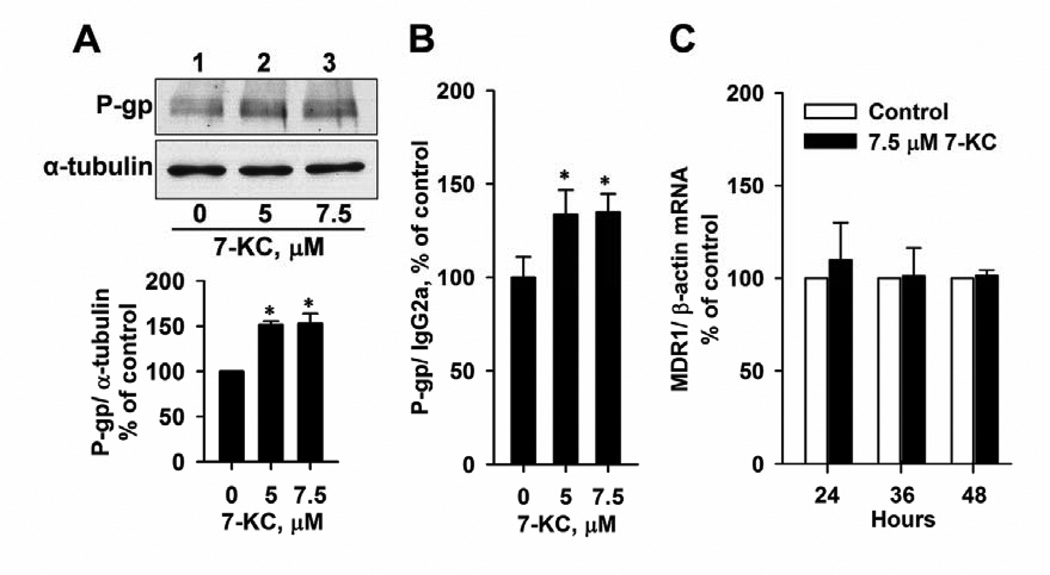
To illustrate the mechanism of P-gp induction by 7-KC, P-gp protein and mRNA levels were analyzed. Immunoblotting analyses of Huh-7 cell lysate showed that exposure to 5 and 7.5 µM 7-KC increased total P-gp protein by 52% and 53%, respectively (Fig. 5A). The flow cytometric detection of immunostained cell-surface P-gp showed that exposure to 5 and 7.5 µM 7-KC increased the surface P-gp protein level by 34% and 35%, respectively (Fig. 5B). However, 7-KC did not affect the MDR1 mRNA level (Fig. 5C). These results suggest that 7-KC up-regulates P-gp expression at a post-transcriptional step. In HuS-E/2 cells, the P-gp protein level was not induced after 48 h-exposure to 7.5 µM 7-KC (90 ± 18% of the control level) (blot not shown).
Fig. 5.
Effects of 7-KC on P-gp protein and mRNA levels in Huh-7 cells. Total and surface P-gp protein levels of cell lysate were analyzed by immunoblotting analysis (A) and immunostaining followed by flow cytometric determination (B), respectively. Cells were exposed to 5 and 7.5 µM 7-KC for 48 h. The band and fluorescence intensities of immune-reacted P-gp were normalized with the respective intensities of the internal controls, α-tubulin and IgG2a. Data are from 3 determinations (*p < 0.05). (C)The P-gp mRNA levels in Huh-7 cells were determined using real-time PCR analysis after exposure of cells to 7.5 µM 7-KC for 24 – 48 h. Data are from 3 experiments with duplicated determinations.
3.7. The main distribution of 7-KC in the non-lipid raft domains in Huh-7 cells
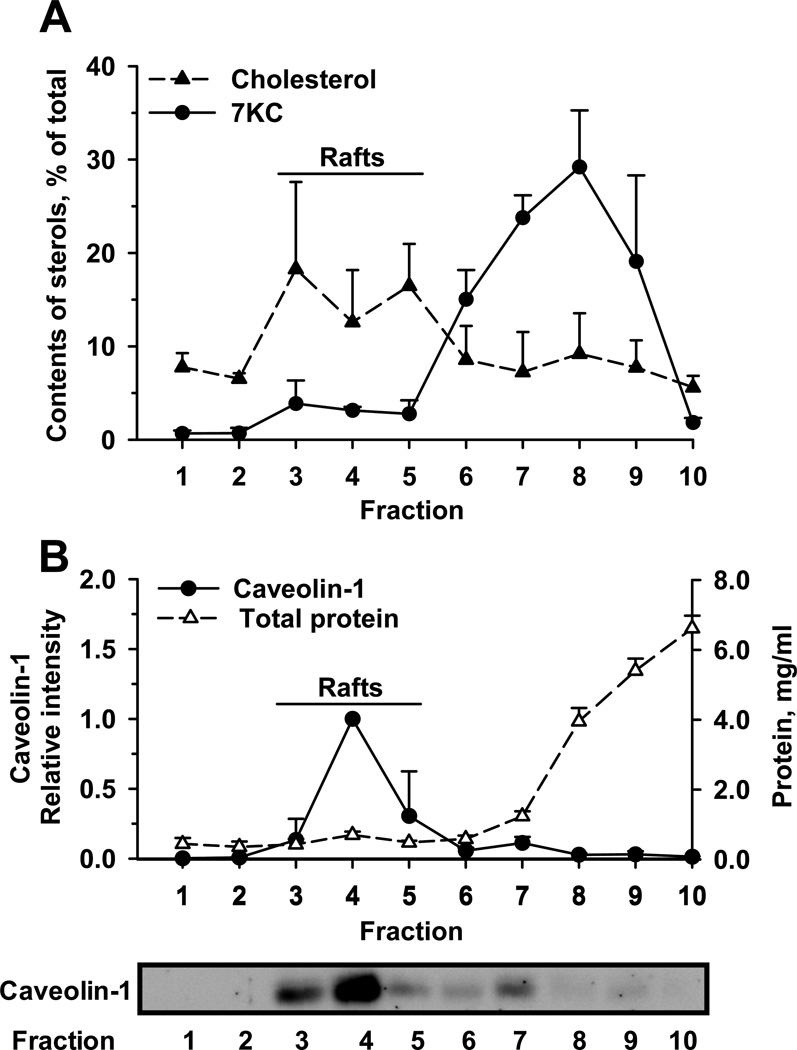
After centrifugation with a sucrose density gradient, 10 fractions were collected from the top (low-density) to the bottom (high-density). After 48-h exposure of Huh-7 cells to 7.5 µM 7-KC, 7-KC was found to distribute mainly into fractions 6 – 9 (Fig. 6A). The cholesterol enriched (Fig. 6A) and caveolin-1 (Fig. 6B) abundant fractions were found in fractions 3–5, indicating the fractions in which the lipid raft domains mainly located. The high density fractions 8–10 contained relatively higher concentrations of total protein than the low density fractions (Fig. 6B). This result revealed that this 7-KC exposure resulted in the main distribution of 7-KC in the non-lipid raft domains in Huh-7 cells.
Fig. 6.
Accumulation of 7-KC in the non-lipid raft domains in 7-KC-treated Huh-7 cells. Huh-7 cells were exposed to 7.5 µM 7-KC for 48 h. After fractionation of cell lysates on a sucrose density gradient, 10 fractions from the top (fraction 1, low density) to the bottom (fraction 10, high density) were collected and subjected to the quantification of 7-KC and cholesterol (A). Data represent the percent of contents of 7-KC and cholesterol in each fraction to their respective total contents in all fractions. The total protein concentration and relative level of caveolin-1 in each fraction were determined (B). The level of caveolin-1 in fraction 4 was defined as 1.0. The representative blot (3.5 µg protein/well loaded) is shown on the bottom. Data are from 3 separate experiments.
3.8. Tthe activation of PI3K/mTOR pathway by 7-KC in Huh-7 but not in HuS-E/2 cells
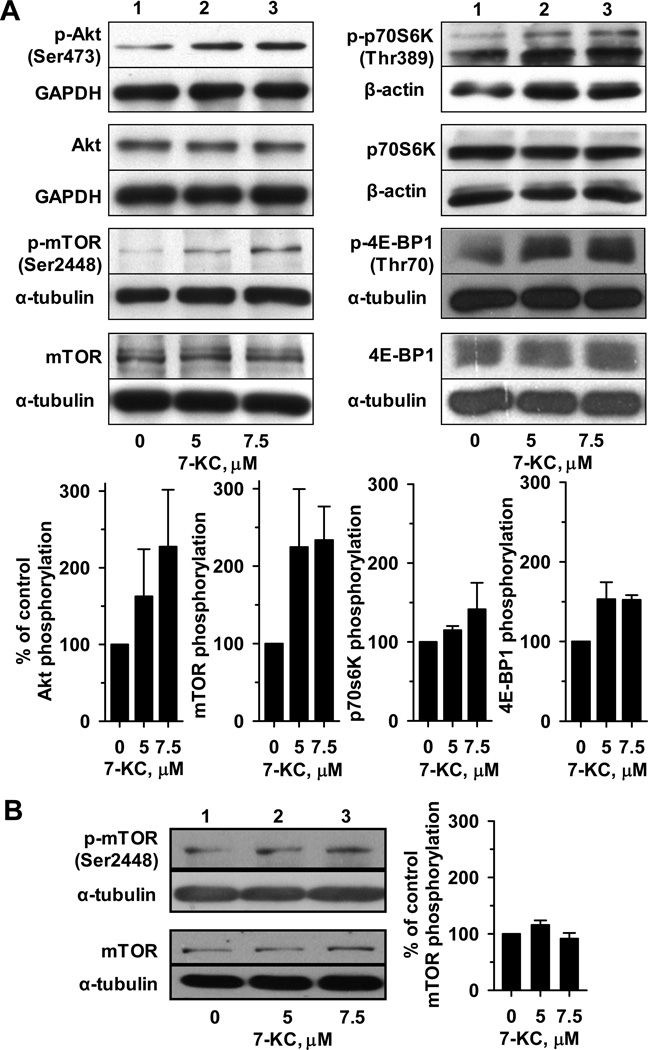
In Huh-7 cells, the phosphorylation status of molecules participating in the PI3K/mTOR pathway was determined by immunoblotting (Fig. 7A). 7-KC at 5 and 7.5 µM stimulated the phosphorylation of Akt and the downstream kinase mTOR. Subsequently, the phosphorylation of two important downstream effectors of mTOR, p70S6K and 4E-BP1, were enhanced. In contrast, in HuS-E/2 cells, the phosphorylation status of mTOR was not enhanced by 48-h exposure to either 5 or 7.5 µM 7-KC (Fig. 7B).
Fig. 7.
Activation of the Akt/mTOR phosphorylation cascade by 7-KC was evident in Huh-7 (A) but not in HuS-E/2 (B) cells. Huh-7 and HuS-E/2 cells were exposed to 5 or 7.5 µM 7-KC for 48 h. Cell lysate (25 µg protein) was subjected to immunoblotting analyses probing the signaling proteins as described in Materials and Methods. The relative band intensities of signaling proteins were normalized with the respective internal controls as indicated. GAPDH: glyceraldehyde 3-phosphate dehydrogenase. The phosphorylation of proteins was evaluated as the ratio of normalized levels of phosphorylated to total protein. Bar graphs show the phosphorylation extent relative to that of control cells and values are the results of 3 determinations.
3.9. Effects of N-acetyl cysteine, oligomycin, and PI3K/mTOR inhibitors on 7-KC-mediated P-gp induction in Huh-7 and HepG2 cells
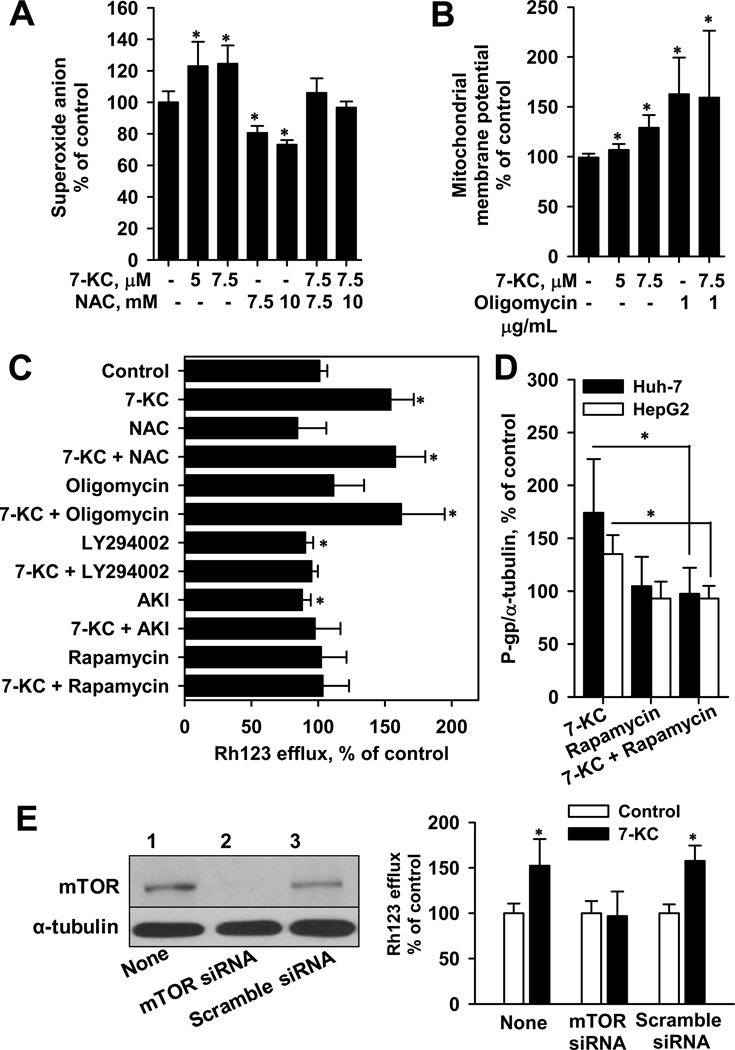
To investigate the upstream signaling of P-gp induction, first, the effects of 7-KC on superoxide anion formation and mitochondrial membrane potential were determined. The 48-h exposure of Huh-7 cells to 7-KC increased cellular superoxide formation, whereas N-acetyl cysteine treatment decreased the constitutive production of superoxide anions (Fig. 8A). The increase of superoxide radicals by 7-KC was eliminated by 30-min pre-treatment with N-acetyl cysteine, a radical scavenger, followed by 48-h co-treatment with 7-KC. Determination of changes in mitochondrial membrane potential using the JC-1 dye showed that 7-KC increased the J-aggregates, suggesting disruption of the proton flux into the mitochondrial matrix (Fig. 8B). The ATP synthase inhibitor oligomycin increased the mitochondrial membrane potential. However, the potential remained high when cells were pre-treated (1 h) with oligomycin and then co-treated (48 h) with 7-KC and oligomycin.
Fig. 8.
The induction of P-gp by 7-KC was dependent on PI3K/mTOR pathway in Huh-7 and HepG2 cells. (A) and (B) show the effects of 7-KC on the superoxide anion generation and mitochondrial membrane potential in Huh-7 cells, respectively. NAC: N-acetyl cystein. Values are the results of 3 or 4 independent experiments with 2 or 3 determinations (*p < 0.05). (C) Effects of NAC (10 mM), oligomycin (1 µg/ml), LY294002 (10 µM), AKI (10 µM), and rapamycin (1 µM) on Rh123 efflux activity induced by 7-KC in Huh-7 cells. Cells were pre-exposed to a scavenger/inhibitor for 1 h prior to the 48-h co-treatment with each of the above-mentioned agents and 7-KC. After co-treatment, cells were exposed to a medium containing 5 µM Rh123 and the P-gp function was determined. Values are the results of 3 independent experiments with 3 or 4 determinations. (D) Effect of rapamycin on the P-gp protein level elevated by 7-KC in Huh-7 and HepG2 cells. P-gp protein levels were analyzed by immunoblotting and the band intensity was determined. Values are the band intensity ratio relative to that in control cells (3 determinations, *p < 0.05). (E) Effect of mTOR knockdown on 7-KC-stimulated (7.5 µM, 48 h) Rh123 efflux activity in Huh-7 cells. Cells were transfected with mTOR siRNA or scramble siRNA. Left panel shows the representative blot confirming the knockdown of mTOR expression. The 7-KC-mediated efflux induction was blocked by mTOR knockdown (right panel). Values are the results of 3 independent experiments (*p < 0.05).
Second, the effects of radical scavengers and signaling inhibitors on 7-KC-elevated efflux activity and protein level of P-gp were studied. Cells were pre-exposed to scavenger/inhibitor for 1 h before 48-h co-treatment with 7-KC. Although oligomycin elevated the mitochondrial membrane potential, as 7-KC did, oligomycin did not stimulate Rh123 efflux. N-acetyl cysteine and oligomycin did not suppress the Rh123 efflux induced by 7-KC. Single treatment with LY294002 (non-selective PI3K inhibitor) and AKI decreased the constitutive efflux function (Fig. 8C), whereas the mTOR inhibitor rapamycin could not affect it. On co-treatment with 7-KC, the LY294002, AKI, and rapamycin decreased the 7-KC-enhanced efflux activity to a basal level. In Huh-7 cells, rapamycin treatment decreased P-gp induction by 7-KC (Fig. 8D). In HepG2 cells, 7-KC-induced P-gp protein level was also decreased by rapamycin. In Huh-7 cells, the transfection of mTOR siRNA knocked down the expression of mTOR protein and suppressed the induction of Rh123 efflux by 7-KC (Fig. 8E). These results revealed that activation of the PI3K/mTOR pathway is essential for P-gp induction by 7-KC in Huh-7 and HepG2 cells.
3.10. Alteration of NADH fluorescence lifetime and the stimulation of lactate production by 7-KC in Huh-7 cells
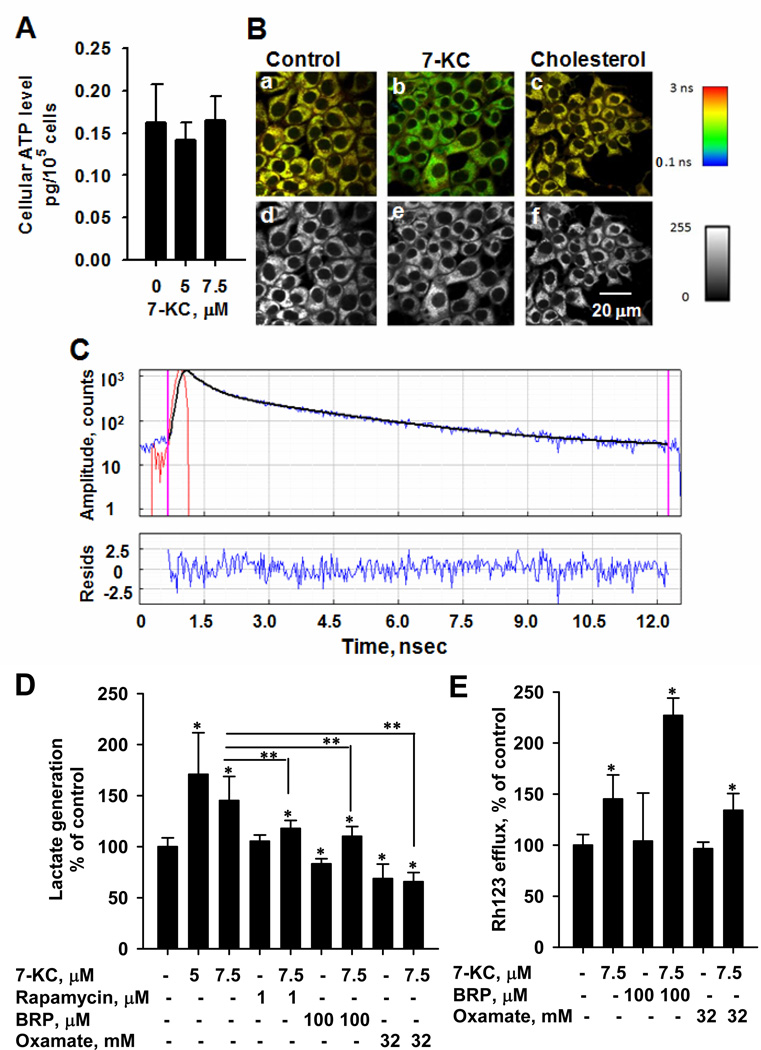
The cellular total ATP level remained unchanged after exposure to 7-KC (Fig. 9A). However, time-resolved fluorescence analysis showed that 7-KC shortened the average lifetime (τ) of NADH, whereas cholesterol had no effect. Representative FLIM microscopy images and time-dependent amplitude changes are shown in Fig.9B and 9C, respectively. The ratio (a1/a2) of free to bound forms of NADH increased after 7-KC exposure, indicating that the decrease of average τ was mainly attributable to the increased fractional contribution of the short lifetime τ1 of free NADH or the decreased τ2 of the protein-bound NADH (Table 1). These changes suggested the perturbation of energetic sources by 7-KC. Consequently, the generation of glycolytic product lactate was investigated to examine the glycolytic shift. The exposure to 7-KC increased lactate production and this increase was reduced by rapamycin (Fig. 9D). Bromopyruvate and sodium oxamate decreased lactate production (Fig. 9D), but none of them reduced 7-KC-induced efflux activity (Fig. 9E). The contribution of glycolytic stimulation to P-gp induction is not obvious.
Fig. 9.
Effects of 7-KC on total ATP level (A), NADH fluorescence lifetime (B and C), and lactate production (D and E) in Huh-7 cells. Cells were exposed to 7.5 µM 7-KC or cholesterol for 48 h. Panel (B) shows the representative multiphoton fluorescence lifetime and intensity images of NADH. The lifetime and intensity are shown in color and gray scales as indicated, respectively. Panel (C) shows the amplitude decay of NADH autofluorescence (blue) as a function of time in vehicle-control cells, the best fit (black), and the instrument response function (red). Residuals (blue) are shown in the bottom panel. Panels (D) and (E) show the effects of inhibitors on lactate production and Rh123 efflux function stimulated by 7-KC, respectively. Cells were pre-exposed to rapamycin or glycolytic inhibitors, bromopyruvate (BRP) and sodium oxamate, at the concentrations as indicated for 1 h and then co-treated with 7-KC for 48 h. The lactate production in control cells was 154 ± 35 nmol/105 cells. Values are the results of 3 experiments with 2 – 3 determinations (*,**p < 0.05).
Table 1.
Changes in fluorescence lifetime and ratio of free NADH to protein-bound NADH as a result of 7-KC or cholesterol treatment of Huh-7 cells.
| Treatment | τ1, ns | τ2, ns | Average τ, ns | a1/a2 |
|---|---|---|---|---|
| Control | 0.53 ± 0.01 | 2.93 ± 0.12 | 2.16 ± 0.09 | 2.80 ± 0.19 |
| 7-KC | 0.57 ± 0.02* | 2.54 ± 0.03* | 1.75 ± 0.03* | 3.81 ± 0.31* |
| Cholesterol | 0.54 ± 0.02 | 2.96 ± 0.06 | 2.18 ± 0.13 | 2.91 ± 0.08 |
Huh-7 cells were exposed to 7.5 µM 7-KC or cholesterol for 48 h. Fluorescence lifetimes of free (τ1) and protein-bound NADH (τ2) and the ratio of their fractional contribution (a1/a2) were determined as described in Materials and Methods. Data represent the mean ± SD of 3 or 4 individual experiments (*p < 0.05).
4. DISCUSSION
Increasing attention is being paid to the unique tumor microenvironment in cancer therapy. Our results showed that 7-KC (at a sub-toxic concentration of 5 and 7.5 µM, 48 h) but not cholesterol stimulated P-gp function in Huh-7 cells. 7-Hydroxycholesterol at a concentration up to 10 µM did not change the efflux function of P-gp. As a result of increased efflux by 7-KC, the accumulation and cell-killing efficacy of doxorubicin were reduced. In another experiment, Huh-7 cells were exposed to 1 µM 7-KC for 4 weeks with the subculture every 3–4 days. However, the Rh123 efflux function was not stimulated by 7-KC (data not shown). The lack of induction by this treatment could be attributed to factors such as inadequate exposure concentration and time-period, the influence of sub-culture process, and the differential effects of acute and chronic exposure. The concentration- and time-dependence of the effect of chronic 7-KC exposure and the P-gp modulation by the other oxysterols will be further investigated.
Compared with cholesterol, 7-KC has higher polarity and is less effective in forming ordered lipid domains and in phase boundary bilayer defects [29]. On the other hand, 7-KC has been reported to activate liver X receptor (LXR) [30], which has a dual function in the regulation of PI3K signaling [31,32]. The activation of PI3K signaling has been linked to lipid raft-associated receptors and kinases, such as epidermal growth factor receptor (EGFR) and Src kinase [33]. It has been reported that 7-KC became incorporated into the sphingolipid/cholesterol-enriched lipid raft domains of the plasma membrane when cells were exposed to 7-KC at a cytotoxic concentration of 50 µM [24]. Cell death was increased and the PI3K/Akt signaling was suppressed. However, our study of 7-KC at a sub-toxic concentration indicated that 7-KC mainly distributed in the non-lipid raft domains and the PI3K/Akt signaling was activated. 7-KC was able to form crystalline membrane domains different from cholesterol, which might be linked to the decreased cell death in aortic smooth muscle cells [34]. However, the association between 7-KC microcrystals and PI3K/mTOR activation has not been demonstrated. The mechanism by which 7-KC activated PI3K/mTOR signaling remains unclear.
Results of chemical inhibition of the signaling pathway, determinations of the phosphorylation cascade, and mTOR siRNA knockdown demonstrate that activation of PI3K/mTOR signaling participates in the 7-KC-mediated induction of P-gp, whereas the contribution of elevated ROS is small. Consistent with the down-regulation reported previously [35,36], our results also showed that constitutive Rh123 efflux activity was decreased by LY294002, AKI, and an inhibitor of extracellular signal-regulated kinase (ERK), PD98059 (data not shown). Contrary to the suppression by LY294002 and AKI, PD98059 did not suppress 7-KC-stimulated Rh123 efflux activity, suggesting that ERK might not be involved in this induction process (data not shown).
The present 7-KC treatment reveals a post-transcriptional induction via the stimulation of the phosphorylation of p70S6K and 4E-BP1, which execute the downstream signal of mTOR and promote the formation of active initiation complex of protein synthesis to accomplish translational regulation [37]. Atorvastatin treatment decreased the blood concentration of 7-KC in patients and the MDR1 mRNA level in HepG2 cells [16,17]. The decrease in 7-KC may not be the main contributor to atorvastatin-mediated down-regulation of P-gp, or atorvastatin may suppress MDR1 mRNA level via different signaling pathways. The post-translational regulation of P-gp has been reported to include phosphorylation through several protein kinases such as protein kinase C and Pim-1 [38]. However, the association between P-gp phosphorylation and efflux function remained inconsistent. To determine the effect of 7-KC on the phosphorylation status of P-gp, imunoblotting analysis of phosphorylated serine on the immunoprecipitated P-gp was performed. Our results showed that phospho-P-gp level that matches the increased total P-gp level was increased by 7-KC but not cholesterol exposure (data not shown). To further identify the contribution of phosphorylation to the functional induction of P-gp by 7-KC, effects of knockdown of protein kinases on the phosphorylation, ubiquitination, protein degradation process, and efflux function of P-gp need to be clarified.
7-KC reduced the cytotoxicity of doxorubicin in another hepatoma cell line HepG2, but not in HuS-E/2 cells. This irresponsiveness might be associated with the lack of 7-KC-mediated mTOR activation in HuS-E/2 cells. Normal and cancer cells exhibit distinct growth properties, including differences in the network/cross-talk among signaling pathways [39]. Our findings provide an evidence for the differential response of hepatoma and normal hepatocyte-derived cells to 7-KC. Huh-7 and HepG2 cells have several differences in their protein profiles, such as the mutation status of p53 [40]. Both p53-dependent and p53-independent induction of P-gp by xenobiotics have been reported in liver cancer cells [41,42]. The induction of P-gp by 7-KC occurred in both Huh-7 and HepG2 cells, suggesting that the p53 mutation in Huh-7 cells did not eliminate its responsiveness to P-gp induction. In our studies, the minimal concentration of 7-KC (5 µM) to show P-gp induction was higher than its blood concentration. However, cells were exposed to 7-KC for a short time-period of 48 h. A further study of chronic exposure to a pathological concentration of 7-KC in vivo is essential to evaluate the contribution of 7-KC to intrinsic chemoresistance in patients. Since 7-KC-mediated P-gp induction was found to be reversible after a 24-h recovery period without 7-KC, the reduction of 7-KC level may have beneficial effects in patients considered to receive chemotherapy.
Both 7-KC and oligomycin elevated the mitochondrial membrane potential, reflecting the reduced level of protons in the mitochondrial matrix. However, 7-KC but not oligomycin stimulated the efflux function of P-gp. Thus, the increased mitochondrial membrane potential could not directly contribute to P-gp induction. The reduced level of protons might result from mitochondrial hyperpolarization caused by ATP import from cytosolic glycolysis and from reverse ATP synthesis via ATP synthase. As a consequence, the total ATP level did not change and cell viability was unaffected. On the other hand, activation of the PI3K/mTOR pathway can also stimulate the aerobic glycolysis pathway and tumor growth [43]. A rapamycin-sensitive stimulation of lactate production by 7-KC was found in Huh-7 cells, supporting the mitochondrial hyperpolarization and glycolytic shift. NADH fluorescence is emitted mainly from mitochondria [19] and the free/bound ratio (a1/a2) of NADH was increased by oxidative phosphorylation inhibitor CoCl2 and by glycolytic inhibitor bromopyruvate [44], suggesting that a1/a2 might not be a selective marker of changes in the cellular energetic machinery. However, τ2 was increased when glycolysis was inhibited and decreased when oxidative phosphorylation was inhibited in cultured cells. Thus, a decrease in τ2 might be a marker of the glycolytic shift. Consistent with the 7-KC-mediated glycolytic stimulation and mitochondrial disruption, τ2 was decreased. Thus, energy generation might be shifted by 7-KC from mitochondrial oxidation to glycolysis, which is known as the Warburg effect [45]. Glycolytic intermediate pyruvate and glycolysis inhibitors (2-deoxy-D-glucose and iodoacetate) have been reported to increase and decrease the expression of P-gp, respectively [46]. However, in our study, neither sodium oxamate nor bromopyruvate suppressed 7-KC-induced efflux activity. P-gp induction by 7-KC may not be linked to enhanced glycolysis.
In summary, our findings demonstrate that 7-KC at a sub-toxic concentration stimulates the efflux function of P-gp, which engenders the reduced cell-killing effect of doxorubicin in Huh-7 cells. The up-regulation of P-gp by 7-KC occurred via the PI3K/mTOR pathway. 7-KC interfered with mitochondrial pH gradient and stimulated glycolysis. All of these events may conduct to the poor prognosis of cancer therapy. Further studies of the chronic effects of oxysterols at a pathological concentration in vivo are warranted.
Acknowledgements
This study was supported mainly by grants V99C1-207 and V101V1-205 from Taipei Veterans General Hospital, Taipei and partly by an institutional grant from the National Research Institute of Chinese Medicine, Taipei.
Abbreviations
- AKI
Akt kinase inhibitor
- 4E-BP1
eukaryotic translation initiation factor (eIF) 4E-binding protein 1
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- FLIM
fluorescence lifetime imaging
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- 7-KC
7-ketocholesterol
- LDH
lactate dehydrogenase
- MDR
multidrug resistance
- mTOR
mammalian target of rapamycin
- MTT
3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium
- NADH
reduced nicotinamide adenine dinucleotide
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- P-gp
P-glycoprotein
- PI3K
phosphatidylinositol 3-kinase
- Rh123
rhodamine 123
- ROS
reactive oxygen species
- RT
reverse transcription
- p70S6K
p70 ribosomal protein S6 kinase.
REFERENCES
- 1.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? The Oncologist. 2010;15(suppl 4):42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 3.Tong AW, Su D, Mues G, Tillery GW, Goldstein R, Klintmalm G, Stone MJ. Chemosensitization of human hepatocellular carcinoma cells with cyclosporine A in poat-liver transplant patient plasma. Clin Cancer Res. 1996;2:531–539. [PubMed] [Google Scholar]
- 4.Soini Y, Virkajarvi N, Raunio H, Paakko P. Expression of P-glycoprotein in hepatocellular carcinoma: a potential marker of prognosis. J Clin Pathol. 1994;49:470–473. doi: 10.1136/jcp.49.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itsubo M, Ishikawa T, Toda G, Tanaka M. Immunohistochemical study of expression and cellular localization of the multidrug resistance gene product P-glycoprotein in primary liver carcinoma. Cancer. 1994;73:298–303. doi: 10.1002/1097-0142(19940115)73:2<298::aid-cncr2820730211>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Grudé P, Conti F, Mennecier D, Louvel A, Houssin D, Weill B, Calmus Y. MDR1 gene expression in hepatocellular carcinoma and the peritumoral liver of patients with and without cirrhosis. Cancer Lett. 2002;186:107–113. doi: 10.1016/s0304-3835(02)00155-6. [DOI] [PubMed] [Google Scholar]
- 7.Nagasue N, Dhar DK, Makino Y, Yoshimura H, Nakamura T. Overexpression of P-glycoprotein in adenomatous hyperplasia of human liver with cirrhosis. J Hepatol. 1995;22:197–201. doi: 10.1016/0168-8278(95)80429-3. [DOI] [PubMed] [Google Scholar]
- 8.Meyer dos Santos S, Weber CC, Franke C, Muller WE, Eckert GP. Cholesterol: coupling between membrane microenvironment and ABC transporter activity. Biochem Biophys Res Comm. 2007;354:216–221. doi: 10.1016/j.bbrc.2006.12.202. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SJ, Lee SD, Chang CF, Wu JC, Tsay SH, Lui WY, Chiang JH, Lo KJ. Hypercholesterolaemia in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1992;7:491–496. doi: 10.1111/j.1440-1746.1992.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 10.Vejux A, Malvitte L, Lizard G. Side effects of oxysterols: cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz J Med Biol Res. 2008;41:545–556. doi: 10.1590/s0100-879x2008000700001. [DOI] [PubMed] [Google Scholar]
- 11.Corradini SG, Micheletta F, Natoli S, Iappelli M, Angelantonio ED, Marco RD, Elisei W, Siciliano M, Rossi M, Berloco P, Attili AF, Diczfalusy U, Iuliano L. High preoperative recipient plasma 7β-hydroxycholesterol is associated with initial poor graft function after liver transplantation. Liver Transpl. 2005;11:1494–1504. doi: 10.1002/lt.20524. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Kitame F, Uemura Y, Ishida N. The regulating effect of cholesterol derivatives isolated from human sera on lymphocyte response to phytohemagglutinin. Tohoku J Exp Med. 1983;140:245–258. doi: 10.1620/tjem.140.245. [DOI] [PubMed] [Google Scholar]
- 13.Ryzlak MT, Fales HM, Russell WL, Schaffner CP. Oxysterols and alcoholic liver disease. Alcohol Clin Exp Res. 1990;14:490–495. doi: 10.1111/j.1530-0277.1990.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 14.Overmoyer BA, McLaren CE, Brittenham GM. Uniformity of liver density and nonheme (storage) iron distribution. Arch Pathol Lab Med. 1987;111:549–554. [PubMed] [Google Scholar]
- 15.Hirayama T, Honda A, Matsuzaki Y, Miyazaki T, Ikegami T, Doy M, Xu G, Lea M, Salen G. Hypercholesterolemia in rats with hepatomas: increased oxysterols accelerate efflux but do not inhibit biosynthesis of cholesterol. Hepatology. 2006;44:602–611. doi: 10.1002/hep.21291. [DOI] [PubMed] [Google Scholar]
- 16.Arca M, Natoli S, Micheletta F, Riggi S, Angelantonio ED, Montali A, Antonini TM, Antonini R, Diczfalusy U, Iuliano L. Increased plasma levels of oxysterols, in vivo markers of oxidative stress, in patients with familial combined hyperlipidemia: reduction during atorvastatin and fenofibrate therapy. Free Rad Biol Med. 2007;42:698–705. doi: 10.1016/j.freeradbiomed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues AC, Curi R, Britto LR, Rebbechi IM, Hirata MH, Bertolami MC, Bernik MM, Dorea EL, Hirata RD. Down-regulation of ABCB1 transporter by atorvastatin in a human hepatoma cell line and in human peripheral blood mononuclear cells. Biochim Biophys Acta. 2006;1760:1866–1873. doi: 10.1016/j.bbagen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Reyes JM, Fermanian S, Yang F, Zhou SY, Herretes S, Murphy DB, Elisseeff JH, Chuck RS. Metabolic changes in mesenchymal stem cells in osteogenic medium measured by autofluorescence spectroscopy. Stem Cells. 2006;245:1213–1217. doi: 10.1634/stemcells.2004-0324. [DOI] [PubMed] [Google Scholar]
- 19.Bird DK, Yan L, Vrotsos KM, Eliceiri KW, Vaughan EM, Keely PJ, White JG, Ramanujam N. Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH. Cancer Res. 2005;65:8766–8773. doi: 10.1158/0008-5472.CAN-04-3922. [DOI] [PubMed] [Google Scholar]
- 20.Aly HH, Watashi K, Hijikata M, Kaneko H, Takada Y, Egawa H, Uemoto S, Shimotohno K. Serum-derived hepatitis C virus infectivity in interferon regulatory factor-7-suppressed human primary hepatocytes. J Hepatol. 2007;46:26–36. doi: 10.1016/j.jhep.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Shieh MJ, Hsu CY, Huang LY, Chen HY, Huang FH, Lai PS. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. J Control Release. 2011;152:418–425. doi: 10.1016/j.jconrel.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Selenite-induced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem. 2003;278:39615–39624. doi: 10.1074/jbc.M304095200. [DOI] [PubMed] [Google Scholar]
- 23.Pan WC, Chen RM, Shen YC, Chen CC, Ueng YF. Suppressive effect of tobacco smoke extracts on oral P-glycoprotein function and its impact in smoke-induced insult to oral epidermal cells. Toxicol Lett. 2009;185:116–123. doi: 10.1016/j.toxlet.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Royer MC, Lemaire-Ewing S, Desrumaux C, Monier S, deBarros JPP, Athias A, Néel D, Lagrost L. 7-Ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E. J Biol Chem. 2009;284:15826–15834. doi: 10.1074/jbc.M808641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescein. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 27.Ghukasyan V, Hsu YY, Kung HS, Kao FJ. Application of fluorescence resonance energy transfer resolved by fluorescence lifetime imaging microscopy for the detection of enterovirus 71 infection in cells. J Biomed Opt. 2007;12:024016–024023. doi: 10.1117/1.2718582. [DOI] [PubMed] [Google Scholar]
- 28.Dordal MS, Winter JN, Atkinson AJ., Jr Kinetic analysis of p-glycoprotein-mediated doxorubicin efflux. J Pharmacol Exp Ther. 1992;263:762–766. [PubMed] [Google Scholar]
- 29.Massey JB, Pownall HJ. The polar nature of 7-ketocholesterol determines its location within membrane domains and the kinetics of membrane microsolubilization by apolipoprotein A-I. Biochemistry. 2005;44:10423–10433. doi: 10.1021/bi0506425. [DOI] [PubMed] [Google Scholar]
- 30.Aye ILMH, Waddell BJ, Mark PJ, Keelan JA. Oxysterols inhibit differentiation and fusion of term primary trophoblasts by activating liver X receptors. Placenta. 2011;32:183–191. doi: 10.1016/j.placenta.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Huwait EA, Greenow KR, Singh NN, Ramji DP. A novel role for c-Jun N-termainal kinase and phosphoinositide 3-kinase in the liver X receptor-mediated induction of macrophage gene expression. Cell Signal. 2011;23:542–549. doi: 10.1016/j.cellsig.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pommier AJC, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, Baron S, Lobaccaro JMA. Liver X receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 33.Arcaro A, Aubert M, Espinosa del Hierro ME, Khanzada UK, Angelidou S, Tetley TD, Bittermann AG, Frame MC, Seckl MJ. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signaling. Cellular Signal. 2007;19:1081–1092. doi: 10.1016/j.cellsig.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Phillips JE, Geng YJ, Mason RP. 7-Ketocholesterol forms crystalline domains in model membranes and murine aortic smooth muscle cells. Atherosclerosis. 2001;159:125–135. doi: 10.1016/s0021-9150(01)00504-4. [DOI] [PubMed] [Google Scholar]
- 35.García MG, Alaniz LD, Russo RIC, Alvarez E, Hajos SE. PI3K/Akt inhibition modulates multidrug resistance and activates NF-κB in murine lymphoma cell lines. Leukemia Res. 2009;33:288–296. doi: 10.1016/j.leukres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther. 2007;6:2092–2102. doi: 10.1158/1535-7163.MCT-07-0148. [DOI] [PubMed] [Google Scholar]
- 37.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breier A, Gibalova L, Seres M, Barancik M, Sulova Z. New insight into P-glycoprotein as a drug target. Anti-cancer Agent Med Chem. 2013;13:159–170. [PubMed] [Google Scholar]
- 39.Jazirehi AR, Wenn PB, Damavand M. Therapeutic implications of targeting the PI3Kinase/AKT/mTOR signaling module in melanoma therapy. Am J Cancer Res. 2012;2:178–191. [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu IC, Tokiwa T, Metcalf RA, Welsh JA, Sun T, Harris CC. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis. 1993;14:987–992. doi: 10.1093/carcin/14.5.987. [DOI] [PubMed] [Google Scholar]
- 41.Mathieu MC, Lapierre I, Brault K, Raymond M. Aromatic hydrocarbon receptor (AhR)-, AhR nuclear translocator- and p53-mediated induction of murine multidrug resistance mdr1 gene by 3-methylcholanthrene and benzo(a)pyrene in hepatoma cells. J Biol Chem. 2001;276:4819–4827. doi: 10.1074/jbc.M008495200. [DOI] [PubMed] [Google Scholar]
- 42.Ros JE, Schuetz JD, Geuken M, Streetz K, Moshage H, Kuipers F, Manns MP, Jansen PLM, Trautwein C, Muller M. Induction of Mdr1b expression by tumor necrosis factor-α in rat liver cells is independent of p53 but requires NF-κB signaling. Hepatology. 2001;33:1425–1431. doi: 10.1053/jhep.2001.24667. [DOI] [PubMed] [Google Scholar]
- 43.Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, Wang Y, Jing Y, Yang H, Chen R, Chang L, Zhang Y, Goto J, Onda H, Chen T, Wang MR, Lu Y, You H, Kwiatkowski D, Zhang H. Mammalian target of rapamycin up-regulation of pyruvate kinase type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skala MC, Riching KM, Bird DK, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, Keely PJ, Ramanujam N. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free NADH in normal and pre-cancerous epithelia. J Biomed Opt. 2007;12:1–19. doi: 10.1117/1.2717503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warburg O. On the origin of cancer cells. Science. 1956;324:1029–1033. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 46.Wartenberg M, Richter M, Datchev A, Günther S, Milosevic N, Bekhite MN, Figulla HR, Aran JM, Pétriz J, Sauer H. Glycolytic pyruvate regulates p-glycoprotein expression in multicellular tumor spheroids via modulation of the intracellular redox state. J Cell Biochem. 2010;109:434–446. doi: 10.1002/jcb.22422. [DOI] [PubMed] [Google Scholar]