Abstract
Background
Epithelial ovarian cancer is diagnosed in 4500 women in the UK each year of whom 1700 will ultimately die of their disease.Of all cases 10% to 15% are diagnosed early when there is still a good possibility of cure. The treatment of early stage disease involves surgery to remove disease often followed by chemotherapy. The largest clinical trials of this adjuvant therapy show an overall survival (OS) advantage with adjuvant platinum-based chemotherapy but the precise role of this treatment in subgroups of women with differing prognoses needs to be defined.
Objectives
To systematically review the evidence for adjuvant chemotherapy in early stage epithelial ovarian cancer to determine firstly whether there is a survival advantage of this treatment over the policy of observation following surgery with chemotherapy reserved for treatment of disease recurrence, and secondly to determine if clinical subgroups of differing prognosis based on histological sub-type, or completeness of surgical staging, have more or less to gain from chemotherapy following initial surgery.
Search methods
We performed an electronic search using the Cochrane Gynaecological Cancer Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL 2011, Issue 3), MEDLINE (1948 to Aug week 5, 2011) and EMBASE (1980 to week 36, 2011). We developed the search strategy using free-text and medical subject headings (MESH).
Selection criteria
We selected randomised clinical trials that met the inclusion criteria set out based on the populations, interventions, comparisons and outcome measures.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality. Disagreements were resolved by discussion with a third review author. We performed random-effects meta-analyses and subgroup analyses.
Main results
Five randomised controlled trials (RCTs), enrolling 1277 women, with a median follow-up of 46 to 121 months, met the inclusion criteria. Four trials were included in the meta-analyses and we considered them to be at a low risk of bias. Meta-analysis of five-year data from three trials indicated that women who received adjuvant platinum-based chemotherapy had better overall survival (OS) than those who did not (1008 women; hazard ratio (HR) 0.71; 95% confidence interval (CI) 0.53 to 0.93). Likewise, meta-analysis of five-year data from four trials indicated that women who received adjuvant chemotherapy had better progression-free survival (PFS) than those who did not (1170 women; HR 0.67; 95% CI 0.53 to 0.84). The trials included in these meta-analyses gave consistent estimates of the effects of chemotherapy. In addition, these findings were robust over time (10-year PFS: two trials, 925 women; HR 0.67; 95% CI 0.54 to 0.84).
Subgroup analysis suggested that women who had optimal surgical staging of their disease were unlikely to benefit from adjuvant chemotherapy (HR for OS 1.22; 95% CI 0.63 to 2.37; two trials, 234 women) whereas those who had sub-optimal staging did (HR for OS 0.63; 95% CI 0.46 to 0.85; two trials, 772 women). One trial showed a benefit from adjuvant chemotherapy among women at high risk (HR for OS 0.48; 95% CI 0.32 to 0.72) but not among those at low/medium risk (HR for OS 0.95; 95% CI 0.54 to 1.66). However, these subgroup findings could be due to chance and should be interpreted with caution.
Authors’ conclusions
Adjuvant platinum-based chemotherapy is effective in prolonging the survival of the majority of patients who are assessed as having early (FIGO stage I/IIa) epithelial ovarian cancer. However, it may be withheld from women in whom there is well-differentiated encapsulated unilateral disease (stage 1a grade 1) or those with comprehensively staged Ib, well or moderately differentiated (grade 1/2) disease. Others with unstaged early disease or those with poorly differentiated tumours should be offered chemotherapy. A pragmatic approach may be necessary in clinical settings where optimal staging is not normally performed/achieved. In such settings, adjuvant chemotherapy may be withheld from those with encapsulated stage Ia grade 1 serous and endometrioid carcinoma and offered to all others with early stage disease.
Medical Subject Headings (MeSH): Antineoplastic Agents [*therapeutic use]; Carboplatin [therapeutic use]; Chemotherapy, Adjuvant [methods]; Cisplatin [therapeutic use]; Disease-Free Survival; Early Detection of Cancer; Melphalan [therapeutic use]; Neoplasm Staging; Ovarian Neoplasms [*drug therapy; pathology; surgery]; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans
BACKGROUND
Description of the condition
Ovarian cancer is the seventh most common cancer among women up to 64 years of age. A woman’s risk of developing ovarian cancer by age 65 years ranges from 0.36% in developing countries to 0.64% in developed countries (GLOBOCAN 2008). Worldwide there are more than 200,000 new cases of ovarian cancer each year, accounting for around 4% of all cancers diagnosed in women. In Europe, ovarian cancer is the leading cause of gynaecological cancer death: just over a third of women are alive five years after diagnosis (Sant 2003), largely because most women with ovarian cancer are diagnosed when the cancer is already at an advanced stage and surgical cure is impossible (Jemal 2008).
Over 85% of ovarian cancers develop in the surface (epithelial) cells of the ovary. There are different types based on microscopic features (histopathological types) of which the most common are serous. Endometrioid, mucinous and clear cell cancers are less common and the malignant Brenner type is rare. Malignant tumours are characterised by their grade: this describes the microscopic pattern of growth (architecture) and cellular features (cytology) and varies from well differentiated (grade G1) to moderately and poorly differentiated (G2 and G3 respectively). Well-differentiated tumours are of better prognosis than G2 or G3 tumours. FIGO staging is used to describe the spread of the disease. FIGO Stage I disease is confined to one or both ovaries and FIGO stage II disease is limited in spread to the true pelvis. FIGO stage I is sub-divided into three stages, Ia-Ic. In stage Ia, disease is confined to one ovary with no involvement of the ovarian surface with no tumour cells in the fluid of the abdominal cavity (negative peritoneal washings); stage Ib indicates similarly encapsulated disease in both ovaries but with no evidence of other spread; stage Ic indicates ovarian cyst rupture or ascites containing malignant cells (Shepherd 1989). FIGO stage II is similarly divided into three sub-stages. Stage IIa indicates spread to the uterus or fallopian tubes; stage IIb indicates spread to other pelvic structures; stage IIc is as for IIa or IIb but also indicates ovarian surface involvement or positive ascites or peritoneal washings. (See Table 1 for full details of FIGO staging.) Fewer than 30% of women present with stage I or II ovarian cancer (Jemal 2008).
Table 1.
Staging of ovarian cancer
| Stage | Description |
|---|---|
| Ia | Disease confined to one ovary with no capsular involvement. Peritoneal washings/cytology negative |
| Ib | Disease confined to both ovaries with no capsular involvement. Peritoneal washings/cytology negative |
| Ic | Disease confined to the ovary/ovaries but ovarian capsulae involved or cyst rupture |
| IIa | Extension to uterus or fallopian tubes |
| IIb | Extension to other pelvic tissues |
| IIc | As for IIa or IIb but one or both ovaries have ruptured capsule or surface tumour; malignant ascites or positive peritoneal washings |
| IIIa | Histologically confirmed microscopic seeding of abdominal peritoneal surfaces and negative retroperitoneal lymph nodes |
| IIIb | Histologically confirmed implants of abdominal peritoneal surfaces less than 2 cm and negative retroperitoneal lymph nodes |
| IIIc | Histologically confirmed implants of abdominal peritoneal surfaces greater than 2 cm or positive retroperitoneal lymph nodes |
| IV | Distant metastases (including liver parenchyma/positive pleural fluid cytology) |
Women with early ovarian cancer should be offered surgery both to remove the disease and to provide accurate staging, which is a key factor in assessing the impact of different treatments in this patient group. The pattern of spread of ovarian cancer is such that small deposits of tumour ‘hidden’ in the upper abdomen and retro-peritoneum can be readily missed. It has been shown that a significant percentage of women will be under-staged if the initial staging surgery is sub-optimal. Accurate staging helps provide better prediction of outcome in individual cases, is an independent prognostic factor for survival in stage I disease (Trimbos 2003; Zanetta 1998) and influences ongoing management.
Recent reports have confirmed a very good prognosis for women with stage Ia disease treated with conservation of the contralateral ovary in order to preserve their fertility (Morice 2001; Schilder 2002). A proportion of patients with stage I disease will be cured by their surgery and it may be that the chance of survival is improved if the surgery is undertaken by a trained gynaecological oncologist (Mayer 1992). There is evidence from a randomised controlled trial (RCT) that systematic pelvic and para-aortic lymphadenectomy will identify more women with lymph node metastases than sampling of suspicious nodes (Maggioni 2006); no survival difference was seen in this trial although it was underpowered to examine this outcome. There is, however, a high incidence of recurrent disease which can be as high as 30% in certain subgroups of women with stage I disease. The challenge is to determine which patients are high risk and would benefit most from additional treatment.
Uncontrolled retrospective studies have identified prognostic factors of importance for this disease. A multivariate analysis of 1545 patients with stage I epithelial ovarian cancer has confirmed tumour grade to be the single most important determinant of survival (Vergote 2001). In addition, capsular involvement or cyst rupture (FIGO stage Ic), were associated with a poorer outcome. The current staging for ovarian cancer does not recognise the prognostic importance of tumour grade.
Another issue relates to the class of ovarian cancers of low malignant potential known as borderline tumours: these neoplasms tend to run a benign course, however adverse prognostic factors are similarly based on histological features. These concerns have prompted calls for a revision of FIGO staging to incorporate the borderline tumours and endorse the importance of tumour grade (Green 2003).
Description of the intervention
Adjuvant treatment is any treatment given after surgical removal of all visible disease in order to reduce the risk of recurrence. Given the significant risk of recurrence in subgroups of patients with completely resected early stage disease, adjuvant treatment is usually considered. The rationale for this treatment is to eradicate any microscopic deposits of tumour that may remain after surgery. Several underpowered clinical trials have examined the merits of adjuvant chemotherapy compared with adjuvant radiotherapy in selected subgroups (Chiara 1994; Hreshchyshyn 1980; Klaassen 1988; Sigurdsson 1982).
A Cochrane review and meta-analysis of individual patient data (AOCTG 1999) confirmed modest two- and five-year survival advantages in women with advanced stage epithelial ovarian cancer who were given platinum-based combination chemotherapy compared with those given combination therapy lacking platinum (hazard ratio (HR) 0.88; 95% confidence interval (CI) 0.79 to 0.98, AOCTG 1999). ICON2 1998 subsequently confirmed equivalent efficacy (and less toxicity) of single-agent carboplatin compared with a combination regimen of cyclophosphamide, doxorubicin and cisplatin (CAP) and recommended it as the standard initial treatment of advanced stage epithelial ovarian cancer. Furthermore, GOG111 1996 demonstrated that, by adding paclitaxel to first-line platinum-based chemotherapy, survival was improved. Hence, the recommended first-line chemotherapy for advanced epithelial ovarian cancer is a platinum agent combined with a taxane. Furthermore, the Gynecologic Cancer InterGroup continues to recommend carboplatin and paclitaxel as the standard comparator arm for trials in ovarian cancer treatment (Thigpen 2011).
Based on the results seen in advanced disease, platinum-based chemotherapy has been adopted for use in early stage disease. Accepted practice in the UK is to offer six cycles of adjuvant chemotherapy (with or without a taxane) to women with stage Ic disease or more. With regard to low-risk disease, the NICE 2011 clinical guideline on ovarian cancer states that adjuvant chemotherapy should not be offered to women with low-risk stage I disease (grade 1 or 2, stage Ia or Ib) if they have undergone optimal staging and should be discussed with women who have had suboptimal staging (NCCC OC 2011).
Why it is important to do this review
An appreciation of the safety of withholding chemotherapy from certain low-risk subgroups of patients with early-stage epithelial ovarian cancer has made possible trials of adjuvant chemotherapy versus observation with treatment on recurrence. Initial trials of this kind have been too small to demonstrate any treatment effect (Bolis 1995; Trope 2000; Young 1990) but more recent collaborative trials have greatly improved the evidence regarding the efficacy of chemotherapy (ACTION 2003; ICON1 2003).
Nevertheless, the precise role of chemotherapy in early stage disease continues to be the subject of some discussion. Some clinicians may be reluctant to recommend platinum-based chemotherapy to certain patients who are unlikely to develop recurrent disease. Various systematic reviews of adjuvant therapy including radiotherapy in early stage epithelial ovarian cancer have been published (Elit 2004; Trope 2007; Winter-Roach 2003). The Trope 2007 review included a post hoc subgroup analysis of adequately versus inadequately staged patient groups and commented in some detail on the strength and weaknesses of the evidence base informing the use of adjuvant platinum-based chemotherapy.
However, there remain unanswered questions regarding adjuvant chemotherapy in early stage ovarian cancer. Clarity is needed on the subgroups of women, if any, who can safely be managed without adjuvant chemotherapy, and whether particular groups of patients have more to gain from it. This updated review aims to collate all the relevant data in the area, including long-term data from previously reviewed trials, to determine the overall benefit of adjuvant chemotherapy in early stage (FIGO stages I/IIa) epithelial ovarian cancer and to give further guidance on which women should receive chemotherapy.
OBJECTIVES
Primary objective
To assess the efficacy of adjuvant chemotherapy in early stage ovarian cancer in terms of overall survival (OS) and progression-free survival (PFS).
Secondary objectives
To determine if there are some patients with early stage disease who are more or less likely to benefit from this treatment (i.e. optimal versus sub-optimal staging, low risk versus high risk).
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with early stage (I/IIa) epithelial ovarian cancer staged at laparotomy.
Types of interventions
Adjuvant chemotherapy versus no adjuvant chemotherapy or placebo.
The term adjuvant used here describes treatment given within three months following surgery, which removed all visible disease.
Types of outcome measures
Primary outcomes
Overall survival (OS) (survival until death from any cause)
Progression-free survival (PFS) or recurrence-free survival (RFS) (for the purposes of this review, PFS and RFS are considered to be the same endpoint)
Secondary outcomes
Disease-specific survival (DSS) (defined as survival until death from ovarian cancer or complications of treatment, with deaths from other causes censored)
- Adverse events, extracted and grouped as:
- ○ haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage)
- ○ gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver, proctitis)
- ○ genitourinary
- ○ skin (stomatitis, mucositis, alopecia, allergy)
- ○ neurological (peripheral and central)
- ○ pulmonary
Search methods for identification of studies
Electronic searches
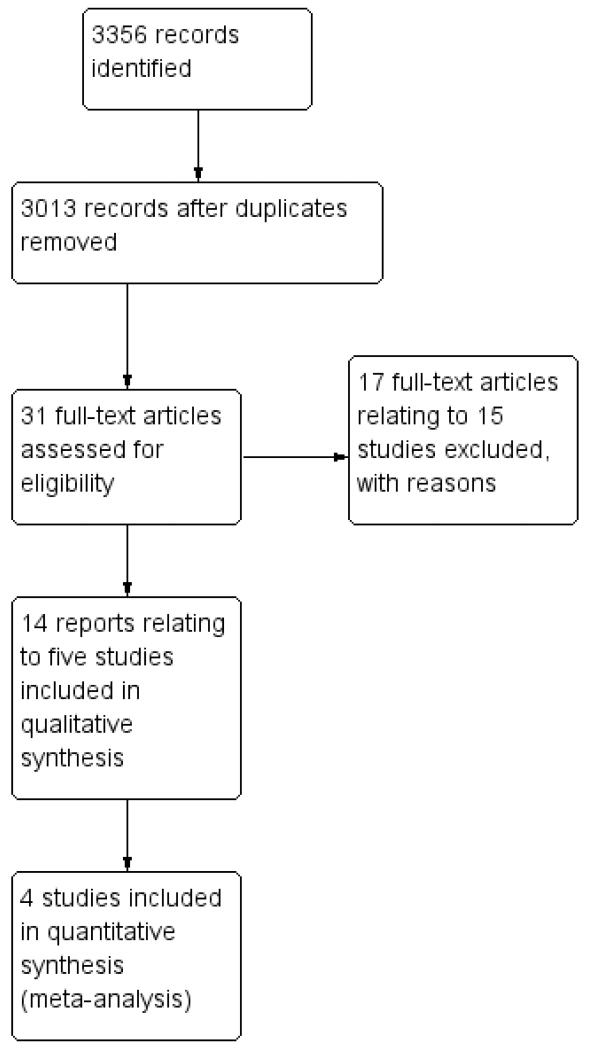
We performed an electronic search using the Cochrane Gynaecological Cancer Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL 2011, Issue 3) (Appendix 1), MEDLINE (1948 to Aug week 5, 2011) (Appendix 2) and EM-BASE (1980 to week 36, 2011) (Appendix 3). This yielded a large number of article titles which two review authors sifted down to a limited number of articles, the full-text versions of which we independently reviewed to select out clinical trials of direct and specific relevance to the review question. (See Figure 1 for search flow diagram). We conducted hand searches of the clinical literature where appropriate to identify additional full-text papers or abstracts of other directly relevant clinical trials. We applied no language restriction.
Figure 1.
Study flow diagram of search results.
Searching other resources
We searched the bibliographies of all relevant papers selected through this strategy. We identified relevant articles on PubMed, and using the ‘related articles’ feature, we carried out a further search for newly published articles. In addition, we searched MetaRegister (http://www.controlled-trials.com/mrct), Physicians Data Query (http://www.nci.nih.gov), http://www.clinicaltrials.gov and http://www.cancer.gov/clinicaltrials/search for ongoing trials. We established personal communication with corresponding authors and clinical experts where possible to enquire about other published or unpublished relevant studies.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database, removed duplicates and two review authors (BWR and HK) examined the remaining references independently. For the 2011 updated search, this was done by TL. We included those studies which clearly did meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors assessed the eligibility of retrieved papers independently (BWR and HK originally and BWR and TL for the update). We resolved disagreements by discussion and documented reasons for exclusion.
Data extraction and management
We designed a specific data extraction form. For included studies, two review authors (BWR and HK) independently extracted data on characteristics of patients, the number recruited to each arm, the completeness of surgical staging, the proportion of the different tumour stages and grades, the balance of prognostic factors achieved and interventions, the dose and duration of chemotherapy given in the treatment arm study quality, duration of follow-up, outcomes and deviations from protocol. Where possible, all data extracted were those relevant to an intention-to-treat analysis, in which participants were analysed in the groups to which they were assigned. We noted the time points at which outcomes were collected and reported. We recorded any adverse effects reported in the trials. Disagreements were resolved by discussion between the review authors. We entered the data into Review Manager 5 software (RevMan 2011) and checked data for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two authors (BWR and HK) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following:
selection bias (random sequence generation; allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data: we considered < 20% attrition to be low risk);
reporting bias (selective reporting of outcomes); and
other possible sources of bias.
For further details see Appendix 4.
Measures of treatment effect
For time-to-event data (OS, DSS and PFS), we extracted the log HR and its variance from trial reports. If these were not given, we attempted to extract the data required to estimate them using Parmar’s methods (Parmar 1998), e.g. number of events in each arm and log-rank P value comparing the relevant outcomes in each arm, or relevant data from Kaplan-Meier survival curves. If it was not possible to estimate the HR, we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of participants assessed per outcome (dichotomous data), in order to estimate a risk ratio (RR). We estimated the number needed to treat to benefit (NNT) by first performing a meta-analysis of the risk difference (RD) and then taking the inverse of the pooled RD.
For other dichotomous outcomes (e.g. adverse events), we extracted the number of patients in each treatment arm who were assessed at endpoint and the number who experienced the outcome of interest. We present dichotomous results as RRs with 95% confidence intervals.
Dealing with missing data
If primary outcome data were not reported, we contacted authors of trial reports. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta-analysis by visual inspection of forest plots and by using the T2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if the I2 statistic was greater than 30% and either T2 was greater than zero, or there was a low P value (< 0.10) in the Chi2 test for heterogeneity. If there was substantial heterogeneity, we investigated the possible reasons for it.
Assessment of reporting biases
Where there were sufficient trials, we examined funnel plots corresponding to meta-analysis of the primary outcomes to assess the potential for publication bias. If these plots suggested that treatment effects were not sampled from a symmetric distribution, as assumed by the random-effects model (REM), we performed a further meta-analyses using fixed-effect models.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2011). We pooled results of studies in a meta-analysis when clinically similar studies were available.
For time-to-event data, we pooled HRs using the generic inverse variance facility.
For any dichotomous outcomes (e.g. adverse events, and numbers of patients who relapsed or died, if it was not possible to treat these outcomes as time-to-event data), we pooled RRs.
We used REM models for all meta-analyses (DerSimonian 1986). If it was inappropriate to pool the data because of clinical heterogeneity, we performed a meta-analysis excluding outlying studies.
Subgroup analysis and investigation of heterogeneity
We planned to do the following subgroup analyses:
type of chemotherapy used; and
optimal/sub-optimal surgical staging, where optimal staging was defined as peritoneal staging plus retroperitoneal node assessment (Table 1).
The outcomes used in subgroup analysis were OS and PFS. Since the only trials with data of satisfactory quality evaluated platinum-based chemotherapy, we did not perform subgroup analysis by type of chemotherapy. In addition, we had planned to perform additional subgroup analyses, to examine the influence of prognostic factors (e.g. clear cell histological subtype, degree of tumour differentiation) and dose of chemotherapy. However, this was not possible, since data were not consistently reported by these subgroups in the included trials and we were unable to obtain individual patient data.
After publication of an abstract reporting the effect of adjuvant chemotherapy compared to no adjuvant chemotherapy in subgroups of high-risk and intermediate/low-risk stage I patients in the ICON1 2003 trial, we decided to present these subgroup data in the review. The definition of these subgroups was as follows:
high risk - Ia grade 3, Ib or Ic grade 2 or 3, any clear cell tumour;
medium risk - Ia grade 2, Ib or Ic grade 1; and
low risk - Ia grade 1.
Subgrouping according to risk was not specified at the protocol stage of this review.
Sensitivity analysis
We performed no sensitivity analyses since all included trials included in the meta-analyses were considered to be at a low risk of bias.
RESULTS
Description of studies
Results of the search
The search strategy identified a total number of 3356 reference hits (Figure 1). The title and abstract screening of these references identified 31 citations (20 trials) as potentially eligible for this review (Table 2). The full- text screening of these 31 references excluded 17 reports relating to 15 trials for the reasons described in the table of Characteristics of excluded studies. The remaining 14 reports (including conference abstracts) pertaining to five RCTs (ACTION 2003; Bolis 1995; ICON1 2003; Trope 2000; Young 1990) met our inclusion criteria and are described in the table of Characteristics of included studies.
Table 2.
Randomised trials of adjuvant treatment: description and quality assessment.
| Study ID | Recruitment period | Staging | Comparison | Randomisation | Intention to treat | 5-year follow-up |
|---|---|---|---|---|---|---|
| Smith 1975 | 1969-74 | No | CT versus RT | Unspecified | No | Incomplete |
| Dembo 1979 | 1971-75 | No | RT versus RT+CT | Stratified | No | Median 52 months |
| Hreshchyshyn 1980 | 1971-78 | No | CT versus RT versus NA | Unspecified | No | No |
| Sigurdsson 1982 | 1975-78 | No | NT versus CT, RT versus CT or (RT + CT) | Stratified, quasi-randomised | No | Yes |
| Sevelda 1987 | 1980-85 | Yes complete in 60.5% | NA versus RT versus (RT + CT) | Unspecified | No | Median 42 months |
| Gronroos 1984 | 1976-78 | No | NA versus RT and NA versus CT | Randomised by birth month (quasi-randomisation) | No | 3-year follow-up |
| Klaassen 1988 | 1975-84 | No | CT versus RT versus IPR | Central telephone | Yes | Median 8 years |
| Sell 1990 | 1981-87 | Complete | RT versus (RT + CT) | Block randomisation | Yes | 4 years |
| Young 1990 | 1976 | Complete | CT versus NA or IPR | Central, computer stratified | Yes | > 6 years |
|
Young 2000
Bell 2006 |
Complete | 3 × CT versus 6 × CT | Central, computerised | Yes | > 6 years | |
| Young 2003 | Complete | CT versus IPR | Central, computerised | Yes | ||
| Vergote 1992 | 1982-88 | Complete | CT versus IPR | Central, computer stratified | Yes | Median 62 months |
| Chiara 1994 | 1985-89 | Complete in 87% | CT versus RT | Central, computerised | Yes | |
| Bolis 1995 | 1983-90 | Complete | CT versus NA or IPR | Central, random generated numbers | Yes | Yes |
| Trope 2000 | 1992-97 | Complete | CT versus NA | Central, computerised | Yes | Median 46 months |
| Kojs 2001 | 1990-96 | Complete | CT versus RT | Method not explicit | Yes | Yes |
| ICON1 2003 | 1990-2001 | Incomplete | CT versus NA | Central computerised | Yes | Median 51 months |
| ACTION 2003 | 1990-2000 | Complete | CT versus NA | Central, computerised | Yes | Median 66 months |
|
Mannel 2011 (GOG 175) |
Complete | CT + maintenance versus CT alone | Central, computerised | Yes | Yes |
Abbreviations: CT = chemotherapy; RT = radiotherapy; IPR = intra-peritoneal radio-isotope therapy; NA = no additional treatment
Included studies
The five included trials, enrolling a total of 1277 participants, compared immediate adjuvant chemotherapy with no immediate adjuvant chemotherapy (Table 3).
Table 3.
Trials of adjuvant chemotherapy versus no further treatment
| Study ID | Patients | Intervention | 5-year survival | Survival/statistics | Adverse effects | Comments |
|---|---|---|---|---|---|---|
| ICON1 2003 | 447 FIGO I-III 93% FIGO stage 1 | Immediate adjuvant platinum-based chemotherapy versus treatment on progression | OS 79% (adjuvant arm) versus70% ( no treatment) | Hazard ratios OS: HR 0.66; 95% CI 0.45 to 0.97; P = 0.03 |
Not reported | Survival improvement with adjuvant therapy |
| ACTION 2003 | 448 FIGO Ia-Ib grade II-III FIGO Ic-IIa FIGO I-IIa clear cell | Immediate adjuvant platinum-based chemotherapy versus treatment on progression Cisplatin dose = 75 mg/m2 Carboplatin dose = 350 mg/m2 |
OS 85% (adjuvant arm) versus 78% (no treatment) | Hazard ratios OS: HR 0.69; 95% CI 0.44 to 1.08; P = 0.10 RFS: HR 0.63; 95% CI 0.43 to 0.92; P = 0.02 |
Not reported | Subgroup analysis showed that non-optimally staged patients in observation arm have significantly worse survival |
| Trope 2000 | 162 highrisk stage I 36% patients had low-volume residual disease | Carboplatin 6 cycles Q28/7 AUC = 7 versus chemo at progression | No difference between arms DFS 70% versus 71%, OS 86% versus 85% | Log rank test DFS P = 0.41 OS P = 0.43 |
Hazard ratios DFS: HR 0.98; 95% CI 0.52 to 1.83 DSS: HR 0.94; 95% CI 0.37 to 2.36 |
Not reported |
| Young 1990 | 48 treatment 44 observation |
Melphalan versus no further treat | DFS 91% versus 98% OS 94% versus 98% | Log rank test DFS P = 0.41 OS P = 0.43 |
Melphalan: 16% had severe myelosuppression. 26% had gastrointestinal side effects. One death: myeloproliferative disorder aplastic anaemia 6 years after completing treatment | Trial under powered to show any real differences |
| Bolis 1995 | 85 FIGO (1976) I A-I B Grade 2 and 3 | Cisplatin 50 mg/m2 × 6 cycles Q28/7 versus no further treatment |
DFS 83% versus 64% OS 88% versus 82% | Hazard ratios DFS: HR 0.50; 95% CI 0.21 to 1.19; P = 0.17 OS: HR 1.20; 95% CI 0.46 to 3.1; P = 0.71 |
Nausea and vomiting in more than two-thirds of patients in cisplatin arm. Severe in less than 10%. Leucopenia 14%; thrombocytopenia 8%; neurological toxicity 6%; renal toxicity 7% | There were patients with residual disease in both arms |
CI = confidence interval; DFS = disease-free survival; OS = overall survival; RFS = recurrence-free survival; AUC = Area under the concentration curve
Young 1990 was the first prospective RCT of adjuvant chemotherapy in early stage ovarian cancer to include a control group that had no immediate post-surgical treatment, with chemotherapy being reserved for treatment of disease recurrence. This American trial published in 1990 was a joint effort of the Gynecologic Oncology Group and the Ovarian Cancer Study Group and randomised women with FIGO 1976 stage Ia and Ib well-differentiated or moderately- differentiated tumours to receive either Melphalan 0.2 mg/Kkg or no chemotherapy. These women were surgically staged via a midline laparotomy to allow thorough assessment of the abdomen and pelvis. A total abdominal hysterectomy, bilateral salpingo-oophorectomy and infracolic omentectomy was performed and biopsies were taken of any peritoneal deposits. Random biopsies of the pelvic and abdominal peritoneum and retroperitoneal lymph node assessment were also performed. This surgical staging routine is most likely to identify occult metastatic disease if present and therefore is optimal. This trial was flawed by the inclusion of 27 women with the Borderline Ovarian Tumour histological sub-type though they were evenly distributed between the two arms of the trial.
The trial enrolled 92 women, randomising 48 to the chemotherapy arm and 44 to the observation-only arm. After randomisation, 11 women (5five in the chemotherapy arm and 6six in the observation-only arm) were deemed ineligible and so 81 women (43 in the chemotherapy arm and 38 in the observation-only arm) were available for analysis. OS and disease-free survival (DFS) were reported. Six women died: two in the chemotherapy arm and four in the observation-only arm. Likewise, six women had disease recurrence: two in the chemotherapy arm and four in the observation-only arm. The authors reported no significant differences between treatment arms in either OS or DFS. Surviving women were followed up for a median of 6six years. HRs were not reported, but Kaplan-Meier plots and log-rank p-P values were presented for both OS and DFS, based on analysis of all eligible women regardless of the treatment they received. Minimum and maximum duration of follow-up were estimated from censoring marks on the Kaplan-Meier plots.
Adverse events were reported in the adjuvant chemotherapy arm but not assessed in the no adjuvant chemotherapy arm.
Bolis 1995 was an Italian multicentre RCT that recruited women with FIGO stage I epithelial ovarian cancer into two trial protocols. In Trial 1, women with Stage Ia and Ib G2 and G3 were randomised to receive either cisplatin (50 mg/m2) for six cycles or to have no further therapy. The authors specified the inclusion of retroperitoneal (pelvic and para-aortic) nodal sampling in the protocol of this trial and therefore staging is considered optimal. In Trial 2, cCisplatin was compared to intra-peritoneal radio-isotope in a higher- risk group of women; this trial was not considered in our review because it did not meet our inclusion criteria.
Trial 1 enrolled 85 women, randomising 41 to the chemotherapy arm and 44 to the observation-only arm. After randomisation, two women (both in the observation-only arm) were deemed ineligible and so 83 women (41 in the chemotherapy arm and 42 in the observation-only arm) were available for analysis. OS and PFS were reported. Seventeen women died: nine in the chemotherapy arm and eight in the observation-only arm. Twenty-one women had disease recurrence: seven in the chemotherapy arm and fourteen 14 in the observation-only arm. The authors reported no significant differences between treatment arms in either OS or PFS. The five-year DFS was 83% for women receiving cisplatin and 64% for the control group; the five-year OS was 87% and 81% in the cisplatin and control groups respectively. Women were followed up for a median of 69 months.
HRs for OS and PFS and their 95% confidence intervals (CI) were reported, adjusted for tumour grade. These were based on analysis of all eligible women according to the treatment allocated by randomisation.
Adverse events were reported in the adjuvant chemotherapy arm but not in the no adjuvant chemotherapy arm.
Trope 2000 was a Scandinavian multicentre RCT in women with high-risk stage I epithelial ovarian cancer, which compared adjuvant carboplatin chemotherapy versus observation with treatment on clinical recurrence. The entry criteria for this trial were: FIGO stage I non-clear cell carcinoma G2 to G3 after a stipulated staging laparotomy via a midline incision with a total abdominal hysterectomy, bilateral salpingo-oophorectomy and an infracolic omentectomy. Peritoneal washings were obtained and a thorough assessment of peritoneal surfaces with biopsy of any suspicious peritoneal or retroperitoneal lesions was performed. A systematic retroperitoneal lymphadenectomy was not stipulated in the surgical staging protocol although this was recommended as being optimal.
This trial had two aims, firstly to determine if there was a survival advantage for patients having adjuvant chemotherapy and secondly to test whether DNA ploidy was an independent prognostic factor in high-risk (non-clear cell) stage I epithelial ovarian cancer. The treatment protocol was with carboplatin intravenously dosed at AUC7 according to Calvert’s formula (Calvert 1989) for six courses.
The trial enrolled 175 women. After randomisation, 13 women were deemed ineligible and so 162 women (81 in each arm) were available for analysis. DSS (i.e. survival of women who did not die of ovarian cancer or complications of treatment) and DFS were reported. Eighteen women died of ovarian cancer: nine in both arms. Thirty-nine women had disease progression: 20 in the chemotherapy arm and 19 in the observation-only arm. The authors reported no significant differences between treatment arms in either DSS or PFS. Women were followed up for a median of 46 months.
Unadjusted HRs for DSS and PFS and their 95% CIs were reported. Multivariate Cox regression confirmed. DNA ploidy, tumour grade and FIGO substage as independent prognostic determinants of DSS.
Adverse events were not reported.
ICON1 2003 was a pragmatic trial of adjuvant platinum- based chemotherapy in early stage epithelial ovarian cancer which recruited women from five countries: United Kingdom, Ireland, Brazil, Italy and Switzerland. Computerised randomisation was done from offices in Milan and London. It was run alongside another collaborative trial, ACTION 2003, and reported simultaneously with it. It was pragmatic about the entry criteria as well as the treatment protocol. Clinicians were asked to recruit women with histologically confirmed invasive epithelial cancer in whom there was some uncertainty of the need for adjuvant chemotherapy. Most women were FIGO stage I although some women had stage II disease. Recommended surgical staging was less stringent in this trial than in the ACTION 2003 trial, with the minimum requirement being for women to have had removal of all visible tumour with a total abdominal hysterectomy and bilateral salpingo-oophorectomy, where appropriate, and omentectomy. The minimal recommendation for ‘peritoneal surgical staging’ means that the women were sub-optimally staged in ICON1 2003.
The majority of women in the treatment group (87%) had carboplatin (AUC5), 11% had cisplatin in combinations and a smaller percentage had other platinum- based regimens.
The trial enrolled 477 women, randomising 241 to the chemotherapy arm and 236 to the observation-only arm. Despite protocol violations, all analyses were on an intention-to-treat (ITT) basis. OS and recurrence-free survival (RFS) after five-year follow-up were reported. One hundred and three women died: 42 in the chemotherapy arm and 61 in the observation-only arm. One hundred and seven women had disease recurrence: 47 in the chemotherapy arm and 60 in the observation-only arm. The authors reported a statistically significant benefit of chemotherapy in terms of both OS and RFS. Surviving women were followed up for a median of 51 months. Unadjusted HRs for OS and PFS and their 95% CIs were reported (HR 0.66; 95% CI 0.45 to 0.97 and 0.65; 95% CI 0.46 to 0.91 respectively). Five-year survival was 79% among women who had chemotherapy compared to 70% among those who did not.
Adverse events were reported in the adjuvant chemotherapy arm but not in the no adjuvant chemotherapy arm.
Longer-term follow-up of this trial, reported in an abstract by Swart 2007, confirmed these results. After median follow-up of 9.2 years, 144 women had died and 168 had disease recurrence. Unadjusted HRs for OS and PFS and their 95% CIs were reported (HR 0.74; 95% CI 0.53 to 1.02 and HR 0.70; 95% CI 0.52 to 0.95 respectively). Ten-year survival was 72% among women who had chemotherapy compared to 64% among those who did not. This abstract also reported the effect of adjuvant chemotherapy, subgrouped by level of risk, namely low/medium risk (Ia, G1 and G2, Ib or Ic, G1) and high risk (Ia, G3, Ib or Ic G2 or G3, any clear cell). Among the high- risk women, those who received adjuvant chemotherapy had significantly better OS and RFS than those who did not receive chemotherapy (HR 0.48; 95% CI: 0.32 to 0.72 and HR 0.52; 95% CI: 0.33 to 0.82 respectively), whereas among low/medium- risk women, there was no significant difference in survival outcomes between treatment arms (HR 0.96; 95% CI: 0.54 to 1.66) and HR 0.96; 95% CI 0.50 to 1.38 respectively).
ACTION 2003 was a trial run at the same time as the ICON1 2003 trial by the European Organisation for Research and Treatment of Cancer (EORTC) collaborators and recruited 448 women. This was a multicentre trial with centralised computer randomisation in Brussels. Nine countries recruited women between November 1990 and January 2000. Entry criteria were more stringent than in the ICON1 2003 trial. The trial was open to women with stage Ia and Ib G2 and G3 (moderate and poorly differentiated tumours), all stage Ic and stage Ia. Surgical staging was also specified and optimal staging to include pelvic and para-aortic retroperitoneal node dissection was strongly recommended. A pre-planned examination of the impact of surgical staging on survival outcome required careful documentation of surgical staging for each case, which was categorizsed as being inadequate, minimal, modified or optimal.
The allowed chemotherapy regimens were single agent or combinations based on either cisplatin at 75 mg/m22 or carboplatin at 350 mg/m2m2. Of the evaluable women who were randomised to receive chemotherapy, 47% had cisplatin in combination with cyclophosphamide and 33% had single-agent carboplatin. Women in the control group had no adjuvant treatment. They were followed -up and chemotherapy was reserved for cases of disease recurrence.
The trial enrolled 448 women, randomising 224 to each arm. Despite protocol violations, all analyses were on an intention-to-treat (ITT) basis. OS and RFS were reported. Seventy-eight women died: 33 in the chemotherapy arm and 45 in the observation-only arm. One hundred women had disease recurrence: 40 in the chemotherapy arm and 60 in the observation-only arm. The authors reported a statistically significant benefit of chemotherapy in terms of RFS and a benefit in terms of OS which was not statistically significant. Women were followed up for a median of 5.5 years.
Unadjusted HRs for OS and RFS and their 95% CIs were reported (HR 0.69; 95% CI 0.44 to 1.08 and HR 0.63; 95% CI 0.43 to 0.92 respectively). Five-year survival was 76% among women who had chemotherapy compared to 68% among those who did not. Multivariate Cox regression confirmed that staging adequacy and tumour grade were statistically significant prognostic factors for both OS and RFS.
Adverse events were not reported.
In a pre-planned sub-group analysis, staging adequacy was dichotomised into optimal and sub-optimal groups. Among the 295 sub-optimally staged women, those who received adjuvant chemotherapy had significantly better OS and RFS than those who did not receive chemotherapy, whereas among the 151 optimally staged women, there was no significant difference in survival outcomes between treatment arms.
Long-term results of this trial (median follow-up of 10.1 years) confirmed the original findings, that optimal surgical staging was associated with better outcomes and the survival benefits of adjuvant chemotherapy were limited to those women with sub-optimal staging (Trimbos 2010).
Summary of included trials
Four of the included trials used cisplatin-based chemotherapy (ACTION 2003; Bolis 1995; ICON1 2003; Trope 2000), while one used melphalan (Young 1990). The trials had some important differences related to inclusion criteria, treatment arm protocols, trial size and results statistic. The three earlier trials (Bolis 1995; Trope 2000; Young 1990) all recruited a small numbers of participants and so may have lacked the statistical power to detect a treatment effect even if one were present. In contrast, the two later trials (ACTION 2003; ICON1 2003; ACTION 2003) were each much larger than preceding trials and since they were run in parallel and reported in a joint analysis, the ‘combined trial’ had sufficient power to demonstrate a treatment effect. Furthermore, while the Bolis 1995 trial protocol specified examination of the retroperitoneal nodal groups at laparotomy, in addition to peritoneal staging, the protocol for ICON1 2003 made no such stipulation. As such the women in the former trial are regarded to have been optimally staged while staging for the ICON1 2003 participants was sub-optimal.
An important difference between ACTION 2003 and the other trials was the predetermined intention of the investigators to examine, in a subgroup, the effect of staging adequacy in either trial arm. Roughly one- third of the women recruited to this trial had more thorough surgical staging (described as optimal as opposed to adequate). The adequacy of staging in the other trials has not been specified but is assumed to be adequate rather than optimal. This is an important difference because it is recognised that more thorough surgical staging (specifically retroperitoneal lymph node dissection) will result in a more accurate identification of women with occult advanced disease and women with disease confined to the ovary.
Excluded studies
Of the 31 full- text references 17 reports relating to 15 trials were excluded for the reasons described in the table of Characteristics of excluded studies.
Risk of bias in included studies
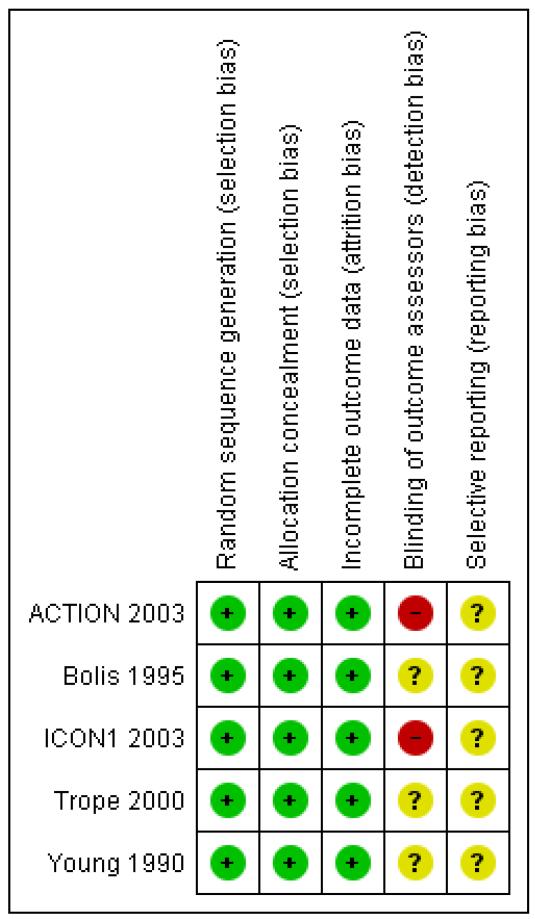
The five included trials were of uniformly good quality (see Characteristics of included studies and Figure 2) except for Young 1990 which had some inconsistencies in reporting (see Effects of interventions below).
Figure 2.
‘Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
All included trials reported adequate randomisation and adequate concealment of allocation. Consequently a balance of prognostic factors was reported by ACTION 2003, ICON1 2003; Trope 2000 and Young 1990; however, Bolis 1995 reported that women in the cisplatin arm were more likely to have poorly differentiated (G3) tumours and less likely to have clear cell histotype.
One trial (ICON1 2003) reported that the trial was open: investigators, patients and trial centre staff were not blinded to treatment allocation after randomisation. None of the other four trials reported blinding of outcome assessors.
ICON1 2003 reported no loss to follow-up after five years; one trial (ACTION 2003) reported 2% loss to follow-up after five years; Trope 2000 reported 7% of women were deemed ineligible after randomisation but that no further participants were lost to follow-up; the remaining two trials reported 2% (Bolis 1995) and 12% (Young 1990) of women were deemed ineligible after randomisation but they did not report whether any subsequent loss to follow-up occurred.
All trials used an intention-to-treat analysis.
Effects of interventions
See: Summary of findings for the main comparison
Four trials (ACTION 2003; Bolis 1995; ICON1 2003; Young 1990) reported OS. One trial (Bolis 1995) reported progression-free survival (PFS); two trials reported recurrence-free survival (RFS) (ACTION 2003; ICON1 2003); two trials reported DFS (Trope 2000; Young 1990); for the purposes of meta-analysis, we assumed that these endpoints referred to the same outcome, measured in the same way, although this may not necessarily be true (Altman 1995). One trial (Trope 2000) reported disease-specific survival (DSS), defined as survival until death from ovarian cancer or from complications of treatment for the disease, with deaths from other causes being censored, and ACTION 2003 reported DSS for the analysis of 10-year data.
We excluded the trial of Young 1990 from all meta-analyses since the data reported in the published report were not internally consistent: table 3 in the trial paper reported one disease recurrence in the chemotherapy group whereas figure 1 in the trial paper showed two disease recurrences in this group; table 3 reported deaths at 35 and 38 months in the chemotherapy group whereas figure 2 showed deaths at 38 and 75 months in this group. This trial evaluated melphalan whereas all other included trials evaluated platinum-based chemotherapy.
The four trials (ACTION 2003; Bolis 1995; ICON1 2003; Trope 2000) that were included in meta-analyses had similar median durations of follow-up: 66, 69, 51 and 46 months respectively. Two trials (ACTION 2003; ICON1 2003) additionally reported the effect of adjuvant chemotherapy after 10 years follow-up. ICON1 2003 subgrouped women by level of risk (Swart 2007; see Subgroup analysis and investigation of heterogeneity), whereas ACTION 2003 subgrouped women by the completeness of staging (optimal and non-optimal) (Trimbos 2010).
Overall survival
Five-year OS was significantly better for women receiving adjuvant chemotherapy than for women in the observation group (three trials, 1008 women; HR 0.71; 95% confidence interval (CI) 0.53 to 0.93), with no heterogeneity between trials (I2 = 0%). This corresponded to a number needed to treat to benefit (NNT) of 17 (95% CI 9 to 100). The trials contributing greatest weight to the analysis were ICON1 2003 (53%) and ACTION 2003 (39%) (Analysis 1.1).
Similarly, the results of the 10-year OS meta-analysis (incorporating ICON1 2003 and ACTION 2003 data) were robust to the five-year findings and showed a significant difference between the two groups in favour of adjuvant chemotherapy (two trials, 925 women; HR 0.74; 95% CI 0.58 to 0.95; Analysis 1.2).
Overall survival, subgrouped by adequacy of surgical staging
We performed meta-analysis of three trials (ACTION 2003; Bolis 1995; ICON1 2003), subgrouped by optimal/sub-optimal surgical staging and excluding two women in the ACTION 2003 trial whose staging status was unknown. At five years, in optimally staged women, there was no significant difference in OS between those who did and did not receive adjuvant chemotherapy (two trials, 234 women; HR 1.22; 95% CI 0.63 to 2.37). In the suboptimally staged subgroup, women who received adjuvant chemotherapy had significantly better OS than those who did not (two trials, 772 women; HR 0.63; 95 % CI 0.46 to 0.85) (Analysis 1.3). For 10-year data, ACTION 2003 reported DSS instead of OS for these subgroups to avoid the bias of intercurrent deaths (see DSS results below).
Overall survival, subgrouped by level of risk
Only one trial subgrouped women according to the level of risk. Among women at low and medium risk, ICON1 2003 showed no significant difference in OS at 10 years between those who did and did not receive adjuvant chemotherapy (HR 0.95; 95% CI 0.54 to 1.66). However, in women at high risk, those receiving adjuvant chemotherapy had significantly better OS at 10 years than those who did not (HR 0.48; 95% CI 0.32 to 0.72). The numbers of women in the low to medium and high-risk groups were not reported (Analysis 1.4).
Progression-free survival
Meta-analysis showed significantly better PFS at five years in women receiving chemotherapy than in women who did not (four trials, 1170 women; HR 0.67; 95% CI 0.53 to 0.84), with no heterogeneity between trials (I2 = 0%). This corresponded to a NNT of 12 (95% CI 7 to 33) (Analysis 1.5). Similarly, at 10 years, PFS was significantly better in the chemotherapy group (two trials, 928 women; HR 0.67; 95% CI 0.54 to 0.84; Analysis 1.6).
Progression-free survival, subgrouped by adequacy of surgical staging
We performed meta-analysis for PFS data at five years, subgrouped by optimal/sub-optimal surgical staging, and excluding two women in the ACTION 2003 trial whose staging status was unknown. Among optimally staged women, this showed no significant difference in PFS between those who did and did not receive adjuvant chemotherapy (two trials, 234 women; HR 0.67; 95% CI 0.36 to 1.22). However, in sub-optimally staged women, those receiving adjuvant chemotherapy had significantly better PFS than those who did not (two trials, 934 women; HR 0.64; 95% CI 0.50 to 0.82) (Analysis 1.7). Ten-year data from ACTION 2003 were robust to these findings (Analysis 1.8)
Progression-free survival, subgrouped by level of risk
Only the ICON1 2003 investigators subgrouped long-term (10-year) PFS data by the level of risk. In women at low and medium risk, ICON1 2003 showed no significant difference in PFS between those who did and did not receive adjuvant chemotherapy (HR 0.96; 95% CI: 0.50 to 1.38); however, in women at high risk, those receiving adjuvant chemotherapy had significantly better PFS than those who did not (HR 0.52; 95% CI 0.33 to 0.82). We were unable to reproduce in RevMan 2011 the 95% CI reported by Swart 2007, since the latter CI was not symmetric on a log scale (Analysis 1.9).
Disease-specific survival
Trope 2000 reported no significant difference in DSS at five years between the adjuvant chemotherapy group and the observation group (one trial, 162 women; HR 0.94; 95% CI 0.37 to 2.37) (Analysis 1.10).
Ten-year follow-up data from ACTION 2003 similarly found no significant difference in DSS between the two groups overall. However, for the subgroup of sub-optimally staged women, DSS was significantly better in the adjuvant chemotherapy group compared with observation (HR 0.58; 95% CI 0.35 to 0.96; Analysis 1.11). In the optimally staged group, chemotherapy provided no significant benefit over observation (one trial, 151 women; HR 1.58; 95% CI 0.61 to 4.09).
Deaths from ovarian cancer
Meta-analysis of three trials (ACTION 2003; Bolis 1995; Trope 2000) assessing 693 women, reported no significant difference in the outcome ‘deaths from ovarian cancer’ at five years between the adjuvant chemotherapy and observation groups (RR 0.76; 95% CI 0.52 to 1.11), with no heterogeneity between trials (I2 = 0%) (Analysis 1.12).
In the only study that reports the 10-year follow-up for this outcome (ACTION 2003), there was no significant difference in deaths from ovarian cancer between the two groups overall. Significantly fewer deaths occurred in the chemotherapy arm of the suboptimally staged subgroup compared with the observation arm (295 women, risk ratio (RR) 0.62; 95% CI 0.40 to 0.97; Analysis 1.13). There was no significant difference in the death rates at 10 years for the optimally staged subgroup.
Adverse events
We were unable to compare the risk of adverse events in women who did and did not receive adjuvant chemotherapy, since none of the trials reported adverse events among women who did not receive adjuvant chemotherapy.
Assessment of reporting bias
Funnel plots were not produced for any outcomes as only four trials contributed data.
Sensitivity analyses
We did not perform sensitivity analyses excluding poor quality trials since all trials reported adequate concealment of allocation and no trials reported blinding of outcome assessors.
DISCUSSION
Summary of main results
See Summary of findings for the main comparison. Five randomised controlled trials (ACTION 2003; Bolis 1995; ICON1 2003; Trope 2000; Young 1990) were identified and met the inclusion criteria, of which four trials evaluating platinum-based chemotherapy (ACTION 2003; Bolis 1995; ICON1 2003; Trope 2000) were of sufficient quality to contribute to a meta-analysis. In total, 1170 women contributed data.
In women with early stage (FIGO I/IIa) epithelial ovarian cancer, those receiving adjuvant chemotherapy had a better five-year OS HR 0.71; 95% confidence interval (CI) 0.53 to 0.93) and progression-free survival (PFS) (HR 0.67; 95% CI 0.53 to 0.84) than those who did not receive adjuvant chemotherapy. This indicates that, at five-year follow-up, almost 30% more women were alive as a result of receiving adjuvant chemotherapy. However, between 9 and 100 women would have to be treated with adjuvant chemotherapy to prevent one death and between 7 and 33 women would have to be treated with adjuvant chemotherapy to prevent one case of disease recurrence. The significant survival benefit of chemotherapy was still evident at 10 years (PFS: two trials, 925 women; HR 0.67; 95% CI 0.54 to 0.84).
However, specific subgroups of women did not benefit from adjuvant chemotherapy. In sub-optimally staged women, adjuvant chemotherapy was associated with a significantly improved OS or PFS but not in optimally staged women. Post hoc evidence from one trial (ICON1 2003) showed that adjuvant chemotherapy was associated with improved PFS and OS in high-risk stage I patients, but not low/medium-risk stage I patients (stage Ia/Ib, grade 1 or 2 and Ic grade 1 as defined by Swart 2007). Based on these findings it may be possible to withhold adjuvant chemotherapy in these subgroups but more evidence is needed.
Overall completeness and applicability of evidence
The large number of women pooled in this meta-analysis gives clear and consistent evidence of the overall benefit of adjuvant chemotherapy for women with early stage ovarian cancer (FIGO stage I/IIa), especially in the women who were sub-optimally staged (all women in ICON1 2003 and two-thirds of those in ACTION 2003 - a total of 772 or 66%). We consider that this subgroup is probably representative of the majority of women treated worldwide for early stage epithelial ovarian cancer.
For women with optimally staged early ovarian cancer, chemotherapy did not prolong survival, and showed no significant benefit in prolonging the time to disease progression, based on a small group of 234 women from two trials, although the point estimate of effect for PFS favoured chemotherapy. Five-year data were consistent with long-term data from the ACTION 2003 trial, however more evidence is needed to corroborate these subgroup findings. The apparent limitation of the benefits of chemotherapy to suboptimally staged women suggests that the real value of adjuvant chemotherapy is in the treatment of occult advanced stage disease. However, some benefit for chemotherapy in optimally staged disease cannot be excluded. For this reason, the authors support the continued practice of offering adjuvant chemotherapy to women staged optimally, who have high-risk histology.
It is possible that the apparent benefits of treatment to subgroups of women with sub-optimal staging and/or high risk were a chance finding. It has been shown that, if an overall treatment effect is statistically significant at the five per cent level (as immediate adjuvant chemotherapy is in our meta-analyses), and the women are divided at random into two similarly sized subgroups, then there is a one in three chance that the treatment effect will be large and statistically significant in one group but irrelevant and non-significant in the other (Peto 1982).
Unfortunately, none of the trials assessed the impact of adjuvant chemotherapy on the quality of life of the women. In addition, adverse events were poorly reported and did not use consistent definitions (e.g. NCI CTCAE v3.0 2006). Only three of the trials reported adverse events (Bolis 1995; ICON1 2003; Young 1990) in women receiving adjuvant chemotherapy; none of the trials reported adverse events in women who did not receive adjuvant chemotherapy.
Quality of the evidence
We consider the evidence for the primary outcomes, OS and PFS, to be of a high quality, and consider the evidence provided by some subgroup analyses to be of a moderate quality (see Summary of findings for the main comparison). Subgrouping according to risk in the ICON1 2003 study was performed post-hoc and the ACTION 2003 trial was not designed to compare different surgical staging procedures, nor were women prospectively stratified by these categories. In addition, the numbers of participants in the ‘optimally staged’ subgroup meta-analyses were small and, in the the risk subgroup meta-analyses, not provided. Subgrouping by risk was performed in only one trial and the limited data, to our knowledge, are only available in the form of a conference abstract (Swart 2007). Hence, further evidence relating to these subgroups, is likely to have an important impact on our confidence in the estimates of effects and may change the estimates.
Potential biases in the review process
The ‘risk’ subgroup was not included in the original protocol of this review and so, by including it later based on the ICON1 2003 trial, may be a potential source of bias. Similarly, the assignment of Trope 2000 and Bolis 1995 to the ‘optimal staging’ subgroup and of ICON1 2003 to ‘sub-optimal staging’ was post hoc and subjective. Since we have downgraded our quality assessment of this evidence, we do not consider the risk of bias to be substantial. To our knowledge there are no other potential biases in the review process.
Agreements and disagreements with other studies or reviews
The role of chemotherapy in early stage epithelial ovarian cancer and the completeness of surgical staging in women with apparent early stage disease are interlinked issues and any discussion of the management of these women must consider both. There is active debate in UK gynaecological oncology circles about lymphadenectomy in early stage epithelial ovarian cancer with many believing in the necessity of a systematic pelvic and para-aortic lymphadenectomy for accurate staging. This is because, when retroperitoneal lymph node dissection is not performed, there is a significant risk of failing to identify occult disease. Since the prognosis for women with para-aortic or pelvic node involvement is worse than for women with true stage I or II disease, any intervention trials with outcomes that group true early stage disease with occult stage IIIa disease will necessarily be very difficult to interpret. The improved survival of women with sub-optimally staged ‘early stage’ ovarian cancer shown in ACTION 2003 is likely to be due to chemotherapy-related treatment of occult disease; thus adjuvant chemotherapy lifted the prognosis of these women to match that of true stage I disease.
The recent NICE guidance (NCCC OC 2011) on the diagnosis and initial treatment of patients with ovarian cancer has taken a pragmatic line in its advice on the role of para-aortic node dissection in ‘early stage’ disease. It does not recommend systematic lymphadenectomy but rather advocates lymph node assessment by palpation and sampling of any suspiciously enlarged nodes. It argues that the morbidity of a comprehensive para-aortic lymphadenectomy cannot be justified.
AUTHORS’ CONCLUSIONS
Implications for practice
Since the finding of early stage epithelial ovarian cancer is often indistinguishable from benign and borderline tumours, it is not surprising that comprehensive staging is infrequently achieved. On this basis, it may be safe practice to recommend adjuvant chemotherapy for the majority of cases of apparent early stage epithelial ovarian cancer. However if staging is comprehensive, it should be possible to identify patients in whom it is safe, if not better, to withhold chemotherapy unless and until it is needed to treat recurrent disease.
A conservative position would be to recommend adjuvant chemotherapy to all women with apparent early stage disease unless they have had comprehensive staging and the histology is not high grade. Patients with well or moderately differentiated encapsulated tumours confined to one ovary, who are optimally staged, should be advised that there is evidence to suggest they will gain limited, if any, survival benefit from adjuvant chemotherapy.
In summary:
Unilateral, encapsulated, well-differentiated serous and endometrioid carcinoma (stage Ia grade 1, non-optimally staged) may be managed without adjuvant chemotherapy.
Stage Ia, and Ib that has been comprehensively staged, well or moderately differentiated (grade 1/2) may be managed without adjuvant chemotherapy.
Poorly or undifferentiated (grade 3) stage Ia/Ib disease should be offered adjuvant chemotherapy.
Non serous histotypes, mucinous and clear cell, should be offered adjuvant therapy.
Implications for research
There are deficiencies in the evidence which can and should be addressed in the context of a collaborative trials.The ACTION 2003 investigators have proposed a trial in which women who are sub-optimally staged are randomised either to have a staging laparotomy or to have adjuvant chemotherapy. The authors propose a trial in apparent early ovarian cancer with two levels of randomisation; the first step would randomise to either optimal staging or peritoneal staging. All patients with high-grade tumours would be recommended adjuvant chemotherapy. In the second step, women with ‘low-risk’ histology in the peritoneal staging arm would be randomly assigned to either adjuvant chemotherapy or observation and those optimally staged would be observed. Such a trial would evaluate firstly whether there is a survival advantage to retroperitoneal node sampling in early stage ovarian cancer and secondly whether a group of women with early stage epithelial ovarian cancer can safely be managed without adjuvant chemotherapy. However, phase 3 trials of early ovarian cancer are difficult to conduct because of the relatively small number of women with early stage disease. Consensus from the 4th Ovarian Cancer Conference of the Gynaecologic Cancer InterGroup recommends that the primary endpoint for these trials is therefore recurrence-free survival (RFS) (Thigpen 2011).
PLAIN LANGUAGE SUMMARY.
Post-surgery (adjuvant) chemotherapy for early stage epithelial ovarian cancer
Chemotherapy with platinum-containing drugs, given after surgery to remove ovarian cancers that have not spread beyond the pelvis, saves lives but is probably unnecessary when the tumour has been surgically proven not to have spread outside of the ovary (stage I), particularly if the specific cell type is not aggressive.
ACKNOWLEDGEMENTS
We thank the staff of the Cochrane Gynaecological Cancer Review Group, in particular Gail Quinn and Clare Jess for their helpful advice and administrative support. We thank Jane Hayes for conducting the comprehensive updated search and Andy Bryant, Newcastle University, for helpful comments on the review. Heather Dickerson contributed to data abstraction and writing the review and performed all statistical analyses for the original review.
SOURCES OF SUPPORT
Internal sources
The Cochrane Collaboration through the Department of Health, UK.
External sources
No sources of support supplied
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Multicentre randomised controlled trial | |
| Participants | 448 FIGO Ia-Ib G2/3, FIGO Ic-IIa, FIGO I-IIa clear cell | |
| Interventions | Immediate platinum-based chemotherapy versus treatment on progression Cisplatin dose = 75 mg/m2 Carboplatin dose = 350 mg/m2 |
|
| Outcomes | DFS and OS Adverse events not reported Median follow-up: 5.5 years |
|
| Notes | Subgroup analysis examined impact of staging adequacy | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on minimisation |
| Allocation concealment (selection bias) | Low risk | Minimisation performed by central co-ordinating centres |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | T: 6/224 (2%) C: 3/224 (1%) |
| Blinding of outcome assessors (detection bias) | High risk | No blinding |
| Selective reporting (reporting bias) | Unclear risk | Intention-to-treat analysis; all pre-specified outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | 85 FIGO (1976) IA-IB Grade 2 and 3 | |
| Interventions | Cisplatin 50 mg/m2 × 6 cycles Q 28/7 versus observation | |
| Outcomes | DFS 83% versus 64% OS 88% versus 82% Adverse events in adjuvant chemotherapy arm: nausea and vomiting in more than 2/3 of patients; but in severe form in less than 10% of courses; leukopenia and thrombocytopenia in 14% of patients but >= Grade 3 in only 1% of patients; no episodes of febrile infection Adverse events in no adjuvant chemotherapy arm: not reported Median follow-up: 69 months |
|
| Notes | Patients with residual disease in both arms | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on tables of random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation by telephone call to co-ordinating centre |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Deemed ineligible after randomisation: T: 0/41 (0%) C: 2/44 (5%) Did not report whether any further loss to follow-up occurred |
| Blinding of outcome assessors (detection bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Intention-to-treat analysis; adverse events in the ‘no adjuvant chemotherapy’ arm were not reported |
| Methods | Multicentre randomised controlled trial | |
| Participants | 447 FIGO I-III 93% FIGO stage I |
|
| Interventions | Immediate platinum-based chemotherapy versus treatment on progression | |
| Outcomes | DFS and OS Adverse events in adjuvant chemotherapy arm: 63/241 (26%) experienced toxicity sufficient to require modification of treatment Adverse events in no adjuvant chemotherapy arm: not reported Median follow-up of surviving women: 51 months (Colombo 2003) Median follow-up: 9.2 years (Swart 2007) |
|
| Notes | Long-term follow-up examined subgroup differences | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on minimisation |
| Allocation concealment (selection bias) | Low risk | Minimisation performed by 2 co-ordinating centres |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | T: 0/241 (0%) C: 0/236 (0%) |
| Blinding of outcome assessors (detection bias) | High risk | No blinding |
| Selective reporting (reporting bias) | Unclear risk | Intention-to-treat analysis; all pre-specified outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | 162 high risk FIGO stage I |
|
| Interventions | Carboplatin 6 cycles Q28/7 AUC = 7 versus treatment at progression | |
| Outcomes | DFS and OS Adverse events not reported Median follow-up: 46 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on tables of random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation by telephone call to co-ordinating centre |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 13/175 (7%) deemed ineligible after randomisation, not reported by treatment arm. No further loss to follow-up |
| Blinding of outcome assessors (detection bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Intention-to-treat analysis; adverse events not reported |
| Methods | Randomised controlled trial | |
| Participants | 92 FIGO stage I | |
| Interventions | Melphalan chemotherapy versus treatment on progression | |
| Outcomes | DFS and OS Adverse events in adjuvant chemotherapy arm: 79% had some degree of myelosuppression; 7 patients (16%) had severe myelosuppression; 5 patients (12%) had platelet count nadirs under 50,000 per cubic mm; 4 patients (9%) had platelet count nadirs under 2000 per cubic mm; no infectious complications related to leukopenia; no bleeding episodes related to thrombocytopenia induced by chemotherapy. 11 patients (26%) reported mild-to-moderate gastric gastrointestinal side effects. No other adverse effects were reported. One patient died 6 years after completing treatment, with a diagnosis of aplastic anaemia; no other myeloprolific disorders or second cancers were seen after > 250 person-years follow-up Adverse events in no adjuvant chemotherapy arm: not reported Median follow-up of surviving women: 6 years |
|
| Notes | Melphalan produced severe myelosuppression | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on computer-generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation by telephone call to co-ordinating centre |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Deemed ineligible after randomisation: T: 5/48 (10%) C: 6/44 (14%) Did not report whether any further loss to follow-up occurred |
| Blinding of outcome assessors (detection bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis; adverse events in ‘no adjuvant chemotherapy’ arm not reported |
C = control; DFS = disease-free survival; ITT = intention-to-treat; OS = overall survival; T = treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chiara 1994 | This RCT compared whole abdominal radiotherapy (WAR) versus CAP chemotherapy |
| Dembo 1979 | A RCT of radiotherapy versus radiotherapy plus chlorambucil |
| Gronroos 1984 | Quasi-randomised trial (by birth month) comparing single or combined chemotherapy agents with radiotherapy or surgery alone in women with epithelial ovarian cancer stages I-IV. Included 150 women with stage I/II epithelial ovarian cancer randomised to 3 groups (surgery only, surgery + CT or surgery + RT). Followed up for 3 years |
| Hreshchyshyn 1980 | This trial compared chemotherapy against radiotherapy and no further treatment. The method of randomisation was not specified and a prognostic balance was not achieved in the different arms of the trial |
| Klaassen 1988 | This trial compared 3 different adjuvant treatments all given after pelvic radiotherapy: melphalan, whole abdominal radiotherapy and intraperitoneal radio-isotope therapy |
| Kojs 2001 | This trial compared adjuvant whole abdominal radiotherapy with CAP (cyclophosphamide, adriamycin and cisplatin) |
| Maggioni 2006 | This was a trial comparing systematic lymphadenectomy with lymph node sampling in apparent early stage ovarian cancer; it was not a trial of adjuvant treatment |
| Mannel 2011 | A randomised trial of maintenance low-dose paclitaxel for 24 weeks versus observation, in completely resected early-stage ovarian cancer patients receiving 3 cycles of chemotherapy (CP). Trial also known as GOG 175 |
| Sell 1990 | In this trial whole abdominal radiotherapy was compared to a combination of pelvic radiotherapy and cyclophosphamide. Additionally the block randomisation method did not achieve prognostic balance between the 2 trial arms |
| Sevelda 1987 | This was a trial of adjuvant radiotherapy versus adjuvant chemo-irradiation in early stage ovarian cancer |
| Sigurdsson 1982 | In this trial melphalan chemotherapy was compared to observation for mucinous stage Ia and Ib tumours, chemotherapy versus radiotherapy compared for non-mucinous stage Ia and Ib and radiotherapy versus chemoradiotherapy were compared in stage Ic-IIc. There was a stratified quasi-randomisation which did not achieve prognostic balance between the various trial arms |
| Smith 1975 | This trial compared melphalan chemotherapy versus whole abdominal radiotherapy; the method of randomisation was unspecified and more patients with stage 1 disease were in the chemotherapy arm |
| Vergote 1992 | This was a methodologically good trial with central computerised randomisation; it compared chemotherapy with intraperitoneal radio-isotope therapy |
| Young 2000 | The comparison was between 3 and 6 cycles of platinum-based adjuvant chemotherapy |
| Young 2003 | This was a trial comparing intraperitoneal radio-isotope therapy with cyclophosphamide and cisplatin chemotherapy after surgery in early stage disease; there was no control arm on observation only |
CAP = cyclophosphamide, adriamycin and cisplatin; CT = chemotherapy; RCT = randomised controlled trial; RT = radiotherapy
DATA AND ANALYSES
Comparison 1.
Adjuvant chemotherapy versus observation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall 5-yr survival | 3 | 1008 | Hazard ratio (Random, 95% CI) | 0.71 [0.53, 0.93] |
| 2 Overall 10-yr survival | 2 | 925 | Hazard Ratio (Random, 95% CI) | 0.74 [0.58, 0.95] |
| 3 Overall 5-yr survival (subgrouped by surgical staging) | 3 | Hazard ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Optimal staging | 2 | 234 | Hazard ratio (Random, 95% CI) | 1.22 [0.63, 2.37] |
| 3.2 Sub-optimal staging | 2 | 772 | Hazard ratio (Random, 95% CI) | 0.63 [0.46, 0.85] |
| 4 Overall 10-yr survival (subgrouped by risk) | 1 | Hazard ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Low/medium risk | 1 | Hazard ratio (Random, 95% CI) | 0.95 [0.54, 1.66] | |
| 4.2 High risk | 1 | Hazard ratio (Random, 95% CI) | 0.48 [0.32, 0.72] | |
| 5 Progression-free 5-yr survival | 4 | 1170 | Hazard ratio (Random, 95% CI) | 0.67 [0.53, 0.84] |
| 6 Progression-free 10-yr survival | 2 | 925 | Hazard Ratio (Random, 95% CI) | 0.67 [0.54, 0.84] |
| 7 Progression-free 5-yr survival (subgrouped by surgical staging) | 4 | Hazard ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Optimal staging | 2 | 234 | Hazard ratio (Random, 95% CI) | 0.67 [0.36, 1.22] |
| 7.2 Sub-optimal staging | 3 | 934 | Hazard ratio (Random, 95% CI) | 0.64 [0.50, 0.82] |
| 8 Progression-free 10-yr survival (subgrouped by surgical staging) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 8.1 Optimal staging | 1 | 151 | Hazard Ratio (Random, 95% CI) | 0.73 [0.38, 1.42] |
| 8.2 Sub-optimal staging | 1 | 295 | Hazard Ratio (Random, 95% CI) | 0.60 [0.41, 0.87] |
| 9 Progression-free 10-yr survival (subgrouped by risk) | 1 | Hazard ratio (Random, 95% CI) | Subtotals only | |
| 9.1 Low/medium | 1 | Hazard ratio (Random, 95% CI) | 0.96 [0.58, 1.59] | |
| 9.2 High | 1 | Hazard ratio (Random, 95% CI) | 0.52 [0.33, 0.82] | |
| 10 Disease-specific 5-yr survival | 1 | Hazard ratio (Random, 95% CI) | Subtotals only | |
| 11 Disease-specific 10-yr survival (subgrouped by surgical staging) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 11.1 Optimal staging | 1 | 151 | Hazard Ratio (Random, 95% CI) | 1.58 [0.61, 4.09] |
| 11.2 Sub-optimal staging | 1 | 295 | Hazard Ratio (Random, 95% CI) | 0.58 [0.35, 0.96] |
| 12 Death from ovarian cancer (5 years) | 3 | 693 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.52, 1.11] |
| 13 Death from ovarian cancer (10 years) | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 13.1 Optimal staging | 1 | 151 | Risk Ratio (M-H, Random, 95% CI) | 1.55 [0.64, 3.79] |
| 13.2 Sub-optimal staging | 1 | 295 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.40, 0.97] |
Analysis 1.1. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 1 Overall 5-yr survival
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 1 Overall 5-yr survival
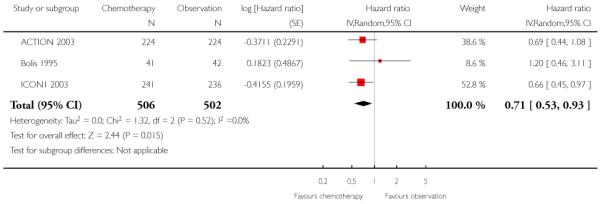 |
Analysis 1.2. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 2 Overall 10-yr survival
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 2 Overall 10-yr survival
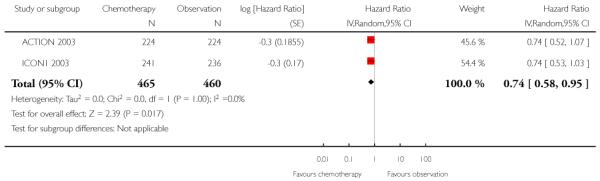 |
Analysis 1.3. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 3 Overall 5-yr survival (subgrouped by surgical staging)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 3 Overall 5-yr survival (subgrouped by surgical staging)
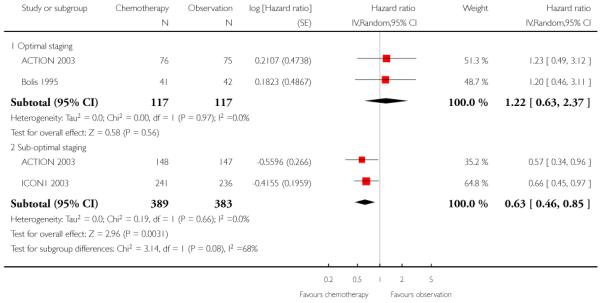 |
Analysis 1.4. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 4 Overall 10-yr survival (subgrouped by risk)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 4 Overall 10-yr survival (subgrouped by risk)
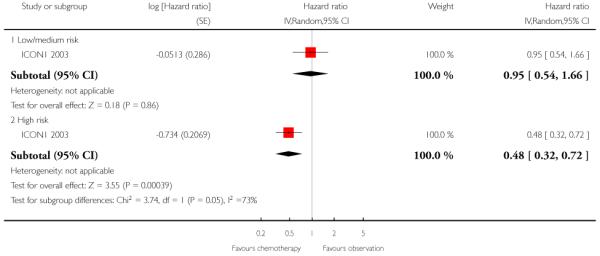 |
Analysis 1.5. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 5 Progression-free 5-yr survival
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 5 Progression-free 5-yr survival
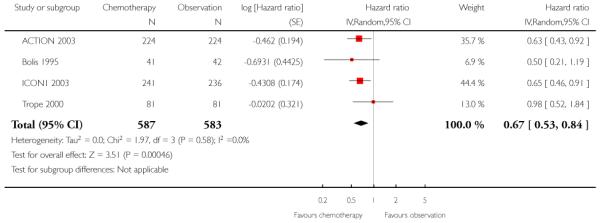 |
Analysis 1.6. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 6 Progression-free 10-yr survival
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 6 Progression-free 10-yr survival
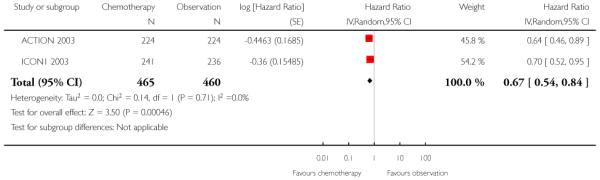 |
Analysis 1.7. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 7 Progression-free 5-yr survival (subgrouped by surgical staging)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 7 Progression-free 5-yr survival (subgrouped by surgical staging)
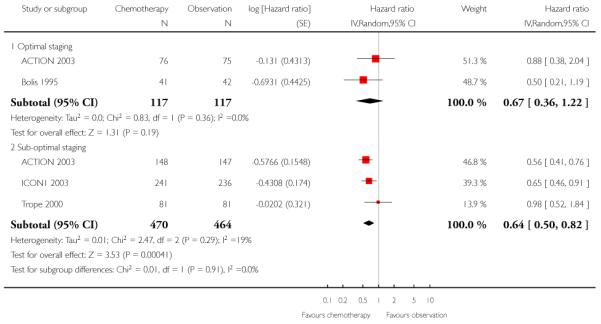 |
Analysis 1.8. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 8 Progression-free 10-yr survival (subgrouped by surgical staging)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 8 Progression-free 10-yr survival (subgrouped by surgical staging)
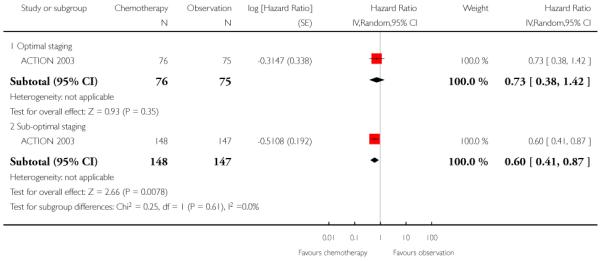 |
Analysis 1.9. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 9 Progression-free 10-yr survival (subgrouped by risk)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 9 Progression-free 10-yr survival (subgrouped by risk)
 |
Analysis 1.10. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 10 Disease-specific 5-yr survival
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 10 Disease-specific 5-yr survival
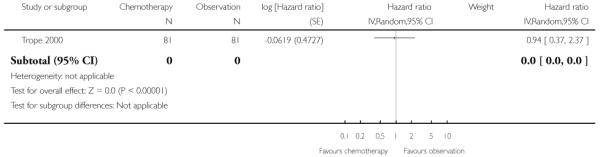 |
Analysis 1.11. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 11 Disease-specific 10-yr survival (subgrouped by surgical staging)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 11 Disease-specific 10-yr survival (subgrouped by surgical staging)
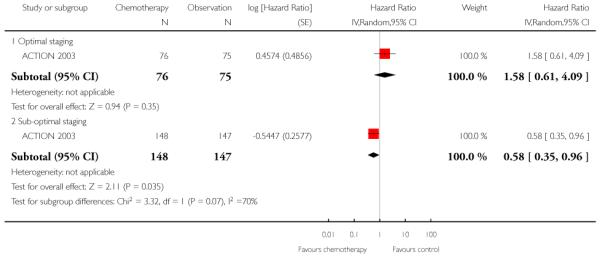 |
Analysis 1.12. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 12 Death from ovarian cancer (5 years)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 12 Death from ovarian cancer (5 years)
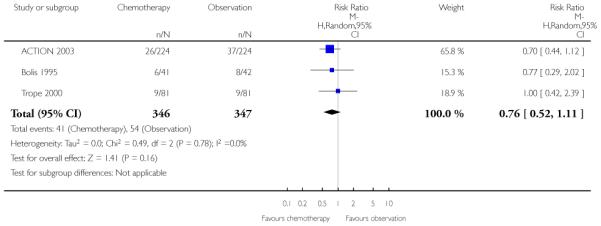 |
Analysis 1.13. Comparison 1 Adjuvant chemotherapy versus observation, Outcome 13 Death from ovarian cancer (10 years)
Review: Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Comparison: 1 Adjuvant chemotherapy versus observation
Outcome: 13 Death from ovarian cancer (10 years)
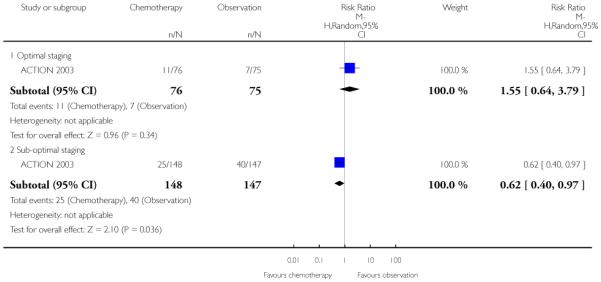 |
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Ovarian Neoplasms explode all trees
#2 ovar* near/5 (cancer* or tumor* or tumour* or neoplas* or carcinoma*or malignan*)
#3 (#1 OR #2)
#4 Any MeSH descriptor with qualifier: DT
#5 MeSH descriptor Antineoplastic Agents explode all trees
#6 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols, this term only
#7 chemotherap*
#8 (#4 OR #5 OR #6 OR #7)
#9 Any MeSH descriptor with qualifier: SU
#10 MeSH descriptor Surgical Procedures, Operative explode all trees
#11 surg* or procedure* or intervention*
#12 (#9 OR #10 OR #11)
#13 (#8 AND #12)
#14 MeSH descriptor Chemotherapy, Adjuvant explode all trees
#15 chemotherap* and adjuvant
#16 (#14 OR #15)
#17 (#13 OR #16)
#18 (#3 AND #17)
This search strategy yielded the following results, including duplicates: CENTRAL = 485 refs.
Appendix 2. MEDLINE search strategy 2011
The 2011 MEDLINE (Ovid) search strategy was conducted from 1948 to September 2011 as follows:
exp Ovarian Neoplasms/
(ovar* adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*)).mp.
1 or 2
drug therapy.fs.
exp Antineoplastic Agents/
Antineoplastic Combined Chemotherapy Protocols/
chemotherap*.mp.
4 or 5 or 6 or 7
surgery.fs.
exp Surgical Procedures, Operative/
(surg* or procedure* or intervention*).mp.
9 or 10 or 11
8 and 12
Chemotherapy, Adjuvant/
(chemotherap* and adjuvant).mp.
14 or 15
13 or 16
3 and 17
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
19 or 20 or 21 or 22 or 23 or 24 or 25
18 and 26
key:
mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier
pt = publication type
ab = abstract
sh = subject heading
This search strategy yielded the following results, including duplicates: total MEDLINE = 997 refs.
Appendix 3. EMBASE search strategy
1 exp ovary tumor/
2 (ovar* adj5 (cancer* or tumor* or tumour* or malignan* or carcinoma* or neoplasm*)).mp.
3 1 or 2
4 exp chemotherapy/
5 exp antineoplastic agent/
6 chemotherap*.mp.
7 4 or 5 or 6
8 exp gynecologic surgery/
9 (surgery or surgical* or procedure* or intervention*).mp.
10 8 or 9
11 7 and 10
12 adjuvant chemotherapy/
13 (adjuvant adj5 chemotherap*).mp.
14 12 or 13
15 11 or 14
16 3 and 15
17 crossover procedure/
18 randomized controlled trial/
19 single blind procedure/
20 random*.mp.
21 factorial*.mp.
22 (crossover* or cross over* or cross-over).mp.
23 placebo*.mp.
24 (doubl* adj blind*).mp.
25 (singl* adj blind*).mp.
26 assign*.mp.
27 allocat*.mp.
28 volunteer*.mp.
29 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30 16 and 29
key:
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
This search strategy yielded the following results, including duplicates: EMBASE = 1630 refs.
Appendix 4. ‘Risk of bias’ assessment
We evaluated risk of bias using the following criteria:
1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it had produced comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. a computer-generated random sequence or a table of random numbers);
high risk of bias (any non-random process, e.g. quasi-randomised: date of birth, clinic ID number or surname);
unclear, e.g. not reported.
2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk (e.g. by telephone randomisation, or use of consecutively numbered, sealed, opaque envelopes);
high risk (e.g. open random number lists or quasi-randomisation such as alternate days, odd/even date of birth, or hospital number, unsealed or non-opaque envelopes);
unclear, e.g. not reported.
3) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as:
low risk;
high risk;
unclear.
4) Loss to follow-up/incomplete outcome data (checking for possible attrition bias)
We recorded the proportion of participants whose outcomes were not reported at the end of the study; we noted if loss to follow-up was not reported. We assessed loss to follow-up as:
low risk, if fewer than 20% of patients were lost to follow-up and reasons for loss to follow-up were similar in both treatment arms;
high risk, if more than 20% of patients were lost to follow-up or reasons for loss to follow-up differed between treatment arms;
unclear, if loss to follow-up was not reported.
5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias. We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review had been reported and analyses were by intention-to-treat);
high risk of bias (where not all the study’s pre-specified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported; analyses not by intention-to-treat);
unclear risk of bias.
6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we had about other possible sources of bias, e.g. an imbalance in baseline/prognostic factors.
HISTORY
Protocol first published: Issue 2, 2004
Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 6 February 2012 | New search has been performed | New author added. |
| 6 February 2012 | New citation required but conclusions have not changed | New search strategy was designed and a search was performed to date (Appendix 2). Eleven additional citations were identified by the updated search (16 September 2011) including nine reports/conference abstracts relating to five previously classified studies. Two reports were added to ‘Studies awaiting classification’ Of the 11 citations identified by the updated search, two studies have been excluded and nine citations added to previously classified studies. Long-term ACTION data have been added to the ‘Data and analyses’ section. |
| 18 May 2011 | Amended | New search performed and methods updated. |
| 2 March 2009 | New citation required but conclusions have not changed | Further editing carried out. |
| 5 June 2008 | Amended | Converted to new review format. |
WHAT’S NEW
Last assessed as up-to-date: 16 January 2012.
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
After publication of an abstract reporting the effect of adjuvant chemotherapy compared to no adjuvant chemotherapy in subgroups of high-risk and intermediate/low-risk women in the ICON1 2003 trial, we decided to present these subgroup data in the review.
As this abstract reported 10-year follow-up of the ICON1 2003 trial, we decided to use the five-year data from this trial in the primary meta-analysis, since this was more consistent with the duration of follow-up of the other included trials, but to use the 10-year data in sensitivity analyses.
Many of the planned subgroup and sensitivity analyses were not possible because relevant data were not reported in original papers and we were unable to obtain these data from authors.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
Adjuvant chemotherapy compared with observation for early stage ovarian cancer
Patient or population: women with stage I/II epithelial ovarian cancer
Settings: hospital and outpatient
Intervention: chemotherapy following surgery
Comparison: observation following surgery
| Outcomes | Hazard ratio (95% CI) Chemotherapy versus observation |
No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Overall 5-yr survival | HR 0.71 (0.53 to 0.93) | 1006 women (three studies) |
⊕⊕⊕⊕ high |
Homogeneous data (I2 = 0%); P = 0.01 |
| Overall 10-yr survival | HR 0.74 (0.58 to 0.95) | 925 women (two studies) |
⊕⊕⊕⊕ high |
Homogeneous data (I2 = 0%);. P = 0.02 |
| Progression-free 5-yr survival | HR 0.67 (0.53 to 0.84) | 1170 women (four studies) |
⊕⊕⊕⊕ high |
Homogeneous data (I2 = 0%); P = 0.0005 |
| Progression-free 10-yr survival | HR 0.67 (0.54 to 0.84) | 925 women (two studies) |
⊕⊕⊕⊕ high |
Homogeneous data (I2 = 0%); P = 0.0005 |
| Overall 5-yr survival: sub-optimal staging* | HR 0.63 (0.46 to 0.85) | 772 women (two studies) |
⊕⊕⊕⊕ high |
Homogeneous data (I2 = 0%); P = 0.003 |
| Progression-free 5-yr survival: sub-optimal staging** | RR 0.64 (0.50 to 0.82) | 934 women (three studies) |
⊕⊕⊕⊕ high |
Homogeneous data (I2 = 19%); P = 0.0004 |
| Overall 5-yr survival: optimal staging* | HR 1.22 (0.63 to 2.37) | 234 women (two studies) |
⊕⊕⊕○ moderate |
Quality of the evidence downgraded to ‘moderate’ due to small subgroup size (I2 = 0%; P = 0.56)* |
| Progression-free 5-yr survival: optimal staging** | RR 0.67 (0.36 to 1.22) | 234 women (two studies) |
⊕⊕⊕○ moderate |
Quality of the evidence downgraded to ‘moderate’ due to small subgroup size (I2 = 0%; P = 0.19) |
CI: confidence interval; HR: hazard ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Tests for subgroup differences between optimal and sub-optimal subgroups for the 5-year OS outcome were Chi2 = 3.14, df = 1 (P = 0.08) and I2 = 68.1%. We consider this to be a significant difference.
Tests for subgroup differences between optimal and sub-optimal subgroups for the 5-year PFS outcome showed that the subgroups were not different with respect to this outcome (P = 0.91; I2 = 0%).
Footnotes
DECLARATIONS OF INTEREST None known.
References to studies included in this review
- ACTION 2003 {published data only} .Timmers PJZ. Understanding the problem of inadequately staging early ovarian cancer. European Journal of Cancer. 2010;46(5):880–4. doi: 10.1016/j.ejca.2009.12.012. [DOI] [PubMed] [Google Scholar]; Trimbos B, Timmers P, Pecorelli S, Coens C, Ven K, van der Burg M, et al. Surgical staging and treatment of early ovarian cancer: long-term analysis from a randomized trial. Journal of the National Cancer Institute. 2010;102(13):982–7. doi: 10.1093/jnci/djq149. [DOI] [PMC free article] [PubMed] [Google Scholar]; Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. Journal of the National Cancer Institute. 2003;95(2):105–12. [PubMed] [Google Scholar]; Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, et al. EORTC-ACTION collaborators European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma. Journal of the National Cancer Institute. 2003;95(2):113–25. [PubMed] [Google Scholar]; Vergote IB, Trimbos BJ, Guthrie D, Parmar M, Bolis G, Mangioni C, et al. Results of a randomized trial in 923 patients with high-risk early ovarian cancer, comparing adjuvant chemotherapy with no further treatment following surgery [abstract] Proceedings of the American Society of Clinical Oncology. 2001;Vol. 20(Part 1):201a. [Google Scholar]
- Bolis 1995 {published data only} .Bolis G, Colombo N, Pecorelli S, Torri V, Marsoni S, Bonazzi C, et al. Adjuvant treatment for early epithelial ovarian cancer: results of two randomised clinical trials comparing cisplatin to no further treatment or chromic phosphate (32P). G.I.C.O.G.: Gruppo Interregionale Collaborativo in Ginecologia Oncologica. Annals of Oncology. 1995;6(9):887–93. doi: 10.1093/oxfordjournals.annonc.a059355. [DOI] [PubMed] [Google Scholar]
- ICON1 2003 {published data only} .Erratum: “International collaborative ovarian neoplasm trial 1: A randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer” (Journal of the National Cancer Institute (2003) vol. 95 (2) (125-32)) Journal of the National Cancer Institute. 2003;95(10):764. doi: 10.1093/jnci/95.2.125. [DOI] [PubMed] [Google Scholar]; Colombo N, Guthrie D, Chiari S, Parmar M, Qian W, Swart AM, et al. International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. Journal of the National Cancer Institute. 2003;95(2):125–32. doi: 10.1093/jnci/95.2.125. [DOI] [PubMed] [Google Scholar]; Colombo N, Pecorelli S. What have we learned from ICON1 and ACTION? International Journal of Gynecological Cancer. 2003;13(Suppl 2):140–3. doi: 10.1111/j.1525-1438.2003.13366.x. [DOI] [PubMed] [Google Scholar]; Colombo N, Trimbos JB, Guthrie D, Vergote I, Mangioni C, Vermorken J, et al. ACTION + ICON1: two parallel randomised phase III trials comparing adjuvant chemotherapy to no adjuvant chemotherapy following surgery in women with high risk early ovarian cancer [abstract] European Journal of Cancer. 2001;37(Suppl 6):276. [Google Scholar]; Swart AC on behalf of ICON collaborators Long-term follow-up of women enrolled in a randomized trial of adjuvant chemotherapy for early stage ovarian cancer (ICON1) Journal of Clinical Oncology. 2007;25(18S):5509. [Google Scholar]
- Trope 2000 {published data only} .Trope C, Kaern J, Hogberg T, Abeler V, Hagen B, Kristensen G, et al. Randomized study on adjuvant chemotherapy in stage I high-risk ovarian cancer with evaluation of DNA-ploidy as prognostic instrument. Annals of Oncology. 2000;11(3):281–8. doi: 10.1023/a:1008399414923. [DOI] [PubMed] [Google Scholar]; Trope C, Kaern J, Vergote I, Hagen B, Rosenberg P, Bertelsen K, et al. Randomized trial on adjuvant carboplatin versus no treatment in Stage I high risk ovarian cancer by the Nordic Ovarian Cancer Study Group (NOCOVA) [abstract] Proceedings of the American Society of Clinical Oncology. 1997;Vol. 16:352a. [Google Scholar]
- Young 1990 {published data only} .Young RC, Walton LA, Ellenberg SS, Homesley HD, Wilbanks GD, Decker DG, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer Results of two prospective randomized trials. New England Journal of Medicine. 1990;322(15):1021–7. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Chiara 1994 {published data only} .Chiara S, Conte P, Franzone P, Orsatti M, Bruzzone M, Rubagotti A, et al. High-risk early-stage ovarian cancer. Randomized clinical trial comparing cisplatin plus cyclophosphamide versus whole abdominal radiotherapy. American Journal of Clinical Oncology. 1994;17(1):72–6. doi: 10.1097/00000421-199402000-00016. [DOI] [PubMed] [Google Scholar]
- Dembo 1979 {published data only} .Dembo AJ, Bush RS, Beale FA, Bean HA, Pringle JF, Sturgeon J, et al. Ovarian carcinoma: improved survival following abdominopelvic irradiation in patients with a completed pelvic operation. American Journal of Obstetrics and Gynecology. 1979;134(7):793–800. [PubMed] [Google Scholar]
- Gronroos 1984 {published data only} .Gronroos M, Nieminen U, Kauppila A. A prospective, randomized, national trial for treatment of ovarian cancer: the role of chemotherapy and external irradiation. European Journal of Obstetrics, Gynaecology and Reproductive Biology. 1984;17(1):33–42. doi: 10.1016/0028-2243(84)90078-9. [DOI] [PubMed] [Google Scholar]
- Hreshchyshyn 1980 {published data only} .Hreshchyshyn MM, Park RC, Blessing JA, Norris HJ, Levy D, et al. The role of adjuvant therapy in stage I ovarian cancer. American Journal of Obstetrics and Gynecology. 1980;138(2):139–45. doi: 10.1016/0002-9378(80)90024-1. [DOI] [PubMed] [Google Scholar]
- Klaassen 1988 {published data only} .Dent SF, Klaassen D, Pater JL, Zee B, Whitehead M. Second primary malignancies following the treatment of early stage ovarian cancer: update of a study by the National Cancer Institute of Canada--Clinical Trials Group (NCIC-CTG) Annals of Oncology. 2000;11(1):65–8. doi: 10.1023/a:1008356806417. [DOI] [PubMed] [Google Scholar]; Klaassen D, Shelley W, Starreveld A, Kirk M, Boyes D, Gerulath A, et al. Early stage ovarian cancer: a randomized clinical trial comparing whole abdominal radiotherapy, melphalan, and intraperitoneal chromic phosphate: a National Cancer Institute of Canada Clinical Trials Group report. Journal of Clinical Oncology. 1988;6(8):1254–63. doi: 10.1200/JCO.1988.6.8.1254. [DOI] [PubMed] [Google Scholar]
- Kojs 2001 {published data only} .Kojs Z, Glinski B, Reinfuss M, Pudelek J, Urbanski K, Kowalska T, et al. Results of a randomized prospective trial comparing postoperative abdominopelvic radiotherapy with postoperative chemotherapy in early ovarian cancer. Cancer Radiotherapie. 2001;5(1):5–11. doi: 10.1016/s1278-3218(00)00022-6. [DOI] [PubMed] [Google Scholar]
- Maggioni 2006 {published data only} .Maggioni A, Benedetti Panici P, Dell’Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. British Journal of Cancer. 2006;95(6):699–704. doi: 10.1038/sj.bjc.6603323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannel 2011 {published data only} .Mannel RS, Brady MF, Kohn EC, Hanjani P, Hiura M, Lee R, et al. A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: a Gynecologic Oncology Group Study. Gynecological Oncology. 2011;122(1):89–94. doi: 10.1016/j.ygyno.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell 1990 {published data only} .Sell A, Bertelsen K, Andersen JE, Stroyer I, Panduro J, Bentzen SM, et al. Randomized study of whole-abdomen irradiation versus pelvic irradiation plus cyclophosphamide in treatment of early ovarian cancer. Gynecologic Oncology. 1990;37(3):367–73. doi: 10.1016/0090-8258(90)90369-v. [DOI] [PubMed] [Google Scholar]
- Sevelda 1987 {published data only} .Sevelda P, Gitsch E, Dittrich C, Haider F, Czerwenka K, Schemper M, et al. Therapeutic and prognostic results of a prospective multicenter ovarian cancer study of FIGO stages I and II. Geburtshilfe Frauenheilkd. 1987;47(3):179–85. doi: 10.1055/s-2008-1035803. [DOI] [PubMed] [Google Scholar]
- Sigurdsson 1982 {published data only} .Sigurdsson K, Johnsson JE, Trope C. Carcinoma of the ovary, stages I and II. A prospective randomized study of the effects of postoperative chemotherapy and radiotherapy. Annales Chirurgiae et Gynaecologiae. 1982;71(6):321–9. [PubMed] [Google Scholar]
- Smith 1975 {published data only} .Smith JP, Rutledge FN, Delclos L, Mayer AR, Chambers SK, Graves E, et al. Results of chemotherapy as an adjunct to surgery in patients with localized ovarian cancer. Seminars in Oncology. 1975;2(3):277–81. [PubMed] [Google Scholar]
- Vergote 1992 {published data only} .Vergote IB, Vergote-De Vos LN, Abeler VM, Aas M, Lindegaard MW, Kjorstad KE, et al. Randomized trial comparing cisplatin with radioactive phosphorus or whole-abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer. 1992;69(3):741–9. doi: 10.1002/1097-0142(19920201)69:3<741::aid-cncr2820690322>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Young 2000 {published data only} .Bell J, Brady MF, Young RC, Walker JL, Look KY, Rose GS, et al. Randomised phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology. 2006;102(3):432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]; Young RC. Three cycles versus six cycles of adjuvant paclitaxel (Taxol)/carboplatin in early stage ovarian cancer. Seminars in Oncology. 2000;27(3 Suppl 7):8–10. [PubMed] [Google Scholar]
- Young 2003 {published data only} .Young RC, Brady MF, Nieberg RK, Long HJ, Mayer AR, Lentz SS, et al. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin--a gynecologic oncology group study. Journal of Clinical Oncology. 2003;21(23):4350–5. doi: 10.1200/JCO.2003.02.154. [DOI] [PubMed] [Google Scholar]
Additional references
- Altman 1995 .Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. British Journal of Cancer. 1995;72:511–8. doi: 10.1038/bjc.1995.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOCTG 1999 .Advanced Ovarian Cancer Trialists Group Chemotherapy for advanced ovarian cancer. Cochrane Database of Systematic Reviews. 1999;Issue 1 doi: 10.1002/14651858.CD001418. [DOI: 10.1002/14651858.CD001418] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell 2006 .Bell J, Brady MF, Young RC, Walker JL, Look KY, Rose GS, et al. Randomised phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology. 2006;102(3):432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Calvert 1989 .Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. Journal of Clinical Oncology. 1989;13(8):2147–8. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- Colombo 2003 .Colombo N, Guthrie D, Chiari S, Parmar M, Qian W, Swart AM, et al. International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. Journal of the National Cancer Institute. 2003;95(2):125–32. doi: 10.1093/jnci/95.2.125. [DOI] [PubMed] [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Elit 2004 .Elit L, Chambers A, Fyles A, Covens A, Carey M, Fung MF. Systematic review of adjuvant care for women with stage I ovarian carcinoma. Cancer. 2004;101(9):1926–35. doi: 10.1002/cncr.20595. [DOI] [PubMed] [Google Scholar]
- GLOBOCAN 2008 .Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. International Agency for Research on Cancer [Internet] Lyon, France: 2010. Available from: http://globocan.iarc.fr. [Google Scholar]
- GOG111 1996 .McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New England Journal of Medicine. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- Green 2003 .Green JA. Early ovarian cancer--time for a rethink on stage. Gynecologic Oncology. 2003;90:235–7. doi: 10.1016/s0090-8258(03)00410-4. [DOI] [PubMed] [Google Scholar]
- Higgins 2011 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.1 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane–handbook.org. [Google Scholar]
- ICON2 1998 .ICON collaborators ICON2: randomised trial of single agent carboplatin against three-drug combination of CAP (cyclophosphamide, doxorubicin, and cisplatin) in women with ovarian cancer. ICON. International Collaborative Neoplasm Study. Lancet. 1998;352:1571–6. [PubMed] [Google Scholar]
- Jemal 2008 .Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA: A Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Mayer 1992 .Mayer AR, Chambers SK, Graves E, Holm C, Tseng PC, Nelson BE, et al. Ovarian cancer staging: does it require a gynecologic oncologist? Gynecologic Oncology. 1992;47:223–7. doi: 10.1016/0090-8258(92)90110-5. [DOI] [PubMed] [Google Scholar]
- Morice 2001 .Morice P, Wicart-Poque F, Rey A, El-Hassan J, Pautier P, Lhommé C, et al. Results of conservative treatment in epithelial ovarian carcinoma. Cancer. 2001;92:2412–8. doi: 10.1002/1097-0142(20011101)92:9<2412::aid-cncr1590>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- NCCC OC 2011 .National Collaborating Centre for Cancer Ovarian cancer: NICE clinical guideline 122. 2011 Apr; http://www.nice.org.uk/nicemedia/live/13464/54268/54268.pdf [PubMed]
- NCI CTCAE v3.0 2006 .National Cancer Institute National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI CTCAE v3.0) 2006 http://www.eortc.be/services/doc/ctc/ctcaev3.pdf
- Parmar 1998 .Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Peto 1982 .Peto R. Statistical aspects of cancer trials. In: Halnan KE, editor. Treatment of Cancer. Chapman and Hall; London: 1982. [Google Scholar]
- RevMan 2011 .The Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Sant 2003 .Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE-3: survival of cancer patients diagnosed 1990-94 - results and commentary. Annals of Oncology. 2003;14(Supplement 5):v61–v118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- Schilder 2002 .Schilder JM, Thompson AM, DePriest PD, Ueland FR, Cibull ML, Kryscio RJ, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecologic Oncology. 2002;87(1):1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- Shepherd 1989 .Shepherd JH. Revised FIGO staging for gynaecological cancer. British Journal of Obstetrics and Gynaecology. 1989;96:889–92. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- Swart 2007 .Swart AC on behalf of ICON collaborators . Journal of Clinical Oncology. Vol. 25. ASCO Annual Meeting Proceedings; Chicago: 2007. Long-term follow-up of women enrolled in a randomized trial of adjuvant chemotherapy in early stage ovarian cancer (ICON1) [Google Scholar]
- Thigpen 2011 .Thigpen T, duBois A, McAlpine J, DiSaia P, Fujiwara K, Hoskins W, the Gynecologic Cancer InterGroup First line therapy in ovarian cancer trials. International Journal of Gynecological Cancer. 2011;21(4):756–62. doi: 10.1097/IGC.0b013e31821ce75d. [DOI] [PubMed] [Google Scholar]
- Trimbos 2003 .Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, et al. EORTC-ACTION collaborators European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma. Journal of the National Cancer Institute. 2003;95(2):113–25. [PubMed] [Google Scholar]
- Trimbos 2010 .Trimbos B, Timmers P, Pecorelli S, Coens C, Ven K, van der Burg M, et al. Surgical staging and treatment of early ovarian cancer: long-term analysis from a randomized trial. Journal of the National Cancer Institute. 2010;102(13):982–7. doi: 10.1093/jnci/djq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope 2007 .Trope C, Kaern J. Adjuvant chemotherapy for early-stage ovarian cancer: review of the literature. Journal of Clinical Oncology. 2007;25(20):2909–20. doi: 10.1200/JCO.2007.11.1013. [DOI] [PubMed] [Google Scholar]
- Vergote 2001 .Vergote I, De Brabanter J, Fyles A, Bertelsen K, Einhorn N, Selvelda P, et al. Prognostic importance of degree of differentiation and cyst rupture in stage 1 invasive epithelial carcinoma. Lancet. 2001;357:176–82. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- Zanetta 1998 .Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Torri V, et al. The accuracy of staging: an important prognostic determinator in stage I ovarian carcinoma. A multivariate analysis. Annals of Oncology. 1998;9(10):1097–101. doi: 10.1023/a:1008424527668. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Winter-Roach 2003 .Winter-Roach B, Hooper L, Kitchener H. Systematic review of adjuvant therapy for early stage (epithelial) ovarian cancer. International Journal of Gynecological Cancer. 2003;13(4):395–404. doi: 10.1046/j.1525-1438.2003.13316.x. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study