Abstract
Polybrominated diphenyl ethers (PBDEs) are routinely found in human tissues including cord blood and breast milk. PBDEs may interfere with thyroid hormone (TH) during development, which could produce neurobehavioral deficits. An assumption in experimental and epidemiological studies is that PBDE effects on serum TH levels will reflect PBDE effects on TH action in tissues. To test whether this assumption is correct, we performed the following experiments. First, five concentrations of diphenyl ether (0–30 mg/kg) were fed daily to pregnant rats to postnatal day 21. PBDEs were measured in dam liver and heart to estimate internal dose. The results were compared with a separate study in which four concentrations of propylthiouracil (PTU; 0, 1, 2, and 3 ppm) was provided to pregnant rats in drinking water for the same duration as for diphenyl ether. PBDE exposure reduced serum T4 similar in magnitude to PTU, but serum TSH was not elevated by PBDE. PBDE treatment did not affect the expression of TH response genes in the liver or heart as did PTU treatment. PTU treatment reduced T4 in liver and heart, but PBDE treatment reduced T4 only in the heart. Tissue PBDEs were in the micrograms per gram lipid range, only slightly higher than observed in human fetal tissues. Thus, PBDE exposure reduces serum T4 but does not produce effects on tissues typical of low TH produced by PTU, demonstrating that the effects of chemical exposure on serum T4 levels may not always be a faithful proxy measure of chemical effects on the ability of thyroid hormone to regulate development and adult physiology.
Polybrominated diphenyl ethers (PBDEs) are persistent organic chemicals and common contaminants in environmental and human tissue samples (1). These chemicals are used as flame retardants in a variety of products, including foam furniture and electrical equipment such as computers and televisions (2), but are also found in food products such as meat, fish, and dairy products (3). Of particular concern is the observation that PBDEs are found in the blood of pregnant women (eg, reference 4), in cord blood, and in breast milk (5) because previous studies indicate that development in rodents (6) and in humans (7, 8) may be particularly sensitive to PBDE exposures.
Some authors propose that PBDEs affect developmental events by interfering with the ability of thyroid hormone (TH) to exert its action in tissues (eg, reference 4); this concept is consistent with the observations that the chemical structures of PBDEs and TH are similar and that other environmental chemicals with this general structure [eg, polychlorinated biphenyls (PCBs)] are also known to interfere with TH action (9, 10). PBDE exposure reduces concentrations of TH in serum in rodents (eg, references 11 and 12). However, different investigators have found both positive (13) and negative (14) associations between serum TH and PBDEs in humans.
An untested underlying assumption is that PBDE effects on blood levels of TH are indicative of PBDE effects on TH action in tissues. It is well known that TH action in tissues is controlled by several steps in addition to the regulation of hormone levels in blood, including the active transport into tissues and cells, conversion of T4 to T3 by specific enzymes, and interacting with multiple nuclear receptor isoforms (15). Thus, it is possible that some chemical exposures, such as PBDEs, can alter the relationship between serum TH levels and the downstream effects on hormone action. Consistent with this concept, PBDE-induced reduction in serum TH is not always associated with an increase in serum TSH in animals (eg, reference 12), although this may be related to dose and/or duration of exposure (12, 16). The failure of PBDE exposure to increased serum TSH would be quite different from the case of exposure to propylthiouracil (PTU). PTU treatment produces a very consistent and dose-dependent decrease in serum TH and a concomitant increase in serum TSH (17, 18). Moreover, the downstream measures of TH action in tissues are tightly linked to serum T4 levels that are controlled by PTU (19–21). Thus, exposure to some classes of exogenous chemicals (eg, PCBs), but not others [eg, PTU, phenobarbital (22)], may result in different relationships between serum T4, serum TSH, and the measures of TH action in tissues. Considering this factor, we sought to test specifically whether the PBDE-induced reduction in the serum TH levels produces downstream effects on TH action that are similar to, or different from, that of the PTU-induced reduction in the serum TH.
Materials and Methods
Animals and experimental design
PBDE experiment
Animal procedures were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and with the approval from the University of Massachusetts Institutional Animal Care and Use Committee. Timed-pregnant Sprague Dawley rats (n = 60; Zivic Miller Laboratories) arrived on gestational day (G) 2. Animals were housed individually in plastic cages, provided continuous food and water, and kept on a 12-hour light, 12-hour dark cycle. From G2 to G6, animals were provided with an untreated vanilla wafer (Keebler miniwafers; Keebler) to train animals to consume the wafer completely. On GD6 animals were weighed and assigned by weight to receive one of five of the following doses of diphenyl ether (DE-71; 0, 0.1, 1, 10, or 30 mg/kg body weight. DE-71 (lot 7550OK20A from the Great Lakes Chemical Corp) was dissolved in toluene (HPLC grade 99.9% pure; Sigma-Aldrich) and calibrated such that each animal would receive 1 μL of solution per gram body weight. Wafers were dosed 24 hours prior to administration and stored in a fume hood to allow complete evaporation of the toluene, and control animals received wafers dosed with toluene alone. Animals were weighed every other day to adjust dosing to changes in weight. Because a number of animals were not pregnant, the group size was reduced to the following: control (n = 8); 0.1 mg/kg (n = 6); 1.0 mg/kg (n = 6); 10.0 mg/kg (n = 7); and 30 mg/kg (n = 9). On postnatal day (P) 4, litters were culled to five male and five female pups where possible. Pups were killed on P21, and trunk blood was collected for serum hormone analysis. The heart, liver, and pituitary were collected, frozen on dry ice, and stored at −80°C. Because some litters were skewed in their sex ratio, we used only male pups.
PTU experiment
This experiment was performed under an Environmental Protection Agency approved Institutional Animal Care and Use Committee protocol to evaluate the impact of graded decrements in serum TH on gene expression in the developing rat brain (21) as well as on oligodendrocyte development (23); the treatment methods are described in detail in these publications. We include measurements from these tissues here not to discover new effects of TH action but rather to demonstrate that known effects of TH insufficiency can be detected in the same assays in which we tested effects of TH insufficiency caused by PBDE exposure. Although performed in Long-Evans rats, the end points of TH action in liver and heart are the same in Long-Evans and Sprague Dawley rats as referenced in Discussion. The liver and heart tissues and serum were collected, frozen, and shipped overnight on dry ice to the University of Massachusetts-Amherst for analysis.
Serum hormone analysis
Total T4 was measured in serum as previously described and validated (24). All experimental samples were evaluated in a single assay. DE-71 did not displace T4 from the antibody (data not shown). Serum total T3 was measured using an RIA kit from MP Biomedicals. Serum free T4 was measured using an RIA kit from MP Biomedicals. Serum TSH was measured using a standard double-antibody assay as described elsewhere (25). All samples were run in duplicate and the intraassay coefficient of variation was 8.0%. The interassay variations ranged from 1% to 10%.
Tissue hormone extraction and measurement
Tissue levels of T4 were measured as described elsewhere (20). Briefly, we used a cold methanol extraction procedure containing iopanoic acid and PTU to block endogenous deiodinases. Trace amounts of 125I-T4 was used to calculate individual extraction efficiency. T4 was measured in the extract according to the methods described above for serum. Data were normalized by protein content.
RNA isolation and quantitative real time PCR
Target mRNAs were isolated and measured according to the procedures described by Giera et al (26). Total RNA was extracted from tissues using Trizol reagent (Invitrogen) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed using the high-capacity cDNA reverse transcription kit (Applied Biosystems) in a reaction volume of 20 μL; the resultant cDNA was diluted 1:1. Quantitative real-time PCR was performed in a 10-μL reaction using 1 μL of cDNA, 400 nM each of forward and reverse primer, and 5 μL Faststart universal SYBR Green master mix (Rox) kit (Roche Diagnostics) on a Mx3000P (Stratagene) using the following cycling conditions: one cycle at 95°C for 10 minutes; 40 cycles at 95°C for 10 seconds, 55–60°C for 20 seconds, and 72°C for 20 seconds. A melting curve was run for each well for single product verification. Primer sequence and annealing temperatures are listed inSupplemental Table 1. All samples were run in duplicate and β-actin was analyzed in parallel duplicate wells.
PBDE residue analysis
Because of the quantity of tissue required from each animal, we measured PBDEs in maternal liver and heart. Individual PBDE congeners were measured as described earlier (27). The details of this procedure are provided in the Supplemental Information.
Statistical analysis
Several precautions were taken to reduce the risk of bias in obtaining our results, and these are described in the Supplemental Material. Real-time PCR data were analyzed using the 2-ΔΔcT method with β-actin as the endogenous reference gene (28). The results were then analyzed using a one-way ANOVA in Prism 6.0 for Mac OSX (GraphPad Software, Inc), and post hoc tests, when appropriate, were performed using Dunnett's test. The Grubb's test (http://www.graphpad.com/quickcalcs/grubbs1.cfm) was used to identify statistical outliers in all data sets. Hormone data also were analyzed by a one-way ANOVA as described. PBDE data were not normally distributed and so were evaluated with an ANOVA on ranks. Because a number of ANOVAs was performed to evaluate the expression of different genes in the same tissue, a more stringent α-level of .025 was applied to reduce experiment-wise error rate.
Results
Maternal and pup body weight and serum hormones
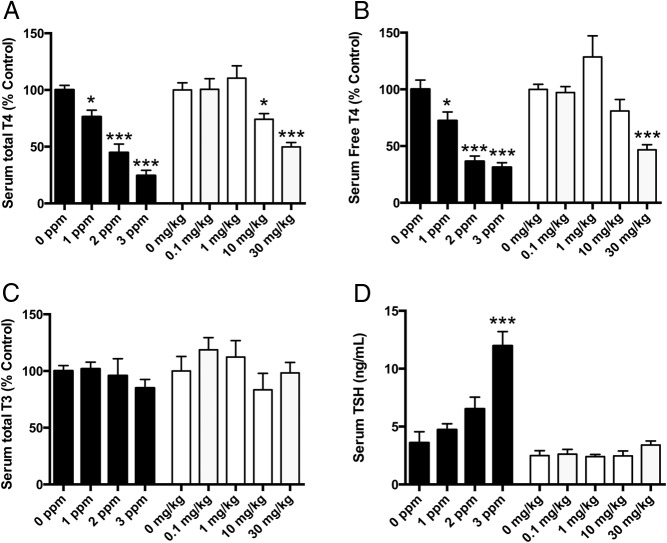
Note that some elements of the PTU experiment have been reported previously (19). Neither maternal nor pup body weight or body weight gain was affected by PTU treatment (Supplemental Table 2). Likewise, pup body weight was unaffected by exposure to PBDE; however, there was a small but significant difference in body weight gain in the dams at the highest dose of PBDE (Supplemental Table 2). Serum total and free T4 was reduced in dams to a similar extent by PTU and by PBDE exposure (Figure 1, A and B). Neither PTU nor PBDE exposure affected serum total T3 (Figure 1C). Serum TSH was elevated in PTU-treated dams but not in dams treated with PBDE (Figure 1D).
Figure 1.
Effects of PTU or PBDE treatment on maternal hormone concentrations. A, Serum total T4 levels in dams at the time the animals were killed on P21, after PTU (solid bars) or PBDE (open bars) treatment. A one-way ANOVA was performed separately for each of the two experiments. For PTU, F3,28 = 27.12; P < .0001; for PBDE, F4,31 = 14.02; P < .001. B, Serum free T4 levels in dams after PTU (solid bars) or PBDE (open bars) treatment. For PTU, F3, 27 = 20.90; P < .0001; for PBDE, F4, 31 = 11.71; P < .0001. C, Serum total T3 in dams treated with PTU (solid bars) or PBDE (open bars) treatment. One-way ANOVA revealed no significant differences among treatment groups. D, Serum TSH in dams treated with PTU (solid bars) or PBDE (open bars). For PTU, a one-way ANOVA demonstrated that TSH levels were significantly increased (F3, 51 = 14.83; P < .0001). This was not the case for PBDE, in which F4,29 = 1.37; P = .27. Bars represent mean ± SEM (n = 5–10 per group). *, P < .05; ***, P < .0001.
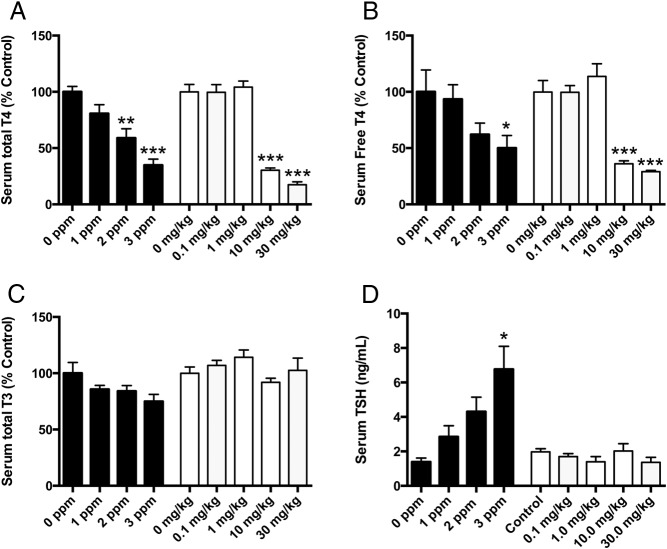
PTU exposure produced a dose-dependent decline in pup serum total and free T4, with 3 ppm PTU producing about a 75% reduction in total T4 and a 50% reduction in serum free T4 (Figure 2, A and B). Likewise, serum total T4 was reduced in PBDE-treated animals but only in those pups treated with 10 or 30 mg/kg, with an 85% decrease in serum total T4 at the highest dose. Similarly, PBDE treatment produced a significant decrease in serum free T4 in pups, with about a 75% reduction in free hormone at 30 mg/kg (Figure 2, A and B). Serum total T3 was not affected by either treatment (Figure 2C). Serum TSH was progressively increased in pups by higher doses of PTU, but serum TSH was not affected by PBDE treatment (Figure 2D).
Figure 2.
Effect of PTU or PBDE treatment on serum hormones in 21-day pups. A, Serum total T4 levels in pups after PTU (solid bars) or PBDE (open bars) treatment. For PTU, F3, 27 = 12.83; P < .0001; for PBDE, F4, 27 = 65.68; P < .0001. B, Serum free T4 levels in pups after PTU (solid bars) or PBDE (open bars) treatment. For PTU, F3, 27 = 3.45; P = .0304; for PBDE, F4, 27 = 27.84; P < .0001. C, Serum total T3 levels were not significantly affected by PTU (solid bars) or PBDE (open bars) treatment. D, Serum TSH in P21 pups after PTU (solid bars) or PBDE (open bars) treatment. For PTU, F3,11 = 4.9; P < .02; for PBDE treatment, F4,24 = 1.13; P = NS. *, P < .05; **, P < .01; ***, P < .0001.
PBDE levels
We performed congener-specific analysis of PBDEs present in maternal liver and heart as described earlier (27). The sum of all PBDE congeners is presented in Table 1. In general, the levels observed in these tissues were similar to those reported earlier despite a very different treatment regimen. The concentration of PBDEs normalized by wet weight was the same for liver and heart but appeared very different when normalized by lipid content because of the different lipid levels in the two tissues.
Table 1.
PBDE Levels in Maternal Liver and Heart
| Treatment, n | Liver |
Heart |
||
|---|---|---|---|---|
| Wet Weight, μg/g | Lipids, μg/g | Wet Weight, μg/g | Lipids, μg/g | |
| Control (n = 4) | 0.011 ± 0.009 | 0.388 ± 0.317 | 0.008 ± 0.003 | 0.361 ± 0.198 |
| 0.1 mg/kg (n = 3) | 0.065 ± 0.00005 | 2.23 ± 0.257 | 0.021 ± 0.006 | 1.02 ± 0.105 |
| 1.0 mg/kg (n = 3) | 0.636 ± 0.046 | 19.9 ± 3.91 | 0.231 ± 0.034 | 9.43 ± 1.28 |
| 10.0 mg/kg (n = 5) | 4.98 ± 1.74a | 142 ± 45.6a | 4.22 ± 1.81a | 211 ± 111a |
| 30.0 mg/kg (n = 3) | 12.4 ± 1.93a | 320 ± 30.6a | 13.1 ± 1.89a | 630 ± 126a |
P = .003.
Treatment effects on TH-responsive genes in liver
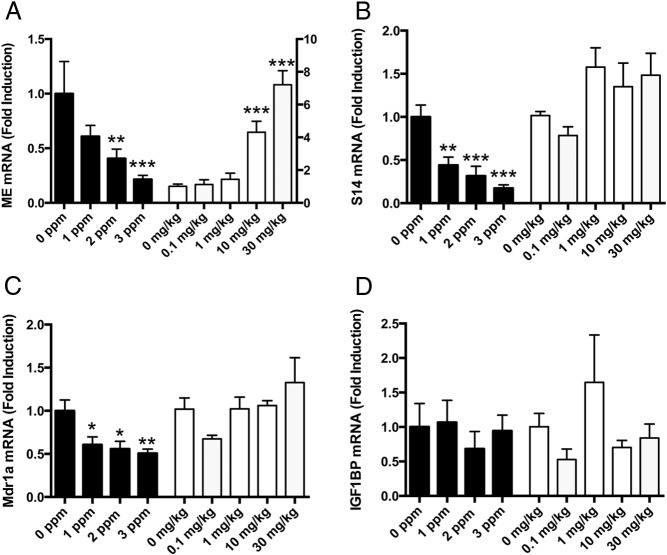
The developing liver is a well-known target of TH action (eg, reference 29). We analyzed the abundance of three positively regulated TH-responsive genes [malic enzyme (ME); spot 14 (S14); and multidrug-resistant gene (Mdr1a)] to monitor the effects of PTU or PBDE treatment on TH action in the liver. In each case, PTU reduced the expression of all three of these genes (Figure 3), consistent with many other literature reports (eg, reference 30). In contrast, ME mRNA levels were significantly increased by PBDE treatment (Figure 3A), whereas S14 and Mdr1a mRNA levels were unaffected by PBDE treatment (Figure 3, B and C). We included an estrogen-responsive gene [IGF binding protein (IGFBP)] to monitor the potential estrogenic effects of PBDEs, but we observed no impact of either PTU or PBDE exposure on the expression of this mRNA (Figure 3D).
Figure 3.
Effect of PTU or PBDE treatment on thyroid hormone action in liver on P21. A, ME mRNA levels were affected by treatment with either PTU (F3,27 = 6.96; P < .001, n = 4–10/group) or PBDE (F4,22 = 25.9; P < .0001, n = 4–7/group). However, PTU treatment significantly reduced whereas PBDE treatment significantly elevated ME mRNA levels. B, S14 mRNA levels were significantly affected by PTU (F3,27 = 9.6; P < .0002, n = 4–10/group) but not by PBDE treatment (F4,23 = 2.5; P = NS, n = 5–7/group). C, Liver MDR1a mRNA levels were significantly affected by PTU treatment (F3,27 = 4.2; P < .01, n = 4–10/group) but not by PBDE treatment (F4,25 = 1.6; P = NS, n = 5–8/group). D, IGFBP mRNA levels in the liver were not affected by either PTU (F3,23 = 0.32; P = NS, n = 4–10/group) or PBDE (F4,27 = 1.85; P = NS, n = 5–8/group) treatment. *, P < .05; **, P < .01; ***, P < .0001.
Treatment effects on TH-responsive genes in heart
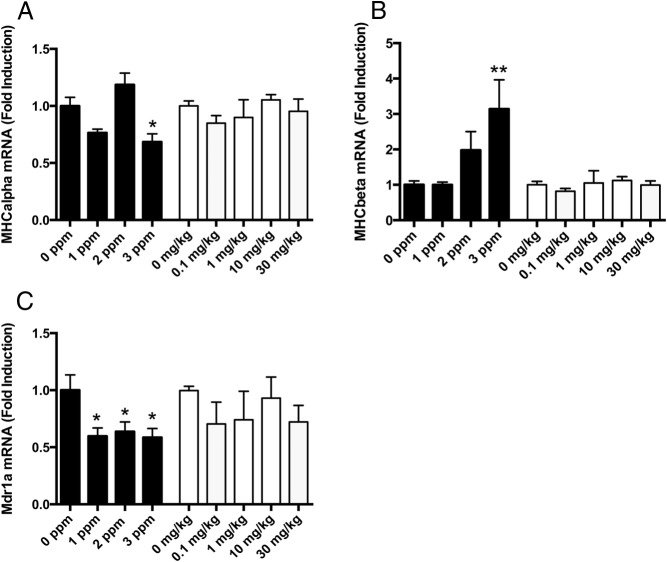
TH does not regulate the same genes in the heart as in the liver; therefore, we chose different TH-responsive genes in the heart to monitor the effects of PTU or PBDE exposure on TH action in this tissue. TH increases the expression of myosin heavy-chain-α (myHC6) but decreases the expression of myosin heavy-chain-β (myHC7) (31), and PTU treatment in this study is consistent with this (Figure 4, A and B). In contrast, neither myHC6 nor myHC7 mRNA levels were affected by PBDE treatment (Figure 4, A and B). As in the liver, Mdr1a mRNA levels were decreased by PTU treatment in the heart but were not affected by PBDE treatment (Figure 4C).
Figure 4.
Effect of PTU or PBDE treatment on thyroid hormone action in heart on P21. A, myHC6 mRNA levels were significantly affected by PTU treatment (F3,28 = 10.4; P < .0001, n = 5–10/group). Post hoc analysis revealed that myHC6 mRNA levels were significantly reduced in animals treated with 3 ppm PTU compared with controls. In contrast, PBDE treatment did not affect myHC6 mRNA levels (F4,27 = 0.83; P = NS, n = 5–8/group). B, myHC7 mRNA levels were significantly affected by PTU treatment (F3,26 = 4.34; P < .01, n = 5–10/group). Post hoc analysis revealed that myHC7 mRNA levels were significantly higher in animals treated with 3 ppm PTU compared with controls. In contrast, myHC7 mRNA levels were not affected by PBDE treatment (F4,27 = 0.51; P = NS, n = 5–8/group). C, MDR1a mRNA levels in heart were significantly reduced by PTU treatment (F3,27 = 3.8; P < .02, 5–10/group). Post hoc analysis showed that all MDR1a mRNA levels were reduced in all PTU-treated animals compared with controls. In contrast, PBDE treatment did not affect MDR1a mRNA levels in heart (F4,25 = 0.8; P = NS, n = 5–8/group).
Treatment effects on tissue TH concentrations in liver and heart
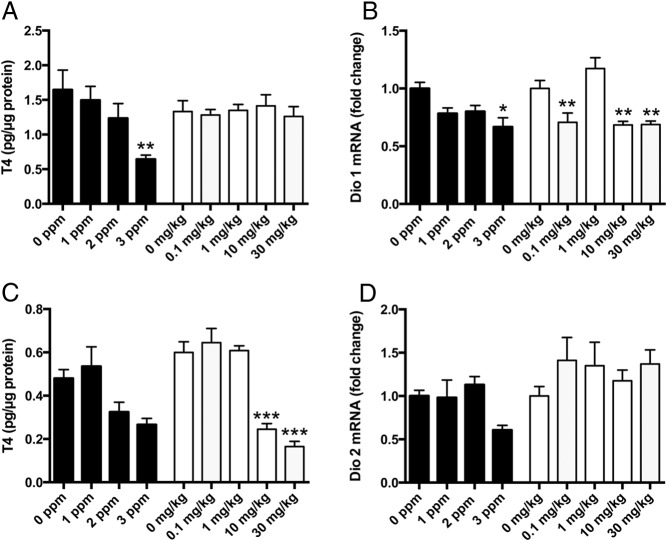
PTU produced a decline in liver T4, but this was not observed in PBDE-treated animals (Figure 5A). In contrast, PBDE- but not PTU-treatment produced a significant decrease in heart T4 levels (Figure 5C). The outer-ring deiodinase expression in the liver (Dio1) was significantly decreased by PTU and by PBDE treatment (Figure 5B), but outer-ring deiodinase expression in heart (Dio2) was not affected by either PTU or PBDE.
Figure 5.
Effects of PTU or PBDE treatment on tissue T4 and deiodinases expression. A, Liver T4 levels were significantly reduced by PTU treatment (F3,26 = 5.09; P < .006, n = 5–9/group) but not by PBDE treatment (F4,27 = 0.18; P = NS, n = 5–8/group). B, PTU exerted a significant effect on T4 levels of the heart (F3,26 = 4.58; P < .01, n = 4–10/group). However, the only significant difference among mean T4 levels was between animals treated with 1 and 3 ppm PTU. In contrast, PBDE treatment very significantly reduced T4 levels in the heart (F4,26 = 26.56; P < .0001, n = 5–8/group). Post hoc analysis revealed that T4 levels in the heart of animals treated with either 10 or 30 mg/kg PBDE were significantly lower than that of control animals. C, Type 1 deiodinase mRNA in the liver was marginally reduced by PTU treatment (F3,24 = 3.38; P < .03, n = 4–9/group) and was limited to animals treated with 3 ppm PTU compared with controls., More robust reductions in D1 mRNA levels were induced by PBDE treatment (F4,27 = 11.01; P < .0001, n = 5–8/group). Post hoc analysis revealed that D1 mRNA levels were significantly lower than control levels in all PBDE-treated animals with the exception of those treated with 1 mg/kg. D, Type 2 deiodinase mRNA was not affected by PTU treatment (F3,25 = 2.69; P = NS, n = 5–9/group) or PBDE treatment (F 4,25 = 1.02; P = NS, n = 4–8/group).
Discussion
We tested the hypothesis that PBDE-induced reductions in serum T4 levels are consistent with canonical downstream effects, ie, up-regulation of TSH due to negative feedback, reductions in target tissue TH concentrations, and subsequent effects on TH-regulated target genes. Our findings demonstrate that this is not the case. The PBDE-induced reduction in both total and free T4 was not associated with an increase in serum TSH [or with TSHβ mRNA in pituitary (data not shown)] or with expected changes in the expression of genes targeted by TH in the liver or heart. Importantly, PBDE reduced the concentration of T4 only in the heart, perhaps indicating that PBDEs can interfere selectively with T4 uptake into tissues. Although we do not fully understand the molecular and cellular events underlying these observations currently, it is clear that the PBDE- and PTU-induced reductions in serum T4 are not physiologically equivalent. The tissue levels of PBDEs observed in this study overlap at the low end with those measured in the human fetal liver (32). Moreover, the concentrations observed in the maternal liver and heart in the present study are somewhat lower than has been reported earlier (27).
Our strategy in this study was to characterize the effect of PBDE on hormone levels first. To show that the PBDE-induced reduction in serum total T4 was sufficient to cause an increase in serum TSH, we compared PBDE treatment with that of PTU. We found that PBDE reduced serum total T4 to a greater extent than did PTU and that PTU caused an increase in serum TSH, whereas PBDE treatment did not. This may have been caused by a difference in the effect of the two chemicals on serum free T4. However, free T4 was similarly reduced by both treatments. Thus, these data suggest that PBDE exposure reduces serum total and free T4 sufficiently to cause an increase in serum TSH but that serum TSH does not rise. An important consideration is whether the analog kit that we used to estimate free T4 underestimated free hormone because PBDEs (and metabolites) can displace T4 from serum binding proteins (33). If this is true, then free T4 may not have been reduced, which would explain why serum TSH did not increase and why liver T4 did not decline. However, this conflicts with the observation that the T4 concentrations declined in the heart in the current study and that PBDEs are pregnane X receptor agonists, inducing enzymes that increase the serum T4 clearance (11, 34). Estimates of free T4 are complicated, and the interpretation is controversial (35).
The next question was whether tissues other than the pituitary gland respond abnormally to PBDE treatment. Our approach to defining the tissue response to low serum T4 was to monitor the expression of TH-regulated mRNAs in those tissues (liver and heart). The strength of this approach is that it takes into consideration all of the ways in which a chemical may alter TH action in tissues, including the effects on the serum hormone level, the selective uptake and metabolism in tissues, and binding to and activating one or both of the TH receptors (TRs) (15). In this way, TH-regulated genes represent an integrated measure of the ability of a chemical to interfere with thyroid hormone action. A weakness in this approach is that TH-regulated genes (eg, S14) are also regulated by other, nonthyroidal pathways and therefore may not provide an unambiguous readout of TH action. This weakness is at least partially ameliorated by the comparison of the effects of PBDE and PTU, by the simultaneous measurement of several TH-regulated genes in two separate tissues, and by the use of several doses of each chemical in a developmental study. It is a potential weakness that the PTU experiment was performed in Long-Evans rats, whereas the PBDE experiment was performed in Sprague Dawley rats. However, PTU blocks the thyroperoxidase enzyme across many species including rodents and humans (36), the molecular end points we captured are regulated by thyroid hormone across species (eg, reference 37), and PBDE treatment in Long-Evans rats produces the same paradoxical effect on the HPT axis as we are showing here (eg, reference 12). Moreover, the value of the PTU limb of the experiment is to provide a direct comparison of the two chemicals in all of our assays because all of the end points measured are known to be regulated by PTU in the manner we replicate here.
A central observation in this study was that PBDE treatment reduced serum total and free T4 but caused an increase in ME expression in the liver instead of the expected decrease. This may reflect that some PBDEs or their metabolites can activate the TR, increasing the expression of TH-regulated genes. There are some reports that hydroxyl-PBDEs can activate the TR (eg, reference 38), so it is possible that this is the mechanism underlying this observation. If so, it is unclear why two other TH-regulated genes in liver (S14 and Mdr1a) and in heart (myHC6 and myHC7) were not similarly affected by PBDE treatment. It is possible that some xenobiotics can interact with the TR in a manner that is dependent on the TR response element sequence as shown by Miyazaki et al (39) or that P450 enzymes present in the liver, but not the heart, metabolize PBDEs to chemical species that can interact with the TR (eg, reference 26).
An alternate hypothesis is that PBDE exposure did not decrease intracellular free T3 in some tissues (ie, liver, heart, pituitary) and therefore did not alter TR activation. If so, it is not clear how both PTU and PBDE exposure can reduce serum free T4, but only PTU reduces intracellular free T3 in tissues. It is possible that PBDE or its metabolites can affect T3 or T4 transporters or deiodinase activity in a way that can account for these observations. If PBDE exposure did not affect intracellular T3 in liver, then ME mRNA levels could have been increased by a nonthyroidal route. This nonthyroidal pathway does not appear to be estrogen mediated because IGFBP, an estrogen-regulated gene in the liver, was not affected by PBDE treatment. We also evaluated measures of TH action in the heart. Because neither ME nor S14 are regulated by TH in heart, we used different but well-known targets of TH action in the heart: myHC6 and myHC7 (40). Our findings in the heart were very similar to those for the liver in that PTU and PBDE exposure produced divergent effects on these two TH-responsive genes.
The mechanism(s) whereby PBDEs can reduce serum TH, but produce variable effects downstream, are not clear. Presumably, PBDEs induce microsomal enzymes that increase T4 clearance, thereby reducing serum T4. But PBDEs also can displace T4 from serum binding proteins (33), which may also account for some of our observations. Perfluorooctanesulfonate also reduces circulating levels of T4 without increasing serum TSH and also can displace T4 from serum binding proteins (41). Chang et al (41) proposed that perfluorooctanesulfonate-induced displacement of T4 from serum binding proteins leads to increased tissue T4 concentrations and a localized increase in TH-regulated gene expression. However, it is not likely that the ability of PBDEs to displace T4 from serum binding proteins can account for the current findings. PBDE exposure not only failed to increase tissue T4 in the liver and heart, but it also caused a significant decrease in the heart T4, yet the expression of only one of three TH-regulated genes evaluated in the liver was altered. Rather, it is more likely that PBDEs (and perhaps other chemicals that demonstrate a similar profile of effects on the thyroid system) can have multiple effects on hormone clearance, tissue uptake, and even receptor activation, producing complex effects on development and adult physiology.
Interestingly, PTU exposure caused a reduction in the liver T4, but PBDE exposure did not; in contrast, both PTU and PBDE exposures reduced T4 levels in the heart. Liver T4 levels declined only in the group treated with 3 ppm PTU, but even 1 ppm PTU produced a significant reduction in the expression of S14 and of Mdr1a in the liver. Therefore, it is likely that tissue T4 represents a very insensitive measure of either serum T4 or TH action in the tissue. However, PBDE exposure clearly decreased T4 levels in heart tissue, but this was not associated with changes in TH-regulated gene expression in the heart. It is unclear whether tissue levels of T3 would have been more informative. Although we did not have sufficient tissues in the current experiment to measure both T4 and T3 (which is much lower in abundance), tissue levels of T3 are not particularly informative of changes in intracellular free T3 (42). Type 1 deiodinase mRNA levels were marginally reduced in the liver by the highest dose of PTU and more robustly by several doses of PBDE. Because Dio1 expression is positively regulated by TH, these data may reflect the measured decreases in serum T4 concentrations for both PTU and PBDE exposures. However, only the highest dose of PTU caused a measurable reduction in liver T4 and PBDE did not reduce liver T4. Thus, there remains a disconnect between changes in serum T4, tissue T4, and downstream gene expression. Furthermore, it is enigmatic that other TH-responsive genes did not respond similarly to PBDE exposure (ie, no change Mdr1a and S14 and dose dependent increases in the expression of ME). In contrast, Dio2 expression in the heart was unaffected by either PBDE or PTU treatment despite reductions in tissue concentrations of T4 in the heart after PBDE exposure.
Overall, these observations that PBDE exposure can reduce serum T4 similarly to the effect of PTU, but that downstream effects are not similar to the effects of PTU, challenge our understanding of the underlying physiology of the thyroid system. PBDEs are believed to reduce serum THs by activating the expression of microsomal enzymes in the liver that cause T4 to be more rapidly cleared from serum (43); in this way, it is analogous to the effects of phenobarbital (44–46). However, although PBDEs can induce the expression of these enzymes (43) and reduce serum T4, the fact that serum TSH is not increased as it is after phenobarbital treatment (47) suggests that PBDEs are not acting in a manner that is completely analogous to that of phenobarbital. Considering these findings, a central conclusion is that serum T4 levels do not necessarily reflect the downstream effects of chemical exposures on the thyroid system. Clearly, additional mechanistic information is required to fully understand the ways in which chemicals may interfere with TH action.
The implications of these findings are significant. First, a general assumption in environmental epidemiology is that relationships between chemical exposures and circulating levels of TH are indicative or even predictive of relationships between chemical exposures and downstream events regulated by TH. Our current results indicate that this assumption may not always be true. This suggestion should motivate the search for sensitive molecular biomarkers of TH action that may help to test that assumption in future studies. In addition, our current results suggest that we may not fully understand the dynamics of the hypothalamic-pituitary-thyroid axis and the ways in which it can be perturbed by chemical exposures. Finally, our current results may provide some insight into why there is so much variability in the results of epidemiological studies of the relationships between chemical exposures (eg, for PCBs) and serum thyroid hormone levels (48). Specifically, chemicals, especially mixtures, may affect the thyroid system at many different points of regulation and create effects that are not easily traced to serum thyroid disruption. Given the importance of thyroid hormone for development and adult health, these issues are important to resolve.
Acknowledgments
DE-71 was kindly provided by Dr Kevin Crofton (US Environmental Protection Agency).
This work was supported in part by US Environmental Protection Agency Science to Achieve Results Grants RD-3213701 (to R.T.Z.) and ES10026 from the National Institute of Environmental Health Sciences (to R.T.Z.).
This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- DE-71
- diphenyl ether
- G
- gestational day
- IGFBP
- IGF binding protein
- Mdr1a
- multidrug-resistant gene
- ME
- malic enzyme
- myHC6
- myosin heavy-chain-α
- myHC7
- myosin heavy-chain-β
- P
- postnatal day
- PBDE
- polybrominated diphenyl ether
- PCB
- polychlorinated biphenyl
- PTU
- propylthiouracil
- S14
- spot 14
- TH
- thyroid hormone
- TR
- TH receptor.
References
- 1. Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs—a review of levels and sources. Int J Hyg Environ Health. 2009;212(2):109–134 [DOI] [PubMed] [Google Scholar]
- 2. Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments—a review on occurrence and human exposure. Environ Pollut. 2012;169:217–229 [DOI] [PubMed] [Google Scholar]
- 3. Schecter A, Haffner D, Colacino J, et al. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite US food samples. Environ Health Perspect. 2010;118(3):357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45(18):7896–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gascon M1, Fort M, Martínez D, et al. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect. 2012;120(12):1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rice DC, Reeve EA, Herlihy A, et al. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29(4):511–520 [DOI] [PubMed] [Google Scholar]
- 7. Eskenazi B, Chevrier J, Rauch SA, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS Study. Environ Health Perspect. 2013;121(2):257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herbstman JB, Sjodin A, Kurzon M, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–248 [DOI] [PubMed] [Google Scholar]
- 10. Zoeller RT. Environmental chemicals targeting thyroid. Hormones (Athens). 2010;9(1):28–40 [DOI] [PubMed] [Google Scholar]
- 11. Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107(1):27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78(1):144–155 [DOI] [PubMed] [Google Scholar]
- 13. Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116(12):1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herbstman JB, Sjodin A, Apelberg BJ, et al. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect. 2008;116(10):1376–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37(1):11–53 [DOI] [PubMed] [Google Scholar]
- 16. Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Perspect. 2008;116(12):1694–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halpern R, Cooper DS, Kieffer JD, et al. Propylthiouracil (PTU) pharmacology in the rat. I. Serum and thyroid PTU measurements by radioimmunoassay. Endocrinology. 1983;113(3):915–920 [DOI] [PubMed] [Google Scholar]
- 18. Cooper DS, Kieffer JD, Halpern R, et al. Propylthiouracil (PTU) pharmacology in the rat. II. Effects of PTU on thyroid function. Endocrinology. 1983;113(3):921–928 [DOI] [PubMed] [Google Scholar]
- 19. Gilbert ME. Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol Sci. 2011;124(2):432–445 [DOI] [PubMed] [Google Scholar]
- 20. Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol. 2010;22(3):153–165 [DOI] [PubMed] [Google Scholar]
- 21. Royland JE, Parker JS, Gilbert ME. A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol. 2008;20(12):1319–1338 [DOI] [PubMed] [Google Scholar]
- 22. Hood A, Klaassen CD. Differential effects of microsomal enzyme inducers on in vitro thyroxine [T(4)] and triiodothyronine [T(3)] glucuronidation. Toxicol Sci. 2000;55(1):78–84 [DOI] [PubMed] [Google Scholar]
- 23. Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology. 2008;149(5):2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect. 2007;115(11):1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thibodeaux JR, Hanson RG, Rogers JM, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci. 2003;74(2):369–381 [DOI] [PubMed] [Google Scholar]
- 26. Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152(7):2909–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bondy GS, Gaertner D, Cherry W, et al. Brominated diphenyl ether (BDE) levels in liver, adipose, and milk from adult and juvenile rats exposed by gavage to the DE-71 technical mixture. Environ Toxicol. 2011;26(6):677–690 [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-δδC(T)] method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 29. Dong H, Yauk CL, Williams A, Lee A, Douglas GR, Wade MG. Hepatic gene expression changes in hypothyroid juvenile mice: characterization of a novel negative thyroid-responsive element. Endocrinology. 2007;148(8):3932–3940 [DOI] [PubMed] [Google Scholar]
- 30. Czyzewska U, Tylicki A, Siemieniuk M, Strumilo S. Changes of activity and kinetics of certain liver and heart enzymes of hypothyroid and T(3)-treated rats. J Physiol Biochem. 2012;68(3):345–351 [DOI] [PubMed] [Google Scholar]
- 31. Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15(2):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from Greater Montreal, QC: a longitudinal study from 1998 through 2006. Environ Health Perspect. 2009;117(4):605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ren XM, Guo LH. Assessment of the binding of hydroxylated polybrominated diphenyl ethers to thyroid hormone transport proteins using a site-specific fluorescence probe. Environ Sci Technol. 2012;46(8):4633–4640 [DOI] [PubMed] [Google Scholar]
- 34. Pacyniak EK, Cheng X, Cunningham ML, Crofton K, Klaassen CD, Guo GL. The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicol Sci. 2007;97(1):94–102 [DOI] [PubMed] [Google Scholar]
- 35. Thienpont LM, Van Uytfanghe K, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Pract Res Clin Endocrinol Metab. 2013;27(5):689–700 [DOI] [PubMed] [Google Scholar]
- 36. Paul KB, Hedge JM, Macherla C, et al. Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology. 2013;312:97–107 [DOI] [PubMed] [Google Scholar]
- 37. Campbell MC, Anderson GW, Mariash CN. Human spot 14 glucose and thyroid hormone response: characterization and thyroid hormone response element identification. Endocrinology. 2003;144(12):5242–5248 [DOI] [PubMed] [Google Scholar]
- 38. Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro. 2011;25(1):257–266 [DOI] [PubMed] [Google Scholar]
- 39. Miyazaki W, Iwasaki T, Takeshita A, Kuroda Y, Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism. J Biol Chem. 2004;279(18):18195–18202 [DOI] [PubMed] [Google Scholar]
- 40. Dillmann WH. Cellular action of thyroid hormone on the heart. Thyroid. 2002;12(6):447–452 [DOI] [PubMed] [Google Scholar]
- 41. Chang SC, Thibodeaux JR, Eastvold ML, et al. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology. 2008;243(3):330–339 [DOI] [PubMed] [Google Scholar]
- 42. Quignodon L, Legrand C, Allioli N, et al. Thyroid hormone signaling is highly heterogeneous during pre- and postnatal brain development. J Mol Endocrinol. 2004;33(2):467–476 [DOI] [PubMed] [Google Scholar]
- 43. Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008;226(3):244–250 [DOI] [PubMed] [Google Scholar]
- 44. Schwartz HL, Bernstein G, Oppenheimer JH. Effect of phenobarbital administration on the subcellular distribution of 125-I-thyroxine in rat liver: mportance of microsomal binding. Endocrinology. 1969;84(2):270–276 [DOI] [PubMed] [Google Scholar]
- 45. Oppenheimer JH, Bernstein G, Surks MI. Increased thyroxine turnover and thyroidal function after stimulation of hepatocellular binding of thyroxine by phenobarbital. J Clin Invest. 1968;47(6):1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernstein G, Artz SA, Hasen J, Oppenheimer JH. Hepatic accumulation of 125I-thyroxine in the rat: augmentation by phenobarbital and chlordane. Endocrinology. 1968;82(2):406–409 [DOI] [PubMed] [Google Scholar]
- 47. Klaassen CD, Hood AM. Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol Pathol. 2001;29(1):34–40 [DOI] [PubMed] [Google Scholar]
- 48. Salay E, Garabrant D. Polychlorinated biphenyls and thyroid hormones in adults: a systematic review appraisal of epidemiological studies. Chemosphere. 2009;74(11):1413–1419 [DOI] [PubMed] [Google Scholar]