Abstract
The three iodothyronine deiodinases (D1, D2, and D3) play major roles in determining the tissue and cellular content of the active thyroid hormone, T3. The D1 and D2 5′-deiodinate T4 to T3 and the D3 5-deiodinates T4 and T3 to inactive forms. 5′-Deiodinase-deficient mice (D1/D2KO) have a mild gross phenotype, whereas D3-deficient mice (D3KO) exhibit significant phenotypic abnormalities of the hypothalamic/pituitary/thyroid axis and other organ systems and are not viable in some background strains. The goal of this study was to perform an initial assessment of the phenotype of mice devoid of all deiodinases (D1/D2/D3KO) and determine whether the marked phenotypic abnormalities of the D3KO mouse are exacerbated or mitigated by the absence of the D1 and D2. Relative to D3KO mutants, survival, growth, and fertility were improved in the D1/D2/D3KO mice, although considerably impaired relative to wild-type and D1/D2KO animals. The triple deiodinase-deficient mice also demonstrated normal brain T3 content at postnatal day 6, normal cerebellar expression of the T3-responsive gene hairless at postnatal day 21, and near normalization of their serum thyroid hormone levels as adults, parameters that are abnormal in either the D3KO or the D1/D2KO mutants. These studies demonstrate that within the supportive environment of a research vivarium, mice lacking all three deiodinases can be bred and survive and that at least some of the phenotypic abnormalities resulting from a deficiency of either the D3 5-deiodinase, or the D1 and D2 5′-deiodinase, are mitigated by the simultaneous lack of all three enzymes.
There is considerable evidence to support the concept that the three iodothyronine deiodinases (D1, D2, and D3) play major roles in determining the content of the active thyroid hormone (TH), T3, in TH-responsive cells (1). The D1, which is highly expressed in liver, kidney, and thyroid, has both 5- and 5′-deiodinase activity. Through its ability to convert T4 to T3, the D1 contributes to the plasma and hence tissue T3 contents (2). In addition, both catalytic activities play a more general role in metabolizing lesser iodothyronines and reclaiming iodide into the circulation (3). The D2, an exclusively 5′-deiodinase that is expressed notably in brain, pituitary, and brown adipose tissue, also contributes to the plasma T3 content (2, 4) and has long been known to generate T3 primarily for local use, and this has been well substantiated by studies in the D2-deficient mouse (D2KO) (5–7). Indeed, in the absence of the D2 the brain T3 content is reduced by as much as 50% (8, 9), and some T3-dependent gene expression patterns are disrupted (8–10). Nevertheless, mice deficient in either or both the D1 and the D2 have a normal serum T3 level attesting to the ability of the hypothalamic/pituitary/thyroid (HPT) axis, in particular the T3-secreting ability of the thyroid gland, to compensate for a complete deficiency of the 5′-deiodinases (9, 11)
On the other hand, the D3, which is exclusively a 5-deiodinase, degrades both T4 and T3 to their inactive metabolites, rT3 and 3,3′-diiodothyronine, respectively. Studies in the D3-deficient mouse (D3KO) strongly suggest that this deiodinase is essential for maintaining appropriate levels of TH in the brain during development (12–14). The importance of both the D2 and the D3 in this regard is well illustrated by the finding that the differentiation of the cochlea is retarded or accelerated, respectively, in D2KO and D3KO mice, and in both genotypes these changes are associated with impaired hearing (6, 15).
In view of the roles the deiodinases are thought to play in TH homeostasis, it was surprising to find that, under standard laboratory conditions, mice lacking one or both of the 5′-deiodinases have only mildly abnormal phenotypes. D1-deficient (D1KO), D2KO, and D1/D2KO mice, all in the C57BL/6 background, grow normally, reproduce, and raise their young appropriately and neurobehavioral changes appear to be minimal (5, 8, 9, 16). In contrast, D3 deficiency results in a mouse that exhibits hyperthyroidism as a neonate, as indicated by a marked elevation in the serum T3 level and brain T3 content during the first few days of life, followed by central hypothyroidism as an adult. In fact, D3 deficiency has proved to be lethal to the neonate in the C57BL/6 strain. The fertility of D3KO mice is severely impaired in several background strains, but viable D3KO mice can be obtained by breeding heterozygous mice in either the 129/Sv strain or a mixed strain such as the CD1. However, even then there is considerable neonatal loss (12, 13).
This study had two goals. First, in view of the important roles the three deiodinases play in controlling intracellular levels of TH, it was of considerable interest to assess the phenotype of a mouse devoid of all deiodinase activity. Second, given the severity of the phenotype of the D3KO, which lacks the ability to inactivate TH, it was of interest to determine whether elimination also of the 5′-deiodinase activating enzymes would mitigate or exacerbate the phenotypic characteristics of the D3KO, including survival, fertility, the impaired HPT axis and altered circulating and intracellular TH levels.
Materials and Methods
Animals
Wild-type (WT), D2KO, double-D1, and D2-deficient (D1/D2KO) mice, mice heterozygous for the inactivated Dio3 gene (D3+/−), double-D2 and D3-deficient (D2/D3KO) mice, and triple-D1, D2-, and D3-deficient (D1/D2/D3KO) mice, all in the C57/BL6 background, were used in this study. The WT, D2KO, D1/D2KO, and D3+/− mice came from our established colonies (5, 9, 17). The D1/D2/D3KO mice were obtained by cross-breeding D1/D2KO mice with D3+/− mice. D2KO/D3+/− mice were obtained by cross-breeding D2KO mice with the D3+/− mice. From breeding pairs of these latter mice, 12 D2/D3KO were obtained. Mice were housed at 22 ± 1°C with controlled lighting (12 h light, 12 h dark), in the barrier section of Dartmouth Medical School's animal research facility. Animal protocols were approved by the Institutional Animal Care and Use Committee of Dartmouth Medical School.
Tissue preparation
For the study in the D1/D2/D3KO mice at postnatal day (P) 21 and P50, only male mice were used. The mice were euthanized with CO2, exsanguinated via the vena cava, and the serum frozen at −80°C for subsequent analyses. The brain was removed, the cerebellum (Cb) separated, and both the Cb and the remaining part of the brain (cerebrum) were frozen at −80 C. The liver and kidneys from the P50 mice were also removed and frozen.
For studies in the D1/D2/D3KO mice at P6, the entire litter and hence both sexes were used. The mice were decapitated, trunk blood was collected, and the serum from two to three mice was pooled for the determination of the T4 level. The brain was removed and dissected into the following areas: cerebral cortex, hypothalamus, Cb, thalamus/hippocampus/striatum (T/H/S), and corpus colliculus/midbrain/pons/medulla (C/M/P/M), and frozen at −80°C for subsequent analyses.
Serum was obtained from adult male D2/D3KO mice as described above
T4 and T3 were extracted from liver, kidneys, and brain areas with 95% methanol containing 0.1 mM propylthiouracil as previously described (18). To release TH from their glucuronide conjugates in the kidney and liver (18, 19), the dried extracts were resuspended in 1 mL of 75 mM sodium phosphate buffer (pH 6.5) and incubated with 100 U of β-glucuronidase (Sigma; type 1X-A from Escherichia coli, sulfatase free) at 37°C for 1 hour. The mixture was then evaporated to dryness.
Total RNA was isolated from the Cb using a commercial RNA isolation reagent (TRIzol solution; Invitrogen), according to the manufacturer's instructions.
Assays for T4 and T3 in serum and tissue samples
Total T4 and T3 concentrations in serum were determined using the Coat-A-Count RIA total T4 and total T3 kits (Diagnostic Systems Laboratories, Inc). The T3 assay was modified for use with mouse serum as previously described (20).
Serum rT3 concentration was measured using our highly sensitive nonequilibrium RIA assay procedures (21). The rT3 antibody was a gift from L. Braverman (22), and ultrapure unlabeled rT3 (Henning Co) was used as the standard. The assay sensitivity was approximately 2 pg/tube.
Mouse serum TSH levels were determined using a highly sensitive double-antibody method, developed by A. F. Parlow, as previously described (5).
The contents of T3 and T4 in brain tissue were determined using the nonequilibrium RIA procedures (21). The T4 and T3 antibodies were obtained from Fitzgerald Industries International, Inc (T3 Ab: catalog number 20-TR45, cross-reactivity with T4 < 0.38%; T4 Ab: catalog number 20-TR40, cross-reactivity with T3 < 7.5%). The assay sensitivity was approximately 2 pg/tube for T3 and 4 pg/tube for T4.
Details of the antibodies are given in Table 1.
Table 1.
Antibodies Used in These Studies
| Protein Target | Antigen Sequence | Antibody Name | Source | Species Raised in | Dilution Used |
|---|---|---|---|---|---|
| T3-BSA | Unknown | Rabbit anti-3,5,3′-triiodothyronine | Fitzgerald Industries International, Inc. Catalog # 20-TR45 | Rabbit polyclonal | 1:30,000 |
| T4-BSA | Unknown | Rabbit anti thyroxine | Fitzgerald Industries International, Inc. Catalog # 20-TR40 | Rabbit polyclonal | 1:7500 |
| rT3-BSA | Unknown | rT3 Ab | Dr L Braverman (gift) | Rabbit circa 1974 | 1:8000 |
Analysis of mRNA levels by real-time PCR
The RNA samples were subjected to deoxyribonuclease treatment, reverse transcription, and real-time PCR using the kits, primers, and methods used in a previous study (20).
Statistical analysis
Statistical significance of the differences in values obtained between two groups was determined by the two-tailed Student's t test. Comparisons among more than two groups were performed by a one-way ANOVA followed by the Fisher's least significant difference test.
Results
Fertility
As previously reported, litter frequency, size, survival and average pup weight in D1KO, D2KO, and D1/D2KO and WT mice are comparable (5, 9, 16). A few breeding pairs of D3+/− mice in the C57BL/6 background have been maintained for several years and only rarely has a D3KO pup survived the perinatal stage and then only to die prior to weaning (12). However, survival of the D3KO is greatly improved when it is in a mixed background, although fertility is still impaired (12, 13). In the present study, by cross-breeding D1/D2KO mice with D3+/− mice, both mutants in the C57BL/6 background, D1/D2/D3KO mice in this background were obtained. Twenty-seven breeding pairs of these D1/D2/D3KO mice were set up over a period of 40 months. Pairs were maintained only until they were 20 weeks old if they did not produce a litter. Pairs that were fertile were maintained for 3 months after their last litter. In contrast to findings with WT, D1KO, D2KO, and D1/D2KO mice, all of which generally produce litters containing on average seven to eight mice, every 3–4 weeks, litter production by the D1/D2/D3KO mice was sporadic and the number of mice in the litter was highly variable. Many litters did not survive the perinatal period, and substantial additional loss occurred prior to weaning. Of the 27 breeding pairs used, five were infertile. The remaining 22 pairs gave birth to 75 litters. All the pups in 20 of these litters died prior to P2. Fifty-five litters yielded 125 pups at weaning for an average of 2.6 ± 0.36 pups per litter. Although it was not possible to obtain an accurate count of the litter size at birth because the dams often consume their dead offspring, it was observed that it was considerably greater than at weaning. Nevertheless, in contrast to the D3KO mouse in the C57BL/6 background, some D1/D2/D3KO mice survived to adulthood and most of them proved to be fertile. Indeed in the mice that did survive past weaning, mortality was not a significant problem; more than 95% of these mice lived until they were used in experiments.
Growth
Growth was impaired in D1/D2/D3KO mice. Although by 16 weeks their weight was within 90% of that of age-matched WT mice, the difference was much greater in younger mice: at 7 and 11 weeks, D1/D2/D3KO mice weighed approximately 57% and 78% of the weight of age-matched WT mice (Figure 1).
Figure 1.
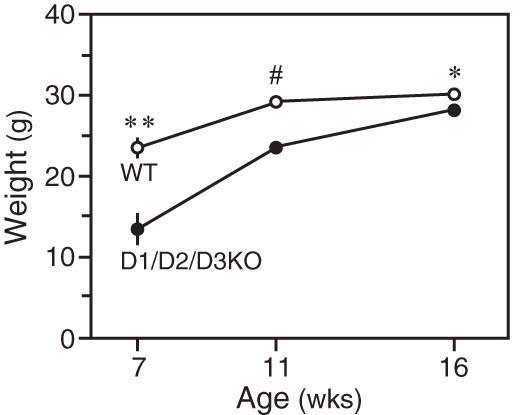
Body weights of WT and D1/D2/D3KO mice at 7 weeks (four mice/group), 11 weeks (five mice/group), and 16 weeks (six mice/group) of age. *, P < .05; **, P < .01; #, P < .001.
Serum hormone levels
Serum T4, T3, and TSH levels in D1/D2KO, D1/D2/D3KO, and D3KO mice expressed as a percentage of that in WT mice are shown in Figure 2. Because the D3−/− mutation is lethal in the C57BL/6 background, our previously published data (12) from D3KO mice in a 129/Sv background are included for the sake of comparison. Although serum TH levels can differ among strains, no significant differences were observed between WT (129/Sv) and WT (C57BL/6) mice (Hernandez, A., unpublished data). The T4 level in the serum of D1/D2/D3KO mice at 21, 35, and 50 days is significantly higher than that in WT mice but lower than that in D1/D2KO mice; at 21 and 50 days, this latter difference is statistically significant. In contrast, the serum T4 level in the D3KO is decreased by more than 95% in weanlings and by 73% in adults. Compared with WT mice, the serum T3 level in the D1/D2/D3KO mice at 21 days is reduced by 25%. However, this decrease is less marked than the 50% reduction observed in the D3KO mice, and by 35 days, the serum T3 level in the D1/D2/D3KO mice is not significantly different from that in WT and D1/D2KO mice. In the D3KO mice at 50 days, the serum T3 level is still reduced, although the reduction is less marked that than seen at 21 days. A comparable increase in the serum TSH level was evident at 35 days and 50 days in both the D3KO and D1/D2/D3KO mice (Figure 2). No rT3 was detected in the serum of the D1/D2/D3KO mice (data not shown), confirming that secretion of rT3 by the thyroid gland is minimal in this species, and thus, rT3 is produced exclusively by the deiodination of T4.
Figure 2.
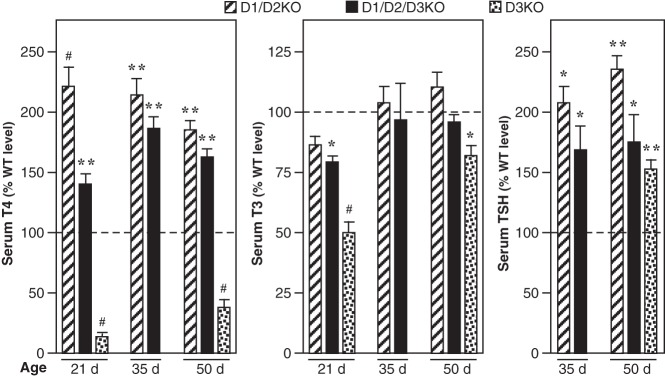
Serum T4, T3, and TSH levels in D1/D2KO, D1/D2/D3KO, and D3KO mice at 21 or 35 and 50 days of age. The D3KO data are included for the purpose of comparison and have been published previously (12). The D3K0 mice are in the 129/Sv background. Values obtained in the mutant mice are expressed as a percentage of the mean value obtained in the corresponding WT mice. Bars represent the mean ± SEM of results obtained in at least four mice/group at P21 and at least eight mice/group at P35 and P50. Statistically significant differences from the WT mice are indicated: *, P < .05; **, P < .01; #, P < .001.
In Figure 3, the serum TH levels in 12 adult double-D2/D3KO (C57BL/6) mice are compared with those in WT (C57BL/6 and 129/Sv) and D3KO (129/Sv) mice. In the D2/D3KO mice, the serum T3 level is maintained, and the serum T4 level, although significantly reduced, is much closer to that in WT mice than that in the D3KO mice.
Figure 3.
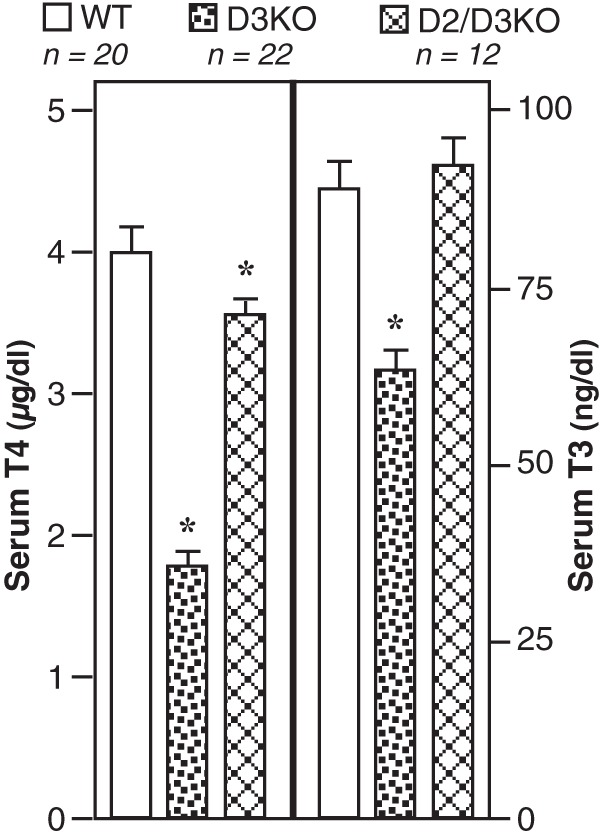
Serum T4 and T3 levels in WT (C57BL/6 and 129/Sv), D3KO (129/Sv), and D2/D3KO (C57BL/6) mice. Bars represent the mean ± SEM. *, Value in mutant mice is significantly different from that in WT mice (P < .01). NB, no significant differences were observed between values obtained in WT(129/Sv) and WR(C57BL/6) mice.
TH contents in liver and kidney
The T4 content, expressed as nanograms per gram of wet weight, was significantly increased in liver but not in the kidney of the adult D1/D2/D3KO mice [WT vs D1/D2/D3KO, liver: 15.0 ± 3.0 vs 33 ± 3.4 (P < .005); kidney: 149 ± 12 vs 185 ± 23 (P = ns)]. No significant differences in the T3 content (nanograms per gram of wet weight) in the liver and kidney of WT and D1/D2/D3KO mice were observed [WT vs D1/D2/D3KO, liver: 19.2 ± 4.3 vs 15.2 ± 3.1 (P = ns); kidney: 26.1 ± 9.9 vs 16.4 ± 2.7 (P = ns)].
Studies in brain
The T4 and T3 contents of cerebrum from WT, D1/D2KO, and D1/D2/D3KO mice are shown in Figure 4. The T4 content of the cerebrum in the D1/D2KO and D1/D2/D3KO mice was much higher than that in WT mice at both ages. The increase was between 2- and 3-fold and was comparable in both genotypes. In contrast, cerebrum T3 content was reduced by approximately 50% in the D1/D2KO mice at both 21 and 50 days. However, when the D3 was also absent, the cerebrum T3 content was not reduced at 21 days and only by 16% at 50 days (P < .05).
Figure 4.
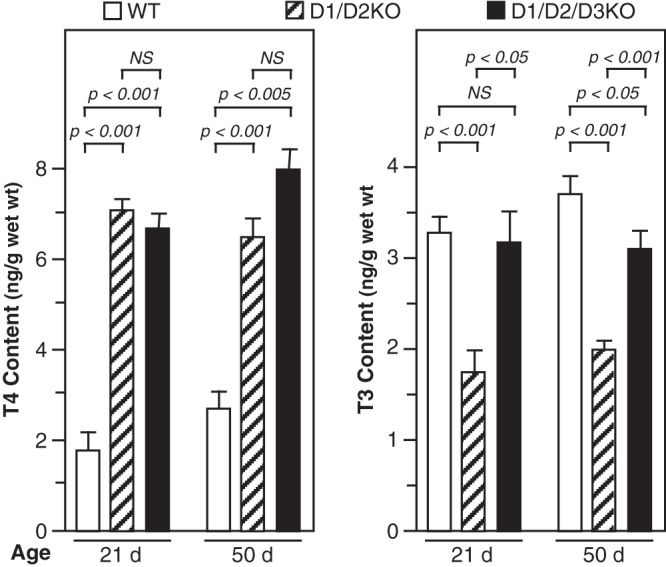
T4 and T3 content in cerebrum of WT, D1/D2KO, and D1/D2/D3KO mice at 21 and 50 days of age. Bars represent the mean ± SEM of results obtained in at least four mice.
Previous studies have shown that in the D1/D2KO mouse, the level of expression of the TH-responsive neuronal gene, Hairless (Hr), in the Cb is significantly reduced at P15 (9). In the present study, it was significantly reduced in this mutant in the Cb at P21. In contrast, it was not reduced in the D1/D2/D3KO. The ratio of Hr RNA to cyclophilin RNA in WT, D1/D2KO, and D1/D2/D3KO was 7.4 ± 0.37, 5.8 ± 0.68, and 6.9 ± 1.01, respectively (WT vs D1/D2KO, P < .05, WT vs D1/D2/D3KO, P = ns).
To determine whether the thyrotoxicity that occurs during the first week postpartum in the D3KO mice (12, 13) is modified when the ability to generate T3 from T4 in the brain and other organs is eliminated, the T4 and T3 contents of several brain areas of WT, D1/D2KO, and D1/D2/D3KO mice were compared in mice at P6. Mean T4 content was slightly higher in all brain areas from D1/D2KO mice vs WT mice, but only in the Cb was the difference significant (Figure 5). In the D1/D2/D3KO mice, the T4 content was substantially increased in all brain areas except the combined C/M/P/M, and compared with that in the corresponding WT brain areas the increases were significant. The differences in the T4 content between the D1/D2KO and the D1/D2/D3KO mice in any of the brain areas did not reach statistical significance.
Figure 5.
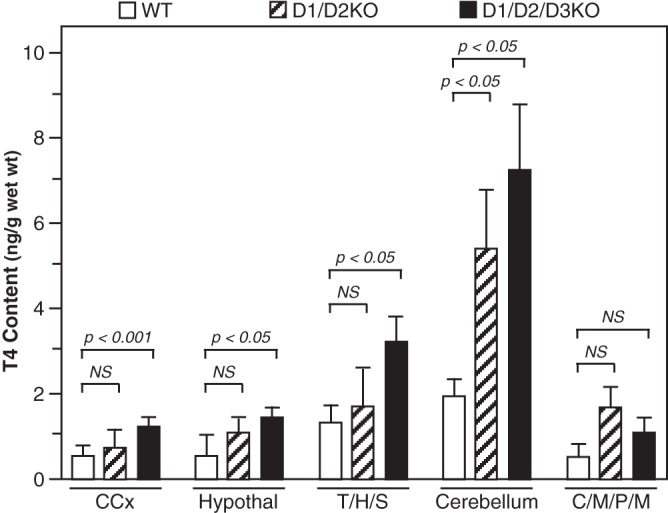
T4 content in brain regions of WT, D1/D2KO, and D1/D2/D3KO mice at P6. Bars represent the mean ± SEM of results obtained in tissues from 11 WT, nine D1/D2KO, and 12 D1/D2/D3KO mice. CCx, cerebral cortex.
In the brain, T3 content at P6 was comparable in all three genotypes across all brain areas except the cerebral cortex. In this area the T3 content was significantly reduced in the D1/D2KO (Figure 6). This reduction was not seen when the mice were also deficient in the D3.
Figure 6.
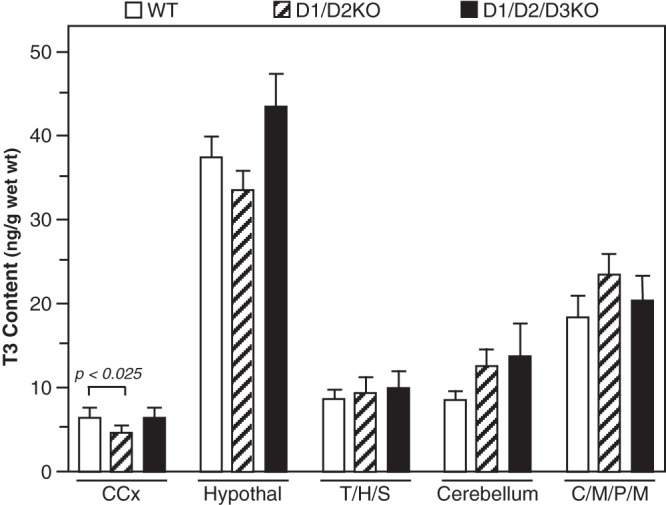
T3 content in the brain regions of WT, D1/D2KO, and D1/D2/D3KO mice at P6. Bars represent the mean ± SEM of results obtained in tissues from 11 WT, nine D1/D2KO, and 12 D1/D2/D3KO mice. CCx, cerebral cortex.
In these P6 mice, the T4 level was determined in four to six pools of serum. The levels in WT, D1/D2KO, and D1/D2/D3KO mice were, respectively, 0.8 ± 0.10, 3.0 ± 1.34, and 3.4 ± 0.52 ng per 100mL (WT vs D1/D2KO and D1/D2/D3KO, P < .001). Insufficient serum precluded determination of the T3 level.
Discussion
These studies indicate that some C57BL/6 mice completely devoid of all iodothyronine deiodinases can survive; can reach within 90% of the weight of a WT mouse; and can conceive, deliver, and raise their young. This phenotype contrasts sharply with the complete lack of survival of the D3KO mouse in this same background strain and with the infertility and marked growth retardation of this mutant in other strains. Furthermore, compared with WT mice, the T3 level was normal or only slightly reduced in serum, normal in brain at P6 and P21, and normal in the adult kidney and liver. Despite these generally normal T3 levels, the phenotype of the D1/D2/D3KO mouse was far from normal. Unlike the D1/D2KO mouse, in which survival, growth, and fertility are no different from WT animals, these functions are significantly impaired in the D1/D2/D3KO mouse. Some of these mice are infertile and when litters are produced, litter size is low and survival is poor. The survival and growth issues occur primarily in the embryonic and neonatal period because D1/D2/D3KO mice that live to P21 and are weaned almost always survive, largely catch up in weight, and appear healthy. Furthermore, survival also occurs in the D2/D3KO mouse in which D1 activity is still present, suggesting that it is the elimination of locally generated T3 from T4 by the D2 in specific tissues that is a mitigating factor.
As previously reported, D3KO mice in the 129/Sv or mixed background strains do generally survive. They exhibit hyperthyroidism during the first few days of life, as indicated by a marked elevation in the serum T3 level and increased brain T3 content and T3-dependent gene expression (12, 13). This is followed by central hypothyroidism starting at approximately P15 (12, 14). These findings provide strong evidence that the D3 plays a critical role in the developing brain by preventing the intracellular T3 content from becoming inappropriately elevated during the fetal and early neonatal period. The other enzyme involved in regulating brain T3 content is the D2. It has been estimated that at least 50% of the T3 in the brain of rodents is generated locally by the D2 (23), and in the D2KO mouse, brain T3 content in neonates at P15 is reduced approximately 50% (20). A comparable reduction is seen also in the D1/D2KO mouse at P15 (9). In view of this, we predicted that the elevated brain T3 content noted in the neonatal D3KO mouse would be attenuated if D2 activity, which is increased in this mutant (12), were also absent, thus resulting in a milder phenotype. This has proved to be the case.
At P6, the T3 content was not increased significantly in any of the brain areas of the D1/D2/D3KO mouse. In fact, the values obtained were not significantly different from those in WT mice. In contrast, the brain T3 content in the D3KO mouse is markedly elevated during the first few days of life (12). The D3 also appears to play a role in controlling brain T3 content in older mice. In confirmation of earlier studies (9), cerebrum T3 content was substantially reduced in the D1/D2KO mice, as was also the expression in the Cb of the T3-dependent gene, Hr. However, when the D3 is also absent, and thus the ability to 5-deiodinate the T3 was removed, the T3 content was either comparable with, or only slightly less than, that in WT mice, and Hr expression in the Cb was not significantly reduced. These findings indicate that a deficiency of 5-deiodinating ability may offset (or rescue) some of the metabolic and phenotypic abnormalities resulting from the lack of 5′-deiodinase processes, and vice versa.
Several caveats, however, limit the application of this concept. First, the extent of molecular and phenotypic assessment of the deiodinase-devoid animals is limited to date. Given differences in both spatial and temporal expression of these enzymes during development and in adulthood, there are likely to be instances in which combined deiodinase deficiencies exacerbate phenotypic abnormalities. In this regard, cochlear development, known to be dependent on a tightly orchestrated pattern of first D3 and later D2 expression in this organ (15), may be severely disrupted by an inability to control the local levels of T3 needed for coordinated patterns of gene expression.
Second, our determinations of T3 content of organs represent average measurements that encompass multiple cell types. This is particularly problematic in the brain where D2 and D3 are expressed in different cell types (D2 predominantly in astroglial cells, D3 in neurons) and in different regions. Thus, deficiencies of these enzymes may have different effects on TH content and action in different cells and brain regions. Notably, however, this was not the case for Hr in the Cb of 21-day-old mice where the expression in the D1/D2/D3 mice was comparable with that in WT animals.
Finally, our initial phenotyping has been conducted on mice housed under standard laboratory conditions of temperature, nutrition, and protection from predatory encounters. Of particular interest will be the response of these animals to alterations in iodine supply, thyroid status, and environmental stress. As in the case of the D2KO mouse that has impaired thermogenesis and hearing, the consequences of total deiodinase deficiency are likely to be severe in terms of survival in natural settings.
In summary, the phenotypic abnormalities noted in the D1/D2/D3KO mouse reinforce the notion that TH metabolism in individual cells and tissues represents an important control point with regard to TH action and that indeed such local control of TH levels is required for optimal metabolic effects of these hormones. However, our observation that many D1/D2/D3KO mice survive, grow, reproduce, and have little gross neurological phenotypic abnormalities leads to the inescapable conclusion that the central HPT axis possesses an intrinsic set point that alone is able to dictate the circulating levels of TH sufficient to assist in directing and regulating major developmental and metabolic processes across most, if not all, organ systems. Nevertheless, the optimal regulation of TH-dependent processes requires the coordination of central synthetic and regulatory processes with local homeostatic mechanisms that allow for titration of TH action at the level of individual cells.
Acknowledgments
This work was supported by National Institutes of Health Grants 5RO1DK79946 and 5R21HD072526.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Cb
- cerebellum
- C/M/P/M
- corpus colliculus/midbrain/pons/medulla
- D1
- deiodinase 1
- D2
- deiodinase 2
- D3
- deiodinase 3
- D1KO
- D1-deficient mouse
- D2KO
- D2-deficient mouse
- D3KO
- D3-deficient mouse
- HPT
- hypothalamic/pituitary/thyroid
- P
- postnatal day
- TH
- thyroid hormone
- T/H/S
- thalamus/hippocampus/striatum.
References
- 1. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen TT, Chapa F, DiStefano JJ., III Direct measurement of the contributions of type I and type II 5′-deiodinases to whole body steady state 3,5,3′-triiodothyronine production from thyroxine in the rat. Endocrinology. 1998;139:4626–4633 [DOI] [PubMed] [Google Scholar]
- 3. St Germain DL, Galton VA, Hernandez A. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150(3):1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. 2005;115(9):2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15(12):2137–2148 [DOI] [PubMed] [Google Scholar]
- 6. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3473–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108(9):1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galton V, Wood E, St Germain EA, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148:3080–3088 [DOI] [PubMed] [Google Scholar]
- 9. Galton VA, Schneider M, Clark AS, St Germain DL. Life without T4 to T3 conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morte B, Ceballos A, Diez D, et al. Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in Mct8 and D2 deficient mice. Endocrinology. 2010;151:2381–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez A, Martinez ME, Croteau W, St Germain DL. Complex organization and structure of sense and antisense transcripts expressed from the DIO3 gene imprinted locus. Genomics. 2004;83(3):413–424 [DOI] [PubMed] [Google Scholar]
- 12. Hernandez A, Martinez E, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christoffolete MA, Arrojo e Drigo R, Gazoni F, et al. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology. 2007;148:954–960 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151:5550–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150:1952–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider MJ, Fiering SN, Thai B, et al. Targeted disruption of the type1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589 [DOI] [PubMed] [Google Scholar]
- 17. Hernandez A, Fiering S, Martinez E, Galton VA, St Germain DL. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology. 2002;143(11):4483–4486 [DOI] [PubMed] [Google Scholar]
- 18. Buitendijk M, Galton VA. Is the kidney a major storage site for thyroxine as thyroxine glucuronide? Thyroid. 2012;22(2):187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galton VA, Pitt-Rivers R. Thyroid hormone metabolism in the kidney. Biochem J. 1959;72:314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galton VA, Wood ET, St Germain EA, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient brain during development. Endocrinology. 2007(148):3080–3088 [DOI] [PubMed] [Google Scholar]
- 21. St Germain DL, Galton VA. Comparative study of pituitary-thyroid hormone economy in fasting and hypothyroid rats. J Clin Invest. 1985;75:679–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roti E, Gnudi A, Braverman LE, et al. Human cord blood concentrations of thyrotropin, thyroglobulin, and iodothyronines after maternal administration of thyrotropin-releasing hormone. J Clin Endo Metab. 1981;53:813–817 [DOI] [PubMed] [Google Scholar]
- 23. Crantz FR, Silva JE, Larsen PR. An analysis of the sources and quantity of 3,5,3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982;110:367–375 [DOI] [PubMed] [Google Scholar]


