Abstract
Monocarboxylate transporter 8 (MCT8) deficiency causes severe X-linked intellectual and neuropsychological impairment associated with abnormal thyroid function tests (TFTs) producing thyroid hormone (TH) deprivation in brain and excess in peripheral tissues. The TH analog diiodothyropropionic acid (DITPA) corrected the TFTs abnormalities and hypermetabolism of MCT8-deficient children but did not improve the neurological phenotype. The latter result was attributed to the late initiation of treatment. Therefore, we gave DITPA to pregnant mice carrying Mct8-deficient embryos to determine whether DITPA, when given prenatally, crosses the placenta and affects the serum TFTs and cerebral cortex of embryos. After depletion of the endogenous TH, Mct8-heterozygous pregnant dams carrying both wild-type (Wt) and Mct8-deficient (Mct8KO) male embryos were given DITPA. Effects were compared with those treated with levothyroxine (L-T4). With DITPA treatment, serum DITPA concentration was not different in the two genotypes, which produced equal effect on serum TSH levels in both groups of pups. In contrast, with L-T4 treatment, TSH did not normalize in Mct8KO pups whereas it did in the Wt littermates and dams despite higher concentration of serum T4. Finally, both treatments similarly modulated the expression of the TH-dependent genes Shh, Klf9, and Aldh1a3 in brain. Thus, the ability of DITPA to cross the placenta, its thyromimetic action on the expression of TH-dependent genes in brain, and its better accessibility to the pituitary than L-T4, as assessed by serum TSH, make DITPA a candidate for the prenatal treatment of MCT8 deficiency.
Mutations in the monocarboxylate transporter 8 (MCT8, SLC16A2) gene, located on the X chromosome, produce in males a syndrome of severe psychomotor retardation and unusual thyroid function tests (TFTs) abnormalities consisting of high serum T3, low rT3, low T4, and normal or slightly elevated TSH (1, 2). The earliest neurological manifestation in the first few months of life is truncal hypotonia with poor head control, progressing to spastic quadriplegia, lack of speech, and poor communication skills. Based on the well-established role of thyroid hormone (TH) in brain development (3), it is assumed that in the absence of MCT8, impaired transport of TH across the blood-brain barrier, and into neurons of the central nervous system have devastating consequences for neural differentiation and proper brain function (4). At the same time, the high serum T3 in MCT8 deficiency increases energy expenditure, resulting in failure to maintain weight despite adequate calorie intake. This hypermetabolic state is generated by tissues that are not predominantly MCT8 dependent for TH transport, including liver and skeletal muscle (5). This coexistence of TH deficiency and excess in patients with MCT8 deficiency (1, 2) has challenged the treatment using simple hormonal replacement.
To circumvent this therapeutic dilemma, TH analogs that exert thyromimetic actions by entering cells independently of MCT8 were sought. Because the TH analog diiodothyropropionic acid (DITPA) corrected the effect of TH deprivation in the central nervous systems of Mct8-deficient (Mct8KO) mice without causing hormonal excess in peripheral tissues (6), DITPA was given on a compassionate basis to four children with MCT8 deficiency for 26–40 months (7). This treatment (2.1–2.4 mg/kg×d), initiated at 8.5–25 months of age, normalized the TFTs and reduced the hypermetabolism and the tendency for weight loss. Although none of the children treated with DITPA developed seizures, there was no measurable improvement in the neurodevelopment deficit. This is likely due to the irreversible neurological damage incurred during fetal and very early neonatal life (7–9) that is not improved by postnatal treatment with DITPA (7). The ability to diagnose MCT8 deficiency prenatally raises the possibility of prenatal treatment of MCT8 deficiency with the potential of improving and even preventing the otherwise-inevitable intellectual and neuropsychiatric disabilities.
To test this possibility, pregnant Mct8 heterozygous (Mct8+/−) mice were treated with DITPA. The capacity of DITPA to cross the placenta and its effects on TFTs and expression of TH-responsive genes in the cerebral cortex of newborn pups were determined. Results suggest that DITPA does cross the placenta and reaches the brain, providing a rationale for prenatal use of DITPA in human mothers carrying a MCT8-deficient fetus.
Materials and Methods
Experimental animals
Procedures carried out on mice were approved by the University of Chicago Institutional Animal Care and Use Committee. Mct8KO mice were generated and housed as described previously (1). All pregnant dams were heterozygous (Mct8+/−), bearing both male wild type (Wt) (Mct8+/y) and Mct8KO (Mct8−/y) littermates used in the study. Experiments were performed on P0 (day of parturition). Genotypes were identified as described previously (1). Endogenous production of TH in pregnant dams and embryos was suppressed, starting at gestational day 10 (E10) with low-iodine diet (Harlan Teklad) and the addition of 0.5% perchlorate and 0.02% methimazole in the drinking water (LoI/MMI/ClO4). Starting at E12 and until delivery, separate groups of pregnant mice, with suppressed endogenous TH production, were given sc, once-daily DITPA [0.3 mg/100g of body weight (BW)×d] or levothyroxine (L-T4) (2 μg/100gBW×d). Based on previous studies, these doses represent replacement regimens for male Wt mice of the same strain with suppressed production of endogenous TH using the same method. Pups from untreated dams (baseline) and pups from dams treated with only LoI/MMI/ClO4 served as controls. Blood samples from dams were obtained on the day of delivery, 20 and 24 hours after the last injection of DITPA, and L-T4, respectively. Pups were anesthetized, and after blood sampling from the jugular vein, were euthanized by decapitation. Tissues were collected, immediately frozen on dry ice, and stored at −80 C. All groups contained 8–12 animals.
Measurement of serum concentrations of DITPA TSH and iodothyronines and tissue content of DITPA
TSH and T4 were measured by radioimmunoassay and DITPA by tandem mass spectroscopy as reported previously (6, 10).
Extraction and measurement of tissue mRNA
Cerebral cortex was collected from male Wt and Mct8KO mice born to untreated dams (baseline), treated with LoI/MMI/ClO4 (MMI), LoI/MMI/ClO4 and L-T4 (MMI+T4), and LoI/MMI/ClO4 and DITPA (MMI+DITPA) to study gene expression. Total RNA was extracted and mRNAs were measured by qPCR as previously described (5). The sequences of primers used to measure sonic hedgehog (Shh), Kruppel-like factor (Klf9), and aldehyde dehydrogenase type 1a3 (Aldh1a3) mRNAs are provided in Supplemental Table 1. The housekeeping gene, RNA polymerase II (RpII), was used as internal control.
Statistical analysis
All results are expressed as mean ± SEM. Statistical analysis of multiple groups were performed using ANOVA. The Student t test was used when there were only two groups to compare. P ≥ .05 was considered not to be significant (NS).
Results
DITPA concentration in serum and content in tissues
To determine whether DITPA crossed the placenta and was equally delivered to embryos of both genotypes, we measured DITPA levels in serum of Wt and Mct8KO littermates at P0 as well as in serum of Mct8+/− dams on the day of parturition. Pups of both genotypes had similar serum DITPA concentrations (P NS). However, they were significantly higher than that of their dams, 9.8-fold and 6.2-fold for Wt mice and Mct8KO mice, respectively; P < .001 (Figure 1A). Thus, DITPA crosses the placenta and is equally delivered to littermates of the two genotypes. Moreover, DITPA shows a longer persistence in serum of newborns than their Mct8+/− dams.
Figure 1.
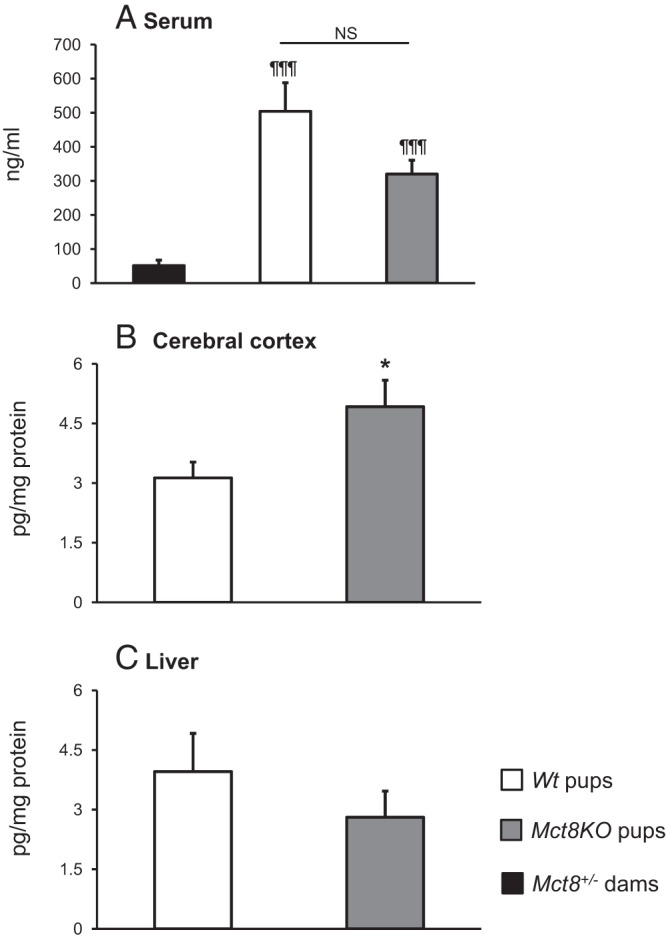
DITPA content in serum and tissues of Mct8+/− dams, and Wt and Mct8KO offspring at P0. Data are expressed as mean ± SE. Statistical differences between Wt and Mct8KO pups are indicated by * above the Mct8KO bars. In panel A, statistical differences from the values in Mct8+/− dams are indicated by ¶ above each value bar. *, P < .05; ¶¶¶, P < .001. NS, not significant (P > .05).
We assessed how DITPA is distributed in central and peripheral tissues of the newborn pups. Mct8KO mice showed a 1.6-fold higher DITPA content in cerebral cortex compared with Wt animals (P < .05, Figure 1B), but not in liver (P NS, Figure 1C). These results demonstrate that DITPA enters into fetal tissues, and, in particular, accumulates in cortex of Mct8KO mice.
Effects of treatments with L-T4 and DITPA on TFTs
We investigated whether and how DITPA, given prenatally, affects TFTs at birth. As previously described (10), at baseline and compared with Wt littermates, Mct8KO newborn mice showed relative hyperthyroidism with lower TSH (P < .001) (Figure 2A) and higher T4 (P < .01) (Figure 2C). The suppression of endogenous TH by administration of LoI/MMI/ClO4 increased the serum TSH levels in newborn pups of both genotypes to an equal degree (P NS, Figure 2A). Compared with TH-deprived animals, treatment with L-T4 produced the expected decrease in serum TSH levels in dams and Wt pups (P < .001), bringing the values to their respective mean baseline levels (P NS). In contrast, L-T4 failed to bring the TSH in Mct8KO pups to pretreatment level, remaining high (P < .001, Figure 2A) despite the higher levels of serum T4 (P < .001, Figure 2C). Contrary to L-T4, DITPA suppressed serum TSH levels to the same extent in the newborn pups of both genotypes (Figure 2A). These results suggest that DITPA given during pregnancy has a better accessibility to the hypothalamo-pituitary feedback system of Mct8KO embryos, and a stronger inhibitory effect on the TSH production than L-T4. Of note, DITPA significantly decreased serum TSH levels (P < .001 vs MMI group) in Mct8+/− dams but not to the same extent as the L-T4 treatment (P < .001 vs MMI) (Figure 2B). Moreover, the DITPA-treated Mct8+/− dams showed higher serum TSH levels compared with both Wt and Mct8KO newborn mice (P < .001).
Figure 2.
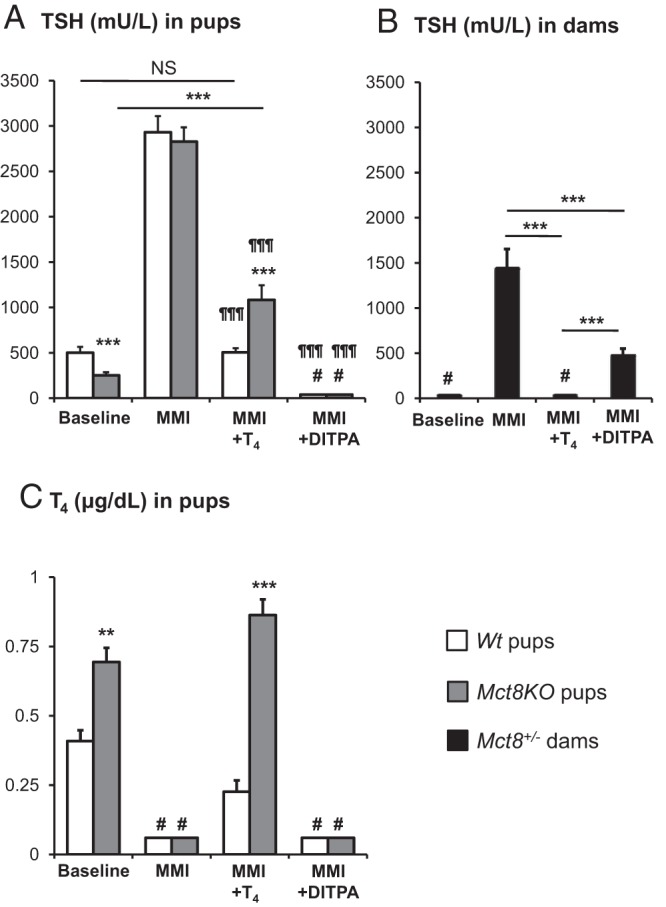
Comparison of the effect of L-T4 and DITPA on serum TSH concentrations of A, Wt and Mct8KO pups at P0; B, Mct8± dams after delivery; and C, on serum T4 levels of Wt and Mct8KO pups at P0. Data are expressed as mean ± SE. Statistical differences between Wt and Mct8KO pups for each treatment group are indicated by * above the Mct8KO bars. In panel A, statistical differences from the values in Mct8+/− dams; in panel B, are indicated by ¶ above each value bar. The symbol # above bars indicates TSH and T4 values suppressed below limits of the assay sensitivity. Treatment with LoI/MMI/ClO4 is indicated as MMI on the abscissa. **, P < .01; ***, P < .001; ¶¶¶, P < .001. #, below the limit of detection of 20 mU/L for TSH and of 0.1 μg/dl for T4.
Effects of DITPA on cerebral cortex of newborn mice
To test the effect of DITPA on cerebral cortex of embryos, we measured the expression of TH-dependent genes at birth (Figure 3). As previously reported (10), and in contrast to adult mice, expression of the genes Shh and Klf9, positively regulated by TH, was higher in untreated (baseline) Mct8KO mice by 2.0-fold (P < .01) and 2.1-fold (P < .001), respectively, compared with the Wt animals. Similarly, the Aldh1a3, a gene negatively regulated by TH, was reduced by 23% (P < .05). As expected, TH deprivation significantly decreased Shh (P < .01) and Klf9 (P < .001) to the same extent in Wt and Mct8KO mice and increased Aldh1a3 (P < .001) mRNA content in both genotypes to the same proportion. Treatment with both L-T4 and DITPA significantly reversed the changes induced by TH deprivation of all three genes (Shh P < .01; Klf9 and Aldh1a3 P < .001 for both L-T4 and DITPA): in L-T4-treated group, Shh, Klf9, and Aldh1a3 genes did not show significant difference between the Wt and Mct8KO mice, whereas DITPA treatment induced a higher Shh gene expression, by 1.8-fold (P < .05), in Mct8KO than in Wt mice. All treatments had the same effect on Klf9 and Aldh1a3 gene expression in both genotypes. These results suggest that DITPA, given prenatally under the current experimental conditions, enters the brain of Mct8KO embryos abolishing, by and large, the differences in gene expression between the genotypes.
Figure 3.
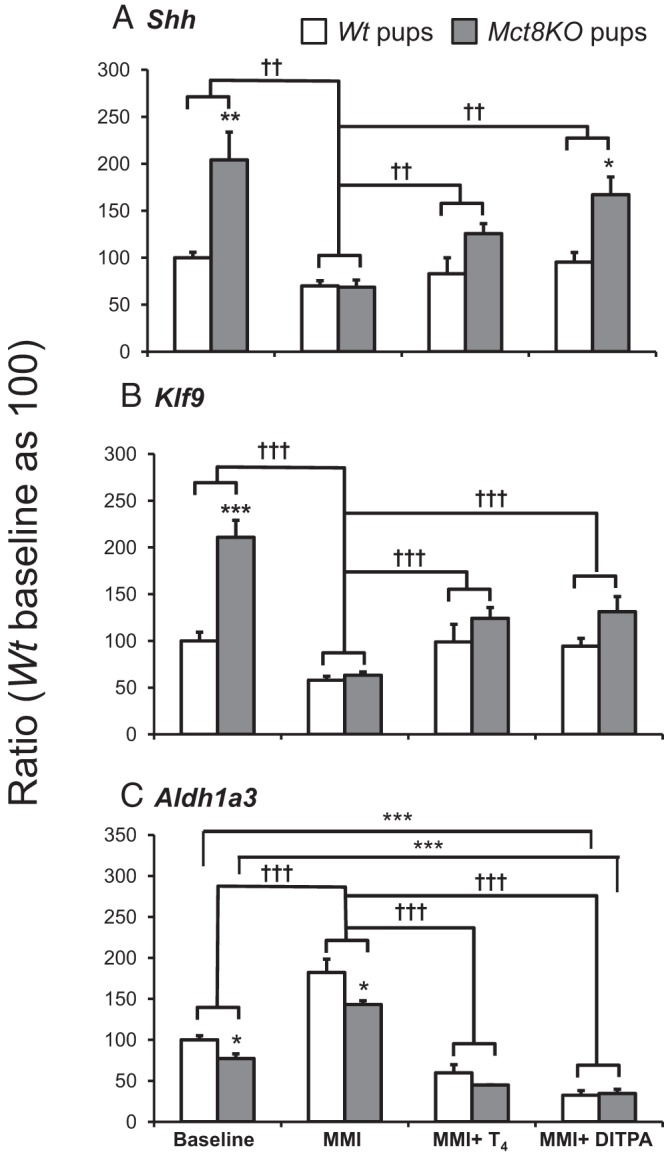
Comparison of the effect of treatment with LoI/MMI/ClO4 alone and in combination with L-T4 and DITPA on expression of A, Shh; B, Klf9; and C, Aldh1a3 genes in cerebral cortex of Wt and Mct8KO mice at P0. Data are expressed as mean ± SE. Statistical differences between Wt and Mct8KO mice for each treatment group are indicated by * above the SE bar of the Mct8KO groups of mice. Statistical differences among different groups are also indicated by * above the horizontal lines indicating the groups being compared. The results of 2-way ANOVA performed to determine the effect LoI/MMI/ClO4 treatment compared with baseline and the effect of T4 or DITPA given the hypothyroid mice regardless of genotype, are indicated by † above the horizontal lines. Treatment with LoI/MMI/ClO4 is indicated as MMI on the abscissa. *, P < .05; ** and ††, P < .01; *** and †††, P < .001.
Discussion
The failure of DITPA to rescue the neurological phenotype of MCT8-deficient patients was ascribed to the late initiation of treatment (7). If true, earlier treatment with DITPA could prevent the brain damage caused by the TH abnormalities during embryonic life. It was with this thought in mind that we conducted the present study to investigate the effects of prenatal exposure to DITPA compared with that of L-T4 on correcting the TFTs abnormalities and TH action in cortex of Mct8KO mice. The choice of L-T4 rather than L-T3 was based on the greater accessibility of L-T4 to brain (6, 11). The major findings in this study are that DITPA crosses the placenta to equally reach the blood circulation of Wt and Mct8KO mice and that prenatal exposure to DITPA has significant effect on serum TSH levels and expression of TH-dependent genes in cerebral cortex of embryos.
Analysis and interpretation of data must take into account the fact that during perinatal life, Mct8KO mice, from embryonic day 17 to postnatal day 3, have high T4 and manifest the effect of central and peripheral tissue TH excess (10). In contrast, adult Mct8KO mice manifest changes typical of central TH deprivation (1, 2, 6).
DITPA suppressed serum TSH levels to the same extent in both genotypes, confirming its thyromimetic effect. On the other hand, the physiological dose of L-T4 was unable to reduce the TSH concentrations in Mct8KO mice to the same level as in Wt mice despite elevated serum T4 levels, confirming the resistance of the hypothalamo-pituitary-axis to L-T4 during the perinatal period of life (10). This resistance was not observed in L-T4-treated Mct8+/− pregnant mice that had suppressed serum TSH levels (Figure 2B). In pregnant mice, 2 μg of L-T4 was enough to suppress the TSH, whereas in Mct8KO adult mice 4 μg did not even normalize TSH levels (6). Similar to the serum, DITPA content in liver did not show statistical difference between the two genotypes of newborn mice, as it occurs in adult mice (6). Contrary to adult mice treated with DITPA (6), we observed a 2.1-fold (P < .05) difference in DITPA levels between cortex and liver in Mct8KO newborn mice and a higher level of DITPA in cortex of Mct8KO mice compared with Wt littermates. Two possible explanations are offered. First, tissue-specific transporters might increase DITPA content in cerebral cortex through an increased influx and/or a decreased efflux. Second, tissue-specific TH-binding proteins could trap DITPA in the cortex. Regarding the latter possibility, we recently showed that, at birth, expression of μ-crystallin, an intracellular TH-binding protein (12), was not significantly different between Wt and Mct8KO mice (10).
Besides the effects on serum TSH levels, the thyromimetic action of DITPA is further confirmed by the response of TH-dependent genes to the treatment. Indeed, Shh, Klf9, and Aldh1a3, during DITPA treatment, showed similar modifications as in the case of L-T4 treatment when compared with the TH-deprived mice: increased expression for the positive Shh and Klf9 genes and decreased expression for the negative Aldh1a3 gene. The fact that Shh gene in DITPA-treated Mct8KO newborn mice showed a significantly higher expression than in the Wt littermates might reflect either the higher concentration of DITPA in cerebral cortex, a greater sensitivity of some genes to DITPA, or an accumulation of DITPA in specific areas of the cortex where the Shh is more expressed. Taking into consideration the suppression of the serum TSH levels, the Aldh1a3 gene expression and the increased DITPA content in cerebral cortex, an excessive dose of the analog seems available to the embryos. The DITPA dose used in this study was physiological to adult male Wt mice and slightly higher than that given to children with MCT8 deficiency (7). Considering the relative hyperthyroid status achieved in the cortex of newborn Mct8KO pups, as indicated by the high levels of Shh mRNA and the suppressed levels of serum TSH and Aldh1a3 mRNA, a lower dose of DITPA could be sufficient.
The capacity of DITPA to cross the placenta, to enter fetal tissues, and to mimic TH action at pituitary and cortical levels in addition to the beneficial effects on the hypermetabolism already described in humans (7), makes this compound particularly suitable for prenatal treatment of MCT8 deficiency in humans. Indeed, another TH analog, 3,3′,5,5′-tetraiodothyroacetic acid, has been recently tested in Mct8-deficient mice during early postnatal life (13). 3,3′,5,5′-tetraiodothyroacetic acid treatment did promote TH-dependent neuronal differentiation in some brain areas. However, it was ineffective in suppressing TRH in the hypothalamus and, in contrast to DITPA, did not significantly ameliorate the peripheral tissue thyrotoxicity of Mct8KO mice (6). On the other hand, treatment with propylthiouracil in combination with T4 that blocks endogenous TH production and D1-mediated conversion of T4 to T3 in peripheral tissues have been tested in older MCT8-deficient children (14–17). This treatment ameliorates the symptoms of thyrotoxicosis without any improvement of the neurological impairments. However, the potential side effects associated with propylthiouracil (18–20) make this treatment less desirable for prenatal treatment of MCT8 deficiency.
The effects that prenatal treatment with DITPA could have on the MCT8 carrier pregnant mother require consideration. When treated with DITPA, Mct8+/− pregnant dams showed less decline in serum TSH levels compared with L-T4-treated dams (Figure 2B). Furthermore, the decline of TSH in Mct8+/− pregnant dams was lesser than that of their litter (Figure 2B vs 2A), suggesting a shorter half-life of DITPA in adult mice, possibly caused by an age-dependent difference in DITPA metabolism. This is supported by the 6- to 9-fold lower serum DITPA level in pregnant dams than their offspring. This decreased effect of DITPA on Mct8+/− pregnant dams provides a predominant effect on the embryos. It allows the administration of relatively small doses to MCT8 heterozygous carriers with optimal effect on the fetus.
Acknowledgments
Present address of A.M.F., Istituto Oncologico Veneto (IOV-IRCCS), Via Gattamelata, 64, 35128 Padova, Italy.
This work was supported in part by Grants DK15070 from the National Institute of Diabetes and Digestive and Kidney Diseases; the Sherman family, Grant SAF2011–25608, Ciberer IS Carlos III, Spain and Esformes Endowment. A.M.F. receives support from “5 per mille” Istituto Oncologico Veneto, Padova, Italy. A.M.D. was supported by a National Institutes of Health Grant F32 DK,91016, and P.G.-I. was a recipient of a Junta de Ampliación de Estudios (JAE), Consejo Superior de Investigaciones Cientificas (CSIC), Spain.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DITPA
- diiodothyropropionic acid
- L-T4
- levothyroxine
- LoI/MMI/ClO4
- low iodine diet/methimazole/perchlorate
- MCT8
- monocarboxylate transporter 8
- NS
- not significant
- TFTs
- thyroid function tests
- TH
- thyroid hormone
- Wt
- wild type.
References
- 1. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043 [DOI] [PubMed] [Google Scholar]
- 2. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122 [DOI] [PubMed] [Google Scholar]
- 4. Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta. 2013;1830:3987–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Cosmo C, Liao XH, Ye H, et al. Mct8-deficient mice have increased energy expenditure and reduced fat mass that is abrogated by normalization of serum T3 levels. Endocrinology. 2013;154:4885–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology. 2009;150:4450–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verge CF, Konrad D, Cohen M, et al. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97:4515–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Visser WE, Visser TJ. Finding the way into the brain without MCT8. J Clin Endocrinol Metab. 2012;97:4362–4365 [DOI] [PubMed] [Google Scholar]
- 9. Fu J, Refetoff S, Dumitrescu AM. Inherited defects of thyroid hormone-cell-membrane transport: Review of recent findings. Curr Opin Endocrinol Diabetes Obes. 2013;20:434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrara AM, Liao XH, Gil-Ibáñez P, et al. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154:2533–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grijota-Martínez C, Díez D, Morreale de Escobar G, Bernal J, Morte B. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology. 2011;152:1713–1721 [DOI] [PubMed] [Google Scholar]
- 12. Vié MP, Evrard C, Osty J, et al. Purification, molecular cloning, and functional expression of the human nicodinamide-adenine dinucleotide phosphate-regulated thyroid hormone-binding protein. Mol Endocrinol. 1997;11:1728–1736 [DOI] [PubMed] [Google Scholar]
- 13. Horn S, Kersseboom S, Mayerl S, et al. Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter mct8. Endocrinology. 2013;154:968–979 [DOI] [PubMed] [Google Scholar]
- 14. Wémeau JL, Pigeyre M, Proust-Lemoine E, et al. Beneficial effects of propylthiouracil plus L-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab. 2008;93:2084–2088 [DOI] [PubMed] [Google Scholar]
- 15. Filho HC, Marui S, Manna TD, et al. Novel mutation in MCT8 gene in a Brazilian boy with thyroid hormone resistance and severe neurologic abnormalities. Arq Bras Endocrinol Metabol. 2011;55:60–66 [DOI] [PubMed] [Google Scholar]
- 16. Visser WE, Vrijmoeth P, Visser FE, Arts WF, van Toor H, Visser TJ. Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clin Endocrinol (Oxf). 2013;78:310–315 [DOI] [PubMed] [Google Scholar]
- 17. Zung A, Visser TJ, Uitterlinden AG, Rivadeneira F, Friesema EC. A child with a deletion in the monocarboxylate transporter 8 gene: 7-year follow-up and effects of thyroid hormone treatment. Eur J Endocrinol. 2011;165:823–830 [DOI] [PubMed] [Google Scholar]
- 18. Hayashida CY, Duarte AJ, Sato AE, Yamashiro-Kanashiro EH. Neonatal hepatitis and lymphocyte sensitization by placental transfer of propylthiouracil. J Endocrinol Invest. 1990;13:937–941 [DOI] [PubMed] [Google Scholar]
- 19. Taylor P, Bhatt S, Gouni R, Quinlan J, Robinson T. A Case of Propylthiouracil-Induced Hepatitis during Pregnancy. Eur Thyroid J. 2012;1:41–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korelitz JJ, McNally DL, Masters MN, Li SX, Xu Y, Rivkees SA. Prevalence of thyrotoxicosis, antithyroid medication use, and complications among pregnant women in the United States. Thyroid. 2013;23:758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]


