Abstract
The anorexigenic adipocyte-derived hormone leptin and the orexigenic hormone ghrelin act in opposition to regulate feeding behavior via the vagal afferent pathways. The mechanisms by which ghrelin exerts its inhibitory effects on leptin are unknown. We hypothesized that ghrelin activates the exchange protein activated by cAMP (Epac), inducing increased SOCS3 expression, which negatively affects leptin signal transduction and neuronal firing in nodose ganglia (NG) neurons. We showed that 91 ± 3% of leptin receptor (LRb) –bearing neurons contained ghrelin receptors (GHS-R1a) and that ghrelin significantly inhibited leptin-stimulated STAT3 phosphorylation in rat NG neurons. Studies of the signaling cascades used by ghrelin showed that ghrelin caused a significant increase in Epac and suppressor of cytokine signaling 3 (SOCS3) expression in cultured rat NG neurons. Transient transfection of cultured NG neurons to silence SOCS3 and Epac genes reversed the inhibitory effects of ghrelin on leptin-stimulated STAT3 phosphorylation. Patch-clamp studies and recordings of single neuronal discharges of vagal primary afferent neurons showed that ghrelin markedly inhibited leptin-stimulated neuronal firing, an action abolished by silencing SOCS3 expression in NG. Plasma ghrelin levels increased significantly during fasting. This was accompanied by enhanced SOCS3 expression in the NG and prevented by treatment with a ghrelin antagonist. Feeding studies showed that silencing SOCS3 expression in the NG reduced food intake evoked by endogenous leptin. We conclude that ghrelin exerts its inhibitory effects on leptin-stimulated neuronal firing by increasing SOCS3 expression. The SOCS3 signaling pathway plays a pivotal role in ghrelin's inhibitory effect on STAT3 phosphorylation, neuronal firing, and feeding behavior.
Ghrelin, a peptide primarily produced in the endocrine cells of the gastric mucosa, acts as an orexigenic hormone to stimulate feeding and growth hormone (GH) secretion by binding to the GH secretagogue receptor (GHS-R) (1–3). Conversely, leptin, an adipocyte-derived hormone, is a negative regulator of feeding and energy metabolism (4–6). Circulating leptin enters the brain by way of the blood-brain barrier (7) and exerts its effects by binding to the long form of the leptin receptor (LRb) (8). LRb receptors are expressed in several regions of the brain, mainly the arcuate nucleus, paraventricular nucleus, and the ventromedial, dorsomedial, and lateral hypothalamus (9). Ghrelin receptors are also expressed in various regions of the brain, including the hypothalamus and nonhypothalamus sites (6), indicating a central role for ghrelin (2).
Research has shown that ghrelin and leptin act in opposition to regulate feeding behavior (2, 10). An intracerebroventricular injection of ghrelin blocks leptin-induced inhibition of food intake (11). In GHS-R-null mice, ghrelin fails to affect the anorexigenic action of leptin, indicating that GHS-R activation directly mediates ghrelin activity (12).
In addition to the hypothalamus, LRb receptors are also expressed in vagal afferent neurons (13, 14). The binding of leptin to LRb receptors stimulates autophosphorylation of Janus activated kinase 2 (JAK2), which in turn activates STAT3 (14–17). Similar to the LRb receptor, the functional ghrelin receptor GHS-R1a is synthesized in the nodose ganglia (NG) and transmitted to the vagal afferent terminals (18). Ghrelin secreted from the stomach may interact with GHS-R1a expressed in these terminals and the resulting signals may be transmitted to the hypothalamus by way of the nucleus of the solitary tract (19). Feeding studies suggest that ghrelin (2) and leptin (20) act by way of vagal afferent pathways to modulate satiety, whereas their receptors in the hypothalamus and other central nonhypothalamic sites are more likely to be involved in regulating long-term feeding behavior and energy metabolism (3, 9).
The mechanisms by which ghrelin exerts inhibitory effects to regulate leptin's action on short-term satiety are unclear. The reported intracellular signaling pathways for ghrelin are complex. Ghrelin has been shown to activate the adenylate cyclase-cAMP-protein kinase A (PKA) signaling pathways in endothelial and smooth muscle cells (21–23). Alternatively, ghrelin may also activate phospholipase C–PKC cascades in dopaminergic neurons (24). Other studies have shown that suppressor of cytokine signaling 3 (SOCS3) plays a pivotal role in the modulation of leptin signaling by inhibiting leptin-activated JAK2 and downstream STAT3 phosphorylation (25). In this manner, SOCS3 negatively regulates hypothalamic leptin signaling (26, 27) and plays an important role in leptin resistance (26). In hypothalamic propiomelanocortin neurons, overexpression of SOCS3 prevented an increase in leptin-stimulated insulin receptor substrate 1/PI3K activity (28) and phospho-STAT3 signaling (29). In contrast, neuronal SOCS3 deficiency enhanced hypothalamic leptin-dependent PI3K signaling (28).
It is conceivable that ghrelin acts by way of SOCS3 pathways to induce leptin resistance. We hypothesize that, in the NG, ghrelin activates the exchange protein activated by cAMP (Epac) by way of a cAMP-dependent pathway, increasing SOCS3 expression, which negatively affects leptin signal transduction and neuronal firing. Using a multilayered approach that included Western blots, patch-clamp electrophysiological studies, and gene silencing techniques in cultured rat NG neurons, as well as in vivo single-cell electrical recordings and feeding studies, we show that ghrelin's inhibitory actions on leptin-stimulated STAT3 phosphorylation and neuronal firing are mediated by way of an Epac–SOCS3 pathway, which contributes to leptin resistance and affects feeding behavior in rats.
Materials and Methods
Animals
All procedures were performed on male Sprague-Dawley rats (160–200 g, Harlan Laboratories) in accordance with National Institutes of Health (NIH) guidelines and as approved by the University Committee on Use and Care of Animals at the University of Michigan.
Immunocytochemistry
Double immunostaining studies were performed on rat NG neurons to identify cells that stained positive for ghrelin receptor GHS-R1a or leptin receptor LRb, and to determine GHS-R1a/LRb colocalization. This procedure has been previously described (13). Briefly, the sections were incubated with 5% normal donkey serum for 1 hour to block nonspecific staining. The primary antibodies to LRb (sc-8325, 1:250, Santa Cruz Biotechnology) and GHS-R1a (sc-10359, 1:100, Santa Cruz Biotechnology) were diluted in PBS containing 2% normal donkey serum, 0.3% Triton X-100, and 0.1% sodium azide and incubated with the sections overnight at 4 C. The coverslips were washed in PBS and then exposed for 20 minutes to species-specific Alexa Fluor 488 (Life Technologies/Molecular Probes) or cyanine 3–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) diluted 1:500 in PBS containing 0.3% Triton X-100. The sections were examined with an Olympus BX2 epifluorescence microscope. Images were stored and analyzed with Photoshop CS2 (Adobe Systems) and an LSM Image Browser (Zeiss).
Isolation and culture of rat NG neurons
The isolation and culture of rat NG neurons was conducted as previously described (14). Experiments were performed on 3–5 male Sprague-Dawley rats (160–170 g). The NG were minced and digested in 4 ml HEPES buffered salt solution containing 1 mg/ml collagenase type 1 (Invitrogen) and 1 mg/ml dispase II (Roche) for 60 minutes at 37 C. The isolated neurons were cultured in an incubator for 72 hours at 37 C in 2 ml DMEM/F-12 media with 10% fetal bovine serum before stimulation with leptin ± ghrelin. STAT3 phosphorylation and SOCS3 and Epac expression in the cell lysates of the NG was analyzed by Western blot analysis.
Lipofectamine transfection with RNA silencing in primary cultured NG neurons
Small interfering RNAs (siRNAs) for SOCS3 (sc-270156), Epac1 (sc-270246), and Epac2 (sc-270233), and a control siRNA (sc-37007) were diluted in Opti-MEM media to a final concentration 15nM. NG neurons at 50–60% confluency were transfected with control siRNA, SOCS3 siRNA, or Epac1/2 siRNA in Opti-MEM and Lipofectamine RNAiMAX (Life Technologies/Invitrogen), as previously described (14). The transfected neurons were incubated for 72 hours in DMEM/F-12 media supplemented with 10% fetal bovine serum. The media were removed and replaced with low-glucose, serum-free DMEM and F-12 medium in equal volumes, supplemented with l-glutamine. The NG neurons were incubated with ghrelin (10nM) for 120 minutes in serum-free DMEM/F12 medium and then stimulated with or without leptin (1nM). After a second wash, lysis buffer (30 μl, Thermo Fisher Scientific) with protease inhibitor was added to the culture dish, and the neurons were incubated for 15 minutes at 4 C. The cell lysates were centrifuged at 10 000 g for 5 minutes. The supernatant (10 μL) was analyzed for protein concentration using a Bio-Rad protein assay kit (Bio-Rad Laboratories) and the remainder was analyzed by Western immunoblotting.
Western immunoblotting
Western immunoblotting was performed as previously described (14). Briefly, protein samples were separated on 10% Ready Gel Tris-HCl (Bio-Rad Laboratories) for 60 minutes at 40 mAmp. The membranes were probed with pSTAT3 antibody (No. 9131), pJAK2 antibody (No. 3776), pMAPK antibody (No. 4370), or SOCS3 antibody (No .2932) from Cell Signaling Technology, p-PI3K antibody (No. 12929, Santa Cruz Biotechnology), or Epac1 antibody (No. 3433–1, Epitomics) at 1:1000 dilution in blocking buffer and incubated overnight at 4 C. The membranes were then incubated with corresponding secondary antibodies in blocking buffer for 1 hour at room temperature. The membranes were exposed to enhanced chemiluminescence buffer for 1 minute and then high-performance chemiluminescence film (GE Healthcare Life Sciences) in the dark. The resulting bands were scanned using a Visioneer OneTouch 9520 Photo Scanner and density of the bands was analyzed using ImageJ (NIH).
Patch-clamp electrophysiological recordings
Whole-cell patch-clamp recordings were performed on cultured NG neurons within 48–72 hours after SOCS3 siRNA transfection, as previously described (14). Duplex siRNA targeting SOCS3 and control siRNA were labeled with Kit-Cy3 as previously described (30) to aid identification of transfected cells. SOCS3 siRNA (sc-270156) and control siRNA (sc-37007) were diluted in Opti-MEM to a final concentration of 15nM and added to the cells. After a 48-hour incubation, whole-cell patch-clamp recordings were performed on Cy3-positive NG neurons at 30 ± 0.5 C. Whole-cell currents were measured using a patch-clamp amplifier (Axopatch 200B, Molecular Devices), digitized (DIGIDATA 1340, Molecular Devices), and recorded on a personal computer using pCLAMP9 (Molecular Devices).
In vivo electrical recordings in single rat NG neurons after electroporation with SOCS3 siRNA
Before the electrical recordings, the rats were randomly divided into two groups of three rats each. The NGs were electroporated with control siRNA (sc-37007) and SOCS3 siRNA (sc-270156). The in vivo electroporation procedure has been previously described (30) The siRNAs (10μM/14 μl) were delivered by square wave electric pulses at 50V/cm and 1 Hz frequency for 20 milliseconds. Transfection efficiency was determined by GFP expression, RT-PCR mRNA quantitation, and Western blot protein expression. Electrical recordings were performed as previously described (30) 5 days after electroporation of the rat NG. Once an NG neuron activated by the electrical vagal stimulation was identified, the response of that neuron to leptin (225 μg/kg, iv) was examined. After a recovery period, the response to ghrelin (25 μg/kg, iv) was recorded, and immediately followed by a second injection of leptin (225 μg/kg, iv). The recordings were repeated in NG neurons from rats electroporated with control and SOCS3 siRNAs. This recording technique has been well established in our previous studies (30, 31).
RT-PCR of SOCS3 mRNA
Total RNA was extracted from cultured NG transfected with control siRNA, SOCS3 siRNA, or Epac siRNA using TRIzol (Invitrogen) according to the manufacturer's instructions. RT was performed using 5 μg of total RNA and the resultant cDNAs were used for PCR. The primer sets to generate SOCS3 mRNA were sense 5′-CTGGCCGCCGCCTCGTCTCGG-3′ and antisense 5′-ACGGCACTCCAGTAGAATCCG-3′, GenBank accession number NM_053565. PCR was performed with Taq DNA polymerase (Promega) through 30 cycles of denaturation (30 s at 94 C), annealing (30 s at 50 C) and extension (30 s at 72 C), followed by a final extension (10 min at 72 C). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. PCR products visualized with ethydium bromide staining and UV light illumination and scanned with an Epson Stylus Photo R2400. Band intensity was analyzed using ImageJ (NIH).
Measurement of plasma ghrelin and leptin levels in fasting rats
Rats (180–200 g) were randomly divided into two groups of five to six rats each. The control rats were fed regular chow, ad libitum, with free access to water. The second group of rats was fasted for 12, 24, 48, or 72 hours, with free access to water. At each time point, the rats in each group were killed. Blood was collected by cardiac puncture and transferred to Becton Dickinson vacutainer tubes (BD Biosciences) containing EDTA. For the ghrelin assay, the tubes contained 1mM p-hydroxymercuribenzoic acid to prevent degradation of acylated ghrelin by proteases. Samples were centrifuged at 3500 rpm for 10 minutes and supernatants were transferred to separate microfuge tubes and 100 μL 0.1 N HCl/ml collected plasma was added to the ghrelin assay tubes. Both sets of tubes were centrifuged at 3500 rpm for 5 minutes. Plasma ghrelin levels (pg/ml) in control and fasted rats were measured at a 1:5 dilution with a ghrelin (rat acylated) enzyme immunoassay (EIA) kit (Cayman Chemical). Plasma leptin levels (pg/ml) were also measured at a 1:5 dilution with a rat EIA kit (R&D Systems).
Measurement of SOCS3 expression in fasting rats after treatment with ghrelin antagonist
Rats (180–200 g) were randomly divided into three groups of four rats each. The control rats were fed regular chow, ad libitum, with free access to water. The second group was fasted for 12 hours with free access to water, and was injected twice with vehicle (saline) during the fasting period. The third group of rats was fasted for 12 hours with free access to water, and were injected twice with 400nM ghrelin antagonist, [D-lys3]-GHRP-6, intraperitoneally at 10 pm (the beginning of the 12-h fast) and at 30 minutes prior to sacrificing the rats after the 12-h fast. The NGs were excised from the control and fasted rats at each time point and homogenized in lysis buffer. SOCS3 expression was determined by Western blot analysis with an SOCS3 antibody (Cell Signaling Technology) and confirmed by RT-PCR analysis, as described earlier.
Feeding studies after electroporation of NG with SOCS3 siRNA
Before initiating the feeding studies, the rats (180–200 g) were randomly divided into two groups of three to five rats each. The right and left NGs were electroporated with control siRNA (sc-37007) or SOCS3 siRNA (sc-270156) (10μM/14 μL). The in vivo electroporation procedure has been previously described (30). Feeding studies were performed 5 days after electroporation. The rats were fasted for 12 hours, beginning at 2200 hours the previous day, with free access to water. The rats were given weighed food the following morning, at which point the cumulative food intake was recorded at 1-hour intervals over 3 hours, as previously described (20). In a separate study, rats were injected with 400nM [D-Lys3]-GHRP-6 (ip), 30 minutes prior to initiation of the feeding study. The feeding study was also conducted as a separate study, under nonfasting conditions in control rats and in rats electroporated with SOCS3 siRNA.
Statistical analysis
For Western blot and RT-PCR studies, the data were normalized for GAPDH or for the respective total protein expression in each experiment and expressed as the fold increase above the unstimulated controls. For pSTAT3, pJAK2, pMAPK, and p-PI3K Western blots, the data were normalized. Results from three to five experiments were used to calculate the means and standard error of the mean. Statistical analysis was performed using a one-way ANOVA with a Dunnett post hoc analysis for multiple comparisons or the nonparametric Kruskal-Wallis test, depending on the study design. Statistical significance was set at P < .05.
Results
GHS-R1a and LRb receptors colocalized in rat NG neurons
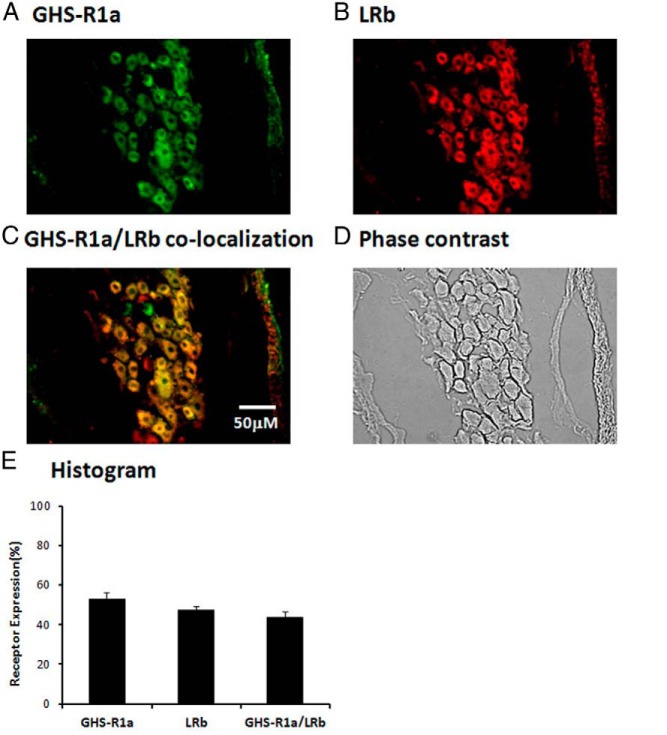
We first determined the cellular distribution of GHS-R1a and LRb in the rat NG neurons. Immunohistochemical staining of normal rat NG showed that 53.1 ± 3% of NG neurons stained positive for GHS-R1 (Figure 1A) and 47.2 ± 6% stained positive for LRb (Figure 1B). GHS-R1a immunoreactivity was present in 91 ± 3% of LRb-bearing NG neurons (Figure 1C). A phase contrast image of the NG demonstates the total number of cells (Figure 1D). A histogram shows the distribution of GHS-R1a and LRb expression in NG neurons (Figure 1E).
Figure 1.
Immunostaining of rat NG neurons demonstrates colocalization of GHS-R1a and LRb. A, Representative photomicrograph of a normal rat NG stained for GHS-R1a shows 53.1 ± 3% of NG neurons stained positive for GHS-R1a. B, 47.2 ± 2% of NG neurons stained positive for LRb. C, 91 ± 3% of LRb-bearing NG neurons contained GHS-R1a. D, Phase contrast image of a normal rat NG to demonstrate the number of cells. E, Histogram shows the distribution of GHS-R1a and LRb expression. The data are representative of three independent experiments.
Ghrelin inhibited leptin-stimulated STAT3 and JAK2 phosphorylation in cultured rat NG neurons
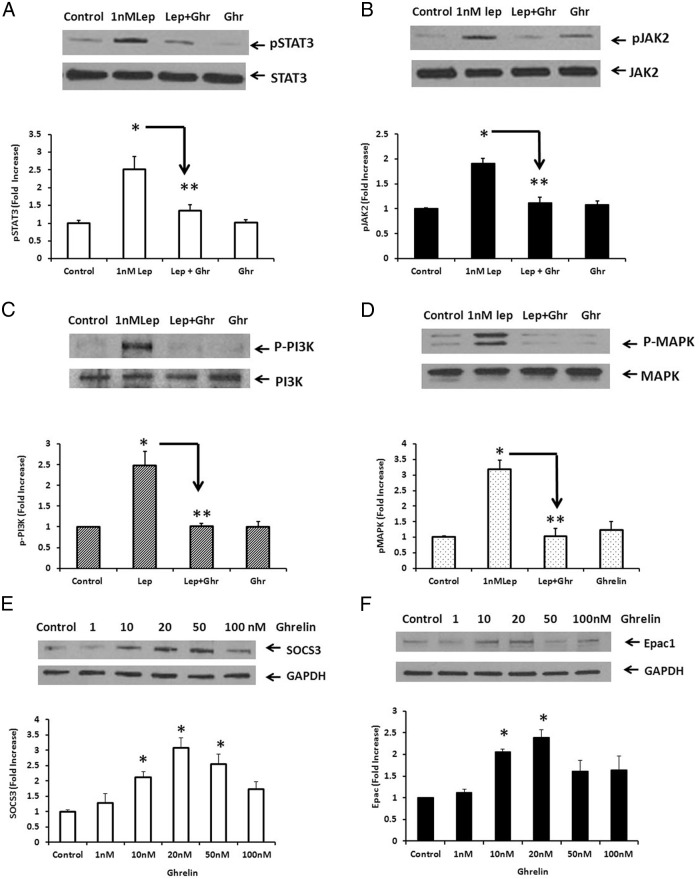
Because it is well known that leptin activates several signal transduction pathways (15), we next determined the effect of ghrelin (10nM) on leptin (1nM) -stimulated STAT3, JAK2, PI3K, and MAPK phosphorylation in cultured rat NG neurons. Our previous studies (14) showed that leptin (0–100nM) stimulated STAT3 phosphorylation in rat NG in a dose-dependent manner; maximal at 1nM. A representative immunoblot (Figure 2A) shows that a 10-minute stimulation with leptin (1nM) caused a 2.52 ± 0.35–fold increase in STAT3 phosphorylation, which was significantly inhibited (> 65%) by ghrelin (10nM) that had been added 60 minutes prior to leptin stimulation. Ghrelin (10nM) alone did not significantly increase STAT3 phosporylation. Because the binding of leptin to the leptin receptor LRb initially stimulates JAK2 phosporylation, before activating STAT3 phosphorylation (15–17), we also examined the effect of ghrelin on leptin-stimulated JAK2 phosphorylation. Leptin (1nM) caused a 1.92 ± 0.24–fold increase in JAK2 phosphorylation (see representative immunoblot, Figure 2B). Ghrelin (10nM) significantly inhibited (> 80%) leptin-stimulated JAK2 phosphorylation. Similarly, leptin (1nM) caused a 2.48 ± 0.33–fold increase in PI3K phosphorylation (Figure 2C) and a 3.19 ± 0.27–fold increase in MAPK phosphorylation (Figure 2D). Ghrelin (10nM) significantly inhibited leptin-stimulated PI3K and MAPK phosphorylation by greater than 80%.
Figure 2.
Effects of ghrelin on leptin-stimulated STAT3 and JAK2 phosphorylation in cultured rat NG neurons. A, Leptin (Lep, 1nM) caused a 2.52 ± 0.35–fold increase in STAT3 phosphorylation, which was significantly inhibited (> 65%) by ghrelin (10nM, Ghr). B, Leptin (1nM) caused a 1.92 ± 0.24–fold increase in JAK2 phosphorylation, which was significantly inhibited (> 80%) by ghrelin (10nM). C, Leptin (10nM) caused a significant (2.48 ± 0.33–fold) increase in PI3K phosphorylation and a significant (3.19 ± 0.27–fold) increase in MAPK phosphorylation (panel D), which was significantly inhibited by ghrelin (10nM) in both studies. The data are representative of five independent experiments. *, P < .05 significantly different from unstimulated control; **, P < .05, significantly different from leptin-stimulated STAT3, JAK2, PI3K, or MAPK phosphorylation. E, Ghrelin-stimulated SOCS3 expression in a dose-dependent manner. NG neurons were stimulated with ghrelin (0–100nM) for 60 minutes. SOCS3 expression was significantly increased at 10, 20, and 50nM. F, Similarly, ghrelin (10 and 20nM) significantly stimulated Epac1 expression. The data are representative of five independent experiments. *, P < .05 suggests statistical significance from unstimulated control.
Ghrelin stimulated Epac1 and SOCS3 expression in a dose-dependent manner
Previous studies of leptin resistance have suggested that SOCS3 acts by a negative feedback mechanism to bind JAK2, preventing its phosphorylation, as well as the downstream activation of STAT3 (25–27). We hypothesized that the binding of ghrelin to GHS-R1a activates G protein Gαs to increase cAMP, which binds to Epac, resulting in increased SOCS3 expression, and ultimately diminishing leptin signal transduction. To determine the signaling pathways involved in ghrelin's inhibitory action, we examined the effect of ghrelin on Epac1 and SOC3 expression in cultured rat NG neurons. NG neurons were stimulated with ghrelin (0–100nM) for 60 minutes, resulting in a biphasic response. SOCS3 expression was significantly increased at 10, 20, and 50nM, with a maximal increase of 3.08 ± 0.31 at 20nM ghrelin (Figure 2E). Similarly, ghrelin-stimulated Epac1 expression was significantly increased at 10 and 20nM, with a maximal increase of 2.39 ± 0.18 at 20nM ghrelin (Figure 2F). The decreased response in SOCS3 or Epac1 expression at 100nM ghrelin may be due to receptor desensitization.
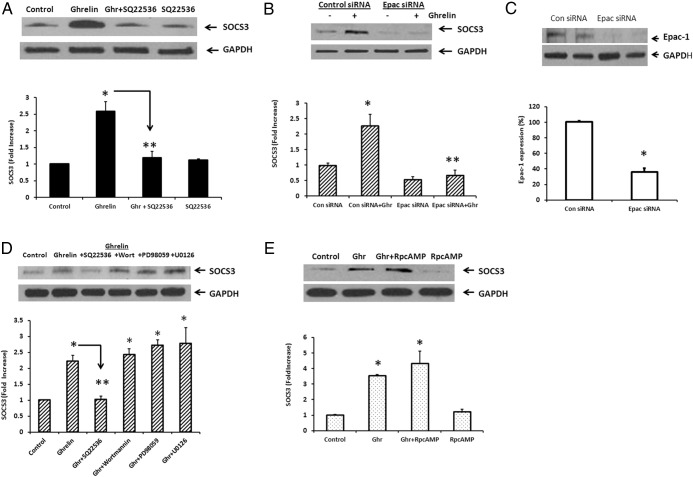
Silencing of Epac1 prevented ghrelin-stimulated SOCS3 expression
To validate our hypothesis, we examined the effect of SQ 22536 (100μM), an adenylate cyclase inhibitor, on ghrelin-stimulated SOCS3 expression. We also transfected cultured NG with Epac1 siRNA and examined its effect on ghrelin-stimulated SOCS3 expression. The cells were stimulated with ghrelin (20nM) for 60 minutes, 72 hours after transfection with Epac1 siRNA. A representative immunoblot (Figure 3A) shows that ghrelin (20nM) caused a 2.59 ± 0.29–fold increase in SOCS3 expression, which was inhibited (> 80%) by SQ 22536 (100μM). SQ 22536 alone had no significant effect on SOCS3 expression. In cells transfected with control siRNA, ghrelin (20nM) caused a significant (2.26 ± 0.37–fold) increase in SOCS3 expression. Ghrelin-stimulated SOCS3 expression in cells transfected with Epac siRNA was significantly inhibited (> 90%) (Figure 3B). Epac siRNA transfection significantly inhibited (> 60%) Epac protein expression in NG (Figure 3C). These results confirm that ghrelin stimulates SOCS3 expression by way of an adenylate cyclase–Epac pathway.
Figure 3.
Effect of inhibitors and Epac siRNA on ghrelin-stimulated SOCS3 expression. A, Representative immunoblot shows that ghrelin (20nM, Ghr) caused a significant (2.59 ± 0.29–fold) increase in SOCS3 expression, which was significantly inhibited (> 80%) by SQ 22536 (100μM). SQ 22536 alone had no significant effect on SOCS3 expression. B, Ghrelin (20nM) caused a 2.26 ± 0.37–fold increase in SOCS3 expression in cells transfected with control siRNA. Ghrelin stimulated SOCS3 expression, which was significantly inhibited (> 90%) by Epac gene silencing. C, Western blot analysis shows that Epac1 expression was significantly inhibited by Epac siRNA (> 60%). D, Effect of PI3K and MAPK inhibitors on ghrelin-stimulated SOCS3 expression. A representative immunoblot shows that ghrelin (20nM, Ghr) caused a significant (2.6 ± 0.21–fold) increase in SOCS3 expression, which was inhibited by > 80% by adenylate cyclase inhibitor SQ 22536 (100μM). The PI3K inhibitor wortmannin (1μM) and the MAPK/MEK inhibitors PD 98059 (30μM) and U0126 (10μM) did not inhibit ghrelin-stimulated SOCS3 expression. E, PKA inhibitor RpcAMP (10μM) did not inhibit ghrelin-stimulated SOCS3 expression. The data are representative of five independent experiments. *, P < .05, significantly different from unstimulated control; **, P < .05, significantly different from ghrelin-stimulated SOCS3 expression.
Effects of PI3K, MAPK, and PKA inhibitors on ghrelin-stimulated SOCS3 expression
Previous studies in cardiac myocytes and endothelial cells (22, 32) have shown that ghrelin can activate the functional GHS-R1 receptor by way of Gαi, leading to activation of the MEK/ERK and PI3K/Akt pathways. We examined the effects of the PI3K inhibitor wortmannin (1μM) and the MAPK inhibitors PD 98059 (30μM) and U0126 (10μM) on ghrelin-stimulated SOCS3 expression. The cells were stimulated with ghrelin (20nM) for 60 minutes in the absence and presence of the inhibitors. A representative immunoblot shows that ghrelin (20nM) caused a significant (2.6 ± 0.21–fold) increase in SOCS3 expression. In contrast with the inhibition observed with adenylate cyclase inhibitor SQ 22536, neither wortmannin, PD 98059, nor U0126 inhibited ghrelin-stimulated SOCS3 expression (Figure 3D). Ghrelin receptor activation of adenylate cyclase can also activate PKA (21). We examined the effect of PKA inhibitor RpcAMP on ghrelin-stimulated SOCS3 expression. RpcAMP (10μM) had no effect on ghrelin-stimulated SOCS3 expression (Figure 3E). The doses of various antagonists were based on previous studies (14, 33, 34). These data suggest that ghrelin stimulates SOCS3 expression by way of the adenylate cyclase–Epac pathway, independent of the PI3K, MAPK/MEK, or PKA signaling cascades.
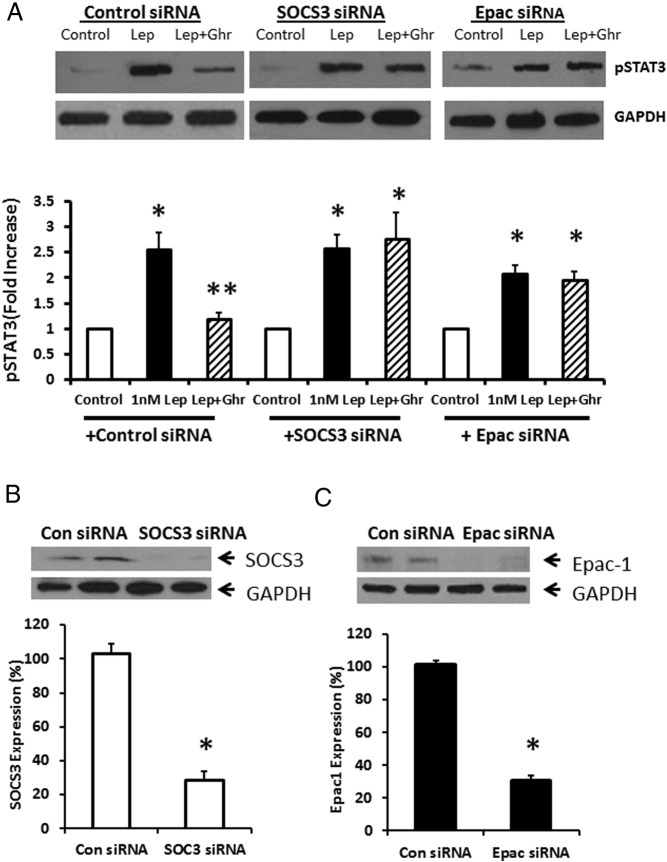
Gene silencing of Epac or SOCS3 abolished ghrelin's inhibitory effect on leptin-stimulated STAT3 phosphorylation
We next transfected cultured NG neurons with control siRNA, SOCS3 siRNA, or Epac siRNA to determine the effect of SOCS3 and Epac gene silencing on the inhibitory effect of ghrelin on leptin-stimulated pSTAT3 phosphorylation. A representative immunoblot (Figure 4A) shows that leptin (1nM) caused a 2.56 ± 0.33–fold increase in STAT3 phosphorylation, which was significantly inhibited (> 70%) by ghrelin (10nM). Transfection of NG neurons with SOCS3 siRNA or Epac siRNA abolished the inhibitory effect of ghrelin on leptin-stimulated STAT3 phosphorylation, but did not alter leptin-stimulated STAT3 phosphorylation. Western blot analysis confirmed successful silencing of SOCS3 expression (Figure 4B) and Epac1 expression (Figure 4C) 5 days after transfection of NG neurons with SOCS3 or Epac siRNA. The data demonstrate that ghrelin exerts its inhibitory action on leptin-stimulated STAT3 phosphorylation by way of the Epac–SOCS3 pathway.
Figure 4.
Effect of ghrelin (10nM) on leptin-stimulated STAT3 phosphorylation after SOCS3 or EPAC gene silencing. A, Leptin (1nM, Lep) caused a 2.56 ± 0.33–fold increase in STAT3 phosphorylation, which was significantly inhibited (> 70%) by ghrelin (10nM, Ghr). Transfection of NG neurons with SOCS3 siRNA or Epac siRNA abolished the inhibitory effect of ghrelin on leptin-stimulated STAT3 phosphorylation, but did not alter leptin (10nM) -stimulated STAT3 phosphorylation. Western blot analysis confirmed successful silencing of B, SOCS3 expression; and C, Epac1 expression; 5 days after transfection of NG neurons with SOCS3 siRNA or Epac siRNA. The data are representative of six independent experiments. *, P < .05, significantly different from unstimulated control; **, P < .05, significantly different from leptin-stimulated STAT3 phosphorylation.
Silencing of SOCS3 reduced ghrelin's action on leptin-stimulated neuronal firing in vitro
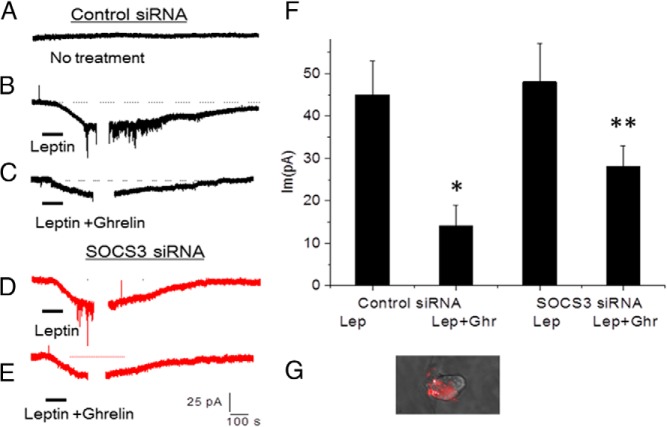
We next determined the effect of ghrelin on leptin-stimulated neuronal firing. Patch-clamp studies were performed on cultured NG neurons stimulated with leptin (10nM) followed by a recovery of the membrane potential. A representative neuronal recording of a rat NG neuron with no treatment (Figure 5A) or stimulated with leptin (10nM) is shown in Figure 5B. Ghrelin (10nM) significantly inhibited the action of leptin (10nM) on neuronal firing (Figure 5C), followed by a recovery of the membrane potential. Silencing SOCS3 expression 48 hours after transfection with SOCS3 siRNA did not alter leptin-stimulated neuronal firing (Figure 5D) but significantly reduced the inhibitory effect of ghrelin (10nM) on leptin-stimulated neuronal firing (Figure 5E). Leptin (10nM) generated an inward current of 45.1 ± 8 pA in 30% of neurons tested. Ghrelin (10nM) significantly inhibited leptin-stimulated neuronal firing, reducing the inward current to 14.1 ± 5 pA. Transfection of NG neurons with SOC3 siRNA reduced ghrelin's inhibitory effect on neuronal firing evoked by leptin to 28.3 ± 5 pA. A summary of the results is shown in Figure 5F. Patch-clamp studies were performed on SOCS3 CY3-siRNA labeled/transfected neurons (Figure 5G).
Figure 5.
Effect of ghrelin (10nM) on leptin-stimulated neuronal firing. A, Representative neuronal recording of rat NG neuron with no treatment after electroporation of control siRNA. B, Representative neuronal recording of a NG neuron stimulated with leptin (10nM, Lep), followed by recovery of the membrane potential. C, representative neuronal recording of rat NG neurons stimulated with leptin (10nM) plus ghrelin (10nM, Ghr), D, Representative neuronal recording of NG neuron stimulated with leptin (10nM), 48 h after transfection with SOCS3 siRNA; and E, representative neuronal recording of NG neuron stimulated with leptin (10nM) plus ghrelin (10nM), 48 h after transfection with SOCS3 siRNA. F, Histogram shows that leptin (10nM) generated an inward current of 45.1 ± 8 pA in 30% of neurons tested. Ghrelin (10nM) significantly inhibited leptin-stimulated neuronal firing, reducing the inward current to 14.1 ± 5 pA. Transfection of NG neurons with SOC3 siRNA partially reversed ghrelin's inhibitory effect on neuronal firing. G, SOCS3 CY3-siRNA transfected/labeled neuron used for patch-clamp studies. The data are representative of six independent experiments. *, P < .05, significantly different from leptin-stimulated neuronal firing. **, Significantly different from leptin-plus-ghrelin–stimulated neuronal firing.
Silencing of SOCS3 prevents inhibitory action of ghrelin on leptin-stimulated nodose neuronal activity in vivo
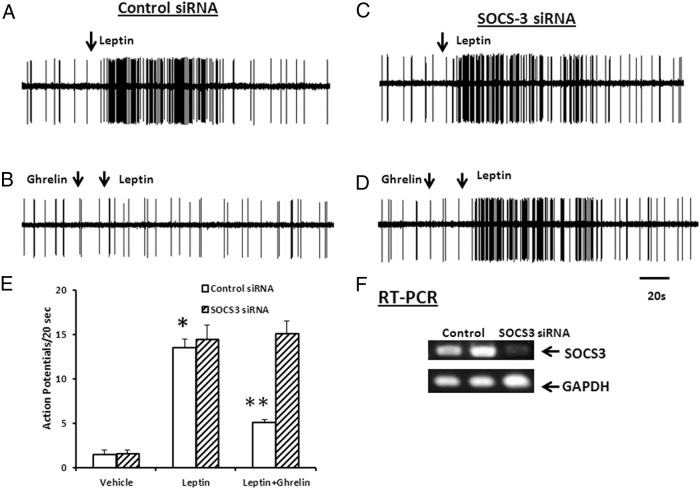
We next determined the effect of SOCS3 silencing on ghrelin's inhibitory action of leptin-stimulated neuronal firing. Rat NG were electroporated with either control siRNA or SOCS3 siRNA and 5 days later, single-unit discharges of vagal primary afferent neurons innervating the gastrointestinal tract were recorded from rat NG, as previously described (13, 31, 35). Data were collected from recordings of single NG neurons in 6 rats. Of 99 neurons tested, 35 responded to leptin (225 μg/kg, iv). All units were either silent or displayed very low spontaneous activity (0–2 impulses per 20 s). As shown in Figure 6A, in rats whose NG were transfected with control siRNA, the infusion of leptin (225 μg/kg, iv) caused the neuronal firing rate to increase from 0 ± 1 to 15.3 ± 3 impulses/dose (P < .05). The administration of ghrelin (25 μg/kg, iv) alone did not alter basal NG firing but significantly inhibited neuronal firing evoked by leptin (225 μg/kg, iv) (n = 12, Figure 6B).
Figure 6.
Silencing of SOCS3 prevents the inhibitory action of ghrelin on leptin-stimulated nodose neuronal activity in vivo. A, Response of NG neuron electroporated with control siRNA to the administration of leptin (225 μg/kg, iv). B, After injection of ghrelin (25 μg/kg, iv), the same neuron failed to respond to leptin. C and D, Silencing the expression of SOCS3 did not affect leptin (225 μg/kg) -stimulated neuronal firing, but it abolished the inhibitory action of ghrelin (25 μg/kg) on leptin stimulation. E, Histogram summarizes NG response to leptin (225 μg/kg) before and after treatment with ghrelin in rats with NG electroporated with either control siRNA or SOCS3 siRNA. F, Success in knocking down SOCS3 gene expression in the NG was validated by RT-PCR analysis. GAPDH was used as the loading control. The data are representative of six independent experiments. *, P < .05, significantly different from basal. **, P < .05, significantly different from leptin-stimulated neuronal firing.
To provide direct evidence that the inhibitory action of ghrelin is mediated by SOCS3 we performed electroporation of the NG with SOCS3 siRNA and plasmid pEGFP-N1 carrying the GFP gene. Single-cell neuronal recordings were performed 5 days after electroporation with SOCS3 siRNA. As shown in Figure 6C, silencing SOCS3 expression did not affect leptin-stimulated neuronal firing (17.5 ± 4 impulses per 20 s) (Figure 6C); however, it abolished the inhibitory action of ghrelin on leptin stimulation (15.6 ± 2 impulses per 20 s) (Figure 6D), confirming that ghrelin acts by way of SOCS3 to inhibit leptin-stimulated neuronal firing. The data are summarized in a histogram (Figure 6E). To determine the success of the SOCS3 gene knockdown, we showed that SOCS3 gene expression was reduced to 45% of control (Figure 6F) 5 days after electroporation, confirming successful knockdown of SOCS3 gene expression in the NG.
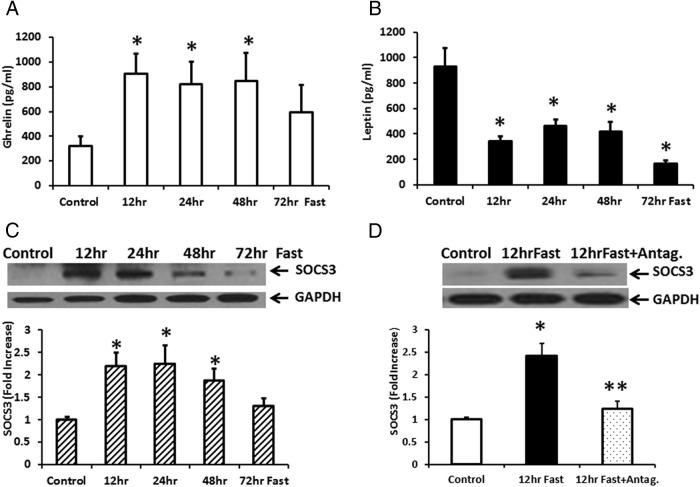
Plasma ghrelin released during prolonged fasting stimulates SOCS3 expression, an effect reversed by treatment with a ghrelin receptor antagonist
Previous studies have reported that plasma ghrelin levels in the rat increase during fasting (36, 37). As shown in Figure 7A, plasma ghrelin levels were significantly increased (> 2-fold) above a basal ghrelin release of 313.1 ± 54 pg/ml at 12, 24, and 48 hours fasting. This was accompanied by a significant decrease in plasma leptin levels after 12–72 hours fasting (Figure 7B). After 12 hours fasting, plasma leptin was reduced from a basal level of 928 ± 144 pg/ml to 339 ± 40 pg/ml. We next determined the effect of increased plasma ghrelin on SOCS3 expression in rat NG. Analysis of SOCS3 expression in the NG showed a significant increase in SOCS3 expression at 12–72 hours fasting (Figure 7C). Treatment with ghrelin receptor antagonist [D-lys3]-GHRP-6 (38–40) at 2200 hours (the beginning of the fasting experiment) and at 0930 hours during the 12-hour fast, significantly inhibited SOCS3 expression (Figure 7D). The data confirm that increased ghrelin stimulates SOCS3 expression in response to fasting.
Figure 7.
Fasting increased ghrelin release in rat plasma and SOCS3 expression in NG; an effect reversed by a ghrelin receptor antagonist. A, Rat plasma ghrelin release was significantly increased by > 2-fold above basal ghrelin release of 313.1 ± 54 pg/ml at 12–48 h fasting. B, Rat plasma leptin was significantly decreased during the 12–72 h fasting from the basal leptin release of 928.5 ± 144 pg/ml. C, Analysis of SOCS3 expression in the NG during fasting showed a significant increase in SOCS3 expression at 12–72 h fasting. D, Treatment of rats with ghrelin receptor antagonist [D-lys3]-GHRP-6 twice on day 1 during the 12-h fast significantly inhibited SOCS3 expression observed during fasting. The data are representative of 4–6 rats in each experimental group. *, P < .05, significantly different from control group. **, P < .05, significantly different from SOCS3 expression after a 12-h fast.
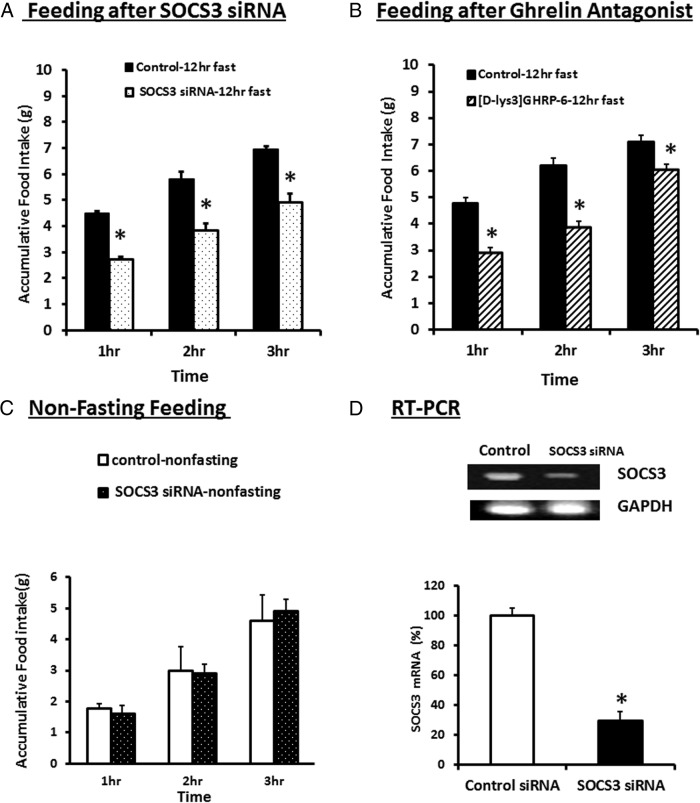
Effect of silencing SOCS3 gene expression on feeding behavior
The feeding studies were performed 5 days after electroporation of the NG with either control siRNA or SOCS3 siRNA. The feeding studies showed that in 12-hour-fasted rats, food intake increased during the first, second, and third hour of feeding (Figure 8A). This effect was significantly reduced after silencing of SOCS3 expression in the NG by electroporation of the left and right NG with SOCS3 siRNA during the first 3 hours of feeding. The ghrelin antagonist, [D-Lys3]-GHRP-6 significantly reduced food intake during the first 3 hrs of feeding (Figure 8B). Feeding studies in nonfasted rats (Figure 8C) showed a reduced intake during the first 3 hours of feeding, compared with food intake after a 12-hour fast. However, under nonfasting conditions, SOCS3 siRNA electroporation had no effect on feeding behavior. This is not unexpected given that plasma ghrelin and NG SOCS3 levels were not increased in nonfasting conditions (Figure 7A and 7C). This suggests that SOCS3 signaling in the NG plays an important role in the regulation of feeding behavior after fasting. Silencing of SOCS3 expression would leave the anorexic action of leptin unopposed and diminish the orexigenic effects of fasting. Note that the success of electroporation was validated by RT-PCR, which demonstrated that SOCS3 siRNA inhibited SOCS3 expression by greater than 70% 5 days after electroporation (Figure 8D).
Figure 8.
Feeding studies in rats electroporated with control siRNA or SOCS3 siRNA or after ghrelin antagonist treatment. A, Food intake increased in the 12-h fasted rats electroporated with control siRNA, during the first 3 h of feeding. This increase was significantly reduced in the group of rats whose NG were electroporated with SOCS3 siRNA during the first 3 h of feeding. B, Ghrelin antagonist 400nM [D-Lys3]-GHRP-6 significantly reduced food intake during the 3 h of feeding compared with the feeding in rats after a 12-h fast. C, Food intake in nonfasted rats was less during the first 3 h of feeding compared with the rats fasted for 12 h. Electroporation of the rat NG with SOCS3 siRNA had no effect on food intake in the nonfasted rats. D, RT-PCR-verified SOCS3 gene knockdown 5 days after electroporation of the NG with control or SOCS3 siRNA. SOCS3 expression was inhibited by > 70% compared with control siRNA electroporation. The data are representative of 3–5 rats in each experimental group. *, P < .05, significantly different from the control fasting group.
Discussion
Gastrointestinal signals that regulate feeding behavior may be mediated by way of neural or hormonal pathways. Studies in rodents have shown that the orexigenic effects of ghrelin are dependent on an intact vagus (19, 41, 42). Vagotomy or administration of capsaicin blocked the effects of ghrelin on feeding and GH secretion. Similarly, perivagal capsaicin abolished the synergistic interaction between leptin and cholecystokinin (CCK) to reduce short-term feeding (20). Thus, ghrelin and leptin seem to interact on multiple levels, including the vago-vagal pathways. In fact, the primary site may be the NG, which are highly sensitive to these peptides. The synergistic increase in STAT3 phosphorylation may be observed with leptin (0.01nM) and CCK (14). In addition, ghrelin released locally from gastric mucosa may act on adjacent vagal afferent fibers innervating the gastroduodenal mucosa. Thus, it seems that ghrelin (2) and leptin (20) act by way of vagal afferent pathways to modulate short-term satiety; whereas their receptors in the hypothalamus and in nonhypothalamus sites in the central nervous system are more likely to be involved in mediating long-term feeding behavior and energy homeostasis (3, 9). The focus of our studies is on the interaction of ghrelin and leptin or CCK at the vagus level to mediate short-term feeding.
This study provides clear evidence that ghrelin inhibits leptin-induced signaling in the NG both in vitro and in vivo. Using dissociated NG, we showed that ghrelin exerted an inhibitory effect on leptin-stimulated STAT3 phosporylation and neuronal firing. We have reported that STAT3 is a key downstream molecule responsible for neuronal excitation evoked by leptin, as silencing of the STAT3 gene abolished leptin-induced neuronal firing (14). STAT3 usually acts by stimulating the transcription of target genes (43), but the rapid electrophysiological effects we observed are not likely to be explained by STAT3-mediated transcription. It is conceivable that STAT3 is involved in modifying the activity of channels or receptors in the NG. To ensure that our in vitro observations were not the result of artifacts arising from culture conditions, we performed in vivo single-unit NG recordings and showed that iv administration of ghrelin significantly inhibited neuronal firing stimulated by leptin. This provides direct evidence that ghrelin interacts directly with leptin at the level of the NG.
Obese humans and animals exhibit leptin resistance. They have a reduced response to endogenous and exogenous leptin, including blunted anorectic responses and reduced STAT3 phosphorylation and neurotransmitter release (26, 44–46). This phenomenon seems to be mediated by feedback regulatory pathways of leptin receptor signaling. Important negative regulators include SOCS3 (25, 26, 55), protein tyrosine phosphatase 1B (47–49), and inflammatory signals such as IKKβ and endoplasmic reticulum stress (50, 51). Among these, SOCS3 seems to play a prominent role in conferring central leptin resistance. In transfected COS cells, forced expression of SOCS3 resulted in inhibition of leptin-induced tyrosine phosphorylation of JAK2 (26). In addition, SOCS3 coimmunoprecipitated with JAK2, indicating that SOCS3 is a leptin-regulated inhibitor of proximal leptin signaling. In mice, SOCS3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity (25). These observations suggest that the level of SOCS3 expression is a critical determinant of leptin sensitivity. SOCS3 may be used by orexigenic molecules such as ghrelin to limit the action of leptin and diminish anorexic signals to the central nervous system during feeding. Our studies showed that the silencing of SOCS3 expression in the NG significantly reversed the inhibitory action of ghrelin in leptin-stimulated neuronal firing.
Research suggests that cAMP suppresses proinflammatory cytokine signaling, such as the IL-6 cascades, which use JAK-STAT3 signaling (52–54). Leptin and its receptor are structurally related to the IL-6 cytokine family (56). It is therefore conceivable that ghrelin acts by way of the cAMP pathway to activate SOCS3 leading to leptin resistance. In endothelial and smooth muscle cells of the human aorta (22, 32) and urinary bladder (57), ghrelin activates the adenylate cyclase–PKA pathways to mediate smooth muscle contraction. We showed that the ghrelin-stimulated increase in SOCS3 was blocked by the adenylate cyclase inhibitor SQ 22536. It is also well known that cAMP acts through Epac, the guanine nucleotide-exchange factor for the small GTPase Rap1, which induces SOCS3 expression in endothelial cells (55) and in the hypothalamus (58). Our study showed that ghrelin stimulated a dose-dependent increase in Epac 1 in the NG, and silencing of Epac1 expression inhibited the ability of ghrelin to stimulate SOCS3. Epac silencing also reversed the inhibitory effects of ghrelin on STAT3 phosphorylation evoked by leptin. These data show that ghrelin exerts its inhibitory action on leptin-stimulated STAT3 phosphorylation by way of the Epac-SOCS3 pathway.
In cardiac myocytes and endothelial cells (22, 32), ghrelin can activate GHS-R1 by way of Gαi, leading to activation of MAPK/ERK and PI3K/Akt pathways. To rule out participation of PI3K or MAPK/MEK pathways, we showed that the PI3K inhibitor wortmannin and the MAPK inhibitors PD 98059 and U0126 did not affect the ability of ghrelin to stimulate SOCS3 expression. Ghrelin receptor activation of adenylate cyclase can also activate PKA (21). We showed that the PKA inhibitor RpcAMP had no effect on ghrelin-stimulated SOCS3 expression. These observations suggest that ghrelin-stimulated SOCS3 activation is independent of PI3K, MEK/MAPK, and PKA signaling pathways. In addition to its interaction with leptin to regulate feeding behavior, ghrelin also attenuates the satiety signals evoked by CCK (59). This inhibitory effect of ghrelin is unlikely to be mediated by SOCS3, given that CCK action was not affected by SOCS3 expression in the NG (unpublished data). Previous studies have reported that functional GHS-R1a and CCK-A receptors coexpress in the NG, and that ghrelin may reverse the inhibitory effect in feeding by decreasing CB-1 and melanin-concentrating hormone expression (60). Alternatively, ghrelin is known to activate several signaling pathways that may alter electrophysiological properties of the NG and result in reduced responsiveness to anorexigenic peptide stimulation. Additional studies are needed to explore these possibilities.
To provide direct evidence that SOCS3 in the NG plays an important role in the regulation of feeding behavior, we silenced SOCS3 gene expression by electroporation of the NG with SOCS3 siRNA. Silencing of SOCS3 in the NG reduced the enhanced food intake that occurred in response to the 24-hour fast. This effect is most likely mediated by ghrelin, which showed a 2-fold elevation in the plasma. Administration of ghrelin receptor antagonist [D-lys3]-GHRP-6 abolished the orexigenic effects of 12-hour fasting (Figure 8B). Hence, it is conceivable that the increased ghrelin release during fasting acts to stimulate eating by inhibiting the anorexigenic effect of leptin through the enhancement of SOCS3 expression in the NG. SOCS3 acts by a negative feedback mechanism to inhibit the action of leptin. Silencing of SOCS3 partially but not completely reversed the orexigenic effect of fasting. This is not unexpected as ghrelin also attenuates satiety signals stimulated by CCK (60), an action not affected by SOCS3 (unpublished result).
We conclude that SOCS3 plays a pivotal role in mediating ghrelin's inhibitory action on leptin-stimulated STAT3 phosphorylation, neuronal firing, and feeding behavior. Ghrelin induces leptin resistance by increasing SOCS3 expression by way of the adenylate cyclase–cAMP-Epac signal transduction pathway, which impairs leptin-stimulated STAT3 phosphorylation and neuronal firing and reverses ghrelin-stimulated food uptake. This pathway provides a novel mechanism to explain the antagonistic action of ghrelin to reduce the satiety action of leptin. It is conceivable that malfunctioning of this signaling pathway may result in eating disorders.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK48419 and P30 DK34933.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Epac
- exchange protein activated by cAMP
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- CCK
- cholecystokinin
- GHS-R
- growth hormone secretagogue receptor
- GHS-R1a
- ghrelin receptor
- JAK2
- Janus activated kinase 2
- LRb
- leptin receptor
- NG
- nodose ganglia
- NIH
- National Institutes of Health
- PKA
- protein kinase A
- siRNA
- Small interfering RNA
- SOCS3
- suppressor of cytokine signaling 3.
References
- 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 2. Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198 [DOI] [PubMed] [Google Scholar]
- 3. Arora S. Anubhuti. Role of neuropeptides in appetite regulation and obesity—a review. Neuropeptides. 2006;40:375–401 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 5. Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leinninger GM, Jo YH, Leshan RL, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311 [DOI] [PubMed] [Google Scholar]
- 8. White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–4071 [DOI] [PubMed] [Google Scholar]
- 9. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nogueiras R, Tovar S, Mitchell SE, et al. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes. 2004;53:2552–2558 [DOI] [PubMed] [Google Scholar]
- 11. Shintani M, Ogawa Y, Ebihara K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232 [DOI] [PubMed] [Google Scholar]
- 12. Perello M, Scott MM, Sakata I, et al. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol. 2012;520:281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Wu X, Zhou S, Owyang C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: Implications for leptin as a regulator of short-term satiety. Am J Physiol Gastrointest Liver Physiol. 2011;300:G217–227 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Heldsinger A, Grabauskas G, Song I, Owyang C. Synergistic interaction between leptin and cholecystokinin in the rat nodose ganglia is mediated by PI3K and STAT3 signaling pathways: Implications for leptin as a regulator of short term satiety. J Biol Chem. 2011;286:11707–11715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav. 2008;94:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology. 2010;151:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frontini A, Bertolotti P, Tonello C, et al. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008;1215:105–115 [DOI] [PubMed] [Google Scholar]
- 18. Sato M, Nakahara K, Miyazato M, Kangawa K, Murakami N. Regulation of GH secretagogue receptor gene expression in the rat nodose ganglion. J Endocrinol. 2007;194:41–46 [DOI] [PubMed] [Google Scholar]
- 19. Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128 [DOI] [PubMed] [Google Scholar]
- 20. Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossi F, Castelli A, Bianco MJ, Bertone C, Brama M, Santiemma V. Ghrelin inhibits contraction and proliferation of human aortic smooth muscle cells by cAMP/PKA pathway activation. Atherosclerosis. 2009;203:97–104 [DOI] [PubMed] [Google Scholar]
- 22. Rossi F, Castelli A, Bianco MJ, Bertone C, Brama M, Santiemma V. Ghrelin induces proliferation in human aortic endothelial cells via ERK1/2 and PI3K/Akt activation. Peptides. 2008;29:2046–2051 [DOI] [PubMed] [Google Scholar]
- 23. Fang H, Hong Z, Zhang J, et al. Effects of ghrelin on the intracellular calcium concentration in rat aorta vascular smooth muscle cells. Cell Physiol Biochem. 2012;30:1299–12309 [DOI] [PubMed] [Google Scholar]
- 24. Shi L, Bian X, Qu Z, et al. Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat Commun. 2013;4:1435–1446 [DOI] [PubMed] [Google Scholar]
- 25. Mori H, Hanada R, Hanada T, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743 [DOI] [PubMed] [Google Scholar]
- 26. Bjørbaek C, El-Haschimi K, Frantz JD, Flier J. S. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065 [DOI] [PubMed] [Google Scholar]
- 27. Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–738 [DOI] [PubMed] [Google Scholar]
- 28. Metlakunta AS, Sahu M, Yasukawa H, et al. Neuronal suppressor of cytokine signaling-3 deficiency enhances hypothalamic leptin-dependent phosphatidylinositol 3-kinase signaling. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1185–R1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301:E187–E195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou SY, Lu Y, Song I, Owyang C. Inhibition of gastric motility by hyperglycemia is mediated by nodose ganglia KATP channels. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G394–G400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Wu X, Yao H, Owyang C. Secretin activates vagal primary afferent neurons in the rat: Evidence from electrophysiological and immunohistochemical studies. Am J Physiol Gastrointest Liver Physiol. 2005;289:G745–752 [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Chen Q, Li G, Ke D. Ghrelin stimulates angiogenesis via GHSR1a-dependent MEK/ERK and PI3K/Akt signal pathways in rat cardiac microvascular endothelial cells. Peptides. 2012;33:92–100 [DOI] [PubMed] [Google Scholar]
- 33. Kohno D, Nakata M, Maekawa F, et al. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251–2263 [DOI] [PubMed] [Google Scholar]
- 34. Markou T, Hadzopoulou-Cladaras M, Lazou A. Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J Mol Cell Cardiol. 2004;37:1001–1011 [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Zhu J, Owyang C. Electrical physiological evidence for high- and low-affinity vagal CCK-A receptors. Am J Physiol. 1999;277:G469–G477 [DOI] [PubMed] [Google Scholar]
- 36. Li RY, Li XS, Shao L, et al. Influence of visceral adiposity on ghrelin secretion and expression in rats during fasting. J Mol Endocrinol. 2009;42:67–74 [DOI] [PubMed] [Google Scholar]
- 37. Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225 [DOI] [PubMed] [Google Scholar]
- 38. Maletínská L, Matyšková R, Maixnerová J, et al. The peptidic GHS-R antagonist [D-Lys(3)]GHRP-6 markedly improves adiposity and related metabolic abnormalities in a mouse model of postmenopausal obesity. Mol Cell Endocrinol. 2011;343:55–62 [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Dong L, Cheng Y, Zhao P. Effects of ghrelin on feeding and interdigestive migrating complex in rats. Scan J Gastroenterology. 2007;42:447–453 [DOI] [PubMed] [Google Scholar]
- 40. Asakawa A, Inui A, Kaga T, Katsuura G, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asakawi A, Inui A, Kaga O, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345 [DOI] [PubMed] [Google Scholar]
- 42. Date Y, Toshinai K, Koda S, et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–3525 [DOI] [PubMed] [Google Scholar]
- 43. Zhang X, Wrzeszczynska MH, Horvath CM, Darnell JE., Jr Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Mol Cell Biol. 1999;19:7138–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889 [DOI] [PubMed] [Google Scholar]
- 45. Enriori PJ., Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194 [DOI] [PubMed] [Google Scholar]
- 46. Flier JS. Neuroscience. Regulating energy balance: The substrate strikes back. Science. 2006;312:861–864 [DOI] [PubMed] [Google Scholar]
- 47. Banno R, Zimmer D, De Jonghe BC, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924 [DOI] [PubMed] [Google Scholar]
- 49. Cook WS, Unger RH. Protein tyrosine phosphatase 1B: A potential leptin resistance factor of obesity. Dev Cell. 2002;2:385–387 [DOI] [PubMed] [Google Scholar]
- 50. Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 51. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: Master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Delgado M, Ganea D. Inhibition of IFN-gamma-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000;165:3051–3057 [DOI] [PubMed] [Google Scholar]
- 54. Gasperini S, Crepaldi L, Calzetti F, et al. Interleukin-10 and cAMP-elevating agents cooperate to induce suppressor of cytokine signaling-3 via a protein kinase A-independent signal. Eur Cytokine Netw. 2002;13:47–53 [PubMed] [Google Scholar]
- 55. Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096 [DOI] [PubMed] [Google Scholar]
- 57. Tolekova AN, Hadzhibozheva PV, Iliev RN, et al. Participation of extracellular Ca(2+) or ghrelin in peptide-mediated contraction of strips from rat urinary bladder. Regul Pept. 2010;162:79–83 [DOI] [PubMed] [Google Scholar]
- 58. Fukuda M, Williams KW, Gautron L, Elmquist JK. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab. 2011;13:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One. 2012;7:e32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: Putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–1297 [DOI] [PubMed] [Google Scholar]