Abstract
The known genetic causes of idiopathic hypogonadotropic hypogonadism (IHH) are often associated with the loss of GnRH neurons, leading to the disruption of the hypothalamic pituitary gonadal axis and subfertility. The majority of IHH cases have unknown origins and likely arise from compound mutations in more than one gene. Here we identify the homeodomain transcription factor ventral anterior homeobox1 (Vax1) as a potential genetic contributor to polygenic IHH. Although otherwise healthy, male and female Vax1 heterozygous (HET) mice are subfertile, indicating dosage sensitivity for the Vax1 allele. Although Vax1 mRNA is expressed in the pituitary, hypothalamus, and testis, we did not detect Vax1 mRNA in the sperm, ovary, or isolated pituitary gonadotropes. Whereas Vax1 HET females produced normal numbers of superovulated oocytes, corpora lutea numbers were reduced along with a slight increase in circulating basal LH and estrogen. The subfertility originated in the hypothalamus in which kisspeptin and GnRH transcripts were altered along with a substantial reduction of GnRH neuron number. Although the pituitary responded normally to a GnRH challenge, diestrus females had reduced LHβ and FSHβ in diestrus. Furthermore, Vax1 HET males had reduced GnRH mRNA and neuron numbers, whereas the pituitary had normal transcript levels and response to GnRH. Interestingly, the Vax1 HET males had an 88% reduction of motile sperm. Taken together, our data suggest that Vax1 HET subfertility originates in the hypothalamus by disrupting the hypothalamic-pituitary-gonadal axis. In addition, male subfertility may also be due to an unknown effect of Vax1 in the testis.
Normosmic idiopathic hypogonadotropic hypogonadism (IHH) and its anosmic counterpart, Kallmann syndrome, are two rare genetic disorders leading to various degrees of subfertility, including complete infertility. This subfertility is often linked to a reduction of GnRH neurons or impairment of the rhythmic release of GnRH. In the last decade, numerous genes have been identified as responsible for these two conditions; however, more than 50% of IHH cases still have unknown origins (1, 2). Genetic mutations known to cause IHH are frequently either autosomal recessive or dominant. Of note, it is becoming increasingly clear that a number of unidentified genetic causes of IHH are the result of compound heterozygosity (2). Compound heterozygosity is particularly hard to detect because it requires identification of mutations in two distinct genes. Despite the difficulty in detecting polygenic IHH, haploinsufficiencies adversely affecting fertility have been reported in both rodents and humans (2–5).
Mutations in genes involved in ventral forebrain development are often associated with IHH because GnRH neurons arise in the olfactory placode and then migrate through the cribriform plate to the hypothalamus. To identify new genes contributing to compound heterozygosity in IHH that would be expected to present with subtle and incomplete IHH phenotypes, we searched published data sets describing knockout (KO) or heterozygote (HET) mice with secondary phenotypic characteristics of IHH (ie, cleft lip) that also involve a gene that is expressed in the ventral forebrain during GnRH neuron development (1, 6). Ventral anterior homeobox 1 (Vax1) KO mice bear numerous characteristics often associated with IHH including the following: 1) involvement in ventral forebrain development, 2) cleft palate/lip, and 3) expression in the hypothalamo-pituitary-gonadal (HPG) axis (7–10). In addition, a study of Vax1 KO mice stated that “no homozygous pups were recovered from the 129/C57BL6 background, suggesting that the mutation is more penetrant in this genetic context” (7), implying an interaction of Vax1 with its genetic environment. Unfortunately, fertility cannot be studied in the Vax1 KO mouse because it dies soon after birth (7, 9, 11).
Vax1 is a homeodomain transcription factor contributing to the formation of the ventral and rostral forebrain of rodents and humans (7, 12, 13). The timing and spatial expression patterns of Vax1 roughly overlap with those of GnRH during embryonic development (www.brain-map.org and H.M. Hoffman, P.L. Mellon personal observations), suggesting that Vax1 could regulate GnRH neuron migration and maturation. Interestingly, in humans, Vax1 mutations are associated with cleft lip (13–15), a characteristic often seen in Kallmann syndrome due to the common transcription factors regulating both lip and ventral forebrain development (16).
Although Vax1 homozygous deletion is lethal, HET mice are viable, overall healthy, and born in Mendelian ratios. In this study, we characterize the reproductive phenotype of Vax1 HET male and female mice. We determined that both genders are subfertile and have significantly reduced numbers of GnRH neurons. Our data support the conclusion that subfertility originates in the hypothalamus, at the level of GnRH neurons. In addition, male subfertility may also include an unknown role of Vax1 in the testis, leading to poor sperm quality.
Materials and Methods
Animals
All animal procedures were performed in accordance with the University of California, San Diego, Institutional Animal Care and Use Committee regulations. Mice were group housed on a 12-hour light, 12-hour dark cycle (on 6:00 am, off 6:00 pm), with ad libitum chow and water. Wild-type (WT) C57BL/6J mice were from Harlan Laboratories. The Vax1 mice (8) were kindly provided by Dr Kapil Bharti (National Institutes of Health, Bethesda, MD). The Vax1 mice were on a C57BL/6;129S1/Sv background. RiboTag (17) and αGSU-iCre (18) mice were on a C57BL/6J background. The mice were killed by a CO2 or isoflurane (Vet One) overdose and decapitation.
Collection of tissues and sperm for RT-PCR
The pituitary, olfactory bulb, ovary, liver, hypothalamus, and testis were collected from 2.8- to 6-month-old mice, snap frozen on dry ice, and stored at −80°C until processed for quantitative RT-PCR (qRT-PCR). Two-millimeter anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) micropunches were collected on a cryostat from 0.5-mm-thick sections as described previously (19). All tissues were collected in the morning. Female mice were in diestrus. To collect sperm RNA, epididymis and vas deferens from 25 mice, as 3 groups of seven to nine mice each, were pooled in Ham's F10 media (Sigma-Aldrich). The epididymis was punctured with a needle, and forceps were used to gently squeeze out sperm from the vas deferens and epididymis. Sperm were dispersed for 10 minutes in Ham's F10 media and then pelleted (800 × g, 10 min, room temperature, 23°C), placed on top of 60% Percoll (Sigma-Aldrich), and centrifuged (800 × g, 20 min, room temperature). The resulting sperm pellet was washed with Ham's F10 and PBS. After each wash, the sperm pellet was collected by centrifugation (800 × g, 5 min, room temperature). Total RNA was extracted by an RNAeasy kit (QIAGEN) with the following modification: the sperm were disrupted in buffer RLT for 10 minutes at room temperature, after which cells were passed five times through a 26-gauge needle, and then RNA extraction proceeded following the manufacturer's instructions.
Plugging efficiency, estrous cyclicity, and fertility assessment
For plug efficiency, a female was housed with a male and plug formation was monitored every morning. A second cohort of virgin Vax1 WT and HET mice were housed in pairs, and the number of litters born and the number of pups per litter were recorded over a period of 120 days. As described previously (20), vaginal smears were performed daily between 9:00 and 11:00 am on 3- to 5-month-old mice by vaginal lavage.
Collection of tissues
Diestrus ovaries were fixed for 4 hours at room temperature and testis O/N (∼16 h) at 4°C. Fixing solution contained 60% ethanol, 30% formaldehyde, and 10% glacial acetic acid. Tissue was paraffin embedded, serially sectioned at 10 μm, and stained with hematoxylin and eosin (H&E; Sigma-Aldrich). Histology was examined and the number of corpora lutea in a single ovary per mouse was recorded blindly by two experimenters (21).
Hormone levels
For serum hormone analysis, mice were killed by isoflurane overdose and blood collected from the abdominal aorta between 9:00 and 11:00 am. Blood was allowed to clot for 1 hour at room temperature and then centrifuged (15 min, 2600 × g). Serum was collected and stored at −20°C before RIA analysis for T, LH, FSH and estradiol (E2) at the Center for Research in Reproduction, Ligand Assay, and Analysis Core, University of Virginia (Richmond, Virginia). Samples were run in singlets. All intraassay coefficients of variance (ACOVs) are based on the variance of samples in the standard curve run in duplicate [LH: lower detection limit: 0.04 ng/mL, intra-ACOV 6.4% and intra-ACOV 8.0%, n = 13; FSH: lower detection limit: 2.0 ng/mL, intra-ACOV 6.9% and inter-ACOV 7.5%, n = 9; E2: lower detection limit: 3.6 pg/mL, intra-ACOV 6% and inter-ACOV 11.4%, n = 55; T: lower detection limit: 9.6 ng/dL, intra-ACOV 4.4% and inter-ACOV 6.4%, n = 58]. Due to the variation in T levels in WT mice, samples were reassayed and comparable results were obtained.
Quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's recommendations. Genomic DNA was eliminated using the DNA-free kit (Applied Biosystems). cDNA was obtained by reverse transcription of total RNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories). cDNA products were detected using an iQ SYBR Green Supermix (Bio-Rad Laboratories) on a qRT-PCR iQ5 real-time detection system (Bio-Rad Laboratories) or the CFX (Bio-Rad Laboratories). cDNA amplification used mouse qRT-PCR primers (Supplemental Table 1). Data were expressed by the 2-δδCT method by normalizing the gene of interest to the housekeeping genes PPia and H2AFz (22). Data are expressed as fold change compared with control or as indicated in the figure legends. Data represent mean fold change ± SEM from a minimum of three independent total RNA samples for each data point.
Immunoprecipitation of pituitary mRNA
Pituitary homogenates were prepared by combining four to five pituitary samples per group of male RiboTag+:αGSU-iCre+ mice of 2–4 months of age or two female diestrus or proestrous pituitaries of 3–4 months. Pituitaries were weighed before Dounce homogenization [2%-3% (wt/vol)] in polysome buffer (50 mM Tris, pH 7.5; 100 mM KCl; 12 mM MgCl2; 1% Nonidet P-40; 1 mM dithiothreitol; 200 U/mL Promega RNasin; 1 mg/mL heparin; 100 μg/mL cycloheximide; Sigma protease inhibitor mixture). Samples were then centrifuged at 10 000 × g for 10 minutes to create a postmitochondrial supernatant. For immunoprecipitations, 100 μL protein G magnetic beads (Dynabeads; Invitrogen) were coupled directly to 2 μL mouse monoclonal anti-HA antibody for 4 hours in homogenization buffer. Antibody-coupled beads were washed three times in homogenization buffer before they were added to homogenates. Supernatants (400 μL) were then added directly to the antibody-coupled Protein G magnetic beads and rotated overnight at 4°C. Samples were placed in a magnet on ice and supernatants recovered before washing the pellets three times for 5 minutes in high-salt buffer (50 mM Tris, pH7.5; 300 mM KCl; 12 mM MgCl2; 1% Nonidet P-40; 1 mM dithiothreitol; 100 μg/mL cycloheximide). To prepare the total RNA, 350 μL of QIAGEN buffer RLT was added to the remaining pellets or to the input samples, respectively. The total RNA was prepared according to the manufacturer's instructions using an RNeasy Mini Plus kit (QIAGEN) and reverse transcribed with an iScript kit (Bio-Rad Laboratories) to generate cDNA. The resulting cDNA was subjected to qRT-PCR. To validate the hemagglutinin antibody, we used a number of controls, which are shown in Results.
Immunohistochemistry
Immunohistochemistry was performed as described previously (23) except that brains were placed overnight in 30% sucrose dissolved in PBS and then cut into coronal 12-μm sections. The GnRH antibody has previously been shown to have excellent specificity under these conditions (20) (see the Supplemental Table 1 for details of the antibodies used). All of the sections between bregma 2.40 and −2.80 were evaluated for GnRH staining in a blinded manner using a microscope. The staining was evaluated in the complete section. To increase the visibility of the GnRH neurons, adjustments of brightness, contrast, and color balance were done with Image J (National Institutes of Health, Bethesda) and applied to the entire image.
GnRH challenge
Baseline tail blood was collected from male and female metestrous/diestrous WT and Vax1 HET littermates. Ten minutes after receiving an ip injection of 1 ng/g GnRH in PBS, a second collection of tail blood was performed. The total volume of blood collected did not exceed 100 μL. Blood was collected between 9:00 am and 12:00 pm and processed as described above, before singlet runs on MILIPLEX (number MPTMAG-49K; Millipore) (LH: lower detection limit: 5.7 pg/mL, intra-ACOV 10.6 and inter-ACOV 6.8%, n = 6).
Ovariectomy and estrogen replacement
As described previously in detail (24), 2- to 3-month-old C57BL/6J mice were ovariectomized and received a sc capsule containing estradiol benzoate (E8515; Sigma-Aldrich) before being killed in the morning (9-:00–10:00 am) or during the LH surge (6:30 pm).
Sperm motility and total sperm count
Epididymides were collected in M2 media at room temperature (Sigma-Aldrich). One epididymis was cut in half and sperm were gently expelled by manual pressing. The numbers of motile and immotile sperm were counted in a hemocytometer 15 minutes after sperm were expelled. To immobilize motile sperm for a total sperm count, the hemocytometer was placed for 5 minutes on a 55°C heat block. The second epididymis was chopped into small pieces and left 30 minutes at room temperature. The solution was filtered through a 70-μM filter (Falcon), and the sperm were diluted in PBS before counting the total number of sperm heads in one epididymis.
Superovulation
WT and Vax1 HET females of 12–14 weeks of age received an ip injection of 10.0 IU/animal of pregnant mare serum (Sigma) at noon. Forty-six hours later, 10 IU of human chorionic gonadotropin (Sigma) were administered ip. Twenty-two hours later, after CO2 asphyxiation and cervical dislocation, fallopian tubes were collected in M2 medium at room temperature. The ampulla were torn to release the oocyte clusters into hyaluronidase M2 medium (Sigma). The fallopian tube was shredded to collect any additional unreleased oocytes. Oocytes were isolated with a fine glass pipette and placed in fresh M2 media then counted. The total and healthy (one cell oocyte) number of oocytes were recorded.
Statistical analysis
Statistical analyses were performed using a Student's t test, Mann-Whitney test, one-way ANOVA, or two-way ANOVA, followed by post hoc analysis by Bonferroni, as indicated in the figure legends, with P < .05 indicated as significant. Male and female mice were not directly compared by statistics in this study but were separated into different data tables.
Results
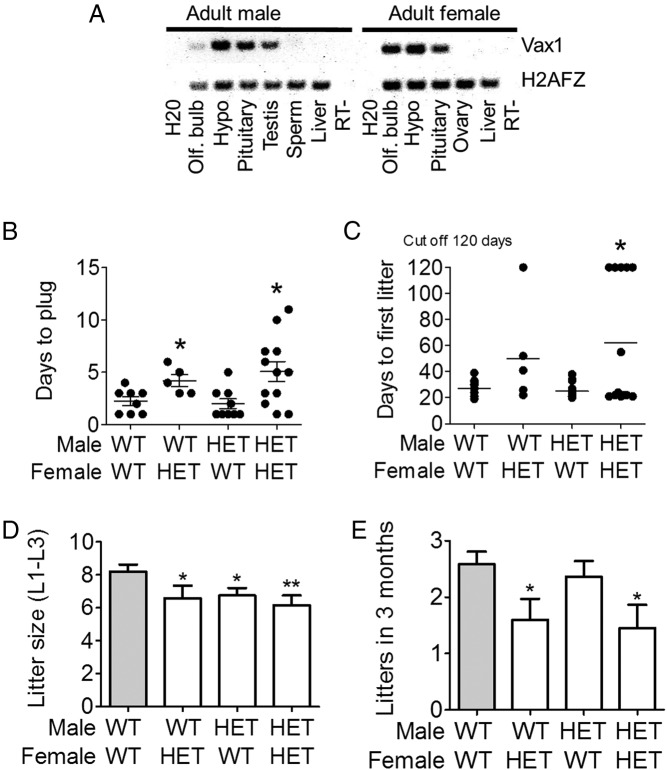
To determine the potential role of Vax1 in mouse fertility, we characterized its expression pattern in relevant organs by RT-PCR (Figure 1A). We confirmed the presence of Vax1 in mouse testis and determined that adult male and female mice expressed Vax1 in the olfactory bulb, hypothalamus and pituitary. No Vax1 mRNA was detected in sperm, liver, or ovary (Figure 1A).
Figure 1.
A, RT-PCR for Vax1 and the housekeeping gene, H2AFz, in various tissues from male and female C57BL/6J mice. B, Fertility assessment of Vax1 HET mice. Virgin inexperienced 10- to 16-week-old male WT and Vax1 HET (WT, HET) mice were cohoused with virgin inexperienced 10- to 16-week-old WT or Vax1 HET female mice and the number of days to first vaginal plug monitored. In a second set of experiments, virgin male and female mice aged 9–11 weeks of age at the start of the experiment were used to determine fertility in Vax1 HETs. C, The number of days to first litter (n = 5–12). D, The average litter size of the first three litters (n = 10–13). E, The number of litters in 3 months were recorded (n = 10–13). Data represent means ± SEM. Statistical analysis was done by Student's t test as compared with WT × WT. *, P < .05; **, P < .01.
Vax1 HET males fathered smaller litters
Pubertal onset in the male was determined by preputial separation (25). We found no difference in age at preputial separation in WT vs Vax1 HET mice (Supplemental Table 2). In accordance with this, adult WT and Vax1 HET males had comparable testis and body weights (Supplemental Table 2). Vax1 HET males copulated with a WT female mouse in the same time frame as WT male mice (Figure 1B). The time to first litter with a WT female was normal (Figure 1C) and the number of litters born in 3 months was normal (Figure 1E). However, the average litter size of the first three litters (L1-L3) from Vax1 HET mice mating with WT or Vax1 HET mice were all significantly reduced as compared with WT × WT matings (Figure 1D).
Reduced motile sperm quality is associated with subfertility in Vax1 HET male mice
Proper sperm generation depends on both the release of LH and FSH from the pituitary as well as the correct functioning of all cell types in the testis (26, 27). To determine the origin of subfertility, we performed H&E staining of WT and Vax1 HET testes. Vax1 HET males had normal testis morphology (Figure 2A). To evaluate sperm quality, we separately prepared the epididymides for either total sperm count or motility. We found that Vax1 HET males had a 39% reduction in total sperm count (Figure 2B). In addition, HET mice had 49% fewer motile sperm than WT (Figure 2C). Thus, there was an 88% reduction in motile sperm in Vax1 HET mice. The reduced sperm motility was not associated with aberrant sperm morphology (Figure 2D).
Figure 2.
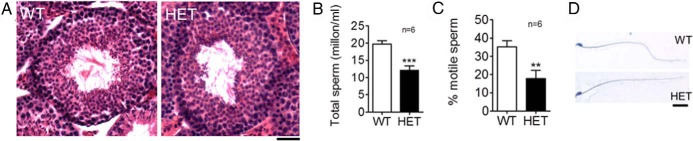
Male Vax1 HET mice have reduced sperm count and motility. A, H&E-stained testes. Scale bar, 10 μm. B, Total sperm count per epididymis, C, Percentage of motile sperm (n = 6). D, H&E-stained sperm. Scale bar, 1 μm. Data represent means ± SEM (Student's t test as compared with control). **, P < .01; ***, P < .001.
Vax1 HET males have normal pituitary gonadotropes but reduced numbers of GnRH neurons
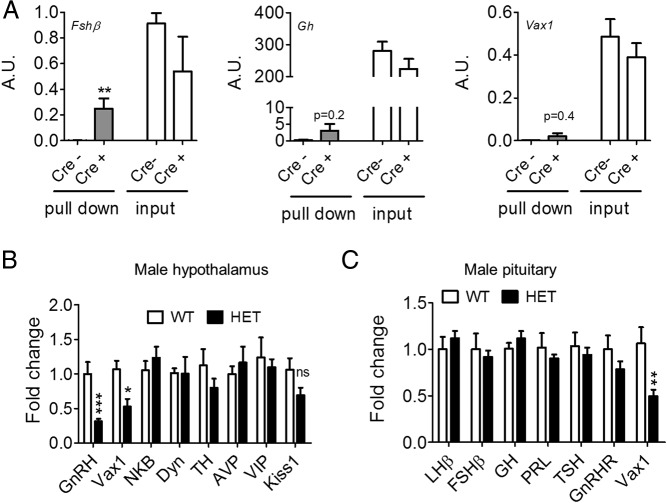
Vax1 is also expressed in the pituitary (Figure 1A). To determine whether it is expressed in gonadotropes, we performed qRT-PCR from gonadotrope-enriched samples obtained from RiboTag immunoprecipitated pituitary collected from adult αGSU-iCre+:RiboTag+ male mice. The data are expressed as arbitrary units and reflect antibody pull down of gonadotropes (Cre+, Figure 3A). As a control for antibody specificity, the input is shown in which no enrichment is obtained in Cre+ samples (Figure 3A, input). To verify that the pull-down enriched our samples with gonadotropes, we used Fshβ as a positive control observing enrichment of Fshβ mRNA in Cre+ samples (Figure 3A, Fshβ, compare Cre− to Cre+). The pull-down was not enriched in somatotropes as evidenced by the lack of Gh mRNA enrichment in the Cre+ pull down (Figure 3A, Gh). No enrichment of Vax1 was detected in gonadotropes from the male pituitary (Figure 3A, Vax1).
Figure 3.
A, Gene transcript levels in gonadotrope pull-down of pituitary from RiboTag male mice crossed with αGSU-icre+ (cre+) and αGSU-icre− (cre−) mice (n = 4). Gene transcript levels of male Vax1 WT and HET were determined by qRT-PCR of hypothalamus (n = 5–8) (B) and pituitary (n = 5–8) (C). All data were analyzed by Student's t test as compared with control. **, P < .01; ***, P < .001. A.U., arbitrary units.
To determine whether Vax1 HET mice had altered gene transcript levels in the hypothalamus and pituitary, we collected these structures from WT and Vax1 HET mice and determined transcript levels of genes known to be involved in GnRH neuron or gonadotrope cell function. qRT-PCR indicated that Vax1 HET mice had a 50% reduction in Vax1 transcript levels in the hypothalamus (Figure 3B). Interestingly, the only other tested gene with altered transcript levels was GnRH, with a 69% reduction (Figure 3B). This led us to count the GnRH neurons. We found that Vax1 HET males had a 71% reduction in the GnRH neuron number (Figure 4, A and B), and very weak to no GnRH staining was detected in the median eminence (not shown). Despite the strong reduction in the GnRH mRNA and GnRH neuron number, we did not detect any significant changes in the pituitary for any of the studied genes (Figure 3C). This is in agreement with the absence of Vax1 mRNA in gonadotropes (Figure 3A) and the circulating levels of FSH, LH, and T, which were comparable between the WT and Vax1 HET males (Figure 5, A–C) as well as the normal GnRH responsiveness of the Vax1 HET pituitary (Figure 4C).
Figure 4.
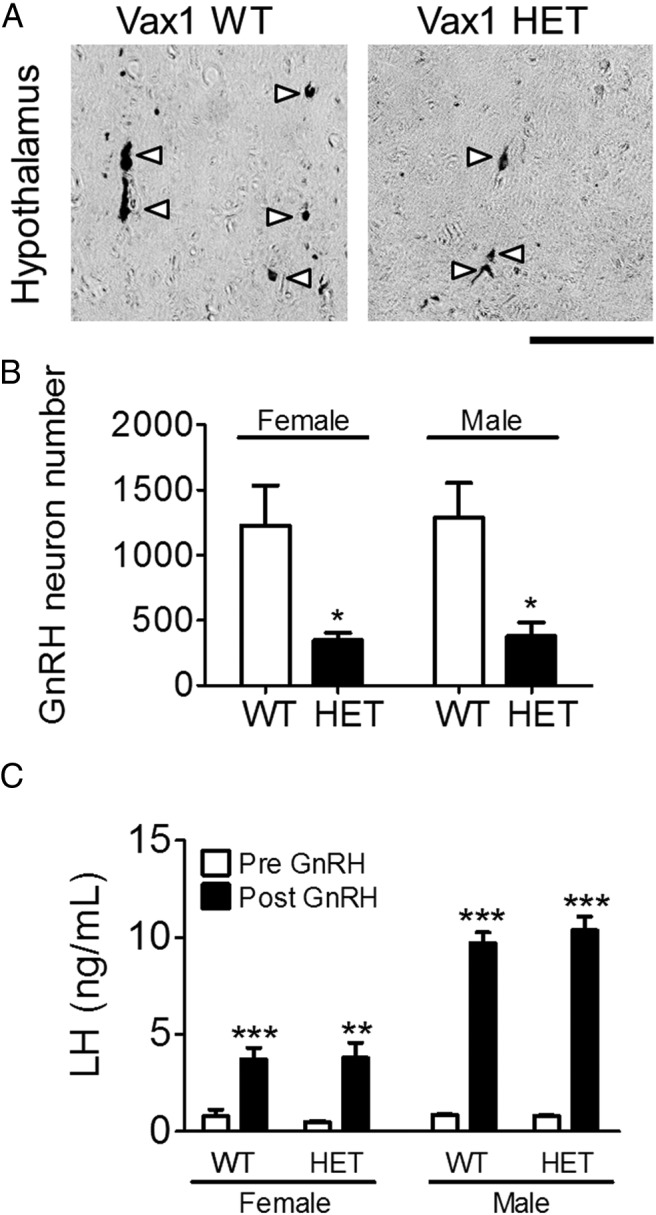
Vax1 adult mice lose more than 50% of GnRH neurons. Immunohistochemistry of GnRH was performed in WT and Vax1 HET mice. A, Illustrative images of GnRH staining in the male hypothalamus. Arrowheads indicate GnRH neurons. Scale bar, 100 μm. B, Total GnRH neuron counts in the adult mouse brain (n = 3–4). Statistical analysis was by Student's t test as compared with WT of the same gender. *, P < .05. C, Serum LH was measured prior to and 10 minutes after a single GnRH challenge in Vax1 diestrous/metestrous females and males. Statistical analysis by two-way ANOVA, as compared before GnRH. **, P < .01; ***, P < .001.
Figure 5.
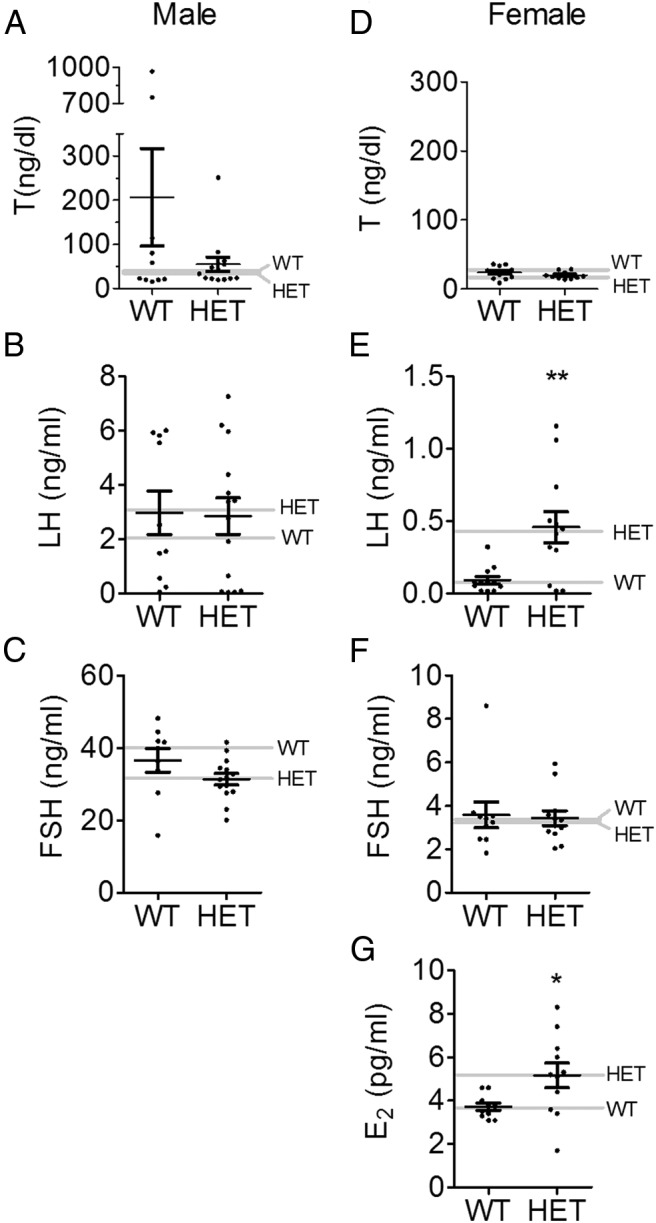
Hormone levels in Vax1 WT and HET mice. Serum was collected from adult (A–C) male and (D–G) female diestrus mice and assayed for T, FSH, LH, and E2. Data represent means ± SEM. Gray line indicates median. Statistical analysis was by Student's t test as compared with WT. *, P < .05; **, P < .01. Male T levels (A) were analyzed by the nonparametric Mann-Whitney test (P = .976).
Vax1 HET females have irregular estrous cycles and are subfertile
Pubertal onset in the female can be determined by vaginal opening, which occurred in the same time frame in WT and Vax1 HET females (Supplemental Table 3). At the age of 2.8–4 months, WT and Vax1 HET diestrus females had comparable body, uterine, and ovary weights (Supplemental Table 3). The time to the first litter of Vax1 HET females was slightly, but not significantly (P = .0826), increased as compared with WT × WT matings (Figure 1C). In contrast, the average litter size of the first three litters produced by Vax1 HET females was significantly reduced (Figure 1D), and Vax1 HET females bore fewer litters in 3 months than WT females (Figure 1E). When mating Vax1 HET males with Vax1 HET females (HET × HET matings), we found that the number of days to first litter, litter size, and the number of litters in 3 months (Figure 1, C–E) were all significantly different from WT × WT matings. It is of note that a great heterogeneity in breeding capacity was seen (Figure 1, B and C), suggesting that the phenotypes studied were semipenetrant. All WT females, whether mating with WT or HET males, were able to produce litters. In contrast, 20% of the Vax1 HET females mating with WT males were unable to produce a litter in 120 days, and 36% of HET × HET matings were infertile.
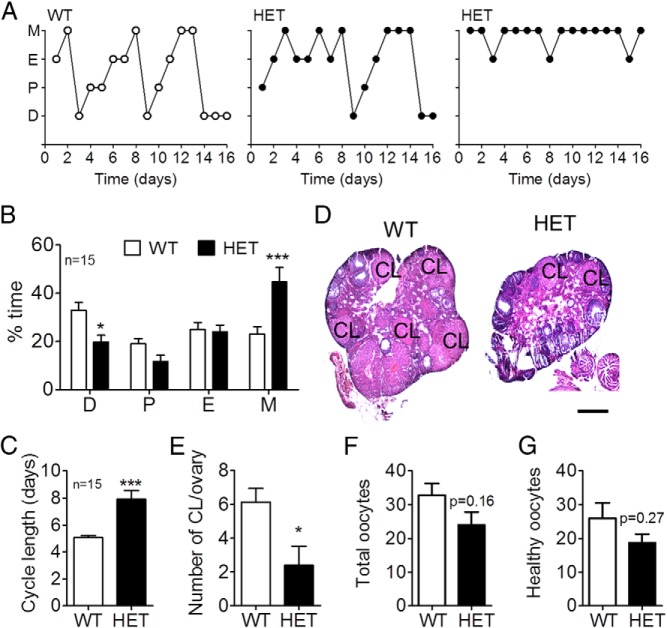
Although Vax1 HET female mice have irregular estrous cycles, they sometimes progress through the cycle in 4–5 days and other times require 10 or more days to complete one cycle (Figure 6A). Despite this great variation, overall, Vax1 HET female mice spent significantly less time in diestrus, more time in metestrus (Figure 6B), and the time to complete one estrous cycle was increased (Figure 6C). Gonadal morphology showed a reduced number of corpora lutea (CL) in Vax1 HET ovaries (Figure 6D, E). Finally, we tested whether the reduced number of CL in HET mice was due to a reduced capacity to ovulate. We superovulated WT and Vax1 HET females and found a nonsignificant tendency for a slight reduction in the capacity of Vax1 HET females to ovulate (Figure 6F) as well as a nonsignificant decrease in healthy ovulated oocytes (Figure 6G).
Figure 6.
A, Estrous cycling was monitored daily in Vax1 WT and HET females. B, Time spent in each stage of the cycle. C, Average cycle length was determined (n = 15). D, H&E-stained ovary for histology evaluation. E, Number of CL in the ovary of diestrous females (n = 5–7). Number of total (F) and healthy (G) oocytes in superovulated Vax1 WT and HET females (n = 3–4) is shown. B, Statistical analysis by two-way ANOVA followed by Bonferroni. *, P < .05; **, P < .01; ***, P < .001 as compared with WT in the same stage of the cycle. C and E–G, Student's t test as compared with control. *, P < .05; ***, P < .001.
Analysis of pituitary gene expression and gonadotropin levels in Vax1 HET females
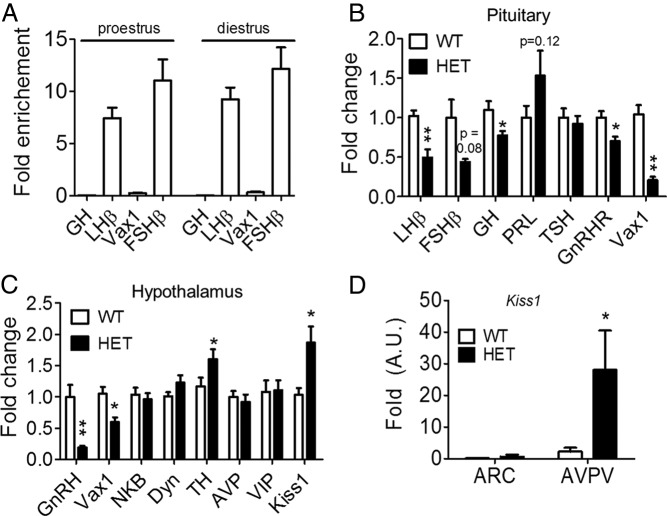
Ovulation requires proper release of FSH and LH from the pituitary as well as estrogen-positive feedback from the ovary to the brain and pituitary (26). To determine whether Vax1 was expressed in female gonadotropes, we performed qRT-PCR from gonadotrope-enriched samples from adult αGSU-iCre+:RiboTag+ proestrous and diestrus female mice. The data in Figure 7A are expressed as fold enrichment as compared with input (17). We observed an enrichment of Fshβ and LHβ, independent of cycle stage (Figure 7A, Fshβ, LHβ, two-way ANOVA, P > .05). The pull-down was not enriched in somatotropes as evidenced by a lack of enrichment of Gh or in Vax1 mRNA (Figure 7A, Gh, Vax1). Despite the absence of Vax1 in gonadotropes (Figure 7A), Vax1 HET diestrus pituitary had reduced Lhβ and Fshβ as well as GnRH receptor levels (Figure 7B). Interestingly, circulating diestrous LH and estrogen were increased (although still within the normal physiological range) (Figure 5, E and G), whereas FSH and T were unchanged at this stage (Figure 5, D and F), indicating a small alteration of the HPG axis. It should be noted that the E2 levels were close to the detection limits of the assay, making the data difficult to interpret. To verify the correlation between the gene transcript levels and circulating hormones, we collected pituitaries in the morning and evening from ovariectomized plus E2 females and measured LHβ transcript levels. Interestingly, although all of the mice produced an LH surge (24), LHβ transcript levels were comparable between the two groups: morning 1.018 ± 0.083 and evening 0.842 ± 0.141 (Student's t test, P = .6112). Finally, pituitary responsiveness to GnRH was tested. Despite the reduction of LHβ transcript levels in Vax1 HET females, they responded normally to a GnRH challenge (Figure 4C).
Figure 7.
A, Gene transcript levels in gonadotrope pull-down of pituitary from RiboTag:αGSU-icre+ diestrous and proestrous females (n = 2–3, each n represents two animals). Female Vax1 WT and HET gene transcript levels were determined by qRT-PCR of pituitary (n = 6–9) (B), hypothalami (n = 6–9) (C), and ARC and AVPV (n = 6–7) (D). Statistical analysis was by Student's t test as compared with control. *, P < .05; **, P < .01.
Vax1 HET females have reduced GnRH and increased Kiss1 mRNA
To identify the origin of Vax1 subfertility, we next performed qRT-PCR of hypothalami from WT and Vax1 HET diestrous females. Similar to Vax1 HET male mice, Vax1 HET females had a strong decrease in hypothalamic GnRH mRNA levels and a 72% reduction in GnRH neuron numbers (Figures 4B and 6C). In addition, Vax1 HET females had a significant increase in hypothalamic Kiss1 mRNA (Figure 7C), which encodes kisspeptin, a neuropeptide necessary to maintain fertility (28).
There are two major hypothalamic kisspeptin populations, one in the ARC and one in the AVPV (29). We used micropunches to separately collect the ARC and AVPV from diestrous WT and HET females. We found that Vax1 HET females had strongly increased Kiss1 mRNA levels in the AVPV but not the ARC (Figure 7D). TH transcript levels were slightly increased in the female hypothalamus (Figure 7C), and this increase was again observed in HET ARC samples (WT: 0.993 ± 0.467; HET: 3.662 ± 1.296, P = .0302), whereas the TH mRNA levels were comparable between the WT and HET AVPV samples (WT: 0.687 ± 0.212; HET: 0.985 ± 0.372, P = .2487).
Discussion
In the last decade, numerous genes have been identified in humans with IHH, showing that lack of GnRH neuron migration, GnRH neuron number reduction, and disruption of the HPG axis gives rise to infertility (1–3, 30, 31). Despite these great advances in the identification of genes associated with IHH, the view that IHH is a monogenic disorder has lately been proven simplistic by the demonstration that gene dosage, as well as compound heterozygosity, is important in IHH (2–5).
Genes involved in ventral forebrain development, the zone that GnRH neurons migrate through during embryogenesis (6), are often also regulators of palate and lip development, supporting the high prevalence of cleft lip in individuals with Kallmann syndrome. The importance of genes involved in ventral forebrain development and GnRH neuron development in IHH agrees with the current study that found a dramatic reduction in the number of GnRH neurons in Vax1 HET mice of both genders along with subfertility. Although IHH can be caused by defects at various levels of the HPG axis, the subfertility of Vax1 HET mice is probably caused by the low number of GnRH neurons. In addition, our data indicate that male subfertility may also be associated with an unknown role of Vax1 in the testis. Of interest, although the reproductive phenotype of an individual with a missense mutation of VAX1 was not reported (13), the study did confirm the importance of VAX1 mutations in cleft lip (13–15), making the screening of VAX1 in IHH clinically relevant.
In the current study, we did not find ectopically localized GnRH neurons, suggesting that the reduction of hypothalamic GnRH neurons is not due to an aberrant migration path. Based on the known role of Vax1 in ventral forebrain development (7), it is plausible that the reduction of GnRH neurons is due to either a reduction in GnRH neurons generated or their loss during migration. In support of the latter hypothesis is the known role of Vax1 as a regulator of midline guidance cues (8) that are involved in the correct migration and number of GnRH neurons (32–34). Because the Vax1 HET mice have reduced numbers of GnRH neurons, it is clear that development and/or migration of GnRH neurons is acutely sensitive to Vax1 gene dosage.
Despite the drastic decrease in GnRH neurons, Vax1 HET males were only modestly subfertile and maintained normal pituitary responsiveness to GnRH as well as gonadotrope-specific transcripts and hormone levels. This supports the impressive robustness of the reproductive system as evidenced by fertility maintenance with as few as 12% GnRH neurons (35) as well as the absence of FSHβ in males (26). Although we do not discount an unidentified role of Vax1 in GnRH neuron function affecting its regulation of the gonadotropes and consequently sperm generation, our data indicate that Vax1 HET male subfertility either has a dual origin in the hypothalamus and testis or could originate in the testis alone in which Vax1 seems to be required for normal sperm generation through an unknown mechanism. Correct sperm generation in mammals depends on LH and FSH action on Leydig and Sertoli cells of the testis to stimulate steroidogenesis and support spermatogenesis. However, based on a recent study that showed absence of Vax1 mRNA in RiboTag-enriched Sertoli and Leydig cells (27) and our finding that it is absent from sperm, it is unlikely that the reduction of motile sperm in Vax1 HET mice is due to Vax1 regulated gene transcription in these cells. It is still unclear where Vax1 is expressed in the testis, except that it is absent from Sertoli and Leydig cells (27) as well as mature sperm (current study). Given that mature sperm do not actively transcribe mRNA, it is plausible that developing sperm cells in the testis express Vax1 during the maturation process. Thus far, we have been unable to determine the localization of Vax1 in the testis. However, with improved Vax1 antibodies, we hope in future studies to be able to determine the origin of the reduced sperm quality in Vax1 HET mice.
Although the Vax1 HET female reproductive phenotype was semipenetrant, overall, Vax1 HET females spent more time in metestrus and less time in diestrus than WT mice, indicating a deregulation of the HPG axis. The variant penetrance of gene knockout on certain characteristics of IHH has previously been observed for the prokineticin 2 gene (30).
Vax1 was not detected in female gonadotropes, supporting the concept that the reduction in gonadotropin transcripts most likely reflects reduced GnRH input to the pituitary. The diminished GnRH input to pituitary resulted in a slight decrease in GnRH receptor expression as well as Lhβ and Fshβ transcript levels. Although activin and inhibin, two regulators of the GnRH receptor, FSH, and LH are essential in normal fertility (36–39), our data do not suggest a role of these peptides in the subfertility of Vax1 HET mice. However, we do show that Vax1 HET mice have a strong reduction in GnRH neuron numbers and GnRH mRNA, which is expected to lead to reduced GnRH secretion at the median eminence. GnRH activation of the GnRH receptor can induce a positive feedback loop, leading to increased GnRH receptor transcription (40–42). Thus, the reduced GnRH input to the pituitary supports the observed reduction in GnRH receptor transcription. Decreased activation of the GnRH receptor decreases LHβ and FSHβ synthesis and release (40–44), and this agrees with the reduction in Lhβ and Fshβ mRNA. Interestingly, we detected slightly higher circulating diestrous LH levels in HET females, at the same stage as reduced LHβ transcript. This is not necessarily contradictory; during the LH surge, when circulating LH peaks, LHβ transcript levels are low. In addition, Vax1 HET female pituitaries maintained their capacity to respond to a GnRH challenge. Taken together, the data strongly support that Vax1 heterozygosity does not affect pituitary function in the female but reduces the capacity of the hypothalamus to generate a correct GnRH output, leading to subfertility.
As shown previously, 12%-34% of GnRH neurons are required for fertility in the mouse (35). Female mice with less than 34% GnRH neurons have irregular cycles and when only 12% of the GnRH population is retained, cycle length is increased and litter size and number are compromised (35). These are all characteristics of the Vax1 HET mice, supporting that the origin of the subfertility is due to the substantial reduction of GnRH neuron numbers in the female. The reduced litter size correlated with reduced corpora lutea in Vax1 HET females. Superovulated females had a nonsignificant decrease in total, as well as healthy, oocytes. This suggests that, although the Vax1 HET females ovulate well, the ovulated oocytes are of a lesser quality, as supported by a tendency of a reduction of healthy oocytes.
Remarkably, in the hypothalamus of Vax1 HET females, we detected increased Kiss1 levels in the AVPV but not the ARC. Kisspeptin is a positive regulator of GnRH neurons (45, 46) and central in maintenance of normal reproductive function (28, 45). Approximately 5% of IHH cases have been associated with kisspeptin-1 receptor mutations (1), making it of particular importance in this study. It is generally accepted that kisspeptin neurons of the ARC mediate negative sex steroid feedback, whereas AVPV kisspeptin neurons are required for positive feedback (47, 48). The increased size of the female AVPV kisspeptin system relative to males is thought to be important in generating the female-specific LH surge (47, 49), in which the proestrus increase of Kiss1 mRNA and neuronal activation in the AVPV are essential in female fertility (50). Based on the data presented, it is not possible to determine the precise cause and functional significance of the increased Kiss1 levels in Vax1 HET diestrus females.
Interestingly, only Vax1 HET diestrus females, but not males, had increased Kiss1 in the AVPV, suggesting three possible alterations. First, an increase in the number of Kiss1-expressing neurons in Vax1 HET female AVPV. We do not think this to be the case because TH levels were comparable between WT and HET in the AVPV, an enzyme coexpressed in almost all Kiss1 neurons (51, 52). Second, advancement in the time of proestrus Kiss1 expression leads to an advancement of the LH surge. A circadian shift of the LH surge has been described in the androgen receptor KO mouse (50); in addition, AVPV Kiss1 is positively regulated by vasopressin, a major neurotransmitter in the suprachiasmatic nucleus (53, 54). A third alteration is a possible up-regulation or enhancement of the positive feedback loop in which E2 is known to act as a positive regulator of AVPV kisspeptin neurons. In support of this third hypothesis, there is a slight increase in diestrus E2 and LH levels, although these values are still low and in the normal physiological range for diestrus mice. Thus, it is not possible to determine if this slight increase in E2 is physiologically relevant for the observed enhancement in the AVPV Kiss1 system.
In conclusion, we have identified Vax1 as a potential new gene participating in polygenic IHH. We propose that heterozygosity of Vax1, along with mutations in other genes, is a plausible contributor to composite IHH. Indeed, the dosage sensitivity to the loss of one allele of Vax1 causes a dramatic decrease in the numbers of GnRH neurons, similar to that seen in haploinsufficient Otx2 heterozygous mice (4). In addition, our data indicate that the origin of the subfertility is different between sexes in Vax1 HET mice, in which female subfertility is due to the strong reduction in GnRH neuron number; male subfertility also includes poor sperm quality associated with an unknown role of Vax1 in the testis.
Acknowledgments
We thank Kapil Bharti (National Institutes of Health) for the Vax1 KO mice; Sally A. Camper and Shannon W. Davis (University of Michigan, Ann Arbor, Michigan) for the αGSU-iCre mice; and Paul S. Amieux (University of Washington, Seattle, Washington) for the RiboTag mice. We also thank Miles F. Wilkinson, Hye-Won Song, and Erica L. Schoeller for assistance with sperm analysis as well as Sunamita S. Leming, Emily A. Witham, Erica C. Pandolfi, Jason D. Meadows, and Ella Kothari for technical assistance. In addition, we thank Erica Schoeller and Kellie Breen for reading the manuscript.
This work was supported by National Institutes of Health (NIH) Grants R01 DK044838, R01 HD072754, and R01 HD020377 (to P.L.M.); Grant R01 HD065856 (to A.S.K.); and Grants R01 HD034283 and R01 HD030428 (to Sally A. Camper, University of Michigan, Ann Arbor, MI). This work was also supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through a cooperative agreement (Grant U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to P.L.M.). P.L.M. was also partially supported by Grants P30 DK063491, P30 CA023100, and P42 ES101337. H.X. was partially supported by Grants F32 HD070579 and T32 DK007044. The Center for Research in Reproduction, Ligand Assay, and Analysis Core, University of Virginia, is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH (Specialized Cooperative Center Program in Reproduction and Infertility Research) Grant U54 HD28934.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ACOV
- assay coefficients of variance
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- CL
- corpora lutea
- E2
- estradiol
- H&E
- hematoxylin and eosin
- HET
- heterozygote
- HPG
- hypothalamo-pituitary-gonadal
- IHH
- idiopathic hypogonadotropic hypogonadism
- KO
- knockout
- qRT-PCR
- quantitative RT-PCR
- Vax1
- ventral anterior homeobox 1
- WT
- wild type.
References
- 1. Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pitteloud N, Quinton R, Pearce S, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walters KA, Allan CM, Jimenez M, et al. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148:3674–3684 [DOI] [PubMed] [Google Scholar]
- 4. Larder R, Kimura I, Meadows J, Clark DD, Mayo S, Mellon PL. Gene dosage of Otx2 is important for fertility in male mice. Mol Cell Endocrinol. 2013;377:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HG, Herrick SR, Lemyre E, et al. Hypogonadotropic hypogonadism and cleft lip and palate caused by a balanced translocation producing haploinsufficiency for FGFR1. J Med Genet. 2005;42:666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316 [DOI] [PubMed] [Google Scholar]
- 7. Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13:3106–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertuzzi S, Hindges R, Mui SH, O'Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev. 1999;13:3092–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soria JM, Taglialatela P, Gil-Perotin S, et al. Defective postnatal neurogenesis and disorganization of the rostral migratory stream in absence of the Vax1 homeobox gene. J Neurosci. 2004;24:11171–11181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallonet M, Hollemann T, Wehr R, et al. Vax1 is a novel homeobox-containing gene expressed in the developing anterior ventral forebrain. Development. 1998;125:2599–2610 [DOI] [PubMed] [Google Scholar]
- 11. Bharti K, Gasper M, Bertuzzi S, Arnheiter H. Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development. 2011;138:873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968 [DOI] [PubMed] [Google Scholar]
- 13. Slavotinek AM, Chao R, Vacik T, et al. VAX1 mutation associated with microphthalmia, corpus callosum agenesis, and orofacial clefting: the first description of a VAX1 phenotype in humans. Hum Mutat. 2012;33:364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butali A, Suzuki S, Cooper ME, et al. Replication of genome wide association identified candidate genes confirm the role of common and rare variants in PAX7 and VAX1 in the etiology of nonsyndromic CL(P). Am J Med Genet A. 2013;161A:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Aquino SN, Messetti AC, Bagordakis E, et al. Polymorphisms in FGF12, VCL, CX43 and VAX1 in Brazilian patients with nonsyndromic cleft lip with or without cleft palate. BMC Med Genet. 2013;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pitteloud N, Meysing A, Quinton R, et al. Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol. 2006;254–255:60–69 [DOI] [PubMed] [Google Scholar]
- 17. Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci USA. 2009;106:13939–13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez-Millan MI, Zeidler MG, Saunders TL, Camper SA, Davis SW. Efficient, specific, developmentally appropriate cre-mediated recombination in anterior pituitary gonadotropes and thyrotropes. Genesis. 2013;51:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Giorgio NP, Semaan SJ, Kim J, et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014;155:1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci. 2011;31:426–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rugh R. The Mouse: Its Reproduction and Development. Oxford, United Kingdom, New York: Oxford University Press; 1990 [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-δδC(T)] method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Clark DD, Gorman MR, Hatori M, Meadows JD, Panda S, Mellon PL. Aberrant development of the suprachiasmatic nucleus and circadian rhythms in mice lacking the homeodomain protein six6. J Biol Rhythms. 2013;28:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Witham EA, Meadows JD, Hoffmann HM, et al. Kisspeptin regulates gonadotropin genes via immediate early gene induction in pituitary gonadotropes. Mol Endocrinol. 2013;27:1283–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303 [DOI] [PubMed] [Google Scholar]
- 26. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204 [DOI] [PubMed] [Google Scholar]
- 27. Sanz E, Evanoff R, Quintana A, et al. RiboTag Analysis of Actively Translated mRNAs in Sertoli and Leydig Cells In Vivo. PLoS One. 2013;8:e66179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 29. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 30. Pitteloud N, Zhang C, Pignatelli D, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007;104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miraoui H, Dwyer AA, Sykiotis GP, et al. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am J Hum Genet. 2013;92:725–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwarting GA, Raitcheva D, Bless EP, Ackerman SL, Tobet S. Netrin 1-mediated chemoattraction regulates the migratory pathway of LHRH neurons. Eur J Neurosci. 2004;19:11–20 [DOI] [PubMed] [Google Scholar]
- 33. Murakami S, Ohki-Hamazaki H, Watanabe K, Ikenaka K, Ono K. Netrin 1 provides a chemoattractive cue for the ventral migration of GnRH neurons in the chick forebrain. J Comp Neurol. 2010;518:2019–2034 [DOI] [PubMed] [Google Scholar]
- 34. Schwarting GA, Kostek C, Bless EP, Ahmad N, Tobet SA. Deleted in colorectal cancer (DCC) regulates the migration of luteinizing hormone-releasing hormone neurons to the basal forebrain. J Neurosci. 2001;21:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone (GnRH) neuron requirements for puberty, ovulation and fertility. Endocrinology. 2008;149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coss D, Mellon PL, Thackray VG. A FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab. 2010;21:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coss D, Thackray VG, Deng CX, Mellon PL. Activin regulates luteinizing hormone β-subunit gene expression through smad-binding and homeobox elements. Mol Endocrinol. 2005;19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cherrington BD, Farmerie TA, Lents CA, Cantlon JD, Roberson MS, Clay CM. Activin responsiveness of the murine gonadotropin-releasing hormone receptor gene is mediated by a composite enhancer containing spatially distinct regulatory elements. Mol Endocrinol. 2005;19:898–912 [DOI] [PubMed] [Google Scholar]
- 39. Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22:253–267 [DOI] [PubMed] [Google Scholar]
- 40. Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci USA. 1995;92:12280–12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231 [DOI] [PubMed] [Google Scholar]
- 42. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pernasetti F, Vasilyev VV, Rosenberg SB, et al. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295 [DOI] [PubMed] [Google Scholar]
- 44. Bedecarrats GY, Kaiser UB. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused L βT2 cells: role of GnRH receptor concentration. Endocrinology. 2003;144:1802–1811 [DOI] [PubMed] [Google Scholar]
- 45. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272 [DOI] [PubMed] [Google Scholar]
- 47. Kauffman AS. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol. 2010;22:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378 [DOI] [PubMed] [Google Scholar]
- 49. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus: sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng XB, Jimenez M, Desai R, et al. Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am J Physiol Endocrinol Metab. 2013;305:E717–E726 [DOI] [PubMed] [Google Scholar]
- 51. Zhang C, Tonsfeldt KJ, Qiu J, et al. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab. 2013;305:E1384–E1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35:111–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]