Abstract
Calcium entry through voltage-dependent Ca2+ channels (VDCCs) is required for pancreatic β-cell insulin secretion. The 2-pore-domain acid-sensitive potassium channel (TASK-1) regulates neuronal excitability and VDCC activation by hyperpolarizing the plasma membrane potential (Δψp); however, a role for pancreatic β-cell TASK-1 channels is unknown. Here we examined the influence of TASK-1 channel activity on the β-cell Δψp and insulin secretion during secretagogue stimulation. TASK-1 channels were found to be highly expressed in human and rodent islets and localized to the plasma membrane of β-cells. TASK-1–like currents of mouse and human β-cells were blocked by the potent TASK-1 channel inhibitor, A1899 (250nM). Although inhibition of TASK-1 currents did not influence the β-cell Δψp in the presence of low (2mM) glucose, A1899 significantly enhanced glucose-stimulated (14mM) Δψp depolarization of human and mouse β-cells. TASK-1 inhibition also resulted in greater secretagogue-stimulated Ca2+ influx in both human and mouse islets. Moreover, conditional ablation of mouse β-cell TASK-1 channels reduced K2P currents, increased glucose-stimulated Δψp depolarization, and augmented secretagogue-stimulated Ca2+ influx. The Δψp depolarization caused by TASK-1 inhibition resulted in a transient increase in glucose-stimulated mouse β-cell action potential (AP) firing frequency. However, secretagogue-stimulated β-cell AP duration eventually increased in the presence of A1899 as well as in β-cells without TASK-1, causing a decrease in AP firing frequency. Ablation or inhibition of mouse β-cell TASK-1 channels also significantly enhanced glucose-stimulated insulin secretion, which improved glucose tolerance. Conversely, TASK-1 ablation did not perturb β-cell Δψp, Ca2+ influx, or insulin secretion under low-glucose conditions (2mM). These results reveal a glucose-dependent role for β-cell TASK-1 channels of limiting glucose-stimulated Δψp depolarization and insulin secretion, which modulates glucose homeostasis.
Elevations in blood glucose stimulate pancreatic β-cell electrical excitability and Ca2+ entry through voltage-dependent Ca2+ channels (VDCCs), which culminates in insulin secretion (1). The activity of VDCCs is controlled by changes in the β-cell Δψp, which is coordinated by the activity of K+ channels (1–3). Closure of the ATP-sensitive K+ channels (KATP) after glucose stimulation results in β-cell Δψp depolarization to a plateau potential from where action potentials (APs) fire (4). When KATP is active, it is responsible for a majority (∼70%) of the total β-cell conductance; thus, other hyperpolarizing K+ currents do not significantly influence the β-cell Δψp under low-glucose conditions (5–7). Whereas under high glucose conditions or when KATP channels are inhibited, other active K+ currents will significantly influence the total β-cell conductance and thus regulate Δψp (5–8). Despite the importance of the Δψp on islet Ca2+ entry and hormone secretion, the background K+ currents that stabilize the Δψp during glucose-induced inhibition of KATP have not been determined (6, 9–15). Although it is known that background K+ currents play an important role in modulating the β-cell Δψp (6), what is not clear is the role of β-cell K2P channels during secretagogue-induced insulin secretion and their respective influence on glucose homeostasis.
The background K+ conductance that stabilizes the β-cell plateau potential resembles the biophysical profile of K2P channels; it is a constitutively active leak current that is voltage and Ca2+ independent (16, 17). When β-cell APs and Ca2+ entry are blocked, the Δψp stabilizes at the plateau potential after a brief hyperpolarization. However, elevations in external K+ depolarizes the plateau potential by reducing the driving force of K+ through K+ channels even after blockade of Ca2+ entry (18–22). The Ca2+-activated K+ channel (Kslow) that polarizes the Δψp and terminates the slow wave of depolarization is not active after Ca2+ channel inhibition; therefore, when the β-cell Δψp reaches the plateau potential after Ca2+ channel inhibition, the Δψp does not fluctuate (15, 18–22). This suggests that an active K+ channel which is not influenced by Ca2+ or AP firing stabilizes the β-cell plateau potential. The β-cell plateau potential is also very stable after KATP inhibition with sulfonylureas and is presumably maintained by a consistent K+ conductance that is non-inactivating (7, 23). This K+ conductance shows similarities to cloned K2P channels that are expressed in β-cells; they are active at all physiological voltages, not regulated by Ca2+, constitutively active and non-inactivating (16, 24). Therefore, K2P channels may play a role in stabilizing the plateau potential of β-cells.
The 2-pore-domain acid-sensitive potassium channel (TASK-1) is the most abundant K+ channel transcript of human pancreatic islets and the second most abundant K+ channel transcript of human β-cells as determined by RNA sequencing (25, 26). TASK-1 channels serve an important role in controlling the Δψp from where APs fire in electrically excitable cells (27–30). For example, TASK-1 channels control hypoglossal motoneuron (HM) excitability; activation of TASK-1 channels reduces HM excitability and TASK-1 channel inhibition increases HM excitability (31). TASK-1 channels are non-inactivating, and allow K+ flux out of the cell at all physiological voltage levels reached in pancreatic β-cells (27–29). Furthermore, TASK-1 channels are regulated by many important signals that have been shown to influence insulin secretion such as G protein signaling through Gq-coupled pathways (32, 33). Therefore, β-cell TASK-1 channel activity may play a role in regulating electrical excitability and secretagogue-stimulated insulin secretion.
Here we investigated the physiological role of TASK-1 channels in mouse and human β-cells. We demonstrate that inhibition of TASK-1 currents significantly depolarizes the β-cell Δψp during glucose stimulation leading to Ca2+ influx, which increases glucose-stimulated insulin secretion (GSIS). Moreover, conditional ablation of mouse β-cell TASK-1 channels was found to improve glucose tolerance by augmenting GSIS. These studies reveal for the first time that β-cell TASK-1 currents serve an important role in regulating glucose homeostasis.
Materials and Methods
Mouse β-cell TASK channel ablation
For β-cell–specific TASK-1 ablation, transgenic mice were used; these are a cross between C57BL/6 mice with a tamoxifen-inducible Cre/ERT expressed in β-cells via an 8.5-kb fragment of the mouse Ins1 promoter (MIP-Cre/ERT, generously provided by Louis Philipson at the University of Chicago) and C57BL/6 mice with exon-2 of the KCNK3 gene floxed (generously provided by Douglas Bayliss at the University of Virginia School of Medicine) (34, 35). For β-cell–specific TASK-3 ablation, transgenic animals were used; these are a cross between MIP-Cre/ERT mice and C57BL/6 mice with exon-2 of the KCNK9 gene floxed (provided by Douglas Bayliss) (34). To induce exon-2 excision of KCNK3 or KCNK9, these mice were treated with tamoxifen (0.15 mg/g body weight for 4 consecutive days) via intragastric gavage. The controls used throughout this paper are MIP-Cre/ERT mice treated with tamoxifen; however, to test tamoxifen induction of KCNK3 or KCNK9 exon-2 excision, the controls were KCNK3 floxed MIP-Cre/ERT mice or KCNK9 floxed MIP-Cre/ERT treated with corn oil.
Chemicals
Chemicals including glucose, Krebs-Ringer buffer (KRB) salts, tolbutamide, amphotericin B, and tetraethylammonium (TEA) were from Sigma. The TASK-1 channel inhibitor A1899 was produced by the Vanderbilt Chemical Synthesis Core as previously described (36).
PCR and quantitative RT-PCR analysis
Genomic DNA from islets was isolated with TRizol (Invitrogen) and used in a PCR with primers that anneal within or spanning exon-2 of KCNK3 or KCNK9 as previously described (34). RNA assessment was performed by the Functional Genomics Shared Resource Laboratory at Vanderbilt (RNA integrity number >7.5, 28S/18S >1.4). Coding DNA was prepared from RNA using the SuperScript 3 First-Strand System (Invitrogen). Quantitative PCR with mouse islet cDNA was performed with the FastStart SYBR Green master mix (Roche) and quantitative real time PCR (QRT-PCR) with human islet cDNA was performed using the One-Step system and TaqMan primers (Applied Biosystems). The experimental data for human islets were normalized to actin and for mouse islets were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or cyclophilin. Relative mRNA expression level differences were assessed by the comparative ΔCt method (37).
Mouse islet and β-cell isolation
Islets were isolated from pancreata of 2- to 4-month-old C57BL/6 mice, using collagenase digestion and Ficoll gradients as previously described (38). Human cadaveric islets from male and female nondiabetic donors were provided by multiple isolation centers organized by the Integrated Islet Distribution Program; the islets all had >85% viability. Female and male islets were plated or dissociated in 0.005% trypsin, placed on glass coverslips, and cultured for 16 hours in RPMI-1640 medium supplemented with 10% fetal calf serum, concentrations of glucose specified, 100 IU/mL penicillin, and 100 mg/mL streptomycin. Cells and islets were maintained in a humidified incubator at 37°C under an atmosphere of 95% air/5% CO2.
Perforated-patch electrophysiology
Patch electrodes (2–4 megaohms) loaded with solution containing (nmol/L) 140 KCl, 1 MgCl2*6H2O, 10 EGTA, and 10 HEPES (pH 7.25 with KOH) and the pore-forming antibiotic amphotericin B were used to record islet attached β-cells (39). Islets were perfused with Krebs-Ringer-HEPES buffer containing (mmol/L) 119 NaCl, 2 CaCl2, 4.7 KCl, 10 HEPES, 1.2 MgSO4, and1.2 KH2 PO4, adjusted to pH 7.35 with NaOH, with the indicated concentrations of glucose and compounds. Cells on the periphery of islets were sealed in voltage clamp at −80 mV, and good access was obtained over several minutes through perforations by amphotericin B (39). After being switched to current clamp, mouse cells that had a resting membrane voltage near −65 mV in 2mM glucose were assumed to be β-cells. To confirm that the human recordings were performed in β-cells, the cells were either post-stained for insulin or the tip of the recording electrode was broken into a microfuge tube and the contents expelled. The contents were assayed for insulin using a human insulin ELISA (ALPCO Diagnostics). If the cells stained positive for insulin or the insulin levels from the recorded cell were significantly elevated compared with the control, then the cell was considered a β-cell.
Whole-cell voltage clamp electrophysiological recordings
TASK-1 channel currents were recorded using whole-cell ruptured patch clamps with an Axopatch 200B amplifier and pCLAMP10 software (Molecular Devices). Patch electrodes (2–4 megaohms) were loaded with intracellular solution containing (mmol/L) 140 KCl, 1 MgCl2·6H2O, 10 EGTA, 10 HEPES, and 5 MgATP, adjusted to pH 7.25 with KOH. Cells were perifused with KRB containing (mmol/L) 97.7 N-methyl-D-glucamine (NMDG), 26 KCl, 25 HEPES, 1.2 MgSO4, 1.2 KH2PO4, 14.4 glucose, and 20 TEA, adjusted to pH 7.35 with NaOH.
Measurement of cytoplasmic calcium
Islets were incubated (20 minutes, 37°C) in KRB supplemented with 2 μmol/L fura-2 acetoxymethyl ester (Molecular Probes). Fluorescence imaging was performed using a Nikon Eclipse TE2000-U microscope equipped with an epifluorescence illuminator (Sutter, Inc), a CCD camera (HQ2; Photometrics Inc), and Nikon Elements software. Cells were perifused at 37°C at a flow of 2 mL/min with appropriate KRB-based solutions that contained glucose concentrations and compounds specified in the figures. The ratios of emitted fluorescence intensities at excitation wavelengths of 340 and 380 nm (F340/F380) were determined every 5 seconds.
Western blot and immunofluorescence analysis
Protein extracts were prepared from islets by extraction with sodium dodecyl sulfate loading buffer (1% sodium dodecyl sulfate, 30 mmol/L Tris-HCl [pH 6.8], 5% β-mercaptoethanol, 5% glycerol, and 0.1% bromophenol blue) with protease inhibitors at 80°C for 10 minutes. After electrophoresis through a 4%–12% denaturing polyacrylamide gel, proteins were prepared as a Western blot on a nitrocellulose membrane (Bio-Rad). Anti–TASK-1 (NeuroMab; this antibody has been extensively validated for TASK-1 channel specificity with knockout tissues; see NeuroMab TASK-1 data sheet, http://neuromab.ucdavis.edu/datasheet/N374_48.pdf) or anti-GAPDH (Rockland Immunochemicals) was used to probe the membrane at 1:250 or 1:700 dilution, respectively, in PBS, 0.1% Tween, and 3% powdered dried milk followed by a horseradish peroxidase-coupled secondary antibody (Jackson ImmunoResearch Laboratories) at 1:5000 in the same solution. The membranes were washed in PBS containing 0.1% Tween between and after antibody incubations; horseradish peroxidase was illuminated using Pico Signal (Pierce) and exposed on Kodak X-Omat Blue film.
Mouse pancreata were fixed in 4% paraformaldehyde and embedded with paraffin as previously described, and 5-μm sections were deparaffinized, rehydrated, and stained overnight with primary antibodies against insulin (1:500; Millipore) and TASK-1 (1:500; NeuroMab). The sections were then washed and stained with secondary antibodies conjugated to Cy3 and DyLight 488 (1:500; Jackson ImmunoResearch Laboratories). Images of the sections were obtained with a Zeiss LSM 710 confocal microscope.
Insulin secretion measurements
Female mouse islets were allowed to recover after isolation overnight in 5.6mM glucose. For insulin measurements, 20 islets (n = 6 mice and n = 4 human islet donors) were incubated with DMEM containing 5.6mM glucose for 4 hours followed by DMEM containing 2mM, 7mM, or 14mM glucose concentrations for 45 minutes. Insulin secretion and total islet insulin content were analyzed using an ELISA-based detection kit (ALPCO Diagnostics), and data are presented as mean ± SEM.
Mouse glucose tolerance testing
Mouse glucose tolerance testing (GTT) was performed as previously described by injecting 2 mg/kg dextrose and monitoring blood glucose at the indicated time points after glucose injection (40). The mice were fed a normal chow diet and treated with tamoxifen at 7 weeks of age and allowed to recover after the last tamoxifen gavage for 2 weeks, and GTT was performed at 10 weeks of age.
Results
TASK-1 channels are functionally expressed in mouse and human β-cells
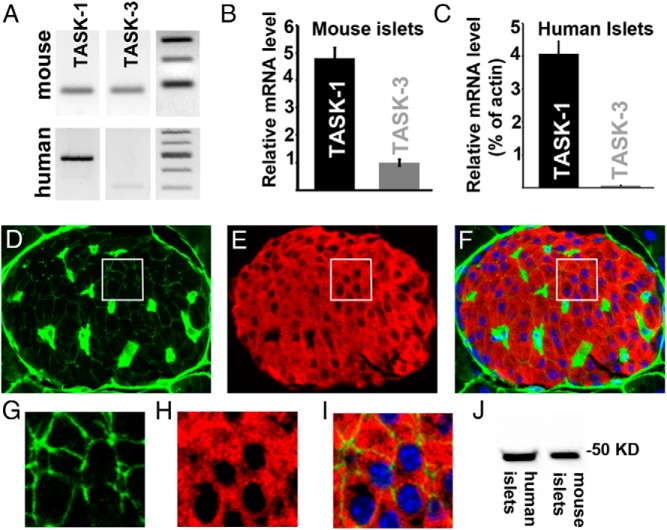
Transcriptome analysis has revealed that TASK-1 RNA is highly expressed in human and mouse islets and β-cells (25, 26). Thus, we characterized TASK-1 channel expression in mouse and human β-cells. Using RT-PCR, we observed that TASK-1 RNA is expressed in both mouse and human islets, which corroborates previous RNA sequencing studies (Figure 1A) (25, 26). The TASK-1–like channel TASK-3 was also detected in human and mouse islets with RT-PCR (Figure 1A). To quantitatively determine TASK-channel RNA levels, QRT-PCR analyses were performed and revealed that TASK-1 was the most abundant TASK channel transcript in both human (4.07% of the level of actin mRNA, n = 8) and mouse islets (Figure 1, B and C). We did not detect TASK-3 RNA in human islets with QRT-PCR and found only modest expression of TASK-3 RNA in mouse islets (Figure 1, B and C). To further examine TASK-1 channel expression, Western blots were performed on human and mouse islet protein samples. TASK-1 protein of the correct molecular mass (∼50 kDa) was detected in both human and mouse islets (Figure 1J). To test the islet-cell localization of TASK-1, immunofluorescence was performed on mouse islet sections. TASK-1 was found in the insulin-positive β-cell membranes of mouse pancreatic islets (Figure 1, D–I). The expanded field of view shown in Figure 1 (G–I) illustrates membrane localization of TASK-1 in mouse β-cells. Moreover, β-cell TASK-1 staining colocalizes with β-catenin, which confirms plasma membrane localization of TASK-1 channels (Supplemental Figure 1). Although staining was also observed in cells that resemble islet vasculature, this paper focused on the function of TASK-1 in the β-cell.
Figure 1.
TASK-1 potassium channels are expressed in pancreatic β-cells. A, RT-PCR detection of TASK-1 and TASK-3 RNA from mouse (top) and human (bottom) islets with DNA ladders on the right. B, QRT-PCR of TASK-1 and TASK-3 RNA expression levels in mouse islets. The data are presented as the relative abundance of TASK-1 to TASK-3 RNA levels that were derived from ΔΔCt values calculated using GAPDH as a housekeeping control. C, TASK-1 and TASK-3 RNA expression levels in human islets determined with TaqMan probes and normalized to actin RNA levels from the same samples. D–F, Immunofluorescence of a mouse pancreatic section for TASK-1 (green), insulin (red), and nuclei (blue) with an overlay of all signals in panel F. G–I, Enlargement of the white box from the images shown in panels D–F with TASK-1 (green), insulin (red), and nuclei (blue). J, Western blot of TASK-1 protein expression run with mouse and human islet protein samples.
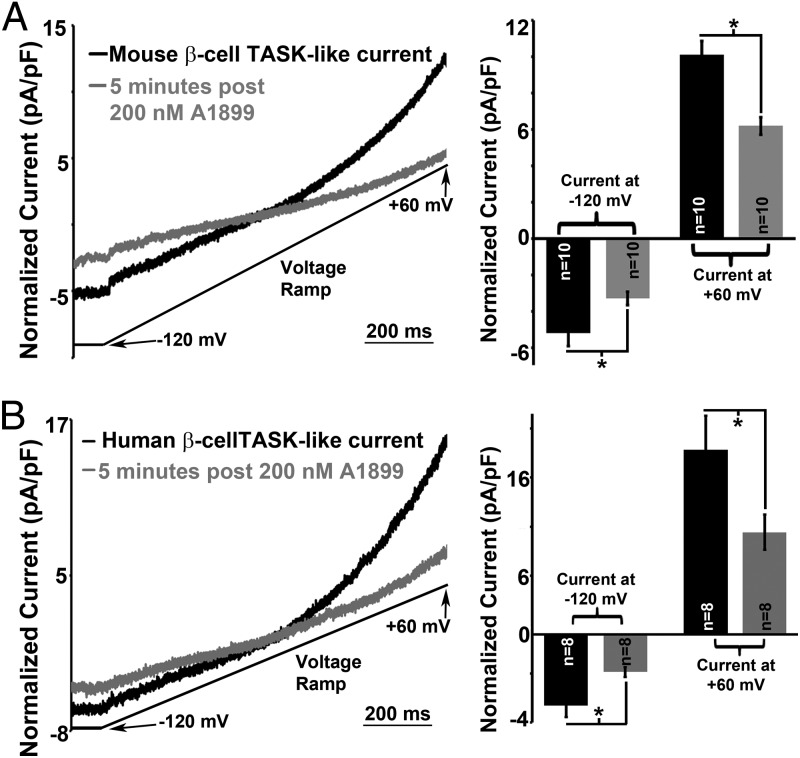
To determine whether β-cell TASK-1 protein generates functional currents, whole-cell voltage clamp recordings were used to measure TASK-1 currents. Currents with biophysical characteristics that correspond to cloned TASK-1 channels were present in mouse and human β-cells (Figure 2, A and C) (31). The β-cell TASK-1–like currents showed slight outward rectification, were insensitive to TEA or tolbutamide, and were also maintained when extracellular Ca2+ was removed (Figure 2, A and C). Thus, the TASK-1–like currents were not mediated via KATP, voltage-gated (Kv), or Ca2+-activated K+ channels. An inhibitor of TASK-1, A1899, was used to test whether the K2P current is mediated via TASK-1 (36). A1899 has been shown to inhibit TASK-1 (IC50 = 7nM) and TASK-3 (IC50 = 70nM). Addition of A1899 to β-cells revealed that TASK-1 is potentially responsible for 38.6% of the K2P currents in mouse β-cells and 44.7% of the K2P currents in human β-cells (Figure 2, B and D) (36).
Figure 2.
Mouse and human β-cells contain functional TASK-1 channels. A, Left panel, Inhibition of mouse β-cell TASK-1 currents with 200nM A1899 (the currents are normalized to cell capacitance; currents recorded in 14mM glucose, 100μM tolbutamide, and 20mM TEA; currents recorded in response to a voltage ramp from −120 to 60 mV over a 1-second interval). Right panel, Average mouse β-cell TASK-1 current amplitude at −120 mV and +60 mV potentials before or 5 minutes after A1899 treatment (±SEM; *, P < .05). B, Left panel, Inhibition of human β-cell TASK-1 currents with 200nM A1899; the currents were normalized to cell capacitance (currents recorded in 14mM glucose, 100μM tolbutamide. and 20mM TEA). Right panel, Average human β-cell TASK-1 current amplitude at −120 mV and +60 mV potentials before or 5 minutes after A1899 treatment (±SEM; *, P < .05).
TASK-1 currents regulate pancreatic β-cell Δψp
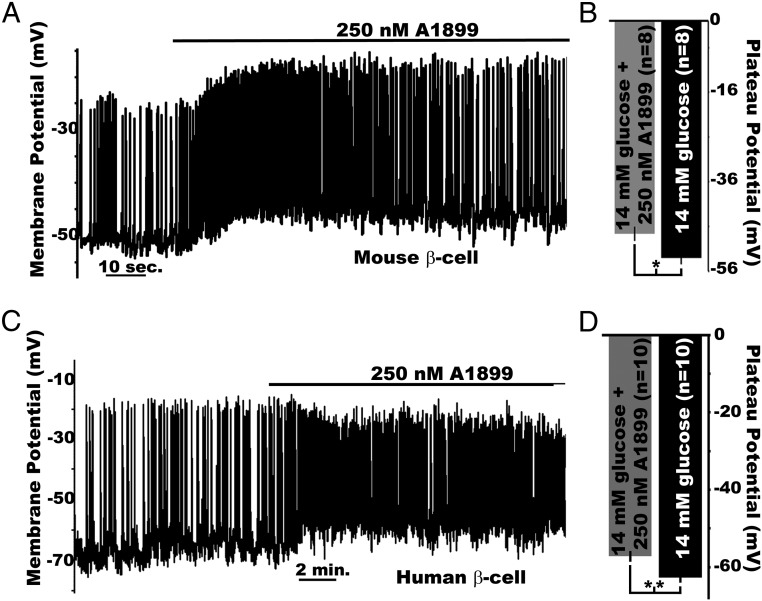
Although the β-cell TASK-1 channels generate small-amplitude K+ currents, modest K+ flux in pancreatic β-cells is predicted to play an important role in modulating the Δψp when KATP is inhibited (5–8). Therefore, β-cell TASK-1 channels were assessed for their influence on Δψp. Inhibition of TASK-1 channels with A1899 caused a significant depolarization (5.4 ± 0.49 mV) of the mouse β-cell Δψp in the presence of high glucose (14mM), which was maintained for the duration of A1899 treatment (Figure 3, A and B). However, A1899 did not significantly change the Δψp of mouse β-cells in low-glucose conditions (2mM, data not shown). Thus, TASK-1 currents depolarized the mouse Δψp when KATP was inhibited during glucose stimulation. Because human β-cell electrical excitability involves a slightly different ion channel cohort than mouse β-cells, we next assessed how human β-cells respond to TASK-1 inhibition. Human β-cells undergoing glucose-stimulated AP firing responded to A1899 treatment with Δψp depolarization (5.5 ± 0.58 mV, Figure 3, C and D). Interestingly, human β-cell AP amplitude was reduced after A1899 treatment. This presumably results from inactivation of voltage-gated sodium (NaV) channels after the Δψp depolarization. These data suggest that electrical excitability is slightly different between human and mouse β-cells after TASK-1 inhibition; however, they both undergo Δψp depolarization in response to A1899.
Figure 3.
TASK-1 currents control the β-cell plateau potential. A, Mouse β-cell electrical activity in response to 14mM glucose alone or with 250nM A1899 (black bar above trace). B, Plateau potential from where mouse β-cell APs fire, averaged for membrane voltage recordings immediately before (black bar) and 5 minutes after A1899 treatment (grey bar; ±SEM; *, P < .05). C, Human β-cell electrical activity recorded in the presence of high glucose alone (14mM) or with 250nM A1899 (black bar above trace). D, Plateau potentials from where human β-cell APs fire, averaged for voltage recordings immediately before (black bar) and 5 minutes after A1899 treatment (grey bar; ±SEM;*, P < .01).
TASK-1 limits glucose-stimulated β-cell calcium influx
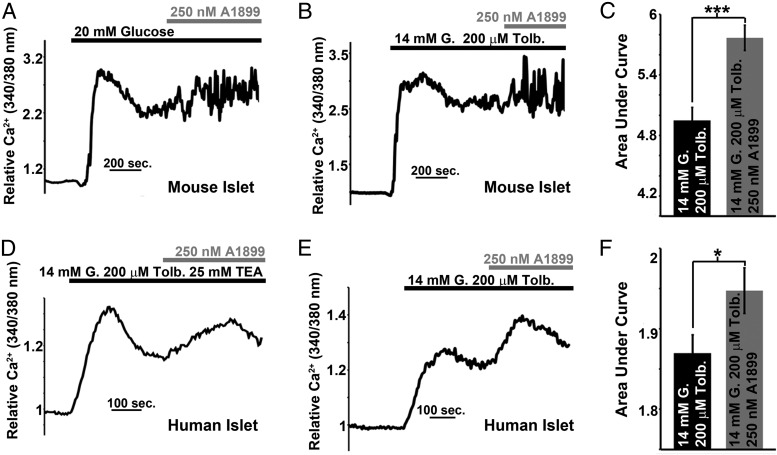
Pancreatic β-cell Ca2+ entry correlates with the degree of Δψp depolarization that occurs during glucose stimulation (41). Therefore, we next assessed mouse and human islet Ca2+ influx in response to TASK-1 inhibition. Although TASK-1 inhibition with A1899 did not significantly modify intracellular Ca2+ levels of mouse islets incubated in low glucose (2mM, data not shown), mouse islets stimulated with high glucose (14mM) showed a significant increase in Ca2+ influx in response to A1899 (Figure 4, A–C). Moreover, when KATP was inhibited with tolbutamide, mouse islets also showed an increase in Ca2+ influx after A1899 induced inhibition of TASK-1 channels (Figure 4, B and C). Although the mouse islet cell population is primarily β-cells (∼80%) and whole-islet Ca2+ responses are representative of β-cell Ca2+ levels, there is a greater proportion of α-cells in human islets (∼30%), and thus, whole human islet Ca2+ responses do not truly represent β-cell Ca2+ levels. Therefore, we tested the role of human TASK-1 channels on islet β-cell function under conditions that limit Ca2+ influx into α-cells. We used tolbutamide-induced KATP inhibition, which has been shown to reduce human islet α-cell Ca2+ influx and stimulate β-cell Ca2+ influx (42). Using this condition, we found an increase in human islet Ca2+ influx with glucose (14mM) and tolbutamide (100μM) stimulation that was significantly enhanced after A1899 treatment (Figure 4, D–F). Furthermore, the ability of A1899 to increase human islet Ca2+ was maintained in the presence of TEA; thus, the elevation of β-cell Ca2+ was not due to block of Kv potassium channels or large conductance Ca2+ activated potassium channels (BK, Figure 4D). These results suggest that human and mouse β-cell TASK-1 channels limit glucose-stimulated Ca2+ influx.
Figure 4.
Mouse and human islet Ca2+ influx is limited by TASK-1 activity. A, Mouse islet intracellular Ca2+ response switching from 2mM glucose to 14mM glucose (black bar) followed by the addition of 250mM A1899 (gray bar). B, Mouse islet Ca2+ response switching from 2mM glucose to 14mM glucose with 100μM tolbutamide (black bar) followed by the addition of 250nM A1899 (gray bar). C, Area under the Ca2+ influx curve of the mouse islets for 5 minutes in 14mM glucose with 100μM tolbutamide (black bar) and for 5 minutes immediately after addition of 250nM A1899 (gray bar; n = 84, ±SEM; ***, P < .001). D, Human islet Ca2+ response switching from 2mM glucose to 14mM glucose with 100μM tolbutamide and 25mM TEA (black bar) followed by the addition of 250nM A1899 (gray bar). E, Human islet Ca2+ response switching from 2mM glucose to 14mM glucose with 100μM tolbutamide (black bar) followed by the addition of 250nM A1899 (gray bar). F, Area under the Ca2+ influx curve of human islets for 5 minutes in 14mM glucose with 100μM tolbutamide (black bar) and for 5 minutes immediately after addition of 250nM A1899 (gray bar; n = 50, ±SEM; *, P < .05).
Conditional ablation of β-cell TASK-1 channels reduces K2P currents
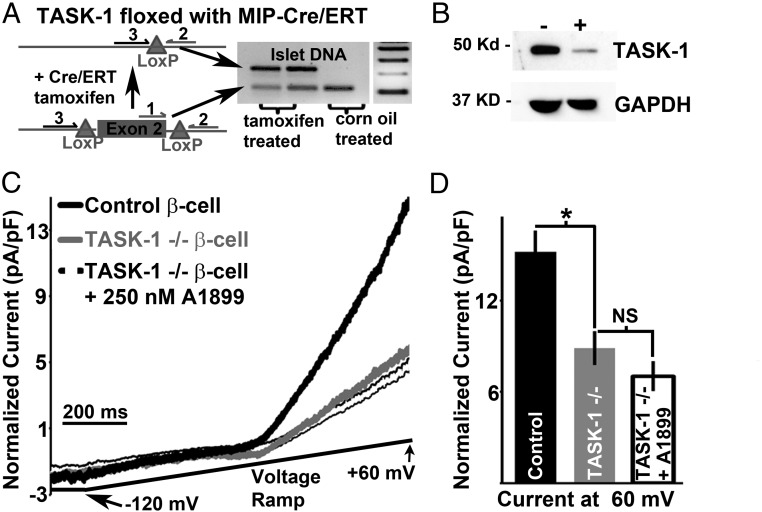
Next we sought to determine an in vivo role for β-cell TASK-1. This was accomplished by developing a mouse model that allows conditional ablation of β-cell TASK-1. These animals were generated by crossing mice with exon-2 of the TASK-1 gene KCNK3 floxed (34) and mice containing an MIP-Cre/ERT transgene (35). Tamoxifen treatment of the KCNK3 floxed MIP-Cre/ERT mice induced excision of exon-2 of β-cell KCNK3 as determined by PCR with genomic DNA isolated from islets of corn oil- or tamoxifen-gavaged mice (Figure 5A). A larger PCR product indicates excision of exon-2, and this is observed only with islet DNA from mice that were treated with tamoxifen (Figure 5A) (34). This results in loss of β-cell TASK-1 protein expression in most pancreatic islet β-cells, which was determined via immunofluorescent staining for TASK-1 and insulin (Supplemental Figure 2). We went on to examine how tamoxifen treatment of these mice influences islet TASK-1 protein levels (Figure 5B). Islet TASK-1 protein levels were significantly reduced in tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice compared with tamoxifen-treated MIP-Cre/ERT mice, which was determined via Western blot analysis (Figure 5B). To ensure that TASK-3 did not compensate for the loss of TASK-1 channels we also designed a mouse model that allows for conditional ablation of TASK-3 and TASK-1. This model was generated by crossing the KCNK3 floxed MIP-Cre/ERT mouse with a mouse that contains a floxed exon-2 of KCNK9, which codes for TASK-3 (Supplemental Figure 1). When treated with tamoxifen, this mouse shows excision of exon-2 of both KCNK3 as well as KCNK9 (Figure 5A and Supplemental Figure 3). Finally, a KCNK9 floxed MIP-Cre/ERT mouse was designed that allows for β-cell TASK-3 ablation alone (Supplemental Figure 3). Therefore, the KCNK3 floxed MIP-Cre/ERT mouse allows conditional β-cell ablation of TASK-1, the KCNK9 floxed MIP-Cre/ERT mouse allows conditional ablation of TASK-3, and the KCNK3 KCNK9 floxed MIP-Cre/ERT mouse allows conditional ablation of TASK-1 and TASK-3.
Figure 5.
Conditional ablation of β-cell TASK-1 reduces K2P current amplitude. A, Islet DNA from floxed KCNK3 MIP-Cre/ERT mice treated with tamoxifen or vehicle (corn oil) and assessed for exon-2 excision via PCR with the primers indicated by numbers 1 to 3. B, Western blot analysis of islet protein probed with TASK-1 and GAPDH antibodies. C, β-Cell TASK-1 currents from KCNK3 floxed MIP-Cre/ERT mice with or without 250nM A1899 treatment (gray trace and black outlined trace) or MIP-Cre/ERT mice (black trace) all treated with tamoxifen (the currents are normalized to cell capacitance; currents recorded in 14mM glucose, 100μM tolbutamide, and 20mM TEA; currents recorded in response to a voltage ramp from −120 mV to 60 mV over a 1-second interval). D, Average TASK-like current amplitude at +60 mV from control β-cells (black bar) and TASK-1–deficient β-cells with and without 250nM A1899 (gray bar and white bar, ±SEM; *, P < .05).
We used these mouse models to examine whether genetic removal of β-cell TASK channels reduces K2P currents. Similar to A1899 inhibition of TASK-1 channels, genetic depletion of β-cell TASK-1 channels caused a significant reduction of β-cell outward K2P currents (Figure 5, C and D). Furthermore, TASK-1–deficient β-cell K2P currents were not inhibited by 250nM A1899 (Figure 5, C and D), which indicates that TASK-3 does not compensate for the loss of β-cell TASK-1. Removal of β-cell TASK-3 channels alone (tamoxifen-treated KCNK9 floxed MIP-Cre/ERT mice) also did not significantly alter K2P currents compared with controls (tamoxifen-treated MIP-Cre/ERT, Supplemental Figure 3). Thus, TASK-1 channels are functionally expressed in mouse β-cells and are responsible for the A1899-sensitive K2P currents.
Ablation of β-cell TASK-1 increases Δψp depolarization and enhances Ca2+ influx
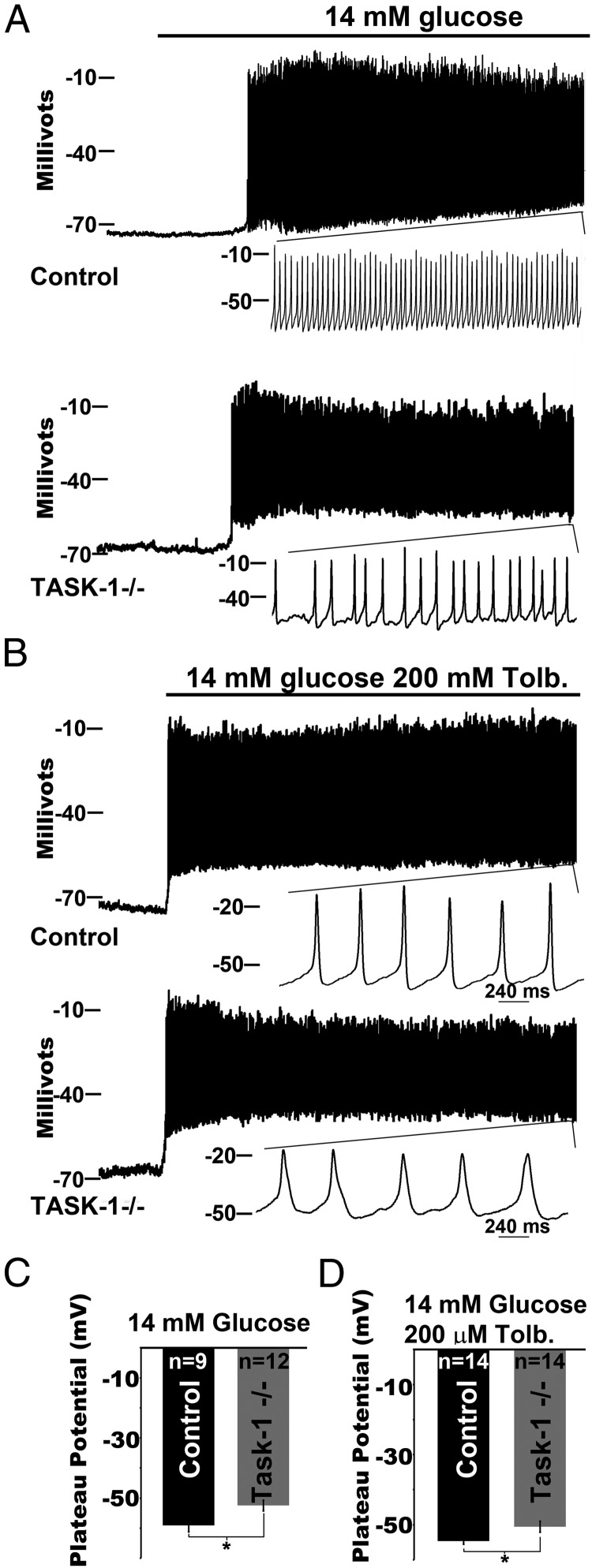
Because TASK-1 channels are active in β-cells, we next assessed whether TASK-1 currents control the β-cell Δψp. Using tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice, we found that loss of β-cell TASK-1 channels resulted in secretagogue-stimulated Δψp depolarization that correlated with the A1899-induced inhibition of TASK-1 channels. Glucose stimulation depolarized the β-cell plateau potential to a greater extent in tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice (−52.5 ± 1.79 mV) compared with controls (tamoxifen-treated MIP-Cre/ERT, −59.1 ± 2.11 mV, Figure 6A). As a control, we also assessed the β-cell plateau potential after removal of TASK-3 (tamoxifen-treated KCNK9 floxed MIP-Cre/ERT mice) and found no change from control β-cells (tamoxifen-treated MIP-Cre/ERT mice, Supplemental Figure 4). Inhibition of KATP with tolbutamide also caused a significantly greater Δψp depolarization of β-cells from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice (−50.6 ± 1.6 mV) compared with tamoxifen-treated controls (MIP-Cre/ERT, −54.6 ± 0.9 mV, Figure 6B). Depolarizing the β-cell plateau potential from where APs fire would be predicted to increase VDCC activity and Ca2+ entry. Indeed, previous studies have found that increasing VDCC activity with BAYK 8644 causes an increase in AP duration and a slowing of AP frequency (43). Interestingly, glucose and/or tolbutamide treatment of β-cells from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice results in a slowing of AP frequency and an increase in AP duration, which is most evident in response to tolbutamide (Figure 6B). Tolbutamide-induced AP firing frequency in tamoxifen-treated KCNK3 floxed MIP-Cre/ERT β-cells (2 ± 0.11 Hz) was significantly slower than in the tamoxifen-treated MIP-Cre/ERT β-cells (2.59 ± 0.2 Hz, P = .014). These data suggest that TASK-1 currents polarize the β-cell plateau potential, which increases AP firing frequency.
Figure 6.
β-Cell TASK-1 ablation results in a depolarization of the plateau potential. A, Electrical activity recorded from control (top trace, β-cells from tamoxifen-treated MIP-Cre/ERT mice) or TASK-1−/− (bottom trace, β-cells from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice) β-cells in response to 14mM glucose (black bar). B, Electrical activity recorded from control (top trace, β-cells from tamoxifen-treated MIP-Cre/ERT mice) or TASK-1−/− (bottom trace, β-cells from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice) β-cells in response to 14mM glucose and 100μM tolbutamide (black bar). C, The plateau potential from where control (black bar) and TASK-1−/− (gray bar) β-cells fire, averaged for membrane voltage recordings 5 minutes after 14mM glucose (±SEM; *, P < .05). D, The plateau potential from where control (black bar) and TASK-1−/− (gray bar) β-cells fire APs, averaged for membrane voltage recordings 5 minutes after 14mM glucose and 100μM tolbutamide (±SEM; *, P < .05).
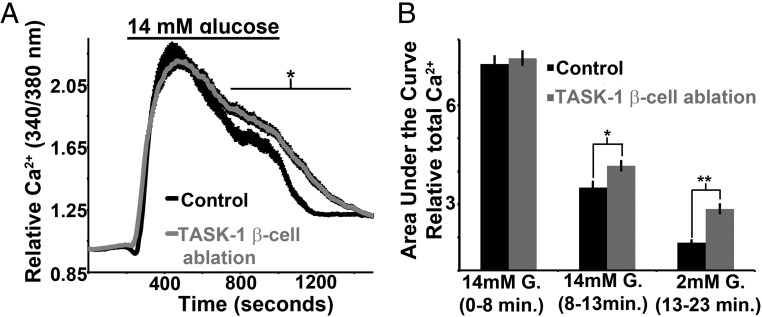
We then assessed whether TASK-1 channel modulation of β-cell excitability also influences glucose-stimulated islet Ca2+ influx. Islets from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice and control mice (MIP-Cre/ERT) showed similar baseline Ca2+ levels in low (2mM) glucose (Figure 7, A and B). After glucose stimulation (14mM), islets from controls and TASK-1 ablation mice reached equivalent Ca2+ levels; however, during prolonged glucose stimulation, the Ca2+ levels were maintained at a significantly greater level in islets from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice compared with controls (tamoxifen-treated MIP-Cre/ERT, Figure 7, A and B). Interestingly, islets with removal of β-cell TASK-1 channels also show a significantly slower return to baseline Ca2+ when switched from high (14mM) to low (2mM) glucose when compared with tamoxifen-treated controls (MIP-Cre/ERT, Figure 7, A and B). Although β-cell TASK-1 limits glucose-stimulated islet Ca2+ influx, there is no change in glucose-stimulated islet Ca2+ influx after removal of β-cell TASK-3 (Supplemental Figure 5). To confirm that A1899 influences islet Ca2+ influx via TASK-1 inhibition, islets from KCNK3 floxed MIP-Cre/ERT mice were assessed for Ca2+ influx with 250nM A1899. TASK-1 ablation resulted in a loss of A1899-induced (250nM) changes in islet Ca2+ with or without ablation of TASK-3 (Supplemental Figure 6). These data suggest that TASK-1 modulates islet Ca2+ influx under conditions when KATP is inhibited. Because Ca2+ entry is coupled to insulin secretion, these data suggest the possibility that TASK-1 may also modulate GSIS.
Figure 7.
Ablation of mouse β-cell TASK-1 results in increased glucose-stimulated Ca2+ influx. A, Mouse islet Ca2+ responses from control (black trace, islets from tamoxifen-treated MIP-Cre/ERT mice, n = 72) and TASK-1 β-cell ablation backgrounds (gray trace, islets from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice, n = 76) switching from 2mM glucose to 14mM glucose (black bar above). B, Area under the Ca2+ influx curve of the control (black bars) and TASK-1 β-cell ablation (gray bars) mouse islets for the first 8 minutes in 14mM glucose, from 8 to 13 minutes in glucose, and for 10 minutes immediately after reduction of glucose from 14mM to 2mM (±SEM; *, P < .05; **, P < .01).
TASK-1 channels modulate glucose homeostasis by limiting β-cell insulin secretion
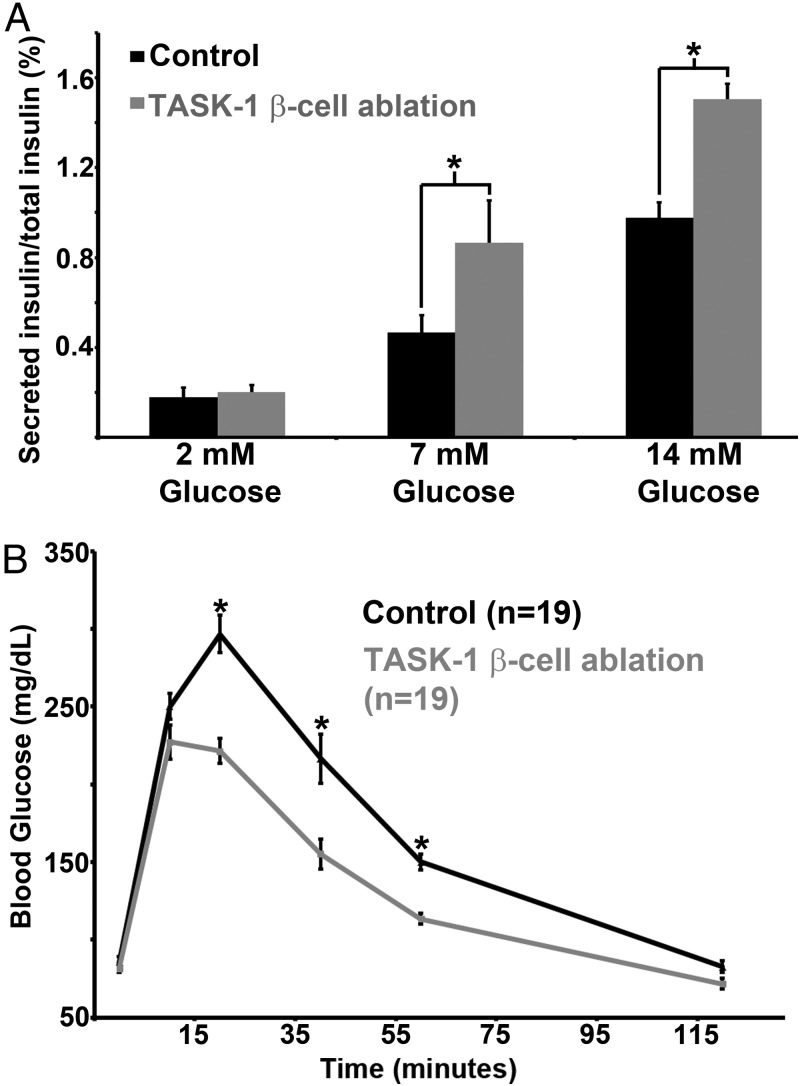
Finally, we examined whether TASK-1 channel modulation of β-cell Ca2+ entry influences insulin secretion and glucose homeostasis. Removal of β-cell TASK-1 significantly enhanced GSIS when islets were switched from 5.6mM glucose to either 7mM or 14mM glucose (Figure 8A). Similarly, inhibition of islet TASK-1 channels with 250nM A1899 caused a significant increase in GSIS (Supplemental Figure 7). However, when β-cell TASK-1–deficient islets were switched from 5.6mM glucose to 2mM glucose, their basal insulin secretion was equivalent to control tamoxifen-treated islets (MIP-Cre/ERT, Figure 8A). Fasting glucose levels were also equivalent in tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice and control mice (tamoxifen-treated MIP-Cre/ERT, Figure 8B). The influence of β-cell TASK channels on glucose homeostasis was also assessed using IP GTTs. Interestingly, removal of β-cell TASK-1 channels resulted in significantly lower blood glucose levels 20 to 60 minutes after ip glucose challenge when compared with control mice (tamoxifen-treated MIP-Cre/ERT, Figure 8B). Because this mouse model specifically ablates β-cell TASK-1 channels, the changes in GTT observed indicate that β-cell TASK-1 channels influence glucose homeostasis. However, removal of β-cell TASK-3 channels does not significantly perturb mouse glucose tolerance when compared with controls (Supplemental Figure 8A). Moreover, β-cell TASK-1 and TASK-3 ablation caused a similar improvement in glucose tolerance observed in mice with TASK-1 ablation alone, which indicates that TASK-3 does not compensate for the loss of TASK-1 (Supplemental Figure 8B). Thus, the data suggest that TASK-1 channels play an important role in limiting β-cell GSIS and thus modulating glucose homeostasis.
Figure 8.
Ablation of mouse β-cell TASK-1 enhances GSIS and increases glucose tolerance. A, Static insulin secretion from control (black bars, islets from tamoxifen-treated MIP-Cre/ERT mice, n = 19) and TASK-1 β-cell ablation (gray bars, islets from tamoxifen-treated KCNK3 floxed MIP-Cre/ERT mice, n = 19) islets in response to 2mM, 7mM, and 14mM glucose solutions. B, GTT on mice with β-cell TASK-1 ablation (gray line, n = 19, tamoxifen-treated female KCNK3 floxed MIP-Cre/ERT mice) and control mice (black line, n = 19, tamoxifen-treated female MIP-Cre/ERT mice) animals (±SEM; *, P < .05).
Discussion
KATP channels hyperpolarize the islet-cell Δψp; however, when KATP is inhibited, the remaining background K+ conductance that stabilizes the islet-cell Δψp from where APs fire has not been characterized. When KATP channels are inhibited after glucose metabolism, the resistance of the β-cell membrane increases; this sensitizes the Δψp to small currents such as those mediated via background K2P K+ channels (44). The results presented here demonstrate that TASK-1 K2P channels play a key role in modulating the plateau potential from where APs fire (45–47). The data suggest that inhibition or ablation of β-cell TASK-1 depolarizes the Δψp only during conditions when KATP channel activity is also inhibited. Thus, mice with loss of β-cell TASK-1 have normal fasting glucose levels but improved glucose tolerance and enhanced GSIS. This results from an increase in glucose-stimulated islet Ca2+ influx after TASK-1 β-cell ablation. Our studies on human and mouse β-cell TASK-1 channels have therefore elucidated an important glucose-dependent role for TASK-1 channels in stabilizing the plateau potential and limiting GSIS.
Like most pancreatic β-cell K+ channels, TASK-1 channels limit Ca2+ influx and insulin secretion (40, 48, 49). Many β-cell K+ channels are activated during the AP and influence AP firing frequency or shape, whereas the TASK-1 channel is constitutively active and limits Δψp depolarization from where APs fire. TASK-1 channel inhibition leads to a ∼5-mV depolarization of the β-cell membrane potential; however, because TASK-1 channels are responsible for only ∼50% of the total K2P current, other K2P channels limit depolarization of the plateau potential in the absence of TASK-1. The plateau potential plays an important role in regulating VDCC activation. Modest depolarization of the plateau potential results in an increase of VDCC activity and Ca2+ influx. However, β-cell VDCCs also show voltage-dependent inactivation after significant depolarization. For example, VDCC inactivation is observed after treatment of β-cells with high extracellular K+ levels, which depolarizes the β-cell plateau potential by ∼20 mV (20, 50, 51). This indicates that K+ channels are responsible for limiting plateau potential depolarization and preventing VDCC inactivation. Thus, we speculate that islet K2P channels limit plateau potential depolarization to help reduce voltage-dependent inactivation of VDCCs.
Although both human and mouse β-cells show depolarization of the plateau potential after TASK-1 inhibition, A1899 causes species-specific effects on β-cell AP firing. Mouse β-cells show a transient increase in AP firing frequency after A1899 treatment followed by stabilization at a slower frequency with longer duration APs; interestingly, human β-cell APs show reduced amplitude after TASK-1 inhibition. Mouse β-cell Δψp depolarization has previously been shown to transiently increase AP firing frequency due to an increase in VDCC activity (43); therefore, the transient increase in mouse β-cell AP firing frequency after A1899 treatment is also probably due to increasing Δψp depolarization and increased VDCC activity. However, increasing VDCC activity eventually increases AP duration and reduces frequency (43), which is observed in β-cells with TASK-1 ablation or TASK-1 channel inhibition after stabilization of AP firing frequency. Glucose stimulation of mouse β-cells results in a transient rapid firing of APs that eventually stabilize at a slower firing frequency. A1899 is added after glucose-induced stabilization of the β-cell AP firing frequency, which results in a transient increase in AP firing frequency. This is not distinguishable in β-cells without TASK-1 immediately after glucose stimulation due to the rapid AP firing frequency observed under these conditions. Both mouse and human islet Ca2+ levels both increase after TASK-1 inhibition, which further indicates that VDCC activity is increased after Δψp depolarization with A1899. VDCCs are responsible for the AP upstroke in mouse β-cells. Previous studies have found that increasing VDCC activity in mouse β-cells results in increased AP duration and decreased frequency (43). Similarly, inhibition or ablation of mouse β-cell TASK-1 channels causes a decrease in AP frequency and increased AP duration, which would be expected after depolarization-induced activation of VDCCs. The human β-cell AP has a slightly different cohort of ion channels involved in the AP upstroke, which is mediated by both VDCCs and NaV channels. Thus, the reduction in human AP amplitude after Δψp depolarization may result from inactivation NaV channels, which is not observed in mouse β-cells because NaV channels are inactive under physiological glucose concentrations. However, mouse neuronal APs that use NaV and VDCC channels do show decreased amplitude after deletion of TASK-1 and TASK-3 channels (30). Inactivation of human β-cell NaV channels leads to VDCCs becoming the primary ion channel responsible for the upstroke of the AP (48). Because the peak Ca2+ current through VDCCs is significantly less than Na+ current through NaVs, VDCC-dependent APs have diminished amplitude compared with NaV-dependent APs (52). Modest depolarization of the human β-cell Δψp may thus serve an important role in shifting the primary channels active during the AP upstroke from NaV to VDCC and thus increase Ca2+ entry and insulin secretion. Therefore, conditions that regulate human β-cell TASK-1 currents may allow for small changes in of the Δψp that will significantly influence Ca2+ influx and insulin secretion.
Because TASK-1 channels regulate human islet insulin secretion, TASK-1 may also regulate human glucose homeostasis. Although there is currently no information about the influence of TASK-1 on human glucose homeostasis, it has recently been reported that mutations in TASK-1 channels cause pulmonary hypertension (PH) (53). PH-causing mutations in TASK-1 produce a channel subunit that acts as a dominant-negative, which results in a loss of TASK-1 channel activity in individuals with these mutations (53). Therefore, islet TASK-1 channels would be inhibited in these PH patients, and future studies are required to test whether their glucose homeostasis is also perturbed (53). TASK-1 is expressed in pulmonary artery smooth muscle cells, and reducing TASK-1 currents in these cells has been proposed to cause PH by modifying vascular tone (54). Interestingly, we find that TASK-1 is also expressed in islet vasculature; thus, TASK-1 may also play a role in regulating blood flow through the islet. It will be of interest to investigate the role of islet vascular TASK-1 channels in relation to the changes in islet vascular tone that occur during glucose stimulation as well as during the pathogenesis of diabetes (55, 56).
Although this is the first report to describe a role for islet K2P channels in modulating glucose homeostasis, other K2P channels are highly expressed in human islets and are also emerging as important regulators of islet function. A recent genome-wide association study found a nonsynonymous coding sequence polymorphism in the K2P encoding gene KCNK16 associated with a predisposition for developing type 2 diabetes (57). This suggests the possibility that defects in islet K2P channel function may influence the pathogenesis of type 2 diabetes (57). Another islet K2P channel, the tandem pore domain weak inward rectifier potassium channel (TWIK-1), has been shown to be expressed in β-cells, but this channel is primarily permeable to Na+ instead of K+ (58). Thus, the TWIK-1 channel modulates β-cell Δψp depolarization instead of polarization. Although TASK-1 is more permeable to K+ than Na+, certain conditions that block TASK-1 channel K+ flux such as acidic extracellular pH cause a collapse of the K+ pore, making it permeable to Na+ (59). Thus, future experiments will determine whether certain physiological stimuli that inhibit TASK-1 channel K+ flux also result in Na+ flux through these channels. This could result in a significant enhancement of Δψp depolarization due to the combined influence of inhibition of K+ efflux and stimulation of Na+ influx. The importance of K2P channels during islet hormone secretion is becoming clear; thus, studies are required to determine whether these channels can be used as potential therapeutic targets for increasing insulin secretion in patients with type 2 diabetes(60).
In conclusion, this study suggests that TASK-1 plays an important role modulating the β-cell plateau potential. TASK-1 channel activity limits Ca2+ influx and insulin secretion during conditions that stimulate β-cell excitability such as glucose stimulation. Thus, TASK-1 polarizes the β-cell plateau potential modulating GSIS and glucose homeostasis.
Acknowledgments
We are grateful to Louis H. Philipson for his support and helpful discussions related to this manuscript. We also thank Douglas Bayliss for the floxed KCNK3 and floxed KCNK9 mice and also for helpful discussions related to data from the manuscript.
This work was supported by National Institutes of Health Grants DK096122 and DK081666 (to D.A.J.) as well as a Pilot and Feasibility grant through the Vanderbilt University Diabetes Research Training Center (P60 DK20593).
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 3729
- AP
- action potential
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GSIS
- glucose-stimulated insulin secretion
- GTT
- glucose tolerance testing
- HM
- hypoglossal motoneuron
- KATP
- ATP-sensitive K+ channels
- KRB
- Krebs-Ringer buffer
- NaV
- voltage-dependent sodium
- Δψp
- plasma membrane potential
- PH
- pulmonary hypertension
- QRT-PCR
- quantitative real time PCR
- TASK-1
- 2-pore-domain acid-sensitive potassium channel
- TEA
- tetraethylammonium
- VDCC
- voltage-dependent Ca2+ channel.
References
- 1. Rorsman P, Bokvist K, Ammälä C, Eliasson L, Renström E, Gäbel J. Ion channels, electrical activity and insulin secretion. Diabetes Metab. 1994;20(2):138–145 [PubMed] [Google Scholar]
- 2. Dean PM, Matthews EK. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970;210(2):255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meissner HP, Henquin JC, Preissler M. Potassium dependence of the membrane potential of pancreatic B-cells. FEBS Lett. 1978;94(1):87–89 [DOI] [PubMed] [Google Scholar]
- 4. Remedi MS, Nichols CG. Hyperinsulinism and diabetes: genetic dissection of beta cell metabolism-excitation coupling in mice. Cell Metab. 2009;10(6):442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravier MA, Nenquin M, Miki T, Seino S, Henquin JC. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology. 2009;150(1):33–45 [DOI] [PubMed] [Google Scholar]
- 6. Gopel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol. 1999;521(Pt 3):717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren J, Sherman A, Bertram R, et al. Slow oscillations of KATP conductance in mouse pancreatic islets provide support for electrical bursting driven by metabolic oscillations. Am J Physiol Endocrinol Metab. 2013;305(7):E805–E817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275(13):9270–9277 [DOI] [PubMed] [Google Scholar]
- 9. Ashcroft FM, Ashcroft SJ, Harrison DE. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988;400:501–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarvin JT, Sachs G, Pace CS. Glucose-induced electrical activity in pancreatic beta-cell: modulation by pH. Am J Physiol. 1981;241(5):C264–C268 [DOI] [PubMed] [Google Scholar]
- 11. Henquin JC. Metabolic control of potassium permeability in pancreatic islet cells. Biochem J. 1980;186(2):541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson CM, Croghan PC, Scott AM, Bangham JA. Potassium and rubidium permeability and potassium conductance of the beta-cell membrane in mouse islets of Langerhans. Q J Exp Physiol. 1986;71(2):205–222 [DOI] [PubMed] [Google Scholar]
- 13. Atwater I, Dawson CM, Ribalet B, Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979;288:575–588 [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang M, Houamed K, Kupershmidt S, Roden D, Satin LS. Pharmacological properties and functional role of Kslow current in mouse pancreatic beta-cells: SK channels contribute to Kslow tail current and modulate insulin secretion. J Gen Physiol. 2005;126(4):353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Göpel SO, Kanno T, Barg S, et al. Activation of Ca(2+)-dependent K(+) channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J Gen Physiol. 1999;114(6):759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duprat F, Lauritzen I, Patel A, Honoré E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007;30(11):573–580 [DOI] [PubMed] [Google Scholar]
- 17. Mathie A, Al-Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol. 2010;588(Pt 17):3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henquin JC, Meissner HP. Effects of theophylline and dibutyryl cyclic adenosine monophosphate on the membrane potential of mouse pancreatic beta-cells. J Physiol. 1984;351:595–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atwater I, Eddlestone GT, Ribalet B, Rojas E. Calcium channel voltage noise across the beta-cell membrane in Islet of Langerhans of the mouse. J Physiol. 1979;291:69P–70P [PubMed] [Google Scholar]
- 20. Willenborg M, Belz M, Schumacher K, Paufler A, Hatlapatka K, Rustenbeck I. Ca(2+)-dependent desensitization of insulin secretion by strong potassium depolarization. Am J Physiol Endocrinol Metab. 2012;303(2):E223–E233 [DOI] [PubMed] [Google Scholar]
- 21. Willenborg M, Hatlapatka K, Ghaly H, Belz M, Panten U, Rustenbeck I. Studies of first phase insulin secretion using imposed plasma membrane depolarization. Front Biosci (Schol Ed). 2011;3:662–679 [DOI] [PubMed] [Google Scholar]
- 22. Meissner HP, Schmeer W. The significance of calcium ions for the glucose-induced electrical activity of pancreatic beta cells. In: Ohnishi ST, Endo M, eds. The Mechanism of Gated Calcium Transport Across Biological Membranes. New York, NY: Academic Press; 1981:157–165 [Google Scholar]
- 23. Hatlapatka K, Willenborg M, Rustenbeck I. Plasma membrane depolarization as a determinant of the first phase of insulin secretion. Am J Physiol Endocrinol Metab. 2009;297(2):E315–E322 [DOI] [PubMed] [Google Scholar]
- 24. Kang D, Choe C, Kim D. Functional expression of TREK-2 in insulin-secreting MIN6 cells. Biochem Biophys Res Commun. 2004;323(1):323–331 [DOI] [PubMed] [Google Scholar]
- 25. Bramswig NC, Everett LJ, Schug J, et al. Epigenomic plasticity enables human pancreatic alpha to β cell reprogramming. J Clin Invest. 2013;123(3):1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8(3):e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16(17):5464–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K(+) channel subunits: kinetic properties and expression in rat heart. Am J Physiol. 1999;277(5 Pt 2):H1669–H1678 [DOI] [PubMed] [Google Scholar]
- 29. Davies LA, Hu C, Guagliardo NA, et al. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci USA. 2008;105(6):2203–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. González JA, Jensen LT, Doyle SE, et al. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30(1):57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25(2):399–410 [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Talley EM, Patel N, et al. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci USA. 2006;103(9):3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weber M, Schmitt A, Wischmeyer E, Döring F. Excitability of pontine startle processing neurones is regulated by the two-pore-domain K+ channel TASK-3 coupled to 5-HT2C receptors. Eur J Neurosci. 2008;28(5):931–940 [DOI] [PubMed] [Google Scholar]
- 34. Mulkey DK, Talley EM, Stornetta RL, et al. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27(51):14049–14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59(12):3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Streit AK, Netter MF, Kempf F, et al. A specific two-pore domain potassium channel blocker defines the structure of the TASK-1 open pore. J Biol Chem. 2011;286(16):13977–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Philipson LH, Rosenberg MP, Kuznetsov A, et al. Delayed rectifier K+ channel overexpression in transgenic islets and beta-cells associated with impaired glucose responsiveness. J Biol Chem. 1994;269(45):27787–27790 [PubMed] [Google Scholar]
- 39. Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37(1):15–26 [DOI] [PubMed] [Google Scholar]
- 40. Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammälä CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 2007;6(3):229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drews G, Krippeit-Drews P, Düfer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–163 [DOI] [PubMed] [Google Scholar]
- 42. MacDonald PE, De Marinis YZ, Ramracheya R, et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5(6):e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lebrun P, Atwater I. Effects of the calcium channel agonist, BAY K 8644, on electrical activity in mouse pancreatic B-cells. Biophys J. 1985;48(6):919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Atwater I, Ribalet B, Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978;278:117–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyazaki S, Taniguchi H, Moritoh Y, et al. Nuclear hormone retinoid X receptor (RXR) negatively regulates the glucose-stimulated insulin secretion of pancreatic β-cells. Diabetes. 2010;59(11):2854–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kovács I, Pocsai K, Czifra G, et al. TASK-3 immunoreactivity shows differential distribution in the human gastrointestinal tract. Virchows Arch. 2005;446(4):402–410 [DOI] [PubMed] [Google Scholar]
- 47. Sirois JE, Lei Q, Talley EM, Lynch C, 3rd, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20(17):6347–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jacobson DA, Mendez F, Thompson M, Torres J, Cochet O, Philipson LH. Calcium-activated and voltage-gated potassium channels of the pancreatic islet impart distinct and complementary roles during secretagogue induced electrical responses. J Physiol. 2010;588(Pt 18):3525–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacDonald PE, Sewing S, Wang J, et al. Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic beta-cells enhances glucose-dependent insulin secretion. J Biol Chem. 2002;277(47):44938–44945 [DOI] [PubMed] [Google Scholar]
- 50. Dawson CM, Atwater I, Rojas E. Potassium-induced insulin release and voltage noise measurements in single mouse islets of Langerhans. J Membr Biol. 1982;64(1–2):33–43 [DOI] [PubMed] [Google Scholar]
- 51. Satin LS, Cook DL. Calcium current inactivation in insulin-secreting cells is mediated by calcium influx and membrane depolarization. Pflugers Arch. 1989;414(1):1–10 [DOI] [PubMed] [Google Scholar]
- 52. Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57(6):1618–1628 [DOI] [PubMed] [Google Scholar]
- 53. Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369(4):351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olschewski A, Li Y, Tang B, et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res. 2006;98(8):1072–1080 [DOI] [PubMed] [Google Scholar]
- 55. Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab. 2010;298(4):E807–E814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dai C, Brissova M, Reinert RB, et al. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes. 2013;62(12):4144–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cho YS, Chen CH, Hu C, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2011;44(1):67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chatelain FC, Bichet D, Douguet D, et al. TWIK1, a unique background channel with variable ion selectivity. Proc Natl Acad Sci USA. 2012;109(14):5499–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma L, Zhang X, Zhou M, Chen H. Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J Biol Chem. 2012;287(44):37145–37153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Investig Drugs. 2007;8(7):555–562 [PubMed] [Google Scholar]