Abstract
Background
Paclitaxel has become a standard drug used in a number of common cancers. At first long infusions were used to reduce the rate of inflow of the drug and as a result reduce the occurrence of hypersensitivity types of allergic reactions. Trials with shorter durations of infusion, and using a cocktail of anti‐allergic drugs to prevent hypersensitivity reactions, some randomised, were begun. These were interpreted as showing that effectiveness of treatment was not lessened by a short infusion time. These studies also appeared to show that some important toxicities were less common with short infusions and that they were more convenient for the patient and the hospital.
Objectives
To assess the effectiveness and toxicity of short versus long infusions of paclitaxel for any advanced adenocarcinoma.
Search methods
We searched the Cochrane Gynaecological Cancer Review Group Specialised Register, The Cochrane Central Register of Controlled Trials (CENTRAL) Issue 1, 2009, MEDLINE and EMBASE up to March 2009. We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included trials and contacted experts in the field, as well as drug companies.
Selection criteria
The review was restricted to randomised controlled trials (RCTs) of single agent paclitaxel or paclitaxel with other drugs, where the only variable was the duration of paclitaxel infusion. The review only includes patients with advanced adenocarcinoma.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Where possible the data were synthesised in meta‐analyses.
Main results
We identified six trials that met our inclusion criteria. The trials compared 3, 24 and 96 hour infusions and one trial examined different schedules (1 versus 3 day). From the included RCTs we found no evidence of a difference between short and long infusions in terms of overall and progression‐free survival and tumour non‐response. In most cases a greater proportion of adverse events and severe toxicity occurred in the 24 hour infusion group compared to the 3 hour group with many of the analyses being highly statistically significant (RR = 0.32, 95% CI 0.22, 0.47, RR = 0.06, 95% CI 0.02, 0.17, RR = 0.59, 95% CI 0.40, 0.88, RR = 0.52, 95% CI 0.28, 0.97 for severe hypersensitivity, febrile neutropenia, sore mouth and diarrhoea outcomes respectively). Although a meta analysis of three trials found that 3 hour infusions were associated with a statistically significant increase in the risk of neurosensory changes compared with 24 hour infusions (RR = 1.26, 95% CI 1.09 to 1.46). Adverses events were not comprehensively reported for any of the other comparisons. Outcomes were incompletely documented and QoL outcomes were not reported in any of the trials. The strength of the evidence is weak in this review as it is based on meta analyses of very few trials or single trial analyses and all trials were at moderate risk of bias and two were published in abstract form only.
Authors' conclusions
Ideally, large, multi‐centre supporting trials are needed as outcomes were incompletely reported in included trials in this review. It may be beneficial to design a multi‐arm trial comparing 3, 24 and 96 hour infusions or maybe looking at different schedules. In the absence of such trials, the decision to offer short or long infusions in advanced adenocarcinoma may need to be individualised, although it certainly appears that women have less toxicity, apart from sensory nerve damage, with a shorter infusion. Efficacy appearing similar regardless of infusion duration.
Keywords: Female; Humans; Adenocarcinoma; Adenocarcinoma/drug therapy; Adenocarcinoma/pathology; Antineoplastic Agents, Phytogenic; Antineoplastic Agents, Phytogenic/administration & dosage; Antineoplastic Agents, Phytogenic/adverse effects; Breast Neoplasms; Breast Neoplasms/drug therapy; Breast Neoplasms/pathology; Drug Administration Schedule; Ovarian Neoplasms; Ovarian Neoplasms/drug therapy; Ovarian Neoplasms/pathology; Paclitaxel; Paclitaxel/administration & dosage; Paclitaxel/adverse effects; Randomized Controlled Trials as Topic
Plain language summary
Comparing the time taken to give paclitaxel (an anticancer drug) in advanced adenocarcinoma
Paclitaxel is derived from Yews (a type of tree), and can be used to treat for several cancers such as lung, womb, ovary and breast. It was initially given by a long infusion (injection) over 24 hours, with premedication to avoid any allergic reactions. It was also thought this method would be more active against tumours. Six randomised trials were included in this review, which found that short (three hour) infusions are more convenient and caused significantly fewer adverse (side) effects (i.e. decreased white blood cell counts, fever, infection or sore mouth). With short‐infusion paclitaxel there is no obvious loss of effectiveness when compared with longer infusions, although further clinical trials are needed to be sure of this.
Background
Paclitaxel, a natural product of Yew trees, is an important drug in the management of cancer. It has an established first‐line role in the management of a number of cancers (McGuire 1996). During the initial development of the drug (phase I studies to find an appropriate dose of the drug) a variety of different doses and schedules were used. When phase II trials began to look for anti‐cancer activity these were required by the National Cancer Institute (NCI, USA) to use long infusion times (24 hours) because of the perceived increased risk of serious hypersensitivity reactions when paclitaxel was given by short infusions (3 hours or less) (Eisenhauer 1994). All infusions were also given with premedication designed to further reduce the risk of hypersensitivity reactions. Initial results from early studies had, however, shown that short infusion times were associated with reduced toxicity to white blood cells, without apparent reduction in anti‐cancer activity (Eisenhauer 1994). Theoretically, a long duration of infusion might be expected to be associated with greater anti‐tumour activity (Huizing 1993).
Subsequently it was felt that it was likely to be safe to use short infusions of paclitaxel provided that it was given with premedication using a combination of three drugs to reduce the risk of a hypersensitivity reaction. A number of randomised clinical trials have compared the effectiveness and toxicity of short versus long infusion times (both with premedication). In general these have been interpreted as showing equal anti‐cancer effectiveness with both long and short infusions and a smaller fall in the white blood count with shorter infusions. Short infusions were also more convenient to all concerned.
Since paclitaxel is an important relatively new anticancer drug, definitive information on the effect of varying the duration of infusion is required as this might alter how effective it is at controlling cancer.
In addition to hypersensitivity reactions, paclitaxel commonly causes side‐effects which can limit its use. These include bone marrow suppression, hair loss, tiredness, nausea and vomiting, muscle pains and damage to nerves that mainly effects sensation. These toxic effects might also vary according to the duration of paclitaxel infusion.
Objectives
To assess the effectiveness and toxicity of short versus long infusions of paclitaxel for any advanced adenocarcinoma. The null hypothesis tested was that infusions of long or short duration have the same effects on efficacy and toxicity.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) were included.
Types of participants
Patients with advanced adenocarcinoma, regardless of type (it was considered that most trials were likely to be in ovarian or breast cancer), receiving chemotherapy with paclitaxel, including patients who had failed prior therapy with other anti‐cancer drugs, or who had received adjuvant chemotherapy or no previous chemotherapy.
Types of interventions
Paclitaxel as a single anti‐cancer drug (used with premedication designed to prevent hypersensitivity reactions in both arms). Only trials comparing infusions of shorter versus longer duration were included.
Types of outcome measures
Primary outcomes
Overall survival (OS): survival until death from all causes.
Secondary outcomes
-
Efficacy:
progression‐free survival (PFS)
-
objective tumour response rate
primary tumour response
overall tumour response
duration of response
Toxicity: classified according to CTCAE 2006: Particular attention was paid to: toxic deaths, neutropenia, granulocytopenia, thrombocytopenia, febrile neutropenia, infection, anaemia, neurotoxicity, arthralgia/myalgia, cardiac effects, hypersensitivity reactions, hair loss, nausea/vomiting, and sore mouth.
Quality of life: measured using a scale that has been validated through reporting of norms in a peer reviewed publication.
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews. The following electronic databases were searched:
The Cochrane Gynaecological Cancer Collaborative Review Group Specialised \trial Register
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 1, 2009
MEDLINE up to March 2009
EMBASE up to March 2009
The CENTRAL, MEDLINE and EMBASE search strategies aiming to identify RCTs comparing low versus high duration infusions of paclitaxel for any advanced adenocarcinoma before March 2009 are presented in Appendix 1, Appendix 2, and Appendix 3 respectively.
Databases were searched from January 1966 until 2001 in the original review and up to March 2009 in this updated version.
All relevant articles found were identified on PubMed and using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and Grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, www.cancer.gov/clinicaltrials and M.D. Anderson Cancer Centre, Gynecologic Oncology Group (GOG) were searched for ongoing trials. The main investigators of one trial identified by searching the grey literature (Holmes 1998) were contacted for further information as it was published in abstract form and we were unable to include any data from the trial in any of the analyses.
Handsearching
The citation list of relevant publications, abstracts of scientific meetings and list of included studies were checked through hand searching and experts in the field contacted to identify further reports trials. Reports of conferences were hand searched in the following sources:
British Journal of Cancer
British Cancer Research Meeting
Annual Meeting of European Society of Medical Oncology (ESMO)
Annual Meeting of the American Society of Clinical Oncology (ASCO)
The reference lists of all eligible trials, key textbooks, and previous systematic reviews were searched for additional trials.
All reports relevant to the review topics were identified on PubMed and the "related article feature" was used for identification of other trials.
Correspondence
Authors of relevant trials were contacted to ask if they knew of further data which may or may not have been published.
Data collection and analysis
Selection of studies
First version of review
Citations were retrieved electronically, de‐duplicated and examined by CW and CG independently. Trials not meeting the inclusion criteria were discarded. Copies of potentially relevant papers were obtained and eligibility assessed independently by CW and CG. Disagreements were resolved by discussion between the two review authors.
Second version of review
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were then removed and the remaining references examined by two review authors (AB, CW) independently. Those studies which clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers were assessed independently by two review authors (AB, CW). Disagreements were resolved by discussion between the two review authors. Reasons for exclusion are documented.
Data extraction and management
For included studies, data were abstracted as recommended in chapter 7 of the Higgins 2011. This included data on publication details (including author, year of publication and journal citation details), setting (including country), study design and methodology, characteristics of patients (inclusion criteria, age, stage, comorbidity, previous treatment, number enrolled in each arm) and interventions (drug dose and duration and concomitant medication, the number of cycles and frequency), risk of bias, duration of follow‐up, outcomes (outcome definition, unit of measurement, upper and lower limits used for scales and whether high or low score is good, number of participants allocated to each intervention group and sample size and missing participant details) and deviations from protocol.
Data on outcomes were extracted as below:
For time to event (OS and PFS) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these were not reported, we estimated them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio (RR).
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in groups to which they were assigned.
The time points at which outcomes were collected and reported were noted.
In this version of the review data were abstracted independently by two review authors (AB, CW) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using the Cochrane Collaboration's tool and the criteria specified in chapter 8 of the Higgins 2011. This included assessment using the following questions and criteria:
Sequence generation
Was the allocation sequence adequately generated?
Yes: e.g. a computer‐generated random sequence or a table of random numbers
No: e.g. date of birth, clinic id‐number or surname
Unclear: e.g. not reported.
Allocation concealment
Was allocation adequately concealed?
Yes: e.g. where the allocation sequence could not be foretold
No: e.g. e.g. allocation sequence could be foretold by patients, investigators or treatment providers
Unclear: e.g. not reported
Blinding
Assessment of blinding was restricted to blinding of outcome assessors, since it would not be possible to blind participants and treatment providers to the different durations of infusion.
Was knowledge of the allocated interventions adequately prevented during the study?
Yes
No
Unclear
Incomplete reporting of outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study; we noted whether or not loss to follow‐up was not reported.
Were incomplete outcome data adequately addressed?
Yes, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
No, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear if loss to follow‐up was not reported
Selective reporting of outcomes
Are reports of the study free of suggestion of selective outcome reporting?
Yes e.g. if review reported all outcomes specified in the protocol
No, otherwise
Unclear, if insufficient information available.
Other potential threats to validity
Was the study apparently free of other problems that could put it at a high risk of bias?
Yes
No
Unclear
In this version of the review the risk of bias tool was applied independently by two reviewers (AB, CW) and differences were resolved by discussion. Results were presented in both a risk of bias graph and a risk of bias summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
For time to event data, we used the HR, where possible.
For dichotomous outcomes, we used the RR.
Dealing with missing data
We did not impute missing outcome data for any outcomes.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Assessment of reporting biases
We were unable to assess reporting bias as only five trials met our inclusion criteria, with at most only three being pooled in any one meta analysis.
Funnel plots corresponding to meta‐analyses in the review were not examined to assess the potential for small study effects as there were only five included trials, with at most only three being pooled in any one meta analysis.
Data synthesis
If sufficient, clinically similar trials were available, their results were pooled in meta‐analyses.
For time‐to‐event data (e.g. OS and PFS), HRs were pooled using the generic inverse variance facility of RevMan 5.
For any dichotomous outcomes (e.g. adverse events, and numbers of patients who relapse or die, if it is not possible to treat these outcomes as time‐to‐event data), the RR was calculated for each trial and these were then pooled.
Random effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Sub‐group analyses were performed, treating each tumour type separately.
Sensitivity analysis
No sensitivity analyses were performed as all included trials were at high risk of bias.
Results
Description of studies
Results of the search
The original search strategy identified 1879 unique references. The title and abstract screening of these references identified four trials as potentially eligible for this review. The updated search strategy identified 392 references in the Specialised Register, 392 in CENTRAL, 869 in MEDLINE and 1069 in EMBASE. When the search results were merged into Endnote and duplicates were removed there were 2139 unique references. The title and abstract screening of these references identified 19 studies as potentially eligible for the review. A number of other randomised trials were excluded at the first sift stage because the chemotherapy drugs and/or doses were not the same in both arms. One potentially eligible trial (in abstract form only (Sulkes 1994)) was excluded as some of the patients were probably included in another report (Peretz 1995) and there were no outcome data. Overall, the full text screening of these 19 studies excluded 14 for the reasons described in the table Characteristics of excluded studies. The remaining five RCTs met our inclusion criteria and are described in the table Characteristics of included studies.
Searches of the grey literature identified one additional relevant trial (Holmes 1998), but this was presented in abstract form only and did not contribute to any of the analyses.
Included studies
The six eligible trials were reported by Eisenhauer 1994; Greco 1995; Holmes 1998; Peretz 1995; Smith 1999; and Spriggs 2007. All trials were multi‐centre apart from Greco 1995 which was a single centre trial (Sarah Cannon Minnie Pearl Cancer Centre) and three of the trials were supported by Bristol‐Myers Squibb with the exception being the trials of Greco 1995, Holmes 1998 and Spriggs 2007 where it was unclear.
The trials differed in the following ways:
Three trials were in breast cancer (Holmes 1998; Peretz 1995; Smith 1999), two in ovarian cancer (Eisenhauer 1994; Spriggs 2007) and the trial of Greco 1995 was in multiple cancer types.
Four trials used standard dose chemotherapy (Eisenhauer 1994; Greco 1995; Peretz 1995; Spriggs 2007), one used high dose chemotherapy (Smith 1999) and the trial of Holmes 1998 used high dose for three hour infusions and standard use for 96 hour infusions.
Four were fully published in peer reviewed journals (Eisenhauer 1994; Greco 1995; Peretz 1995; Spriggs 2007), while the other two were available only as abstracts (Holmes 1998; Peretz 1995).
Detailed descriptions of these trials are given below and in table Characteristics of included studies.
Trials comparing 3 versus 24 hour infusions
Eisenhauer 1994
The trial of Eisenhauer 1994 was a 2 x 2 factorial trial which randomised 407 women with ovarian cancer to either 3 or 24 hour paclitaxel infusions and to either a 135 mg/ m2 or 175 mg/ m2 dose of paclitaxel with 391 (96%) patients eligible and assessable for toxicity and 382 (94%) were eligible for response. Each of the two sets of factorial groups was well balanced with respect to most characteristics, apart from the proportion of patients who had progressed on their most recent chemotherapy regime. This was higher in the 3‐hour group than the 24‐hour group. The way that toxicity was reported varied according to the type of toxicity (platelets: grade IV, white cells: grade III or greater and others any grade or undefined). There were worries over hypersensitivity reactions at the time so these are given prominence. Patients in the trial did not routinely receive colony stimulating factors. Follow‐up duration was short when this trial was reported, though many relapses and deaths occurred in the first year.
Peretz 1995
In the trial of Peretz 1995, 521 patients with relapsed breast cancer were randomised to 3 or 24 hour infusions of 175mg/ m2 paclitaxel as a single agent. Data for all end points are reported in this study. Data available for this trial was in abstract only and lacked detail. The total number of eligible patients was presented, but the number randomised to each arm was not given. Attempts to get further information from the lead author of the abstract and Bristol‐Myers Squibb failed. A published abstract was also found giving details of Israeli experience of 3 and 24 hour infusions in both breast and ovarian cancer (Sulkes 1994). It seems likely that the breast cancer patients were included in the Peretz 1995 trial, although there is no cross reference to confirm this. In addition no data on outcomes were presented, so Sulkes 1994 was not included in this review.
Peretz 1995 compared the paclitaxel dose described above every 21 days in women with advanced, usually previously treated, breast cancer. Dose escalation was allowed in both arms. Two‐thirds of patients were pretreated with anthracyclines, 24% being resistant to anthracyclines. Colony stimulating factors were not routinely used in either arm, but more patients in the 3 hour arm had dose escalations (65% versus 34%, P < 0.001).
The method of collecting toxicity data was not defined and the scales used were also undefined though it seems likely that they are four point scales. The criteria for response are undefined and it is not clear whether there was independent assessment of response. Full details on the eligibility of patients is not available. Data on time to progression is available, but there were no data on OS.
Smith 1999
The trial of Smith 1999 randomised 563 women with breast cancer to either 3 or 24 hour infusions of high‐dose paclitaxel (250 mg/ m2). In this study, patients receiving the longer duration infusion were given prophylactic G‐CSF, designed to stop the white blood cell count falling, in an attempt to reduce the risk of infection in patients with a low granulocyte count. Patients receiving the shorter infusion of paclitaxel received G‐CSF only if they had such an episode of infection. Data from this trial could be used for all of the end points of the review apart from that of white cell toxicity and infection. Data on the effect of the different durations of infusion of paclitaxel on white cells could have been misleading because of the different polices regarding the use of G‐CSF in the two trial arms.
Trial comparing 3 versus 96 hour infusions
Holmes 1998
The Holmes 1998 trial planned to accrue 226 eligible patients with measurable‐evaluable metastatic breast cancer (MBC), but it was unclear how many were actually randomised or analysed when the results were presented. The trial is reported in abstract form only and lacked any sort of detail. Women were randomised to receive either paclitaxel 250 mg/m2 for 3 hour infusions or 140 mg/m2 for 96 hour infusions repeated every 21 days. Granulocyte colony‐stimulating factor (G‐CSF) was added only if women experienced neutropenic fever or infection then dose‐reduction. Patients with MBC were stratified by (1) doxorubicin‐sensitivity (doxorubicin‐resistant: progression during treatment for MBC or within 6 months after adjuvant doxorubicin) and (2) number of prior regimens (inclusive of adjuvant: 0 or 1 versus 2 or 3).
We attempted to get further information from the lead author of the abstract but as of March 2011 there was no response. The abstract concluded that there was no significant difference in overall response (OR), duration or survival and that OR was low (possibly due to stringent OR requirements (20% metabolic response) and the fact the trial was a multicenter trial). Toxicity was evaluable in 123 patients treated from March 1994 to October 1995 (data not shown). The trial reported that the 96 hour arm had fewer toxic effects, but that this was less convenient. Furthermore, they added that these data do not justify the extra logistical support required for 96 hour infusion.
Trials comparing 24 hour versus longer infusion schedules
Greco 1994
The Greco 1995 trial randomised 56 women with advanced cancer, either resistant or refractory, to initial standard therapy or with an untreated primarily resistant tumour type. Before randomisation, patients were stratified according to performance status, primary disease site, and previous chemotherapy. The trial included 17 (30.50%) women with breast cancer, 16 (28.5%) with non‐small cell lung cancer (NSCLC), nine (16%) with ovarian cancer, five (9%) with small cell lung cancer and nine (16%) women had other cancer types. Paclitaxel was infused as a single dose of 135 mg/m2 over 1 hour or divided into 3 doses infused over 1 hour on 3 consecutive days. There was no dose escalation and patients did not receive prophylactic granulocyte colony‐stimulating factor (G‐CSF).
Spriggs 2007
The Spriggs 2007 trial randomised 293 women (of which 280 were eligible) with sub‐optimal stage III or IV epithelial ovarian cancer, fallopian tube or primary peritoneal cancer. The trial regimens were allocated from randomly permuted blocks of treatments with an equal number of each study treatment within each block. The trial included 92 (33%) women with performance status of 0, 155 (55%) with a status of 1 and 33 (12%) women had a status of 2. Women received six cycles of cisplatin and either paclitaxel 135 mg/m2 during 24 hours or paclitaxel 120mg/m2 during 96 hours. Colony stimulating factors were not routinely used in either arm and there were no dose escalations.
Patients in all five included trials received prophylactic medication to reduce the risk of hypersensitivity reactions.
Reporting of outcome data
Efficacy
Two trials reported OS and three reported PFS. We estimated the HR for OS and PFS for the comparison of 3 versus 24 hour infusions in the trial of Smith 1999 and the HR for PFS in the Eisenhauer 1994 trial. We extracted the exact log rank P‐value from the Kaplan‐Meier plots and the total number of reported deaths and cases of progression in each group and used the methods of Parmar 1998. The trial of Spriggs 2007 that compared 24 versus 96 hour infusions explicitly reported adjusted HRs and 95% confidence intervals (CIs) for OS and PFS.
The HR for OS and PFS in the trial of Spriggs 2007 was adjusted for: initial measurable disease status (present versus absent), performance status (0 versus 1 versus 2), histology (clear cell or mucinous adenocarcinoma versus other cell types), and stage of disease (III versus IV).
For the distribution of these factors at baseline for each trial by treatment arm see the table Characteristics of included studies.
Overall tumour response was reported in all five trials. Specific time points at which this was assessed was not reported in any of the trials, but the range of cycle length of chemotherapy was mentioned. Most trials reported a range of cycles of between one and eight, with most trials reporting up to six cycles. The majority of cycles given were three weekly. We analysed in terms of tumour non‐response rather than tumour response, so that there was a consistent reference group and RRs favouring 3 hour (3 versus 24 hour comparison) and 24 hour (24 versus 96 hour comparison) infusions were consistently on the left of the line of no effect on the forest plots.
Toxicity
Four trials (Eisenhauer 1994; Greco 1995; Smith 1999; Spriggs 2007) reported acute toxicity in detail though the type of scale used was not described and different cut off points were used and information was frequently already combined by site or grade. Extracted data were grouped in the reported grades and pooled since toxicity is unlikely to be confounded by the tumour type. Late toxicity was not commented on in any of the trials. WHO.
Individual trials in this review did not report the type of toxicity scale used and each reported the data differently, apart from the trials of Greco 1995 and Spriggs 2007 which used the ECOG and GOG scales respectively. All used scales with a four point system.
Quality of life
Quality of life data were reported in only one trial (Eisenhauer 1994). Patients were given a score out of ten based on a published five item questionnaire for cancer patients (Spitzer 1981). Baseline scores were compared with scores after 6 months.
Excluded studies
Fourteen references were excluded (numerous other ones were nested into some of the included studies as they were duplicate publications or commentaries), after obtaining the full text, for the following reasons:
Three trials (Atad 1997; Connelly 1996; Kudelka 1999) did not include a comparison of short versus long duration infusions of paclitaxel.
Two references (Boddy 2000; Keung 1993) included abstracts of other possible included trials from the title and abstract sift (Boddy 2000 was an abstract of the trial of Boddy 2001 and Keung 1993 was an abstract of the Huizing 1993 trial).
Three crossover trials (Boddy 2001; Jennens 2003; Rischin 1996) were excluded as the primary outcomes in this review were OS and PFS. The trials of Boddy 2001 and Rischin 1996 also had a pharmacokinetic focus.
Four trials (Calvert 1999; Gianni 1995; Huizing 1993; Mross 2002) did not report outcome measures specified in our protocol and appeared to have a pharmacokinetic focus. The trial of Gianni 1995 also seemed to vary carboplatin dose as well as duration of paclitaxel.
One reference (Nannan 1999) reported a study that did not appear to be an RCT. The study compared 1 hour versus 3 hour infusion but had a pharmacokinetic focus. Tumour response was reported but five of the seven women in the 3 hour regimen crossed over to the 96 hour regimen.
The Sulkes 1994 reference appeared to discuss women with breast cancer who had already been reported in the trial of Peretz 1995 (a co‐author on this paper). In addition no data on outcomes was reported.
For further details of all the excluded studies see the table Characteristics of excluded studies.
Risk of bias in included studies
All six trials were at high risk of bias: they satisfied no more than three of the criteria that we used to assess risk of bias ‐ see Figure 1, Figure 2.
1.
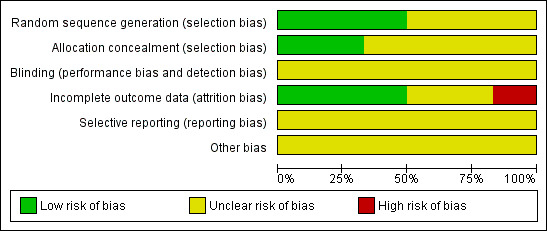
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
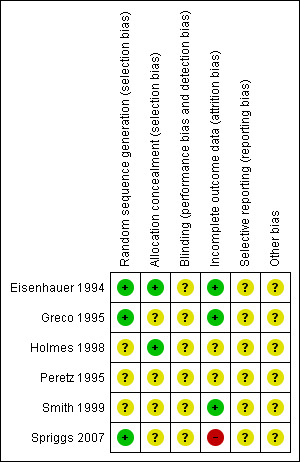
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Three trials (Eisenhauer 1994; Greco 1995; Spriggs 2007) reported the method of generation of the sequence of random numbers used to allocate women to treatment arms, but only the trial of Eisenhauer 1994 reported concealment of this allocation sequence from patients and healthcare professionals involved in the trial. While the abstract of Holmes 1998 did not provide details of the method of sequence generation, the authors did report that patients were randomised at a central data management office so concealment was likely to be adequate. It was unclear whether the healthcare professionals who assessed disease progression were blinded in any of the trials. It was also unclear whether the trials reported all the outcomes that they assessed or whether any additional biases were present. In three of the trials (Eisenhauer 1994; Greco 1995; Smith 1999) more than 80% of women who were enrolled were assessed at endpoint, it was unclear in two trials that presented only an abstract (Holmes 1998; Peretz 1995) and in the trial of Spriggs 2007 less than 80% of the women enrolled were assessed at endpoint.
Effects of interventions
For dichotomous outcomes, we were unable to estimate a RR for comparisons of treatments if one or both treatment groups experienced no events, as in the hypersensitivity outcome comparing 1 and 3 day infusion schedules. We did however compute the RR in Analysis 4.1 as the default continuity correction in RevMan for the 3 versus 24 hour comparison of women with febrile neutropenia in the trial of Eisenhauer 1994 was satisfactory. This was due to the percentage in the long duration arm being significantly higher than in the short duration arm, meaning that a small increment added to the zero count still adequately demonstrated the magnitude of the difference (0 out of 187 versus 24 out of 204 in the 3 hour and 24 hour groups respectively).
4.1. Analysis.
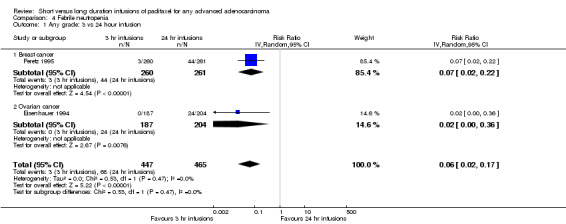
Comparison 4 Febrile neutropenia, Outcome 1 Any grade: 3 vs 24 hour infusion.
Since only a small number of trials were included in meta‐analyses, funnel plots were not examined.
Survival
Overall survival (risk of death)
3 versus 24 hour infusion
Using a HR to compare the survival experience of women in the two treatment groups, the trial of Smith 1999 found no statistically significant difference in the risk of death between the 3 hour and 24 hour infusion groups (HR = 0.97, 95% CI 0.78 to 1.20, Analysis 1.1).
1.1. Analysis.
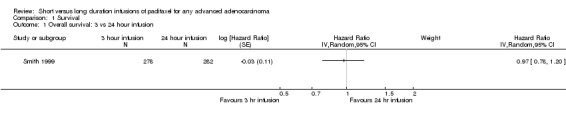
Comparison 1 Survival, Outcome 1 Overall survival: 3 vs 24 hour infusion.
24 versus 96 hour infusion
The trial of Spriggs 2007 found no statistically significant difference in overall survival between the 24 hour and 96 hour infusion groups, after adjustment for important prognostic factors (HR = 1.17, 95% CI 0.90 to 1.52, Analysis 1.2).
1.2. Analysis.

Comparison 1 Survival, Outcome 2 Overall survival: 24 vs 96 hour infusion.
Progression‐free survival
3 versus 24 hour infusion
Meta‐analysis of two trials (Eisenhauer 1994; Smith 1999), assessing 942 participants, found no statistically significant difference in the number of women with disease progression between the 3 hour and 24 hour infusion groups (HR = 0.84, 95% CI 0.58 to 1.23, Analysis 1.3). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent substantial heterogeneity (I2 = 73%).
1.3. Analysis.
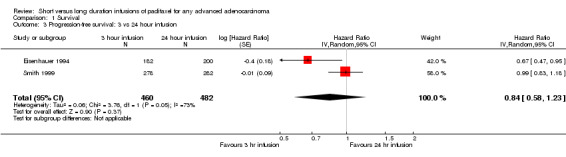
Comparison 1 Survival, Outcome 3 Progression‐free survival: 3 vs 24 hour infusion.
24 versus 96 hour infusion
The trial of Spriggs 2007 found no statistically significant difference in the number of women with disease progression between the 24 hour and 96 hour infusion groups, after adjustment for important prognostic factors (HR = 1.00, 95% CI 0.78 to 1.28, Analysis 1.4).
1.4. Analysis.
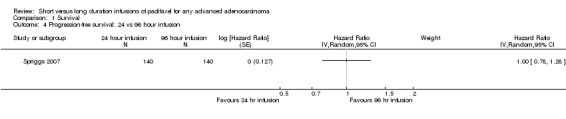
Comparison 1 Survival, Outcome 4 Progression‐free survival: 24 vs 96 hour infusion.
Tumour non‐response
We meta‐analysed tumour non‐response rather than tumour response, so that there was a consistent reference group and RRs favouring 3 hour (3 versus 24 hour comparison) and 24 hour (24 versus 96 hour comparison) infusions were consistently on the left of the line of no effect on the forest plots.
Non‐response was defined as treatment having no effect on the tumour. Complete and partial response were grouped together and were deemed 'response'.
Overall tumour non‐response
3 versus 24 hour infusion
Meta‐analysis of three trials (Eisenhauer 1994; Peretz 1995; Smith 1999), assessing 1423 participants, found no statistically significant difference (although the results approached borderline significance) in the number of women with overall tumour non‐response between the 3 hour and 24 hour infusion groups (RR = 1.07, 95% CI 0.98 to 1.17, Analysis 2.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent moderate heterogeneity (I2 = 37%). The conclusions were similar in subgroups that compared 3 hour and 24 hour infusions for patients with breast cancer and ovarian cancer.
2.1. Analysis.
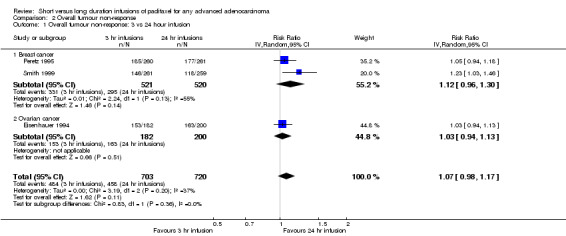
Comparison 2 Overall tumour non‐response, Outcome 1 Overall tumour non‐response: 3 vs 24 hour infusion.
24 versus 96 hour infusion
The trial of Spriggs 2007 found no statistically significant difference in overall tumour non‐response between the 24 hour and 96 hour infusion groups (RR = 1.25, 95% CI 0.83 to 1.90, Analysis 2.2).
2.2. Analysis.

Comparison 2 Overall tumour non‐response, Outcome 2 Overall tumour non response: 24 vs 96 hour infusion.
1 versus 3 day schedule
The trial of Greco 1995 found no statistically significant difference in overall tumour non‐response between the 1 and 3 day schedules (RR = 0.88, 95% CI 0.67 to 1.14, Analysis 2.3).
2.3. Analysis.

Comparison 2 Overall tumour non‐response, Outcome 3 Overall tumour non‐response: 1 vs 3 day schedule.
Neutropenia or granulocytopenia
3 versus 24 hour infusion
Meta‐analysis of two trials (Eisenhauer 1994; Peretz 1995), assessing 912 participants, found that 3 hour infusions were associated with a large and statistically significant decrease in the risk of neutropenia or granulocytopenia compared with 24 hour infusions (RR = 0.32, 95% CI 0.22 to 0.47, Analysis 3.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent considerable heterogeneity (I2 = 77%). The conclusions were similar in subgroups that compared 3 hour and 24 hour infusions for patients with breast cancer and ovarian cancer (see analyses 3.1.1 and 3.1.2). The evidence from the breast cancer trial of high dose paclitaxel (Smith 1999) was not included in the meta analysis because of the different patterns of use of G‐CSF in the two arms of the trial (G‐CSF was given prophylactically in the 24 hour infusion arm to reduce the effect of the high dose of paclitaxel on the white cell blood count).
3.1. Analysis.
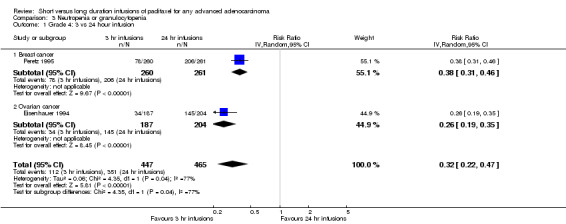
Comparison 3 Neutropenia or granulocytopenia, Outcome 1 Grade 4: 3 vs 24 hour infusion.
Febrile neutropenia
3 versus 24 hour infusion
Data from the two assessable trials above also found that 3 hour infusions were associated with a large and statistically significant decrease in the risk of febrile neutropenia compared to 24 hour infusions (RR = 0.06, 95% CI 0.02 to 0.17, Analysis 4.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%). The conclusions were similar in subgroups that compared 3 hour and 24 hour infusions for patients with breast cancer and ovarian cancer (see analyses 4.1.1 and 4.1.2).
Oral mucositis (sore mouth)
3 versus 24 hour infusion
Meta‐analysis of two trials (Eisenhauer 1994; Peretz 1995), assessing 912 participants, found that 3 hour infusions were associated with a statistically significant decrease in the risk of oral mucositis compared with 24 hour infusions (RR = 0.59, 95% CI 0.40 to 0.88, Analysis 5.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent substantial heterogeneity (I2 = 70%). The conclusions were similar in the trial of Peretz 1995 which included women with breast cancer (see analysis 5.1.1), but not in the trial of Eisenhauer 1994 as there was no statistically significant difference in the risk of mucositis between the 3 and 24 hour infusion groups for women with ovarian cancer (see analysis 5.1.2).
5.1. Analysis.
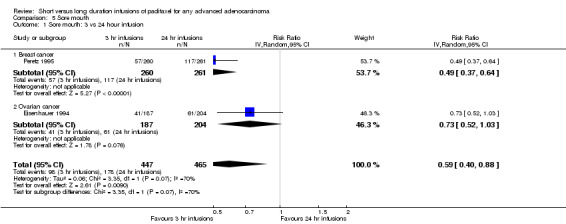
Comparison 5 Sore mouth, Outcome 1 Sore mouth: 3 vs 24 hour infusion.
Nausea and vomiting
3 versus 24 hour infusion
Meta‐analysis of two trials (Eisenhauer 1994; Smith 1999), assessing 948 participants, found no statistically significant difference in the risk of nausea or vomiting between the 3 hour and 24 hour infusion groups (RR = 0.75, 95% CI 0.24 to 2.35, Analysis 6.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent considerable heterogeneity (I2 = 80%). The conclusions were similar in the trial of Eisenhauer 1994 which included women with ovarian cancer (see analysis 6.1.2), but not in the trial of Smith 1999 as there was a statistically significant decrease in risk of nausea or vomiting in the 3 hour infusion group compared to the 24 hour group for women with breast cancer (see analysis 6.1.1).
6.1. Analysis.
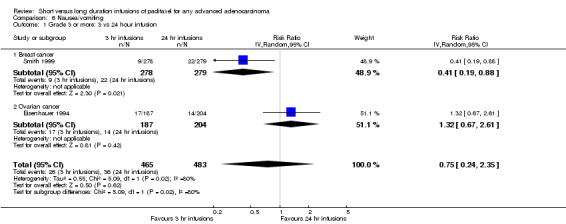
Comparison 6 Nausea/vomiting, Outcome 1 Grade 3 or more: 3 vs 24 hour infusion.
Neurosensory change
3 versus 24 hour infusion
Meta‐analysis of three trials (Eisenhauer 1994; Peretz 1995; Smith 1999), assessing 1,469 participants, found that 3 hour infusions were associated with a statistically significant increase in the risk of neurosensory changes compared with 24 hour infusions (RR = 1.26, 95% CI 1.09 to 1.46, Analysis 7.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent moderate heterogeneity (I2 = 34%). The conclusions differed in the breast cancer and ovarian cancer subgroups where statistical significance was not reached at the 5% level in the ovarian cancer trial of Eisenhauer 1994 (see analysis 7.1.1 and see analysis 7.1.2).
7.1. Analysis.
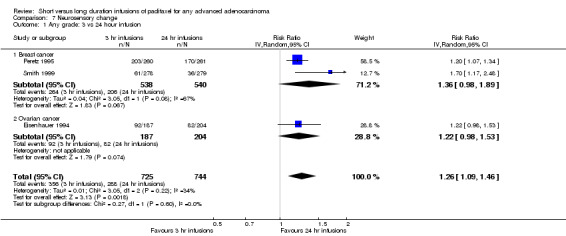
Comparison 7 Neurosensory change, Outcome 1 Any grade: 3 vs 24 hour infusion.
Grade III or IV toxicity was uncommon at standard doses, but was more common at the high doses used in Smith (Smith 1999).
Muscle, joint and bone pain
3 versus 24 hour infusion
Meta‐analysis of two trials (Eisenhauer 1994; Smith 1999), assessing 948 participants, found no statistically significant difference in the risk of Muscle, joint and bone pain between the 3 hour and 24 hour infusion groups (RR = 1.07, 95% CI 0.92 to 1.23, Analysis 8.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%). The conclusions were similar in subgroups that compared 3 hour and 24 hour infusions for patients with breast cancer and ovarian cancer (see analyses 8.1.1 and 8.1.2).
8.1. Analysis.
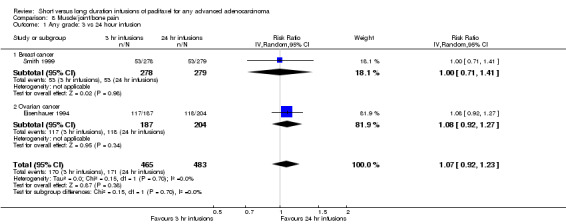
Comparison 8 Muscle/joint/bone pain, Outcome 1 Any grade: 3 vs 24 hour infusion.
Hair loss
3 versus 24 hour infusion
Meta‐analysis of two trials (Eisenhauer 1994; Smith 1999), assessing 948 participants, found no statistically significant difference in the risk of alopecia between the 3 hour and 24 hour infusion groups (RR = 0.95, 95% CI 0.81 to 1.12, Analysis 9.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent considerable heterogeneity (I2 = 80%). The conclusions were similar in the trial of Eisenhauer 1994 which included women with ovarian cancer (see analysis 9.1.2), but not in the trial of Smith 1999 as there was a statistically significant decrease in risk of alopecia in the 3 hour infusion group compared to the 24 hour group for women with breast cancer (see analysis 9.1.1).
9.1. Analysis.
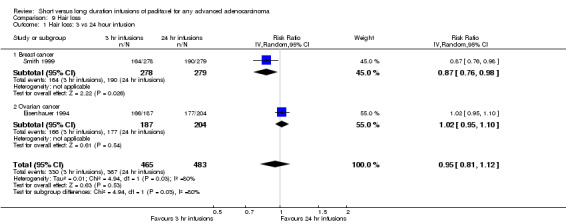
Comparison 9 Hair loss, Outcome 1 Hair loss: 3 vs 24 hour infusion.
24 versus 96 hour infusion
The trial of Spriggs 2007 found no statistically significant difference in the risk of alopecia between the 24 hour and 96 hour infusion groups (RR = 0.95, 95% CI 0.82 to 1.10, Analysis 9.2).
9.2. Analysis.

Comparison 9 Hair loss, Outcome 2 Hair loss: 24 vs 96 hour infusion.
1 versus 3 day schedule
In the trial of Greco 1995 all 28 women on each infusion schedule experienced alopecia (Analysis 9.3).
9.3. Analysis.
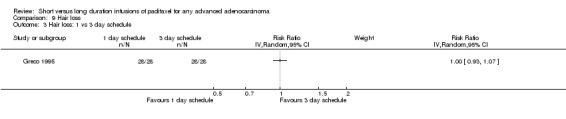
Comparison 9 Hair loss, Outcome 3 Hair loss: 1 vs 3 day schedule.
Hypersensitivity
3 versus 24 hour infusion
Clinically significant hypersensitivity reactions, although a special concern at the time of the Eisenhauer trial (Eisenhauer 1994) were uncommon (1.3% of patients). Meta‐analysis of two trials (Eisenhauer 1994; Smith 1999), assessing 948 participants, found no statistically significant difference in the risk of hypersensitivity between the 3 hour and 24 hour infusion groups (RR = 1.86, 95% CI 0.63 to 5.52, Analysis 10.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%). The conclusions were similar in subgroups that compared 3 hour and 24 hour infusions for patients with breast cancer and ovarian cancer (see analyses 10.1.1 and 10.1.2).
10.1. Analysis.
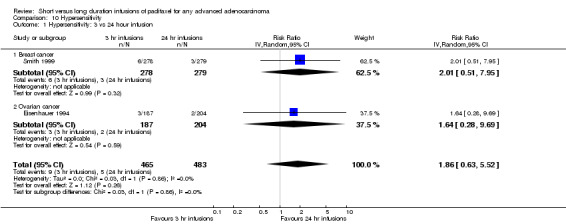
Comparison 10 Hypersensitivity, Outcome 1 Hypersensitivity: 3 vs 24 hour infusion.
1 versus 3 day schedule
The trial of Greco 1995 found no statistically significant difference in the risk of hypersensitivity between the 1 and 3 day infusion schedules. The trial reported only one hypersensitivity reaction and this was in a woman on the 3 day schedule.
Diarrhoea
3 versus 24 hour infusion
Meta‐analysis of two trials (Peretz 1995; Smith 1999), assessing 1078 participants, found that 3 hour infusions were associated with a statistically significant decrease in the risk of diarrhoea compared with 24 hour infusions (RR = 0.52, 95% CI 0.28 to 0.97, Analysis 11.1). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent moderate heterogeneity (I2 = 32%). There were substantially more women in the trial of Peretz 1995 who experienced diarrhoea than in the trial of Smith 1999.
11.1. Analysis.
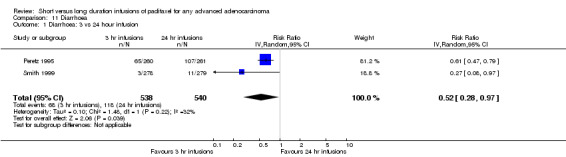
Comparison 11 Diarrhoea, Outcome 1 Diarrhoea: 3 vs 24 hour infusion.
1 versus 3 day schedule
The trial of Greco 1995 found no statistically significant difference in the risk of diarrhoea between the 1 and 3 day infusion schedules (RR = 4.00, 95% CI 0.48 to 33.58, Analysis 11.2). Only five woman in the trial experienced diarrhoea; four women on the 1 day schedule and one on the 3 day schedule.
11.2. Analysis.
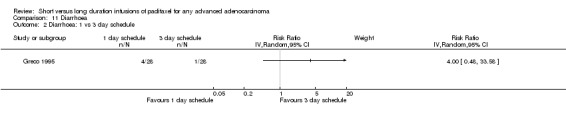
Comparison 11 Diarrhoea, Outcome 2 Diarrhoea: 1 vs 3 day schedule.
Toxicity associated deaths
3 versus 24 hour infusion
Only Smith 1999 reported deaths associated with toxicity. In this trial there was no statistically significant difference in the risk of death from toxicity between the 3 and 24 hour infusion groups (RR = 1.76, 95% CI 0.52 to 5.93, Analysis 12.1).
12.1. Analysis.

Comparison 12 Toxicity associated deaths, Outcome 1 Toxicity associated deaths: 3 vs 24 hour infusion.
Anaemia
24 versus 96 hour infusion
The trial of Spriggs 2007 found that 24 hour infusions were associated with a marginally statistically significant decrease in the risk of anaemia compared with 96 hour infusions (RR = 0.91, 95% CI 0.84 to 1.00, Analysis 13.1).
13.1. Analysis.

Comparison 13 Anemia, Outcome 1 Anemia: 24 vs 96 hour infusion.
Cardiac events
24 versus 96 hour infusion
The trial of Spriggs 2007 found no statistically significant difference in the risk of a cardiac event between the 24 hour and 96 hour infusion groups (RR = 1.04, 95% CI 0.64 to 1.69, Analysis 14.1).
14.1. Analysis.

Comparison 14 Cardiac events, Outcome 1 Cardiac events: 24 vs 96 hour infusion.
Infection
24 versus 96 hour infusion
The trial of Spriggs 2007 found no statistically significant difference in the risk of infection between the 24 hour and 96 hour infusion groups (RR = 1.07, 95% CI 0.54 to 2.13, Analysis 15.1).
15.1. Analysis.

Comparison 15 Infection, Outcome 1 Infection: 24 vs 96 hour infusion.
Quality of life
24 versus 96 hour infusion
Quality of life data were reported in only one of the trials (Eisenhauer 1994). Patients were given a score out of ten based on a published five item questionnaire for cancer patients (Spitzer 1981). Baseline scores were compared with scores after six months. No significant differences were found in the time to worsening of quality of life of patients when the 3 hour and 24 hour infusion groups were compared.
Discussion
Summary of main results
A number of observations can be made from the data from the individual trials. These can be considered under two headings, efficacy and toxicity:
Efficacy
Although the pooled data for infusions of 3 versus 24 hours are presented these should be interpreted with great caution since there is clinical heterogeneity and assumptions have been made in extracting the data for the Peretz 1995 trial. There was no statistical heterogeneity. In none of the trials was there a striking advantage for one paclitaxel infusion duration over another in any of the measures of efficacy reported. However, where there were statistically significant advantages these favoured the longer infusion. Longer infusions of high dose paclitaxel in breast cancer (Smith 1999) resulted in a significantly higher response rate, though there were no significant differences in event‐free survival or OS. In the trial of Peretz 1995 there was an insignificant difference in response rate, but there was a significantly longer survival for the 24 hour infusion, though this was lost when the data were adjusted for prognostic factors (details not given). The trial of Eisenhauer 1994 is in a different tumour site (ovarian cancer) and response rates and median times to progression were not significantly different between the two arms. There was, however, an overall trend for women receiving three hour infusions of paclitaxel to have a longer PFS interval. In contrast, there was no evidence of a significant difference in OS between the two infusion durations.
Two trials (Greco 1995; Spriggs 2007) tested shorter infusions versus infusions of 3 or 4 days. The trials are very different in several ways. Greco 1995 included a wide variety of tumour types and used a short single infusion and compared this with short infusions on three consecutive days. Spriggs 2007 only included gynaecological cancers, but compares a 24 hour infusion with a 96 hour infusion. Duration of the infusion did not appear to have a significant effect on efficacy in the two trials, but doses varied between the different arms making any conclusion difficult. The trial of Holmes 1998 used a high dose of paclitaxel (250mg/M2) as a 3 hour infusion and compared this to 140mg/M2 as a 96 hour infusion. This trial has only been presented in abstract form and its conclusion, that there was no difference in efficacy cannot be substantiated.
Toxicity
There is an internal consistency in the results and the pooled data are probably more reliable as tumour type is unlikely to effect toxicity. Though the trial of Smith 1999 cannot be used (there were different policies for the use of G‐CSF in the two arms), the other two trials clearly show that shorter durations of paclitaxel infusions produced significantly less neutropenia and fewer episodes of febrile neutropenia, infection and sore mouth. This is shown despite the use of more dose escalations with the 3 hour infusion than the 24 hour arm in the Peretz 1995 trial. Neurotoxicity, in contrast, was significantly less common in the 24 hour infusion arms. It is difficult to combine this data as it is presented separately as sensory and motor neuro‐toxicity in the Smith trial and combined in the other two. It is not clear which grades are being presented in the Peretz 1995 trial. All grades and three or greater are presented in the Eisenhauer 1994 trial. The incidence of hypersensitivity is not significantly different in the two infusion durations being tested in any of the trials. Other side effects appeared unaffected by the duration of the paclitaxel infusions.
There were no major reported differences in toxicity in the trials of Greco 1995 and Spriggs 2007 for the comparisons of shorter infusions versus infusions of 3 or 4 days, but the different designs limit any conclusions. The data on a subset of patients is not presented in the Holmes 1998 trial and it is not possible to tell how much less toxicity there may have been in the patients receiving the 96 hour infusion.
Overall completeness and applicability of evidence
This review appears to include all of the available randomised trials, but some of the evidence is only available in abstract form. The data is principally from trials sponsored by Bristol‐Myers Squibb. Though initial trials of paclitaxel used 24 hour infusions, 3 hour infusions have become the commonly used standard. Definitive evidence on the efficacy and toxicity of longer versus shorter duration infusions are only likely to change current practice if there are major changes in outcomes with a particular infusion duration. The findings of this review confirm known differences in toxicity that are dependent on duration of infusion. There is no clear evidence between 3 hour versus 24 hour infusions in terms of overall and progression‐free survival and tumour non‐response. The data is from RCTs, but is applicable to a general population of women with ovarian cancer. There is no evidence that efficacy is different for 24 hour versus 96 hour infusions or 1 day versus 3 day treatments. However, there are insufficient data to make any reliable conclusions for these comparisons. The findings suggest that there is no rationale for changing the current practice of using three hour infusions.
Quality of the evidence
The meta‐analyses of the RCTs trials that have compared different duration of paclitaxel infusions should be interpreted with caution, since the trials were clinically heterogeneous. Some trials are confounded by variations in the dose of paclitaxel or other drugs rendering them un interpretable. The three trials that use the same dose of paclitaxel in both arms, are difficult to combine as two are in breast cancer (Peretz 1995; Smith 1999) and one is in ovarian cancer (Eisenhauer 1994). The two breast cancer trials use very different doses of paclitaxel. Meta‐analysis of toxicity data is complicated by the use of different or unidentified toxicity scales and the presentation of data using different cut‐off points on the scales. In addition, one trial (Smith 1999), had different policies for G‐CSF use in the two arms of the trial.
The results of this meta‐analysis must remain speculative because of the potential problems of combining trials where different tumours and different chemotherapy doses are used. The failure to present the numbers of patients randomised to each arm of the Peretz 1995 trial means that the results presented must in addition be an estimate. The clearest conclusion is that three hour infusions of paclitaxel are associated with a very much lower incidence of neutropenia, febrile neutropenia and sore mouth than 24 hour infusions. This finding was well known, but is emphasised when the data from the two eligible trials are combined. Similarly, it has previously been accepted that 24 hour infusions of paclitaxel cause less nerve toxicity. This finding is confirmed in the synthesis of the data (though combining the data is complicated by different reporting methods). There is no clear evidence of differences in other side‐effects associated with 3 or 24 hour infusions.
The tentative finding that the overall and primary tumour response rates were rather higher for 24 hour infusions is, however, more controversial. Data for OS, PFS and/or progression event‐free survival do not show benefit for either duration of infusion. In contrast, there is some evidence that OS was slightly longer with a 24 hour infusion. The conventional view has been that there is no difference in the effectiveness of paclitaxel regardless of whether it is used as an infusion given over 3 or 24 hours. The data in this review suggest that the question remains open. While no claim can be made that the anti‐tumour effectiveness of a 24 hour infusion is greater than a 3 hour infusion, the review shows that the effect of longer infusion duration on effectiveness requires further data. At the very least, data from the Peretz 1995 trial should be made available for inclusion in an update of this review. From the data available, any difference in efficacy, if it exists, is likely to be modest and it may well require more patients than are included in the 3 eligible trials comparing 3 and 24 hour infusions to show a clear result.
The three trials (Greco 1995; Holmes 1998; Spriggs 2007) reporting the results of shorter infusion versus 3 or 4 day infusions cannot be reliably interpreted because of major differences in patient inclusion, different doses in the two arms, different total dose, and use of varying duration of infusion as well as a single infusion compared to three daily infusions
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were sifted and data extracted by at least two reviewers independently. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias i.e. studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as the analyses were restricted to meta‐analyses of a small number of trials or single trials.
The reliability and interpretability of the review is reduced by the inclusion of different tumour types and different doses of paclitaxel. Analysis of toxicity data is effected by the use of different scales and cut points.
Agreements and disagreements with other studies or reviews
The overall conclusions of this review are in keeping with data from non‐randomised phase two trials, though these have generally been small in size. The data from these RCTs are more reliable than any comparable non‐randomised evidence.
Authors' conclusions
Implications for practice.
A short duration infusion such as a three hour infusion of paclitaxel is more convenient for patients, doctors and nurses and health care systems. There appears to be evidence that in general, short infusions cause less toxicity and have a lesser effect on white blood cells. This means that there is a lower risk of infection and need for hospital admission and also makes it easier to combine paclitaxel with other anti‐cancer drugs.
From the included RCTs we found no evidence of a difference between short and long infusions in terms of survival. There was a non‐significant increase in risk of the tumour not responding to treatment when shorter infusions were compared to longer ones. In most cases more adverse events and severe toxicity occurred in the longer infusion groups with many of the analyses being highly statistically significant. In the absence of QoL data and given the fact that there was no statistically significant differences in overall and progression‐free survival or tumour response rates, it may be sensible to consider short infusions in favour of long infusions as patients would endure less toxicity. However, sensory nerve damage was more common in women receiving three hour infusions and this may be dose‐limiting for some patients. In this review, comparisons were restricted to meta analyses of very few trials or single trial analyses and many included trials were of insufficient size. Therefore the decision to offer short or long infusions in advanced adenocarcinoma may need to be individualised. The uncertainty regarding any impact on survival should be discussed openly with the women.
Trials comparing shorter infusions with three or four day infusions showed no major differences in outcome, but cannot be relied on as there is major clinical heterogeneity.
Implications for research.
Updated data for all the six RCTs should be made available for a further systematic review. Ideally, large, multi‐centre supporting trials need to be designed as outcomes were incompletely reported in included trials in this review. These trials should include a mixture of breast and ovarian cancer patients and should perform a thorough subgroup analysis within the trial. Outcomes such as overall and progression‐free survival should be reported as well as other important outcomes such as tumour response, quality of life and severe adverse events and toxicity. It may be beneficial to design a multi‐arm trial comparing 3, 24 and 96 hour infusions or maybe looking at different schedules as in the trial of Greco 1995. However, in the absence of more compelling evidence, it is unlikely that further large trials comparing different durations of infusion will be conducted. The current use of paclitaxel in combination with other drugs, means that the reduction in white cell toxicity associated with three hour infusions becomes a more important factor. The use of a 24 hour or other longer infusions may require dose reductions in paclitaxel or other drugs, and/or the use of G‐CSF, in order to maintain acceptable toxicity. The published data on shorter infusions versus 3 or 4 days infusions does not appear to justify further research.
What's new
| Date | Event | Description |
|---|---|---|
| 20 April 2015 | Review declared as stable | No additional studies expected on this topic. |
History
Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 3 December 2013 | Amended | Text amendment |
| 29 March 2011 | New search has been performed | New search conducted in March 2009 and authors amended. |
| 29 March 2011 | New citation required but conclusions have not changed | New trials identified and included. |
| 19 April 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Gail Quinn and Clare Jess for their contribution to the editorial process and Jane Hayes for help with search strategies and for running the searches. We also thank William P McGuire (Gynecologic Oncology Group, USA) for help in identifying potentially eligible trials and Bristol‐Myers Squibb for help in finding eligible trials. We also thank Iveta Simera and Clive Grafton for their contribution to the original review.
Appendices
Appendix 1. CENTRAL search strategy
Updated CENTRAL Issue 1 13‐03‐2009
MeSH descriptor Adenocarcinoma explode all trees
adenocarcinoma*
malignant next adenoma*
(#1 OR #2 OR #3)
MeSH descriptor Paclitaxel explode all trees
paclitaxel
abi next 007
abraxane
anzatax
asotax
bristaxol
nsc next 125973
onxol
paxene
praxel
taxol
xytotax
(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17)
(#4 AND #18)
Appendix 2. MEDLINE search strategy
Updated Medline Ovid 2001‐Feb week 4 2009
exp Adenocarcinoma/
adenocarcinoma*.mp.
malignant adenoma*.mp.
1 or 2 or 3
exp Paclitaxel/
paclitaxel.mp.
"abi 007".mp.
abraxane.mp.
anzatax.mp.
asotax.mp.
bristaxol.mp.
nsc 125973.mp.
onxol.mp.
paxene.mp.
praxel.mp.
taxol.mp.
xytotax.mp.
5 or 6 or 7 or 8 or 9 or 10 or11 or 12 or 13 or 14 or 15 or 16 or 17
4 and 18
"randomized controlled trial".pt.
"controlled clinical trial".pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
19 and 28
Animals/
Humans/
30 not (30 and 31)
29 not 32
key: mp=title, original title, abstract, name of substance word, subject heading word, pt=publication type, ab=abstract, fs=floating subheading
Appendix 3. EMBASE search strategy
Updated Embase Ovid 2001‐2009 week 10
exp Adenocarcinoma/
adenocarcinoma*.mp.
malignant adenoma*.mp.
1 or 2 or 3
exp Paclitaxel/
paclitaxel.mp.
"abi 007".mp.]
abraxane.mp.
anzatax.mp.
asotax.mp.
bristaxol.mp.
nsc 125973.mp.
onxol.mp.
paxene.mp.
praxel.mp.
taxol.mp.
xytotax.mp.
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
4 and 18
exp Controlled Clinical Trial/
randomized.ab.
placebo.ab.
dt.fs.
randomly.ab.
trial.ab.
groups.ab.
20 or 21 or 22 or 23 or 24 or 25 or 26
19 and 27
exp Animal/
Human/
29 not (29 and 30)
28 not 31
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name, pt=publication type, fs=floating subheading, ab=abstract
Data and analyses
Comparison 1. Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival: 3 vs 24 hour infusion | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Overall survival: 24 vs 96 hour infusion | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 Progression‐free survival: 3 vs 24 hour infusion | 2 | 942 | Hazard Ratio (Random, 95% CI) | 0.84 [0.58, 1.23] |
| 4 Progression‐free survival: 24 vs 96 hour infusion | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
Comparison 2. Overall tumour non‐response.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall tumour non‐response: 3 vs 24 hour infusion | 3 | 1423 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.98, 1.17] |
| 1.1 Breast cancer | 2 | 1041 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.96, 1.30] |
| 1.2 Ovarian cancer | 1 | 382 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 2 Overall tumour non response: 24 vs 96 hour infusion | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 Overall tumour non‐response: 1 vs 3 day schedule | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 3. Neutropenia or granulocytopenia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Grade 4: 3 vs 24 hour infusion | 2 | 912 | Risk Ratio (IV, Random, 95% CI) | 0.32 [0.22, 0.47] |
| 1.1 Breast cancer | 1 | 521 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.31, 0.46] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.19, 0.35] |
Comparison 4. Febrile neutropenia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any grade: 3 vs 24 hour infusion | 2 | 912 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.02, 0.17] |
| 1.1 Breast cancer | 1 | 521 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.02, 0.22] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.36] |
Comparison 5. Sore mouth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sore mouth: 3 vs 24 hour infusion | 2 | 912 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.40, 0.88] |
| 1.1 Breast cancer | 1 | 521 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.37, 0.64] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.52, 1.03] |
Comparison 6. Nausea/vomiting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Grade 3 or more: 3 vs 24 hour infusion | 2 | 948 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.24, 2.35] |
| 1.1 Breast cancer | 1 | 557 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.19, 0.88] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 1.32 [0.67, 2.61] |
Comparison 7. Neurosensory change.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any grade: 3 vs 24 hour infusion | 3 | 1469 | Risk Ratio (IV, Random, 95% CI) | 1.26 [1.09, 1.46] |
| 1.1 Breast cancer | 2 | 1078 | Risk Ratio (IV, Random, 95% CI) | 1.36 [0.98, 1.89] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.98, 1.53] |
Comparison 8. Muscle/joint/bone pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any grade: 3 vs 24 hour infusion | 2 | 948 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.92, 1.23] |
| 1.1 Breast cancer | 1 | 557 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.71, 1.41] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.92, 1.27] |
Comparison 9. Hair loss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hair loss: 3 vs 24 hour infusion | 2 | 948 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.81, 1.12] |
| 1.1 Breast cancer | 1 | 557 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.76, 0.98] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 2 Hair loss: 24 vs 96 hour infusion | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 Hair loss: 1 vs 3 day schedule | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 10. Hypersensitivity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersensitivity: 3 vs 24 hour infusion | 2 | 948 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.63, 5.52] |
| 1.1 Breast cancer | 1 | 557 | Risk Ratio (IV, Random, 95% CI) | 2.01 [0.51, 7.95] |
| 1.2 Ovarian cancer | 1 | 391 | Risk Ratio (IV, Random, 95% CI) | 1.64 [0.28, 9.69] |
Comparison 11. Diarrhoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea: 3 vs 24 hour infusion | 2 | 1078 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 2 Diarrhoea: 1 vs 3 day schedule | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 12. Toxicity associated deaths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Toxicity associated deaths: 3 vs 24 hour infusion | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 13. Anemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anemia: 24 vs 96 hour infusion | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 14. Cardiac events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac events: 24 vs 96 hour infusion | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 15. Infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection: 24 vs 96 hour infusion | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eisenhauer 1994.
| Methods | RCT of 2 x 2 factorial design. | |
| Participants | 407 patients with histologically documented progressive epithelial ovarian cancer previously treated with either one or two platinum containing chemotherapy regimens. Of all eligible patients (N = 391): Median age in the trial was 57 years in both infusion groups. There were 158 (%) women with performance status 0, 166 (%) women with status 1 and 67 (%) with status 2. Histological cell types were as follows: Serous: 222 (%), Mucinous: 23 (%), Endometroid: 42 (%), Clear cell: 16 (%), Other: 88 (%). |
|
| Interventions | Single agent paclitaxel at standard dose (135 versus 175 mg/m2) and two durations of infusion (3 versus 24 hours). | |
| Outcomes | Toxicity, response, time to progression, overall survival and quality of life. | |
| Notes | Trial randomised 407 patients, of which 391 were eligible. Data in this trial can be used for all the end points of the review. 106/407 (26%) women were still alive at the end of the study. There was no statistically significant difference between the two infusion groups (P = 0.3). Median times to progression in the two infusion duration groups were similar (17 versus 16 weeks), but the three hour group showed an overall trend for a longer progression‐free interval (P = 0.07). At the time of the reported analysis 27 patients were still alive. Median survival for the three hour infusion group was 51 weeks and that for the 24 hr infusion group was 48 weeks. Any degree of hypersensitivity reaction were similar for the two groups (45% versus 42% respectively). White blood cell suppression was common and was clearly related to the duration of paclitaxel infusion. Only grade IV neutropenia was reported (24 hour infusion 71% and three hour infusion 18%). Only nine patients discontinued paclitaxel because of side‐effects (four low white cell count, three hypersensitivity reactions, one sore mouth and one pulmonary oedema). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation lists were generated by the biostatistics and data management department at Bristol‐Myers Squibb". |
| Allocation concealment (selection bias) | Low risk | "On identification of an eligible patient, the study investigator completed an eligibility checklist and reported this information by telephone or facsimile to one or two regional randomisation sites". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 382/407 (94%) for response and 391/407 (96%) for toxicity. 3 hr infusion: 182/195 (93%) and 187/195 (96%) patients were assessed for response and toxicity respectively. 24 hr infusion: 200/212 (94%) and 204/212 (96%) patients were assessed for response and toxicity respectively. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Greco 1995.
| Methods | RCT | |
| Participants | 56 women with advanced cancer either resistant or refractory to initial standard therapy or with an untreated primarily resistant tumour type. Median age in the trial was 57 years (Range: 30 to 73 years). 39 (69%) women had an Eastern Cooperative Oncology Group performance status of 1. Tumour types were as follows: Breast cancer: 17 (30.5%), Non‐small cell lung cancer (NSCLC): 16 (28.5%), Ovarian cancer: 9 (16%), Small cell lung cancer: 5 (9%), Colorectal cancer: 2 (3.5%), Other: 7 (12.5%). |
|
| Interventions | Paclitaxel 135 mg/m2 infused as a single dose over 1 hour or divided into 3 doses infused over 1 hour on 3 consecutive days. | |
| Outcomes | Toxicity, objective response. | |
| Notes | There were no serious acute hypersensitivity reactions with either paclitaxel schedule. 7/28 responders had received 1 day schedule 4/28 responders had received 3 day schedule |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before randomization, patients were stratified according to performance status ... primary disease site, and previous chemotherapy. They were then randomized by a random card system". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed for response: 56/56 (100%) "All patients were evaluable for the toxicity assessment ... After two courses, 48 of the 56 patients were evaluable for response. The other eight patients were considered treatment failures". |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Holmes 1998.
| Methods | RCT Trial Hypothesis: Antineoplastic activity of paclitaxel in metastatic breast cancer (MBC) is schedule‐dependent; infusion by 96 hr has more antineoplastic activity than by 3 hr. |
|
| Participants | Eligible patients had measurable‐evaluable MBC and usual requirements for trials. Patients were stratified by (1) doxorubicin‐sensitivity (doxorubicin‐resistant: progression during treatment for MBC or within 6 months after adjuvant doxorubicin) and (2) number of prior regimens (inclusive of adjuvant: 0 or 1 versus 2 or 3). | |
| Interventions | Paclitaxel 250 mg/m2/3‐hr (usual premeds) or 140 mg/m2/96‐hr (no premed) repeated every 21 days. G‐CSF added only for neutropenic fever or infection then dose‐reduction. | |
| Outcomes | Toxicity, objective response, survival | |
| Notes |
Conclusions: (1) No significant difference in overall response (OR), duration, survival. (2) OR low‐possibly due to: a) stringent OR requirements (20% MR); b) multicenter trial. (3) These data do not justify the extra logistical support required for 96‐hr infusion. Supported by grant CA 45809. Toxicity by arm reported ASCO 1996: Toxicity in 123 evaluable patients treated from March 1994 to October 1995 (data not shown). The 96‐hr arm had fewer toxic effects, but was less convenient. Trial planned accrual of 226 patients and from 1996 continued to compare the efficacy of these two schedules. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Randomized at central data management office". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Peretz 1995.
| Methods | RCT. | |
| Participants | 521 patients with relapsed breast cancer | |
| Interventions | Single agent paclitaxel at standard dose (175mg/ m2). 3 versus 24 hour infusion. | |
| Outcomes | Toxicity, response, time to progression | |
| Notes | Abstract only, only total randomised given, no breakdown by arm. We have assumed 1:1 randomisation in this trial, but this may be misleading and should be interpreted with caution since the outcomes are reported as crude numbers rather than percentages. Objective responses were reported in 29% of women receiving an infusion of three hours duration compared with 32% of women having a 24 hour infusion. Median time to progression was 3.8 months for the three hour infusion compared to 4.6 months for a 24 hour infusion (P =0.02). Median overall survival was 9.8 months for the three hour infusion compared with 13.4 months for the 24 hour infusion (P = 0.02). After adjustment for prognostic factors these differences were not significant (time to progression P =0.08, survival P =0.10). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but no further details are reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Smith 1999.
| Methods | RCT. | |
| Participants | 563 patients with metastatic or locally advanced breast cancer Age at entry in the trial was as follows: <= 49 years: 197 (35%), >= 50 years: 366 (65%). There were 349 (62%) women with a normal performance status and 214 (38%) with a symptomatic status. Disease stage was as follows: IIIB: 92 (16%), IV: 471 (84%). |
|
| Interventions | High dose single agent paclitaxel (250 mg/ m2). 3 versus 24 hour infusion. | |
| Outcomes | Primary and overall tumour response, event‐free survival (progressive disease, relapse or death), survival, toxicity, compliance. | |
| Notes | Patients receiving the longer duration infusion were given prophylactic G‐CSF in an attempt to reduce the risk of infection in patients with a low granulocyte count. Patients receiving the shorter infusion of paclitaxel only received G‐CSF if they had such an episode of infection. 176/278 women died in the 3 hour infusion group and 184/282 women died in the 24 hour infusion group. 241/278 women either had progressive disease, relapsed or died in the 3 hour infusion group compared to 251/282 women in the 24 hour infusion group. Median time to death was 21.1 months (18.2‐24.2 months) and 21.9 (19.6 to 23.6 months) months in the 3 hour and 24 hour infusion groups respectively. Median time to first event was 6.3 months (5.4‐7.4 months) and 7.2 (6.1 to 8.3 months) months in the 3 hour and 24 hour infusion groups respectively. None of these differences were statistically significant, even when adjusted for prognostic variables (survival P = 0.96 and event‐free survival P = 0.95). The primary tumour response rates were 41% for the three hour infusion and 51% for the 24 hour infusion (P = 0.03 and P = 0.02 when adjusted for significant factors in a logistic regression analysis). The figures for overall responses were 44% for the three hour infusion and 54% for the 24 hour infusion (unadjusted P value = 0.02 and adjusted P = 0.02). There were 11 deaths due to adverse events (10 in the first four cycles), a number of which were due to infection. Seven of these were in patients receiving the three hour infusion and four with the 24 hour infusion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 hr infusion: 258/279 (92%) patients were assessable for primary tumour response, 261/279 (94%) patients for overall tumour response and 278/279 (99%) patients were assessed for progression‐free survival, overall survival and toxicity. 24 hr infusion: 255/284 (90%) patients were assessable for primary tumour response, 259/284 (91%) patients for overall tumour response and 282/284 (99%) patients were assessed for progression‐free and overall survival. 279/284 (98%) patients were assessed for toxicity. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Spriggs 2007.
| Methods | RCT | |
| Participants | 293 women with sub‐optimal stage III or IV epithelial ovarian cancer, fallopian tube or primary peritoneal cancer. Of the 280 assessable patients: Median age in the trial was 58.3 years in the 24 hour infusion group and 60.2 years in the 96 hour infusion group (Range in trial: 24.4 to 85.8 years). 92 (33%) women had a performance status of 0, 155 (55%) had status of 1 and 33 (12%) women had a status of 2. Histological cell types were as follows: Serous adenocarcinoma: 211 (75.25%), Mucinous adenocarcinoma: 3 (1%), Endometroid adenocarcinoma: 19 (7%), Clear cell adenocarcinoma: 11 (4%), adenocarcinoma unspecified: 6 (2%), Mixed epithelial: 17 (6%), Undifferentiated adenocarcinoma: 9 (3.25%), Other: 4 (1.5%). |
|
| Interventions | Six cycles of cisplatin and either paclitaxel 135 mg/m2 during 24 hours or paclitaxel 120mg/m2 during 96 hours. | |
| Outcomes | Progression‐free and overall survival, response, toxicity. | |
| Notes | 293 women were randomised, but 13 women were deemed ineligible. 140 in each arm were assessable. Hazard ratio for PFS was adjusted for initial measurable disease status, performance status, histology and stage of disease. Hazard ratio for OS was adjusted for measurable disease status and additionally adjusted using the same variables as for PFS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study regimens were allocated from randomly permuted blocks of treatments with an equal number of each study treatment within each block". |
| Allocation concealment (selection bias) | Unclear risk | "The assigned study treatment for each patient remained concealed until the patient was registered successfully". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | % analysed: 280/293 (96%) for survival outcomes, 276/293 (94%) for toxicity, but only 181/293 (62%) for response. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atad 1997 | No short versus long duration infusions of paclitaxel comparison and study appears to be a case series. |
| Boddy 2000 | Abstract of Boddy 2001 trial. |
| Boddy 2001 | Cross over trial with a pharmacokinetic focus. |
| Calvert 1999 | Trial does not report outcome measures as specified in protocol. The trial compares 1 hr versus 3 hr infusion but had a pharmacokinetic focus. |
| Connelly 1996 | No short versus long duration infusions of paclitaxel comparison. |
| Gianni 1995 | Trial seemed to vary carboplatin dose as well as duration of paclitaxel and had a pharmacokinetic focus. |
| Huizing 1993 | Trial does not report outcome measures as specified in protocol. The trial compares 3 hr versus 24 hr infusion but had a pharmacokinetics focus. |
| Jennens 2003 | Cross over trial. |
| Keung 1993 | Abstract of Huizing 1993 trial. |
| Kudelka 1999 | No short versus long duration infusions of paclitaxel comparison. |
| Mross 2002 | Trial does not report outcome measures as specified in protocol. The trial compares 1 hr versus 3 hr infusion but had a pharmacokinetics focus. |
| Nannan 1999 | Does not appear to be an RCT. The study compared 1 hr versus 3 hr infusion but had a pharmacokinetic focus. Tumour response was reported but five of the seven women in the 3 hr regimen crossed over to the 96 hr regimen as it was prespecified that patients not responding to the 3 hr schedule were permitted to cross over. |
| Rischin 1996 | Cross over trial with a pharmacokinetic focus. |
| Sulkes 1994 | Israeli experience of trials of duration of infusion of paclitaxel. Includes both breast (69 women) and ovarian cancer (38 women), but the breast cancer patients are likely to have been included in the report by Peretz (a co‐author of this paper). In addition no data on outcomes were presented. |
Differences between protocol and review
The following methods were specified in the protocol but not implemented as we found only six trials that met our inclusion criteria. None of the trials reported continuous outcomes such as quality of life or had multiple treatment groups. All trials were at high risk of bias so we did not conduct sensitivity analysis around quality. There was also a insufficient number of trials to assess the potential for small study effects such as publication bias.
The methods specified below may be required when a future update is carried out.
Data extraction and management
For continuous outcomes (e.g. quality of life measures), we planned to extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
Measures of treatment effect
For continuous outcomes (e.g. QoL measures), we planned to use the mean difference between treatment arms.
Assessment of reporting biases
Funnel plots corresponding to meta‐analysis of the primary outcome were examined to assess the potential for small study effects such as publication bias. If these plots suggested that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, further meta‐analyses were performed using fixed effects models.
Data synthesis
For continuous outcomes (e.g. QoL measures), the mean differences between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardised mean difference will be pooled.
If any trials have multiple treatment groups, the ‘shared’ comparison group will be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group will be treated as independent comparisons.
If possible, studies making different comparisons will be synthesised using the sub‐group methods of Bucher 1997.
Sensitivity analysis
We will perform sensitivity analyses, excluding studies which do not report adequate (i) concealment of allocation, (ii) blinding of the outcome assessor.
Contributions of authors
CW ‐ Originated review question, designed review, extracted data, wrote review. AB ‐ designed review, extracted data, performed analyses, wrote review.
Sources of support
Internal sources
Medical Research Council, UK.
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Declarations of interest
Chris Williams has acted as an expert witness on behalf of Napro pharmaceuticals in court proceedings between Bristol‐Myers Squibb and Napro regarding the patent of paclitaxel.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Eisenhauer 1994 {published data only}
- Eisenhauer EA, Bokkel Huinink W W, Swenerton KD, Gianni L, Myles J, Burg MEL, et al. European‐Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high‐dose versus low‐dose and long versus short infusion. Journal of Clinical Oncology 1994;12(12):2654‐66. [DOI] [PubMed] [Google Scholar]
- LhommÚ C, Eisenhauer E, Swenerton K, SouliÚ P, Armand J, Bokkel Huinink, et al. Essai randomisÚ europÚen et canadien comparent 2 doses et 2 durÚes de perfusion de Taxol chez des patientes traitÚes pour cancer de l'ovarie (CO) rÚsistant au platine. (ABSTRACT). Bulletin du cancer 1994;81(6):560. [Google Scholar]
- Swenerton K, Eisenhauer E, Bokkel Huinink W, Myles J, Mangioni C, Burg M, et al. Taxol in relapsed ovarian cancer: high vs low dose and short vs long infusion. Proceedings of the American Society of Clinical Oncology 1993;12:256, Abstract 810. [Google Scholar]
- Bokkel Huinink W W, Eisenhauer E, Swenerton K. Preliminary evaluation of a multicenter, randomized comparative study of TAXOL (paclitaxel) dose and infusion length in platinum‐treated Ovarian cancer. Canadian‐European Taxol Cooperative Trial Group A2 ‐ 0305‐7372. Cancer Treatment Reviews 1993;19 Suppl C:79‐86. [DOI] [PubMed] [Google Scholar]
- Bokkel Huinink W, Swenerton K, Eisenhauer E, Onetto N, Winograd B. Toxicity of taxol: a European‐Canadian trial of high vs low dose and short vs long infusion in ovarian cancer coordinated by the NCI Canada clinical trials group (NCIC CTG). Annals of Oncology 1992;3 Suppl 5:101, Abstract 390. [Google Scholar]
Greco 1995 {published data only}
- Greco F A, Hainsworth J D. One‐hour paclitaxel infusion schedules: a phase I/II comparative trial. Seminars in Oncology 1995;22(3 Suppl 6):118‐23. [PubMed] [Google Scholar]
- Greco F A, Hainsworth J D. Paclitaxel (Taxol): phase I/II trial comparing 1‐hour infusion schedules. Seminars in Oncology 1994;21(5 Suppl 8):3‐8. [PubMed] [Google Scholar]
- Hainsworth J D, Greco F A. Paclitaxel administered by 1‐hour infusion. Preliminary results of a phase I/II trial comparing two schedules. Cancer 1994;74(4):1377‐82. [DOI] [PubMed] [Google Scholar]
- Hainsworth J D, Hopkins L, Thomas M, Greco F A. Taxol administered by one hour infusion: preliminary results of a phase I/II study comparing two dose schedules. Proceedings of the American Society of Clinical Oncology 1994;13:155, Abstract 413. [Google Scholar]
- Hainsworth J D, Raefsky E L, Greco F A. Paclitaxel administered by a 1‐hour infusion: A phase I‐II trial comparing two schedules. The Cancer Journal from Scientific American 1995;1(4):281‐7 T3 ‐ Comment in: Cancer J Sci Am. 1995 Nov‐Dec;1(4):250‐1. PMID: 9166484.. [PubMed] [Google Scholar]
Holmes 1998 {published data only}
- Holmes FA, Valero V, Buzdar AU, Booser DJ, Winn R, Tolcher A, Seidman A, Goodwin W, Bearden J, Baysinger L, Hortobagyi GN, Arbuck SA. FINAL RESULTS: RANDOMIZED PHASE III TRIAL OF PACLITAXEL BY 3‐HR VERSUS 96‐HR INFUSION IN PATIENTS (PT) WITH MET BREAST CANCER (MBC). THE LONG & SHORT OF IT (Meeting abstract).. American Society of Clinical Oncology (ASCO) Annual Meeting. 1998.
- Holmes FA, Valero V, Walters R, Buzdar AU, Booser DJ, Winn R, Tolcher A, Seidman A, Goodwin W, Bearden J, Whealin H, Hortobagyi GN, Arbuck SG. Phase III trial of paclitaxel (P) administered over 3‐ or 96‐hr for metastatic breast cancer (MBC). Proceedings of the American Society of Clinical Oncologists 1996;15:106. [Google Scholar]
Peretz 1995 {published data only}
- Peretz T, Sulkes A, Chollet P, Gelman K, Paridaens R, Gorbonuva V, et al. A Multicentre, Randomised Study of Two Schedules Of Paclitaxel (PTX) In Patients With Advanced Breast Cancer (ABC). European Journal of Cancer (proceedings of ECCO 8). 1995; Vol. 31A (Suppl 5):Abstract 345.
Smith 1999 {published data only}
- Mamounas E, Brown A, Smith R, et al. Effect of the taxol duration of infusion in advanced breast cancer (ABC): results from NSAPBB‐26 trial comparing 3‐ to 24‐hr infusion of high dose taxol. Proc Am Soc Clin Oncol 1998;17:101a. [Google Scholar]
- Mamounas E, Brown A, Smith R, Lembersky B, Fisher B, Wickerham DL, Wolmark N, Atkins J, Shibata H, Baez L, DeFusco P, Davila E, Thirlwell M, Bearden J, Tipping S, Scholnik A. EFFECT OF TAXOL DURATION OF INFUSION IN ADVANCED BREAST CANCER (ABC): RESULTS FROM NSABP B‐26 TRIAL COMPARING 3‐ TO 24‐HR INFUSION OF HIGH‐DOSE TAXOL (Meeting abstract). ASCO Annual Meeting. 1998.
- Smith RE, Brown AM, Mamounas EP, Anderson SJ, Lembersky BC, Atkins JH, et al. Randomized trial of 3‐hour versus 24‐hour infusion of high‐dose paclitaxel in patients with metastatic or locally advanced breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B‐26. Journal of Clinical Oncology 1999;17(11):3403‐4411. [DOI] [PubMed] [Google Scholar]
Spriggs 2007 {published data only}
- Spriggs D R, Brady M F, Vaccarello L, Clarke Pearson D L, Burger R A, Mannel R, et al. Phase III randomized trial of intravenous cisplatin plus a 24‐ or 96‐hour infusion of paclitaxel in epithelial ovarian cancer: a Gynecologic Oncology Group Study. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 2007;25(28):4466‐71. [DOI] [PubMed] [Google Scholar]
- Spriggs D R, Brady M, Rubin S, Hanley M, Copeland L J, Clarke Pearson D, et al. A phase III randomized trial of cisplatin and paclitaxel administered by either 24 hour or 96 hour infusion in patients with selected stage III or stage IV epithelial ovarian cancer (GOG162). Proceedings of the American Society of Clinical Oncology 2004;23:449, Abstract 5004. [Google Scholar]
References to studies excluded from this review
Atad 1997 {published data only}
- Atad J, Steiner M, Saggy S, Borovik R, Abramovici H. Prolonged taxol combination chemotherapy in recurrent advanced epithelial ovarian cancer. International Journal of Gynecological Cancer 1997;7 Suppl 2:92, Abstract P237. [Google Scholar]
Boddy 2000 {published data only}
- Boddy A V, Griffin M J, Wright J G, Sludden J A, Thomas H D, Fishwick K, et al. Paclitaxel and carboplatin dose‐intensity and duration of infusion in the treatment of ovarian cancer. British Journal of Cancer 2000;83 Suppl 1:79, Abstract P 203. [Google Scholar]
Boddy 2001 {published data only}
- Boddy A V, Griffin M J, Sludden J, Thomas H D, Fishwick K, Wright J G, et al. Pharmacological study of paclitaxel duration of infusion combined with GFR‐based carboplatin in the treatment of ovarian cancer. Cancer Chemotherapy & Pharmacology 2001;48(1):15‐21. [DOI] [PubMed] [Google Scholar]
Calvert 1999 {published data only}
- Calvert A H, Ghokul S, Al Azraqi A, Wright J, Lind M, Bailey N, et al. Carboplatin and paclitaxel, alone and in combination: dose escalation, measurement of renal function, and role of the p53 tumor suppressor gene. Seminars in Oncology 1999;26(1 Suppl 2):90‐4. [PubMed] [Google Scholar]
Connelly 1996 {published data only}
- Connelly E, Markman M, Kennedy A, Webster K, Kulp B, Peterson G, et al. Paclitaxel delivered as a 3‐hr infusion with cisplatin in patients with gynecologic cancers: unexpected incidence of neurotoxicity A2 ‐ 0090‐8258. Gynecologic Oncology 1996;62(2):166‐8. [DOI] [PubMed] [Google Scholar]
Gianni 1995 {published data only}
- Gianni L, Kearns C M, Giani A, Capri G, Vigano L, Lacatelli A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. Journal of Clinical Oncology 1995;13(1):180‐90. [DOI] [PubMed] [Google Scholar]
Huizing 1993 {published data only}
- Huizing M T, Keung A C, Rosing H, Kuij V, Bokkel Huinink W W, Mandjes I M, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum‐pretreated ovarian cancer patients. Journal of Clinical Oncology 1993;11(11):2127‐35. [DOI] [PubMed] [Google Scholar]
Jennens 2003 {published data only}
- Jennens R, Rischin D, Yuen K, Toner G, Millward M. Comparison of neutropenia in a randomized, crossover trial of 3‐, 6‐, and 24‐h infusions of paclitaxel. Gynecologic Oncology 2003;91(1):190‐3. [DOI] [PubMed] [Google Scholar]
Keung 1993 {published data only}
- Keung A C, Kaul S, Pinedo H M, Bokkel Huinink W W, Beijen J H. Pharmacokinetics of Taxol® given by 3‐h or 24‐H infusion to patients with ovarian carcinoma. Proceedings of the American Society of Clinical Oncology 1993;12:130, Abstract 321. [Google Scholar]
Kudelka 1999 {published data only}
- Kudelka A P, Verschraegen C F, Shen Y, Gonzalez De Leon C, Edwrads C L, Freedman R S, et al. Long‐term results and pharmacokinetics of high‐dose paclitaxel in patients with refractory epithelial ovarian carcinoma. International Journal of Gynecological Cancer 1999;9(1):44‐53. [DOI] [PubMed] [Google Scholar]
Mross 2002 {published data only}
- Mross K, Haring B, Hollander N, Mielke S, Behringer D, Massing U, et al. Comparison of 1‐hour and 3‐hours paclitaxel infusion pharmacokinetics: results from a randomized trial. Onkologie 2002;25(6):503‐8. [DOI] [PubMed] [Google Scholar]
Nannan 1999 {published data only}
- Nannan Panday V R, Bokkel Huinink W W, Vermorken J B, Rosing H, Koopman F J, Swart M, et al. Pharmacokinetics of paclitaxel administered as a 3‐hour or 96‐hour infusion. Pharmacological Research 1999;40(1):67‐74. [DOI] [PubMed] [Google Scholar]
Rischin 1996 {published data only}
- Rischin D, Webster L K, Millward M J, Linahan B M, Toner G C, Woollett A M, et al. Cremophor pharmacokinetics in patients receiving 3‐, 6‐, and 24‐hour infusions of paclitaxel A2 ‐ 0027‐8874. Journal of the National Cancer Institute 1996;88(18):1297‐301. [DOI] [PubMed] [Google Scholar]
Sulkes 1994 {published data only}
- Sulkes A, Beller U, Peretz T, Shacter J, Hornreich G, McDaniel C, Winograd B. Taxol: initial Israeli experience with a novel anticancer agent. Israeli Journal of Medical Sciences 1994;30(1):70‐8. [PMID:7908013] [PubMed] [Google Scholar]
Additional references
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group. 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
Eisenhauer 1994
- Eisenhauer EA, Bokkel Huinink WW, Swenerton KD, et al. European‐Canadian randomised trial of paclitaxel in relapsed ovarian cancer: high‐dose versus low‐dose and long versus short infusion. Journal of Clinical Oncology 1994;12:2654‐66. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
McGuire 1996
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. The New England journal of medicine 1996;334:1‐6. [DOI] [PubMed] [Google Scholar]
McGuire 1999
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Long‐term follow‐up of GOG‐111: a randomized trial comparing cisplatin combined with cyclophosphamide or paclitaxel in patients with stage III or IV epithelial ovarian cancer. International Journal of Gynecological Cancer 1999;9(Suppl 1):8‐9. [Google Scholar]
NCI 2004
- Surveillance EaERSP. SEER Public‐Use 1973‐2001. National Cancer Institute 2004.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Piccart 2000
- Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin‐paclitaxel versus cisplatin‐cyclophosphamide in women with advanced epithelial ovarian cancer: three‐year results. Journal of the National Cancer Institute 2000;92(9):699‐708. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spitzer 1981
- Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients. J Chronic Disease 1981;34:585‐598. [DOI] [PubMed] [Google Scholar]
WHO
- WHO Toxicity Criteria. http://www.fda.gov/cder/cancer/toxicityframe.htm.


