Abstract
Background
This is an updated version of the original Cochrane review published in Issue 2, 2007. The role of radiotherapy (both pelvic external beam radiotherapy (EBRT) and vaginal intracavity brachytherapy (VBT)) in stage I endometrial cancer following hysterectomy remains controversial.
Objectives
To assess the efficacy of adjuvant radiotherapy following surgery for stage I endometrial cancer.
Search methods
We searched The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Specialised Register to end‐2005 for the original review, and extended the search to January 2012 for the update.
Selection criteria
We included randomised controlled trials (RCTs) that compared post‐operative adjuvant radiotherapy (either EBRTor VBT, or both) versus no radiotherapy or VBT in women with stage I endometrial cancer.
Data collection and analysis
Two review authors independently assessed trials and extracted data to a specifically designed data collection form. The primary outcome was overall survival. Secondary outcomes were endometrial cancer‐related deaths, locoregional recurrence and distant recurrence. Meta‐analyses were performed using Cochrane Review Manager Software 5.1.
Main results
We included eight trials. Seven trials (3628 women) compared EBRT with no EBRT (or VBT), and one trial (645 women) compared VBTwith no additional treatment. We considered six of the eight trials to be of a high quality. Time‐to‐event data were not available for all trials and all outcomes.
EBRT (with or without VBT) compared with no EBRT (or VBT alone) for stage I endometrial carcinoma significantly reduced locoregional recurrence (time‐to‐event data: five trials, 2965 women; Hazard Ratio (HR) 0.36, 95% Confidence Interval (CI) 0.25 to 0.52; and dichotomous data: seven trials, 3628 women; Risk Ratio (RR) 0.33, 95% CI 0.23 to 0.47). This reduced risk of locoregional recurrence did not translate into improved overall survival (time‐to‐event data: five trials, 2,965 women; HR 0.99, 95% CI 0.82 to1.20; and dichotomous data: seven trials, 3628 women; RR 0.98, 95% CI 0.83 to 1.15) or improved endometrial cancer‐related survival (time‐to‐event data: five trials, 2965 women; HR 0.96, 95% CI 0.72 to 1.28; and dichotomous data: seven trials, 3628 women; RR 1.02, 95% CI 0.81 to 1.29) or improved distant recurrence rates (dichotomous data: seven trials, 3628 women; RR 1.04, 95% CI 0.80 to1.35).
EBRT did not improve survival outcomes in either the intermediate‐risk or high‐risk subgroups, although high‐risk data were limited, and a benefit of EBRT for high‐risk women could not be excluded. One trial (PORTEC‐2) compared EBRT with VBT in the high‐intermediate risk group and reported that VBT was effective in ensuring vaginal control with a non‐significant difference in loco‐regional relapse rate compared to EBRT (5.1% versus 2.1%; HR 2.08, 95% CI 0.71 to 6.09; P = 0·17). In the subgroup of low‐risk patients (IA/B and grade 1/2), EBRT increased the risk of endometrial carcinoma‐related deaths (including treatment‐related deaths) (two trials, 517 women; RR 2.64, 95% CI 1.05 to 6.66) but there was a lack of data on overall survival. We considered the evidence for the low‐risk subgroup to be of a low quality.
EBRT was associated with significantly increased severe acute toxicity (two trials, 1328 patients, RR 4.68, 95% CI 1.35 to 16.16), increased severe late toxicity (six trials, 3501 women; RR 2.58, 95% CI 1.61 to 4.11) and significant reductions in quality of life scores and rectal and bladder function more than 10 years after randomisation (one trial, 351 women) compared with no EBRT.
One trial of VBT versus no additional treatment in women with low‐risk lesions reported a non‐significant reduction in locoregional recurrence in the VBT group compared with the no additional treatment group (RR 0.39, (95% CI 0.14 to 1.09). There were no significant differences in survival outcomes in this trial.
Authors' conclusions
EBRT reduces the risk of locoregional recurrence but has no significant impact on cancer‐related deaths or overall survival. It is associated with significant morbidity and a reduction in quality of life. There is no demonstrable survival advantage from adjuvant EBRT for high‐risk stage I endometrial cancer, however, the meta‐analyses of this subgroup were underpowered and also included high‐intermediate risk women, therefore we cannot exclude a small benefit in the high‐risk subgroup. EBRT may have an adverse effect on endometrial cancer survival when used to treat uncomplicated low‐risk (IA/B grade 1/2) endometrial cancer. For the intermediate to high‐intermediate risk group, VBT alone appears to be adequate in ensuring vaginal control compared to EBRT. Further research is needed to guide practice for lesions that are truly high risk. In addition, the definitions of risk should be standardised.
Keywords: Female; Humans; Endometrial Neoplasms; Endometrial Neoplasms/pathology; Endometrial Neoplasms/radiotherapy; Endometrial Neoplasms/surgery; Neoplasm Staging; Radiotherapy, Adjuvant; Radiotherapy, Adjuvant/methods; Randomized Controlled Trials as Topic; Survival Analysis
Plain language summary
Adjuvant radiotherapy for stage I endometrial cancer
Women with stage I (early) endometrial cancer have a low risk of recurrence of their disease. Less than 10% of women treated with surgery alone have a recurrence after surgery. This risk is significantly higher (and may be double) for some women with high risk factors including aggressive cell types (grade 3) and deep invasion of the muscle (stage IC). External beam radiotherapy (EBRT) after surgery reduces the risk that the cancer will initially recur in the pelvis by around two‐thirds compared to surgery alone, but does not reduce the risk of death.
EBRT carries an inherent risk of lasting treatment‐related side‐effects and routine use should be avoided in stage I endometrial cancer. However, from the available evidence, we cannot exclude the possibility of EBRT benefiting women with high‐risk stage I disease. Vaginal brachytherapy (VBT) appears to be useful in reducing locoregional recurrence and may be associated with less side‐effects than EBRT.
Summary of findings
Summary of findings for the main comparison. Summary of findings: EBRT versus no EBRT for stage I endometrial cancer.
| EBRT compared with No EBRT for stage I endometrial cancer | ||||||
|
Patient or population: women with stage I endometrial cancer Settings: hospital Intervention: EBRT (with or without VBT) Comparison: No EBRT (or VBT alone) | ||||||
| Outcomes | Population with illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No EBRT | EBRT | |||||
| Death from all causes | Stage I overall |
HR 0.99 (0.82 to 1.20) HR 1.05 [0.85 to 1.31) HR 0.91 (0.60 to 1.39) |
2628 women
(five trials) 2560 women (five trials) 334 women (two trials) |
⊕⊕⊕⊕
high ⊕⊕⊕⊕ high ⊕⊕⊕⊝ moderate |
Dichotomous meta‐analysis included seven trials (one of low quality) and produced similar results (3628 women; RR 0.98, 95% CI 0.83 to 1.15). Evidence downgraded as GOG 99 HIR data were used. |
|
| 151 per 1000 | 149 per 1000 (124 to 181) | |||||
| Intermediate risk | ||||||
| 135 per 1000 |
142 per 1000 115 to 177) |
|||||
| High risk | ||||||
| 269 per 1000 |
245 per 1000 (161 to 374) |
|||||
| Endometrial carcinoma‐related death | Stage I overall |
HR 0.96 (0.72 to 1.28) RR 2.64 (1.05 to 6.66) HR 1.03 (0.70 to 1.51) HR 0.84 (0.51 to 1.40) |
2965 women
(five trials) 517 women (two trials) 2560 women (five trials) 334 women (two trials) |
⊕⊕⊕⊕
high ⊕⊕⊝⊝ low ⊕⊕⊕⊕ high ⊕⊕⊕⊝ moderate |
Evidence downgraded due to the small number of deaths (16 versus 6), inclusion of Aalders 1980 and LIR women from GOG 99, and the wide confidence interval. ** Evidence downgraded as GOG 99 HIR data were used. |
|
| 78 per 1000 | 75 per 1000 (56 to 100) | |||||
| Low risk | ||||||
| 23 per 1000 | 61 per 1000 (24 to 153) | |||||
| Intermediate risk | ||||||
| 67 per 1000 | 69 per 1000 (52 to 101) | |||||
| High risk | ||||||
| 214 per 1000 | 179 per 1000 (109 to 300) | |||||
| Locoregional recurrence | Stage I overall | HR 0.36 (0.25 to 0.52) | 2965 women (five trials) | ⊕⊕⊕⊕ high | Dichotomous meta‐analysis included seven trials and produced similar results (3628 women, RR 0.33, 95% CI 0.23 to 0.47). | |
| 75 per 1000 | 27 per 1000 (19 to 39) | |||||
| Severe acute toxicity | 4 per 1000 |
19 per 1000 (5 to 65) |
RR 4.68 (1.35 to 16.16) | 1328 women (two trials) |
⊕⊕⊕⊕ high | The control group included women who underwent vaginal brachytherapy. |
| Severe late toxicity | 14 per 1000 |
36 per 1000 (23 to 58) |
RR 2.58 (1.61 to 4.11) | 3501 women (six trials) | ⊕⊕⊕⊕ high | The control group included women who underwent vaginal brachytherapy. |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; HR: Hazard Ratio; LIR: low‐intermediate risk; HIR: high‐intermediate risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
* The assumed risk is based on the mean control group risk across included studies for each outcome.
**Despite the low quality of the evidence, further EBRT research is not warranted in this low risk group.
EBRT: external beam radiotherapy, VBT: vaginal brachytherapy
Background
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews (Issue 2, 2007).
Description of the condition
Cancer of the endometrium is one of the commonest gynaecological cancers in the western world. Seventy‐five per cent of women affected are postmenopausal. Ninety per cent of tumours are endometrioid adenocarcinomas. Other pathologies include adenosquamous, serous, clear cell carcinoma and mixed mesenchymal. Adenocarcinomas usually present early with postmenopausal bleeding. The commonest modes of spread are invasion of the myometrium and lymphatic spread to the external and common iliac lymph nodes and the para‐aortic lymph nodes. Distant metastases generally occur late but occur more frequently with higher grade and more advanced disease (DiSaia 1985).
Most endometrial cancers are diagnosed at stage I disease. Stage I endometrial cancer is confined to the body of the uterus (Creasman 2001). The staging system has undergone revision since these quoted studies have been reported. For the purposes of this review, we use the (pre‐2010) FIGO staging which defines stage IA as cancer that does not invade the myometrium and is confined to the endometrium; stage IB as cancer that invades less than one half of the muscle wall of the uterus; stage IC is cancer that invades more than one half of the muscle wall of the uterus (Table 2). The initial treatment for stage I disease is usually surgery involving a hysterectomy and bilateral salpingo‐oophorectomy (BSO). The decision to give adjuvant radiotherapy depends on whether the women have high risk factors including the stage of disease, depth of myometrium invasion, grade of the tumour, lymphovascular invasion, the age of the woman and her co‐morbidities. The site of first relapse is usually the upper vagina, or vaginal vault, and this can be reduced by adjuvant postoperative radiotherapy.
1. FIGO stage I of the corpus uteri.
| Stage | Description |
| IA | Tumour limited to endometrium |
| IB | Invasion of less than half the myometrium |
| IC | Invasion equal to or more than half myometrium |
Adjuvant hormonal therapy was used to treat endometrial cancer in the past but the advantages were offset by disadvantages (Martin‐Hirsch 2010). Adjuvant platinum‐based chemotherapy improves survival for women with high‐risk cancers and may have added value when used before or after external beam radiotherapy (EBRT) (Johnson 2011). Adjuvant chemoradiation is currently under investigation (PORTEC‐3).
In order to stage the disease accurately, a pelvic and /or para‐aortic lymphadenectomy is often performed to determine the need for adjuvant therapy. Two recent randomised trials of systematic pelvic lymphadenectomy versus no lymphadenectomy for stage I endometrial cancer showed no evidence of benefit in terms of overall or disease‐free survival in the lymphadenectomy group (Kitchener 2009; Panici 2008). Lymphadenectomy is no longer recommended in women with stage I endometrial carcinoma outside of clinical trials (Kitchener 2009), unless it will directly influence management.
Description of the intervention
Both pelvic EBRT and vaginal intracavitary brachytherapy (VBT) carry the risks of acute toxicities and long‐term complications (Creutzberg 2001). Although the acute side effects of pelvic radiotherapy ‐ on the irradiated skin, gastro‐intestinal tract and genitourinary tract ‐ settle down in the majority of patients following treatment, over 20% of patients continue to have persistent mild (grade 1) complications including urgency, abdominal cramps, diarrhoea, vaginal dryness and stenosis which may affect their quality of life. Around 3% of women develop severe long‐term complications, mostly of the gastrointestinal tract (Creutzberg 2001). Radiotherapy may be associated with a slightly increased risk of second neoplasms (Berrington de Gonzalez 2011; Kumar 2009).
Our original review combined data from four randomised trials of EBRT (with or without VBT) versus no EBRT (Aalders 1980, GOG 99, PORTEC‐1, Soderini 2003) and found no significant difference in overall or endometrial cancer‐related survival, despite reduced locoregional recurrence in the EBRT group (Kong 2007a). Since then, the trend has moved away from EBRT towards the less toxic VBT for local control. The use of vaginal radiotherapy for early endometrial cancer is now more common than EBRT in some countries (Naumann 2007) and several clinicians advocate using VBT alone, even for high‐risk endometrial cancer (Atahan 2008; McCloskey 2010).
How the intervention might work
Several treatment options for stage I endometrial cancer are in practice, including:
adjuvant pelvic EBRT,
adjuvant VBT,
a surveillance policy.
Adjuvant pelvic EBRT is designed to irradiate sites of potential micrometastatic cancer that remains in the region of the pelvis after surgery. This includes: the vaginal vault, parametrial ligaments and draining lymph nodes. Vaginal intracavity brachytherapy is designed to treat only the vagina and to decrease radiation effect on the gastrointestinal tract and the urinary tract. A surveillance policy is designed to spare the toxicity of adjuvant treatment, keeping radiotherapy in reserve to salvage any pelvic relapses of the cancer that may occur.
Why it is important to do this review
A recent review, with meta‐analyses including new data, concluded that EBRT cannot be routinely recommended as routine treatment to improve survival in early endometrial cancer (ASTEC/EN.5). However, new trials comparing EBRT and VBT and VBT to no additional treatment have since been reported. Hence there is a need for an updated review on the benefits and risks of adjuvant radiotherapy to guide the clinical management of stage I endometrial cancer. We considered meta‐analyses of trials comparing adjuvant radiotherapy with chemotherapy beyond the scope of this review.
Objectives
To assess the efficacy of adjuvant radiotherapy (both EBRT and VBT) when used following surgery for stage I endometrial cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing surgery followed by radiotherapy versus surgery alone or with VBT.
Types of participants
Women with stage I endometrial cancer who had been treated surgically with a hysterectomy and bilateral oophorectomy, with or without pelvic and para‐aortic lymphadenectomy.
Not all women had a complete assessment of the pelvic and para‐aortic lymph nodes. The status of pelvic and para‐aortic lymph nodes included was either:
known negative from lymphadenectomy, or
unknown.
Although the lack of a diagnostic lymphadenectomy was not an exclusion criteria, each trial (or centre) had a consistent practice and criteria for lymphadenectomy and the randomisation should have ensured that the arms of the trial were balanced.
Types of interventions
Surgery with the addition of either none or one or both of the following, with the intention to start within three months of surgery.
EBRT to the pelvis or para‐aortic nodes, or both.
VBT.
Types of outcome measures
Primary outcomes
Overall survival
Secondary outcomes
Locoregional recurrence
Distant recurrence
Endometrial cancer deaths
Data on acute and late toxicity were recorded where possible.
Search methods for identification of studies
Electronic searches
The following electronic databases were originally searched from 1966 to 2005; the searches were revised and run again in May 2011 and January 2012 for the updated review as follows:
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2011, Issue 4) (Appendix 1).
MEDLINE (to January 2012) (Appendix 2).
EMBASE (to January 2012) (Appendix 3).
The Specialised Register of the Cochrane Gynaecological Cancer Review Group (CGCRG).
Searching other resources
We searched the reference lists of the relevant papers for further studies and sought papers in all languages. In addition, we searched the Meta‐register and its links for ongoing trials. We attempted to contact the main investigators of the relevant past and ongoing trials for further information (e.g. unpublished trials, interim results) and most responded generously.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by the electronic searches to a database by IS and removed duplicate citations and obvious irrelevant materials. For the original review, three review authors selected studies independently (AK, Chris Williams and Mandy Collingwood, see Acknowledgements) and then compared notes. For the updated review, AK, NJ and TL selected trials. Differences between authors were resolved by discussion. The inclusion and exclusion criteria were as follows (see also the Criteria for considering studies for this review above).
Inclusion criteria: RCTs comparing surgery and radiotherapy with surgery alone (with or without VBT) for stage I endometrial cancer.
Exclusion criteria: non‐randomised trials, trials of pre‐operative radiotherapy, trials of sarcoma or mixed histology, those where the data on sarcoma cannot be separated out, trials where one or more of the groups contained fewer than 10 women, and trials of radiotherapy versus other active treatment such as chemotherapy or hormonal therapy.
Data extraction and management
For the original review, three review authors independently extracted data from the included trials using pre‐specified data collection forms; for the updated review, this was done by AK and TL. Any discrepancies in data extraction were resolved by discussion. For all trials included in the analysis, we collected and analysed data relating to numbers of patients, characteristics of patients and their disease, including histology and grading. We recorded the extent of surgery and details of the dose, fractionation and mode of delivery of the EBRT and brachytherapy. We noted variations between radiation treatments within trials.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies by using The Cochrane Collaboration's tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included the following assessment.
Selection bias: random sequence generation and allocation concealment.
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data.
Reporting bias: selective reporting of outcomes.
Other possible sources of bias.
Two review authors applied the 'Risk of bias' tool independently (AK, TL) and differences were resolved by discussion or by appeal to a third review author (NJ). Results are summarised in the 'Risk of bias' graph and a 'Risk of bias' summary. Results of meta‐analyses are interpreted in light of the findings with respect to the risk of bias.
Measures of treatment effect
For time‐to‐event data (e.g. overall survival, endometrial carcinoma‐related death), we attempted to extract hazard ratios (HR) and their associated variances. Where this was not possible, we extracted individual (dichotomous) patient data. Dichotomous data (e.g. incidence of women with locoregional recurrence, distant recurrence or death related to endometrial carcinoma) are presented as summary risk ratios (RRs) with 95% confidence intervals (CIs).
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero, or there was a low P value (< 0.10) in the Chi² test for heterogeneity.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used the methods described by Parmar 1998 to estimate HRs where possible from Kaplan Meier curves and used the generic inverse facility of RevMan 2011 to combine the data. We used the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986). The random‐effects summary was treated as the average range of possible treatment effects and is presented as the average treatment effect with 95% CIs, and the estimates of T² and I². We calculated the absolute risk and number needed to treat (NNT), where possible.
Subgroup analysis and investigation of heterogeneity
The initial protocol stated that we would carry out subgroup analysis by prognostic factors if possible. As the definitions and inclusion of patients with high risk factors varied between the studies, we grouped women according to the investigators' definitions of intermediate‐risk and high‐risk. Where this was not possible, we defined women as intermediate‐risk if they had stage IC or grade 3, and high risk if they had stage IC and grade 3, since these two factors consistently correlate strongly with prognosis.
As standard radiotherapy protocols for stage I endometrial cancer may include VBT, we anticipated heterogeneity and grouped trials by control group for the purposes of investigation and clarification as follows:
EBRT versus no additional treatment;
EBRT versus no additional treatment (with women also receiving VBT balanced across groups); and
EBRT versus VBT.
Neither subgroup analyses was specified at protocol stage.
Sensitivity analysis
We performed sensitivity analyses where there was a risk of bias associated with the quality of some of the included trials, or where the risk of bias was unclear i.e. Soderini 2003 and Aalders 1980. In addition, we performed sensitivity analysis where potential clinical heterogeneity existed without statistical heterogeneity e.g. with PORTEC‐2.
Results
Description of studies
Results of the search
For the original review, reviewers selected 33 records as relevant; of these, four studies (13 records) were included and 20 studies were excluded. For the updated review, 2,165 records were identified by the updated search (2006 to January 2012) rendering 1,806 after de‐duplication. We selected 20 records as relevant, retrieved the full text, and assessed these studies independently; of these, four studies (11 records) were included and nine studies were excluded. Therefore, for this updated version of the review, eight studies (24 records),were 'included' and 29 studies/records were excluded.
Included studies
We included eight trials in our meta‐analyses (Aalders 1980; ASTEC/EN.5; GOG 99; PORTEC‐1; PORTEC‐2; Soderini 2003; Sorbe 2009; Sorbe 2011). ASTEC/EN.5 consists of combined data from two trials. Four RCTs were included in the original Cochrane review (Aalders 1980; GOG 99; PORTEC‐1; Soderini 2003) and we added a further four trials to the updated review (ASTEC/EN.5; PORTEC‐2; Sorbe 2009; Sorbe 2011). We obtained additional data from Soderini 2003 after contacting the lead investigator (see Characteristics of included studies for more details).
These trials included 4,273 evaluable women: Aalders 1980 = 540 women; ASTEC/EN.5 = 905 women; GOG 99 = 392 women; PORTEC‐1 = 714 women; PORTEC‐2 = 427 women; Soderini 2003 = 123 women; Sorbe 2009 = 645 women and Sorbe 2011 = 527 women. All included trials compared pelvic EBRT with no EBRT except for Sorbe 2009, which compared VBT with no additional treatment. PORTEC‐2 compared EBRT with VBT alone. In three trials, VBT was given to all women (Aalders 1980; Sorbe 2011) or some women (ASTEC/EN.5), such that VBT was balanced between intervention and control groups.
All women in PORTEC‐1, PORTEC‐2 and Aalders 1980 underwent a total abdominal hysterectomy (TAH) and bilateral salpingo‐oophorectomy (BSO) without routine lymphadenectomy. In PORTEC‐1 and PORTEC‐2, abdominal exploration was done and any suspicious lymph nodes were removed. In ASTEC/EN.5, lymphadenectomy as part of surgical staging was not a requirement for randomisation and 29.4% of women underwent lymphadenectomy, which was balanced across groups. In GOG 99 and Soderini 2003, all women underwent pelvic and para‐aortic lymphadenectomy. Women in Sorbe 2009 and Sorbe 2011 underwent TAH, BSO, appendectomy, node sampling of enlarged nodes and peritoneal washings; lymphadenectomy was not routine.
All eight trials studied stage I endometrial cancer. Sorbe 2009 only included women with stage IA and IB. Women with stage IB and grade 1 cancer were included in Aalders 1980 and GOG 99. GOG 99 included women with any degree of myometrial invasion with adenocarcinoma of any grade (i.e. stage IB and above). They also included women with occult stage IIA and IIB, although both arms have almost equal proportion of these women (approximately 9.5%). In general, the baseline characteristics were well balanced in the two arms of the three trials. GOG 99 defined a high‐intermediate risk subgroup of patients after randomisation as: 1) grade 2 or 3, presence of lymphovascular space invasion (LVSI), and stage IC; 2) age ≥ 50 with any two risk factors listed above; 3) age ≥ 70 with any risk factor listed above.
PORTEC‐1 included women of intermediate‐risk (IB grade 2) and high‐intermediate‐risk defined by the following: age > 60, stage IC grade 1 or 2; or age > 60, stage IB grade 3. Thirty‐one percent of women were intermediate‐risk (and also some low‐risk), balanced across groups. Women with Stage IB grade 1 or IC grade 3 lesions were excluded.
PORTEC‐2 included women of high‐intermediate risk only, defined as age > 60 years and stage IC grade 1 or 2 disease, or stage IB grade 3 disease, or stage IIA disease (excluding grade 3). Women with stage II cancer (11.5%) were balanced across groups.
ASTEC/EN.5 included stage I and IIA with intermediate‐risk or high‐risk features including: stage IA and IB grade 3, IC all grades, papillary serous or clear cell histology all stages; overall, 4.9% of patients were stage II (4.1% stage IIA) and were balanced across the two groups. 'High risk' included all papillary serous and clear cell subtypes, all other subtypes in IC (grade 3) and IIA (grade 3), and all women in stage IIB.
Sorbe 2011 included intermediate‐risk women defined as: FIGO Stage I (surgical staging); endometrioid histological type; the presence of Grade 3 or deep myometrial infiltration or DNA aneuploidy; nuclear Grade l‐2; pathologically negative lymph nodes; and negative abdominal cytology. The last two points were optional.
The median length of follow‐up of women at the time of analysis was: ASTEC/EN.5 = 58 months, GOG 99 = 69 months, PORTEC‐1 = 52 months, PORTEC‐2 = 45 months, Sorbe 2009 = 68 months (mean; range = 2 to 151 months), Aalders 1980 = median not stated (range of 3 to 10 years), Soderini 2003 = 48 months and Sorbe 2011 = 62 months.
PORTEC‐1 has published 8, 10 and 15‐year follow‐up data (Creutzberg 2003, Scholten 2005, Nout 2011 and Creutzberg 2011).
For further details see Characteristics of included studies.
Excluded studies
See Characteristics of excluded studies.
We excluded 29 studies, 14 because they were not RCTs.
We excluded 12 RCTs (three of which are duplicate trials, so nine RCTs in total) because either the randomisation was not between adjuvant radiotherapy versus no radiotherapy (De Palo 1993; Garzetti 1994; Maggi 1993; Maggi 2006), unacceptable methods of randomisation were used (Marchetti 1985; Piver 1979) or the intervention was pre‐operative radiotherapy which was an exclusion criteria (Weigensberg 1984).
Hogberg 2010 compared chemotherapy and radiotherapy with radiotherapy alone (included data from EORTC 55991, MaNGO ILIADE‐III and NSGO‐EC‐9501).
GOG 122 and GOG 150 compared chemotherapy with radiotherapy for advanced endometrial carcinoma and stage I‐IV carcinosarcoma of the uterus respectively; Sagae 2005 and Susumu 2008 reported the same trial comparing radiotherapy with chemotherapy.
Haie Meder 1995 and Sorbe 2005 compared two different VBT doses. Haie Meder 2004 is a long‐term follow‐up report of Haie Meder 1995.
Risk of bias in included studies
See 'Risk of bias' tables in Characteristics of included studies and Figure 1.
1.
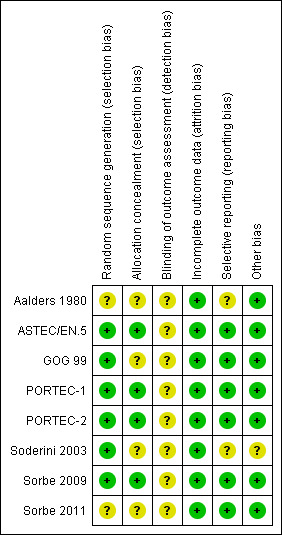
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We considered the following five trials to be of high quality and at a low risk of bias: ASTEC/EN.5; GOG 99; PORTEC‐1; PORTEC‐2; Sorbe 2009. In these studies, the methods of randomisation were clearly described, analyses were by intention‐to‐treat and there was minimal loss to follow‐up. GOG 99 did not describe allocation concealment and lost 24 women to follow‐up at a median of 50 months (6%). PORTEC‐1 lost one woman to follow‐up. Due to the nature of the intervention, patients and treatment providers were not blind to group allocation in any of the trials, however, outcome assessor blinding was described in three trials (ASTEC/EN.5; PORTEC‐2; Sorbe 2009).
The published report of Sorbe 2011 lacked methodological details, specifically with regard to the method of randomisation, allocation concealment and blinding, however all expected outcomes and attrition were reported. We provisionally considered this trial to be at moderate to low risk of bias pending additional details from the authors.
We considered Aalders 1980 and Soderini 2003 to be at moderate and high risk of bias respectively. Trial methodology was not adequately described in either, however Aalders 1980 reported loss to follow‐up (none) and baseline characteristics were comparable.
Soderini 2003 was only ever published as an abstract. We decided to include it following a personal communication with Dr Soderini confirming that a computer‐generated table had been used to randomise patients and that there had been no loss to follow‐up. In addition, Dr Soderini provided some additional data and a copy of the poster presentation from the 2003 ESGO conference where the data had originally been presented. According to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the evidence for the presence of several types of reporting biases, such as publication bias, demonstrates the need to search comprehensively for studies that meet the eligibility criteria for a Cochrane review. Guidelines for including unpublished data such as these are contained in the handbook.
Since Aalders 1980 and Soderini 2003 did not report time‐to‐event data, they are not represented in any of the time‐to‐event meta‐analyses in this review. However, where we included their data in our dichotomous meta‐analyses, we performed sensitivity analyses to assess their impact. Where meta‐analyses combined data from Soderini 2003 with only one other trial, we presented the meta‐analyses with subtotals only. On sensitivity analyses, Soderini 2003 had little impact on any of the findings, with its weighting limited to 2.8% to 4.3% of all the analyses except for the lymphadenectomy subgroup analysis, where it contributed 21% of the data.
Effects of interventions
See: Table 1
1. EBRT (with or without VBT) versus no EBRT (or VBT alone): All women (five‐year data)
Seven trials (3628 evaluable women) contributed data to the outcomes for this comparison (Aalders 1980; ASTEC/EN.5; GOG 99; PORTEC‐1; PORTEC‐2; Soderini 2003; Sorbe 2011). The meta‐analyses included published, unpublished and synthesised (time‐to‐event) data. Aalders 1980 and Soderini 2003 did not contribute data to time‐to‐event analyses.
a. Death from all causes (overall survival)
There was no significant difference in overall survival time between the EBRT treatment group and the no EBRT group (time‐to‐event data; five trials, 2965 women; Hazard ratio (HR) 0.99, 95% confidence interval (CI) 0.82 to 1.20; Analysis 1.1; Figure 2) or survival rates (dichotomous data; seven trials, 3628 women; risk ratio (RR) 0.98, 95% CI 0.83 to 1.15; Analysis 1.2). Dichotomous meta‐analysis included data from Soderini 2003 (3.2% weighting). Whilst these data introduced heterogeneity into the EBRT versus no additional treatment subgroup, they did not significantly impact the overall findings (five trials, 2,965 women; RR 0.98; 95% CI 0.82 to 1.17 without Soderini 2003). There was no heterogeneity of data across the exploratory subgroups (EBRT versus no additional treatment, EBRT versus no additional treatment (VBT balanced across groups) and EBRT versus VBT) (I² = 0%).
1.1. Analysis.
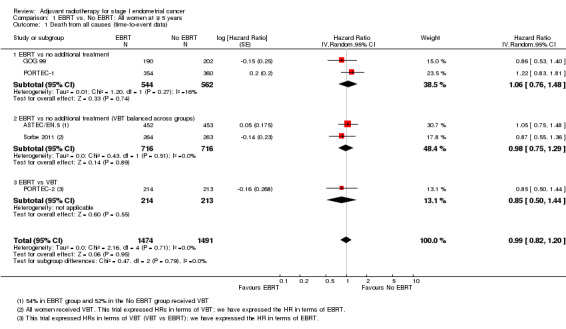
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 1 Death from all causes (time‐to‐event data).
2.
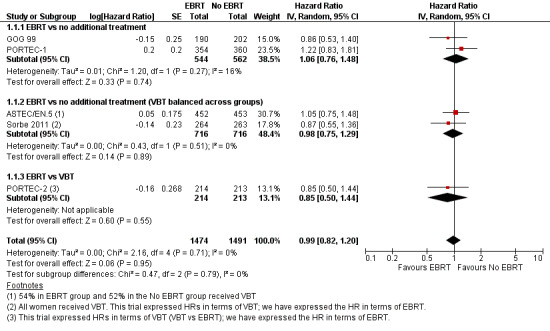
Forest plot of comparison: 1 EBRT vs. No EBRT: All patients at 5 years, outcome: 1.1 Death from all causes (time‐to‐event data).
1.2. Analysis.
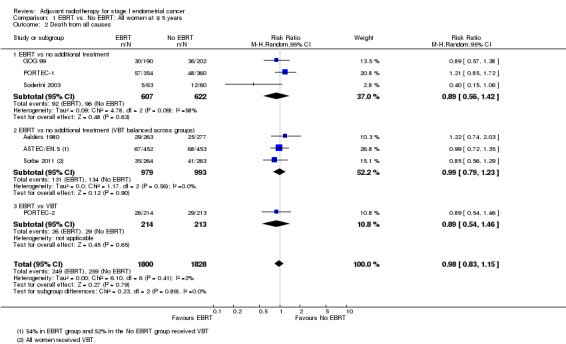
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 2 Death from all causes.
b. Endometrial cancer deaths
There was no significant difference in cancer‐specific survival between the EBRT group and the no EBRT group using time‐to‐event data (five trials, 2965 women; HR 0.96, 95% CI 0.72 to 1.28; Analysis 1.3) or dichotomous data (seven trials, 3628 women; RR 1.02, 95% CI 0.81 to 1.29; Analysis 1.4). Data were not heterogeneous and tests for subgroup differences were not significant. The combined weight of Soderini 2003 and Aalders 1980 accounted for 21.7% and their inclusion in the meta‐analysis had little impact on the risk ratio (five trials, 2965 women; RR 0.97, 95%CI 0.71 to 1.33 without these two trials).
1.3. Analysis.
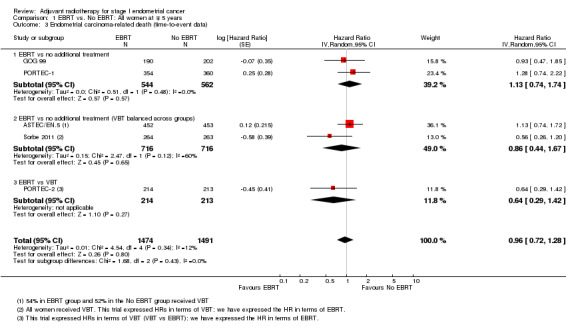
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 3 Endometrial carcinoma‐related death (time‐to‐event data).
1.4. Analysis.
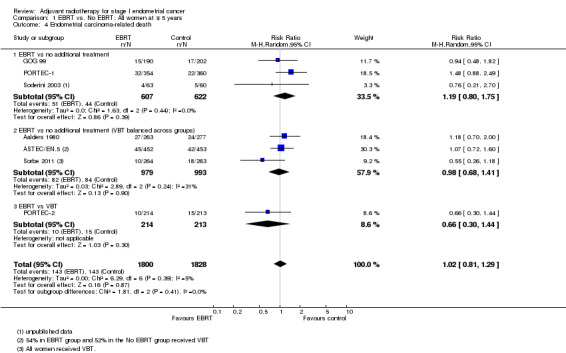
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 4 Endometrial carcinoma‐related death.
c. Locoregional recurrence
External beam pelvic radiotherapy significantly reduced locoregional recurrence. This applied to both time‐to‐event data (five trials, 2965 women; HR 0.36, 95% CI 0.25 to 0.52; Analysis 1.5) and dichotomous data (seven trials, 3628 women; RR 0.33, 95% CI 0.23 to 0.47; Analysis 1.6). The latter translates to a 67% reduction in the risk that the first relapse will be locoregional (95% CI 53% to 77%) with EBRT. There was no evidence of heterogeneity (I² = 0%).
1.5. Analysis.
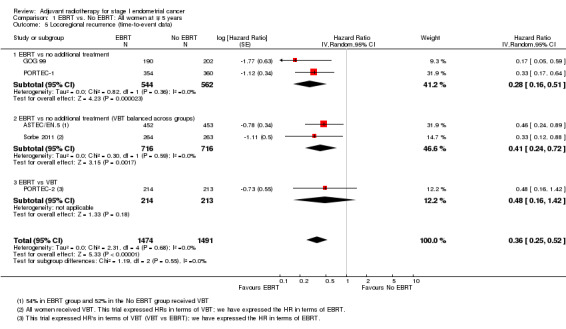
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 5 Locoregional recurrence (time‐to‐event data).
1.6. Analysis.
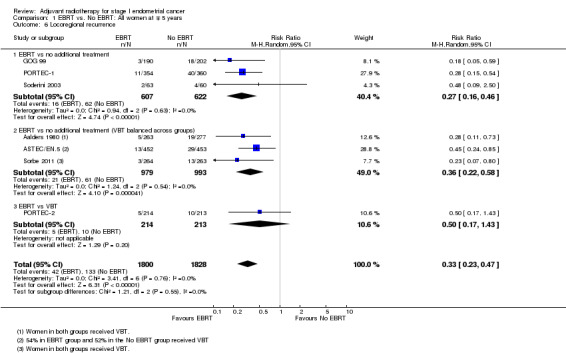
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 6 Locoregional recurrence.
d. Distant recurrence
Meta‐analysis of dichotomous data showed no significant difference between treatment and control groups (seven trials, 3628 women; RR 1.04, 95% CI 0.80 to 1.35; Analysis 1.8). The RR was 1.03 (95% CI 0.77 to 1.38) without Soderini 2003 (2.8% weighting). Only three trials (GOG 99; PORTEC‐2; Sorbe 2011) had time‐to‐event data for this outcome (1346 women; HR 0.71, 95% CI 0.46 to 1.09; Analysis 1.7).
1.8. Analysis.
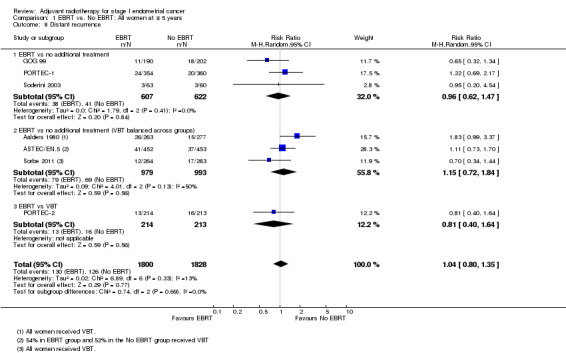
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 8 Distant recurrence.
1.7. Analysis.
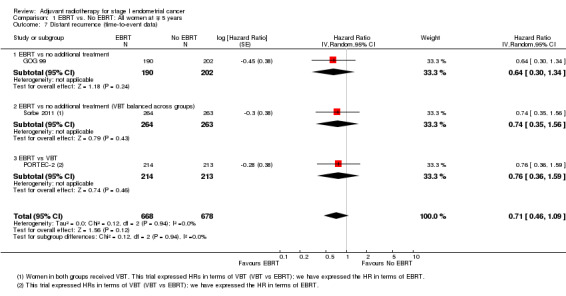
Comparison 1 EBRT vs. No EBRT: All women at ∓ 5 years, Outcome 7 Distant recurrence (time‐to‐event data).
2. EBRT versus no EBRT: Low‐risk women (IA/B and Grade 1/2)
Only two trials contributed data to the meta‐analyses of EBRT for low‐risk women (Aalders 1980, GOG 99). We included GOG 99 data defined by investigators as 'low‐intermediate risk'; in this trial, endometrial cancer deaths included treatment‐related deaths and deaths from unknown cause. EBRT increased the risk of endometrial carcinoma‐related death (two trials, 517 women; RR 2.64,95% CI 1.05 to 6.66, Analysis 2.2).
2.2. Analysis.

Comparison 2 EBRT vs. No EBRT: Low risk women (IA/B and grade 1/2), Outcome 2 Endometrial carcinoma‐related deaths.
Only GOG 99 data were available for the outcome of 'death from all causes' and so meta‐analysis could not be done (Analysis 2.1), however, the RR for GOG 99 data alone were 1.03 (95% CI 0.51 to 2.08).
2.1. Analysis.
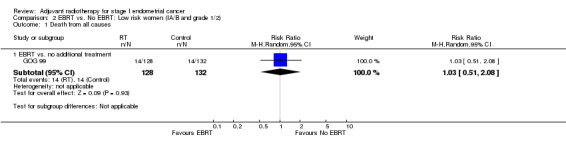
Comparison 2 EBRT vs. No EBRT: Low risk women (IA/B and grade 1/2), Outcome 1 Death from all causes.
3. EBRT versus no EBRT: Intermediate‐risk women (as defined by investigators; OR Stage IC OR Grade 3)
We included low‐intermediate risk data from GOG 99 in these subgroup analyses. The PORTEC‐2 data used were reported as 'true high‐intermediate risk' data (N = 366), including some unpublished data. There was no significant difference in overall survival (time‐to‐event data: five studies, 2,560 women; HR 1.05, 95% CI 0.85 to 1.31; Analysis 3.1; and dichotomous data: seven studies, 2944 women; RR 0.98, 95% CI 0.82 to 1.18; Analysis 3.2). Soderini 2003 had a weighting of 3.6% in the dichotomous meta‐analysis and had little impact on the results (six studies, 2,821 women; RR 1.02; 95% CI 0.84 to 1.23 without Soderini 2003 data).
3.1. Analysis.
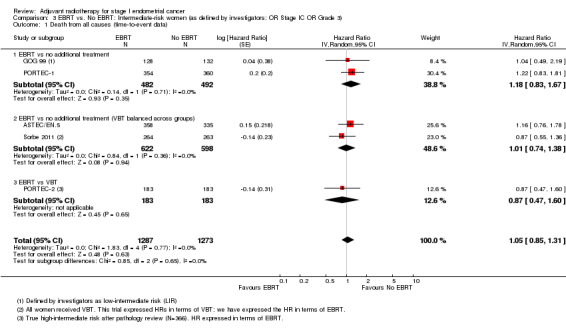
Comparison 3 EBRT vs. No EBRT: Intermediate‐risk women (as defined by investigators; OR Stage IC OR Grade 3), Outcome 1 Death from all causes (time‐to‐event data).
3.2. Analysis.
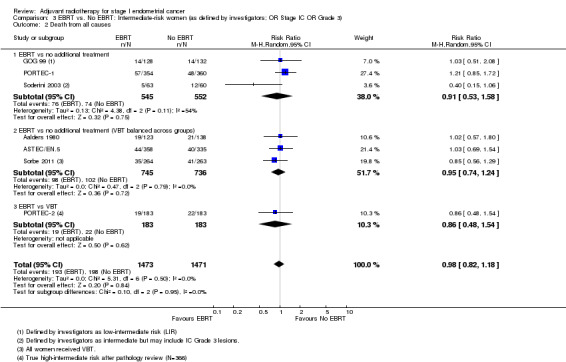
Comparison 3 EBRT vs. No EBRT: Intermediate‐risk women (as defined by investigators; OR Stage IC OR Grade 3), Outcome 2 Death from all causes.
There was no significant difference in endometrial cancer‐related deaths (time‐to‐event data: five trials, 2560 women; HR 1.03, 95% CI 0.70 to 1.51; Analysis 3.3; dichotomous data: six trials, 2821 women; RR 1.08, 95% CI 0.77 to 1.51; Analysis 3.4).
3.3. Analysis.

Comparison 3 EBRT vs. No EBRT: Intermediate‐risk women (as defined by investigators; OR Stage IC OR Grade 3), Outcome 3 Endometrial cancer‐related deaths (time‐to‐event data).
3.4. Analysis.
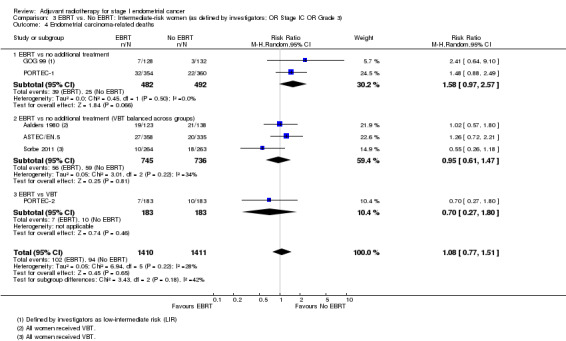
Comparison 3 EBRT vs. No EBRT: Intermediate‐risk women (as defined by investigators; OR Stage IC OR Grade 3), Outcome 4 Endometrial carcinoma‐related deaths.
If we excluded GOG 99 from the time‐to‐event meta‐analyses (which included low‐intermediate data), the HRs for overall survival (HR 1.06, 95% CI 0.84 to 1.32) and endometrial‐cancer specific survival (HR 0.97, 95% CI 0.66 to 1.41) were similar to those above.
One trial (PORTEC‐2) compared EBRT with VBT in the high‐intermediate risk group and reported that VBT was effective in ensuring vaginal control with a non‐significant difference in loco‐regional relapse rate compared to EBRT (5.1% versus 2.1%; HR 2.08, 95% CI 0.71 to 6.09; P = 0·17).
4. EBRT versus no EBRT: High‐risk women (as defined by investigators; OR Stage IC AND Grade 3)
We included high‐intermediate risk women from GOG 99 in these meta‐analyses. There was no significant difference in overall survival (time‐to‐event data: two trials, 334 women; HR 0.91, 95% CI 0.60 to 1.39; Analysis 4.1; and dichotomous data: three trials, 429 women; RR 0.88, 95% CI 0.63 to 1.22; Analysis 4.2). Likewise, there was no significant difference in endometrial cancer‐specific survival (time‐to‐event data: two trials, 334 women; HR 0.84, 95% CI 0.51 to 1.40, Analysis 4.3; and dichotomous data: three trials, 429 women; RR 0.80, 95% CI 0.54 to 1.18, Analysis 4.4) .
4.1. Analysis.
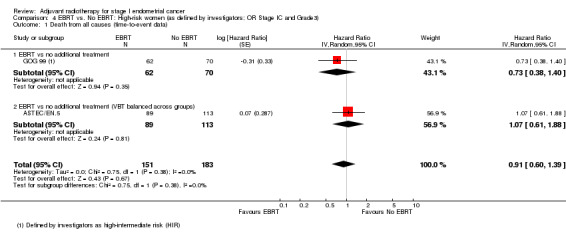
Comparison 4 EBRT vs. No EBRT: High‐risk women (as defined by investigators; OR Stage IC and Grade3), Outcome 1 Death from all causes (time‐to‐event data).
4.2. Analysis.
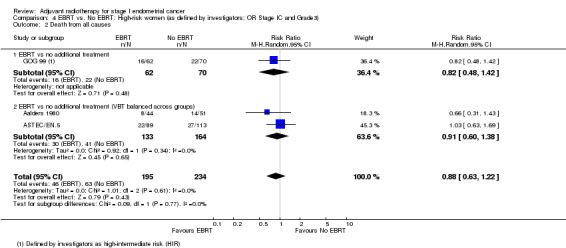
Comparison 4 EBRT vs. No EBRT: High‐risk women (as defined by investigators; OR Stage IC and Grade3), Outcome 2 Death from all causes.
4.3. Analysis.
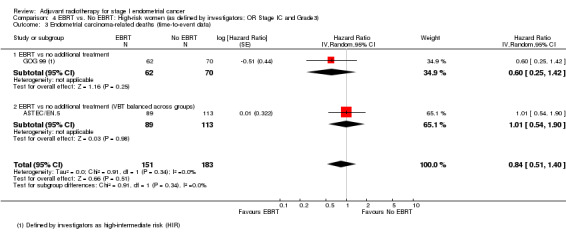
Comparison 4 EBRT vs. No EBRT: High‐risk women (as defined by investigators; OR Stage IC and Grade3), Outcome 3 Endometrial carcinoma‐related deaths (time‐to‐event data).
4.4. Analysis.
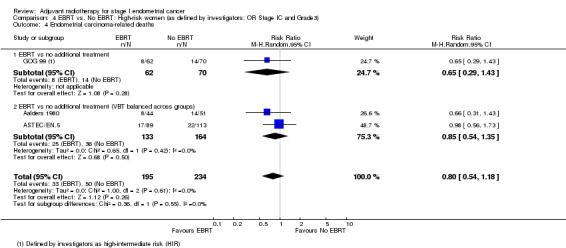
Comparison 4 EBRT vs. No EBRT: High‐risk women (as defined by investigators; OR Stage IC and Grade3), Outcome 4 Endometrial carcinoma‐related deaths.
If we excluded GOG 99 data from the dichotomous meta‐analyses, two trials remained (297 women; ASTEC/EN.5; Aalders 1980) giving a RR for OS of 0.91 (95% CI 0.60 to 1.38) and a RR for endometrial cancer‐specific survival of 0.85 (95% CI 0.54 to 1.35).
5. ERBT versus no EBRT: Intermediate‐risk and high‐risk women
There was no significant difference between intermediate‐risk and high‐risk subgroups with regard to death from all causes (Analysis 5.1; tests for subgroup differences: I² = 0%, P = 0.56) or endometrial carcinoma‐related deaths (Analysis 5.2; tests for subgroup differences: I² = 24%, P = 0.25). However, data on high‐risk women were limited.
5.1. Analysis.
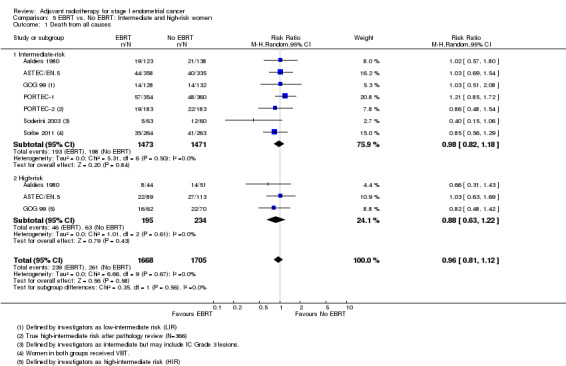
Comparison 5 EBRT vs. No EBRT: Intermediate and high‐risk women, Outcome 1 Death from all causes.
5.2. Analysis.
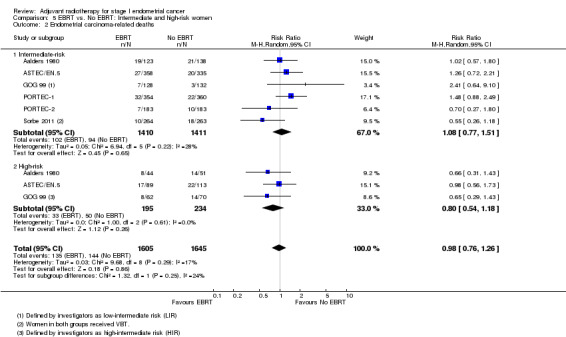
Comparison 5 EBRT vs. No EBRT: Intermediate and high‐risk women, Outcome 2 Endometrial carcinoma‐related deaths.
6. EBRT versus no EBRT: All lymphadenectomy women (pelvic and para‐aortic)
Three trials contributed data to these subgroup analyses: ASTEC/EN.5, GOG 99 and Soderini 2003. There was no significant difference in overall survival between EBRT and no EBRT in the subgroup of women with pelvic and para‐aortic lymphadenectomy (three trials, 781 women; RR 0.87, 95% CI 0.51 to 1.47; Analysis 6.2). Soderini 2003 decreased the RR in the direction of EBRT (21% weighting) and, when we excluded these data, the RR for overall survival was 1.07 (95% CI 0.69 to1.65; two trials, 658 women).
6.2. Analysis.
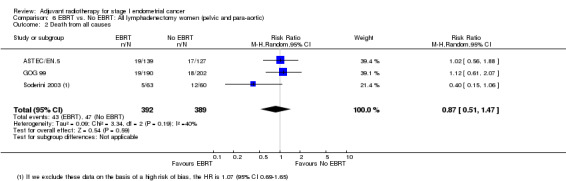
Comparison 6 EBRT vs. No EBRT: All lymphadenectomy women (pelvic and para‐aortic), Outcome 2 Death from all causes.
Two trials (ASTEC/EN.5 and Soderini 2003) reported RRs for endometrial cancer‐specific survival for this subgroup (Analysis 6.4) and two trials (GOG 99 and Soderini 2003) reported 'all recurrences' ( Analysis 6.5). Results are presented as subtotals only.
6.4. Analysis.
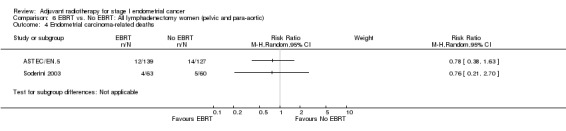
Comparison 6 EBRT vs. No EBRT: All lymphadenectomy women (pelvic and para‐aortic), Outcome 4 Endometrial carcinoma‐related deaths.
6.5. Analysis.
Comparison 6 EBRT vs. No EBRT: All lymphadenectomy women (pelvic and para‐aortic), Outcome 5 All recurrences.
7. EBRT versus no EBRT: All women (long‐term follow‐up data)
PORTEC‐1 reported follow‐up data at eight years (Creutzberg 2003), 10 years (Scholten 2005) and 15 years post‐randomisation (Creutzberg 2011). These follow‐up data essentially convey a consistent effect over time, i.e. that EBRT reduces locoregional recurrence but has no value in reducing deaths overall or deaths related to endometrial‐cancer in women with low and intermediate‐risk endometrial cancer. For 10‐year overall survival, meta‐analysis of dichotomous data from PORTEC‐1 and Aalders 1980 yields a RR 1.26 (95% CI 1.03 to 1.54) Analysis 7.1.
7.1. Analysis.
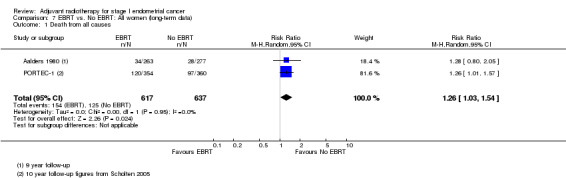
Comparison 7 EBRT vs. No EBRT: All women (long‐term data), Outcome 1 Death from all causes.
8. VBT versus no additional treatment
Only one trial (Sorbe 2009), studied VBT versus no additional treatment in low‐risk women only. There was no significant difference in overall survival between the women who received VBT versus those who received no additional treatment in this study (RR 1.09, 95% CI 0.56 to 2.11) or in endometrial cancer‐related deaths (645 women, RR 1.43, 95% CI 0.46 to 4.46). However, there was a non‐significant reduction in loco‐regional recurrence in the VBT group compared with the no additional treatment group (RR 0.39, 95% CI 0.14 to 1.09; Analysis 8.2).
8.2. Analysis.
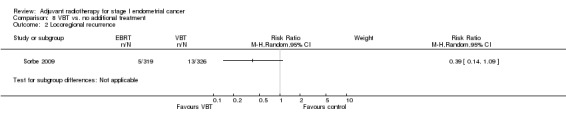
Comparison 8 VBT vs. no additional treatment, Outcome 2 Locoregional recurrence.
9. EBRT versus no EBRT: Adverse effects
Severe acute toxicity (G3/4) was significantly more frequent in the EBRT group than the no EBRT group (two trials, 1328 women; RR 4.68, 95% CI 1.35 to 16.16, Analysis 9.2), as was severe late toxicity (Grade 3/4) (six trials, 3,501 women; RR 2.58, 95% CI 1.61 to 4.11, Analysis 9.4).
9.2. Analysis.
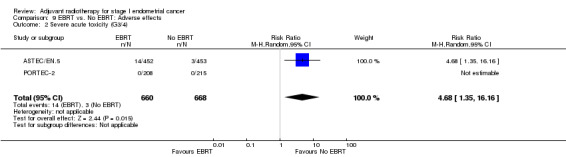
Comparison 9 EBRT vs. No EBRT: Adverse effects, Outcome 2 Severe acute toxicity (G3/4).
9.4. Analysis.
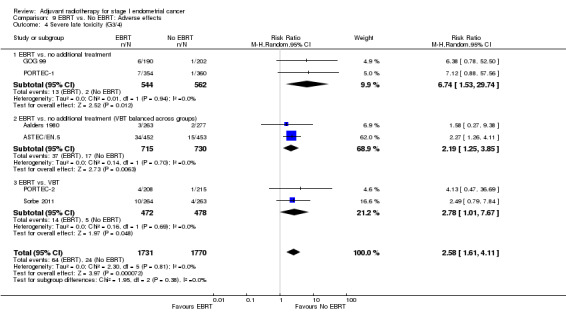
Comparison 9 EBRT vs. No EBRT: Adverse effects, Outcome 4 Severe late toxicity (G3/4).
Two women in the EBRT group of GOG 99 and two in Aalders 1980 died from radiation‐related complications involving intestinal injury.
10. EBRT versus no EBRT: Quality of life and long‐term adverse effects
PORTEC‐1 has published long‐term data. Nout 2011 evaluated health‐related quality of life more than a decade after treatment with the Dutch version of the Short Form 36‐Item (SF‐36) questionnaire. Symptoms were assessed with a modified version of the EORTC PR25 module for bowel and bladder symptoms. The OV28 and CX24 modules assessed sexual function. The median follow‐up was 13.3 years. Three hundred and fifty‐one women were confirmed to be alive with correct address and 246 (70%) returned the questionnaire. Women who had received radiotherapy had significantly (P < 0.01) higher rates of urinary incontinence, diarrhoea, and faecal leakage that limited their daily activities. The clinical significance is illustrated by use of incontinence materials by women more than 10 years after radiotherapy compared with no additional treatment (day and night use, 42.9% versus 15.2% respectively; P < 0 .001). Random allocation to radiotherapy was associated with lower SF‐36 scores on the scales "physical functioning" (P = 0.004), "role‐physical" (P=0.003) and "bodily pain" (P = 0.009). There was no detectable difference in reported sexual function scores.
Discussion
Summary of main results
For our original review (see Appendix 5 for original results) several major EBRT studies in endometrial carcinoma were ongoing (ASTEC/EN.5; PORTEC‐2; Sorbe 2009; Sorbe 2011). Now that these high quality trials have been published, we are able to give more definitive answers regarding adjuvant radiotherapy in stage I endometrial carcinoma.
See Table 1. Eight trials contributed data to this review. Adjuvant EBRT significantly reduced the risk that the first recurrence would be in the field of radiation but had no significant effect on overall survival, endometrial cancer‐related survival or distant metastases. There were no survival benefits from ERBT for any of the main subgroup populations, namely, low‐risk, intermediate‐risk, high‐risk and women who underwent pelvic and para‐aortic lymphadenectomy, although we downgraded the evidence for the low‐risk and the high‐risk subgroups to low and moderate quality respectively. The long‐term follow‐up data from PORTEC‐1 suggest that these findings hold true over time (Creutzberg 2004; Nout 2011; Scholten 2005).
Only one trial compared VBT to no additional treatment in women with low‐risk disease (Sorbe 2009). This study found that both locoregional and distant recurrences are low in low‐risk women and postoperative VBT did not improve survival. However, there was a non‐significant reduction in locoregional recurrence in the VBT group. EBRT increased the risk of endometrial carcinoma‐related death in the low‐risk group, possibly due to the inclusion of data from treatment‐related deaths, however, this could be due to chance. We have downgraded the quality of this evidence due to the limited data, inclusion in the meta‐analysis of Aalders 1980 (an older study with some methodological short‐comings), and inclusion of low‐intermediate risk data from GOG 99. Since the loco‐regional recurrence rate in this subgroup is low and not significantly improved by VBT, VBT is probably not required in these women. We do not believe that further EBRT research is warranted in this low‐risk group.
There is insufficient evidence to draw conclusions about VBT in intermediate and high‐risk women: One trial compared EBRT with VBT in intermediate‐risk women (PORTEC‐2) and VBT was given to all women (Aalders 1980; Sorbe 2011) or some women (ASTEC/EN.5) in two trials, such that VBT was balanced between intervention and control groups. VBT was effective in ensuring vaginal control in PORTEC‐2 although the locoregional relapse was slightly higher (statistically not significant) with VBT than with EBRT. Sorbe 2011 reported a significant difference in locoregional relapse in favour of the EBRT plus VBT group; however, women in this group experienced significantly more severe late side‐effects. PORTEC‐2 and Sorbe 2011 recommend that VBT is used as the adjuvant treatment of choice for women with endometrial carcinoma of intermediate‐risk (Sorbe 2011) and high‐intermediate risk (PORTEC‐2), respectively. Due to a lack of data, it is not yet known whether the use of VBT will have a survival benefit over no treatment for intermediate‐risk and high‐risk stage I women, although it is unlikely to yield a survival benefit since there is no survival benefit with EBRT. In addition, whilst VBT appears useful to reduce the risk of vaginal recurrence, it is not known whether it reduces pelvic side wall recurrence. Further evidence, comparing VBT with no additional treatment in intermediate‐risk and high‐risk women is desirable, however, since 5‐year loco‐regional recurrence rates were 14% in the "no treatment" group of PORTEC‐1, such trials may be unethical.
Regarding toxicity, EBRT was associated with significantly more severe acute toxicity and late toxicity (grade 3 and 4) that no ERBT. A few women were reported to have died from radiotherapy‐related complications.
Overall completeness and applicability of evidence
This updated review confirms the main findings of the previous review as well as the conclusions from major randomised studies in women with stage I endometrial carcinoma, i.e. adjuvant EBRT reduces locoregional recurrence but does not improve overall survival or endometrial cancer‐related deaths; however, we found insufficient evidence to draw conclusions about the high‐risk subgroup and cannot exclude a benefit for EBRT in this subgroup. In addition, based on low‐quality evidence, our findings suggest that EBRT may have an adverse effect on cancer‐specific survival in low‐risk women. Therefore, whilst we agree with the current practice and recommendation from ASTEC/EN.5 that routine EBRT should not be recommended in women with stage I endometrial carcinoma regardless of risk factors, further evidence is needed to guide practice for women who are truly high‐risk. To our knowledge, no studies have assessed the benefits and risks of adjuvant VBT versus no additional treatment in women with intermediate‐risk or high‐risk stage I endometrial cancer. We are, therefore, unable to draw conclusions about whether VBT is necessary in these women.
Quality of the evidence
This updated review has included several high‐quality randomised studies, including ASTEC/EN.5, GOG 99, PORTEC‐1, PORTEC‐2 and Sorbe 2009. Sorbe 2011 lacked some methodological details but since all expected outcomes were reported and groups had similar baseline characteristics, we considered this trial at moderate to low risk of bias, pending more details. We acknowledged that Soderini 2003 and Aalders 1980 are of poorer quality and may have higher risk of bias but we have carried out sensitivity analysis by excluding these trials to assess their impact. We downgraded the evidence relating to the high‐risk group of women as these meta‐analyses consisted of data from two or three trials, one of which was Aalders 1980. Furthermore, we included high‐intermediate data from GOG 99 and not true high‐risk data (Table 1).This means that, for high‐risk women, further research may have an important impact on our confidence in the estimate of effect and may change the estimate. We consider the evidence pertaining to EBRT in the low‐risk subgroup to be of a low quality.
Potential biases in the review process
Although our initial protocol stated that we would carry out subgroup analysis by prognostic factors if possible, we did not predefine these subgroups. The prognostic factors considered differed between included trials as did the definitions, and inclusion of women, for the various risk subgroups. We grouped women according to investigators definitions or, if this was not possible, we defined women as intermediate‐risk if they had stage IC or grade 3, and high‐risk if they had stage IC and grade 3. In GOG 99 we could not separate the low‐intermediate risk from low‐risk and thus this group was included in both groups; their high‐intermediate risk data were included in our high‐risk subgroup. This may be considered inappropriate and we therefore, presented results with and without these data. However, the difference in definitions and lack of individual patient data to separate women into the different risk‐defined subgroups may have introduced bias. In the next update, we plan to analyse data from women in the ASTEC/EN.5 and PORTEC‐1 trials who satisfy the GOG 99 criteria for high‐risk data, pending the availability of these data from the investigators. This may reduce the potential for bias in this subgroup. We urge investigators planning and conducting trials in early endometrial cancer to standardise the definitions of risk.
Since writing the protocol for this review, VBT has become the standard adjuvant intervention for early endometrial cancer at many centres; therefore, for the updated review we decided to group trials according to control group (e.g. no additional treatment or VBT), for the purposes of clarity, and to investigate potential heterogeneity between trial interventions. There was no significant heterogeneity between these subgroups, however, they consisted of one to three trials only and any differences or similarities between them might have resulted from bias.
It may be argued that PORTEC‐2 should not be included in meta‐analyses of EBRT versus no EBRT as this trial directly compares EBRT with VBT, therefore VBT is not balanced between groups. We considered VBT to be equivalent to the 'no treatment' or 'control' group and, for the purposes of clarity, we distinguished this trial in a separate subgroup. Sensitivity and subgroup analyses showed that the results were statistically similar when PORTEC‐2 data were excluded, even for loco‐regional recurrence; furthermore, we detected no statistical heterogeneity when these data were included.
For the risk subgroups, Aalders 1980 reported endometrial cancer‐related deaths only and not overall survival. However, since the overall data for the two survival outcomes were similar in this trial, we included these cancer‐specific data in the overall survival subgroup meta‐analyses. Whilst this may not be entirely appropriate, sensitivity analyses revealed that it had little impact on the dichotomous findings. Furthermore, Aalders 1980 did not report time‐to‐event data and therefore was not included in the main (time‐to‐event) survival meta‐analyses.
Agreements and disagreements with other studies or reviews
The results of this review agree with the ASTEC/EN.5 review, although ASTEC/EN.5 excluded Aalders 1980, on the basis that it was undertaken before the introduction of FIGO staging, and Soderini 2003, on the basis that it was only published as an abstract. Neither Soderini 2003 nor Aalders 1980 presented time‐to‐event data. However, we included these lower quality trials and performed sensitivity analyses to assess the impact of including their data on the review findings. With regard to the outcome 'endometrial carcinoma‐related deaths' (Analysis 1.4), their combined weight accounted for 21.7% and their inclusion had little impact on the overall risk ratio (seven trials, 3628 women; RR 1.02, 95% CI 0.81 to 1.29; versus five trials, 2965 women; RR 0.97, 95%CI 0.71 to 1.33). Soderini 2003 contributed a 2.8% weighting to the 'death from all causes' outcome and, similarly, had a negligible impact on the results (RR 0.98 versus RR 1.00).
The original Cochrane review found a trend towards a survival benefit in high‐risk women (1C G3), however, the ASTEC/EN5 data now dominate these meta‐analyses of the high‐risk subgroup (weighting of 45% to 65%). These new data have shifted the survival HRs in the direction of no difference.
Since adjuvant EBRT does not improve survival from stage I endometrial cancer, even, it appears, in the high‐risk group, it raises questions as to whether other treatment modalities such as chemotherapy (CT) and targeted therapies may be more effective than radiotherapy in improving survival. Our review was not predefined to answer the question of adjuvant chemotherapy in endometrial carcinoma; this intervention has been reviewed separately (Johnson 2011). In the Johnson 2011 review, data from four trials comparing conventional adjuvant platinum based combination chemotherapy to radiotherapy were pooled (GOG 122; GOG 150; Maggi 2006; Susumu 2008); meta‐analyses showed a statistically significant improvement in survival rates (RR 0.76, 95% CI 0.62 to 0.92) and progression‐free survival (HR 0.80, 95% CI 0.66 to 0.97) in favour of chemotherapy.
PORTEC‐3, a randomised phase III trial comparing concurrent chemoradiation and adjuvant CT with pelvic radiation alone in high‐risk and advanced stage endometrial carcinoma, is currently ongoing.
Several targeted therapies are now emerging for endometrial cancer, including mTOR inhibitors, EGFR inhibitors, and fibroblast growth factor receptor 2 inhibitor, which are beyond the scope of this review. Further details can be found in Zagouri 2010.
Authors' conclusions
Implications for practice.
This updated systematic review confirms that although EBRT decreases locoregional recurrence, it does not decrease overall deaths or deaths related to endometrial cancer in stage I endometrial cancer, regardless of their risk factors. Although a benefit for high‐risk stage I disease cannot be excluded, we agree with the recommendations of ASTEC/EN.5 investigators, namely that routine EBRT cannot be recommended to improve survival in women with stage I endometrial carcinoma, and that VBT may be preferable for local control in intermediate and high‐intermediate risk women. In women with low‐risk disease, adjuvant radiotherapy may have an adverse effect on endometrial cancer‐related survival compared to no additional treatment.
Implications for research.
Due to the relatively good prognosis of women with stage 1 endometrial cancer, large numbers of participants are needed to conduct sufficiently powered RCTs to detect any differences in survival that may be present. The number of women with high‐risk stage I endometrial cancer who have participated in trials of adjuvant EBRT is relatively small. The apparent lack of any survival advantage in this group does not exclude the possibility of a small advantage. Our sample size means that our analyses lack power and there may be a place for more trials of EBRT in this selected group. In addition, since adjuvant radiotherapy may not be shown to increase survival in women with stage I endometrial cancer including the high‐risk group, there is a need to investigate other treatment modalities which may improve outcome for the high‐risk women. This includes chemotherapy and targeted therapies. The role of adjuvant concurrent chemoradiation followed by adjuvant chemotherapy is currently being investigated in endometrial cancer patients with high risk factors (PORTEC‐3).
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2018 | Amended | Next stage expected date amended. |
| 28 June 2018 | Review declared as stable | The original question has been answered and the review conclusions will not change with additional studies. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 1 March 2012 | Amended | Minor amendments made to the discussion and conclusions. |
| 26 January 2012 | New citation required and conclusions have changed | Data from additional studies included (ASTEC/EN.5; PORTEC‐2; Sorbe 2009; Sorbe 2011) and the 15‐year follow‐up of PORTEC‐1. Five studies excluded. Search updated from June 2011 to January 2012. No additional studies identified. |
| 16 January 2012 | New search has been performed | Search updated (January 2006 to May 2011). Ten new studies added to awaiting classification section: Kitchener 2009; Sorbe 2005; ASTEC/EN.5; PORTEC‐2 (two papers), Sorbe 2009; Susumu 2008; Takin 2011; Hogberg 2010; Dickler 2010; Sorbe 2011. |
| 19 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank the following people for their contributions to the updated review:
Carien Creutzberg and Remi Nout for providing additional data from PORTEC‐1 and PORTEC‐2.
Alejandro Soderini for providing additional unpublished information and data from Soderini 2003.
Bengt Sorbe for providing additional unpublished information from Sorbe 2009.
Jane Hayes from the CGCRG for performing the updated search.
Andy Bryant for assisting with some of the statistical methods and data synthesis used in the review.
Katharine Tylko‐Hill for providing consumer feedback.
The CGCRG editors, Gail Quinn and Clare Jess, for their administrative support.
Carien Creutzberg, Remi Nout and Nick Reed for providing critical appraisal as part of the peer‐review process.
We would like to thank the following people for their contributions to the original review:
Chris Williams (CW) for co‐writing the protocol and review.
Mandy Collingwood (MC) for selecting studies and co‐writing the protocol.
Iveta Simera (IS) for performing the original search.
Paul Cornes (PC) for critical comments on the review.
Astrid Scholten and Carien Creutzberg for providing raw data from PORTEC‐1 and Pierre Martin‐Hirsch for expert criticism.
Vivien Garner of the Cochrane Gynaecological Cancer Review Group (CGCRG) for obtaining papers.
Ann Marie Swart, Claire Amos, Max Parmar and Elizabeth Eisenhausuer from The ASTEC study group for contacting GOG 99 on behalf of our group and providing the information on the ASTEC study.
Heather Dickinson for her critical comments on the methods of the review as well as statistical input on the analysis and helpful textual amendments.
Melanie Powell, Consultant Clinical Oncologist at St. Bartholomew's Hospital, London and Mary Quigley, Consultant Clinical Oncologist at Oldchurch Hospital, Romford for reviewing the initial draft of this review.
We are grateful to the Department of Health (UK) for funding the CGCRG and for access to The Cochrane Library and statistical expertise.
Appendices
Appendix 1. CENTRAL search strategy
Issue 4, 2011
#1 MeSH descriptor Endometrial Neoplasms explode all trees #2 endometr* #3 uter* near/5 (body or corp*) #4 (#2 OR #3) #5 cancer* or neoplas* or malignan* or carcinom* or tumor* or tumour* or adenocarcinoma* #6 (#4 AND #5) #7 (#1 OR #6) #8 MeSH descriptor Radiotherapy explode all trees #9 radiotherap* #10 radiation #11 irradiat* #12 brachytherap* #13 external beam #14 teletherap* #15 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16 (#7 AND #15)
Appendix 2. MEDLINE search strategy
To January Week 1 2012
1 exp Endometrial Neoplasms/ 2 endometr*.mp. 3 (uter* adj5 (body or corp*)).mp. 4 2 or 3 5 (cancer* or neoplas* or malignan* or carcinom* or tumor* or tumour* or adenocarcinoma*).mp. 6 4 and 5 7 1 or 6 8 exp Radiotherapy/ 9 radiotherap*.mp. 10 radiation.mp. 11 irradiat*.mp. 12 brachytherap*.mp. 13 external beam.mp. 14 teletherap*.mp. 15 or/8‐14 16 7 and 15 17 randomized controlled trial.pt. 18 controlled clinical trial.pt. 19 randomized.ab. 20 placebo.ab. 21 radiotherapy.fs. 22 randomly.ab. 23 trial.ab. 24 groups.ab. 25 or/17‐24 26 16 and 25 27 (animals not (humans and animals)).sh. 28 26 not 27
key: mp=title, original title, abstract, name of substance word, subject heading word, pt=publication type, ab=abstract, fs= floating subheading, sh=Medical Subject Heading
Appendix 3. EMBASE search strategy
To January week 2 2012
1 exp Endometrium Tumor/ 2 endometr*.mp. 3 (uter* adj5 (body or corp*)).mp. 4 3 or 2 5 (cancer* or neoplas* or malignan* or carcinom* or tumor* or tumour* or adenocarcinoma*).mp. 6 4 and 5 7 6 or 1 8 exp Radiotherapy/ 9 radiotherap*.mp. 10 radiation.mp. 11 irradiat*.mp. 12 brachytherap*.mp. 13 external beam.mp. 14 teletherap*.mp. 15 or/8‐14 16 7 and 15 17 exp Controlled Clinical Trial/ 18 randomized.ab. 19 placebo.ab. 20 rt.fs. 21 randomly.ab. 22 trial.ab. 23 groups.ab. 24 or/17‐23 25 24 and 16
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name, fs‐ floating, subheading, ab=abstract
Appendix 4. Risk of bias assessment
For the updated review, two reviewe authors (AK and TL) independently assessed the risk of bias of each included study using the criteria outlined in the Cochrane handbook for Systematic Reviews of Interventions (Higgins 2009).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator),
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) or,
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we had about other possible sources of bias and assessed each study as at:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
Appendix 5. Results and conclusions of the 2007 version of this review
Results
The meta‐analysis of four trials included 1770 patients (Aalders 1980; GOG 99; PORTEC‐1; Soderini 2003). The addition of pelvic external beam radiotherapy to surgery reduced locoregional recurrence, a relative risk (RR) of 0.28 (95% confidence interval (CI) 0.17 to 0.44, P < 0.00001), which is a 72% reduction in the risk of pelvic relapse (95% CI 56% to 83%) and an absolute risk reduction of 6% (95% CI of 4% to 8%). The number needed to treat (NNT) to prevent one locoregional recurrence is 16.7 patients (95% CI 12.5 to 25). The reduction in the risk of locoregional recurrence did not translate into either a reduction in the risk of distant recurrence or death from all causes or endometrial cancer death. A subgroup analysis of women with multiple high risk factors (including stage 1c and grade 3) showed a trend toward the reduction in the risk of death from all causes and endometrial cancer death in patients who underwent adjuvant external beam radiotherapy.
Authors' conclusions
Patients with stage I endometrial carcinoma have different risks of local and distant recurrence depending on the presence of risk factors including stage 1c, grade 3, lymphovascular space invasion and age. Though external beam pelvic radiotherapy reduced locoregional recurrence by 72%, there is no evidence to suggest that it reduced the risk of death. In patients with multiple high risk factors, including stage 1c and grade 3, there was a trend towards a survival benefit and adjuvant external beam radiotherapy may be justified. For patients with only one risk factor, grade 3 or stage 1c, no definite conclusion can be made and data from ongoing studies (ASTEC; Lukka) are awaited. External beam radiotherapy carries a risk of toxicity and should be avoided in stage 1 endometrial cancer patients with no high risk factors.
Data and analyses
Comparison 1. EBRT vs. No EBRT: All women at ∓ 5 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes (time‐to‐event data) | 5 | 2965 | Hazard Ratio (Random, 95% CI) | 0.99 [0.82, 1.20] |
| 1.1 EBRT vs no additional treatment | 2 | 1106 | Hazard Ratio (Random, 95% CI) | 1.06 [0.76, 1.48] |
| 1.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 1432 | Hazard Ratio (Random, 95% CI) | 0.98 [0.75, 1.29] |
| 1.3 EBRT vs VBT | 1 | 427 | Hazard Ratio (Random, 95% CI) | 0.85 [0.50, 1.44] |
| 2 Death from all causes | 7 | 3628 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.15] |
| 2.1 EBRT vs no additional treatment | 3 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 2.2 EBRT vs no additional treatment (VBT balanced across groups) | 3 | 1972 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.23] |
| 2.3 EBRT vs VBT | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 3 Endometrial carcinoma‐related death (time‐to‐event data) | 5 | 2965 | Hazard Ratio (Random, 95% CI) | 0.96 [0.72, 1.28] |
| 3.1 EBRT vs no additional treatment | 2 | 1106 | Hazard Ratio (Random, 95% CI) | 1.13 [0.74, 1.74] |
| 3.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 1432 | Hazard Ratio (Random, 95% CI) | 0.86 [0.44, 1.67] |
| 3.3 EBRT vs VBT | 1 | 427 | Hazard Ratio (Random, 95% CI) | 0.64 [0.29, 1.42] |
| 4 Endometrial carcinoma‐related death | 7 | 3628 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.29] |
| 4.1 EBRT vs no additional treatment | 3 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.80, 1.75] |
| 4.2 EBRT vs no additional treatment (VBT balanced across groups) | 3 | 1972 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| 4.3 EBRT vs VBT | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.30, 1.44] |
| 5 Locoregional recurrence (time‐to‐event data) | 5 | 2965 | Hazard Ratio (Random, 95% CI) | 0.36 [0.25, 0.52] |
| 5.1 EBRT vs no additional treatment | 2 | 1106 | Hazard Ratio (Random, 95% CI) | 0.28 [0.16, 0.51] |
| 5.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 1432 | Hazard Ratio (Random, 95% CI) | 0.41 [0.24, 0.72] |
| 5.3 EBRT vs VBT | 1 | 427 | Hazard Ratio (Random, 95% CI) | 0.48 [0.16, 1.42] |
| 6 Locoregional recurrence | 7 | 3628 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.23, 0.47] |
| 6.1 EBRT vs no additional treatment | 3 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.16, 0.46] |
| 6.2 EBRT vs no additional treatment (VBT balanced across groups) | 3 | 1972 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.58] |
| 6.3 EBRT vs VBT | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.17, 1.43] |
| 7 Distant recurrence (time‐to‐event data) | 3 | 1346 | Hazard Ratio (Random, 95% CI) | 0.71 [0.46, 1.09] |
| 7.1 EBRT vs no additional treatment | 1 | 392 | Hazard Ratio (Random, 95% CI) | 0.64 [0.30, 1.34] |
| 7.2 EBRT vs no additional treatment (VBT balanced across groups) | 1 | 527 | Hazard Ratio (Random, 95% CI) | 0.74 [0.35, 1.56] |
| 7.3 EBRT vs VBT | 1 | 427 | Hazard Ratio (Random, 95% CI) | 0.76 [0.36, 1.59] |
| 8 Distant recurrence | 7 | 3628 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.35] |
| 8.1 EBRT vs no additional treatment | 3 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.62, 1.47] |
| 8.2 EBRT vs no additional treatment (VBT balanced across groups) | 3 | 1972 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.72, 1.84] |
| 8.3 EBRT vs VBT | 1 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.40, 1.64] |
Comparison 2. EBRT vs. No EBRT: Low risk women (IA/B and grade 1/2).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 EBRT vs. no additional treatment | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.51, 2.08] |
| 2 Endometrial carcinoma‐related deaths | 2 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.05, 6.66] |
| 2.1 EBRT vs. no additional treatment | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [0.64, 9.10] |
| 2.2 EBRT vs. no additional treatment (VBT balanced across groups) | 1 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.80, 10.42] |
Comparison 3. EBRT vs. No EBRT: Intermediate‐risk women (as defined by investigators; OR Stage IC OR Grade 3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes (time‐to‐event data) | 5 | 2560 | Hazard Ratio (Random, 95% CI) | 1.05 [0.85, 1.31] |
| 1.1 EBRT vs no additional treatment | 2 | 974 | Hazard Ratio (Random, 95% CI) | 1.18 [0.83, 1.67] |
| 1.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 1220 | Hazard Ratio (Random, 95% CI) | 1.01 [0.74, 1.38] |
| 1.3 EBRT vs VBT | 1 | 366 | Hazard Ratio (Random, 95% CI) | 0.87 [0.47, 1.60] |
| 2 Death from all causes | 7 | 2944 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 2.1 EBRT vs no additional treatment | 3 | 1097 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.58] |
| 2.2 EBRT vs no additional treatment (VBT balanced across groups) | 3 | 1481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.24] |
| 2.3 EBRT vs VBT | 1 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.54] |
| 3 Endometrial cancer‐related deaths (time‐to‐event data) | 5 | 2560 | Hazard Ratio (Random, 95% CI) | 1.03 [0.70, 1.51] |
| 3.1 EBRT vs no additional treatment | 2 | 974 | Hazard Ratio (Random, 95% CI) | 1.40 [0.84, 2.33] |
| 3.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 1220 | Hazard Ratio (Random, 95% CI) | 0.84 [0.42, 1.72] |
| 3.3 ERBT vs VBT | 1 | 366 | Hazard Ratio (Random, 95% CI) | 0.70 [0.27, 1.84] |
| 4 Endometrial carcinoma‐related deaths | 6 | 2821 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.51] |
| 4.1 EBRT vs no additional treatment | 2 | 974 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.97, 2.57] |
| 4.2 EBRT vs no additional treatment (VBT balanced across groups) | 3 | 1481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.47] |
| 4.3 EBRT vs VBT | 1 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.27, 1.80] |
Comparison 4. EBRT vs. No EBRT: High‐risk women (as defined by investigators; OR Stage IC and Grade3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes (time‐to‐event data) | 2 | 334 | Hazard Ratio (Random, 95% CI) | 0.91 [0.60, 1.39] |
| 1.1 EBRT vs no additional treatment | 1 | 132 | Hazard Ratio (Random, 95% CI) | 0.73 [0.38, 1.40] |
| 1.2 EBRT vs no additional treatment (VBT balanced across groups) | 1 | 202 | Hazard Ratio (Random, 95% CI) | 1.07 [0.61, 1.88] |
| 2 Death from all causes | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 2.1 EBRT vs no additional treatment | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.48, 1.42] |
| 2.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.60, 1.38] |
| 3 Endometrial carcinoma‐related deaths (time‐to‐event data) | 2 | 334 | Hazard Ratio (Random, 95% CI) | 0.84 [0.51, 1.40] |
| 3.1 EBRT vs no additional treatment | 1 | 132 | Hazard Ratio (Random, 95% CI) | 0.60 [0.25, 1.42] |
| 3.2 EBRT vs no additional treatment (VBT balanced across groups) | 1 | 202 | Hazard Ratio (Random, 95% CI) | 1.01 [0.54, 1.90] |
| 4 Endometrial carcinoma‐related deaths | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.18] |
| 4.1 EBRT vs no additional treatment | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.29, 1.43] |
| 4.2 EBRT vs no additional treatment (VBT balanced across groups) | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.35] |
Comparison 5. EBRT vs. No EBRT: Intermediate and high‐risk women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes | 7 | 3373 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.12] |
| 1.1 Intermediate‐risk | 7 | 2944 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 1.2 High‐risk | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 2 Endometrial carcinoma‐related deaths | 6 | 3250 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.26] |
| 2.1 Intermediate‐risk | 6 | 2821 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.51] |
| 2.2 High‐risk | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.18] |
Comparison 6. EBRT vs. No EBRT: All lymphadenectomy women (pelvic and para‐aortic).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes (time‐to‐event data) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Death from all causes | 3 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.47] |
| 3 Endometrial carcinoma‐related deaths (time‐to‐event data) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Endometrial carcinoma‐related deaths | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 All recurrences | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
6.1. Analysis.
Comparison 6 EBRT vs. No EBRT: All lymphadenectomy women (pelvic and para‐aortic), Outcome 1 Death from all causes (time‐to‐event data).
6.3. Analysis.
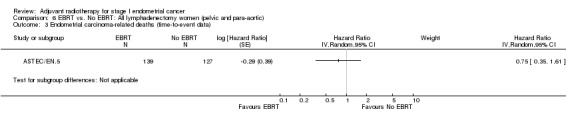
Comparison 6 EBRT vs. No EBRT: All lymphadenectomy women (pelvic and para‐aortic), Outcome 3 Endometrial carcinoma‐related deaths (time‐to‐event data).
Comparison 7. EBRT vs. No EBRT: All women (long‐term data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes | 2 | 1254 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.03, 1.54] |
| 2 Endometrial carcinoma‐related deaths | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Locoregional recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
7.2. Analysis.
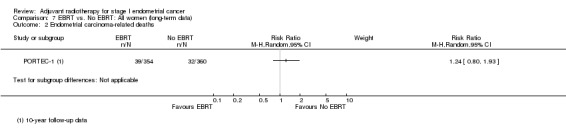
Comparison 7 EBRT vs. No EBRT: All women (long‐term data), Outcome 2 Endometrial carcinoma‐related deaths.
7.3. Analysis.
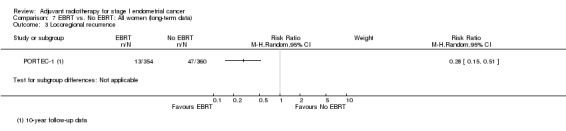
Comparison 7 EBRT vs. No EBRT: All women (long‐term data), Outcome 3 Locoregional recurrence.
Comparison 8. VBT vs. no additional treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from all causes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Locoregional recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Distant recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Endometrial carcinoma‐related death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
8.1. Analysis.
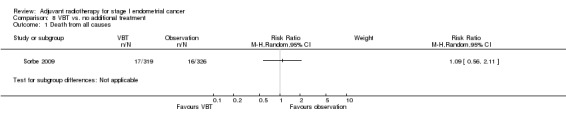
Comparison 8 VBT vs. no additional treatment, Outcome 1 Death from all causes.
8.3. Analysis.
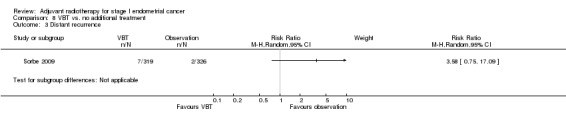
Comparison 8 VBT vs. no additional treatment, Outcome 3 Distant recurrence.
8.4. Analysis.
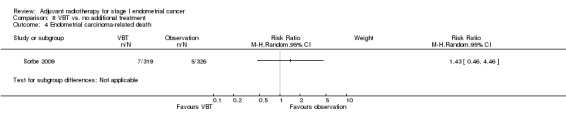
Comparison 8 VBT vs. no additional treatment, Outcome 4 Endometrial carcinoma‐related death.
Comparison 9. EBRT vs. No EBRT: Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute toxicity (all grades) | 2 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [1.49, 5.92] |
| 2 Severe acute toxicity (G3/4) | 2 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 4.68 [1.35, 16.16] |
| 3 Late toxicity (all grades) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Severe late toxicity (G3/4) | 6 | 3501 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [1.61, 4.11] |
| 4.1 EBRT vs. no additional treatment | 2 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 6.74 [1.53, 29.74] |
| 4.2 EBRT vs. no additional treatment (VBT balanced across groups) | 2 | 1445 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.25, 3.85] |
| 4.3 EBRT vs. VBT | 2 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.01, 7.67] |
9.1. Analysis.
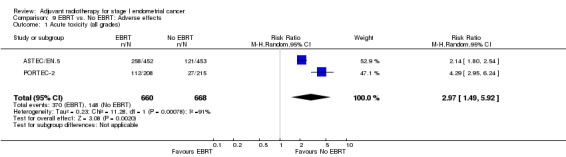
Comparison 9 EBRT vs. No EBRT: Adverse effects, Outcome 1 Acute toxicity (all grades).
9.3. Analysis.
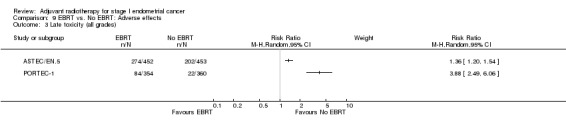
Comparison 9 EBRT vs. No EBRT: Adverse effects, Outcome 3 Late toxicity (all grades).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aalders 1980.
| Methods | Methods of randomisation not specified. Application of ITT analysis was not mentioned. | |
| Participants | Women with Stage 1 endometrial cancer following TAH and BSO. Also included women with Stage IB and grade 1 tumour. | |
| Interventions | All had intravaginal radium. Intervention group received further pelvic RT but not the control group. Follow‐up was 3‐10 years. | |
| Outcomes | Pelvic radiotherapy reduced vaginal and pelvic recurrences (1.9% versus 6.9%, P < 0.001 but not overall survival rate/5‐year survival). | |
| Notes | Only patients with grade 3 and Stage IC tumour might have benefited from pelvic radiotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Histological material "reassessed without reference to the clinical course" suggests that some measure of assessor blinding was applied. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women lost to follow‐up. Baseline characteristics comparable. |
| Selective reporting (reporting bias) | Unclear risk | Application of ITT were not described. |
| Other bias | Low risk | Baseline characteristics comparable. |
ASTEC/EN.5.
| Methods | Two multicentre randomised trials combined data From July 1996 to March 2005, ASTEC recruited participants from UK, Poland, Norway and New Zealand; EN.5 recruited participants from Canada, Australia and USA. Randomisation was computer‐generated with central allocation via telephone. | |
| Participants | 789 ASTEC participants and 116 EN.5 participants with histologically confirmed intermediate‐risk (stage IA and IB Grade 3, IC and IIA Grade 1 and 2) or high‐risk (IC and IIA Grade 3, and IIB) early‐stage endometrial cancer. Lymphadenectomy was not required. Women with positive lymph nodes were eligible for ASTEC but not EN.5. Randomisation was based on the local pathology report. VBT was allowed if the centre's policy was to offer it to all stage I and IIa women, irrespective of group allocation. | |
| Interventions | EBRT (40‐46 Gy in 20‐25 daily fractions) versus no additional treatment until recurrence (NAT), with or without VBT. | |
| Outcomes | Primary outcome was overall survival. Secondary outcomes were disease‐specific survival, disease‐specific recurrence, recurrence‐free survival, isolated loco‐regional recurrence, and treatment toxicity. Median follow‐up was 58 months. | |
| Notes | Overall, group size (452 EBRT and 453 NAT) and baseline characteristics were similar, except for small imbalance in proportion of high‐risk women (25% in NAT versus 20% in ERBT group). 52% in NAT group and 54% in EBRT group received VBT. 5% of ASTEC women received other adjuvant treatment, balanced between groups. 92% in the EBRT group received the allocated treatment; 2% in the observation group received EBRT. Analysis was by ITT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation via telephone. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of cause of death was made by the chief investigator blinded to the treatment group (ASTEC) and was classified as treatment‐related, disease‐related treatment and disease‐related, or other (non‐endometrial cancer, non‐treatment‐related). Participants and other personnel were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low level of missing data since all assessed for primary outcome. |
| Selective reporting (reporting bias) | Low risk | All expected pre‐specified outcomes reported. Analysis by ITT. |
| Other bias | Low risk | Baseline data were generally balanced between the two groups, except for a small imbalance in the proportion of high‐risk women, with 25% of those in the observation group classified as high risk compared with 20% in the external beam radiotherapy group. |
GOG 99.
| Methods | A balanced block randomisation scheme was used. 448 women randomised; 56 women were excluded from the ITT analysis on the basis that they were ineligible either because of inadequate staging or because of histology or FIGO stage. | |
| Participants | Women with Stage IB and IC, also IIA (occult) and IIB (occult) and had TAH and BSO and selective bilateral pelvic, and para‐aortic lymphadenectomy with removal of any enlarged or suspicious nodes. | |
| Interventions | Women were randomised to either whole pelvic RT (190 women) or no additional therapy (202 women). Median follow‐up was 56 months with 9% followed for less than 2 years. | |
| Outcomes | Pelvic RT reduced pelvic and vaginal recurrences but not the overall survival as pelvic recurrences are often effectively treated with second‐line therapy. | |
| Notes | A high intermediate risk group (HIR) was defined as those with (1) moderate to poorly differentiated tumour, presence of lymphovascular invasion, and outer third myometrial invasion; (2) age 50 or greater with any two risk factors listed above; or (3) age of at least 70 with any risk factor listed above. RT had greatest impact on preventing recurrence in this subgroup. Statistically significant differences were seen in frequency and severity of haematological, GI, GU and cutaneous toxicities in favour of the control group (P < 0.001). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A balanced block randomisation scheme was used. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. EBRT group size smaller than control group but post‐randomisation exclusions were made without knowledge of outcome data. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Analysis by ITT. |
| Other bias | Low risk | High quality trial. |
PORTEC‐1.
| Methods | Multicentre RCT. Centre‐blocked randomisation by telephone was done at the trial office with variable block sizes and was stratified by radiation oncology centre and depth of myometrial invasion. ITT analysis was used. | |
| Participants | Women with stage 1 endometrial carcinoma (grade 1 with deep myometrial invasion, grade 2 with any invasion or grade 3 with superficial invasion). All had TAH and BSO without lymphadenectomy. | |
| Interventions | Women were randomised to pelvic RT or no further treatment. Intravaginal brachytherapy was not given. Follow‐up was 5‐7 years. | |
| Outcomes | Pelvic RT reduced locoregional recurrence (4% versus 14%, P < 0.001) but not overall survival or endometrial‐cancer‐related death. Treatment‐related complications occurred in 25% of RT women and in 6% of the control group. | |
| Notes | No formal subgroup analysis according to depth of myometrial invasion and grade was done. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centre‐block randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation by telephone. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 714/715 women evaluated. |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all pre‐specified outcomes reported. |
| Other bias | Low risk | High quality trial. |
PORTEC‐2.
| Methods | Multicentre, open‐label, non‐inferiority computer‐randomised trial undertaken in The Netherlands between May 2002 and Sept 2006. Assigned via Internet with an application trial online process (TOP). Stratified by stage, centre, brachytherapy (low‐dose versus high‐dose) and patient age. Investigators blinded to treatment group. Analysis by ITT. | |
| Participants | 427 women with stage I or IIA endometrial cancer with high‐intermediate features namely, 1) age greater than 60 years and stage IC grade 1 or 2 disease, or stage 1B grade 3 disease; and (2) stage IIA disease, any age (apart from grade 3 with greater than 50% myometrial invasion). | |
| Interventions | Pelvic EBRT (46 Gy in 23 fractions; n = 214) versus VBT (21 Gy high‐dose rate in three fractions, or 30 Gy low‐dose rate; n = 213). | |
| Outcomes | Primary: vaginal recurrence. Secondary: overall survival, disease‐free survival, toxicity and QOL. | |
| Notes | Included 49 Stage IIA women (23 versus 26 respectively). Baseline characteristics comparable. Median follow‐up 45 months (range 18 to 78). Rates of acute grade 1–2 gastrointestinal toxicity were significantly lower in the VBT group than in the EBRT group at completion of radiotherapy (12·6% [27/215] versus 53·8% [112/208]). Central pathology review of 367 (86%) women had been completed at the time of analysis (183 [86%] women in EBRT group and 184 [86%] in VBT group). After review, 34 (8%) women had features of high‐risk disease (19 [9%] in EBRT group versus 15 [7%] in VBT group); 27 (6%) were low risk, and therefore in retrospect ineligible (12 [6%] versus 15 [7%]). Analysis of outcomes of the 366 women (86%) who remained high‐intermediate risk (true high‐intermediate risk) at review confirmed the findings of the ITT analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐randomised. |
| Allocation concealment (selection bias) | Low risk | Internet allocation via trial online process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. No missing data. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. ITT analysis. |
| Other bias | Low risk | High quality trial. Patient and tumour characteristics were well balanced between groups. |
Soderini 2003.
| Methods | Only an abstract. Randomisation by computer‐generated table. Analyses by ITT and no loss to follow‐up (personal communication 21/6/11). | |
| Participants | Women with intermediate risk (1bG2‐3 ‐1c) endometrioid endometrial carcinoma. all women had TAH‐BSO, pelvic‐para‐aortic lymphadenectomy and peritoneal washings. | |
| Interventions | Women were randomised to pelvic RT 50 Gy or no RT. | |
| Outcomes | Overall survival, progression‐free survival, locoregional recurrence and distant recurrence. | |
| Notes | Initially only an abstract was available. However, Dr Soderini confirmed via personal communication that analysis was by ITT, there was no loss to follow‐up and that women were randomised via a computer‐generated table after complete surgical staging. Median follow‐up was 48 months (range 12‐84 months). EC‐related deaths were 4/63 (RT) versus 5/60 (no RT). Attempts to publish the data apparently halted when Dr Soderini changed jobs in 2005. In addition to providing us with limited unpublished data, he sent us a copy of the original poster presentation (ESGO meeting in Brussels, 2003). Individual data on intermediate risk and high‐risk women were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table (personal communication). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up (personal communication). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details but analysis done by ITT (personal communication). |
| Other bias | Unclear risk | Insufficient details. |
Sorbe 2009.
| Methods | Multicentre European RCT; 650 recruited between Jan 1995 to Dec 2004, 5 excluded due to inclusion criteria not being met. Stratification was done per centre and group allocation was concealed by closed envelopes. | |
| Participants | 645 women with low‐risk, Stage IA‐IB endometrial cancer of Grade 1‐2. | |
| Interventions | Surgery and VBT (319 women) versus surgery alone (326 women). Surgery included TAH and BSO, node sampling and peritoneal washing. Lymphadenectomy was performed at one of the six participating centres. Time between surgery and VBT was 4 to 8 weeks; total dose ranged from 18 to 40 Gy. VBT given on outpatient basis. | |
| Outcomes | Overall survival, locoregional recurrence and toxicity. | |
| Notes | Mean follow‐up was 68 months (range 2‐151 months). Baseline characteristics were comparable. Four vaginal recurrences occurred in the VBT group versus. 10 in the control group. Late treatment side‐effects were mainly grade 1‐2 (EORTC criteria). Vaginal side‐effects were worse in the VBT group (28 versus 5, P = 0.00004). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation with stratification by centre. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were blind to group allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Analyses by ITT. |
| Other bias | Low risk | High quality trial. |
Sorbe 2011.
| Methods | RCT conducted at five Swedish centres. 562 women recruited between 1997 to 2008. Scanty methodological details provided in report. | |
| Participants | 527 women with 'medium‐risk' endometrial cancer defined as: FIGO Stage I (surgical staging); endometrioid histological type; the presence of Grade 3 or deep myometrial infiltration or DNA aneuploidy; nuclear Grade l‐2; pathologically negative lymph nodes; and negative abdominal cytology. Last two points were optional for this study and data were not available for all cases. Lymphovascular space invasion was not regularly included in the pathology reports at the participating centres and was not included in the definition of the medium‐risk group. Primary surgery consisted of TAH, BSO, appendectomy, node sampling of enlarged nodes, and peritoneal washings with cytology. Routine lymphadenectomy was not performed. | |
| Interventions | EBRT+VBT (264 women) versus VBT only (263 women). Time to intervention was between 4‐8 weeks post‐surgery. All VBT was given as on an outpatient basis (EQD2 total dose of 19.5 to 23.5 Gy at 5mm). EBRT was given as five fractions per week to a total dose of 46 Gy. | |
| Outcomes | Primary: Locoregional recurrence and overall survival. Secondary: recurrence‐free survival, recurrence‐free interval, cancer‐specific survival and toxicity. QOL assessed at baseline, 3 months and one year will be presented in a separate report. |
|
| Notes | Attrition: 14 patients not eligible, 15 patients withdrawn for personal reasons and five lost to follow‐up, therefore 34/562 excluded (21 in EBRT/VBT versus 14 in the VBT group). Median follow‐up = 62 months (range 12 to 138 months). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised' ‐ no details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 562 enrolled; 34/562 excluded from analyses including five lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes and all expected outcomes of interest reported. |
| Other bias | Low risk | Medium to low risk. Participant and tumour characteristics were similar between groups (FIGO Stage IA and DNA aneuploidy slightly higher in EBRT+VBT). Trial methodology unclear. Additional methodological details requested from authors. |
BSO: bilateral salpingo‐oophorectomy EBRT: external beam radiotherapy GI: gastro‐intestinal GU: genito‐urinary ITT: intention‐to‐treat NAT: no additional treatment QOL: quality of life RT: radiotherapy TAH: total abdominal hysterectomy VBT: vaginal brachytherapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Algan 1996 | Not RCT. |
| Atzinger 2001 | A review, not RCT. |
| Carey 1995 | Not RCT. |
| De Palo 1993 | Although it is a RCT, primary aim of this study was to assess hormonotherapy. All high‐risk women received RT. |
| Dickler 2010 | Not RCT. Women treated with electronic brachytherapy alone or combined with EBRT. |
| DiSaia 1985 | Not RCT. |
| Eltabbakh 1997 | Not RCT. |
| Garzetti 1994 | RCT of EBRT versus medroxy‐progesterone acetate and Tamoxifen. Outcome was NKC immune assay. |
| GOG 122 | RCT comparing CT with RT for advanced endometrial cancer. |
| GOG 150 | RCT comparing CT with RT for stage I‐IV carcinosarcoma of the uterus. |
| Haie Meder 1995 | RCT comparing two doses of VBT (abstract). |
| Haie Meder 2004 | RCT comparing two doses of VBT (long‐term follow‐up of Haie Meder 1995). |
| Hogberg 2010 | RCT comparing radiotherapy with CT versus radiotherapy without CT. |
| Kang 1992 | Not RCT. Inclusion of stage IIIA women. |
| Kucera 1990 | Not RCT. |
| Maggi 1993 | 1. Although it is a RCT, randomisation was between RT and CT. 2. Inclusion of stage II and III endometrial cancer patients. |
| Maggi 2006 | RCT with comparison between adjuvant CT and adjuvant RT in women with high risk endometrial carcinoma (stage IC, IIG3 and III). Data for IC not extractable separately. |
| Marchetti 1985 | Same study as Piver 1979, see Piver 1979 for reason of exclusion. |
| Meerwaldt 1990 | Not RCT. |
| Orr 1997 | Not RCT. |
| Piver 1979 | Randomisation was unacceptable as there was no direct randomisation between the three arms. It was more like two separate trials in two different periods that were combined into one. Each trial in the 2 separate periods involved a pre‐operative RT arm. Pre‐operative RT as an intervention was excluded from our search criteria. Same study as Marchetti 1985. |
| Poulsen 1996 | Not RCT. |
| Rubin 1995 | Reference to GOG trials. |
| Sagae 2005 | The study compared RT with CT, not adjuvant RT versus no RT. |
| Sorbe 2005 | RCT comparing two different VBT doses. |
| Susumu 2008 | RCT of EBRT versus CT. |
| Takin 2011 | Not RCT. EBRT compared with observation. |
| Touboul 2001 | Not RCT. |
| Weigensberg 1984 | Excluded because the randomisation was between intracavity radiation versus EBRT, rather than randomisation between surgery versus RT. In addition, stage 1 was according clinical staging, which is not often accurate and that radiation given before surgery (pre‐op radiotherapy) which is an exclusion criteria and would have altered the pathology obtained after surgery. |
CT: chemotherapy EBRT: external beam radiotherapy NKC: natural killer cell RCT: randomised controlled trialRT: radiotherapy VBT: vaginal brachytherapy
Differences between protocol and review
The original review stated the 'Types of studies' as: RCTs comparing surgery followed by adjuvant radiotherapy versus surgery alone. For the updated review, we added 'or with vaginal brachytherapy'.
For the update, included trials were grouped according to the control group (e.g. no additional treatment or VBT), for the purposes of investigating potential heterogeneity and for providing clarity to the reader. See Subgroup analysis and investigation of heterogeneity.
In the original review, endometrial cancer‐specific survival data from Aalders 1980 were also used for the 'Death from all causes' outcome. For the update, this was not done.
Contributions of authors
For the updated review, AK, NJ and TL selected relevant articles from the search findings. AK and TL assessed the relevant trials for quality and extracted data. TL entered the data and edited the review. AK, NJ and TL updated the text. HK reviewed the work and provided critical comment.
For the original review, MC & CW wrote the text of the protocol. AK, CW & MC selected relevant articles from the search findings. IS compiled the search strategy and carried out the searches. AK & IS assessed the relevant trials for quality and extracted the data. NJ provided additional references and critical comment. HK initiated the review, provided papers and gave clinical advice. CW & NJ edited the review.
Sources of support
Internal sources
NHS R & D programme, UK.
Medical Research Council, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aalders 1980 {published data only}
- Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstetrics and Gynecology 1980;56(4):419‐27. [PubMed] [Google Scholar]
- Onsrud M, Kolstad P, Normann T. Postoperative external pelvic irradiation in carcinoma of the corpus stage I: a controlled clinical trial. Gynecologic Oncology 1976;4(2):222‐31. [DOI] [PubMed] [Google Scholar]
ASTEC/EN.5 {published data only}
- ASTEC/EN.5 Study Group: Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta‐analysis. The Lancet 2009;373(9658):137‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. The Lancet 2009;373(9658):125‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOG 99 {published data only}
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. Erratum to “A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study” [Gynecol. Oncol. 92 (2004) 744–751]. Gynecologic Oncology 2004;94(1):241‐2. [DOI] [PubMed] [Google Scholar]
- Keys HM, Roberts JA, Brunetto VL, Ziano RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group Study. Gynecologic Oncology 2004;92(3):744‐51. [DOI] [PubMed] [Google Scholar]
- Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study. Cancer 2006;107(12):2786‐91. [DOI] [PubMed] [Google Scholar]
PORTEC‐1 {published data only}
- Burger MP, Mol BW. Treatment for patients with stage‐1 endometrial carcinoma (comment on: Lancet. 2000 Apr 22;355(9213):1404‐11. Lancet 2000;356(9232):853‐4. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, Koper PCM, Lybeert MLM, Warlam Rodenhuis CC. A prospective randomized trial to assess the effect of adjuvant radiotherapy in medium risk pT1 endometrial carcinoma. International Journal of Gynecological Cancer 1995;5 (Supl.1):2 Abstract 8. [Google Scholar]
- Creutzberg CL, Nout RA, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. PORTEC Study Group. Fifteen‐year radiotherapy outcomes of the randomized PORTEC‐1 trial for endometrial carcinoma. International Journal of Radiation Oncology, Biology, Physics 2011;15(81):e631‐8. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage‐1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355(9213):1404‐11. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám‐Rodenhuis CC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecologic Oncology 2003;89(2):201‐9. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám‐Rodenhuis CC, et al. The morbidity of treatment for patients with Stage I endometrial cancer: results from a randomized trial. International Journal of Radiation Oncology, Biology, Physics 2001;51(5):1246‐55. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, Putten WL, Wárlám‐Rodenhuis CC, Bergh AC, Winter KA, Koper PC, et al. Outcome of high‐risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. Journal of Clinical Oncology 2004;22(7):1234‐41. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, Putten WLJ, Koper PCM, et al. Randomized trial on postoperative radiotherapy in pT1 endometrial carcinoma. International Journal of Gynecological Cancer. 1999; Vol. 9 Suppl 1:63 Abstract B5.
- Look KY. Who benefits from radiotherapy in treatment of endometrial cancer and at what price? (comment on: Lancet. 2000 Apr 22;355(9213):1404‐11). Lancet 2000;355(9213):1381‐2. [DOI] [PubMed] [Google Scholar]
- Nout RA, Poll‐Franse LV, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. Long‐term outcome and quality of life of patients with endometrial carcinoma treated with of without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC‐1) trial. Journal of Clinical Oncology 2011;29(13):1692‐700. [DOI] [PubMed] [Google Scholar]
- Scholten AN, Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. Postoperative radiotherapy for Stage 1 endometrial carcinoma: long‐term outcome of the randomized PORTEC trial with central pathology review. International Journal of Radiation Oncology, Biology, Physics 2005;63(3):834‐8. [DOI] [PubMed] [Google Scholar]
PORTEC‐2 {published and unpublished data}
- Nout RA, Putter H, Jürgenliemk‐Schulz IM, Jobsen JJ, Lutgens LC, Steen‐Banasik EM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC‐2 trial. Journal of Clinical Oncology 2009;27(21):3547‐56. [DOI] [PubMed] [Google Scholar]
- Nout RA, Smit VTHBM, Putter H, Jürgenliemk‐Schulz IM, Jobsen JJ, Lutgens LCHW, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high‐intermediate risk (PORTEC‐2): an open‐label, non‐inferiority, randomised trial. The Lancet 2010;375(9717):816‐23. [DOI] [PubMed] [Google Scholar]
Soderini 2003 {published and unpublished data}
- Soderini A, Anchezar JP, Sardi JE. Role of adjuvant radiotherapy (RT) in intermediate risk (1b G2‐3‐1C) endometrioid carcinoma (EC) after extended staging surgery (ESS). Preliminary reports of a randomised trial. International Journal of Gynecological Cancer 2003;13 (Supp 1), Abstract P0147:78. [Google Scholar]
Sorbe 2009 {published and unpublished data}
- Sorbe B, Nordström B, Mäenpää J, Kuhelj J, Kuhelj D, Okkan S, et al. Intravaginal brachytherapy in FIGO stage I low‐risk endometrial cancer: a controlled randomized study. International Journal of Gynecological Cancer 2009;19(5):873‐8. [DOI] [PubMed] [Google Scholar]
Sorbe 2011 {published data only}
- Sorbe B, Horvath G, Andersson H, Boman K, Lundgren C, Pettersson B. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium‐risk endometrial carcinoma ‐ a prospective randomised study. International Journal of Radiation Oncology, Biology, Physics 2011 Jun 13, issue Epub ahead of print. [PUBMED: 21676554] [DOI] [PubMed]
References to studies excluded from this review
Algan 1996 {published data only}
- Algan O, Tabesh T, Hanlon A, Hogan WM, Boente M, Lanciano RM. Improved outcome in patients treated with postoperative radiation therapy for pathologic stage I/II endometrial cancer. International Journal of Radiation Oncology, Biology, Physics 1996;35(5):925‐33. [DOI] [PubMed] [Google Scholar]
Atzinger 2001 {published data only}
- Atzinger A. Is there a benefit of postoperative pelvic irradiation in patients with FIGO stage I endometrial carcinoma?. Strahlenther Onkology 2001;177(2):112‐3. [PubMed] [Google Scholar]
Carey 1995 {published data only}
- Carey MS, O'Connell GJ, Johanson CR, Goodyear MD, Murphy KJ, Daya DM, et al. Good outcome associated with a standardized treatment protocol using selective postoperative radiation in patients with clinical stage I adenocarcinoma of the endometrium. Gynecologic Oncology 1995;57(2):138‐44. [DOI] [PubMed] [Google Scholar]
De Palo 1993 {published data only}
- Palo G, Mangioni C, Periti P, Vecchio M, Marubini E. Treatment of FIGO (1971) stage I endometrial carcinoma with intensive surgery, radiotherapy and hormonotherapy according to pathological prognostic groups. Long‐term results of a randomised multicentre study. European Journal of Cancer 1993;29A(8):1133‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickler 2010 {published data only}
- Dickler A. Puthawala MY, Thropay JP, Bhatnagar A, Schreiber G. Prospective multi‐centre trial utilizing electronic brachytherapy for the treatment of endometrial cancer. Radiation Oncology 2010;5(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiSaia 1985 {published data only}
- DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in Stage 1 endometrium cancer. American Journal of Obstetrics and Gynecology 1985;151:1009‐15. [DOI] [PubMed] [Google Scholar]
Eltabbakh 1997 {published data only}
- Eltabbakh GH, Piver MS, Hempling RE, Shin KH. Excellent long‐term survival and absence of vaginal recurrences in 332 patients with low‐risk Stage I endometrial adenocarcinoma treated with hysterectomy and vaginal brachytherapy without formal staging lymph node sampling: Report of a prospective trial. International Journal of Radiation Oncology, Biology, Physics 1997;38(2):373‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garzetti 1994 {published data only}
- Garzetti GG, Ciavattini A, Muzzioli M, Goteri G, Fabris, N, Valensise H, et al. The relationship of clinical‐pathologic status and adjuvant treatment with natural killer cell activity in stage I and II endometrial carcinoma. Acta Obstetricia et Gynecologica Scandinavica 1994;73(8):652‐7. [DOI] [PubMed] [Google Scholar]
GOG 122 {published data only}
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole‐abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2006;24(1):36‐44. [DOI] [PubMed] [Google Scholar]
GOG 150 {published data only}
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin‐ifosfamide and mesna (CIM) as post‐surgical therapy in stage I‐IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology 2007;107(2):177‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haie Meder 1995 {published data only}
- Haie‐Meder C, Kramar A, Castaigne D, Lambin P, Bouzy J, Duvillard P, et al. Comparison of two low‐dose rates in vaginal prophylactic pre‐operative (PO) brachytherapy (BT) for stage I‐II endometrial carcinoma: results of a randomized trial. International Journal of Radiation Oncology, Biology, Physics 1995;32(Suppl1):223. [Google Scholar]
Haie Meder 2004 {published data only}
- Haie‐Meder C, Kramar A, Boussairi H, Bouzy J, Briot E, Castaigne D, et al. Comparison of two low dose rates in vaginal prophylactic pre‐operative brachytherapy for patients with stage I‐II endometrial carcinoma: long term results of a randomized trial. International Journal of Gynecological Cancer 2004;14:150‐1. [Google Scholar]
Hogberg 2010 {published data only}
- Hogberg T, Signorelli M, Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer ‐ results from two randomised studies. European Journal of Cancer 2010;46(13):2422‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 1992 {published data only}
- Kang HJ, Sarin P, Lee HN, Saxena VS, Reddy S. Does treating whole abdomen improve survival in patients with clinically early stage endometrial and positive peritoneal cytology?. Gynecologic Oncology. 1992; Vol. 46:263‐4, Abstract 16.
Kucera 1990 {published data only}
- Kucera H, Vavra N, Weghaupt K. Benefit of external irradiation in pathologic stage I endometrial carcinoma: a prospective clinical trial of 605 patients who received postoperative vaginal irradiation and additional pelvic irradiation in the presence of unfavorable prognostic factors. Gynecologic Oncology 1990;38(1):99‐104. [DOI] [PubMed] [Google Scholar]
Maggi 1993 {published data only}
- Maggi R, Cagnazzo G, Atlante G, Marinaccio M. Risk groups and adjuvant therapy in surgical staged endometrial cancer patients. A randomized multicentre study comparing chemotherapy with radiation therapy. International Journal of Gynecological Cancer. 1999; Vol. 9 Suppl 1:85 Abstract B73.
- Maggi R, Cucchi L, Restelli C, Delzotti F, Frigoli A. Post surgical treatment of endometrial cancer: GICOG trial. International Journal of Gynecological Cancer. 1993; Vol. 3 Suppl 1:46 Abstract 166.
Maggi 2006 {published data only}
- Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high‐risk endometrial carcinoma: results of a randomised trial. British Journal of Cancer 2006;95:266‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchetti 1985 {published data only}
- Marchetti DL, Piver MS, Tsukada Y, Reese P. 100% Prevention of vaginal recurrence in patients with stage 1, grade 1 or 2 and <50% myometrial invasion treated by TAH BSO and postoperative vaginal radium. Proceedings of American Society of Clinical Oncology 4 March 1985;4:113. [C‐439] [Google Scholar]
Meerwaldt 1990 {published data only}
- Meerwaldt JH, Hoekstra CJ, Putten WL, Tjokrowardojo AS, Koper PC. Endometrial adenocarcinoma, adjuvant radiotherapy tailored to prognostic factors. International Journal of Radiation Oncology Biology, Physics 1990;18:299‐304. [DOI] [PubMed] [Google Scholar]
Orr 1997 {published data only}
- Orr JW Jr, Holimon JL, Orr PF. Stage I corpus cancer: Is teletherapy necessary?. American Journal of Obstetrics and Gynaecology 1997;176(4):777‐89. [DOI] [PubMed] [Google Scholar]
Piver 1979 {published data only}
- Marchetti DL, Piver MS, Tsukada Y, Reese P. 100% prevention of vaginal recurrence in patients with stage I, grade 1 or 2 and < 50% myometrial invasion treated by TAH BSO and postoperative vaginal radium. Proceedings of ASCO 1985;4:113. [Google Scholar]
- Piver MS, Yazigi R, Blumenson L, Tsukada Y. A prospective trial comparing hysterectomy, hysterectomy plus vaginal radium, and uterine radium plus hysterectomy in stage I endometrial carcinoma. Obstetrics and Gynaecology 1979;54(1):85‐9. [DOI] [PubMed] [Google Scholar]
Poulsen 1996 {published data only}
- Poulsen HK, Jacobsen M, Bertelsen K, Andersen JE, Ahrons S, Bock J, et al. from The Danish Endometrial Cancer Group (DEMCA). Adjuvant radiation therapy is not necessary in the management of endometrial carcinoma stage I, low‐risk cases. International Journal of Gynecological Cancer 1996;6(1):38‐43. [Google Scholar]
Rubin 1995 {published data only}
- Rubin SC, Barakat RR. Treatment of high‐risk endometrial cancer should include post‐operative radiation. International Journal of Gynecological Cancer 1995;5 Suppl 1:4 Abstract 13. [Google Scholar]
Sagae 2005 {unpublished data only}
- Sagae S. Randomized phase III trial of whole pelvic radiotherapy vs cisplatin‐based chemotherapy in patients with intermediate risk endometrial carcinoma. Proceedings of American Society of Clinical Oncology Annual Meeting. 2005.
Sorbe 2005 {published data only}
- Sorbe B, Straumits, Karlsson L. Intravaginal high‐dose‐rate brachytherapy for stage I endometrial cancer: a randomized study of two dose‐per‐fraction levels. International Journal of Radiation Oncology, Biology, Physics 2005;62(5):1385‐9. [DOI] [PubMed] [Google Scholar]
Susumu 2008 {published data only}
- Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin‐based combined chemotherapy in patients with intermediate‐ and high‐risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecologic Oncology 2008;108(1):226‐33. [DOI] [PubMed] [Google Scholar]
Takin 2011 {published data only}
- Takin S, Gungor M, Orta F, Oztuna D. Does postoperative radiotherapy provide any survival advantage over observation in stage IC endometrial cancer after comprehensive surgical staging?. European Journal of Obstetrics Gynecology and Reproductive Biology 2011;154(2):200‐4. [DOI] [PubMed] [Google Scholar]
Touboul 2001 {published data only}
- Touboul E, Belkacemi Y, Buffat L, Deniaud Alexandre E, Lefranc JP, Lhuillier P, et al. Adenocarcinoma of the endometrium treated with combined irradiation and surgery: study of 437 patients. International Journal of Radiation Oncology, Biology, Physics 2001;50(1):81‐97. [DOI] [PubMed] [Google Scholar]
Weigensberg 1984 {published data only}
- Weigensberg IJ. Preoperative radiation therapy in Stage I endometrial adenocarcinoma. II. Final report of a clinical trial. Cancer 1984;53(2):242‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Atahan 2008
- Atahan IL, Ozyar E, Yildiz F, Ozyigit G, Genc M, Ulger S, et al. Vaginal high dose rate brachytherapy alone in patients with intermediate to high risk stage I endometrial carcinoma after radical surgery. Internationl Journal of Gynecological Cancer 2008;18:1294‐9. [DOI] [PubMed] [Google Scholar]
Berrington de Gonzalez 2011
- Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncology 2011;12:353‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creasman 2001
- Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz APM, et al. Carcinoma of the corpus uteri. Journal of Epidemiology and Biostatistics 2001;6(1):45‐86. [PubMed] [Google Scholar]
Creutzberg 2001
- Creutzberg C, Putten W, Koper P, Lybert M, Jobsen J, Warlam‐Rodenhuis C, et al. The morbidity of treatment for patients with Stage I endometrial cancer: results from a randomized trial. International Journal of Radiation Oncology, Biology , Physics 2001;51(5):1246‐55. [DOI] [PubMed] [Google Scholar]
Creutzberg 2003
- Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám‐Rodenhuis CC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecologic Oncology 2003;89(2):201‐9. [DOI] [PubMed] [Google Scholar]
Creutzberg 2004
- Creutzberg CL, Putten WL, Warlam‐Rodenhuis CC, Bergh AC, Winter KA, Koper PC, et al. Outcome of High‐risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. Journal of Clinical Oncology 2004;22(7):1234‐41. [DOI] [PubMed] [Google Scholar]
Creutzberg 2011
- Creutzberg CL, Nout RA, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. PORTEC Study Group. Fifteen‐year radiotherapy outcomes of the randomized PORTEC‐1 trial for endometrial carcinoma. International Journal of Radiation Oncology, Biology, Physics 2011;15(81):3631‐8. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Vol. Available from www.cochrane‐handbook.org.
Johnson 2011
- Johnson N, Bryant A, Miles T, Hogberg T, Cornes P. Adjuvant chemotherapy for endometrial cancer after hysterectomy. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD003175.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitchener 2009
- Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. The Lancet 2009;373(9658):125‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2009
- Kumar S, Shah JP, Bryant CS, Awonuga AO, Imudia AN, Ruterbusch JJ, et al. Second neoplasms in survivors of endometrial cancer: impact of radiation therapy. Gynecologic Oncology 2009;113(2):233‐9. [DOI] [PubMed] [Google Scholar]
McCloskey 2010
- McCloskey SA, Tchabo NE, Malhotra HK, Odunsi K, Rodabaugh K, Singhal P, et al. Adjuvant vaginal brachytherapy alone for high risk localised endometrial cancer as defined by three major randomised trials of adjuvant pelvic radiation. Gynecologic Oncology 2010;116:404‐7. [DOI] [PubMed] [Google Scholar]
Naumann 2007
- Naumann RW, Coleman RL. The use of adjuvant radiation therapy in early endometrial cancer by members of the Society of Gynecologic Oncologists in 2005. Gynecologic Oncology 2007;105(1):7‐12. [DOI] [PubMed] [Google Scholar]
Nout 2011
- Nout RA, Poll‐Franse LV, Lybeert ML, Wárlám‐Rodenhuis CC, Jobsen JJ, Mens JW, et al. Long‐term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC‐1) trial. Journal of Clinical Oncology 2011;29(13):1692‐700. [DOI] [PubMed] [Google Scholar]
Panici 2008
- Panici PB, Basile S, Maneschi F, Lissoni AA, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early‐stage endometrial carcinoma: Randomized clinical trial. Journal of the National Cancer Institute 2008;100(23):1707‐16. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
PORTEC‐3
- Creutzberg CCL. Randomized Phase III Trial Comparing Concurrent Chemoradiation and Adjuvant Chemotherapy With Pelvic Radiation Alone in High Risk and Advanced Stage Endometrial Carcinoma: PORTEC‐3. Clinical Trials Register Ongoing trial; Vol. NCT00411138.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Scholten 2005
- Scholten AN, Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. Postoperative radiotherapy for Stage 1 endometrial carcinoma: long‐term outcome of the randomized PORTEC trial with central pathology review. International Journal of Radiation Oncology, Biology, Physics 2005;63(3):834‐8. [DOI] [PubMed] [Google Scholar]
Zagouri 2010
- Zagouri F, Bozas G, Kafantari E, Tsiatas M, Nikitas N, Dimopoulos MA, et al. Endometrial cancer: what is new in adjuvant and molecularly targeted therapy?. Obstetrics and Gynecology International 2010: 749579; Vol. Epub 2010 Feb 2. [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Kong 2007a
- Kong AA, Johnson N, Cornes P, Simera I, Collingwood M, Williams C, et al. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD003916.pub2] [DOI] [PubMed] [Google Scholar]
Kong 2007b
- Kong A, Simera I, Collingwood M, Williams C, Kitchener H. Adjuvant radiotherapy for stage I endometrial cancer: systematic review and meta‐analysis. Annals of Oncology 2007;18:1595‐604. [DOI] [PubMed] [Google Scholar]


