Abstract
Purpose
This study was designed to investigate the intratumoral uptake of hollow gold nanospheres (HAuNS) after hepatic intra-arterial (IA) and intravenous (IV) injection in a liver tumor model.
Materials and Methods
Fifteen VX2 tumor-bearing rabbits were randomized into five groups (N=3 in each group) that received either IV 64Cu-labeled PEG-HAuNS (IV-PEG-HAuNS), IA 64Cu-labeled PEG-HAuNS (IA-PEG-HAuNS), IV cyclic peptide (RGD)-conjugated 64Cu-labeled PEG-HAuNS (IV-RGD-PEG-HAuNS), IA RGD-conjugated 64Cu-labeled PEG-HAuNS (IA-RGD-PEG-HAuNS), or IA 64Cu-labeled PEG-HAuNS with lipiodol (IA-PEG-HAuNS-lipiodol). The animals underwent PET/CT 1 hour after injection, and uptake expressed as percentage of injected dose per gram of tissue (%ID/g) was measured in tumor and major organs. The animals were euthanized 24 hours after injection, and tissues were evaluated for radioactivity.
Results
At 1 hour after injection, animals in the IA-PEG-HAuNS-lipiodol group showed significantly higher tumor uptake (P < 0.001) and higher ratios of tumor-to-normal liver uptake (P < 0.001) than those in all other groups. The biodistribution of radioactivity 24 hours after injection showed that IA delivery of PEG-HAuNS with lipiodol resulted in the highest tumor uptake (0.33 %ID/g; P < 0.001) and tumor-to-normal liver ratio (P < 0.001) among all delivery methods. At 24 hours, the IA-RGD-PEG-HAuNS group showed higher tumor uptake than the IA-PEG-HAuNS group (0.20 %ID/g vs. 0.099 %ID/g; P < 0.001).
Conclusion
Adding iodized oil to IA-PEG-HAuNS maximizes nanoparticle delivery to hepatic tumors and therefore may be useful in targeted chemotherapy and photoablative therapy. PET/CT can be used to noninvasively monitor the biodistribution of radiolabeled HAuNS after IV or IA injection.
Keywords: Hollow gold nanospheres, liver tumor, intraarterial injection, PET/CT, copper-64, lipiodol
INTRODUCTION
Minimally invasive interventional radiology techniques, including transarterial interventions and percutaneous ablation, have become mainstays in the treatment of many patients with liver cancer [1–3]. However, use of these techniques is limited by incomplete elimination of cancer cells; therefore, there remains an urgent need to develop treatment approaches targeting liver tumors. Although recent advances in nanotechnology offer promise for selective delivery of anticancer drugs to cancer cells, efficient delivery to target sites remains challenging [4–6]. Increased drug uptake in solid tumors is often achieved by either passive targeting, via enhanced permeability and retention, or active targeting, in which a homing ligand is attached to the nanoparticles to facilitate their selective binding to tumor cells or tumor stroma. Uptake may also be enhanced by directly injecting nanoparticles into a tumor-feeding artery. To date, only one group has reported the distribution of drug-loaded nanoparticles after their intra-arterial (IA) injection in an animal model [7, 8]. Those studies found that hepatic IA injection of doxorubicin-loaded nanoparticles resulted in substantially higher concentrations of the drug in the liver tumors than did IA injection of free doxorubicin. However, to the best of our knowledge, no direct comparison between IA and intravenous (IV) injection of gold nanoparticles has been done in a clinically relevant, large animal liver tumor model.
Lipiodol selectively enters and is retained by liver tumors when injected into the hepatic artery [9–11], and has been used as a vehicle for delivery of cytotoxic and radiotherapeutic drugs [12–15]. Although arterial embolization with a mixture of ferromagnetic nanoparticles and lipiodol has been used in animal models of selective hyperthermia [16, 17], tumor uptake after IA injection of gold nanoparticles mixed with lipiodol has not been reported previously.
Hollow gold nanospheres (HAuNS) measure approximately 40 nm in diameter, possess a hollow interior suitable for large payloads of drugs, and display tunable surface plasmon resonance in near-infrared regions. Efficient photothermal effects of HAuNS have been applied in cancer therapy, including targeted photothermal ablation [18, 19], tumor site-specific RNA interference [20], and near-infrared light-activated drug release [21].
The in vivo pharmacokinetic and tissue distribution properties of HAuNS are of great interest clinically because of their potential for cancer imaging and therapy. Preliminary studies in rodents demonstrated that imaging with positron emission tomography (PET) may facilitate quantitative assessment of distribution of HAuNS radiolabeled with 64Cu [20]. However, before HAuNS can be used in cancer patients, some critical issues need to be addressed, including evaluation of methods to maximize tumor uptake of HAuNS and development of imaging modalities to noninvasively evaluate their biodistribution.
In this study we conjugated cyclic arginine-glycine-aspartic acid (RGD) peptides with the HAuNS to target the αvβ3 integrin receptors. The αvβ3 integrin is a heterodimeric cell surface receptor that is overexpressed in the tumor neovasculature and shows a high binding affinity for RGD sequences [22, 23]. Various RGD analogs have been developed to target the tumor vasculature and have shown to improve tumor uptake in in-vitro and in-vivo [24, 25].
Rabbits were used in the current study because the rabbit vasculature is large enough to allow selective hepatic arterial catheterization. An orthotopic VX2 tumor is a hypervascular tumor [26] supplied predominantly by the hepatic artery [27]. In addition, angiogenic blood vessels in VX2 tumors express high levels of αvβ3 integrin [28]. The purpose of the current study was to test the hypothesis that liver tumor uptake of HAuNS could be enhanced by use of one or more methods of targeting tumor cells, specifically the first of these methods uses IA rather than IV administration to reduce systemic clearance of the drug; the second method is to coat the HAuNS with the αvβ3 integrin-targeting cyclic peptide RGD (arginine-glycine-aspartic acid) to enhance uptake by angiogenic tumor; and the third method is to add iodized oil to the HAuNS to improve uptake by liver tumor. We tested the hypothesis in a rabbit model of liver cancer using orthotopically inoculated cells; to localize and quantify uptake of HAuNS, we used positron emission tomography/computed tomography (PET/CT).
MATERIALS AND METHODS
All amino acid derivatives and coupling reagents were purchased from Novabiochem (San Diego, Calif.), and 1,4,7,10-tetraazacyclododecane-1,4,7-tris(t-butyl acetate)-10-acetate-mono-(N-hydroxylsuccinimide ester) was purchased from Macrocyclics (Dallas, Tex.). All other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, Mo.). 1,4,7,10-Tetraazacyclododecane-1,4,7-tris(acetic acid)-10-[acetic acid-N-(2-(2-(2-aminoethoxy)ethoxy)ethyl)-lipoic acid mono amide] (DOTA-LA) was synthesized according to a previously reported method [20].
Synthesis of HAuNS
c(RGDfK)-PEG-HAuNS conjugate was synthesized according to a previously reported method [29]. Briefly, cyclic RGD peptide c(RGDfK) was conjugated to N-hydroxysuccinimidyl-PEG-S-acetylthioacetate (NHS-PEG-SATA; molecular weight, 5000), and the resulting product was treated with hydroxylamine (0.5 M) in phosphate-buffered saline (PBS) [30]. HAuNS were synthesized by the cobalt nanoparticle-mediated reduction of chloroauric acid according to previously reported procedures [18, 31]. HAuNS (8.5×1012 particles/mL) were mixed with argon-purged aqueous solution containing c(RGDfK)-PEG-SH (50 μg/mL) to yield c(RGDfK)-PEG-HAuNS.
Conjugation of RGD to HAuNS and 64Cu Radiolabeling
DOTA-LA was labeled with 64Cu prior to introduction to HAuNS. 64Cu-DOTA-LA was then mixed with an aqueous solution of HAuNS (400 μL, ~2 × 1013 particles) at room temperature for 4 hours to produce 64Cu-HAuNS. A mixture of c(RGDfK)-PEG-SH (200 μg in 40 μL water) and methoxy-PEG5000-SH (180 μL, 5 mg/mL) was incubated with 64Cu-HAuNS at room temperature overnight to yield 64Cu-labeled RGD-PEG-HAuNS. The radiolabeled nanoparticles (64Cu-RGD-PEG-HAuNS) were purified and concentrated by centrifugation at 8000 rpm for 5 minutes. 64Cu-labeled PEGylated HAuNS (64Cu-PEG-HAuNS) was similarly synthesized by mixing 200 μL of methoxy-PEG5000-SH (5 mg/mL) with 64Cu-HAuNS at room temperature overnight [20]. The overall radiochemical yield for both 64Cu-PEG-HAuNS and 64Cu-RGD-PEG-HAuNS was 97%, and specific activity for both 64Cu-labeled nanoparticles was 690–750 MBq/nmol nanoparticles. Previous studies have established that after 24 h incubation in serum, 64Cu-labeled HAuNS lost about 4.8% of associated radioactivity [20].
Animals
A total of 15 adult New Zealand white rabbits (mean weight, 4.04 ± 0.31 kg) were included in this study. All experimentation involving animals was approved by the Institutional Animal Care and Use Committee. Animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International and in accordance with regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, and the National Institutes of Health.
Tumor Inoculation
For tumor inoculation, each animal was sedated with an intramuscular injection of buprenorphine (0.15 mg) (Buprenex; Bedford Laboratories, Bedford, Ohio), and anesthesia was induced with isoflurane (5%) in oxygen (1.5 L/min) administered via oxygen mask. The abdomen was shaved and prepared for aseptic surgery. VX2 tumor was inoculated in the left lobe of the liver as described elsewhere [32].
Study Groups
Rabbits were randomized to 5 groups (n = 3 in each group) that received either IV 64Cu-PEG-HAuNS (IV-PEG-HAuNS), IA 64Cu-PEG-HAuNS (IA-PEG-HAuNS), IV RGD-conjugated 64Cu-PEG-HAuNS (IV-RGD-PEG-HAuNS), IA RGD-conjugated 64Cu-PEG-HAuNS (IA-RGD-PEG-HAuNS), or IA 64Cu-PEG-HAuNS mixed with 0.4 ml of lipiodol (Ethiodol; Savage Laboratories, Melville, NY) (IA-PEG-HAuNS-lipiodol).
64Cu-HAuNS Administration
Ten days after inoculation, each rabbit was sedated and anesthetized as described above. For each rabbit receiving IA HAuNS, the right groin was prepared for aseptic surgery. A small incision was made, and the right common femoral artery was isolated. A 4-Fr introducer sheath (Cook Inc., Bloomington, Ind.) was placed into the vessel through a small arteriotomy. A 2.8-Fr microcatheter (EmboCath Plus; BioSphere Medical, Rockland, Mass.) was introduced through the sheath, and advanced into the proper hepatic artery and positioned just above the origin of the gastroduodenal artery. Approximately 23.31 ± 6.67 MBq (0.63 ± 0.18 mCi) of each 64Cu-labeled PEG-HAuNS formulation in 1 mL of saline mixed with 0.4 mL of contrast medium (Visipaque 320; GE Healthcare, Princeton, N.J) was slowly injected through the catheter with fluoroscopic monitoring. At the end of the injection, the catheter and sheath were removed, the femoral artery was ligated, and the incision was closed. For each rabbit receiving IV-PEG-HAuNS, approximately 25.16 ± 5.92 MBq (0.68 ± 0.16 mCi) of 64Cu-HAuNS in 1.0 mL of PBS was administered via a 22-gauge winged IV catheter (BD Medical, Sandy, Utah) placed in a marginal ear vein, and the catheter was then flushed with 2.0 mL of saline.
PET Imaging and Analysis
PET/CT imaging studies were performed using Discovery ST-16 PET/CT system (GE Healthcare). Helical CT scans were obtained from head to mid-thighs (120 kVp, 300 mA, 13.5-mm/rotation table speed). For the IA injection groups, whole-body PET scans were conducted at 1 hour after 64Cu-HAuNS injection. For the IV injection groups, a series of whole-body PET scans were conducted for the first 60 minutes after 64Cu-HAuNS injection. PET images were reconstructed using standard vendor-provided reconstruction algorithms, and corrected for attenuation. To ensure that the measured injected dose was accurate in terms of activity measured by PET, we cross-calibrated the dose calibrator (CRC-15R; Capintec, Inc., Ramsey, N.J.) with the PET camera [33]. Regional dynamic and whole-body reconstructed PET/CT data were converted to Digital Imaging and Communications in Medicine (DICOM) 3.0, part 10 file format and transferred to a HERMES workstation (version 2.2; Nuclear Diagnostics AB, Stockholm, Sweden). The regions of interest were generated and sampled using a previously described technique [34]. Data were decay-corrected and expressed as the percentage of injected dose per gram of tissue (%ID/g).
Biodistribution at 24 hours after Injection
At 24 hours after IA or IV injection of imaging agent, the animals were euthanized with an IV dose of Beuthanasia-D (70 mg/kg; Schering-Plough Animal Health Corp., Kenilworth, NJ). A complete necropsy was performed, and liver tumors and samples of the normal liver, bile, spleen, kidneys, lungs, and hind limb skeletal muscle were removed. Triplicate samples from each of the collected fluids and tissues were weighed and evaluated for radioactivity using a Packard Cobra gamma counter (GMI, Inc., Ramsey, Minn.). Uptake of 64Cu-HAuNS was calculated as the percentage of injected dose per gram of tissue (%ID/g).
Intratumoral Distribution of HAuNS
The formalin-fixed tumor tissues were embedded in paraffin and cut into 5-μm slices. After antigen retrieval with proteinase K (20 μg/ml in tris-EDTA buffer, pH 8.0) (Fisher Scientific, Pittsburgh, Pa.), two slices from each animal were stained with mouse anti-human CD31 monoclonal antibody (Dako, Carpinteria, Calif.) at 1:40 dilution at 4°C overnight. The sections were washed with PBS, and stained with Alexa Fluor 594-conjugated goat anti-mouse immunoglobulin (Invitrogen, Carlsbad, Calif.) for 30 minutes. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich Co.). The slices were examined under an Axio Observer.Z1 fluorescence microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany). HAuNS were visualized under dark-field condenser [19].
Statistical Analysis
One-way analyses of variance followed by Bonferroni’s post-hoc comparison tests were performed in all statistical analyses. Comparisons of tissue uptake were performed using GraphPad prism software (Graphpad Software, Inc., La Jolla, Calif.). Multiple comparisons with Tukey-adjusted P-values were conducted to investigate pairwise differences using SAS v. 9.1.3 (SAS Institute Inc., Cary, N.C.). For unbalanced data, the Tukey-Kramer method was used to correct P-values. Differences between groups were considered statistically significant if P < 0.05.
RESULTS
Synthesis and Characterization of HAuNS Conjugates
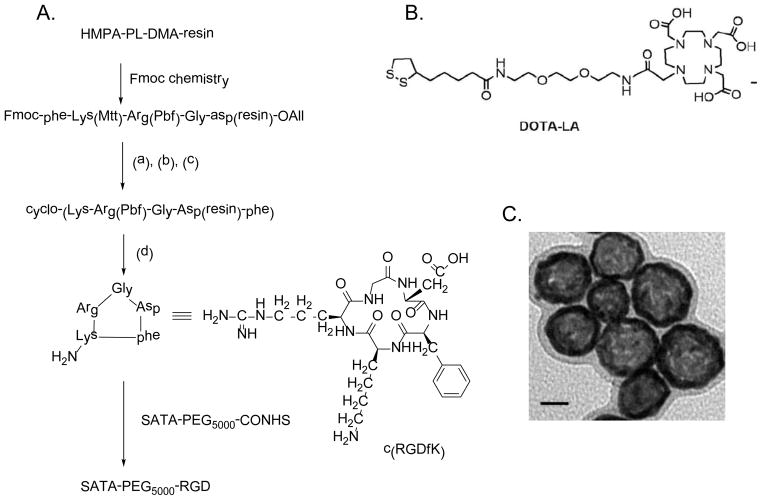
Figures 1 depicts the synthesis of SATA-PEG5000-RGD precursor used to introduce RGD to HAuNS and the structure of the DOTA-LA chelator used for radiolabeling with 64Cu. Transmission electron microscopy showed that RGD-PEG-HAuNS had an average diameter of ~40 nm (size range, 44.7±1.1 nm, zeta potential −16 mAV) (Fig. 1C). PEG-HAuNS without RGD were found to have a similar size and morphology when examined under transmission electron microscopy. The radiolabeling efficiency of the nanoparticles analyzed using instant thin-layer chromatography was >97%. After decay of the 64Cu-labeled RGD-PEG-HAuNS, amino acid quantification showed an average of about 66 RGD peptides conjugated with each particle.
Figure 1.
(A) Scheme for synthesis of SATA-PEG5000-RGD. Reagents and conditions: (a) Pd0[P(C6H5)3]4 (3 eq), CHCl3/AcOH/NMM (37/2/1, v/v/v); (b) 20% piperidine in DMF; (c) PyBOP/HOBt/DIPEA (3/3/6 eq) in DMF; (d) TFA/DCM/TES (1/97/2, v/v/v). (B) Structure of 1,4,7,10-tetraazacyclododecane-1,4,7-tris(acetic acid)-10-[acetic acid-N-(2-(2-(2-aminoethoxy)ethoxy)ethyl)-lipoic acid mono amide] (DOTA-LA). (C) Transmission electron microscopy image of RGD-PEG-HAuNS. Bar, 20 nm.
Our preliminary labeling results showed if the DOTA-LA was conjugated to the HAuNS first followed by 64Cu labeling, the labeling efficiency was low. This is probably because the residual trace amount of Co2+ introduced during the synthesis of HAuNS competed for the chelation of 64Cu2+ with DOTA. The reversed labeling procedure, i.e. labeling DOTA-LA first with 64Cu followed by nanoparticle conjugation, led to high radiolabeling efficiency. The nanoparticles were centrifuged at 8,000 rpm for 10 min. The supernatant was discarded and water was added to the pellet to suspend the particles. This washing procedure was repeated for three times to ensure complete removal of un-conjugated SATA-PEG5000-RGD and radioligand 64Cu-DOTA-LA.
PET/CT Imaging
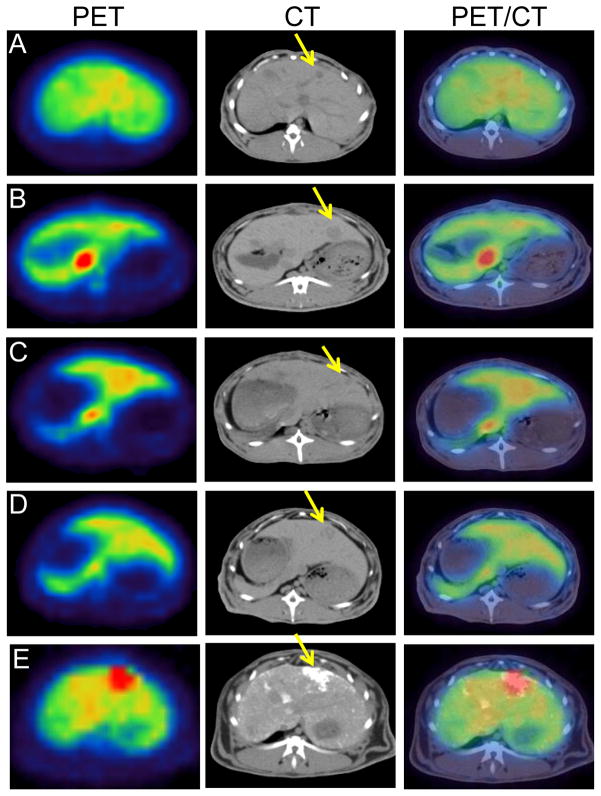
PET/CT revealed whole-body biodistribution patterns after IV or IA injection of each 64Cu-labeled PEG-HAuNS (Fig. 2). At 1 hour after injection, relatively high accumulations of radioactivity were visualized in the liver, kidneys, urinary bladder, heart, and large blood vessels in each group. The spleen and orbital soft tissues showed moderate radioactivity accumulation. The hepatic VX2 tumor had highest accumulation of 64Cu-PEG-HAuNS at 1 hour after IA injection when the nanoparticles were mixed with lipiodol prior to injection. In contrast, in all the other groups, the tumors had less 64Cu-HAuNS accumulation than the normal liver and thus were delineated as defects in the normal liver tissue (Figs 2 and 3).
Figure 2.
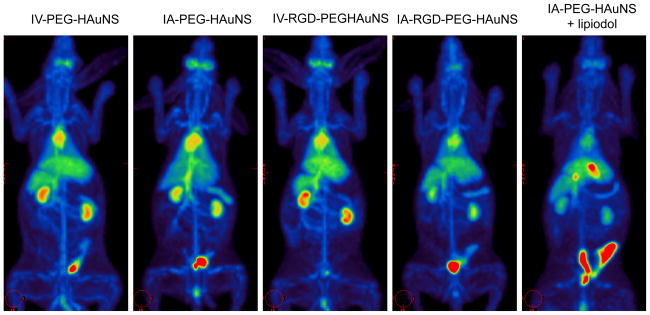
Representative whole-body PET/CT images of rabbits bearing hepatic VX2 tumors at 1 hour after injection of each 64Cu-labeled PEG-HAuNS.
Figure 3.
Representative axial PET/CT images of rabbits bearing hepatic VX2 tumors at 1 hour after injection of 64Cu-labeled PEG-HAuNS in different groups.
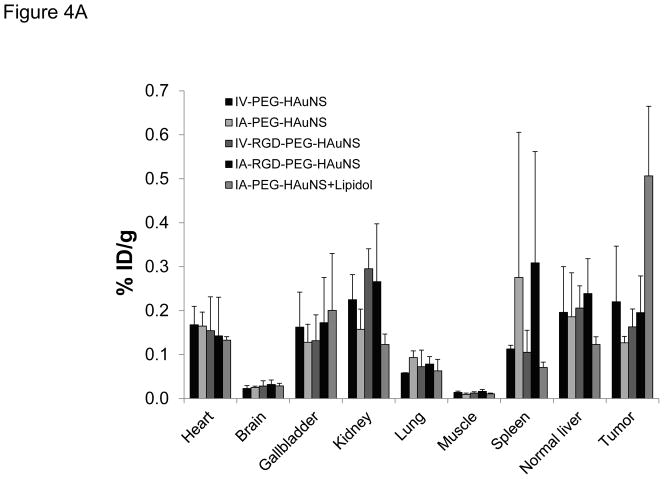
In vivo biodistribution data based on PET image analysis obtained at 1 hour after IV or IA injection of each 64Cu-labeled PEG-HAuNS are shown in Figure 4A and Table 1. Consistent with the imaging findings, rabbits in the IA-PEG-HAuNS-lipiodol group showed the highest level of radioactivity in the hepatic VX2 tumor (0.50 ± 0.16 %ID/g); this level was significantly higher (P < 0.01) than those seen in the other groups (0.22 ± 0.12 %ID/g in IV-PEG-HAuNS, 0.13 ± 0.01 %ID/g in IA-PEG-HAuNS, 0.16 ± 0.04 %ID/g in IV-RGD-PEG-HAuNS, and 0.20 ± 0.08 %ID/g in IA-RGD-PEG-HAuNS), which did not significantly differ from each other. Overall, all the groups showed high levels of radioactivity in the liver, gallbladder, kidneys, and heart and negligible activity in the muscle and brain.
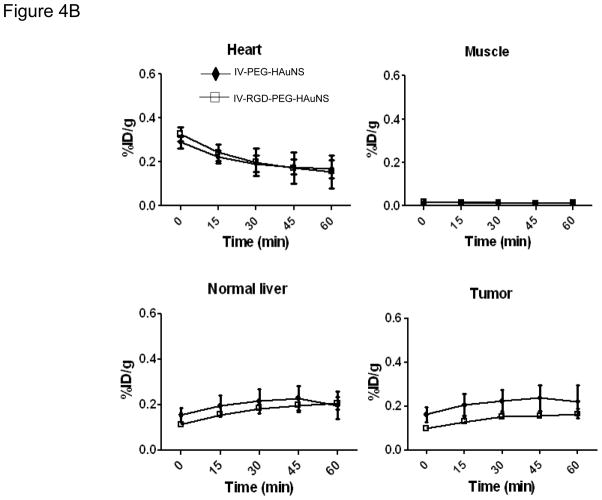
Figure 4.
(A) In vivo biodistribution in rabbits bearing hepatic VX2 tumors at 1 hour after injection of each 64Cu-labeled PEG-HAuNS. (B–E) Time-activity curves of heart (B), muscle (C), normal liver (D), and tumor (E) from hepatic VX2 tumor-bearing rabbits over a 60-minute period after intravenous injection. The data are presented as mean ± SD.
Table 1.
PET Activity in different tissues at 60 minutes
| PET Activity (%ID/g; mean ± Std) | ||||||
|---|---|---|---|---|---|---|
| Groups/ Tissue | IV-PEG- HAuNS | IA-PEG- HAuNS | IV-RGD-PEG- HAuNS | IA-RGD-PEG- HAuNS | IA-PEG- HAuNS+Lipidol | P values |
| Heart | 0.1679 ± 0.0417 | 0.1650 ± 0.0317 | 0.1546 ± 0.0767 | 0.1425 ± 0.0879 | 0.1328 ± 0.0081 | 0.9465 |
| Brain | 0.0229 ± 0.0065 | 0.0252 ± 0.0032 | 0.0285 ± 0.0118 | 0.0320 ± 0.0103 | 0.0287 ± 0.0060 | 0.7705 |
| Gallbladder | 0.1624 ± 0.0797 | 0.1280 ± 0.0411 | 0.1316 ± 0.0589 | 0.1725 ± 0.1030 | 0.2004 ± 0.1298 | 0.8441 |
| Kidney | 0.2249 ± 0.0570 | 0.1575 ± 0.0461 | 0.2953 ± 0.0454 | 0.2660 ± 0.1315 | 0.1229 ± 0.0237 | 0.0802 |
| Lung | 0.0582 ± 0.0005 | 0.0931 ± 0.0154 | 0.0726 ± 0.0376 | 0.0785 ± 0.0169 | 0.0630 ± 0.0261 | 0.5408 |
| Muscle | 0.0138 ± 0.0031 | 0.0096 ± 0.0029 | 0.0125 ± 0.0029 | 0.0162 ± 0.0045 | 0.0107 ± 0.0018 | 0.1849 |
| Spleen | 0.1129 ± 0.0082 | 0.2755 ± 0.3304 | 0.1051 ± 0.0502 | 0.3088 ± 0.2530 | 0.0707 ± 0.0120 | 0.5091 |
| Normal Liver | 0.1962 ± 0.1039 | 0.1859 ± 0.1003 | 0.2059 ± 0.0505 | 0.2389 ± 0.0792 | 0.1230 ± 0.0174 | 0.4627 |
| Tumor | 0.2201 ± 0.1268 | 0.1269 ± 0.0146 | 0.1631 ± 0.0408 | 0.1954 ± 0.0836 | 0.5067 ± 0.1583 | 0.0064 |
The ratio of tumor-to-normal liver uptake was 4.17 ± 1.35 in the IA-PEG-HAuNS-lipiodol group, which was significantly higher than that in the other groups (1.11 ± 0.06 in IV-PEG-HAuNS, 0.81 ± 0.35 in IA-PEG-HAuNS, 0.79 ± 0.08 in IV-RGD-PEG-HAuNS, and 0.81 ± 0.15 in IA-RGD-PEG-HAuNS; P < 0.01). The tumor-to-muscle uptake ratio was also significantly higher in the IA-PEG-HAuNS-lipiodol group (48.01 ± 15.57) than in the other four groups (17.37 ± 13.09 in IV-PEG-HAuNS, P < 0.003; 14.16 ± 4.75 in IA-PEG-HAuNS, P < 0.006; 13.54 ± 4.57 in IV-RGD-PEG-HAuNS, P < 0.005; and 12.74 ± 6.21 in IA-RGD-PEG-HAuNS, P < 0.05).
The time-activity curves of heart, normal liver, muscle, and tumor over the first 60 minutes for IV-PEG-HAuNS and IV-RGD-PEG-HAuNS groups are presented in Figure 4B–E. The heart exhibited high levels of radioactivity immediately after injection; the activity gradually decreased over the 60-minute period. The activities in both normal liver and hepatic VX2 tumor for the IV-PEG-HAuNS group gradually increased for 45 minutes and then decreased slightly at 60 minutes. Conversely, in the IV-RGD-PEG-HAuNS group, activity in the hepatic VX2 tumor gradually increased for 60 minutes after injection. Tissue uptake did not significantly differ between these two groups for all organs analyzed at all time points.
Tissue Radioactivity at 24 Hours
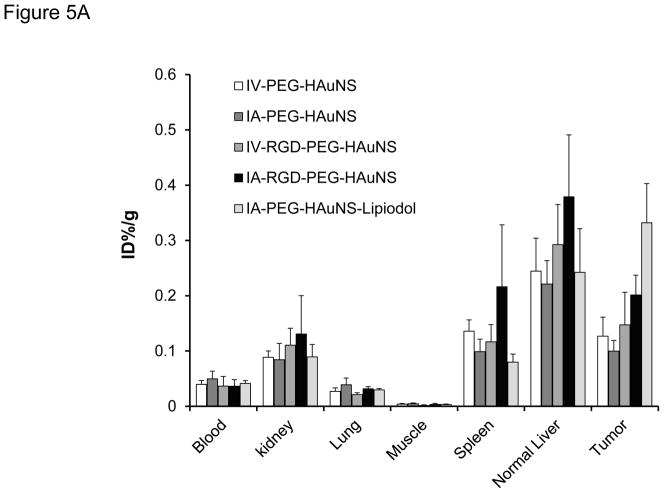
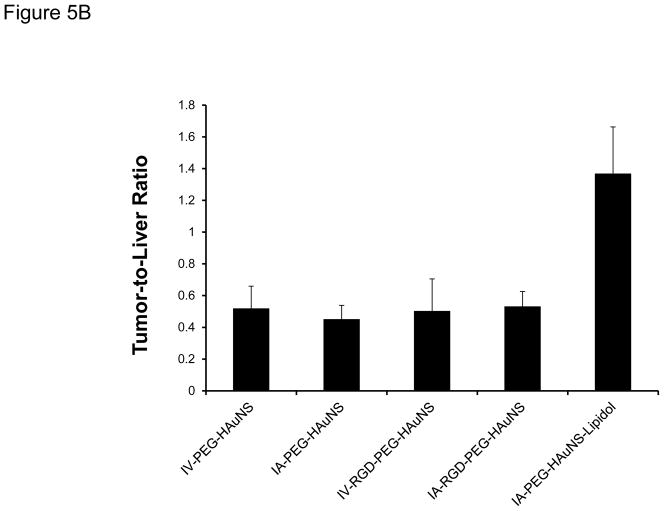
The biodistribution of radioactivity in the tissues excised at 24 hours after injection (Fig. 5A and Table 2) showed that IA-PEG-HAuNS-lipiodol had the highest tumor uptake (0.33 %ID/g) among all the delivery methods (P < 0.001). The tumor-to-normal liver ratio in the IA-PEG-HAuNS-lipiodol group was about 2.7 times that in the other groups (P < 0.001; Fig. 5B). The other IA and IV injections did not significantly differ with respect to tumor uptake (P = 0.81). The IA-RGD-PEG-HAuNS group showed significantly higher tumor uptake than the IA-PEG-HAuNS group (0.20 %ID/g vs. 0.099 %ID/g; P < 0.01). However, tumor uptake did not significantly differ between the IV-RGD-PEG-HAuNS and IV-PEG-HAuNS groups (P = 0.92). In all groups, liver continued to show high activity while the spleen and kidneys showed moderate activity. Lungs and muscles showed low activity. High activity was also observed in the bile, particularly for the IA-RGD-PEG-HAuNS and IA-PEG-HAuNS-lipiodol groups, indicating that a significant amount of HAuNS was sequestered via the hepatobiliary route.
Figure 5.
(A) Biodistribution analysis of selected tissues from rabbits injected with 64Cu-labeled PEG-HAuNS and sacrificed at 24 hours after IA or IV injection. Data are presented as mean ± SD of %ID/g. (B) Tumor-to-liver uptake ratio of the %ID/g values 24 hours after IV or IA injection of PEG-HAuNS. Data are expressed as mean ± SD.
Table 2.
Radioactivity in different tissues at 24 hours
| Tissue Radioactivity (%ID/g; mean ± Std) | ||||||
|---|---|---|---|---|---|---|
| Groups/ Tissue | IV-PEG- HAuNS | IA-PEG- HAuNS | IV-RGD- PEG-HAuNS | IA-RGD- PEG- HAuNS | IA-PEG- HAuNS+Lipidol | P values |
| Blood | 0.0397 ± 0.0071 | 0.0498 ± 0.0137 | 0.0366 ± 0.0175 | 0.0364 ± 0.0118 | 0.0413 ± 0.0051 | 0.7316 |
| Bile | 0.1156 ± 0.0330 | 0.0761 ± 0.0277 | 0.0537 ± 0.0019 | 0.2003 ± 0.1224 | 0.2128 ± 0.0508 | 0.1849 |
| Kidney | 0.0887 ± 0.0114 | 0.0844 ± 0.0295 | 0.1107 ± 0.0307 | 0.1307 ± 0.0695 | 0.0895 ± 0.0223 | 0.5850 |
| Lung | 0.0270 ± 0.0064 | 0.0391 ± 0.0120 | 0.0212 ± 0.0035 | 0.0317 ± 0.0038 | 0.0297 ± 0.0025 | 0.0657 |
| Muscle | 0.0039 ± 0.0013 | 0.0047 ± 0.0014 | 0.0016 ± 0.0011 | 0.0033 ± 0.0020 | 0.0031 ± 0.0010 | 0.0831 |
| Spleen | 0.1360 ± 0.0203 | 0.0989 ± 0.0227 | 0.1167 ± 0.0313 | 0.2164 ± 0.1119 | 0.0798 ± 0.0147 | 0.2302 |
| Normal Liver | 0.2445 ± 0.0597 | 0.2212 ± 0.0423 | 0.2925 ± 0.0726 | 0.3789 ± 0.1120 | 0.2424 ± 0.0788 | 0.2022 |
| Tumor | 0.1270 ± 0.0343 | 0.1000 ± 0.0191 | 0.1473 ± 0.0591 | 0.2014 ± 0.0359 | 0.3319 ± 0.0711 | <0.0001 |
Intratumor Distribution
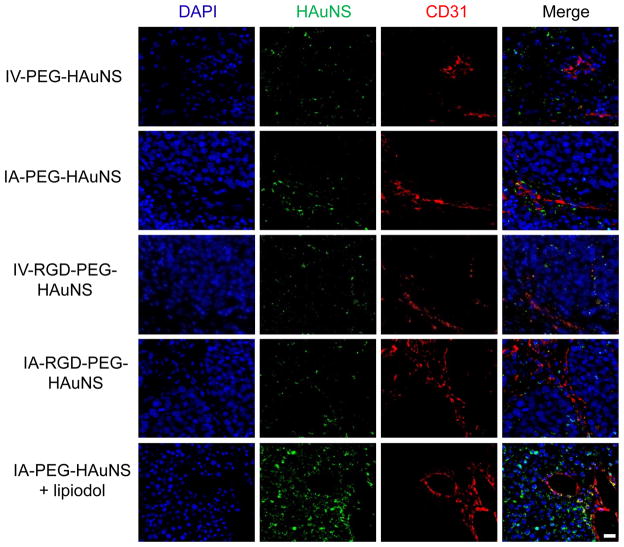
Dark-field and fluorescence microscopic findings in the different study groups are presented in Figure 6. Dark-field microscopy showed the highest intensity of gold in the IA-PEG-HAuNS-lipiodol group, with the other four groups showing similar lower intensities. Whereas the HAuNS were clustered around the blood vessels in the IA-PEG-HAuNS and IA-RGD-PEG-HAuNS groups, HAuNS in the IA-PEG-HAuNS-lipiodol group were seen throughout the tumor. Co-registration of gold particles and nuclear staining was also noted in the IA-PEG-HAuNS-lipiodol group, suggesting intracellular localization of the HAuNS.
Figure 6.
Representative images of intratumoral distribution of 64Cu-labeled PEG-HAuNS 24 hours after IV or IA injection. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). The scattering signal of PEG-HAuNS (green pseudocolor) was observed under a dark-field condenser. Tumor vessels were immunostained with mouse anti-human CD31 monoclonal antibody (red). Bar, 20 μm.
DISCUSSION
On the basis of these results, we conclude that liver tumor targeting of HAuNS can be augmented by using IA administration and by mixing the nanoparticles with iodized oil. Our findings were supported both by the PET images acquired at 1 hour after injection and by quantification of tissue radioactivity using the “cut-and-count” method at 24 hours after injection. Moreover, lipiodol appeared to facilitate more uniform intratumoral distribution of HAuNS and possibly internalization of HAuNS as well, as revealed by combined fluorescence and dark-field microphotography. To the best of our knowledge, this is the first published report that IA injection with lipiodol significantly increases the uptake of HAuNS in VX2 tumors in rabbit livers.
Transcatheter arterial chemoembolization with an emulsion of lipiodol and various anticancer drugs is widely used in clinical practice for the treatment of hypervascular liver tumors, particularly hepatocellular carcinoma (HCC) [15]. A number of experimental and clinical reports on hepatic arterial embolization performed with iodized oil have shown that iodized oil selectively enters and is retained by liver tumors when it is injected into the hepatic artery [9–11, 35]. Because of the preferential uptake into tumor tissue, lipiodol has been used as a vehicle for the targeted delivery of cytotoxic or radiotherapeutic drugs via the intra-arterial route. Although the mechanism for selective concentration and retention of lipiodol in HCC is unclear, several hypotheses have been proposed; these include increased blood flow and increased permeability of tumor vessels, decreased kupferr cell population in the tumor and lack of functional local lymphatics [36, 37]. These mechanisms may have been responsible for the increased uptake of HAuNS in VX2 tumors when they were mixed with lipiodol before IA injection.
In a rabbit subcutaneous VX2 tumor model, Lijowski et al. [38] found that systemically administered αvβ3-targeted 99mTc-gadolinium nanoparticles (270 nm in diameter) produced a higher tumor-to-muscle uptake ratio than nontargeted nanoparticles. Interestingly, we did not observe any differential uptake between PEG-HAuNS and RGD-PEG-HAuNS at any time point after IV injection, possibly because of preferential uptake of the nanoparticles in the liver’s reticuloendothelial system. Conversely, in the IA injection groups, although no difference in tumor uptake was noted at 1 hour after injection, tumor uptake at 24 hours was higher in the animals given IA-RGD-PEG-HAuNS than in those given IV-PEG-HAuNS. This increased uptake of IA-RGD-HAuNS but not IV-HAuNS at 24 hours is interesting, and the cause of this difference remains to be elucidated. The data suggest that, over time, the extraction of RGD-HAuNS from the perfusion blood pool after IA injection by αvβ3 integrin-expressing angiogenic blood vessels may significantly increase the tumor uptake of RGD-PEG-HAuNS compared with nontargeting PEG-HAuNS. Further studies are needed to assess long-term uptake and retention of nanoparticles in tumors and major organs.
Clinical application of nanoparticles for cancer therapy requires an understanding of the pharmacokinetics and organ distribution of nanoparticles after systemic or regional administration. PET is a noninvasive imaging tool that can quantify the uptake of systemically injected nanoparticles at the target site with high sensitivity in real time [39]. In the present study, whole-body PET/CT permitted interrogation of the dynamics, distribution, and clearance of HAuNS after IV and IA injection in a noninvasive fashion. Also, the use of a combined PET/CT system that permits co-registration of PET images with axial CT images allowed us to localize the radiolabeled HAuNS with a high anatomic accuracy.
From the PET/CT images, it is evident that both 64Cu-labeled RGD-PEG-HAuNS and nontargeted PEG-HAuNS accumulated in the organs of the hepatobiliary and renal systems, which contributed to the clearance of HAuNS from the circulation. The uptake and clearance through the kidney/bladder may be explained by the presence of a small fraction of HAuNS nanoparticles with sizes less than 5 nm (below the renal clearance threshold), decoupling of a small amount of free 64Cu-DOTA from the surface of HAuNS, and/or transchelation of 64Cu with endogenous metal ions. Bone activity was also observed in the whole body PET/CT images (Fig. 2). This may be attributed to trace amount of free 64Cu detached from 64Cu-labeled nanoparticles. It is also possible that some HAuNS nanoparticles were taken up by macrophages in the bone marrow. If this is the case, potential bone marrow toxicity should be considered when translating HAuNS into the clinic. Analysis of first-hour dynamic imaging data in the IV injection groups revealed slow clearance from the blood and a gradual increase of both nontargeted and targeted HAuNS in the liver and the tumor. These data support earlier findings that PEGylated HAuNS displayed a long blood half-life in mice [40]. The current PET/CT images also revealed no appreciable uptake of HAuNS in the brains of rabbits, which is consistent with previous micro-PET imaging results in mice [20] and suggests that PEG-HAuNS cannot penetrate the blood-brain barrier.
Efficient photothermal effects of HAuNS have been successfully applied in cancer therapy, including targeted photothermal ablation [18, 19], tumor site-specific RNA interference [20], and near-infrared light-activated drug release [21]. However, effective use of these therapies requires selective delivery of HAuNS to the tumor. Using iodized oil and IA administration of PEG-HAuNS resulted in higher levels of nanoparticle uptake and higher tumor-to-normal liver uptake ratios than those attained using IV and other IA administration strategies, even those involving targeting of cell surface receptors. Further studies are required to assess the long-term retention and clearance of IA injection of PEG-HAuNS with lipiodol before clinical studies can be initiated. Our results also showed that noninvasive PET imaging of 64Cu-labeled HAuNS may be useful for tracking the in vivo distribution of HAuNS after IV and hepatic IA injection and for quantitatively estimating tumor uptake for thermal and other therapies. PET visualization of tumor uptake of nanoparticles may also help determine the optimal timing and application of photothermal therapy.
Our study has limitations that must be acknowledged. We could not perform dynamic evaluation in the IA injection groups because of the time required for transferring the animals from the angiographic suite to the PET/CT scanner. Also, we did not study the intratumoral nanoparticle concentrations more than 24 hours after injection; hence, we cannot say whether the nanoparticles are retained in the tumor beyond that time. Additional studies to assess the long-term retention of HAuNS in hepatic tumors after various routes of administration are in progress.
In conclusion, using iodized oil and IA administration of PEG-HAuNS resulted in higher levels of nanoparticle uptake and higher tumor-to-normal liver uptake ratios than those attained using IV and other IA administration strategies, even those involving targeting of cell surface receptors. Our results also showed that noninvasive PET imaging of 64Cu-labeled HAuNS may be useful for tracking the in vivo distribution and for quantitative estimation of tumor uptake of HAuNS after IV and hepatic IA injection.
Acknowledgments
This research was supported in part by the National Institutes of Health grants CA119387 and GM092599, by a grant from the John S. Dunn Research Foundation.
REFRENCES
- 1.Buijs M, Vossen JA, Frangakis C, Hong K, Georgiades CS, Chen Y, Liapi E, Geschwind JF. Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization--single-center experience. Radiology. 2008;249:346–354. doi: 10.1148/radiol.2483071902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong K, Georgiades CS, Geschwind JF. Technology insight: Image-guided therapies for hepatocellular carcinoma--intra-arterial and ablative techniques. Nat Clin Pract Oncol. 2006;3:315–324. doi: 10.1038/ncponc0512. [DOI] [PubMed] [Google Scholar]
- 3.Ridge JA, Collin C, Bading JR, Hancock C, Conti PS, Daly JM, Raaf JH. Increased adriamycin levels in hepatic implants of rabbit Vx-2 carcinoma from regional infusion. Cancer Res. 1988;48:4584–4587. [PubMed] [Google Scholar]
- 4.Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-Based Cancer Therapeutics-Promise and Challenge-Lessons Learned Through the NCI Alliance for Nanotechnology in Cancer. Pharm Res. 2010;28:273–278. doi: 10.1007/s11095-010-0214-7. [DOI] [PubMed] [Google Scholar]
- 5.Maeng JH, Lee DH, Jung KH, Bae YH, Park IS, Jeong S, Jeon YS, Shim CK, Kim W, Kim J, Lee J, Lee YM, Kim JH, Kim WH, Hong SS. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 2010;31:4995–5006. doi: 10.1016/j.biomaterials.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 6.van Vlerken LE, Duan Z, Little SR, Seiden MV, Amiji MM. Augmentation of therapeutic efficacy in drug-resistant tumor models using ceramide coadministration in temporal-controlled polymer-blend nanoparticle delivery systems. AAPS J. 12:171–180. doi: 10.1208/s12248-010-9174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JH, Ling R, Yao Q, Wang L, Ma Z, Li Y, Wang Z, Xu H. Enhanced antitumor efficacy on hepatoma-bearing rats with adriamycin-loaded nanoparticles administered into hepatic artery. World J Gastroenterol. 2004;10:1989–1991. doi: 10.3748/wjg.v10.i13.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JH, Wang L, Ling R, Li Y, Wang Z, Yao Q, Ma Z. Body distribution of nanoparticle-containing adriamycin injected into the hepatic artery of hepatoma-bearing rats. Dig Dis Sci. 2004;49:1170–1173. doi: 10.1023/b:ddas.0000037807.96064.99. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Novell JR, Winslet MC, Hobbs KE. Iodized oil in the treatment of hepatocellular carcinoma. Br J Surg. 1994;81:1563–1571. doi: 10.1002/bjs.1800811105. [DOI] [PubMed] [Google Scholar]
- 10.Okayasu I, Hatakeyama S, Yoshida T, Yoshimatsu S, Tsuruta K, Miyamoto H, Kimula Y. Selective and persistent deposition and gradual drainage of iodized oil, Lipiodol in the hepatocellular carcinoma after injection into the feeding hepatic artery. Am J Clin Pathol. 1988;90:536–544. doi: 10.1093/ajcp/90.5.536. [DOI] [PubMed] [Google Scholar]
- 11.Takaki Y, Kaminou T, Shabana M, Ihaya T, Otsubo K, Ogawa T. Suitable blending method of lipiodol-cisplatin in transcatheter arterial embolization for hepatocellular carcinoma: evaluation of sustained release and accumulation nature. Hepatogastroenterology. 2008;55:202–206. [PubMed] [Google Scholar]
- 12.Clouse ME, Stokes KR, Kruskal JB, Perry LJ, Stuart KE, Nasser IA. Chemoembolization for hepatocellular carcinoma: epinephrine followed by a doxorubicin-ethiodized oil emulsion and gelatin sponge powder. J Vasc Interv Radiol. 1993;4:717–725. doi: 10.1016/s1051-0443(93)71956-9. [DOI] [PubMed] [Google Scholar]
- 13.Garin E, Denizot B, Noiret N, Lepareur N, Roux J, Moreau M, Herry JY, Bourguet P, Benoit JP, Lejeune JJ. 188Re-SSS lipiodol: radiolabelling and biodistribution following injection into the hepatic artery of rats bearing hepatoma. Nucl Med Commun. 2004;25:1007–1013. doi: 10.1097/00006231-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Garin E, Noiret N, Malbert C, Lepareur N, Roucoux A, Caulet-Maugendre S, Moisan A, Lecloirec J, Herry JY, Bourguet P. Development and biodistribution of 188Re-SSS lipiodol following injection into the hepatic artery of healthy pigs. Eur J Nucl Med Mol Imaging. 2004;31:542–546. doi: 10.1007/s00259-003-1402-z. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–786. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 16.Ho D, Sun X, Sun S. Monodisperse magnetic nanoparticles for theranostic applications. Acc Chem Res. 2011;44:875–882. doi: 10.1021/ar200090c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacroix LM, Huls NF, Ho D, Sun X, Cheng K, Sun S. Stable single-crystalline body centered cubic Fe nanoparticles. Nano Lett. 2011;11:1641–1645. doi: 10.1021/nl200110t. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin Cancer Res. 2009;15:876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ, Li C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther. 2008;7:1730–1739. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Zhang G, Zhang R, Flores LG, 2nd, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010;70:3177–3188. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Wang W, Wu Q, Ke S, Houston J, Sevick-Muraca E, Dong L, Chow D, Charnsangavej C, Gelovani JG. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl Med Biol. 2006;33:349–358. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Ke S, Wu Q, Charnsangavej C, Gurfinkel M, Gelovani JG, Abbruzzese JL, Sevick-Muraca EM, Li C. Near-infrared optical imaging of integrin alphavbeta3 in human tumor xenografts. Mol Imaging. 2004;3:343–351. doi: 10.1162/15353500200404148. [DOI] [PubMed] [Google Scholar]
- 24.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 25.Ryppa C, Mann-Steinberg H, Biniossek ML, Satchi-Fainaro R, Kratz F. In vitro and in vivo evaluation of a paclitaxel conjugate with the divalent peptide E-[c(RGDfK)2] that targets integrin alpha v beta 3. Int J Pharm. 2009;368:89–97. doi: 10.1016/j.ijpharm.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 26.Geschwind JF, Ramsey DE, Cleffken B, van der Wal BC, Kobeiter H, Juluru K, Hartnell GG, Choti MA. Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Intervent Radiol. 2003;26:111–117. doi: 10.1007/s00270-002-2524-6. [DOI] [PubMed] [Google Scholar]
- 27.Burgener FA, Violante MR. Comparison of hepatic VX2-carcinomas after intra-arterial, intraportal and intraparenchymal tumor cell injection. An angiographic and computed tomographic study in the rabbit. Invest Radiol. 1979;14:410–414. doi: 10.1097/00004424-197909000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Lijowski M, Zhang H, Partlow KC, Caruthers SD, Kiefer G, Gulyas G, Athey P, Scott MJ, Wickline SA, Lanza GM. Imaging of Vx-2 rabbit tumors with alpha(nu)beta3-integrin-targeted 111In nanoparticles. Int J Cancer. 2007;120:1951–1957. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Melancon MP, Xiong C, Huang Q, Elliott A, Song S, Zhang R, Flores LG, 2nd, Gelovani JG, Wang LV, Ku G, Stafford RJ, Li C. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Wu Q, Pasuelo M, McMurray JS, Li C. Probing for integrin alpha v beta3 binding of RGD peptides using fluorescence polarization. Bioconjug Chem. 2005;16:729–734. doi: 10.1021/bc049763s. [DOI] [PubMed] [Google Scholar]
- 31.Schwartzberg AM, Olson TY, Talley CE, Zhang JZ. Synthesis, characterization, and tunable optical properties of hollow gold nanospheres. Journal of Physical Chemistry B. 2006;110:19935–19944. doi: 10.1021/jp062136a. [DOI] [PubMed] [Google Scholar]
- 32.Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909–3913. [PubMed] [Google Scholar]
- 33.Tian M, Ogawa K, Wendt R, Mukhopadhyay U, Balatoni J, Fukumitsu N, Uthamanthil R, Borne A, Brammer D, Jackson J, Mawlawi O, Yang B, Alauddin M, Gelovani JG. Whole-body biodistribution kinetics, metabolism, and radiation dosimetry estimates of 18F-PEG6-IPQA in nonhuman primates. J Nucl Med. 2011;52:934–941. doi: 10.2967/jnumed.110.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nye JA, Schuster DM, Yu W, Camp VM, Goodman MM, Votaw JR. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med. 2007;48:1017–1020. doi: 10.2967/jnumed.107.040097. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita Y, Takahashi M, Fujimura N, Kan M. Clinical evaluation of hepatic artery embolization: comparison between Gelfoam and Lipiodol with anticancer agent. Radiat Med. 1987;5:61–67. [PubMed] [Google Scholar]
- 36.Ohishi H, Uchida H, Yoshimura H, Ohue S, Ueda J, Katsuragi M, Matsuo N, Hosogi Y. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology. 1985;154:25–29. doi: 10.1148/radiology.154.1.2981114. [DOI] [PubMed] [Google Scholar]
- 37.Raoul JL, Heresbach D, Bretagne JF, Ferrer DB, Duvauferrier R, Bourguet P, Messner M, Gosselin M. Chemoembolization of hepatocellular carcinomas. A study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992;70:585–590. doi: 10.1002/1097-0142(19920801)70:3<585::aid-cncr2820700308>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Lijowski M, Caruthers S, Hu G, Zhang H, Scott MJ, Williams T, Erpelding T, Schmieder AH, Kiefer G, Gulyas G, Athey PS, Gaffney PJ, Wickline SA, Lanza GM. High sensitivity: high-resolution SPECT-CT/MR molecular imaging of angiogenesis in the Vx2 model. Invest Radiol. 2009;44:15–22. doi: 10.1097/RLI.0b013e31818935eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pressly ED, Rossin R, Hagooly A, Fukukawa K, Messmore BW, Welch MJ, Wooley KL, Lamm MS, Hule RA, Pochan DJ, Hawker CJ. Structural effects on the biodistribution and positron emission tomography (PET) imaging of well-defined (64)Cu-labeled nanoparticles comprised of amphiphilic block graft copolymers. Biomacromolecules. 2007;8:3126–3134. doi: 10.1021/bm700541e. [DOI] [PubMed] [Google Scholar]
- 40.Lu W, Huang Q, Ku G, Wen X, Zhou M, Guzatov D, Brecht P, Su R, Oraevsky A, Wang LV, Li C. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials. 2010;31:2617–2626. doi: 10.1016/j.biomaterials.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]