Abstract
Difficulty in inhibition or cognitive control is a common and significant sequela of pediatric traumatic brain injury (TBI). The present study used functional MRI to examine one specific inhibitory function, interference control, in 11 adolescents, aged 12–16 years, (mean age, 15.7 years) with TBI who were at least 1 year postinjury and 11 age-matched typically developing control participants (TC) (mean age, 15.2 years). Participants completed a Counting Stroop task with 2 main conditions: (1) a neutral condition requiring the counting of animal words and (2) an interference condition in which mismatched number words were counted. Both TBI and TC adolescents activated similar networks of brain regions relevant to interference control, but the TBI group showed higher levels of activation relative to the TC group in multiple brain areas within this network, including predominantly right frontal and parietal regions. Findings of greater activation of the relevant neural network in the TBI group are consistent with recent fMRI findings using other interference control paradigms with individuals with a history of TBI.
Keywords: Childhood brain disorder, Inhibition, Executive functions, Brain imaging, Adolescent, Head injury
INTRODUCTION
Traumatic brain injury (TBI) is one of the most common causes of acquired disability in childhood and adolescence (Keenan & Bratton, 2006), with the highest peak in incidence of TBI occurring during the adolescent and young adult years (Langlois, Rutland-Brown, & Thomas, 2006). Adolescence also constitutes a critical time for brain development (Casey, Tottenham, Liston, & Durston, 2005) and an important transitional period during which the child is expected to assume increasing responsibility for self-management (Crosnoe & Trinitapoli, 2008; Wigfield & Eccles, 1994). Deficits in executive functions (EF) are commonly observed in pediatric TBI (Max et al., 2005a, 2005b; Max, Manes, et al., 2005). In general, children with severe TBI display poorer performance on EF measures, including attention, working memory, behavioral inhibition, and cognitive control, than less-severely injured or healthy control children (e.g., Anderson, Catroppa, Morse, Haritou, & Rosenfeld, 2005; Anderson, Fenwick, Manly, & Robertson, 1998; Catroppa & Anderson, 2005; Ewing-Cobbs et al., 1998; Yeates et al., 2005). Deficits in EF may help explain the discrepancy between seemingly normal performance on many standard non-EF neurocognitive measures (as in areas of verbal and non-verbal abilities), despite observed impairments in everyday social and academic functioning (Anderson et al., 2005; Catroppa & Anderson, 2003; Ewing-Cobbs, Prasad, Landry, Kramer, & DeLeon, 2004). Intact EF skills play an important role in interpersonal, school, and vocational success (Levin & Hanten, 2005; Pennington, 1994; Schachar, Levin, Max, Purvis, & Chen, 2004).
One EF domain in which children and adolescents with TBI have shown deficits concerns inhibitory or interference control. Whereas cognitive control has been used to refer broadly to the ability to select, sustain, filter, or optimize information processing (e.g., Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004), regulate and reconcile our goals and actions (e.g., Aarts, Roelofs, & van Turennout, 2008), and monitor and resolve conflicts (e.g., Posner & Rothbart, 2007), interference control refers to the specific ability to select information from competing sources to minimize interference or conflict (e.g., Aarts et al., 2008; Fan, Flombaum, McCandliss, Thomas, & Posner, 2003; Levin, Hanten, & Li, 2009). Thus, a better understanding of the neural substrates of interference control has the potential to inform our understanding of one of the core deficits arising from TBI in childhood and adolescence.
Extant studies using functional magnetic resonance imaging (fMRI) to examine EF and TBI have focused largely on adults (e.g., Chen, Johnston, Collie, McCroy, & Ptito, 2007; Mayer et al., 2009; McAllister et al., 1999, 2001; Newsome, Scheibel, Steinberg, et al., 2007; Scheibel et al., 2007, 2009; Smits et al., 2009), with many focusing on working memory as a domain of interest. A few fMRI studies have examined attention and working memory functions in pediatric TBI but did not focus on interference control (e.g., Kramer, Chiu, Shear, & Wade, 2009; Kramer et al., 2008; Newsome, Scheibel, Hunter, et al., 2007; Newsome et al., 2008). Scheibel and colleagues (2007, 2009) investigated interference control in adults with TBI at 3 months post-injury using a stimulus response compatibility task. In this task, participants pressed keys on the side indicated by the direction of arrows on the screen (neutral) or on the side opposite to that indicated by the arrows (interference). These authors reported higher levels of inhibition-related activation (i.e., incompatible minus compatible) in adults with TBI relative to adults with orthopedic injuries (OI) across an extensive range of brain regions including the left precentral gyrus, midline cingulate region, medial frontal, middle frontal, and superior frontal gyri bilaterally. Participants with more severe TBI had higher levels of activation than those with milder injuries or OI. Higher levels of activation were reported to be associated with higher levels of task performance in the incompatible condition in participants with TBI (e.g., Scheibel et al., 2009). Smits and colleagues (2009) studied interference control in healthy controls and in adult patients (age 18–50) who suffered minor head injuries, as defined by GCS scores of 13–15 with accompanying post-concussive symptoms, approximately 1 month post-injury using the Counting Stroop task developed by Bush and colleagues (1998, 1999). In this task, participants were shown several words on screen and asked to count the number of words and respond in two critical conditions. The words were non-number words (e.g., animal names) in the Neutral condition, whereas in the Interference condition, there were number names that mismatched the number of words on screen (e.g., “four four” or “two two two two”). For the Interference minus Neutral contrast, Smits and colleagues observed overall activation broadly in lateral prefrontal areas (including superior, middle, and inferior frontal gyrus) with rightward bias, medial superior frontal gyrus, right inferior and superior parietal lobules, and other posterior parietal areas. In addition, activation levels in the insula, inferior frontal gyrus, pre- and post-central gyrus on the left, as well as posterior parietal cortex, anterior cingulate, and posterior cingulate bilaterally were associated with the number of self-reported post-concussion symptoms (with control =0 symptoms), with higher activation levels corresponding to greater numbers of post-concussion symptoms.
The current study sought to examine the neural substrates associated with interference control in adolescents with complicated mild to severe TBI relative to a cohort of age and sex-matched healthy controls. Consistent with Scheibel and colleagues (2007), we hypothesized that adolescents with and without TBI would activate similar frontal and parietal regions when engaging interference control processes during the Interference condition to a greater extent than during the Neutral condition, but that the adolescents with TBI would demonstrate greater activation of brain regions supporting interference control compared to controls.
METHODS
Participants
Participants with TBI, who were in ongoing behavioral intervention studies, were identified as eligible for participation based on current age and time since injury. All adolescent participants with TBI were required to have sustained a TBI requiring overnight hospitalization with a lowest Glasgow Coma Scale score of ≤12, or a GCS of 13–15 accompanied by abnormalities on imaging. Functional imaging was conducted ≥12 months post-injury because we were interested in the longer-term effects of TBI on developing brain networks during adolescence. Eighteen consented to be enrolled in the study, but 1 was unable to be scheduled during the period of the study, 1 declined to complete the scan, 3 were excluded due to metal in their bodies, and 2 were excluded due to excessive motion (>3 mm, see fMRI method below), leaving a total of 11 participants (mean age, 15.7 years; range, 12–16 years) with usable fMRI data. A typically developing comparison cohort of 15 adolescents (TC group) matched with the TBI group on sex, handedness, and race/ethnicity, were recruited through an institutional review board-approved electronic notice to employees of a large, urban hospital. Individuals who expressed interest in participation were screened for eligibility and matched to the adolescents with TBI until 15 eligible healthy adolescents consented to participate. Of this group of 15, 2 had excessive motion during the fMRI session under the same criterion, 1 declined to finish a scan, and Counting Stroop data for 1 participant was lost due to equipment malfunction, leaving 11 participants (mean age, 15.2 years; range, 12–16 years), with usable fMRI data. Eligibility criteria for both groups included English as the primary language spoken in the home. Adolescents were excluded if they had a diagnosis of significant developmental disability (i.e., placement in a special classroom and IQ <70), significant psychiatric/behavioral disturbances (e.g., bipolar, major depression, autism), or extreme vision and hearing deficits. Additional exclusion criteria for the control group included any prior diagnosis of a neurological disorder or any indications of a history of TBI. Both the intervention project and the current imaging study were approved by the Institutional Review Board. Thus, a total of 33 participants completed informed consent to participate in the study, 28 were assessed and provided behavioral data, and 22 (67%) yielded behavioral and usable fMRI data. Those with usable fMRI data did not differ from others who were excluded in terms of age, IQ, injury severity, or self- or parent-report of EF skills.
Procedures
After informed consent was obtained, adolescents completed a brief neuropsychological battery including an abbreviated assessment of intelligence and measures of various aspects of EF. Participants then completed several fMRI tasks including the Counting Stroop, described in the present report.
Cognitive and behavioral functioning
Receptive vocabulary was assessed using the Peabody Picture Vocabulary Test-Fourth Edition (PPVT-IV, Dunn & Dunn, 2007). Single word reading skills, which have been used as a proxy for pre-injury cognitive status (Orme, Johnstone, Hanks, & Novack, 2004), were measured using the Wide Range Achievement Test-Fourth Edition (WRAT-4, Wilkinson & Robertson, 2006). The Working Memory (WM) and Processing Speed (PS) Indices from the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV, Wechsler, 2003) were administered to assess domains of EF known to be vulnerable to TBI. Interference control was assessed using the Color-Word Interference Test of the Delis-Kaplan Executive Function System (D-KEFS, Delis, Kaplan, & Kramer, 2001). In this Stroop-like task, the participant is presented with a color word that is printed in a font color that is different from the word and asked to name the color of the font. Parents and adolescents completed the Behavior Rating Inventory of Executive Function (BRIEF, Gioia, Isquith, & Guy, 2000) and BRIEF-self report (BRIEF-SR, Guy, Isquith, & Gioia, 2004), respectively.
Stimuli and behavioral tasks used for fMRI
In the variant of the Counting Stroop Task used in the present study (cf. Bush et al., 1998, 1999), one to four identical words were presented in a vertical column on the screen on each trial of 1.5 s. Participants were asked to note the number of words that appeared on the screen and press one of four corresponding response buttons on a button box. For example, if the word appeared four times on the screen, the correct response would be “4.” The Interference condition contained all number words (i.e., “one,” “two,” “three,” or “four”), with each appearing 2 to 5 times in each block in a random manner. The Neutral condition contained names of common animals (i.e., “dog,” “cat,” “mouse,” or “bird”), again each appearing 2 to 5 times in each block in a random manner. The two conditions were balanced for the number and length of the words. There were fourteen trials in each of the two conditions. Additionally, we also included a third, baseline fixation condition. In this condition, participants were asked to monitor a row of “+” signs presented at the center of the screen, and the number of “+” signs on screen changed every 2 s (randomly from two to six “+” signs). The baseline condition was included for the following reasons. First, if the interference control comparison (Interference minus Neutral) turned out to be non-significant or sub-threshold in either group, a baseline condition may allow us to check whether the effect was genuine or whether it was due to equipment failure, subject non-compliance, or the presence of other artifacts. Second, if a group difference in the interference control comparison was observed (as in Smits et al., 2009), it might have been difficult to interpret, without a baseline condition, because this between-group difference might have been due to different levels of activation in the Neutral condition (relative to some baseline state) across groups. Thus, a baseline condition was selected that would engage attention to stimuli and interference control as little as possible to accentuate its difference from the Neutral and Interference conditions. Participants were given one example of each condition during a pre-scan training session, and all indicated understanding of the instructions. No further training was provided. Video and audio monitoring were used to observe whether there were any problems during the administration of the task and track whether participants appeared to be responding appropriately. Stimulus administration and response logging was accomplished using the experimental software E-Prime with magnet compatible goggles and a response system.
fMRI data acquisition and analyses
MR scanning was performed on a 3 Tesla Philips Achieva MR scanner. A T2*-weighted, gradient-echo EPI sequence was used for fMRI scans (repetition time/echo time [TR/ TE] =2000/30 ms, field of view [FOV] =240 × 240 mm, matrix =80 × 80, slice thickness =4 mm, flip angle =90°). Forty-one slices were acquired at 210 time points for a total imaging time of 432 s, beginning with a 12 s (6 volumes) fixation period to allow for T1 relaxation effects that were subsequently discarded before statistical post-processing of the fMRI data. Functional imaging time consisted of six cycles, each 66-s long with fixation periods of 12 s and condition periods of 21 s alternating with one another (i.e., fixation-neutral-fixation-interference). A T1-weighted, three-dimensional (3D) MPRAGE whole brain scan was also obtained for anatomical co-registration (TR/TE =8.2/3.7 ms, FOV =25 cm × 25 cm, matrix =256 × 256, slice thickness =1 mm, scan time =355 s) before the functional scans.
Following Schmithorst and Holland (2007), fMRI image post-processing was accomplished using in-house software written in IDL™ (ITT visual information solutions). The reconstructed EPI data were corrected for drift using quadratic baseline correction on a pixel-by-pixel basis (Hu, Le, Parrish, & Erhard, 1995; Le & Hu, 1996), co-registered to the reference volume to reduce the effects of motion artifacts using a pyramid iterative algorithm (Thevenaz, Ruttimann, & Unser, 1998), and transformed into Talairach coordinates using landmarks (anterior commissure, posterior commissure, inter-hemispheric plane, and bounding volume) obtained from the T1-weighted anatomic images (Talairach & Tournoux, 1988) using a linear affine transformation shown previously to be valid for individuals 5 to 18 years of age (Muzik & Chugani, 2000; Wilke, Schmithorst, & Holland, 2002). During the co-registration procedure, which produced one point-estimate of movement in 3-D space per time point, excessive motion was defined as a median voxel displacement of 3 mm. The fMRI data from four participants were deemed to show excessive motion for >10% of the EPI data set from Counting Stroop and were subsequently discarded. This cutoff was selected to allow for inclusion of as many data sets as possible, while excluding poor data that were unusable even after motion correction. Once subjects who exceeded criteria for excessive movement were excluded, the TBI and TC group did not differ in mean movement, median movement, and proportion of time points where movement exceeded 3 mm, all ps >.11.
For each participant, Pearson’s partial correlation analyses were computed on a voxel-wise basis between EPI data and task reference functions corresponding to the Neutral (21 s), Interference (21 s), and Control (12 +12 =24 s) conditions, using the motion correction parameter as a covariate. All task reference functions were box-car convolved with a canonical hemodynamic response function. A 6-s delay was applied to the reference function to allow for the canonical hemodynamic response to peak. Correlation coefficients were then transformed into Z-score maps using Fisher’s Z-transformation. Group level random effects analyses were performed on these Z-maps from individual participants in the context of the general linear model (GLM). A post-processing filter (full-width, half maximum [FWHM] =8 mm) was then applied before significant regions of activation on a voxel-by-voxel basis were identified (Worsley & Friston, 1995), generating statistical parameter maps. A clustering threshold of 100 contiguous voxels (corresponding to 3600 mL of brain tissue in the Talairach space) was used unless otherwise stated (Xiong, Gao, Lancaster, & Fox, 1995) to improve visualization of the parameter maps and to reduce the severity of the corrections that were made for multiple comparisons. Based on the method of Ledberg, Akerman, and Roland (1998), Monte Carlo simulations were used to estimate corrected p values and performed in the following manner. The spatial autocorrelations present in the fit residuals were used to estimate the intrinsic smoothness in the data. “Null” activation maps were generated from spatially auto-correlated Gaussian noise generated using the previously found smoothness estimates and post-processing parameters (e.g., threshold intensity, cluster size, and exogenous spatial filtering). The simulations were repeated, and the corrected p values estimated by computing the proportion of null maps with spurious activated clusters detected. Monte-Carlo simulation was performed to assure p <.001 after adjusting for multiple comparisons. For each cluster, the Talairach coordinates of the pixel that showed the maximum Z-value before filtering (i.e., the maxima) is reported here.
RESULTS
Group Comparisons on Demographic, Cognitive, and Behavioral Data
Table 1 provides the demographic, cognitive, and behavioral data for the 22 adolescents with usable fMRI data. The mean Glasgow Coma Scale score for the TBI group was 12.6 (SD =3.6). Eight of 11 children had complicated mild TBI characterized by GCS scores of 13–15 with abnormal imaging. The other three children (1 severe) had GCS scores of <13 with no abnormalities on clinical CT or MRI at the time of injury. The average time since injury was 1.8 years (SD =0.45). The groups were comparable with respect to age, family income, race/ethnicity, sex, and handedness. Adolescents in the TC group showed numerically better performance on the WRAT and PPVT than the adolescents with TBI (with effect sizes indexed by Cohen’s d =0.68 and 0.41, respectively), but this difference did not reach statistical significance (both ps >.10). There were also no significant group differences on the WISC PSI and WMI, although effect size for WISC WMI (Cohen’s d =0.68) was in the medium range. Groups were also comparable on the D-KEFS Color-Word Inhibit task. Adolescents with TBI had significantly higher levels of parent-rated executive dysfunction (i.e., on the BRIEF) than did the TC group, although means in both groups were within the normal range. Parents of adolescents with TBI rated their children as having significantly greater difficulties with shifting from one task to another, behavior regulation, and meta-cognition than the TC group, all ps <.05.
Table 1.
Demographic characteristics and neuropsychological performance by group
| Measure | TBI (n =11*) | Control (n =11*) | t/Chi Square | p | Cohen’s d |
|---|---|---|---|---|---|
| Age | 15.66 (1.01) | 15.23 (1.54) | .77 | .45 | .33 |
| Family income | 6.64 (4.78) | 8.00 (2.79) | −.82 | .42 | .35 |
| Male sex | 7 (64%) | 6 (55%) | .19 | .67 | |
| Right handed | 11 (100%) | 9 (82%) | 2.20 | .14 | |
| Race | |||||
| Caucasian | 9 (82%) | 10 (91%) | .39 | .53 | |
| African American | 2 (18%) | 1 (9%) | |||
| PPVT-4 | 102.54 (14.5) | 107.73 (9.9) | −.98 | .34 | .41 |
| WRAT-4 | 101.91 (18.7) | 114.64 (18.6) | −1.60 | .13 | .68 |
| WISC-IV PSI | 107.64 (15.2) | 105.45 (17.0) | .32 | .75 | .13 |
| WISC-IV WM | 98.90 (10.2) | 107.73 (15.2) | −1.55 | .14 | .68 |
| BRIEF GEC | 53.09 (13.3) | 42.73 (8.7) | 2.16 | .05 | .92 |
| BRIEF MI | 53.55 (12.3) | 43.64 (9.3) | 2.13 | .05 | .91 |
| BRIEF BRI | 52.00 (12.8) | 42.73 (6.2) | 2.17 | .05 | .92 |
| BRIEF Inhibit | 54.00 (13.6) | 45.64 (6.4) | 1.84 | .09 | .79 |
| BRIEF Shift | 49.36 (9.4) | 41.45 (5.2) | 2.45 | .03 | 1.04 |
| BRIEF-SR GEC | 53.18 (11.6) | 48.73 (10.1) | .96 | .35 | .41 |
| BRIEF-SR MI | 55.00 (12.6) | 48.00 (11.1) | 1.38 | .18 | .59 |
| BRIEF-SR BRI | 50.27 (10.4) | 49.45 (10.7) | .18 | .86 | .08 |
| BRIEF-SR Inhibit | 49.64 (10.3) | 48.64 (11.1) | .22 | .93 | .09 |
| BRIEF-SR Shift | 49.64 (11.2) | 49.55 (9.0) | .02 | .98 | .01 |
| D-KEFS CW Inhibit | 10.55 (2.5) | 9.82 (1.5) | .83 | .42 | .35 |
| Counting Stroop | |||||
| % Corr. Neutral | 93.73 (4.9) | 96.55 (2.5) | −1.7 | .11 | .73 |
| % Corr. Interference | 83.27 (8.6) | 87.27 (11.5) | −.92 | .39 | .39 |
| RT Neutral | 813 (73) | 746 (79) | 2.08 | .05 | .88 |
| RT Interference | 892 (78) | 832 (98) | 1.59 | .13 | .68 |
| Accuracy Diff. | 10.45 (8.8) | 9.27 (9.9) | .30 | .77 | .11 |
| RT Diff. | 79 (35) | 86 (52) | −.39 | .70 | .16 |
Note. TBI =Traumatic Brain Injuries; TC =Typically Developing Controls; PPVT =Peabody Picture Vocabulary Test Version 4; WRAT =Wide Range Achievement Test 4th Edition; WISC =Wechsler Intelligence Scale for Children 4th Edition; PSI =Processing Speed Index; WMI =Working Memory Index; BRIEF =Behavior Rating Inventory of Executive Function; GEC =Global Executive Composite; SR =Self Report; BRI =Behavior Regulation Index; MI =Metacognition Index DKEFS CW =Delis-Kaplan Executive Function System Color Word Interference Task; RT =median reaction time.
Only participants who completed all tests and contributed useable functional magnetic resonance imaging (fMRI) data are included here.
Counting Stroop: Behavioral and fMRI results
Performance data for the Counting Stroop task were recorded during the fMRI scan. All but one participant showed higher accuracy and faster median reaction time in the Neutral condition than the Interference condition. Mean accuracy for participants with TBI was not significantly lower than that for participants in the TC group, all ps >.11. Median reaction time (RT) calculated for each participant, excluding data from all error trials, showed that adolescents with TBI were slower than those in the TC group in the Neutral condition, t(20) =2.08, p =.05, but not in the Interference condition, t(20) =1.59, p >.13. Focusing on interference control, defined as the difference between the Neutral and the Interference conditions, the two groups were well matched and not different in RT Difference (79 ms for the TBI group and 86 ms for the TC group) or in Accuracy Difference (10.4% for the TBI group and 9.3% for the TC group), both ps >.7 and Cohen’s d <0.17. Note that the magnitude of the interference control effects in RT and accuracy were numerically larger than has been demonstrated in studies of adults (range of RT Diff. =29 to 46 +ms, see Bush et al., 2003; range of Accuracy Diff. =0 to 3%, see Bush et al., 1998, 1999).
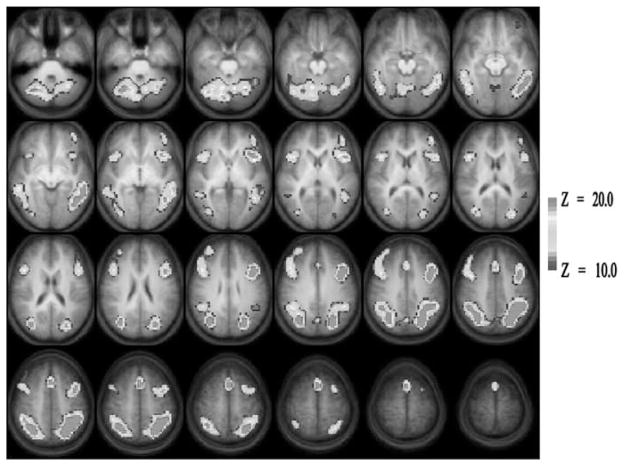
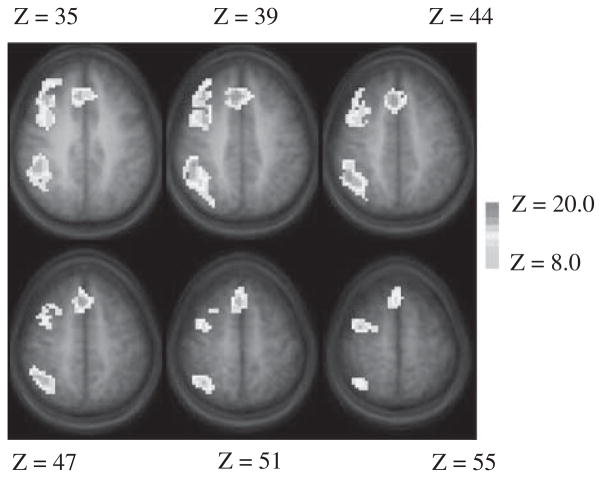
Figure 1 presents the composite Z-score map of brain regions that were significantly more active during the Interference condition compared to the Neutral condition in the entire sample of 22 participants. Predominantly bilateral activation was seen in broad extent of the brain. These include dorsal as well as ventrolateral prefrontal cortices, anterior cingulate, posterior parietal cortex, inferior and middle temporal gyri, lingual and fusiform gyrus, cerebellum, and other occipital regions. Group-related differences in brain activity were computed on a voxel-wise basis and are displayed in Figure 2 with the Talairach coordinate of the centroids of significant differences listed in Table 2. Participants in the TBI group had higher levels of interference-control related activation relative to the TC group in frontal and parietal areas including the right middle frontal gyrus (MFG, Brodmann area [BA] 6), medial frontal areas including dorsal anterior cingulate (BA 32) and frontal cortex (BA 8), and right dorsolateral prefrontal cortex (BA 9). Activation differences were also seen in the right parietal areas including the superior (BA 7) and inferior (BA 40) parietal lobule. There were no areas in which participants in the TC group demonstrated greater inhibition-related activation than participants in the TBI group.
Fig. 1.
Brain activation map for the Interference Control comparison across the entire group of participants. Only positive activation foci (Interference >Neutral) are shown here. Images are horizontal slices 4 mm apart and start at Z =–29 mm (top left) to Z =+63 mm (bottom right). Images are in radiological convention: left side of the images corresponds to the right hemisphere. Image parameters are as follows: nominal Z threshold =10.0, cluster size =100, corrected p <.001 for multiple comparisons.
Fig. 2.
Group difference map in the Interference Control comparison. The traumatic brain injury (TBI) group had significantly higher levels of activation in multiple brain regions relative to the typically developing control participants (TC) group (warm color). The TC group had no regions with higher levels of activation than the TBI group. Nominal Z threshold =8.0, cluster size =100, corrected p <.001 for multiple comparisons.
Table 2.
Regions of interest (ROIs) showing significantly different levels of activation between adolescents with TBI (TBI) and typically developing controls (TC) in the interference minus neutral contrast during the counting stroop task
| ROI | Brodmann areas | Talairach coordinates
|
Peak Z | ||
|---|---|---|---|---|---|
| x | y | z | |||
| TBI > TC | |||||
| R Middle frontal gyrus | 6 | 34 | 3 | 39 | 3.1 |
| R Middle frontal gyrus | 6 | 34 | −3 | 55 | 3.2 |
| R Medial frontal gyrus | 8 | 4 | 21 | 47 | 4.2 |
| R Medial frontal gyrus | 8 | 7 | 30 | 39 | 4.8 |
| Anterior cingulate cortex | 32 | 4 | 24 | 39 | 2.3 |
| R Precentral gyrus | 9 | 37 | 24 | 35 | 3.6 |
| R Inferior parietal lobule | 40 | 40 | −33 | 35 | 4.4 |
| R Superior parietal lobule | 7 | 32 | −57 | 51 | 2.5 |
Given that the imaging findings showed that the TBI group had a larger difference in the Interference minus Neutral contrast than the TC group, whereas the groups were largely comparable on measures (both RT diff. and Accuracy diff) indexing interference control (Interference minus Neutral) on the Stroop task, it is possible that the between-group difference in imaging might have arisen from between-group differences in the Neutral condition (relative to baseline). We, therefore, repeated the above analyses with the Neutral minus Fixation Baseline contrast. At the same nominal thresholds (i.e., Z =8.0, cluster size =100), no activation differences between groups were detected anywhere. Only when the nominal threshold was made much less stringent (i.e., to Z =4.0, cluster size =50) was there a trend toward a larger Neutral minus Baseline contrast in the TC group than the TBI group, but the few scattered foci were found around the left parietal areas (BA 7 and 40), the cerebellum, and left dorsolateral prefrontal areas; none of these areas overlapped with the ones identified in Figure 2. At this more lenient threshold, there were no areas in which the TBI group showed a larger Neutral minus Baseline difference than the TC group. Thus, imaging data did not provide support for the notion that group differences in the interference control contrast was due solely to differences in the Neutral minus Baseline Fixation contrast.
DISCUSSION
The findings from this study are consistent with our hypotheses that adolescents with TBI would activate similar networks of brain regions during the Counting Stroop task relative to typically developing controls, but would demonstrate stronger activation of brain regions supporting inhibition. The regions with higher levels of activation in the TBI group are in the frontal and parietal cortices (including BA 6, 8, 32, 7, 9, & 40) and right-lateralized and belong to the overall neural network that supports interference control more broadly (Figure 1). Our findings are consistent with extant studies that observed higher levels of activation in adults with TBI relative to controls (either healthy adults or adults with less severe TBI) when they engage in interference control in similar frontal and parietal areas (Scheibel et al., 2007, 2009; Smits et al., 2009).
It is particularly noteworthy that this altered neural activation pattern persists in our sample of adolescents with TBI despite their participation in ongoing behavioral intervention, relatively normal level of performance on the interference control task, and at least 12 months post-injury. Whether the observation of increased activation is specific to adolescents with complicated mild TBI remains to be determined, since the adults with TBI with GCS in the mild range in Smits and colleagues (2009) were studied only 1-month post-injury. It should also be noted that other fMRI studies in adults with TBI have reported a more complicated relationship between levels of brain activation and injury status or severity that may vary depending on task difficulty or time since injury. For example, fMRI studies have also reported higher or lower levels (or even absence) of activation in brain regions in adults with TBI relative to controls in working memory tasks that depended for instance on working memory load (e.g., Chen et al., 2004; McAllister et al., 1999, 2001). In addition, brain activation in right superior frontal areas initially showed a reduced level of activation in adults with TBI compared to healthy controls, but activation increased in this area over a 6-month period such that the between-group differences were much reduced (e.g., Sanchez-Carrion et al., 2008).
The current study demonstrates the feasibility of assessing interference control with fMRI in adolescents following TBI using healthy adolescents as controls. The adolescents with TBI successfully completed the scanning protocol and were largely equivalent to healthy controls on task performance with minimal training before scanning, as well as on most demographic measures. In conjunction with other studies using healthy controls (e.g., Smits et al., 2009), the results provide preliminary evidence that the altered pattern of brain activation in individuals with TBI may not be limited to cases where OI participants were used as a comparison standard (e.g., Scheibel et al., 2007, 2009). In the current study, we focused on examining interference control in adolescents with TBI within a relatively restricted range of age and IQ that was generally comparable to the healthy controls. Future investigations using a more diverse sample with a broad GCS range could also examine the relationship between GCS and brain activation in children as has been done for adults with TBI.
Care must be taken in generalizing from the current findings given the small and heterogeneous nature of the sample of adolescents with TBI. All but three children with TBI had injuries that would be termed complicated mild. Moreover, although most measures of cognitive performance fell within the average range overall, they exhibit considerable variability across participants, which was not directly examined in the current study. The effect size estimates (Cohen’s d) for some neuropsychological and behavioral measures, including estimates of overall cognitive ability, were indicated in the “moderate” to “large” range numerically, which suggests that statistically significant differences between groups may emerge with a larger sample size. The small sample size and the issue of insufficient statistical power clearly limit the generalizability of results from the present preliminary study to the broader population.
Future research is needed to determine the neural substrates of inhibition and cognitive control in children with more significant cognitive and behavioral impairment following TBI. Additionally, the current study examined only one aspect of inhibition at a single point in time during the chronic phase of recovery. Additional studies will be required to understand the neural substrates of other aspects of inhibition and related executive functions, whether the process of recovery results in neural remodeling over time, and how neural functioning relates to functional outcomes. Nonetheless, this study provides important new information about neural differences in the network subserving interference control and inhibition following TBI in adolescence.
Acknowledgments
This work was supported in part by (1) NIH grant RO1-MH073764 from the National Institute of Mental Health; (2) H133G050239 from the National Institute on Disability and Rehabilitation Research in the Department of Education, and (3) EMS/Trauma grant from the Ohio Department of Public Safety. The information in this manuscript is an original contribution to the literature and has not previously been published, electronically or in print. The authors certify that we know of no conflict of interest, and we adhere to the ethical guidelines of the Helsinki Declaration. We thank three anonymous reviewers for providing numerous helpful comments and suggestions for improving the manuscript. The order of the first two authors was determined by a coin toss.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. The Journal of Neuroscience. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Attentional and processing skills following traumatic brain injury in early childhood. Brain Injury. 2005;19:699–710. doi: 10.1080/02699050400025281. [DOI] [PubMed] [Google Scholar]
- Anderson V, Fenwick T, Manly T, Robertson I. Attentional skills following traumatic brain injury in childhood: A componential analysis. Brain Injury. 1998;12:937–949. doi: 10.1080/026990598121990. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The multi-source interference task: A validation study with fMRI in individual subjects. Molecular Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting stroop: An interference task specialized for functional neuroimaging – validation study with functional MRI. Human Brain Mapping. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. Children’s attentional skills 2 years post-traumatic brain injury. Developmental Neuropsychology. 2003;23:359–373. doi: 10.1207/S15326942DN2303_3. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. A prospective study of the recovery of attention from acute to 2 years following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2005;11:84–98. doi: 10.1017/S1355617705050101. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Collie A, McCroy P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:1231–1238. doi: 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Crosnoe R, Trinitapoli J. Shared family activities and the transition from childhood into adolescence. Journal of Research on Adolescence. 2008;18:23–48. doi: 10.1111/j.1532-7795.2008.00549.x. [DOI] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test (PPVT4) 4. Minneapolis, MN: NCS Pearson, Inc; 2007. [Google Scholar]
- E-Prime [Computer software] Sharpsburg, PA: Psychological Software Tools, Inc; [Google Scholar]
- Ewing-Cobbs L, Prasad M, Fletcher JM, Levin HS, Miner ME, Eisenberg HM. Attention after pediatric traumatic brain injury: A multidimensional assessment. Child Neuropsychology. 1998;4:35–48. doi: 10.1076/chin.4.1.35.3194. [DOI] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Landry SH, Kramer L, DeLeon R. Executive functions following traumatic brain injury in young children: A preliminary analysis. Developmental Neuropsychology. 2004;26:487–512. doi: 10.1207/s15326942dn2601_7. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC. Behavior rating of executive function (BRIEF) Child Neuropsychology. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Guy SC, Isquith PK, Gioia GA. BRIEF-SR: Behavior rating inventory of executive function–Self-report version. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magnetic Resonance in Medicine. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Keenan HT, Bratton SL. Epidemiology and outcomes of pediatric traumatic brain injury. Developmental Neuroscience. 2006;28:256–263. doi: 10.1159/000094152. [DOI] [PubMed] [Google Scholar]
- Kramer ME, Chiu CY, Shear PK, Wade SL. Neural correlates of verbal associative memory and mnemonic strategy use following childhood traumatic brain injury. Journal of Pediatric Rehabilitation Medicine. 2009;2:255–271. doi: 10.3233/PRM-2009-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ME, Chiu CY, Walz NC, Holland SK, Yuan W, Karunanayaka P, Wade SL. Long-term neural processing of attention following early childhood traumatic brain injury: fMRI and neurobehavioral outcomes. Journal of the International Neuropsychological Society. 2008;14:424–435. doi: 10.1017/S1355617708080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2006. Retrieved from CDC website: www.cdc.gov/ncipc/pub-res/tbi../tbi%20in%20the%20us_jan_2006.pdf. [Google Scholar]
- Le TH, Hu X. Retrospective estimation and correction of physiological artifacts in fMRI by direct extraction of physiological activity from MR data. Magnetic Resonance in Medicine. 1996;35:290–298. doi: 10.1002/mrm.1910350305. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33:79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Li X. The relation of cognitive control to social outcome after paediatric TBI: Implications for intervention. Developmental Neurorehabilitation. 2009;12:320–329. doi: 10.3109/17518420903087673. [DOI] [PubMed] [Google Scholar]
- Max JE, Manes FF, Robertson BAM, Mathews K, Fox PT, Lancaster J. Prefrontal and executive attention network lesions and the development of attention-deficit/hyperactivity symptomatology. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:443–450. doi: 10.1097/01.chi.0000156661.38576.0f. [DOI] [PubMed] [Google Scholar]
- Max JE, Schachar RJ, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, Landis J. Predictors of attention-deficit/hyperactivity disorder within 6 months after pediatric traumatic brain injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2005a;44:1032–1040. doi: 10.1097/01.chi.0000173293.05817.b1. [DOI] [PubMed] [Google Scholar]
- Max JE, Schachar RJ, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, Landis J. Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2005b;44:1041–1049. doi: 10.1097/01.chi.0000173292.05817.f8. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, Yeo RA. Auditory orienting and inhibition of return in mild traumatic brain injury: A fMRI study. Human Brain Mapping. 2009;30:4152–4166. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuro-image. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC. Statistical parametric mapping: Assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Hunter JV, Wang ZJ, Chu Z, Li X, Levin HS. Brain activation during working memory after traumatic brain injury in children. Neurocase. 2007;13:16–24. doi: 10.1080/13554790601186629. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Steinberg JL, Troyanskaya M, Sharma RG, Rauch RA, Levin HS. Working memory brain activation following severe traumatic brain injury. Cortex. 2007;43:95–111. doi: 10.1016/S0010-9452(08)70448-9. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Steinberg JL, Scheibel RS, Troyanskaya M, Chu Z, Hanten G, Levin HS. Effects of traumatic brain injury on working memory-related brain activation in adolescents. Neuropsychology. 2008;22:419–425. doi: 10.1037/0894-4105.22.4.419. [DOI] [PubMed] [Google Scholar]
- Orme DR, Johnstone B, Hanks R, Novack T. The WRAT-3 reading subtest as a measure of premorbid intelligence among persons with brain injury. Rehabilitation Psychology. 2004;49:250–253. [Google Scholar]
- Pennington BF. The working memory function of the prefrontal cortices: Implications for developmental and individual differences in cognition. In: Haith MM, Benson J, Roberts RJ, Pennington BF, editors. Future oriented processes in development. Chicago: University of Chicago Press; 1994. pp. 243–289. [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of the prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carrion R, Fernandez-Espejo D, Junque C, Falcon C, Bargallo N, Roig T, Vendrell P. A longitudinal fMRI study of working memory in severe TBI patients with diffuse axonal injury. Neuroimage. 2008;43:421–429. doi: 10.1016/j.neuroimage.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Schachar R, Levin HS, Max JE, Purvis K, Chen S. Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: Do preinjury behavior and injury severity predict outcome? Developmental Neuropsychology. 2004;25:179–198. doi: 10.1207/s15326942dn2501&2_10. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Levin HS. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabilitation and Neural Repair. 2007;21:36–45. doi: 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, Levin HS. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. Journal of Neurotrauma. 2009;26:1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35:406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, van der Lugt A. Postconcussion syndrome after minor head injury: Brain activation of working memory and attention. Human Brain Mapping. 2009;30:2789–2803. doi: 10.1002/hbm.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotactic atlas of the human brain. New York, NY: Thieme; 1988. [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Transactions in Image Processing. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Test for Children (WISC-IV) 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Wigfield A, Eccles J. Middle grades schooling and early adolescent development: An introduction. Journal of Early Adolescence. 1994;14:102–106. doi:0.1177/027243169401400202. [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide range achievement test (WRAT-4) 4. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited – Again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3:287–301. doi: 10.1002/hbm.460030404. [DOI] [Google Scholar]
- Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, Drotar D. Long-term attention problems in children with traumatic brain injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:574–584. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]