Abstract
Purpose of the Review
To highlight recent research on amino acid sensing and signaling and the role of amino acid transporters in the regulation of human skeletal muscle protein metabolism.
Recent Findings
The mechanisms that sense amino acid availability and activate mechanistic target of rapamycin complex 1 (mTORC1) signaling and protein synthesis are emerging, with multiple new proteins and intracellular amino acid sensors recently identified. Amino acid transporters have a role in the delivery of amino acids to these intracellular sensors and new findings provide further support for amino acid transporters as possible extracellular amino acid sensors. There is growing evidence in human skeletal muscle that amino acid transporter expression is dynamic and responsive to various stimuli, indicating amino acid transporters may have a unique role in the regulation of human skeletal muscle adaptation.
Summary
There is a clear need to further examine the role of amino acid transporters in human skeletal muscle and their link to cellular amino acid sensing and signaling in the control of protein metabolism. A better understanding of amino acid transport and transporters will allow us to optimize nutritional strategies to accelerate muscle health and improve outcomes for clinical populations.
Keywords: transceptor, mTORC1, protein synthesis, amino acid sensing and signaling
INTRODUCTION
Skeletal muscle is a very plastic tissue, capable of adapting to a variety of external and internal stimuli in a very specific manner [1]. For instance, it is well known that resistance exercise training produces dramatic increases in muscle size and strength, whereas chronic periods of inactivity result in muscle atrophy and reduced muscle function. The significance of muscle adaptation extends beyond changes in physical function, as muscle size is correlated with several clinically relevant outcomes, including rehabilitation time, hospital length of stay, and susceptibility to injury and illness [2,3]. The consequences of chronic, even sometimes intermittent, exposure to stimuli that result in the loss of muscle mass and function (i.e., inactivity, hospitalization, inflammation, malnutrition) become much more adverse in clinical populations and older adults whom are already at risk for muscle related dysfunction, physical dependence, and poor health outcomes [4]. Because muscle adaptation is largely governed by changes in muscle protein metabolism, a concerted effort has been made by several investigators to better understand the mechanisms that regulate muscle protein metabolism in an effort to identify therapeutic targets and thus maximize the effect of various strategies designed to increase, preserve, or restore muscle size and function.
An emerging role for amino acid transporters in the regulation of protein metabolism has evolved over recent years. Pioneering work by several research labs [5-11] have substantially increased our understanding of the mechanisms through which specific classes of amino acid transporters not only facilitate the movement of amino acids across a cell membrane, but also the mechanisms through which these transporters and the influx of amino acids into the cell may be linked to the regulation of protein metabolism (for reviews see [12,13]). These findings have provided, and continue to provide, a solid foundation from which to begin to identify the therapeutic importance of amino acid transport and transporters in the regulation of human skeletal muscle adaptation and will be critical to maximize therapeutic strategies to increase muscle health and physical function. In addition, more data are demonstrating a dynamic response in the expression levels of select amino acid transporters in human skeletal muscle following a variety of stimuli (Table 1).
Table 1.
Amino acid transporters implicated in the regulation of protein metabolism and a summary of the mRNA response of these transporters to anabolic stimuli in human skeletal muscle.
| Common Name | System; Description | Gene | Amino Acid Substrates | Amino Acid Ingestion |
Resistance Exercise |
Resistance Exercise + Amino Acids |
|||
|---|---|---|---|---|---|---|---|---|---|
| Young | Older | Young | Older | Young | Older | ||||
| LAT1 | System L; Large Neutral AA Transporter | SLC7A5 | His, Met, Leu, Ile, Val, Phe, Tyr, Trp, Gln | ↑ | ↑ | ↑ | ↑ * | ↑ ↑ | ↑ |
| SNAT2 | System A; Sodium Coupled AA Transporter | SLC38A2 | Gly, Pro, Ala, Ser, Cys, Gln, Asn, His, Met | ↑ | ↔ | ↔ | ↑ | ↑ ↑ | ↑ |
| PAT1 | System Imino; Proton Coupled AA Transporter | SLC36A1 | Pro, Gly, Ala | ↑ | ? | ↑ | ↑ |
↔
†
↑ |
↔ |
| CAT1 | System y+; Cationic AA Transporter | SLC7A1 | Arg, Lys, His | ? | ? | ↑ | ↑ | ↑ | ↑ |
Arrows represent an increase (↑) or no change (↔) from basal values for human skeletal muscle mRNA expression. Double arrows (↑↑) indicate a greater overall response in the specific amino acid transporter relative to resistance exercise without post exercise amino acid ingestion. Representative change data are from [37,40,44-46].
indicates greater response in older vs. young
? indicates no available data
indicates data from two studies with different findings.
Please see [51,52] for more detailed information regarding amino acid transporter nomenclature and transport systems.
Thus, the goal of this review is to highlight new findings regarding the mechanisms of amino acid sensing and signaling and the role of amino acid transporters in the regulation of muscle protein metabolism. We will then focus our discussion on data highlighting the responsiveness of select amino acid transporters to various stimuli in human skeletal muscle. For the specific mechanisms through which amino acid transporters function to transport amino acids, please refer to the following in depth reviews [12,21,22].
AMINO ACID SENSING AND SIGNALING MECHANISMS IN PROTEIN METABOLISM
A precise understanding of the mechanisms involved in regulating protein metabolism in response to amino acid availability is necessary to maximize our understanding of the role of amino acid transport and transporters as it relates to both the loss of muscle size and strength and the development of therapies for human skeletal muscle. In animal and human skeletal muscle an increase in amino acid availability regulates muscle protein metabolism largely through the stimulation of the mechanistic (a.k.a., mammalian) target of rapamycin complex 1 (mTORC1) signaling pathway [23-25]. Stimulation of the mTORC1 signaling pathway by amino acids results in an increase in the rate of muscle protein synthesis [23,26] and perhaps a reduction in autophagy [27,28]. In human skeletal muscle, this increase in muscle protein synthesis following amino acid ingestion lasts approximately 2-3 hours before returning to basal values [29]. Similarly, ingesting amino acids after exercise enhances muscle protein synthesis in human skeletal above that elicited independently by exercise or amino acid ingestion [30]. While the ability for amino acids to stimulate mTORC1 and muscle protein synthesis is well described, less is known about the specific mechanisms through which an increase in amino acid availability activates mTORC1.
Great strides have been made to begin to unravel some of these mechanisms, and work from the Sabatini laboratory has identified that mTORC1 activation by amino acids requires the recruitment of mTORC1 to the lysosomal surface where mTORC1's direct upstream activator, Rheb, is located [9,10]. This recruitment of mTORC1 to the lysosomal membrane is mediated by heterodimers of Rag GTPases [5,31]. In particular, increased amino acid availability stimulates a shift in the GDP and GTP loading of the Rag heterodimer, which facilitates the binding of the Rag heterodimer to mTORC1 and the recruitment of mTORC1 to the lysosomal surface [5]. The subsequent “docking” of mTORC1 to the lysosome appears to require a complex of proteins referred to as the ragulators [10]. The ragulators provide the anchoring mechanism between the Rag GTPase heterodimer bound to mTORC1 and the lysosomal membrane, which allows for the activation of mTORC1 [10]. More recent work has also identified a negative regulator of the recruitment of mTORC1 to the lysosomal surface. Specifically, when amino acid sufficiency is low a protein subcomplex referred to as GATOR1 (GTPase activating protein [GAP] activity toward Rags) has been shown to inactivate the Rag heterodimer complex, which inhibits the ability for the Rag GTPase heterodimer to recruit mTORC1 to the lysosomal surface. This inhibition of the Rag GTPase heterodimer by GATOR1 is removed by a second protein subcomplex, GATOR2, and removal of the inhibitory effects of GATOR1 by GATOR2 is required for amino acid induced mTORC1 activation [32].
Two intracellular amino acid sensing components have also recently been identified, leucyl-tRNA synthetase [33] and vacuolar H+ ATPase [9], that appear to have a role in mediating the amino acid-induced localization of mTORC1 to the lysosomal surface. The leucyl-tRNA synthetase appears to be sensitive to intracellular leucine, and in the presence of elevated leucine concentrations leucyl-tRNA synthetase seems to migrate to the lysosome where it facilitates the proper nucleotide loading of the Rag GTPase heterodimer complex [33], which (through the mechanisms described above) stimulates mTORC1 activity. The vacuolar H+ ATPase, on the other hand, is housed in the lysosomal membrane and has been identified as a critical component of the amino acid sensing machinery on the lysosomal surface. The vacuolar H+ ATPase appears to be sensitive to amino acid accumulation within the lysosome, and through direct interactions with the ragulator-Rag complex stimulates mTORC1 activation in the presence of increased amino acid concentrations within the lysosomal lumen [9]. In addition, overexpression of proton-assisted amino acid transporter (PAT) 1, an amino acid transporter found in the lysosomal membrane that exports amino acids from the lysosomal lumen into the cytosol, has been shown to inhibit mTORC1 stimulation in the presence of amino acids [9], further highlighting lumenal amino acid accumulation as an integral component of amino acid sensing and signaling to mTORC1. The presence of these intracellular amino acid sensing mechanisms and their link to mTORC1 activation highlight amino acid transport into the muscle cell is likely an important mechanism in the ability for amino acids to stimulate protein synthesis. Indeed, inhibiting the actions of specific amino acid transporters has been shown to inhibit mTORC1 signaling in the presence of elevated amino acids [8,34,35], however, future research is necessary to more precisely uncover the link between the role of specific amino acid transporters and these intracellular sensing mechanisms.
In addition to their role as transport systems to facilitate the delivery of amino acids to intracellular amino acid sensors, select amino acid transporters have been identified to have dual functions as “transceptors” [12], most notably sodium coupled neutral amino acid transporter 2 (SNAT2). The potential role of SNAT2 as an amino acid transceptor in mammalian cells was eloquently demonstrated in a recent study by Pinilla et al. [36]. These researchers incubated MCF-7 human breast cancer cells with Me-AIB, a competitive inhibitor of SNAT2 substrates, to diminish intracellular accumulation of SNAT2 substrates as well as branched chain amino acids (via L-type amino acid transporter 1 [LAT1] tertiary active transport that requires intracellular accumulation of SNAT2 substrates for the counter transport of branched chain amino acids into the cell [7]). While the diminished intracellular amino acid supply reduced cell proliferation, cell growth was still observed. Further, incubation with Me-AIB did result in increased S6K1 phosphorylation, a marker of mTORC1 activity, indicating that the intracellular accumulation of SNAT2 substrates are not required for mTORC1 stimulation. Instead, these data suggest that perhaps the binding of amino acids to SNAT2, or the conformational change of SNAT2 during amino acid influx, triggers a signaling cascade that stimulates mTORC1 activation, a process that may include SNAT2 binding to myosin light-chain kinase (MLCK) II [36]. Consequently, in addition to transporting amino acids across the cell membrane, SNAT2 (and likely other transporters) may also serve as sensing mechanisms to monitor changes in extracellular amino acid availability and perhaps prime the cell for a subsequent influx of amino acids.
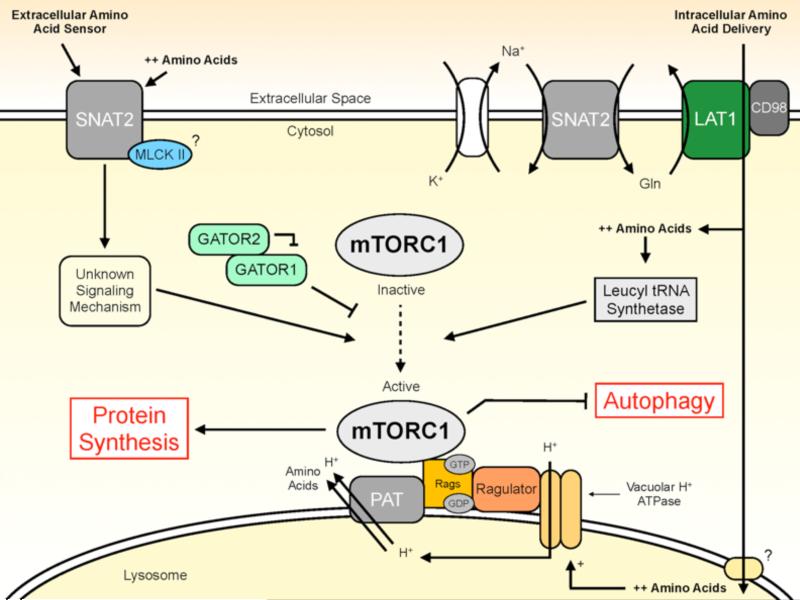
Although there appears to be some debate, the stimulatory effects of amino acids on mTORC1 signaling and protein metabolism are likely manifested in part through various mechanisms that respond to changes in both intracellular and extracellular amino acid availability [12,29]. Recent findings highlight that amino acid transporters could have an integral role in responding to changes in both intracellular and extracellular amino acid pools. As summarized in Figure 1, amino acid transporters likely have a pivotal role in the delivery of amino acids to intracellular sensing mechanisms and select amino acid transporters may have dual functions as extracellular amino acid sensors through transceptor signaling. In addition, PAT1 has been implicated in mTORC1 activation through a yet to be defined process that doesn't appear to require the actual transport of amino acids into the cell [37], but instead may be linked to the activation of mTORC1 at the lysosomal membrane [38]. Certainly more work is necessary to uncover additional amino acid sensing and signaling mechanisms and this research focus will only add to the emerging evidence suggesting amino acid transport and transporters have an important role in the regulation of protein metabolism.
Figure 1.
Schematic of the mechanisms regulating protein metabolism in response to increased amino acid availability and the role of amino acid transporters. GATOR, GTPase activating protein [GAP] activity toward Rags; Gln, glutamine; LAT1, system L amino acid transporter; MLCK II, myosin light-chain kinase II; mTORC1, mechanistic target of rapamycin complex 1; PAT1, proton-assisted amino acid transporter 1; RAGs, Ras-related GTPases; SNAT2, system A amino acid transporter.
THE EMERGING ROLE OF AMINO ACID TRANSPORTERS IN THE CONTROL OF HUMAN SKELETAL MUSCLE PROTEIN METABOLISM
Two of the most powerful stimulators of protein synthesis in human skeletal muscle are nutrient ingestion (e.g., protein/amino acids and insulin) and exercise. Indeed, it is well documented that both of these stimuli facilitate an increase in amino acid transport into the muscle, which provides both a stimulus (as described above) and the substrate for increased rates of protein synthesis. More recently, added focus has been given to explore and identify how various stimuli affect the expression of select amino acid transporters (Table 1) and what role the response of these amino acid transporters may have in the regulation of muscle protein metabolism and muscle adaptation.
Amino Acid Ingestion and Amino Acid Transporters in Skeletal Muscle
The first study to characterize amino acid transporter expression in human skeletal muscle following amino acid ingestion was performed in 2010 by Drummond et al. [18]. Concomitant with the increase in amino acid transport into the muscle, in a group of healthy young individuals we observed an increase in the skeletal muscle mRNA expression of select amino acid transporters. In particular, while the mRNA expression of many transporters were analyzed in that study, the most notable increases were observed in the mRNA expression of LAT1, SNAT2 and PAT1, which are amino acid transporters thought to have key roles in mTORC1 signaling and muscle protein synthesis/muscle growth [6,8,34,35,39]. Further, this study also indicated that the upregulation of these amino acid transporters may have occurred downstream of mTORC1 signaling, perhaps through activating transcription factor 4 (ATF4), which is a well described regulator of amino acid transporter expression that has been linked to mTORC1 signaling [11,40]. These findings indicate that the upregulation of amino acid transporters in this case serves as an important adaptive response to improve the intracellular delivery of amino acids into skeletal muscle.
More recently, we investigated the effect of short-term bed rest on the protein anabolic response of older adults to essential amino acid ingestion in an effort to obtain insight into the mechanisms contributing to muscle atrophy with inactivity [16]. We observed an impaired skeletal muscle protein synthesis response to essential amino acid ingestion following one week of bed rest, indicating that the loss of muscle size and strength during bed rest may be facilitated, in part, through an impaired muscle protein synthesis response to nutrients. Further, this impaired protein synthesis response was associated with a blunted response in mTORC1 signaling and amino acid transporter protein content. More recently, a similar blunted muscle protein synthesis response to protein ingestion was also observed in older adults following a 14 day period of reduced daily physical activity (step counts) [41]. Although amino acid transporters were not examined in that study, based on the collective findings of these two studies it is interesting to speculate that perhaps an inability for nutrient ingestion to elicit an increase in amino acid transporter content during inactivity may reduce the sensitivity of skeletal muscle to amino acid ingestion, and thus the impaired muscle protein synthesis response following inactivity may be manifested, in part, through alterations in amino acid transport and transporter function. Given the link between reduced physical activity and hospitalization in older adults [42,43], more work is necessary to better delineate the role of skeletal muscle amino acid transporters in this impaired muscle protein synthesis response to nutrients. Such insight could serve in the development of nutritional strategies to counter the deleterious effects of inactivity on muscle health and function in older adults.
Resistance Exercise and Amino Acid Transporters in Skeletal Muscle
The expression of amino acid transporters in human skeletal muscle has also been investigated in response to resistance exercise [14,15,17], and these studies demonstrate that resistance exercise increases the expression of select amino acid transporters in the skeletal muscle of both young and older individuals. In addition, similar to the time course of muscle protein synthesis, the upregulation of amino acid transporters following resistance exercise is much more sustained relative to the increase following amino acid ingestion [17,18], at least in young adults. This sustained upregulation of amino acid transporters following resistance exercise could serve to sensitize skeletal muscle to nutrients for a prolonged period of time [44]. Further, in a recent microarray analysis, skeletal muscle amino acid transport was identified as an important biological process related to muscle size and strength following 12 weeks of resistance exercise training. Specifically, in a relatively large cohort of young and older adults Raue et al. [45] determined that the skeletal muscle mRNA expression of various amino acid transporters in response to the first and last exercise bout of the 12 week training program was related to gains in muscle size and strength. This relationship between amino acid transporter expression and human skeletal muscle growth is supported by other recent in vivo models of mammalian muscle growth/development [46]. Collectively, there is growing evidence supporting a unique role for skeletal muscle amino acid transporters in the adaptive response to resistance exercise.
Although both young and older adults experience an increase in skeletal muscle amino acid transporter expression following resistance exercise, the mechanisms facilitating the increase in amino acid transporter expression may differ between young and older adults. In particular, in young adults the upregulation of skeletal muscle amino acid transporter expression is concomitant to an increase in mTORC1 signaling and nuclear ATF4 protein expression [17], in which ATF4 is a known regulator of amino acid transporter expression [11,40]. In contrast, older adults do not appear to experience the same increase in mTORC1 signaling and nuclear ATF4 protein expression following resistance exercise. Instead, an increase in the phosphorylated (Y705) nuclear protein expression of signal transducer and activator of transcription 3 (STAT3) is observed in older adults following resistance exercise [17]. STAT3 has been shown to be associated with the upregulation of SNAT2 in response to inflammatory markers [47], indicating that the upregulation of amino acid transporters following resistance exercise in older adults could be mediated through a stress response to the exercise bout. Further, this potential stress mediated upregulation of skeletal muscle amino acid transporters in response to resistance exercise may provide a maximal stimulus following acute exercise, as we have demonstrated that ingesting essential amino acids shortly following a bout of resistance exercise appears to enhance skeletal muscle amino acid transporter expression only in young adults [15].
CONCLUSIONS
A significant amount of work in recent years has begun to unravel the mechanisms through which cells sense amino acid availability and regulate protein synthesis (Figure 1). Amino acid transporters likely represent an important link in the ability for amino acids to stimulate cellular protein synthesis. Not only do amino acid transporters facilitate the delivery of amino acids to intracellular amino acid sensors, but more data are beginning to indicate that various amino acid transporters may have a dual “transceptor” function. There is also mounting evidence in human skeletal muscle demonstrating that the expression level of select amino acid transporters is highly dynamic and responsive to various stimuli (Table 1) and that changes in these amino acid transporters are associated with amino acid sensing, signaling, and muscle growth and atrophy. Thus, amino acid transporters represent a very important mechanism regulating changes in human skeletal muscle protein synthesis, and perhaps even a rate-limiting step in the process of amino acid induced stimulation of skeletal muscle protein metabolism. Certainly, in human skeletal muscle this research focus remains in its infancy, however, continuing to build upon the strong mechanistic foundation derived from various cell and animal models will provide tremendous insight into the role of amino acid transport and transporters in amino acid sensing and signaling and the regulation of human skeletal muscle protein metabolism. This translational research effort should provide a basis to maximize nutritional therapeutic strategies aimed to improve skeletal muscle health and physical function.
KEY POINTS.
Multiple proteins, protein complexes, and intracellular amino acid sensors appear necessary to facilitate the activation of mTORC1 in the presence of elevated intracellular amino acid availability.
Amino acid transporters, in general, may have dual roles both as amino acid delivery systems to intracellular amino acid sensors and as extracellular amino acid sensors/receptors.
The expression of amino acid transporters in human skeletal muscle is dynamic and responsive to a variety of stimuli, and changes in human skeletal muscle amino acid transporter expression has been associated with changes in muscle size.
There is a need for more translational research combining mechanistic research efforts with in vivo human studies to accelerate the development of nutritional therapeutic strategies to improve muscle health and function in older adults and clinical populations.
ACKNOWLEGDMENTS
This work was supported by NIH/National Institute of Arthritis and Musculoskeletal and Skin Disease R01AR049877, NIH/National Institute on Aging P30AG024832 and R01AG030070, and NIH Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachy LD, editor. Handbook of Physiology: Skeletal Muscle. American Physiological Society; Bethesda, MD: 1983. pp. 555–630. [Google Scholar]
- 2.Suetta C, Magnusson SP, Rosted A, et al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients--a controlled, randomized study. J Am Geriatr Soc. 2004;52:2016–2022. doi: 10.1111/j.1532-5415.2004.52557.x. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 4.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heublein S, Kazi S, Ogmundsdottir MH, et al. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird FE, Bett KJ, MacLean C, et al. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297:E822–829. doi: 10.1152/ajpendo.00330.2009. [DOI] [PubMed] [Google Scholar]
- 8.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoncu R, Bar-Peled L, Efeyan A, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancak Y, Bar-Peled L, Zoncu R, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem. 2007;282:16744–16753. doi: 10.1074/jbc.M610510200. [DOI] [PubMed] [Google Scholar]
- 12.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends in molecular medicine. 2012;18:524–533. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churchward-Venne TA, Burd NA, Mitchell CJ, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Dickinson JM, Drummond MJ, Coben JR, et al. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr. 2013;32:273–280. doi: 10.1016/j.clnu.2012.07.009. [Recent work highlighting that aging may alter the response of amino acid transporters to an anabolic stimulus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Drummond MJ, Dickinson JM, Fry CS, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–1122. doi: 10.1152/ajpendo.00603.2011. [Important work demonstrating that muscle atrophy following bed rest is associated with an impaired amino acid transporter response to essential amino acid ingestion.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond MJ, Fry CS, Glynn EL, et al. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond MJ, Glynn EL, Fry CS, et al. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poncet N, Taylor PM. The role of amino acid transporters in nutrition. Curr Opin Clin Nutr Metab Care. 2013;16:57–65. doi: 10.1097/MCO.0b013e32835a885c. [DOI] [PubMed] [Google Scholar]
- 21.Goberdhan DC, Ogmundsdottir MH, Kazi S, et al. Amino acid sensing and mTOR regulation: inside or out? Biochem Soc Trans. 2009;37:248–252. doi: 10.1042/BST0370248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor PM. Amino acid transporters: eminences grises of nutrient signalling mechanisms? Biochem Soc Trans. 2009;37:237–241. doi: 10.1042/BST0370237. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson JM, Fry CS, Drummond MJ, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 25.Suryawan A, Jeyapalan AS, Orellana RA, et al. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita S, Dreyer HC, Drummond MJ, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Molecular biology of the cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glynn EL, Fry CS, Drummond MJ, et al. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R533–540. doi: 10.1152/ajpregu.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glynn EL, Fry CS, Drummond MJ, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970–1976. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72:551S–557S. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]
- 31.Kim E, Goraksha-Hicks P, Li L, et al. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Bar-Peled L, Chantranupong L, Cherniack AD, et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [This study identified a new octomeric protein complex, referred to as GATOR, as an important complex regulation Rag GTPase activity and mTORC1 activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Han JM, Jeong SJ, Park MC, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [Important work describing the role of Leucyl-tRNA synthetase as in intracellular amino acid sensor implicated in activating mTORC1.] [DOI] [PubMed] [Google Scholar]
- 34.Evans K, Nasim Z, Brown J, et al. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol. 2007;18:1426–1436. doi: 10.1681/ASN.2006091014. [DOI] [PubMed] [Google Scholar]
- 35.Hyde R, Hajduch E, Powell DJ, et al. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 36.Pinilla J, Aledo JC, Cwiklinski E, et al. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci (Elite Ed) 2011;3:1289–1299. doi: 10.2741/e332. [DOI] [PubMed] [Google Scholar]
- 37.Goberdhan DC, Meredith D, Boyd CA, et al. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development. 2005;132:2365–2375. doi: 10.1242/dev.01821. [DOI] [PubMed] [Google Scholar]
- 38*.Ogmundsdottir MH, Heublein S, Kazi S, et al. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One. 2012;7:e36616. doi: 10.1371/journal.pone.0036616. [This work presents additional insight into the many roles that various amino acid transporters might have in the regulation of protein metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christie GR, Hajduch E, Hundal HS, et al. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem. 2002;277:9952–9957. doi: 10.1074/jbc.M107694200. [DOI] [PubMed] [Google Scholar]
- 40*.Luo JQ, Chen DW, Yu B. Upregulation of amino acid transporter expression induced by L-leucine availability in L6 myotubes is associated with ATF4 signaling through mTORC1-dependent mechanism. Nutrition. 2013;29:284–290. doi: 10.1016/j.nut.2012.05.008. [Recent work highlighting the role of ATF as a mechanism facilitating the upregulation of amino acid transporters in muscle cells.] [DOI] [PubMed] [Google Scholar]
- 41.Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98:2604–2612. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 42.Fisher SR, Kuo YF, Graham JE, et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Archives of internal medicine. 2010;170:1942–1943. doi: 10.1001/archinternmed.2010.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher SR, Kuo YF, Sharma G, et al. Mobility After Hospital Discharge as a Marker for 30-Day Readmission. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls252. In Press. [Epub ahead of print] doi:10.1093/gerona/gls1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burd NA, West DW, Moore DR, et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 45*.Raue U, Trappe TA, Estrem ST, et al. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol. 2012;112:1625–1636. doi: 10.1152/japplphysiol.00435.2011. [This microarray work in human skeletal muscle identified amino acid transport as an important biological process related to muscle growth following 12 weeks of resistance exercise training.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Suryawan A, Nguyen HV, Almonaci RD, et al. Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids. 2012 doi: 10.1007/s00726-012-1326-7. In Press. [Epub ahead of print] doi:10.1007/s00726-012-1326-7. [Additional data highlighting an important role for amino acid transporters in regulating mammalian muscle growth processes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297:C1228–1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]