Abstract
Mutations in the GBA1 gene, encoding the enzyme glucocerebrosidase, cause the lysosomal storage disorder, Gaucher disease (GD), and are associated with the development of Parkinson's disease (PD) and other Lewy body disorders. Interestingly, GBA1 variants are the most common genetic risk factor associated with PD. While clinical studies argue a strong case towards a link between GBA1 mutations and the development of PD, mechanistic insights have been lacking. Here, we review recent findings that have provided some biochemical evidence to bridge this relationship, focusing on the molecular link between two proteins, α-synuclein and glucocerebrosidase, involved in PD and GD, respectively.
Keywords: Gaucher disease, Parkinson's disease, lysosome, amyloid
Introduction
Glucocerebrosidase (abbreviated GCase), a 497-residue lysosomal hydrolase, cleaves the β-glucosyl linkage of glucosylceramide (GlcCer) to glucose and ceramide [1]. To date, over 300 mutations (point mutations, deletions, insertions, etc.) have been identified that result in the loss of GCase activity, either by insufficient protein translation, protein misfolding, or incorrect trafficking to the lysosome, resulting in the buildup of the substrate GlcCer [2, 3]. The relationship between the type of mutation in GCase and the clinical phenotypes are unclear. Clinical observations reveal three types of GD, non-neuronopathic (type 1), the most common, and neuronopathic (type 2 and 3) disease [1]. In non-neuronopathic cases, most organs and tissues are affected, whereas in neuronopathic types, the nervous system is implicated. The type and symptoms can vary widely amongst individuals ranging from enlarged liver and spleen to brain problems such as seizures and dementia. Enzyme replacement therapy involving periodic administration of recombinant GCase is the only effective treatment in type 1 patients [4].
PD is an age-related degenerative disorder of the central nervous system that results in neuron death causing symptoms such as tremor, stiffness, slowness, and difficulty with posture [5]. One hallmark of the disease is the presence of cytoplasmic inclusions called Lewy bodies (LBs), enriched in insoluble fibrillar α-synuclein (α-syn) aggregates and lipid deposits that develop in surviving neurons [6, 7]. Five missense α-syn mutations, A30P, E46K, H50Q, G51D and A53T [8–12] and gene duplications or triplications [13, 14] are strongly associated with early-onset PD, although the specific pathogenic role of α-syn remains to be determined. Recent clinical and genetic studies now point to a new association between mutations in the GBA1 gene and the development of PD [15–17]. Since α-syn is predominantly degraded by lysosomes, in part by chaperone-mediated autophagy [18] and GCase is a lysosome hydrolase, a connection between these two proteins and their associated diseases is intriguing.
Relationship between α-Syn and GCase
The relevance of GD to PD comes from the observation that GD patients and heterozygous GBA1 mutation carriers are at an increased risk of developing PD [19]. In fact, clinical studies have shown that PD patients are over five times more likely to carry a mutation in the GBA1 gene [15]. Conversely, GD and GBA1 mutation carriers have an increased risk of developing PD, although the vast majority of such individuals do not get the disease. Post-mortem analysis of GD patients and carriers with PD symptoms harbor LBs enriched with mutant GCase and α-syn [20]. Moreover, GBA1 carriers had more corticol LBs compared to those without mutations. Analysis of PD brains with GBA1 mutations showed a significant reduction in GCase activity in the substantia nigra (SN), a site with greatest α-syn concentrations in PD [21]. This reduction in activity was also accompanied by a decreased level of GCase. Interestingly, GCase activity was also reduced in the SN of sporadic PD brains [21].
Based on clinical and genetic evidence, two models have been proposed for the observed association, a GBA1 mutant mediated loss-of-function and a toxic gain-of-function. Several GBA mutants (e.g. N370S and L444P) overexpressed in neuronal cells displayed elevated levels of endogenous α-syn in a time- and dose-dependent manner [22]. Here, the levels of α-syn did not correlate with GCase enzyme activity, but were dependent on amount of transfected mutant cDNA and protein synthesized [22]. These data would imply a toxic gain-of-function mechanism, which is independent of GCase activity. In contrast, wild-type GCase knockdown experiments in primary neurons using shRNA by lentiviral infection caused a dramatic increase in the levels of oligomeric α-syn [23]. Furthermore, enzymatic inhibition of GCase by conduritol B epoxide (CBE) in human neuroblastoma cells and the SN of mice revealed elevated levels of α-syn, supporting a loss-of-function mechanism [24]. However, this observation has been disputed, since treatment of CBE in another in vivo model does not increase α-syn protein levels [25].
Several GD mouse models that have identical mutants to those found in humans have been studied to look at the effect on α-syn levels and pathology [26]. Interestingly, a heterozygous Gaucher (D409V) mouse displaying a ~50% reduction in GCase activity showed accumulation of α-syn aggregates, but more importantly, no change in substrate levels in the brain were apparent, implying a toxic gain-of-function [27]. This result is analogous to human carriers of heterozygous GBA1 mutations that show no consistent evidence of substrate accumulation. Based on tissue culture and animal models, it is conceivable that either mechanism plays a role in PD susceptibility, or in combination may exert a more pronounced age-related effect.
Studies demonstrating a reciprocal relationship between GCase and α-syn have paved the way in finding treatments for both GD and GBA1 mutation carriers with PD. Interests outside of enzyme replacement therapy have been strategies to increase GCase trafficking and activity. For example, it was demonstrated recently that viral vector-mediated increase in wild-type GCase levels can reverse PD-related features in a GD mouse model [28, 29]. Here, GCase delivery into the brains of mice reduces the accumulation of both substrate and α-syn. Furthermore, overexpression of GCase in A53T α-syn mice reduced the levels of α-syn. These studies suggest that enhancing GCase activity is a potential therapeutic strategy for synucleinopathies with or without GBA1 mutations. Other strategies include small molecule chaperones such as Ambroxol [30] and isofagomine [31]. These competitive inhibitors of GCase bind to the misfolded protein, correcting folding to permit passage through the ER to the lysosome. A more recent report highlights a new class of non-inhibitory chaperones, pyrazolpyrimidines [32], which were shown to offer an advantage over all other small molecule chaperones in not inhibiting GCase activity. Another complimentary strategy is the use of histone deacetylase inhibitors, which are known to affect the heat shock gene response by limiting the deacetylation of the chaperone HSP90, causing a reduced recognition of misfolded mutant GCase [33, 34].
Mechanistic Insights into GBA1 Associated PD
A mechanistic link between GBA1 mutations and α-syn has been the subject of recent investigations, which have provided some new perspectives. One possible scenario is misfolded and accumulated GCase leads to insufficient α-syn degradation by either disrupting the ubiquitin/proteosomal pathway or causing impairment of lysosome function, resulting in α-syn aggregation. Alternatively, misfolded GCase is degraded, leading to loss of enzyme activity and buildup of substrate, influencing lipid homeostasis. This in turn, could lead to disruption of α-syn membrane binding and enhance aggregation. While both scenarios have gained community support, each has its own pitfalls. For example, some PD patients have GBA1 null mutations, a finding in conflict with a gain-of-function mechanism. A strong argument against loss-of-function is that most GD patients do not get PD, despite having low levels of GCase.
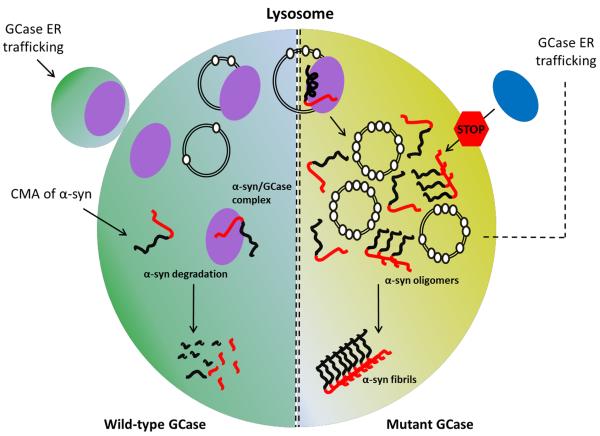
One study discovered a physical interaction existed between recombinant α-syn and GCase under lysosomal-like conditions [35]. Here, fluorescently labeled α-syn at the C-terminus (residue 136), when co-incubated GCase with at pH 5.5, showed significant spectral changes, indicating binding of the C-terminal region of α-syn to GCase. Dissociation constants ranging from 1–20 μM were reported where protein/enzyme complex formation is modulated by the ionic strength of the solution. This interaction was abolished under physiological buffer conditions of pH 7.4. Nuclear magnetic resonance (NMR) spectroscopy revealed residues 118–137 on α-syn as the site of GCase interaction. Immunoprecipitation results of brain samples taken from PD patients showed endogenous GCase and α-syn coimunoprecipitated at pH 5.5 but not at 7.4, in accord with fluorescence and NMR data obtained from recombinant proteins. Additionally, immunofluorescence imaging of neuronal cells over-expressing GCase and α-syn showed co-localization of the proteins in the lysosome. These compelling results offer new mechanistic insights into the connection between these two proteins, where one could envisage an interaction that has a beneficial consequence under normal physiological conditions (Figure 1). A further extension to these findings showed that a common GD-related mutant, N370S, which has reduced enzymatic activity, had reduced affinity for α-syn, suggesting a weakened interaction could perturb the system, thereby promoting α-syn accumulation (Figure 1).
Figure 1. Proposed molecular links between α-syn and GCase.
In normal functioning lysosomes (left, green), wild-type GCase (purple) enters the lysosome via vesicle transport from the endoplasmic reticulum (ER)/Golgi apparatus. α-Syn is trafficked to the lysosome by chaperone mediated autophagy (CMA) and translocated across the membrane. Upon lysosomal entry, GCase hydrolyses the substrate, GlcCer (open white circles), on intralysosomal vesicles, while α-syn is targeted for proteolytic degradation. At the same time, an α-syn/GCase complex may form, a result from the physical interaction of the C-terminal domain of α-syn and GCase. This interaction could have a beneficial effect by promoting lysosomal degradation of α-syn, or inhibiting its aggregation propensity. In compromised lysosomes (right, yellow) with mutant GBA1 (blue), several scenarios may occur. GCase mutations (blue), resulting in its decrease in activity or levels, cause the buildup of GlcCer (note more open circles) on intralysosomal vesicles, which in turn accelerate and stabilize α-syn aggregates en route to fibril formation. Here, one might infer that this type of mechanism could limit α-syn degradation, while stabilized oligomers compromise the integrity of the lysosome. Additionally, α-syn could accumulate in the ER/Golgi and block the trafficking of residual GCase that might otherwise reach the lysosome (not shown). This in turn would exacerbate the situation by further increasing substrate levels and stabilizing α-syn oligomers, resulting in further inhibition of GCase trafficking. Alternatively, the buildup of intralysosomal vesicles and α-syn could strengthen a membrane bound GCase/α-syn interaction, which would have an inhibitory effect on residual GCase, resulting in further loss of activity.
A follow up study extended this physical interaction by considering how membranes would influence the α-syn/GCase complex, since membranes are pertinent for both enzyme activity and α-syn conformation [36, 37]. Here, comparable affinities were shown between the two proteins on and off the membrane; however, when membrane associated, this physical interaction involved a larger α-syn region. This shift included residues that were N-terminal to the binding region seen in membrane-free solution. Interestingly, α-syn was shown to act as a potent GCase inhibitor only when membrane bound, adopting α-helical structure, with a reported IC50 in the submicromolar range. This observation offers a mechanistic extension into the physical connection of these two proteins. Mutations that decrease GCase levels or weaken the α-syn interaction would increase the levels of α-syn and GlcCer and thereby may facilitate α-syn/GCase complex formation on the membrane, which would in turn lead to a secondary loss in GCase activity through α-syn inhibition (Figure 1). While this physical interaction offers a new perspective, other pathological factors are likely involved, since only a small fraction of GD patients and carriers develop PD.
A different mechanistic connection between these two proteins was recently reported [23]. Here, it was shown that depletion of enzyme in primary cultures and human iPS neurons compromised lysosomal protein degradation, leading to an increase in α-syn aggregation levels. Biochemical analysis revealed an increase in oligomeric α-syn in GCase depleted neurons, suggesting this may be a fundamental consequence of GBA1 mutations. In vitro analysis using recombinant monomeric α-syn showed that GlcCer affected its aggregation propensity, causing a prolonged lag phase of fibril growth only at acidic pH. Although ill-defined in this study, the authors speculate that under these conditions, pre-fibrillar oligomeric species are stabilized, which in turn may be an important step in the pathology of the disease (Figure 1). In addition, it was shown that overexpressing α-syn inhibits intracellular trafficking and lysosomal activity of endogenous GCase in neurons. These data imply that decreased wild-type GCase activity may influence the development of sporadic synucleinopathies.
Collectively, the authors propose a positive feedback loop mechanism where GCase depletion in the lysosome causes buildup of the substrate GlcCer, which in turn stabilizes formation of toxic α-syn oligomers. The buildup of α-syn further blocks the ER-golgi trafficking of GCase leading to further GlcCer accumulation and α-syn aggregation (Figure 1).
Concluding Remarks
While mechanistic insights are emerging into the connection between α-syn and GCase dysfunction, further data are warranted to support or refute these notions. Furthermore, it is known that other lysosomal proteins such as lysosomal integral membrane protein type-2 (LIMP-2) [38, 39] and Saposin C (SapC) [40], which were not discussed in this review, play a role in GCase trafficking and enzymatic activity, respectively. For example, SapC, an essential activator for the hydrolysis of GlcCer by GCase in lysosomes, is believed to physically associate with both GCase and the phospholipid membrane [40]. Interestingly, SapC levels are increased in the spleens of patients with GD, possibly to compensate for loss in GCase protein levels by raising residual GCase activity. Furthermore, SapC deficiency produces clinical phenotypes similar to GD, despite normal GCase levels [40]. As for LIMP-2, which is a specific binding partner of GCase, transporting it to the lysosome from the trans-golgi network, mutations in the gene are implicated in myoclonic epilepsy which is part of the GD phenotypic variation [38, 41]. On a final note, the protease, cathepsin D (CatD), has been identified as the main lysosomal enzyme to degrade α-syn [42], resulting in C-terminal truncations that have been identified in isolated lysosomes [42]. Therefore, one might speculate that up-regulating CatD may alleviate some of the symptoms associated with PD and other synucleinopathies. These data imply that all three proteins should be considered as modifiers in GD and PD. Particularly, the interplay between them may be important, as patients with identical GBA1 mutations display different phenotypes. Clearly, future investigations are needed to evaluate their roles in GD and PD progression.
Acknowledgments
Funding This work was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute.
Abbreviations used
- GD
Gaucher disease
- PD
Parkinson's disease
- GCase
glucocerebrosidase
- Glcer
glucosylceramide
- LBs
Lewy bodies
- α-syn
- SN
substantia nigra
- CBE
conduritol B epoxide
- LIMP-2
lysosomal integral membrane protein-2
- SapC
saposin C
- CatD
Cathepsin D
References
- 1.Butters TD. Gaucher disease. Curr. Opin. Chem. Biol. 2007;11:412–418. doi: 10.1016/j.cbpa.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: Mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum. Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 3.Grabowski GA. Gaucher disease: Gene frequencies and genotype/phenotype correlations. Genet. Test. 1997;1:5–12. doi: 10.1089/gte.1997.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski GA. Lysosomal storage disease 1 - phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee VMY, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological α-synuclein: New targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Uversky VN, Eliezer D. Biophysics of Parkinson's disease: Structure and aggregation of α-synuclein. Curr. Protein Pept. Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part ii: α-Synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 8.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, DiIorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 9.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa EG, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 10.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel α-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, Verny C, Brice A, French Parkinsons Dis Genet S. G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 2013;73:459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 12.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nature Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 13.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. α-Synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 14.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841–841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 15.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang NY, Lee YN, Lee HJ, Kim YS, Lee SJ. Glucocerebrosidase, a new player changing the old rules in Lewy body diseases. Biol. Chem. 2013;394:807–818. doi: 10.1515/hsz-2012-0322. [DOI] [PubMed] [Google Scholar]
- 18.Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of α-synuclein in vivo. J. Biol. Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol. Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Wong KD, Sidransky E, Verma A, Mixon TH, Sandberg GD, Wakefield LK, Morrison A, Lwin A, Colegial C, Allman JM, Schiffmann R. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Gegg ME, Burke D, Heales SJR, Cooper JM, Hardy J, Wood NW, Schapira AHV. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen V, Sardi P, Ng J, Xu YH, Sun Y, Tomlinson JJ, Kolodziej P, Kahn I, Saftig P, Woulfe J, Rochet JC, Glicksman MA, Cheng SH, Grabowski GA, Shihabuddin LS, Schlossmacher MG. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 23.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning-Bog AB, Schule B, Langston JW. α-Synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: A biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30:1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Dermentzaki G, Dimitriou E, Xilouri M, Michelakakis H, Stefanis L. Loss of β-glucocerebrosidase activity does not affect α-synuclein levels or lysosomal function in neuronal cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sardi SP, Singh P, Cheng SH, Shihabuddin LS, Schlossmacher MG. Mutant GBA1 expression and synucleinopathy risk: First insights from cellular and mouse models. Neurodegener. Dis. 2012;10:195–202. doi: 10.1159/000335038. [DOI] [PubMed] [Google Scholar]
- 27.Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li LY, Stanek LM, Passini MA, Grabowski GA, Schlossmacher MG, Sidman RL, Cheng SH, Shihabuddin LS. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sardi SP, Clarke J, Viel C, Chan M, Tamsett TJ, Treleaven CM, Bu J, Sweet L, Passini MA, Dodge JC, Yu WH, Sidman RL, Cheng SH, Shihabuddin LS. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schapira AHV, Gegg ME. Glucocerebrosidase in the pathogenesis and treatment of Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3214–3215. doi: 10.1073/pnas.1300822110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendikov-Bar I, Maor G, Filocamo M, Horowitz M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol. Dis. 2013;50:141–145. doi: 10.1016/j.bcmd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Liou B, Xu YH, Quinn B, Zhang WJ, Hamler R, Setchell KDR, Grabowski GA. Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease. J. Biol. Chem. 2012;287:4275–4287. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patnaik S, Zheng W, Choi JH, Motabar O, Southall N, Westbroek W, Lea WA, Velayati A, Goldin E, Sidransky E, Leister W, Marugan JJ. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J. Med. Chem. 2012;55:5734–5748. doi: 10.1021/jm300063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CZ, Rahimpour S, Lu J, Pacak K, Ikejiri B, Brady RO, Zhuang ZP. Histone deacetylase inhibitors increase glucocerebrosidase activity in Gaucher disease by modulation of molecular chaperones. Proc. Natl. Acad. Sci. U. S. A. 2013;110:966–971. doi: 10.1073/pnas.1221046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady RO, Yang CZ, Zhuang ZP. An innovative approach to the treatment of Gaucher disease and possibly other metabolic disorders of the brain. J. Inherit. Metab. Dis. 2013;36:451–454. doi: 10.1007/s10545-012-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, Sidransky E, Lee JC. α-Synuclein interacts with glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J. Biol. Chem. 2011;286:28080–28088. doi: 10.1074/jbc.M111.237859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound α-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol. Genet. Metab. 2013;108:56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cookson MR. A feedforward loop links Gaucher and Parkinson's diseases? Cell. 2011;146:9–11. doi: 10.1016/j.cell.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin XY, Brondyk W, Van Patten S, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Velayati A, DePaolo J, Gupta N, Choi JH, Moaven N, Westbroek W, Goker-Alpan O, Goldin E, Stubblefield BK, Kolodny E, Tayebi N, Sidransky E. A mutation in SCARB2 is a modifier in Gaucher disease. Hum. Mutat. 2011;32:1232–1238. doi: 10.1002/humu.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamargo RJ, Velayati A, Goldin E, Sidransky E. The role of saposin C in Gaucher disease. Mol. Genet. Metab. 2012;106:257–263. doi: 10.1016/j.ymgme.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellen S, Gupta N, Velayati A, Choi J, Stubblefield B, Sidransky E, Tayebi N. Are mutations in LIMP-2 associated with myoclonic epilepsy in patients with Gaucher disease? Mol. Genet. Metab. 2010;99:S17–S17. [Google Scholar]
- 42.Sevlever D, Jiang PZ, Yen SHC. Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]