Abstract
The dopamine D1 receptor–D3 receptor (D1R-D3R) heteromer is being considered as a potential therapeutic target for neuropsychiatric disorders. Previous studies suggested that this heteromer could be involved in the ability of D3R agonists to potentiate locomotor activation induced by D1R agonists. It has also been postulated that its overexpression plays a role in L-dopa–induced dyskinesia and in drug addiction. However, little is known about its biochemical properties. By combining bioluminescence resonance energy transfer, bimolecular complementation techniques, and cell-signaling experiments in transfected cells, evidence was obtained for a tetrameric stoichiometry of the D1R–D3R heteromer, constituted by two interacting D1R and D3R homodimers coupled to Gs and Gi proteins, respectively. Coactivation of both receptors led to the canonical negative interaction at the level of adenylyl cyclase signaling, to a strong recruitment of β-arrestin-1, and to a positive cross talk of D1R and D3R agonists at the level of mitogen-activated protein kinase (MAPK) signaling. Furthermore, D1R or D3R antagonists counteracted β-arrestin-1 recruitment and MAPK activation induced by D3R and D1R agonists, respectively (cross-antagonism). Positive cross talk and cross-antagonism at the MAPK level were counteracted by specific synthetic peptides with amino acid sequences corresponding to D1R transmembrane (TM) domains TM5 and TM6, which also selectively modified the quaternary structure of the D1R-D3R heteromer, as demonstrated by complementation of hemiproteins of yellow fluorescence protein fused to D1R and D3R. These results demonstrate functional selectivity of allosteric modulations within the D1R-D3R heteromer, which can be involved with the reported behavioral synergism of D1R and D3R agonists.
Introduction
Most evidence indicates that, as for family C G protein–coupled receptors (GPCRs), family A GPCRs can form homodimers and heteromers. Homodimers seem to be a predominant species with potential dynamic formation of higher-order oligomers, particularly tetramers (Milligan, 2013; Ferré et al., 2014). Although monomeric GPCRs can activate G proteins, the pentameric structure constituted by one GPCR homodimer and one heterotrimeric G protein may provide a main functional unit at the plasma membrane, and oligomeric entities can be viewed as multiples of dimers (Ferré et al., 2014). It still needs to be resolved whether GPCR heteromers are preferentially heterodimers or whether they are mostly constituted by heteromers of homodimers. Allosteric mechanisms determine a multiplicity of possible unique pharmacological properties of GPCR homomers and heteromers. Some general mechanisms seem to apply, particularly at the level of ligand-binding properties (Ferré et al., 2014). Furthermore, in addition to ligand-binding properties, unique properties for each GPCR oligomer emerge in relation to different intrinsic efficacy of ligands for different signaling pathways (functional selectivity) (Ferré et al., 2014). Previous studies have provided evidence for the expression of dopamine D1 receptor (D1R) and D3 receptor (D3R) heteromers in mammalian transfected cells and suggested some biochemical findings are related to D1R-D3R receptor oligomerization. Those findings include a D3R agonist-mediated increase in the affinity of D1R agonists and a potentiation of D1R agonist-mediated signaling trough adenylyl cyclase (Fiorentini et al., 2008; Marcellino et al., 2008). It was hypothesized that these receptor-receptor interactions could underlie some behavioral findings, such as the selective synergistic locomotor activation of D1R and D3R agonists observed in reserpinized mice (Marcellino et al., 2008).
Demonstration of the functional significance of receptors heteromers is becoming an important goal in GPCR research. One main reason is their possible use as targets for drug development, because of their unique biochemical properties. Molecular or chemical tools that destabilize the quaternary structure of the heteromer can be used to ascertain a biochemical property of the GPCR heteromer (Ferré et al., 2009, 2014). This can be achieved by introducing mutations of key determinant residues at the oligomerization interfaces or using competing peptides with the sequence of specific receptor domains putatively involved in receptor oligomerization (Hebert et al., 1996; Baneres and Parello, 2003; Azdad et al., 2009; Pei et al., 2010; He et al., 2011).
In the present study, evidence was obtained for a minimal tetrameric stoichiometry of the heteromer, comprised of D1R and D3R homodimers able to couple to Gs and Gi proteins, respectively. By using selective peptides with the sequence of specific transmembrane domains (TM) of the D1R, we were able to demonstrate ligand-induced allosteric interactions in the D1R-D3R heteromer (positive cross talk and cross-antagonism) that constitute specific biochemical characteristics of the D1R-D3R heteromer. These allosteric interactions selectively modulated mitogen-activated protein kinase (MAPK) signaling and were also observed at the level of β-arrestin-1 recruitment, but not at the level of G protein coupling or adenylyl cyclase signaling. The results demonstrate the existence of functional selectivity of allosteric modulations within the D1R-D3R heteromer. These allosteric modulations can have implications for the treatment of several neuropsychiatric disorders, because the D1R-D3R heteromer is being considered as a target for drug development in Parkinson’s disease and drug addiction (Fiorentini et al., 2010; Ferré et al., 2010, 2014).
Materials and Methods
CODA-RET Assay.
The cDNAs for human D1R, D3R, and Gαs short were obtained from www.cdna.org. D1R and D3R were tagged with signal peptide (Guo et al., 2003), followed by a Myc epitope tag for D1R (SM hereafter) or Flag epitope tag for D3R (SF hereafter) using standard molecular biology procedures. The cDNAs encoding full-length Renilla luciferase 8 (Rluc; provided by S. Gambhir, Standford University, Standford, CA) or hemitruncated proteins corresponding to fragments L1 (amino acids 1–229) or L2 (amino acids 230–311) were fused in frame C terminus of SM-D1R or SF-D3R (in the case of SF-D3R following a linker sequence between the receptor and the luciferase) in the pcDNA3.1 vector. The following human G protein constructs were used: Gαi1-mVenus with mVenus inserted at position 91, Gαs short-mVenus (Gαss-mVenus) with mVenus inserted at position 154, untagged Gβ1, and untagged Gγ2. All the constructs were confirmed by sequencing analysis. Several constructs were shared by J. Javitch (Columbia University, New York, NY; D1R Rluc split constructs), C. Gales at INSERM (Toulouse, France; Gαi1 construct), and N. Lambert (Georgia Regents University, Augusta, GA; Gαss construct). A constant amount of plasmid cDNA (15 μg) was transfected into human embryonic kidney (HEK) cells (HEK-293) using polyethylenimine (PEI; Sigma-Aldrich, St. Louis, MO) in a 1 to 3 ratio in 10-cm dishes. Cells were maintained in culture with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. The transfected amount and ratio among the receptor-L1, receptor-L2, Gα, Gβ1, and Gγ2 were optimized by testing various ratios of plasmids encoding the different sensors. Experiments were performed ∼48 hours post-transfection.
Complemented donor-acceptor resonance energy transfer (CODA-RET) uses a Gα protein fused to yellow fluorescent protein variant (mVenus) as an acceptor for energy transfer from protein fused to Rluc and was measured by a bioluminescence resonance energy transfer (BRET) process, as previously described (Guo et al., 2008). As shown in the cartoons from Fig. 1, bimolecular complementation of two different receptor-fused hemiproteins corresponding to the N-teminal and the C-terminal domains of Rluc was used as BRET donor. RET took place between complemented luciferase complex and Gα-mVenus. Receptor ligand-induced changes in BRET were measured. Briefly, cells were harvested, washed, and resuspended in phosphate-buffered saline. Approximately 200,000 cells/well were distributed in 96-well plates, and 5 μM coelenterazine H (substrate for luciferase) was added to each well. One minute after addition of coelenterazine H, ligands were added to each well. Antagonists were added 15 minutes before the addition of agonist. The fluorescence of the acceptor was quantified (excitation at 500 nm and emission at 540 nm for 1-second recording) 2 minutes after a ligand was added in a Mithras LB940 (Berthold Technologies, Bad Wilbad, Germany). In parallel, the BRET signal from the same batch of cells was determined as the ratio of the light emitted by mVenus (510–540 nm) over that emitted by Rluc8 (485 nm). Results are expressed as the BRET change produced by the corresponding drug minus BRET in the absence of the drug. Data and statistical analysis was performed with Prism 5 (GraphPad Software, La Jolla, CA).
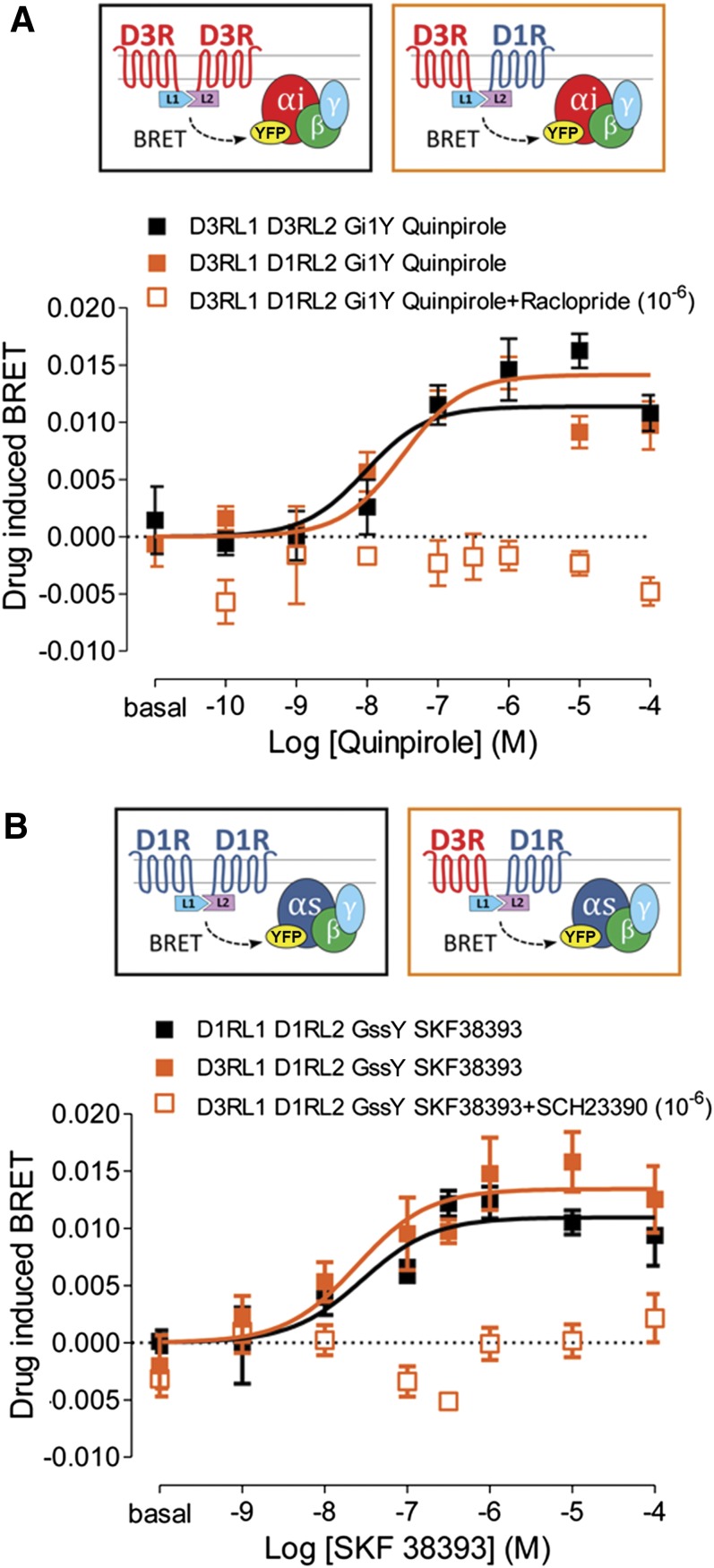
Fig. 1.
Gs and Gi protein coupling to D1R-D3R heteromer determined by CODA-RET experiments. BRET between complemented hemitruncated Rluc (L1 and L2 are the N-terminal and the C-terminal domains of Rluc, respectively) and G protein-YFP, as indicated in the cartoons at the top, was determined in HEK-293 cells expressing the following: (A) Gαi-YFP (Gi1Y), D3R-L1 and D3R-L2 (black symbols), or D1R-L2 (orange symbols) or (B) Gαss-YFP (GssY), D1R-L2 and D1R-L1 (black symbols), or D3R-L1 (orange symbols). Cells were preincubated for 15 minutes at room temperature with medium (filled symbols) or with 1 μM D3R antagonist raclopride (A, orange open symbol) or the D1R antagonist SCH 23390 (B, orange open symbol) before the addition of increasing concentrations of D3R agonist quinpirole (A) or D1R agonist SKF 38393 (B). To the BRET values for each agonist concentration was subtracted the BRET value in the absence of ligands. Values are means ± S.E.M. (n = 3–5).
Fusion Proteins.
Sequences encoding amino acid residues 1–155 and 155–238 of the Venus variant of yellow fluorescence protein (YFP) and amino acids residues 1–229 and 230–311 of Rluc protein were subcloned in the pcDNA3.1 vector to obtain YFP and Rluc hemitruncated proteins. The human cDNAs for D1R and D3R, cloned into pcDNA3.1, were amplified without their stop codons using sense and antisense primers harboring unique EcoRI and BamHI. The amplified fragment corresponding to D1R was subcloned to be in-frame with restriction sites of pcDNA3.1-Rluc, pcDNA3.1-cRluc, pcDNA3.1-nRluc, pcDNA3.1-cYFP, or pcDNA3.1-nYFP to give plasmids that express D1R fused to Rluc or to hemitruncated proteins on the C-terminal end of the receptor (D1R-cRluc, D1R-nRluc, D1R-cYFP, or D1R-nYFP). To obtain a plasmid that expresses D1R fused to Rluc on the C-terminal end of the receptor (D1R-Rluc), the cDNAs for D1R were amplified without their stop codons using sense and antisense primers harboring unique HindIII and BamHI, and the amplified fragment was subcloned to be in-frame with restriction sites of a Rluc-expressing vector (pRluc-N1; PerkinElmer, Wellesley, MA). The amplified fragment corresponding to D3R was subcloned to be in-frame with BamHI and EcoRI restriction sites of pcDNA3.1-cYFP or pcDNA3.1-nYFP to give plasmids that express D3R fused to YFP or to hemitruncated proteins on the C-terminal end of the receptor (D3R-YFP, D3R-cYFP, and D3R-nYFP). The receptor-fusion protein expression and function were tested by confocal microscopy and by second messengers, extracellular signal-regulated kinases (ERK)1/2 phosphorylation, or cAMP production, as described previously (Marcellino et al., 2008; Ferrada et al., 2009). Human β-arrestin-1-Rluc6, cloned in the pcDNA3.1 RLuc vector (pRLuc-N1 PerkinElmer), was generously provided by M. Castro (Santiago de Compostela University, Galicia, Spain).
Cells and Cell Clones.
To obtain cells expressing D1R, D3R, or D1R-D3R, we first cloned D1R-Rluc, D3R-YFP, or D3R-YFP-D1R-Rluc plasmids in a vector containing a FLP-FRT site, a hygromicin resistance gene, and, only in the vector containing both receptors, an internal ribosomal entry site placed between receptors. These vectors were cotransfected in HEK-293 cells with the Flp recombinase expression vector pOG44 (1 µg/9 µg) to obtain FLP-FRT-HEK stable cell lines expressing D1R-Rluc (D1R cells), D3R-YFP (D3R cells), or both receptors (D1R-D3R cells). Transfection was performed using lipofectamine (Invitrogen, Paisley, Scotland) method following the instructions of the supplier. Twenty-four hours after transfection, the selection antibiotic was added (1000 µg/ml hygromicin B; Invitrogen). Antibiotic-resistant clones were isolated, and, after an appropriate number of passages, stable cell lines were selected and characterized by radioligand binding, as indicated in Results.
Cell Culture and Transient Transfection.
Wild-type HEK-293 cells and D1R, D3R, and D1R-D3R clones were grown in Dulbecco’s modified Eagle’s medium (Gibco, Paisley, Scotland) supplemented with 2 mM L-glutamine, 100 μg/ml sodium pyruvate, 100 U/ml penicillin/streptomycin, minimum essential medium nonessential amino acids solution (1/100), and 5% (v/v) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA). For cell clones, the corresponding selection antibiotic was added in the culture medium (300 µg/ml hygromicin B). All cells were maintained at 37°C in an atmosphere of 5% CO2. HEK-293 cells growing in 6-well dishes were transiently transfected with the corresponding fusion protein cDNA by the PEI method (Carriba et al., 2008). To control the cell number, sample protein concentration was determined using a Bradford assay kit (Bio-Rad, Munich, Germany) and bovine serum albumin dilutions as standards. For adenylyl cyclase 5 (AC5) transfection, HEK-293 cells growing in 6-well dishes were transiently transfected with a plasmid encoding for AC5 by the lipofectamine (Invitrogen) method following the instructions of the supplier. AC5 cDNA (Robinson and Caron, 1997) was generously provided by Y. Ishikawa and K. Suita (Yokohama City University, Yokohama, Japan).
Fluorescence Complementation Assays.
After 48 hours of transient transfection with 1 μM cDNA encoding for D3R-nYFP and D1R-cYFP, D1R-nYPF and D3R-cYFP, D3R-cYFP and D3R-nYFP, or D1R-cYFP and D1R-nYFP, HEK-293 cells were treated or not with the indicated TAT peptides (4 μM) for 60 minutes at 37°C. To quantify protein-reconstituted YFP expression, cells (20 μg protein) were distributed in 96-well microplates (black plates with a transparent bottom; Porvair, King’s Lynn, UK), and fluorescence was read in a Fluoro Star Optima Fluorimeter (BMG Labtechnologies, Offenburg, Germany) equipped with a high-energy xenon flash lamp, using a 10-nm bandwidth excitation filter at 400-nm reading. Protein fluorescence expression was determined as fluorescence of the sample minus the fluorescence of cells not expressing the fusion proteins (basal). Cells expressing D1R-cVenus and nVenus or D3R-nVenus and cVenus showed a fluorescence level not different from nontransfected cells.
BRET and BRET with Bimolecular Luminescence and Fluorescence Complementation Assays.
For BRET and BRET with bimolecular luminescence and fluorescence complementation assays, HEK-293T cells were transiently cotransfected with a constant amount of cDNA encoding for proteins fused to RLuc, nRLuc, or cRLuc, and with increasing amounts of the cDNA corresponding to proteins fused to YFP, nYFP, or cYFP (see figure legends). To quantify protein-YFP expression or complemented YFP expression, cells (20 μg protein) were distributed in 96-well microplates (black plates with a transparent bottom) and fluorescence was read in a Fluo Star Optima Fluorimeter (BMG) equipped with a high-energy xenon flash lamp, using a 10-nm bandwidth excitation filter at 400-nm reading. Relative fluorescence values were given as fluorescence of the sample minus the fluorescence of cells expressing the BRET donor alone. For BRET measurements, the equivalent of 20 μg cell suspension was distributed in 96-well microplates (Corning 3600, white plates; Sigma-Aldrich), and 5 μM coelenterazine H (Molecular Probes, Eugene, OR) was added. After 1 minute for BRET or after 5 minutes for BRET with bimolecular luminescence and fluorescence complementation, the readings were collected using a Mithras LB 940 that allows the integration of the signals detected in the short-wavelength filter at 485 nm (440–500 nm) and the long-wavelength filter at 530 nm (510–590 nm). To quantify protein-RLuc or protein-reconstituted RLuc expression, luminescence readings were also performed after 10 minutes of adding 5 μM coelenterazine H. The net BRET is defined as [(long-wavelength emission)/(short-wavelength emission)] − Cf, where Cf corresponds to [(long-wavelength emission)/(short-wavelength emission)] for the donor construct expressed alone in the same experiment. BRET is expressed as milli-BRET units (net BRET × 1000).
β-Arrestin-1 Recruitment.
Arrestin recruitment was determined by BRET experiments in HEK-293T cells 48 h after transfection with the indicated amounts of cDNA corresponding to D1R, D3R-YFP, and β-arrestin-1-Rluc. Cells (20 μg total protein from a cell suspension per well in 96-well microplates) were not treated or treated for 15 minutes with the indicated antagonists, and 5 µM coelenterazine H was added before stimulation with the agonist for 10 minutes. BRET between β-arrestin 1-Rluc and D3R-YFP was determined, as above.
ERK1/2 Phosphorylation.
Cell clones were cultured overnight in serum-free medium before the addition of any agent and were not treated or treated with the indicated TAT peptides TM5, TM6, and TM7 (4 μM) for 60 minutes at 37°C. When indicated, cells were treated overnight with 10 ng/ml pertussis toxin (PTX) or 100 ng/ml cholera toxin (CTX). After that, cells were incubated with the indicated antagonist for 15 minutes at 37°C and then activated for 7 minutes with the indicated agonist. Cells were rinsed with ice-cold phosphate-buffered saline and lysed by the addition of 500 μl ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 50 mM NaF, 150 mM NaCl, 45 mM β-glycerophosphate, 1% Triton X-100, 20 μM phenyl-arsine oxide, 0.4 mM NaVO4, and protease inhibitor cocktail). The cellular debris was removed by centrifugation at 13,000g for 5 minutes at 4°C, and the protein was quantified by the bicinchoninic acid method using bovine serum albumin dilutions as standard. To determine the level of ERK1/2 phosphorylation, equivalent amounts of protein (10 μg) were separated by electrophoresis on a denaturing 10% SDS-polyacrylamide gel and transferred onto polyvinylidene fluoride membranes. Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) was then added, and the membrane was rocked for 90 minutes. The membranes were then probed with a mixture of a mouse anti–phospho-ERK1/2 antibody (1:2500; Sigma-Aldrich) and rabbit anti-ERK1/2 antibody that recognizes both phosphorylated and nonphosphorylated ERK1/2 (1:40,000; Sigma-Aldrich) for 3 hours minimum or overnight. The 42- and 44-kDa bands corresponding to ERK1 and ERK2 were visualized by the addition of a mixture of IRDye 800 (anti-mouse) antibody (1:10,000; Sigma-Aldrich) and IRDye 680 (anti-rabbit) antibody (1:10,000; Sigma-Aldrich) for 1–2 hours and scanned by the Odyssey infrared scanner (LICOR Biosciences). Band densities were quantified using the scanner software and exported to Excel (Microsoft, Redmond, WA). The level of phosphorylated ERK1/2 isoforms was normalized for differences in loading using the total ERK1/2 protein band intensities.
Radioligand-Binding Experiments.
Binding experiments were performed with cell membrane suspensions (0.2 mg protein/ml) at 25°C in 50 mM Tris-HCl buffer, pH 7.4, containing 10 mM MgCl2 or with this buffer also supplemented with 120 mM NaCl and 5 mM KCl for raclopride binding. For saturation experiments, membranes were incubated with increasing free concentrations of the D1R antagonist [3H](R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH 23390; 70.5 Ci/mmol; PerkinElmer, Boston, MA) or with increasing free concentrations of the D3R antagonist [3H]raclopride (81.9 Ci/mmol; PerkinElmer). Membranes were incubated with ligands providing enough time to achieve stable equilibrium for the lower ligand concentrations. Nonspecific binding was determined in the presence of 30 µM nonlabeled ligand. Free and membrane-bound ligand were separated by rapid filtration of 500-μl aliquots in a cell harvester (Brandel, Gaithersburg, MD) through Whatman GF/C filters embedded in 0.3% PEI that were subsequently washed for 5 seconds with 5 ml ice-cold Tris-HCl buffer. The filters were incubated with 10 ml Ultima Gold MV scintillation cocktail (PerkinElmer) overnight at room temperature, and radioactivity counts were determined using a Tri-Carb 2800TR scintillation counter (PerkinElmer) with an efficiency of 62%. Protein was quantified by the bicinchoninic acid method (Pierce Chemical, Rockford, IL) using bovine serum albumin dilutions as standard. Saturation curves were analyzed by nonlinear regression, using the commercial Grafit software (Erithacus Software, Berkeley, CA). Binding data were fitted to the two-state dimer receptor model using the equations previously described for noncooperative ligands in saturation curves (Casadó et al., 2007; Ferré et al., 2014).
Adenylyl Cyclase Assay.
A whole cell-cyclic AMP accumulation assay was used with stably transfected HEK-293 cells expressing D3R or both D1R and D3R. In some experiments, cells were transiently transfected with AC5 (2 µg cDNA). Cells were grown, as described above, and, the day of the assay, medium was removed and substituted by a Hank’s balanced salt solution buffer. Then cells were incubated simultaneously with the indicated D1R and D3R agonists and antagonists 17 minutes at room temperature. To stop the reaction, incubation buffer was aspirated and 0.1 M HCl was added. Determination of cAMP levels was performed using an enzyme-linked immunosorbent assay kit (Enzo Life Sciences, Farmingdale, NY) following the protocol suggested by the provider. The adenylyl cyclase assay was also used in experiments with Chinese hamster ovary cell lines stably transfected with adenosine A1 receptors (A1R) or A2A receptors (A2AR) (Orru et al., 2011) to provide positive controls of the effect of CTX and PTX in experiments of MAPK signaling.
Results
Ligand-Induced Changes in Gs and Gi Protein Coupling to the D1R-D3R Heteromer.
HEK-293 cells were transiently transfected with D3R and D1R or with D3R fused to complementary Rluc hemitruncated proteins and Gαi1 fused to YFP (Fig. 1A). An increase in BRET signal was observed as a function of the D3R agonist quinpirole concentration. The results showed that quinpirole induces Gαi1 coupling to D3R in the D3R-D3R homomer (Fig. 1A, black squares) and the D1R-D3R heteromer (Fig. 1A, filled orange squares) with close potencies (log EC50, M = −7.5 ± 0.3 and −8.0 ± 0.3, respectively). The agonist-induced response was inhibited by the D3R antagonist raclopride (1 μM) (Fig. 1A, open orange squares). In HEK-293 cells transiently transfected with D3R and D1R or D1R fused to complementary Rluc hemitruncated proteins and Gαs short fused to YFP (Fig. 1B), the D1R agonist (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide (SKF 38393) induced a dose-dependent increase in BRET values. SKF 38393 promoted Gss coupling to D1R in the D1R-D1R homomer (Fig. 1B, black squares) and the D1R-D3R heteromer (Fig. 1B, filled orange squares) with close potencies (log EC50, M = −7.5 ± 0.3 and −7.6 ± 0.4, respectively). The effect of SKF 38393 was counteracted by the D1R antagonist SCH 23390 (1 μM) (Fig. 1B, open orange squares). Control experiments also showed that D1R-D1R and D3R-D3R homomers do not couple to Gi1 and Gss, respectively (data not shown). These results indicate that both Gi and Gs are coupled to D1R-D3R heteromers.
We then evaluated the possible effects of agonists or antagonists binding to one of the receptors in the heteromer on agonist-induced G protein coupling in the partner receptor (cross talk or cross-antagonism). In HEK-293 cells transiently transfected with D3R and D1R fused to the complementary Rluc hemitruncated proteins and Gi1 or Gss coupled to mVenus, SKF 38393- or quinpirole-induced increases in BRET were determined after the addition of agonists or antagonists of the other receptor unit in the heteromer. For quinpirole-induced Gi1 coupling to D1R-D3R heteromers, the addition of 1 μM SKF 38393 or 1 μM SCH 23390 did not cause any significant change in EC50 values deduced from concentration-response BRET curves (Table 1). Similarly, EC50 values for SKF 38393-induced Gss coupling were not changed significantly after addition of 1 μM quinpirole or 1 μM raclopride (Table 1). These results indicate that D1R and D3R in the D1R-D3R heteromer couple to their preferred G protein subtype irrespective of simultaneous binding of ligands to the other receptor unit in the heteromer. They also support that a simultaneous coupling to Gs and Gi proteins in the D1R-D3R heteromer can take place upon simultaneous binding of D1R and D3R ligands.
TABLE 1.
Effect of partner receptor ligands on agonist-induced G protein interaction to D1R-D3R heteromers
CODA-RET experiments were performed in human embryonic kidney-293 cells expressing dopamine receptor type 3 (D3R)-L1 and dopamine receptor type 1 (D1R)-L2 (D3R and D1R fused to the N-terminal and C-terminal hemitruncated Renilla luciferase 8 proteins, respectively) and Gαi1-yellow fluorescence protein (YFP) or Gαss-YFP. Bioluminescence resonance energy transfer between complemented hemitruncated Renilla luciferase 8 and G protein-mVenus was determined as a function of increasing concentrations of quinpirole or (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide (SKF 38393) in the absence or in the presence of 1 μM SKF 38393, (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH 23390), quinpirole, or raclopride, as indicated in Table 1. From the dose-response curves, EC50 values were calculated, and one-way analysis of variance, followed by post hoc Newman-Keuls test, did not show significant differences between EC50 values.
| Receptor | G Protein | Treatment | Log EC50 (M) (mean ± S.E.M.) | n |
|---|---|---|---|---|
| D3R-L1 + D1R-L2 | Gαi1-YFP | Quinpirole | −8.0 ± 0.3 | 3 |
| Quinpirole + SKF 38393 | −8.3 ± 0.2 | 3 | ||
| Quinpirole + SCH 23390 | −7.9 ± 0.3 | 3 | ||
| Gαss-YFP | SKF 38393 | −7.6 ± 0.3 | 8 | |
| SKF 38393 + quinpirole | −8.0 ± 0.2 | 3 | ||
| SKF 38393 + raclopride | −7.2 ± 0.4 | 8 |
D1R and D3R Oligomerize as Heteromers of Homodimers.
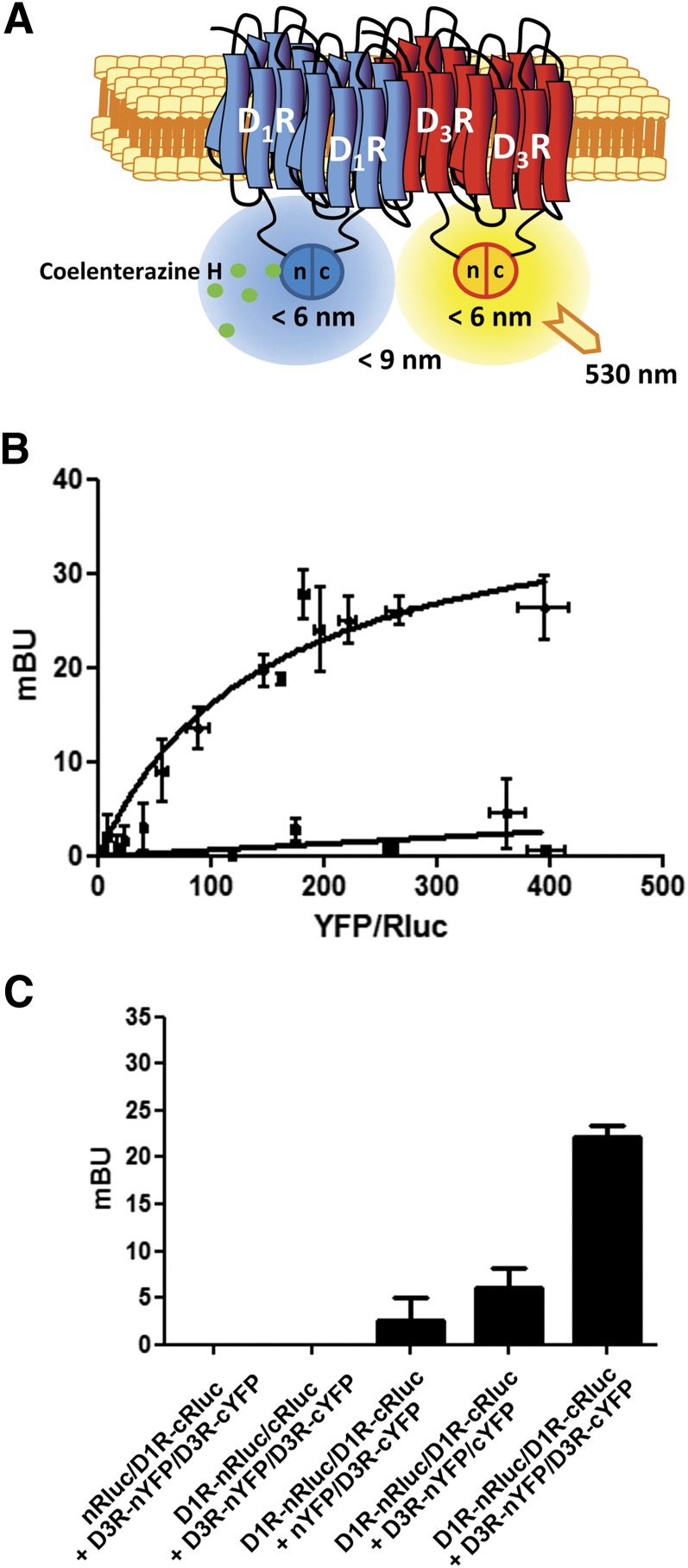
Because it would be difficult for two GPCR protomers to simultaneously accommodate two trimeric G protein molecules due to steric hindrance (Maurice et al., 2011), the results of CODA-RET experiments fit with the proposed model of receptor heteromers comprised of two different homodimers (Ferré et al., 2014). To test this hypothesis, we combined bimolecular fluorescence complementation with BRET (Kerppola, 2006; Robitaille et al., 2009). For this purpose, D1R was fused to the N-terminal or to the C-terminal hemitruncated protein of Rluc (D1R-nRluc and D1R-cRluc) that only upon coexpression and complementation can act as a BRET donor (Fig. 2A). The BRET acceptor protein was obtained upon complementation of the D3R fused to the N-terminal portion of the YFP Venus protein (D3R-nYFP) and the D3R fused to the C-terminal hemitruncated YFP (D3R-cYFP) (Fig. 2A). When all four receptor constructs were transfected in the cell, we obtained a positive and saturable BRET signal (Fig. 2B). As a negative control, only low and linear BRET was obtained in cells expressing D1R-nRluc, D1R-cRluc, and the cannabinoid CB1 receptor (CB1R) fused to the two YFP hemitruncated proteins (CB1R-nYFP and CB1R-cYFP) (Fig. 2B). Further negative controls included independent experiments replacing each receptor fused to its hemitruncated protein with the same nonfused (soluble) hemitruncated protein (Fig. 2C). These results demonstrate that D1R-D3R heteromers arrange as heterotetramers constituted by D1R and D3R homodimers.
Fig. 2.
Tetrameric structure of D1R-D3R heteromers. (A) Schematic representation of BRET and bimolecular fluorescence complementation of D1R-nRluc with D1R-cRluc and D3R-nYFP with D3R-cYFP. (B) BRET saturation curve was obtained in HEK-293 cells transfected with 0.75 μg cDNA corresponding to D1R-cRluc and D1R-nRluc and increasing amounts of cDNA corresponding to D3R-nYFP and D3R-cYFP (equal amounts for each construct). As a negative control, low and linear BRET was obtained in HEK-293 cells transfected with 0.75 μg cDNA corresponding to D1R-cRluc and D1R-nRluc and increasing amounts of cDNA corresponding to CB1R-nYFP and CB1R-cYFP (equal amounts for each construct). Milli-BRET units (mBU) are represented versus the ratio between the fluorescence of the acceptor and the luciferase activity of the donor (YFP/Rluc). Data are mean ± S.D. of three different experiments grouped as a function of the amount of BRET acceptor. (C) Positive BRET obtained in HEK-293 cells transfected with the cDNA corresponding to D1R-cRluc (0.75 μg), D1R-nRluc (0.75 μg), D3R-nYFP (1 μg), and D3R-cYFP (1 μg) was compared with that obtained in cells in which the cDNA corresponding to each one of the receptors fused to its hemitruncated protein was replaced by the same amount of cDNA corresponding to the nonfused hemitruncated protein. Values are means ± S.E.M. of three different experiments.
Adenylyl Cyclase Signaling in the D1R-D3R Heteromer.
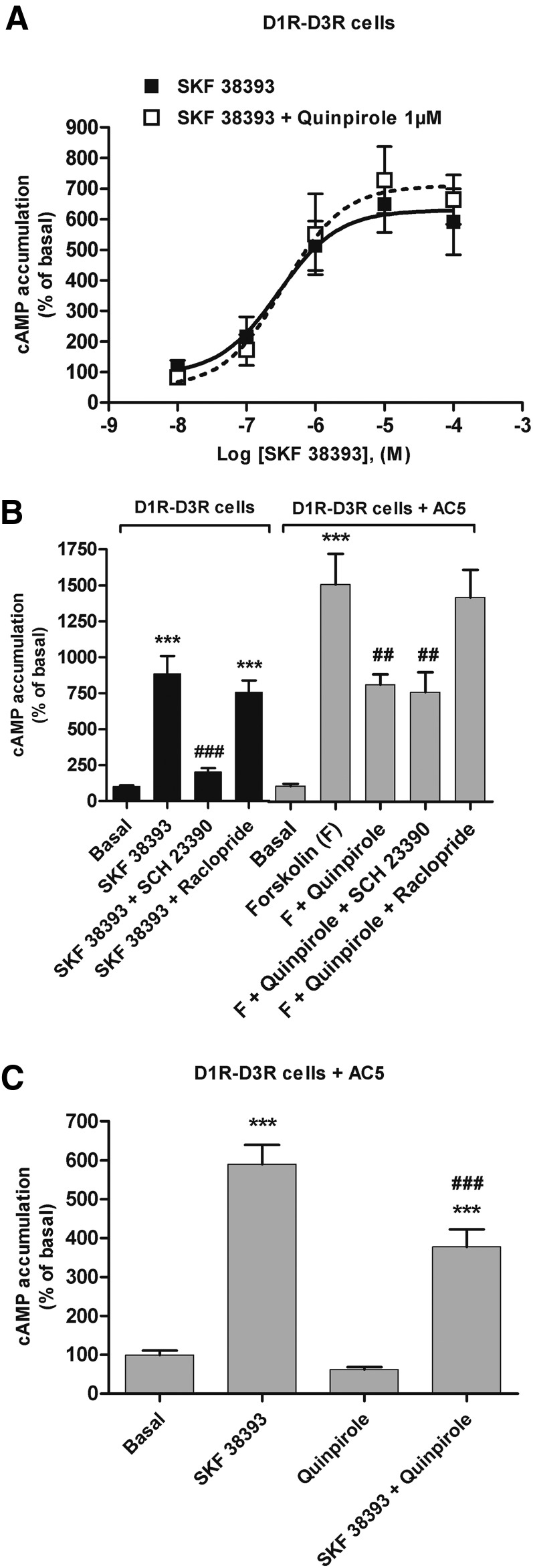
A cell line with stable expression of D1R fused to Rluc and D3R fused to YFP (D1R-D3R cells) was developed. Control cell lines with D1R fused to Rluc (D1R cells) and D3R fused to YFP (D3R cells) were also obtained. Saturation experiments with [3H]raclopride showed similar Bmax values in both D3R and D1R-D3R cells (in means ± S.E.M.: 1.1 ± 0.1 and 0.80 ± 0.04 pmol/mg protein, respectively). Saturation experiments with [3H]SCH 23390 also showed similar Bmax values in both D1R and D1R-D3R cells (0.9 ± 0.02 and 0.80 ± 0.01 pmol/mg protein, respectively). D1R-D3R cells had therefore very similar expression of D1R and D3R, as expected from an internal ribosomal entry site construction. D1R-D3R cells showed stable and measurable BRET values (BRET ratios) due to heteromerization because emission at 510–540 nm after the addition of coelenterazine H was significantly greater in D1R-D3R cells than in D1R cells (BRET ratio in means ± S.E.M.: 0.714 ± 0.005 and 0.687 ± 0.007 milli-BRET units, respectively; Student’s t test: P = 0.002; n = 30).
SKF 38393 promoted a concentration-dependent cAMP accumulation in D1R-D3R cells (Fig. 3A). However, quinpirole did not produce any significant effect on forskolin-induced cAMP accumulation (data not shown). Furthermore, quinpirole (1 μM) did not modify the increase in cAMP levels produced by SKF 38393 (Fig. 3A; EC50 of 340 and 290 nM with and without quinpirole, respectively). Previous studies have shown that D3R can only inhibit the activity of AC5, which has been reported not being expressed in HEK-293 cells (Robinson and Caron, 1997). In agreement, upon transient transfection of AC5 in D1R-D3R cells, a significant decrease of forskolin-induced cAMP accumulation by quinpirole (1 μM) could be demonstrated (Fig. 3B, gray bars). The cAMP accumulation promoted by SKF 38393 (1 μM) and the inhibition of forskolin-induced cAMP accumulation promoted by quinpirole (1 μM) were counteracted by SCH 23390 (10 μM) and raclopride (10 μM), respectively (Fig. 3B). No cross-antagonism was observed, because raclopride did not revert the effect of SKF 38393, nor did SCH 23390 revert the effect of quinpirole (Fig. 3B). These results mirrored those obtained with CODA-RET experiments, indicating that agonist-induced G protein–mediated signaling by one of the receptor units in the heteromer was not counteracted by antagonist binding to the other receptor unit. The lack of influence of allosteric interactions in the heteromer on adenylyl cyclase signaling was also seen upon coactivation of both receptors. Upon transfection with AC5, a canonical Gi-Gs–mediated negative interaction at the level of adenylyl cyclase signaling was observed in D1R-D3R cells, by which 1 μM quinpirole significantly decreased cAMP accumulation induced by 1 μM SKF 38393 (Fig. 3C). These results indicate that both Gi and Gs proteins can simultaneously signal upon coactivation of both receptors in the D1R-D3R heteromers. Moreover, the results do not support positive cross talk at the level of adenylyl cyclase signaling as a biochemical property of D1R-D3R heteromerization (Fiorentini et al., 2008).
Fig. 3.
Adenylyl cyclase signaling in D1R-D3R cells. cAMP accumulation was determined in D1R-D3R cells. (A) Cells were stimulated with increasing concentrations of the D1R agonist SKF 38393 in the absence (white symbols) or the presence (black symbols) of 1 μM quinpirole. (B) Cells were not transfected (black columns) or transfected (gray columns) with AC5. Cells were pretreated or not for 15 minutes with 10 μM D3R antagonist raclopride or D1R antagonist SCH 23390 prior to stimulation with SKF 38393 (1 μM) or quinpirole (1 μM) plus forskolin (1 μM). Values represent means ± S.E.M. (n = 4–11) of the percentage of cAMP accumulation relative to basal levels found in transfected or nontransfected cells. (C) Cells transfected with AC5 were stimulated with SKF 38393 (1 μM), quinpirole (1 μM), or both. Values are expressed as percentage over basal and are means ± S.E.M. (n = 7–10). Significant differences were calculated by one-way analysis of variance with post hoc Newman-Keuls test: ***P < 0.001 versus basal values; ###P < 0.001 versus SKF 38393; ##P < 0.01 versus forskolin.
Allosteric Interactions in the D1R-D3R Heteromer Modulate MAPK Signaling.
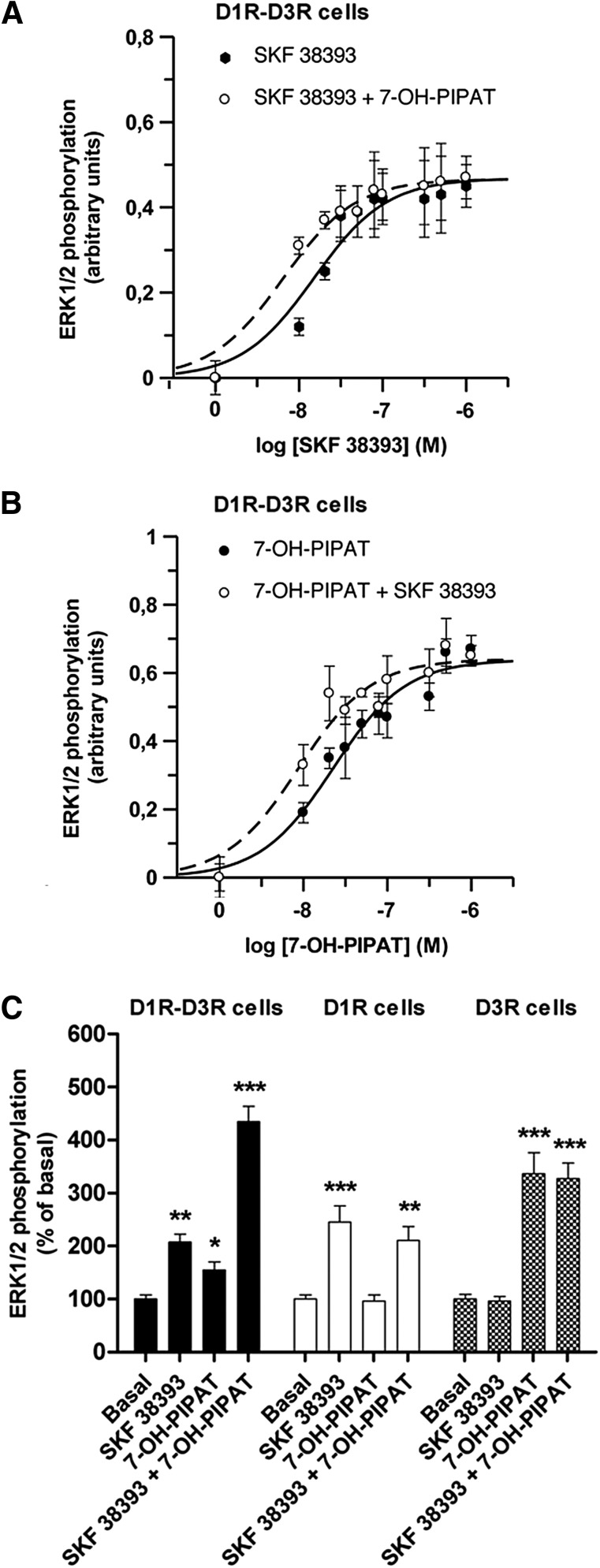
In D1R-D3R cells, the D1R agonist SKF 38393, or the D3R agonists quinpirole (data not shown) and (RS)-trans-7-hydroxy-2-[N-propyl-N-(3′-iodo-2′-propenyl)amino]tetralin maleate (7-OH-PIPAT), produced MAPK activation (increase in ERK1/2 phosphorylation) in a concentration-dependent manner (Fig. 4, A and B, solid line: ERK1/2 phosphorylation after subtraction of basal values). Interestingly, coactivation with 0.1 μM SKF 38393 and 0.1 μM 7-OH-PIPAT produced a strong activating effect (Fig. 4C, black bars), indicating a synergistic positive cross talk. In fact, a significant shift to the left in the SKF 38393 concentration-response curve was obtained with the addition of 0.1 μM 7-OH-PIPAT (more than twofold increase in potency; see legend to Fig. 1A for EC50 values and statistical analysis). Furthermore, a significant shift to the left in the 7-OH-PIPAT concentration-response curve was also observed with the addition of 0.1 μM SKF 38393 (more than twofold increase in potency; see legend to Fig. 1B for EC50 values and statistical analysis). This demonstrates that the potency of an agonist for either D1R or D3R increases by agonist binding to the partner receptor. The positive cross talk was not due to nonspecific effects of the ligands because, in cells only expressing D1R, the effect of SKF 38393 was not modified by 7-OH-PIPAT (Fig. 4C, white bars), and in cells only expressing D3R the effect of 7-OH-PIPAT was not changed in the presence of SKF 38393 (Fig. 4C, hatched bars).
Fig. 4.
Agonist-induced ERK1/2 phosphorylation in D1R, D3R, or D1R-D3R cells. (A) ERK1/2 phosphorylation was determined in D1R-D3R cells stimulated with increasing concentrations of the D1R agonist SKF 38393 in the absence (solid line; EC50 = 15 ± 3 nM; n = 3) or in the presence (broken line; EC50 = 6 ± 1 nM; n = 3) of the D3R agonist 0.1 μM 7-OH-PIPAT. (B) ERK1/2 phosphorylation was determined in D1R-D3R cells stimulated with increasing concentrations of 7-OH-PIPAT in absence (solid line; EC50 = 24 ± 4 nM; n = 3) or presence (broken line; EC50 = 9 ± 2 nM; n = 3) of 0.1 μM SKF 38393. (A) and (B) Values are expressed as the means ± S.E.M., subtracting basal values from control untreated cells (A and B, solid lines) and subtracting the values obtained with 7-OH-PIPAT alone (A, broken line) or SKF 38393 alone (B, broken line). Significant differences were obtained between EC50 values of SKF 38393 and SKF 38393 plus 7-OH-PIPAT (A) and between EC50 values of 7-OH-PIPAT and 7-OH-PIPAT plus SKF 38393. Student’s t test: P < 0.05 in both cases. (C) ERK1/2 phosphorylation was determined in D1R-D3R cells (black columns), D1R cells (white columns), or D3R cells (hatched columns). Cells were not treated (basal) or treated with SKF 38393 (0.1 μM) and 7-OH-PIPAT (0.1 μM) alone or in combination. Values are expressed as percentage over basal and are means ± S.E.M. (n = 6). Significant differences were calculated by one-way analysis of variance with post hoc Newman-Keuls test: *P < 0.05, **P < 0.01, and ***P < 0.001 versus basal values.
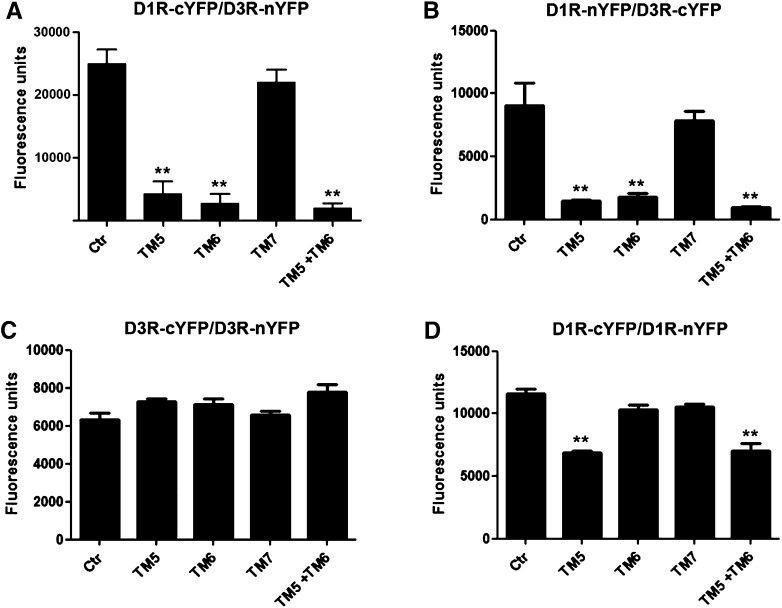
HIV TAT peptides fused to D1R TM 5, 6, and 7 peptides (TM5, TM6, and TM7) were tested for their ability to destabilize the D1R-D3R heteromer and, consequently, the energy transfer in BRET experiments in D1R-D3R cells (which express D1R-Rluc and D3R-YFP). Surprisingly, and at odds with previously published studies (Borroto-Escuela et al., 2010), the application of peptides (4–40 μM for 60 minutes) significantly inhibited the enzymatic activity of Rluc, with strong reduction of bioluminescence values. Reductions about 50% were obtained at peptide concentrations of 40 μM, which, in our hands, invalidates BRET as a method to evaluate modifications of receptor heteromer structure induced by synthetic hydrophobic TM-like peptides. As an alternative method to BRET, we studied the ability of the TM-like peptides to destabilize D1R-D3R heteromers by fluorescence complementation experiments. HEK-293 cells were transfected with D3R fused to the YFP N-terminal fragment (nYFP) and D1R fused to the YFP C-terminal fragment (cYFP). Fluorescence could be detected after YFP reconstitution, due to a close receptor-receptor interaction (25,000 ± 5000 fluorescence units/20 μg protein). When cells were treated for 1 hour with 4 μM D1R TM peptides, TM5 and TM6 alone or in combination, but not TM7, a significant decrease of YFP fluorescence was found (Fig. 5A). Complementation was also achieved, albeit with less efficiency, with cells expressing D1R fused to nYFP and D3R fused to cYFP (Fig. 5B). Again, fluorescence was significantly decreased with D1R TM5 and TM6, but not with TM7 (Fig. 5B). As controls for peptide selectivity, complementation experiments were performed in cells expressing D3R fused to cYFP and nYFP. None of the peptides modified D3R-cYFP/D3R-nYFP complementation (Fig. 5C). Moreover, complementation experiments were performed in cells expressing D1R fused to cYFP and nYFP. In these cells, D1R TM5, but not TM6, also decreased D1R-cYFP/D1R-nYFP complementation (Fig. 5D), although the effect was less pronounced than for D1R-cYFP/D3R-nYFP and D3R-cYFP/D1R-nYFP complementation. Of note, in preliminary experiments to develop the assay, it was observed that TM peptides (4–40 μM) did not produce a significant change of fluorescence in cells expressing YFP Venus alone or cells expressing D3R or D1R fused to the entire YFP Venus.
Fig. 5.
Effect of D1R TAT-TM-like peptides on D1R-D3R heteromerization detected by fluorescence complementation experiments. HEK-293 cells expressing (A) D3R fused to the YFP N-terminal fragment (nYFP) and D1R fused to the YFP C-terminal fragment (cYFP); (B) D1R fused to nYFP and D3R fused to cYFP; (C) D3R fused to cYFP and D3R fused to nYFP; and (D) D1R fused to cYFP and D1R fused to nYFP. Fluorescence at 530 nm was determined in cells treated or not treated (control or Ctr) for 1 hour with 4 μM D1R TM peptide TM5, TM6, or TM7 alone or a combination of TM5 plus TM6. Values are expressed as percentage over the fluorescence detected in the absence of peptides and are means ± S.E.M. (n = 4). Significant differences were calculated by one-way analysis of variance with post hoc Newman-Keuls test: **P < 0.01 versus control.
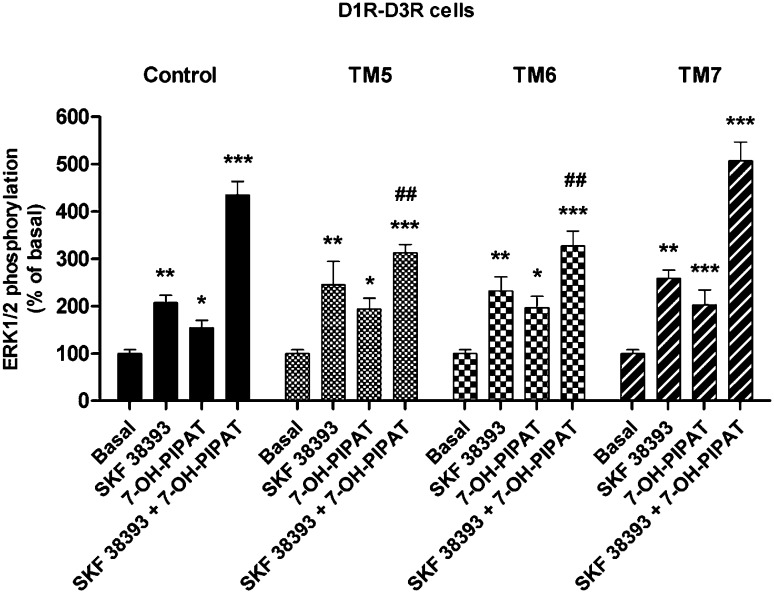
Together, these results indicate that selective peptides can disrupt the quaternary structure of the D1R-D3R heteromer, and could potentially be used as tools to explore its biochemical properties. Thus, D1R-D3R cells were treated for 1 hour with 4 μM D1R TM5, TM6, or TM7 prior to stimulation with 0.1 μM 7-OH-PIPAT or SKF 38393 alone or in combination, and ERK1/2 phosphorylation was determined. Significantly, in D1R-D3R cells, incubation with D1R TM5 and TM6, but not TM7, disrupted the positive cross talk of SKF 38393 plus 7-OH-PIPAT (Fig. 6). These results indicate that the positive cross talk between D1R and D3R agonist at the level of MAPK signaling is a specific biochemical property of the D1R-D3R heteromer, because it can only be observed upon the appropriate quaternary structure of the heteromer.
Fig. 6.
Effect of D1R TAT-TM-like peptides on agonist-induced ERK1/2 phosphorylation in D1R-D3R cells. Cells were not treated (black columns) or treated for 1 hour with 4 μM TM5, TM6, or TM7 before stimulation with the D1R agonist SKF 38393 (0.1 μM), the D3R agonist 7-OH-PIPAT (0.1 μM), or both. Values are expressed as percentage over basal and are means ± S.E.M. (n = 6). Significant differences were calculated by one-way analysis of variance with post hoc Newman-Keuls test. *P < 0.05, **P < 0.01, and ***P < 0.001 versus basal values; ##P < 0.01 as compared with SKF 38393 + 7-OH-PIPAT in the control group.
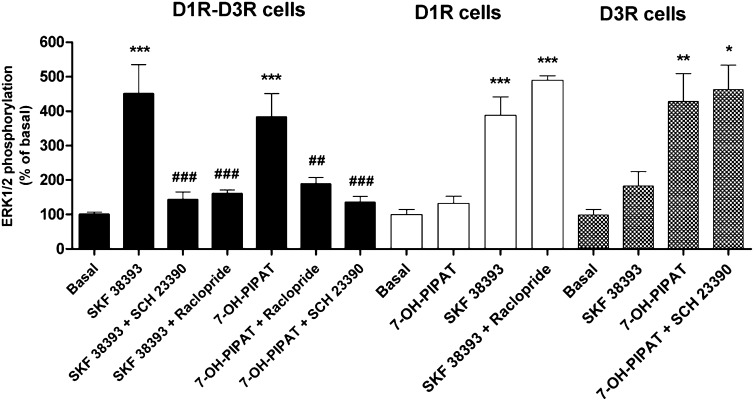
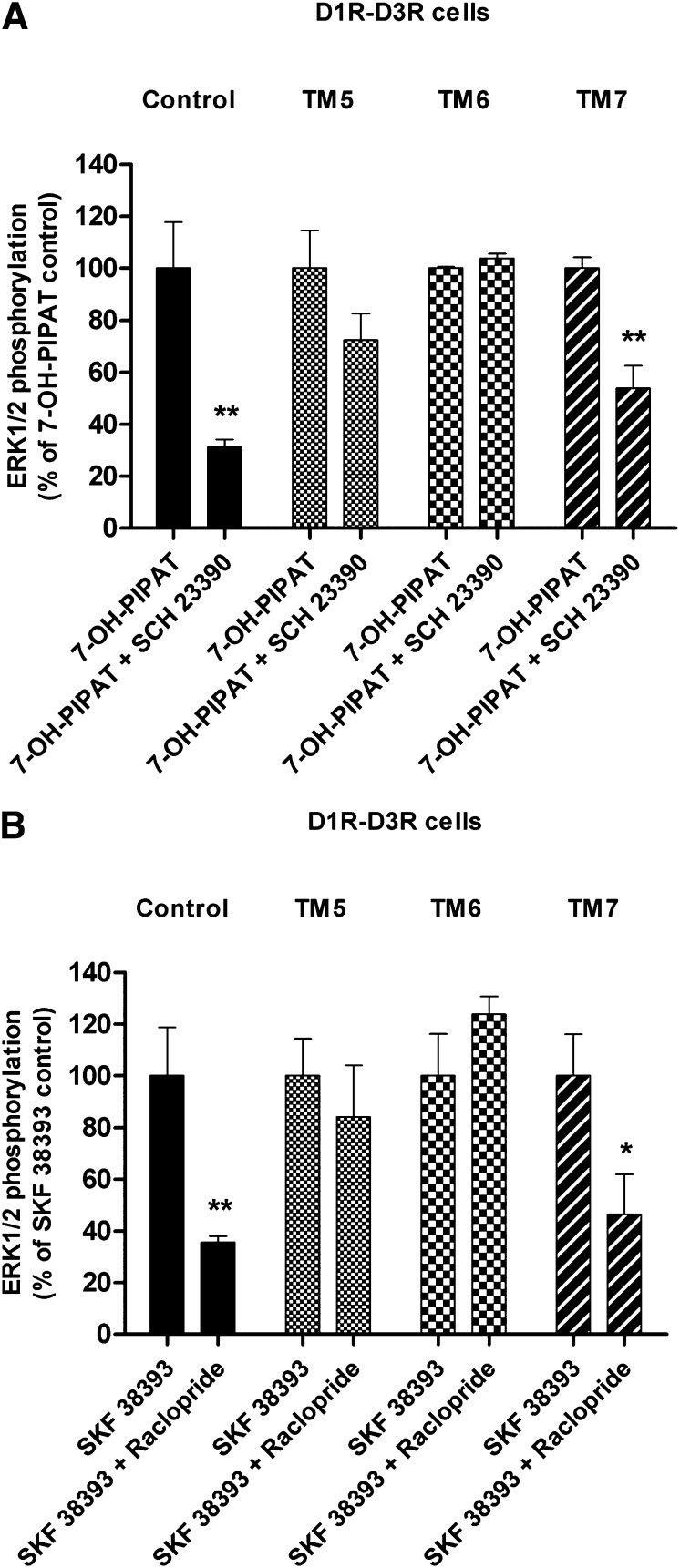
Cross-antagonism at the MAPK level was also found to be a biochemical property of the D1R-D3R heteromer. In D1R-D3R cells, the D1R antagonist SCH 23390 (1 μM) not only antagonized ERK1/2 phosphorylation induced by 0.1 μM SKF 38393, but also by 0.1 μM 7-OH-PIPAT (Fig. 7, black bars). Similarly, the D3R antagonist raclopride (1 μM) antagonized the effect of both SKF 38393 and 7-OH-PIPAT (Fig. 7, black bars). Cross-antagonism could not be explained by a lack of ligand specificity, because raclopride (1 μM) did not counteract ERK1/2 phosphorylation induced by 0.1 μM SKF 38393 in D1R cells (Fig. 7, white bars), and SCH 23390 (1 μM) did not modify ERK1/2 phosphorylation induced by 0.1 μM 7-OH-PIPAT in D3R cells (Fig. 7, hatched bars). Again, incubation for 1 hour with 4 μM D1R TM5 and TM6, but not TM7, disrupted the cross-antagonism of SCH 23390 on 7-OH-PIPAT–induced ERK1/2 phosphorylation in D1R-D3R cells (Fig. 8A) and of raclopride on SKF 38393-induced ERK1/2 phosphorylation (Fig. 8B). These results demonstrate that allosteric interactions between ligands (positive cross talk and cross-antagonism) in the D1R-D3R heteromer modulate MAPK signaling.
Fig. 7.
Effect of D1R and D3R antagonists on agonist-induced ERK1/2 phosphorylation in D1R-D3R cells. ERK1/2 phosphorylation was determined in D1R-D3R cells (black columns), D1R cells (white columns), or D3R cells (hatched columns). Cells were pretreated for 15 minutes with 1 μM D3R antagonist raclopride or D1R antagonist SCH 23390 prior to stimulation with 0.1 μM D1R agonist SKF 38393 or D3R agonist 7-OH-PIPAT. Values are expressed as percentage over basal and are means ± S.E.M. (n = 5–6). Significant differences were calculated by one-way analysis of variance with post hoc Newman-Keuls test. *P < 0.05, **P < 0.01, ***P < 0.001 versus basal values; ##P < 0.01 and ###P < 0.001, as compared with SKF 38393 or 7-OH-PIPAT.
Fig. 8.
Effect of D1R TM peptides on cross-antagonism of agonist-induced ERK1/2 phosphorylation in D1R, D3R, or D1R-D3R cells. ERK1/2 phosphorylation was determined in cells not treated (control) or treated for 1 hour with 4 μM TM5, TM6, or TM7. Cells were incubated for 15 minutes with 1 μM D1R antagonist SCH23390 (A) or D3R antagonist raclopride (B) before stimulation with 0.1 μM D3R agonist 7-OH-PIPAT (A) or the D1R agonist SKF 38393 (B). Values are expressed as percentage of agonist-induced phosphorylation in control conditions and are means ± S.E.M. (n = 4–5). Significant differences were calculated by Student’s t test: *P < 0.05, **P < 0.01 versus 7-OH-PIPAT or SKF 38393-treated control cells in each condition (A or B).
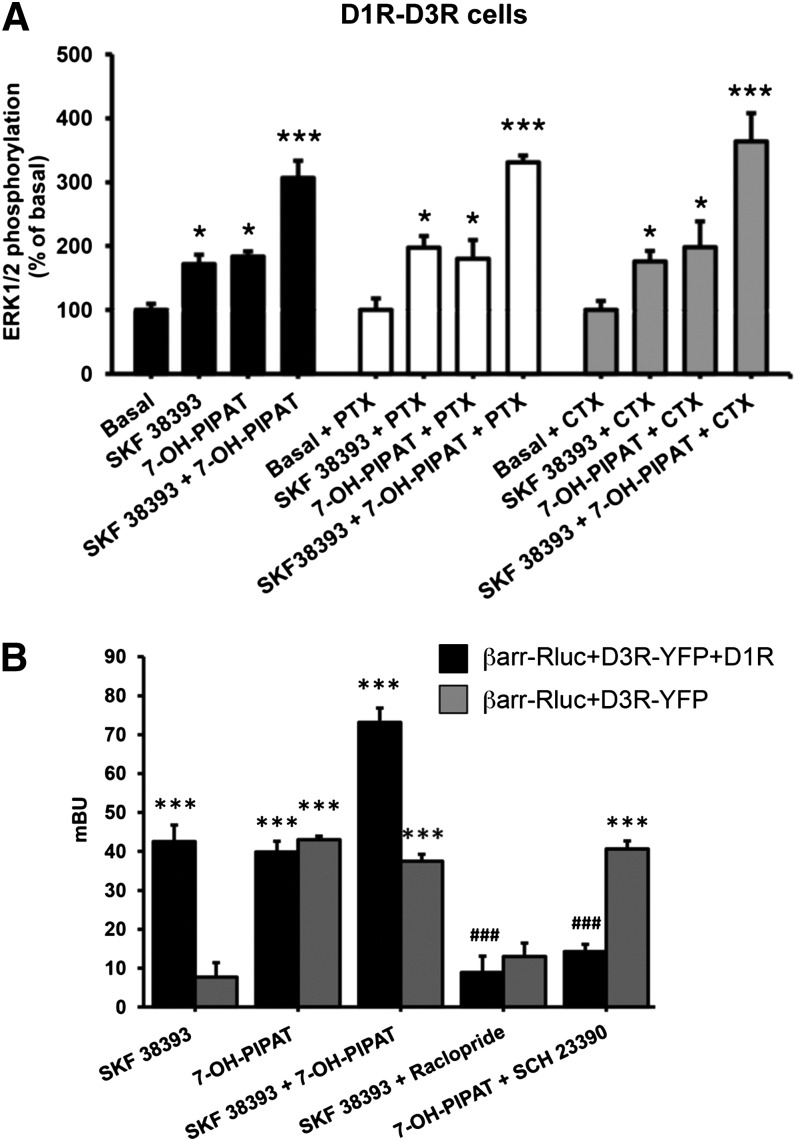
The results described above indicate the existence of a functional selectivity of allosteric interactions in the D1R-D3R heteromer, because they are not involved in ligand-induced G protein coupling or G protein–mediated signaling (adenylyl cyclase), but are involved in MAPK activation. To demonstrate that agonist-induced ERK1/2 phosphorylation is G protein–independent, we performed experiments in the presence of PTX and CTX. In D1R-D3R cells, ERK1/2 phosphorylation induced by SKF 38393 or 7-OH-PIPAT was unchanged after treatment with CTX or PTX, and neither toxin modified the positive cross talk between D1R and D3R agonists (Fig. 9A). Parallel control experiments using the same conditions were performed in Chinese hamster ovary cell lines expressing A1R or A2AR (Orru et al., 2011) to provide positive controls of the effect of CTX and PTX. In A1R-expressing cells, PTX, but not CTX, counteracted the ability of an A1R agonist (N-cyclopentyladenosine) to inhibit forskolin-induced cAMP accumulation. In A2AR-expressing cells, CTX, but not PTX, counteracted the ability of an A2AR agonist (4-[2-[[6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoicacid hydrochloride; CGS 21680) (data not shown). We then explored whether the allosteric changes observed at the level of MAPK signaling could also be observed at the level of β-arrestin-1 recruitment, because β-arrestin recruitment-dependent MAPK signaling has been well established for several GPCRs (Kovacs et al., 2009). In cells expressing D3R fused to YFP, β-arrestin-1-Rluc, and D1R, a strong recruitment of β-arrestin-1 was observed in BRET experiments upon coadministration of SKF 38393 and 7-OH-PIPAT. Furthermore, cross-antagonism of SKF 38393-induced β-arrestin-1 recruitment with raclopride and 7-OH-PIPAT–induced β-arrestin-1 recruitment with SCH 23390 was also demonstrated (Fig. 9B, black bars). Importantly, β-arrestin-1 recruitment was induced not only with the D3R agonist, 7-OH-PIPAT, but also with the D1R agonist SKF 38393, indicating recruitment of β-arrestin by the D1R-D3R heteromer unit (Fig. 9B, black bars). As a negative control, in cells expressing D3R fused to YFP and β-arrestin-1-Rluc, but not D1R, SKF 38393 was not able to induce β-arrestin-1 recruitment. Finally, either D1R agonists or antagonists did not significantly modify 7-OH-PIPAT–induced recruitment of β-arrestin-1 (Fig. 9B, gray bars). These results indicate that allosteric interactions in the D1R-D3R heteromer selectively modulate G protein–independent MAPK signaling and β-arrestin-1 recruitment.
Fig. 9.
G protein–independent mechanisms of the allosteric modulations in the D1R-D3R heteromer. (A) D1R-D3R cells were treated overnight with vehicle (black columns), with 10 ng/ml pertussis toxin (PTX, white columns), or with 100 ng/ml cholera toxin (CTX, gray columns). Cells were not stimulated (basal) or stimulated with 0.1 µM D1R agonist SKF 38393 or the D3R agonist 7-OH-PIPAT alone or in combination. Values are expressed as percentage over basal and are means ± S.E.M. (n = 5). (B) HEK-293T cells were transfected with the cDNA corresponding to D3R-YFP (1 µg cDNA) and β-arrestin-1-Rluc (0.5 µg cDNA) in the absence (gray columns) or presence of D1R (1.5 µg cDNA) (black columns). Forty-eight hours post-transfection, cells were treated with vehicle or 1 μM D3R antagonist raclopride or the D1R antagonist SCH23390 before stimulation with 0.1 μM 7-OH-PIPAT or SKF 38393, and β-arrestin-1 recruitment was measured by BRET experiments. Values are means ± S.E.M. (n = 7–8). Significant differences were calculated by one-way analysis of variance with post hoc Newman-Keuls test: *P < 0.05, ***P < 0.001 versus basal values; (B) ###P < 0.001 as compared with agonist alone.
Discussion
Increasing attention is being given to the D1R-D3R heteromer as a potential therapeutic target for a variety of neuropsychiatric disorders, including Parkinson’s disease and drug addiction (Fiorentini et al., 2010; Ferré et al., 2010, 2014). From our study, three major conclusions can be made. First, D1R-D3R heteromers arrange as heterotetramers consisting of D1R and D3R homodimers able to couple to Gs and Gi proteins, respectively. This arrangement promotes the canonical negative interaction at the level of adenylyl cyclase signaling. Second, allosteric interactions between ligands take place within the D1R-D3R heteromer that allows selective modulation of G protein–independent MAPK signaling and β-arrestin-1 recruitment. Third, peptides competing for GPCR TM domains provide useful tools to probe GPCR heteromerization.
A D3R agonist-mediated potentiation of D1R agonist-mediated signaling through adenylyl cyclase was initially suggested as a biochemical property of the D1R-D3R heteromer (Fiorentini et al., 2008). It was also suggested that a main allosteric mechanism responsible for this putative biochemical property is a D3R agonist-mediated increase in the affinity of D1R agonists (Marcellino et al., 2008). In the present study, we wanted first to evaluate whether changes in G protein coupling could also be involved. The recently introduced CODA-RET assay specifically allows detecting ligand-induced conformation changes in the receptor–G protein interface of a defined G protein–coupled receptor oligomer (Urizar et al., 2011). Using this technique, we could then demonstrate that, upon agonist binding, D1R interacts with Gs and D3R interacts with Gi when forming homomers or heteromers. It has been argued that two protomers in a GPCR oligomer are insufficient to simultaneously accommodate two trimeric G protein molecules (Maurice et al., 2011). Our results using CODA-RET experiments fit with the proposed model of receptor heteromers comprised of two different homodimers, each able to signal with their preferred G protein (Ferré et al., 2014). In fact, in the present study, a heterotetrameric structure of the D1R-D3R heteromer could be demonstrated with BRET experiments using double bimolecular complementation of Rluc and YFP.
We could not confirm the previously reported existence of a positive cross talk at the level of adenylyl cyclase in HEK-293 cells expressing D1R and D3R, by which a D3R agonist potentiates D1R agonist-induced adenylyl cyclase activation (Fiorentini et al., 2008). In fact, CODA-RET experiments in transiently transfected cells showed that, in the D1R-D3R receptor heteromer, a D3R agonist does not modify the potency of a D1R agonist to induce a Gs conformational change. Furthermore, in agreement with previous studies, D3R activation did not inhibit forskolin-induced cAMP accumulation in HEK-293 cells (Robinson and Caron, 1997). This is related to the fact that D3R can only inhibit the activity of AC5, which is not expressed in HEK-293 cells (Robinson and Caron, 1997). When AC5 was cotransfected to D1R-D3R cells, D3R activation was not only able to inhibit forskolin- but also D1R agonist-induced cAMP accumulation. Therefore, differences in AC5 expression could explain at least part of the discrepancies between the previously reported study (Fiorentini et al., 2008) and the present results. In the brain, D1R and D3R are mostly colocalized in the striatum, in GABAergic efferent neurons that express AC5 as the predominant type of adenylyl cyclase (Lee et al., 2002). Our results therefore suggest that in the brain, if D1R-D3R heteromers are significantly represented, the Gi-dependent D3R-mediated activation should lead to a reduced Gs-dependent D1R-mediated activation of adenylyl cyclase signaling.
One important question is, therefore, what is the mechanism involved in the synergistic effects of D1R and D3R agonists observed at the behavioral level (Marcellino et al., 2008). Either D1R-D3R heteromers are not involved or the behavioral results depend on signaling pathways other than adenylyl cyclase activation. We did find a positive cross talk of D1R and D3R agonists, but at the level of MAPK signaling. Furthermore, a strong recruitment of β-arrestin-1 was observed in BRET experiments upon coadministration of SKF 38393 and 7-OH-PIPAT. The positive cross talk at the MAPK level was counteracted by the same peptides that modified the quaternary structure of the heteromer (D1R TM5 and TM6, but not TM7), as demonstrated in YFP-complementation experiments. This indicates that a selective positive cross talk of D1R and D3R receptor agonists at the MAPK level represents a biochemical property of the D1R-D3R receptor heteromer. The synthetic peptide strategy could not be used in β-arrestin-1 recruitment BRET-based experiments because of their direct inhibitory effects on Rluc activity (see Results). Nevertheless, the ability of the D1R agonist to promote β-arrestin-1-Rluc recruitment to D3R-YFP in the heteromer indicates its dependence on D1R-D3R receptor heteromerization. D1R-D3R heteromer-mediated MAPK signaling and β-arrestin-1 recruitment could therefore be major players involved in the synergistic motor-activating effects of D1R and D3R agonists (Marcellino et al., 2008).
Cross-antagonism has been previously suggested to be a biochemical property of several receptor heteromers, such as for D1R-histamine H3 receptor and α α1B and β1 adrenergic receptor–dopamine D4 receptor heteromers (Gonzalez et al., 2012; Moreno et al., 2014). Cross-antagonism would imply an allosteric interaction between ligands by which an antagonist of one receptor in the heteromer blocks the agonist-induced activation of the partner receptor in the heteromer. Although it is difficult to envision a mechanism for cross-antagonism that does not depend on the existence of direct intermolecular receptor interactions, dependence on the right quaternary structure should be established to unequivocally demonstrate that cross-antagonism is a biochemical property of a receptor heteromer. In the present study, with the use of selective disrupting peptides, we provide direct evidence for cross-antagonism as a biochemical property of the D1R-D3R heteromer. Again, this biochemical property was only selective for MAPK signaling, and it was not observed in cAMP experiments in D1R-D3R cells or CODA-RET experiments in transiently transfected cells. Cross-antagonism at the MAPK level could be secondary to an allosteric modulation at the level of β-arrestin-1 recruitment, in which cross-antagonism was also observed. Thus, G protein–independent and β-arrestin–dependent MAPK signaling has been well established for several GPCRs (Kovacs et al., 2009). In fact, CTX and PTX treatments could not counteract ERK1/2 activation induced by D1R and D3R agonists, respectively. Altogether, the present study indicates the existence of a functional selectivity of the allosteric interactions in the D1R-D3R heteromer, which modulate G protein–independent and β-arrestin–dependent signaling, but not G protein activation.
The differential modulation of G protein–dependent and independent signaling in D1R-D3R heteromers observed in the present study is difficult to reconcile with the idea that a main biochemical property of the D1R-D3R receptor heteromer is the ability of D3R activation to increase the affinity of D1R agonists (Fiorentini et al., 2008; Marcellino et al., 2008). In that case, D3R agonists should have also potentiated D1R-mediated Gs protein activation and adenylyl cyclase signaling (which we did not observe either in the presence or the absence of AC5). Instead, we observed identical concentration-response curves of D1R agonist-induced Gs conformational changes in transiently transfected cells and cAMP accumulation in D1R-D3R cells with or without concomitant exposure to the D3R agonist. Our results therefore indicate that the previously reported changes in affinity of D1R agonists upon D3R activation are not observable in all experimental conditions and might not only be dependent on D1R-D3R heteromerization.
The present study confirms the validity of the synthetic-peptide approach. It is at least safe to say that it can be successfully used to demonstrate that a biochemical finding corresponds to a biochemical property of a GPCR oligomer. The ability of selective peptides to simultaneously induce a modification of the quaternary structure of the D1R-D3R heteromer (alteration of YFP complementation) and to disrupt specific allosteric interactions of D1R and D3R ligands allowed establishing these as biochemical properties of the heteromer. Furthermore, comparing the effects of the same D1R TM peptides on the quaternary structure of D1R-D3R, D1R-D1R, and D3R-D3R oligomers allowed making some inferences about the oligomeric interfaces. The D1R TM5 and TM6 peptides significantly modified the quaternary structure of D1R-D3R. Interestingly, D1R TM5 peptide was also able to reduce D1R-D1R complementation, whereas TM6 and TM7 had no effect. This would suggest that D1R TM5 forms part of the D1R-D3R heteromer and D1R-D1R homomer interfaces. Importantly, D1R TM5- and TM6-mediated functional disruption was specific for the allosteric interactions between D1R and D3R ligands modulating MAPK signaling, without affecting the direct signaling mediated by D1R or D3R agonists, suggesting that D1 TM5 and TM6 are part of the conduit of those allosteric interactions.
Functional selectivity has gained more attention in GPCR research, because it can provide the means to obtained drugs with less secondary effects, avoiding nonwanted side effects associated with one of the signaling pathways (Reiter et al., 2012). Disentangling the intimate molecular mechanisms and conformational changes involved in the allosteric interactions in the D1R-D3R heteromer might therefore bring new aspects in the field. It has been postulated that the D1R-D3R heteromer constitutes an important functional unit in the brain and that it plays a role in several neuropsychiatric disorders, such as L-dopa–induced dyskinesia and drug addiction (Ferré et al., 2010, 2014), conditions in which there is upregulation of D3R (Staley and Mash, 1996; Ferré et al., 2010). As already shown in preclinical models of both conditions, dopamine D3R antagonists should be particularly useful, according to our study, by blocking both D3R- and D1R-mediated β-arrestin-1–dependent MAPK signaling in the striatal D1R-D3R heteromer. Our study suggests that evaluation of the effects of different ligands in D1R-D3R heteromer-expressing cell lines may provide new tools for the understanding of the pathophysiology and treatment of these disorders.
Acknowledgments
The authors thank Jasmina Jiménez for technical assistance; Dr. Jonathan Javitch (Columbia University), Dr. Celine Gales (INSERM), and Dr. Nevin Lambert (Georgia Regents University) for generously sharing various plasmid constructs for BRET experiments; Dr. Yoshitomi Ishikawa and Dr. Kenji Suita (Yokohama City University) for AC5 cDNA; and Dr. Marian Castro (Santiago de Compostela University) for β-arrestin-1-Rluc6 cDNA.
Abbreviations
- A1R
adenosine A1 receptor
- A2AR
A2A receptor
- AC5
adenylyl cyclase type 5
- BRET
bioluminescence resonance energy transfer
- CB1R
CB1 receptor
- CODA-RET
complemented donor-acceptor resonance energy transfer
- CTX
cholera toxin
- D1R
dopamine receptor type 1
- D3R
dopamine receptor type 3
- ERK
extracellular signal-regulated kinase
- GPCR
G protein–coupled receptor
- HEK
human embryonic kidney
- MAPK
mitogen-activated protein kinase
- 7-OH-PIPAT
(RS)-trans-7-hydroxy-2-[N-propyl-N-(3′-iodo-2′-propenyl)amino]tetralin maleate
- PEI
polyethylenimine
- PTX
pertussis toxin
- Rluc
Renilla luciferase 8
- SCH 23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- SKF 38393
(±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide
- TM
transmembrane domain
- YFP
yellow fluorescence protein
Authorship Contributions
Participated in research design: Guitart, Lluís, Casadó, McCormick, Mallol, Canela, Ferré.
Conducted experiments: Guitart, Navarro, Moreno, Yano, Cai, Sánchez-Soto, Kumar-Barodia, Naidu, Mallol, Cortés.
Performed data analysis: Guitart, Navarro, Moreno, Yano, Cai, Sánchez-Soto, Cortés, Casadó, Lluís, McCormick, Ferré.
Wrote or contributed to the writing of the manuscript: Ferré, Casadó, McCormick, Lluís, Guitart, Navarro, Moreno, Yano.
Footnotes
This work was supported by intramural funds of the National Institutes of Health National Institute on Drug Addiction; grants from Michael J. Fox Foundation for Parkinson’s Research [RRIA 2010], Spanish Ministerio de Ciencia y Tecnología [SAF2011-23813 and SAF2009-07276], and Government of Catalonia [2009-SGR-12]; and a grant [CB06/05/0064] from Centro de Investigación Biomédica en Red Sobre Enfermedades Neurodegenerativas. P.J.M. was supported through a Ramón y Cajal fellowship.
References
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. (2009) Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology 34:972–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banères JL, Parello J. (2003) Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol 329:815–829 [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Gómez-Soler M, Corrales F, Marcellino D, Narvaez M, Frankowska M, Flajolet M, Heintz N, et al. (2010) Characterization of the A2AR-D2R interface: focus on the role of the C-terminal tail and the transmembrane helices. Biochem Biophys Res Commun 402:801–807 [DOI] [PubMed] [Google Scholar]
- Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, Cortés A, Mallol J, Fuxe K, Canela EI, et al. (2008) Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods 5:727–733 [DOI] [PubMed] [Google Scholar]
- Casadó V, Cortés A, Ciruela F, Mallol J, Ferré S, Lluis C, Canela EI, Franco R. (2007) Old and new ways to calculate the affinity of agonists and antagonists interacting with G-protein-coupled monomeric and dimeric receptors: the receptor-dimer cooperativity index. Pharmacol Ther 116:343–354 [DOI] [PubMed] [Google Scholar]
- Ferrada C, Moreno E, Casadó V, Bongers G, Cortés A, Mallol J, Canela EI, Leurs R, Ferré S, Lluís C, et al. (2009) Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol 157:64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, et al. (2009) Building a new conceptual framework for receptor heteromers. Nat Chem Biol 5:131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Lluis C, Lanciego JL, Franco R. (2010) Prime time for G-protein-coupled receptor heteromers as therapeutic targets for CNS disorders: the dopamine D₁-D₃ receptor heteromer. CNS Neurol Disord Drug Targets 9:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin J-P, Guitart X. (2014) G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66:413–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. (2008) Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol 74:59–69 [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Spano P, Missale C. (2010) Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Curr Opin Pharmacol 10:87–92 [DOI] [PubMed] [Google Scholar]
- González S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, Borycz J, Ortiz J, Lluís C, Franco R, et al. (2012) Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol Psychiatry 17:650–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA. (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem 278:4385–4388 [DOI] [PubMed] [Google Scholar]
- Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J 27:2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, et al. (2011) Facilitation of μ-opioid receptor activity by preventing δ-opioid receptor-mediated codegradation. Neuron 69:120–131 [DOI] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. (1996) A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem 271:16384–16392 [DOI] [PubMed] [Google Scholar]
- Kerppola TK. (2006) Visualization of molecular interactions by fluorescence complementation. Nat Rev Mol Cell Biol 7:449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. (2009) Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 17:443–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Hong JH, Choi IY, Che Y, Lee JK, Yang SD, Song CW, Kang HS, Lee JH, Noh JS, et al. (2002) Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J Neurosci 22:7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, et al. (2008) Identification of dopamine D1-D3 receptor heteromers: indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem 283:26016–26025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P, Kamal M, Jockers R. (2011) Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol Sci 32:514–520 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2013) The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization. Mol Pharmacol 84:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Moreno-Delgado D, Navarro G, Hoffmann HM, Fuentes S, Rosell-Vilar S, Gasperini P, Rodríguez-Ruiz M, Medrano M, Mallol J, et al. (2014) Cocaine disrupts histamine H3 receptor modulation of dopamine D1 receptor signaling: σ1-D1-H3 receptor complexes as key targets for reducing cocaine’s effects. J Neurosci 34:3545–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Bakešová J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, Lluís C, Cortés A, Franco R, Casadó V, et al. (2011) Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS One 6:e16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. (2010) Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med 16:1393–1395 [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. (2012) Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52:179–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. (1997) Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Mol Pharmacol 52:508–514 [DOI] [PubMed] [Google Scholar]
- Robitaille M, Héroux I, Baragli A, Hébert TE. (2009) Novel tools for use in bioluminescence resonance energy transfer (BRET) assays. Methods Mol Biol 574:215–234 [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100–6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar E, Yano H, Kolster R, Galés C, Lambert N, Javitch JA. (2011) CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol 7:624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]