Abstract
The Nef protein enhances human immunodeficiency virus type 1 (HIV-1) replication through an unknown mechanism. We and others have previously reported that efficient HIV-1 replication in activated primary CD4+ T cells depends on the ability of Nef to downregulate CD4 from the cell surface. Here we demonstrate that Nef greatly enhances the infectivity of HIV-1 particles produced in primary T cells. Nef-defective HIV-1 particles contained significantly reduced quantities of gp120 on their surface; however, Nef did not affect the levels of virion-associated gp41, indicating that Nef indirectly stabilizes the association of gp120 with gp41. Surprisingly, Nef was not required for efficient replication of viruses that use CCR5 for entry, nor did Nef influence the infectivity or gp120 content of these virions. Nef also inhibited the incorporation of CD4 into HIV-1 particles released from primary T cells. We propose that Nef, by downregulating cell surface CD4, enhances HIV-1 replication by inhibiting CD4-induced dissociation of gp120 from gp41. The preferential requirement for Nef in the replication of X4-tropic HIV-1 suggests that the ability of Nef to downregulate CD4 may be most important at later stages of disease when X4-tropic viruses emerge.
The accessory gene Nef is a key virulence factor of primate lentiviruses. Nef markedly enhances viral replication and progression to AIDS in simian immunodeficiency virus (SIV)-infected macaques (34). In humans, a small subset of long term nonprogressors harbor human immunodeficiency virus type 1 (HIV-1) mutants encoding defective Nef genes (35, 45, 57). The absence of high viral loads associated with Nef-defective virus infection suggests that Nef promotes virus replication in vivo. In vitro, Nef enhances HIV-1 replication in primary T lymphocytes and macrophages (24, 47, 63, 65). Although the precise mechanism by which Nef enhances replication in primary T lymphocytes is unknown, we and others have reported that CD4 downregulation by Nef is strongly linked to HIV-1 replication in primary T lymphocytes, peripheral blood mononuclear cells (PBMC), and tonsillar histocultures (29, 43).
CD4 is the major receptor for HIV-1, HIV-2, and SIV (22, 37). Infected cells downregulate CD4 following HIV infection (22, 37). HIV-1 encodes three gene products, Env, Vpu, and Nef, that downregulate cell surface CD4 expression (for a review, see reference 38). At late times following infection, Env gp160 precursors bind and sequester newly formed CD4 molecules in the endoplasmic reticulum (7, 20, 32, 64). Vpu then interacts directly with the cytoplasmic tail of gp120-bound CD4, targeting this complex to the cellular proteasomal degradation pathway (8, 13, 71). Thus, both Env and Vpu function by preventing nascent CD4 from reaching the plasma membrane. Nef, in contrast, is expressed immediately following infection and stimulates the internalization and degradation of CD4 molecules already present on the surface of the infected cell prior to Env and Vpu expression (2, 12, 59). Nef interacts simultaneously with the cytoplasmic tail of CD4 and members of the cellular clathrin endocytic machinery, such as adaptor proteins (9, 19, 27, 30, 41), β-cop (5, 56), and/or vacuolar ATPase (42, 44). Nef thus directs infected cell surface CD4 to cellular endocytic pathways and to its subsequent degradation in lysosomes. The importance of CD4 downregulation for HIV-1 replication is supported by the observation that HIV replicates poorly in cells engineered to overexpress CD4 (17, 46). However, the mechanism by which CD4 downregulation by Nef enhances HIV replication in primary T cells is unknown.
In this study, we demonstrate that Nef markedly enhances the infectivity of progeny virions released from primary human T cells by (indirectly) stabilizing the association of gp120 with gp41. Interestingly, R5-tropic HIV-1, unlike X4-tropic HIV, replicated efficiently in the absence of a functional nef gene, and the Nef-defective progeny virions contained normal quantities of gp120. We further show that Nef prevents CD4 incorporation of both X4- and R5-tropic virions, suggesting that the greater sensitivity of X4-tropic Env to CD4-mediated interference determines the specific requirement for Nef in replication of X4-tropic HIV-1.
MATERIALS AND METHODS
Cells.
293T cells for virus production and HeLa-CD4/LTR-lacZ (P4/CCR5+) cells for infectivity assays were maintained in Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% fetal bovine serum (Atlanta Biologicals) and penicillin-streptomycin (Cellgro).
Highly purified, activated primary CD4+ T lymphocytes were prepared as described previously (43, 67), with a few modifications. Mononuclear cells were purified from buffy coats of the blood of seronegative donors by banding onto Ficoll-Hypaque (Amersham Biosciences; 30 min, 800 × g, 25°C). CD4+ cells were then isolated with two successive rounds of positive selection with anti-CD4 Dynabeads and subsequent release using Detachabead (Dynal Biotech). A 2:1 bead/cell ratio was used in both rounds to enhance cell purity. Homogeneity of cells (>99% CD4+ T cells) was confirmed by staining with CD3- and CD4-specific monoclonal antibodies (BD Pharmingen) and analysis by flow cytometry (data not shown). Purified CD4+ cells were subsequently activated with bacterial superantigen staphylococcal enterotoxin B (SEB; 100 ng/ml) and mitomycin C-killed PBMC from another donor (10:1 PBMC/CD4+ cell ratio). T cells were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (HyClone), MEM amino acids, l-glutamine, MEM vitamins, sodium pyruvate (Gibco), and penicillin plus streptomycin (Cellgro). Three days following stimulation, cells were split 1:2 in medium containing interleukin-2 (IL-2; NIH AIDS Research and Reference Reagent Program; 200 U/ml final concentration). Cultures were then split 1:2 every 2 days in IL-2 medium and infected with HIV-1 at 7 days poststimulation. Despite slightly different purification and activation protocols from our earlier study, X4-tropic HIV still required Nef and Nef-mediated CD4 downregulation for efficient replication in these activated CD4+ T cells (data not shown).
HIV-1 proviral constructs.
The X4-tropic proviral constructs R8 and Nef-defective R8ΔN were described previously (28). R8-based chimeras R8BaL and R8AD8 express the Env genes of the R5-tropic isolates BaL and AD8. R8BaL and R8BaLΔN, respectively, were created by substituting the SalI-to-BamHI fragment from the isolate BaL into the corresponding sites of R8 and R8ΔN as described previously (68). R8AD8 was derived from the viral construct pNL(AD8), a gift from E. Freed (26). R8AD8 and R8AD8ΔN were constructed by transferring the SalI-BamHI region of pNL(AD8) into the corresponding sites of R8 and R8ΔN. The X4-tropic HIV-1 proviral clones R7 and R7ΔN were described previously (2). SF2 and SF2ΔN were a gift from M. Tobiume and M. Matsuta. X4-tropic pNL4-3 and pNL4-3ΔX and R5-tropic clone pYU2 were obtained from the NIH AIDS Research and Reference Reagent Program. R5-tropic clones JR-CSF and nef-defective JR-CSFΔX were gifts from Jerome Zack (33). The YU2b chimera, containing the BssHII-XhoI fragment of YU2 inserted into the equivalent sites of R7, and YU2bΔN, containing an end-filled XhoI site within the Nef gene, were gifts from M. Feinberg and were described previously (47).
Chimeric NL4-3 proviruses containing alternate V3 loop regions, 123-74 (X4), 10-26 (R5), and 49-5 (R5), were gifts from B. Chesebro and were described previously (14). These viruses contain the V3 loops of an X4-tropic primary isolate, R5-tropic JR-CSF, and R5-tropic BaL, respectively. Nef-defective clones of these viruses, 123-74ΔX, 10-26ΔX, and 49-5ΔX, were created by end-filling of the XhoI site in Nef, producing a frameshift in the Nef open reading frame. Absence of Nef expression was confirmed by immunoblot analysis of viral lysates. R8-based viral clones expressing the Nef mutant proteins T80A, LL165AA, and DD175AA were constructed as described previously (43). Coreceptor usage of all cloned viruses was verified by differential infection of P4/CCR5− and P4/CCR5+ indicator cells (see below).
Virus production in 293T and primary T lymphocytes.
Virus stocks for replication analyses were produced via transient transfection of proviral plasmids in 293T cells, as previously described (1). Supernatants from transfected cells were clarified by filtration through 0.45-μm syringe filters, and aliquots were frozen at −80°C. Viruses were normalized for p24 antigen content by p24 enzyme-linked immunosorbent assay (ELISA) (70) prior to infection of primary T cells. Virus stocks were thawed once prior to use.
HIV-1 was also produced in primary T cells as follows. Activated CD4+ T cells (106) were inoculated with high-titer stocks (500 ng of p24) of 293T-derived viruses using centrifugal inoculation (2 h, 900 × g, 25°C) (52). Two days later, infected cells were collected using low-speed centrifugation. Cells were washed once with phosphate-buffered saline (PBS) and resuspended in fresh IL-2-containing medium. Three and 6 days later, virus supernatants were harvested and centrifuged to remove cell contaminants. Following removal of samples for p24 and infectivity assays, supernatants were used immediately for subsequent purification by velocity sedimentation.
Replication assays.
HIV-1 replication was analyzed as previously described (43). Briefly, 2 ng of each 293T-produced virus was inoculated onto 80,000 activated T cells in IL-2-containing medium (200 μl). At 2- or 3-day intervals, samples (100 μl) of the culture supernatants were removed and replenished with IL-2-containing medium. HIV-1 in the supernatants was quantified by p24-specific capture ELISA.
Single-cycle HIV-1 infection assays.
The infectivity of 293T-produced and primary T cell-produced virus was quantified using a HeLa-CD4/LTR-lacZ indicator cell line, P4/CCR5+, as described previously (3, 11). These cells stably express the primary HIV receptor CD4, the coreceptor CCR5, and an integrated lacZ reporter gene downstream of the HIV-1 long terminal repeat (LTR), as well as endogenous CXCR4. Dilutions of virus were inoculated onto 40,000 cells/well in 48-well dishes in the presence of DEAE-dextran (20 μg/ml). Expression of β-galactosidase in infected cells was visualized by staining with the colorimetric substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside 48 h postinfection. Infected cells were quantified by analysis of digital images of the stained cultures using NIH Image software. Infectivity values were calculated as the number of blue cells per nanogram of p24 of the input virus.
Soluble CD4 (sCD4) inhibition experiments were performed as follows. Viruses (harvested from 293T cells) were incubated with increasing amounts of sCD4 (Progenics Pharmaceuticals, Inc.) for 1 h at 37°C prior to addition of virus to target P4/CCR5+ cells. Dilutions of these viruses were added in the presence of the indicated concentrations of sCD4 to P4/CCR5+ cells, and infection was determined as described above.
Velocity gradient purification of HIV-1 particles.
To obtain highly purified HIV-1 for quantitative analysis of virion-associated gp120, gp41, and CD4, we employed 6-to-18% linear iodixinol (Optiprep) velocity centrifugation gradients, similar to a previously described method (25). Following centrifugation at 35,000 rpm for 1.5 h (210,000 × g maximum; SW41 Beckman), fractions (1 ml) were collected. PBS (0.5 ml) was added to each fraction to reduce the solution density, and virions were pelleted at 100,000 × g for 20 min in a Beckman TLA-55 rotor. After removal of the supernatants, HIV-1 pellets were lysed by resuspension in ELISA sample diluent (10% calf serum, 0.5% TX-100 in PBS), and the lysates were assayed for p24, gp120, gp41, and CD4 by antigen capture ELISA.
Quantitative ELISAs for gp120, gp41, and CD4.
For the gp120 capture ELISA, polyclonal sheep anti-gp120 peptide (4 μg/ml; Cliniqa Inc.) was used as a gp120-specific capture antibody to coat Immunolon II plates (Dynex). Plates were subsequently blocked for 1 h using 5% bovine calf serum in PBS and washed. Dilutions of the virus lysates in ELISA sample diluent were added and incubated for 2 h at 37°C. Following washing, the human gp120-specific monoclonal 2G12 (0.67 μg/ml; AIDS Research and Reference Reagent Program) was added and allowed to bind for 1 h at 37°C. Polyclonal horseradish peroxidase (HRP)-conjugated goat antibody specific for human immunoglobulin G (0.2 μg/ml; Pierce) was used as a detector for 1 h at 37°C, and the signal was revealed using TMB (3,3,′,5,5′-tetramethylbenzidine) substrate (Kirkegaard & Perry Laboratories). Dilutions of purified gp120IIIB (NIH AIDS Research and Reference Reagent Program) were used to construct a standard curve. As a control to test whether primary T-cell-derived CD4 might interfere with the detection of gp120 on purified virions, we incubated virions with sCD4 (1 μg/ml) and assayed the lysates for gp120 by ELISA. The results demonstrated that the gp120 ELISA was not significantly affected by the presence of sCD4.
For the gp41 capture ELISA, the mouse anti-gp41 monoclonal antibody (MAb) Chessie 8 (5 μg/ml; NIH AIDS Research and Reference Reagent Program) was used for capture. The gp41-specific human MAb 5F3 (0.67 μg/ml; NIH AIDS Research and Reference Reagent Program) was utilized as a primary detector antibody and anti-human HRP (0.2 μg/ml; Pierce) was used as a secondary antibody. As a standard for comparison, we used dilutions of clarified 293T-produced HIV-1. Dilutions of an HIV-1 viral lysate were used to construct a standard curve, assuming a 1:1 stoichiometry and 3:1 weight ratio of gp120 to gp41 associated with purified 293T-derived virions.
For the CD4 capture ELISA, we used a mouse anti-CD4-specific MAb (4 μg/ml; Trinity Biotech) as a capture antibody. This antibody was chosen for its inability to block HIV infection in order to permit efficient capture of CD4 whether or not bound to gp120. As primary and secondary antibodies, we utilized the polyclonal CD4-specific antiserum 806 (1:25,000 dilution; NIH AIDS Research and Reference Reagent Program) and HRP-conjugated anti-rabbit (0.2 μg/ml; Pierce), respectively. Recombinant sCD4 was utilized to construct a standard curve for quantitation.
RESULTS
Coreceptor-dependent requirement for Nef in HIV-1 replication in activated primary CD4+ T cells.
Our laboratory previously reported that CD4 downregulation by Nef markedly enhances the replication of HIV-1 derived from an X4-tropic molecular clone (43). During the course of that study, we also observed that a virus encoding the env gene from the R5-tropic BaL isolate replicated efficiently in the absence of a functional nef gene. Based on these findings, we hypothesized that the requirement for Nef in HIV-1 replication depends on viral coreceptor usage. To test this hypothesis, we assayed the replication of a variety of cloned X4- and R5-tropic nef+ and nef-defective viruses. As a cell culture system for our studies, we utilized highly purified CD4+ T cells activated with bacterial superantigen (SEB) and mitomycin C-killed allogeneic PBMC (43, 67). This culture system minimizes variability that may arise from CD8+ T cells and macrophages, which secrete factors that influence HIV-1 replication (16, 69). Furthermore, the use of a T-cell receptor ligand (SEB) and costimulation with antigen-presenting cells likely represents a more physiological method of T-cell activation than nonspecific phytohemagglutinin (PHA)-mediated cross-linking of T-cell surface molecules. These cells were preferable to PHA-activated PBMC, owing to the greater HIV-1 replication and the strong reproducible effect of Nef observed with this culture system.
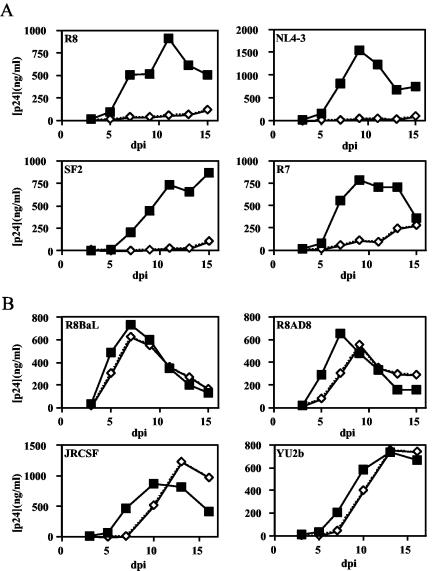
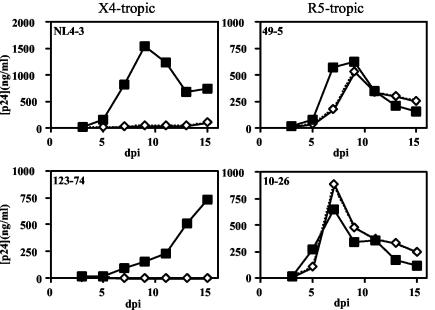
The X4-tropic isolates R8, NL4-3, R7, and SF2 replicated well in activated CD4+ T cells (Fig. 1A); however, the nef-defective mutants of these viruses replicated poorly. By contrast, the R5-tropic viruses R8BaL, R8AD8, JR-CSF, and YU2b replicated efficiently irrespective of their nef genotype, with only slight delays in the replication of the nef-defective viruses (Fig. 1B). To determine whether the differential requirement of Nef for replication of X4- and R5-tropic viruses resulted from alternative coreceptor usage and not other genetic disparities (such as in other regions of env or vpu) that may compensate for the absence of the Nef protein, we examined three additional chimeric viruses for their requirement for Nef for efficient replication. Each chimera (123-74, 49-5, and 10-26) is genetically identical to the X4-tropic NL4-3 virus except for the V3 loop of gp120, which determines coreceptor utilization. We observed that, although the X4-tropic NL43 and 123-74 viruses required Nef for replication, R5-tropic chimeras 10-26 and 49-5 did not (Fig. 2). Thus, the requirement for Nef in HIV replication maps to the V3 loop region of gp120.
FIG. 1.
Nef is specifically required for efficient replication of X4-tropic HIV-1 in activated primary CD4+ T cells. Wild-type and nef-defective X4-tropic (A) and R5-tropic (B) virus clones were cultured in activated highly purified CD4+ T lymphocytes as described in Materials and Methods. Results shown are the averages of duplicate growth curves from a representative of two experiments. Filled squares, nef+ HIV-1; open diamonds, nef-deficient HIV-1.
FIG. 2.
The requirement for Nef in HIV-1 replication maps to the V3 loop of gp120. NL4-3-based V3 loop chimeras conferring different tropisms were cultured in activated CD4+ primary T lymphocytes as described in Materials and Methods. Results shown are the averages of duplicate growth curves from a representative of eight experiments. Filled squares, nef+ HIV-1; open diamonds, nef-deficient HIV-1.
Requirement for Nef for optimal infectivity of X4-tropic virus produced in T lymphocytes.
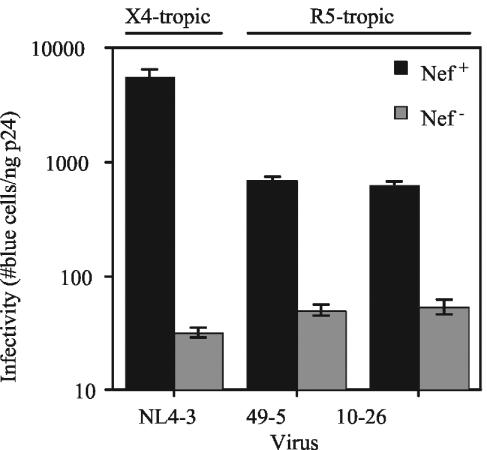
CD4 downregulation by Nef markedly enhances the replication of X4-tropic HIV-1 in primary T lymphocytes (29, 43). Removal of cell surface CD4 during virion production is likely advantageous to the progeny HIV virion particle and its ability to infect subsequent cells (17, 39). We hypothesized that Nef, by downregulating CD4, enhances the infectivity of HIV-1 particles released from primary T cells, therefore promoting viral spread. To test this, wild-type and nef-defective HIV-1 particles were harvested from T cells infected at high multiplicity, and infectivity was determined by titration on a HeLa-CD4/LTR-lacZ indicator cell line (P4/CCR5+). We found that Nef increased the infectivity of the X4-tropic NL4-3 virus by 170-fold (Fig. 3). In contrast, Nef only moderately enhanced the infectivity of the R5-tropic viruses 49-5 and 10-26 (13.5- and 11.5-fold, respectively). These effects were similar in magnitude to the 10-fold effect of Nef typically observed for HIV-1 produced in CD4-negative cells (1, 3, 15, 48, 53) (see Table S1 in the supplemental material). Coreceptor-specific infectivity differences were also observed for R8 and R8BaL (120- and 24-fold, respectively). As a control, we assayed the infectivity of the X4- and R5-tropic viruses when produced by transfection of 293T cells with proviral DNA. Although the ability of Nef to enhance the infectivity of virions produced in 293T cells was highly dependent on the viral strain, we found that there was no correlation between the magnitude of CD4-independent infectivity enhancement by Nef and viral coreceptor usage of the viruses used throughout this study (see Table S2 in the supplemental material). We thus conclude that Nef preferentially enhances the infectivity of X4-tropic virus produced in CD4+ primary T lymphocytes.
FIG. 3.
Nef is required for optimal infectivity of X4-tropic virions released from HIV-1-infected primary T lymphocytes. Viruses were harvested 5 days following initial inoculation, and infectivity was analyzed on P4/CCR5+ reporter cells as described in Materials and Methods. Values shown are the number of blue cells per nanogram of p24 of input virus. Results shown are the mean values and standard deviations and are a representative of six experiments. Filled bars, nef+ HIV-1; shaded bars, nef-deficient HIV-1.
Nef enhances the gp120 content of X4-tropic virions produced in primary T lymphocytes.
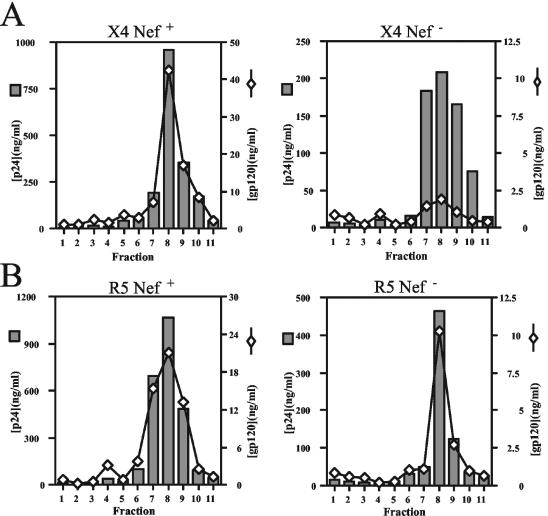
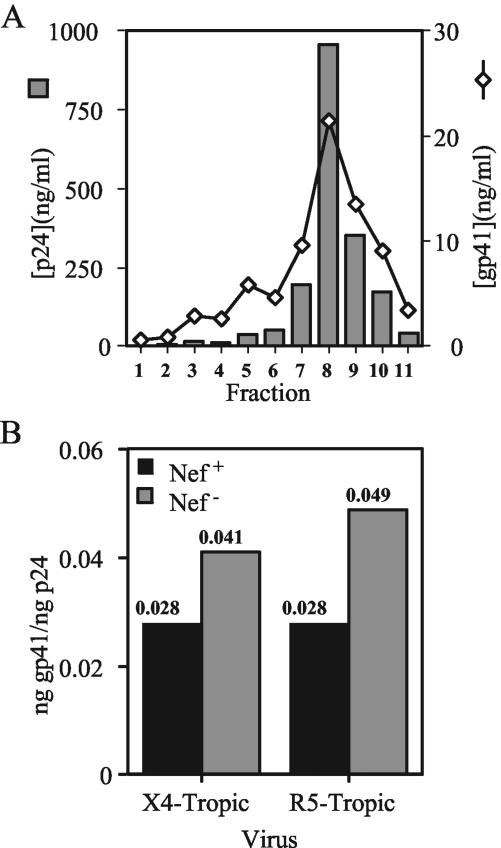
CD4 may bind to Env on the surface of the cell and prevent incorporation of Env into progeny virions, thereby impairing HIV-1 infectivity (4, 17, 39). By downregulating CD4, Nef may prevent CD4-induced inhibition of Env incorporation. We therefore asked whether Nef affects the levels of Env incorporation into virions released from primary T cells. To test this, we quantified the levels of virion-associated HIV-1 Env proteins by antigen capture ELISAs. To ensure that the virus samples were free of cell-derived microvesicles that could confound the analyses, we utilized velocity gradient sedimentation to purify virions from the supernatants of infected T lymphocytes. Following pelleting of virions in gradient fractions and subsequent lysis of the pellets, we performed p24-specific capture ELISAs to determine the virus quantities in the fractions. For both X4-tropic NL4-3 and R5-tropic 49-5, the majority of virions were found in fractions 7 to 10 (Fig. 4). Analysis of the fractions by gp120-specific ELISA revealed that gp120 was present in the same fractions as p24. There was a marked decrease in gp120 per p24 content across the gradient of X4-tropic NL4-3 Nef-defective virus (Fig. 4A). When total gp120 in peak fractions was calculated and divided by the p24 content of these fractions, we found that there was 5.8-fold more gp120 per p24 in purified X4-tropic NL4-3 than the nef-defective NL4-3ΔX. Similar differences were observed with the R8 and R8ΔN viruses (>4-fold [data not shown]). In contrast, identical quantities of gp120 were present on wild-type and Nef-defective 49-5 virions (Fig. 4B). Similar results were observed in purified 10-26 and R8BaL virus (1.4- and 1.3-fold, respectively [data not shown]). Thus, Nef did not significantly affect the gp120 levels of R5-tropic HIV-1 derived from primary CD4+ T cells. Although the data shown are from a single representative experiment, the results from four individual experiments are presented in Table 1. In contrast, nef+ and nef-defective virions contained identical quantities of gp120 when harvested from CD4-negative 293T cells, irrespective of the coreceptor use of the virus (data not shown). We conclude that Nef specifically enhances the gp120 levels of X4-tropic virions produced in primary T cells.
FIG. 4.
Nef enhances the gp120 content of X4-tropic virions purified from primary T lymphocytes. X4-tropic (A) and R5-tropic (B) viruses were purified 8 days postinfection, and ELISAs specific for p24 and gp120 were performed as described in Materials and Methods. Shown is a representative of four experiments. Values shown are nanograms per milliliter for both p24 and gp120. Shaded bars, p24; open diamonds, gp120.
TABLE 1.
Nef greatly enhances virion infectivity and gp120 content and prevents CD4 incorporation of X4-tropic, NL4-3 virions produced in CD4+ primary T lymphocytes
| Expt no.a | Virus | Infectivity | Fold difference (nef+/nef defective) | gp120/p24 | gp41/p24 | CD4/p24 |
|---|---|---|---|---|---|---|
| 1 | NL4-3 (nef+) | 360 ± 34 | 40 | 0.0450 | 0.028 | 0.0013 |
| NL4-3 (nef defective) | 9 ± 1.3 | 0.0077 | 0.041 | 0.0060 | ||
| 49-5 (nef+) | 77 ± 12 | 11 | 0.0220 | 0.028 | 0.0013 | |
| 49-5 (nef defective) | 6.9 ± 0.9 | 0.0220 | 0.049 | 0.0045 | ||
| 10-26 (nef+) | 160 ± 12 | 13 | 0.0580 | 0.040 | 0.0017 | |
| 10-26 (nef defective) | 12 ± 0.7 | 0.0400 | 0.044 | 0.0035 | ||
| 2 | NL4-3 (nef+) | 970 ± 130 | 190 | 0.0110 | NDb | 0.0055 |
| NL4-3 (nef defective) | 5.2 ± 1.3 | 0.0027 | ND | 0.0440 | ||
| 49-5 (nef+) | 210 ± 11 | 25 | 0.0190 | ND | 0.0008 | |
| 49-5 (nef defective) | 8.3 ± 1.0 | 0.0170 | ND | 0.0022 | ||
| 10-26 (nef+) | 330 ± 81 | 18 | 0.0430 | ND | 0.0011 | |
| 10-26 (nef defective) | 18 ± 3.0 | 0.0370 | ND | 0.0027 | ||
| 3A | NL4-3 (nef+) | 5,500 ± 880 | 170 | 0.0120 | 0.017 | 0.0022 |
| NL4-3 (nef defective) | 32 ± 2.9 | 0.0052 | 0.014 | 0.0120 | ||
| 49-5 (nef+) | 680 ± 54 | 14 | 0.0380 | 0.024 | 0.0020 | |
| 49-5 (nef defective) | 50 ± 5.4 | 0.0330 | 0.027 | 0.0038 | ||
| 10-26 (nef+) | 620 ± 43 | 11 | 0.0610 | 0.023 | 0.0013 | |
| 10-26 (nef defective) | 54 ± 7.8 | 0.0560 | 0.032 | 0.0040 | ||
| 3B | NL4-3 (nef+) | 1,500 ± 260 | 140 | 0.0110 | 0.0170 | 0.0028 |
| NL4-3 (nef defective) | 11 ± 1.1 | 0.0047 | 0.0094 | 0.0140 | ||
| 49-5 (nef+) | 310 ± 25 | 12 | 0.0290 | 0.0290 | 0.0047 | |
| 49-5 (nef defective) | 26 ± 0.7 | 0.0230 | 0.0380 | 0.0090 | ||
| 10-26 (nef+) | 320 ± 49 | 15 | 0.0480 | 0.0200 | 0.0024 | |
| 10-26 (nef defective) | 22 ± 4.1 | 0.0490 | 0.0260 | 0.0050 |
Experiments shown are the results of three independent experiments consisting of four donors total. While experiments 1 and 2 were independent, experiments 3A and 3B were performed together in parallel.
ND, not determined.
The decreased levels of gp120 on Nef-defective X4-tropic HIV-1 particles suggested either that Nef is required for incorporation of the Env complex into virions or that Nef stabilizes gp120 on the surface of X4-tropic virions. To distinguish between these possibilities, we determined the gp41 levels on virions purified from T cells. Due to difficulties in obtaining sufficient quantities of Nef-defective X4-tropic HIV-1 particles from primary T cells for Western blotting, we established a sensitive gp41-specific capture ELISA (see Materials and Methods). Using this assay, we observed that gp41 peaked at the p24-containing gradient fractions (Fig. 5A). We calculated the total quantities of gp41 in peak virus fractions and normalized them to total p24. The results revealed that nef-defective NL4-3 virions contained slightly elevated (1.5-fold) levels of gp41 relative to levels in nef+ virions (Fig. 5B). We observed similar results for the R5-tropic 49-5 and 10-26 viruses (1.8- and 1.1-fold, respectively) (Fig. 5B and data not shown). The results of additional experiments analyzing virion-associated gp41 levels are presented in Table 1. By contrast, Nef did not affect the gp41 levels of these viruses when harvested from CD4-negative 293T cells (data not shown). We conclude that Nef has only minimal effects on the levels of gp41 on HIV-1 particles produced in CD4+ primary T cells irrespective of the coreceptor usage of the virus. Thus, the reduced gp120 content of nef-defective, X4-tropic virions appears to result from dissociation of gp120 from gp41.
FIG. 5.
Nef does not affect gp41 content in X4- and R5-tropic virions purified from primary T lymphocytes. ELISAs were performed on gradient fractions containing purified T-cell virus as described in Materials and Methods. (A) Levels of gp41 from a representative purification of NL4-3 virions. Shaded bars, p24; open diamonds, gp120. (B) Total gp41 in peak fractions was divided by total p24 to calculate virion-associated gp41. Shown are the results of a representative of four experiments. Filled bars, nef+ HIV-1; shaded bars, nef-deficient HIV-1.
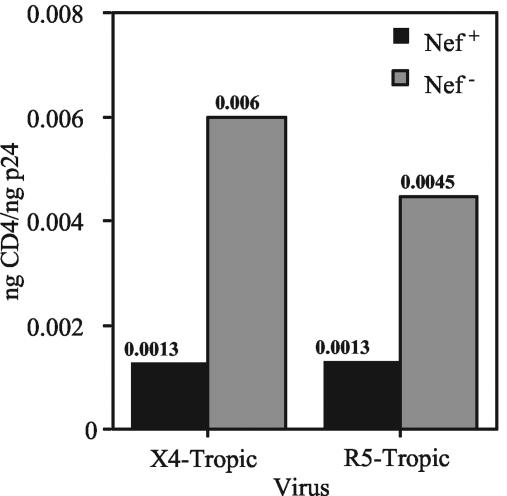
Nef prevents CD4 incorporation into X4- and R5-tropic HIV-1 virions.
We and others previously showed that HIV-1 replication is strongly linked to the ability of Nef to downregulate CD4 (29, 43). Incorporation of CD4 into HIV-1 particles could result in gp120 shedding and/or interfere with its function, thereby inhibiting replication (17, 39). We therefore asked whether Nef prevents CD4 incorporation into HIV-1 particles harvested from primary T cells. To quantify levels of CD4 on nef+ and nef-defective HIV-1 particles, we established a sensitive, CD4-specific ELISA and assayed lysates of gradient-purified virions. The results demonstrated that expression of Nef resulted in decreased levels of virion-associated CD4, irrespective of coreceptor usage of the virus (4.6- and 3.4-fold for NL4-3 and 49-5, respectively) (Fig. 6). Nef also inhibited CD4 incorporation into 10-26 virions, but by only 2.1-fold (data not shown). Thus, the effect of Nef on CD4 incorporation was somewhat strain dependent, suggesting that CD4 incorporation can be affected by determinants in Env.
FIG. 6.
Nef prevents incorporation of CD4 into X4- and R5-tropic virions produced in CD4+ primary T lymphocytes. CD4 capture ELISAs were performed on gradient-purified virions as described in Materials and Methods. The total of the CD4 in peak fractions was divided by the total p24 to calculate virion-associated CD4. Shown are the results of a representative of four experiments. Filled bars, nef+ HIV-1; shaded bars, nef-deficient HIV-1.
To further examine the connection between Nef, CD4, and gp120, we used the results from the CD4 and gp120 assays to calculate the ratio of CD4 to gp120 on the purified virions. As shown in Table 2, X4- and R5-tropic virions contained low ratios of CD4 to gp120 when the viruses expressed Nef. However, the Nef-defective variant of NL4-3 exhibited a high ratio of CD4 to gp120. The ratio suggests that on a molar basis, the level of CD4 actually exceeded that of gp120 on these virions. By contrast, both of the R5-tropic viruses maintained low CD4/gp120 ratios even in the absence of Nef. These results suggest that both the low levels of gp120 and the high levels of CD4 on nef-defective X4-tropic HIV-1 particles are responsible for the poor infectivity of these virions.
TABLE 2.
Ratios of CD4 to gp120 on HIV-1 cultured in CD4+ primary T cells
| Virus | CD4/p24 | gp120/p24 | CD4/gp120 | Fold difference CD4/gp120 (nef defective/nef+) |
|---|---|---|---|---|
| NL4-3 (nef+) | 0.00130 | 0.0445 | 0.029 | 27 |
| NL4-3 (nef defective) | 0.00601 | 0.0077 | 0.78 | |
| 49-5 (nef+) | 0.00131 | 0.0216 | 0.061 | 3.3 |
| 49-5 (nef defective) | 0.00447 | 0.0223 | 0.20 | |
| 10-26 (nef+) | 0.00167 | 0.0576 | 0.029 | 3.0 |
| 10-26 (nef defective) | 0.00352 | 0.040 | 0.088 |
Nef-mediated CD4 downregulation enhances progeny virion gp120 content and prevents CD4 incorporation.
Multiple functions of Nef, including CD4 downregulation and interaction with cellular signaling pathways, are important for optimal HIV replication in activated PBMC and CD4+ primary T lymphocytes (18, 29, 43, 60, 72). Although the large defects in the infectivity and gp120 content of X4-tropic, Nef-defective HIV virions produced in primary T cells likely result from the incapacity of these viruses to downregulate cell surface CD4, it is entirely possible that the absence of another function of Nef is responsible for these defects. To test whether these virion defects are indeed linked to CD4 downregulation, we extended our analysis to Nef mutants selectively deficient in this activity.
We found that the CD4 downregulation-deficient Nef point mutants, LL165AA and DD175AA, were quantitatively similar to Nef-defective virions when produced in CD4+ T cells (Table 3). These mutants not only replicated poorly (reference 43 and data not shown), but also had low gp120 content, higher levels of CD4, and were poorly infectious relative to wild-type virions. As a control, CD4 downregulation-competent T80A mutant virions were found to be comparable to the wild-type virions when produced in primary T cells. This mutant was impaired for infectivity enhancement when produced in 293T cells (43). However, T80A virions produced in primary T cells had similar gp120 content and CD4 levels relative to wild-type virions. Together, these data suggest that CD4 downregulation by Nef is required for (i) optimal infectivity, (ii) efficient gp120 incorporation, and (iii) reduced CD4 incorporation when X4-tropic virions are produced in primary CD4+ T lymphocytes.
TABLE 3.
CD4 downregulation by Nef enhances gp120 and decreases CD4 content of X4-tropic HIV produced in CD4+ primary T lymphocytes
| Expt no.a | Virus | Infectivity | Fold difference (nef+/nef mutant) | gp120/p24 | gp41/p24 | CD4/p24 |
|---|---|---|---|---|---|---|
| 1A | R8 (nef+) | 2,500 ± 370 | 0.039 | 0.030 | 0.0012 | |
| R8 (nef defective) | 40 ± 9 | 63 | 0.019 | 0.026 | 0.0077 | |
| LL165AA | 540 ± 160 | 4.6 | 0.024 | 0.025 | 0.0048 | |
| DD175AA | 440 ± 51 | 5.7 | 0.021 | 0.027 | 0.0058 | |
| T80A | 880 ± 280 | 2.8 | 0.037 | 0.032 | 0.0014 | |
| 1B | R8 (nef+) | 6,000 ± 1,500 | 0.055 | 0.030 | 0.0019 | |
| R8 (nef defective) | 50 ± 8 | 120 | 0.023 | 0.031 | 0.0170 | |
| LL165AA | 340 ± 130 | 18 | 0.016 | 0.021 | 0.0099 | |
| DD175AA | 250 ± 55 | 24 | 0.032 | 0.024 | 0.0058 | |
| T80A | 1,300 ± 260 | 4.6 | 0.047 | 0.027 | 0.0021 | |
| 2A | R8 (nef+) | 2,700 ± 210 | 0.030 | 0.041 | 0.0084 | |
| R8 (nef defective) | 80 ± 10 | 34 | 0.010 | 0.032 | 0.0390 | |
| LL165AA | 290 ± 27 | 9.3 | 0.014 | 0.030 | 0.0240 | |
| DD175AA | 400 ± 36 | 6.8 | 0.012 | 0.024 | 0.0170 | |
| T80A | 580 ± 9 | 4.7 | 0.021 | 0.029 | 0.0075 | |
| 2B | R8 (nef+) | 6,300 ± 240 | 0.035 | 0.029 | 0.0025 | |
| R8 (nef defective) | 90 ± 15 | 70 | 0.013 | 0.027 | 0.0140 | |
| LL165AA | 410 ± 32 | 15 | 0.013 | 0.024 | 0.0120 | |
| DD175AA | 720 ± 110 | 8.8 | 0.013 | 0.018 | 0.0074 | |
| T80A | 1,900 ± 78 | 3.3 | 0.026 | 0.030 | 0.0036 |
Experiments shown are the results of two separate donors (A and B) for two independent experiments.
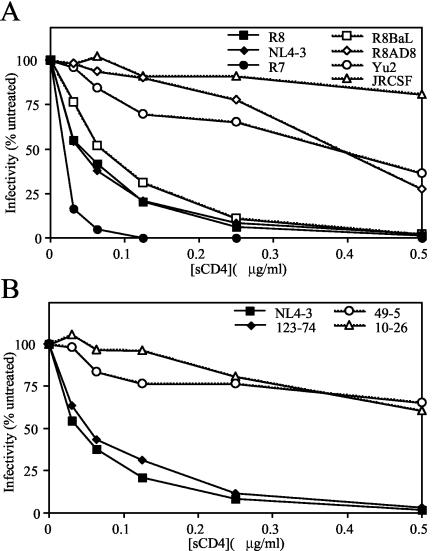
Infection by X4-tropic HIV-1 is more sensitive to inhibition by sCD4.
Nef-defective virions contained higher levels of CD4 than wild-type HIV-1. Infection by HIV-1 laboratory strains (predominately X4 tropic) is more susceptible to inhibition by sCD4 than are HIV-1 primary isolates (which are normally R5 tropic) (21, 49, 62). We hypothesized that virion-associated CD4 more potently inhibits infection by X4-tropic viruses, thereby preferentially inhibiting replication of nef-defective X4-tropic replication. To test this hypothesis, we quantified infection of P4/CCR5+ cells following incubation of X4- and R5-tropic viruses with sCD4. As shown in Fig. 7A, X4-tropic isolates produced in 293T cells were considerably more sensitive to inhibition of infectivity by sCD4 than R5-tropic isolates. An apparent exception was the R5-tropic virus R8BaL, which was more sensitive to sCD4 than the other R5-tropic viruses. Similar results were observed when viruses were produced in primary CD4+ T cells. Although the viruses analyzed in Fig. 7A expressed Nef, the absence of Nef expression did not affect the sCD4 sensitivity of the X4-tropic R8 or R5-tropic R8BaL viruses, whether the virions were produced in 293T cells or primary CD4+ T cells (data not shown).
FIG. 7.
sCD4 preferentially inhibits infection by X4-tropic HIV-1. X4- and R5-tropic viral isolates (A) and V3 loop mutants (B) were produced in 293T cells. Virions were treated with the indicated concentrations of sCD4 and assayed for infection on P4/CCR5+ reporter cells as described in Materials and Methods. Values are expressed as a percentage of the infectivity of the corresponding untreated virus. Shown is a representative of up to three experiments. Filled symbols, X4-tropic HIV-1; open symbols, R5-tropic HIV-1.
To determine whether the sensitivity to sCD4 is determined by the V3 loop of gp120, we tested the infectivity of the V3 loop chimeric viruses NL4-3, 123-74, 49-5, and 10-26 following preincubation with sCD4 (Fig. 7B). We observed that the X4-tropic virus chimeras NL4-3 and 123-74 exhibited greater sensitivity to sCD4 than the R5-tropic chimeric viruses 49-5 and 10-26, whether virions were produced in 293T (Fig. 7B) or CD4+ primary T cells (data not shown). Nef protein expression did not affect sCD4 sensitivity of X4-tropic NL4-3 and R5-tropic 49-5 and 10-26 virions (data not shown). We conclude that the sensitivity to sCD4 is modulated by sequences in the V3 loop of gp120.
DISCUSSION
In this study, we investigated the mechanism by which Nef promotes HIV-1 replication in primary CD4+ T lymphocytes. Nef strongly enhanced the infectivity of X4-tropic HIV-1. Nef also markedly increased the levels of gp120 associated with HIV-1 particles. However, Nef did not significantly alter the levels of virion-associated gp41, indicating that Nef stabilizes the association between gp120 and gp41 on the cell and/or viral membrane. We also found that Nef-defective HIV-1 strains that utilize CCR5 as a coreceptor, unlike CXCR4-dependent viruses, replicate efficiently in activated primary T cells. Nef-defective, R5-tropic virions exhibited normal infectivity and gp120 content when released from these cells, further supporting a functional connection between reduced virion gp120, reduced infectivity, and impaired replication of Nef-defective, X4-tropic HIV-1. Nef also prevented CD4 incorporation into HIV-1 virions from primary T cells, irrespective of the coreceptor usage of the virus.
Some of our data are in contrast with those in a study by Papkalla and coworkers (54), who reported that Nef is required for replication of both X4- and R5-tropic HIV-1 in PHA-activated PBMC. In contrast, we observed that R5-tropic HIV-1 replicated efficiently in the absence of Nef in purified CD4+ T cells activated by superantigen and killed antigen-presenting cells (mitomycin C-treated PBMC). These seemingly contradictory observations may arise from differences in the culture systems used, including differences in activation or the presence of cells in PBMC that may specifically affect the replication of R5-tropic HIV-1, including CD8+ T cells, macrophages, and dendritic cells. For our studies, the purified cell system offered advantages over PHA-activated PBMC, including more-robust HIV-1 replication and a highly reproducible requirement of Nef for the growth of X4-tropic viruses. The purified cell system also appeared less prone to donor-to-donor variability than experiments in activated PBMC cultures. Although we do not yet know the precise cause of the differences between our results and those of Papkalla et al., identification of a specific cell type in PBMC that regulates the requirement of Nef for replication of R5-tropic viruses may represent an interesting goal for future studies.
Our results revealed that X4-tropic nef-defective virus produced in primary T lymphocytes was markedly impaired for infectivity. We propose that, through removal of CD4 from the cell surface, Nef enhances viral infectivity by facilitating the incorporation of gp120 into nascent virions and by preventing virion incorporation of CD4. Similar conclusions were reached in studies by Lama and coworkers (4, 39), who showed that expression of CD4 in 293T and Jurkat cells inhibited the incorporation of Env into HIV-1 particles and that Nef overcame the inhibition by downregulating CD4 expression. Our findings extend these conclusions to a physiologically relevant cell type, primary CD4+ T cells. The magnitude of the infectivity decrease appears to be sufficient to account for the impaired replication of Nef-defective HIV-1. Although Nef also enhances the infectivity of cell-free HIV-1 produced in the absence of CD4 (1, 3, 15, 48, 53), we previously concluded that CD4 downregulation is the major activity of Nef required for HIV-1 replication in cultured primary T cells. This conclusion was based on previous studies of Nef point mutants, in which the efficiency of replication correlated strongly with the ability of Nef to downregulate CD4 but not with CD4-independent infectivity enhancement (29, 43). The infectivity of X4- and R5-tropic HIV-1 is enhanced by Nef to a similar extent when produced in cells lacking CD4 (reference 54 and results presented herein). Thus, the ability of R5-tropic HIV-1 to replicate efficiently in the absence of Nef further reinforces the conclusion that CD4-independent infectivity enhancement plays a relatively minor role in HIV-1 replication.
In this study, we found that Nef-defective HIV-1 particles contained significantly lower gp120 levels when the Env was specific for the CXCR4 coreceptor. The differential loss of gp120 between X4- and R5-tropic HIV-1 may be due to differences in the inherent stability of the gp120-gp41 complex, or to differences in gp120 conformational changes induced upon CD4 binding. Previous studies have shown that laboratory-adapted strains of HIV-1, which are generally X4-tropic, readily shed gp120 upon addition of soluble CD4 and are more sensitive to inhibition by sCD4 (31, 36, 50, 61). The preferential inhibition of X4-tropic HIV-1 by sCD4 is also in general agreement with the hypothesis that cellular CD4 is more detrimental for replication of X4-tropic HIV-1. However, virions containing gp120 from the R5-tropic isolate BaL were sensitive to sCD4, but like the other R5-tropic viruses examined, this virus replicated efficiently in the absence of Nef. This finding indicates the lack of a strict correlation between the degree of sensitivity to sCD4 and the requirement for Nef in HIV-1 replication. We observed similar CD4 surface expression levels of the CXCR4- and CCR5-expressing cells in our T cell cultures (see Fig. S1 in the supplemental material), suggesting that the specific infectivity and gp120 defects of X4-tropic nef-defective virions are not due to increased CD4 levels of the CXCR4+ cell population. It remains possible that other factors present on infected cells, such as the specific coreceptor, may contribute to the apparent resistance of R5-tropic gp120 to the inhibitory effects of cellular CD4.
In addition to the inhibitory effect of CD4 on cell-free virus, it is also possible that during continuous viral replication, HIV-1 transmission may be more efficient for R5-tropic HIV-1. Recent studies have shown that R5-tropic HIV-1 is less sensitive to some inhibitors of fusion, such as T-20, and that this resistance is associated with faster fusion kinetics and higher gp120 affinity for coreceptor (23, 58). During continuous replication, HIV-1 may spread through multiple modes, including cell-to-cell transmission as well as infection by cell-free virions. Considering that X4- and R5-tropic Env proteins mediate fusion at different rates, it is likely that the mechanisms by which HIV-1 spreads through a culture of T cells depend on the particular coreceptor used for entry. It is also possible that the CD4+ T cells infected by X4- and R5-tropic viruses represent distinct cell subsets (6, 40) and that the requirement for Nef depends on the producer cell. We have thus far been unable to demonstrate a differential effect of CD4 expression on the infectivity of X4- and R5-tropic HIV-1 when viruses are produced from 293T cells (unpublished data). Thus, the specific requirement for Nef in X4-tropic HIV-1 may be unique to HIV-1 released from primary T cells.
Our finding that Nef is not required for efficient replication of R5-tropic HIV-1 in primary T cells has implications for HIV-1 pathogenesis. Recent studies have demonstrated that various activities of Nef are more highly conserved in HIV-1 and SIV isolates recovered early or late following initial infection of the host. Analysis of the Nef genes isolated from infected individuals and monkeys have shown that major histocompatibility complex (MHC) class I downregulation is a common feature of Nef isolates present at early stages of infection (10, 51). In contrast, CD4 downregulation is more highly conserved in Nef genes from late stages of HIV and SIV infection (4, 55) and in AIDS patients (10, 66). Together, these studies suggest that while MHC I downregulation by Nef is important for HIV-1 persistence during the early asymptomatic phase of HIV-1 infection, downregulation of CD4 is more important for HIV-1 replication during late symptomatic stages of HIV-1 disease, when X4-tropic HIV-1 strains emerge. Thus, CD4 downregulation may be more strongly correlated with isolates present at late stages due to the requirement of this activity for replication of X4-tropic viruses. Because R5-tropic HIV-1 predominates early in infection of the host, CD4 downregulation may be less important during this stage due to the ability of R5-tropic HIV-1 to replicate in T cells despite the lack of Nef. These data also suggest that therapeutic strategies targeting Nef may be best accomplished by treatments that target Nef's ability to downregulate MHC I, thereby suppressing the ability of Nef to promote immune evasion. However, management of HIV-1 disease may also be facilitated by development of therapeutics that block CD4 downregulation, thereby preventing the emergence of X4-tropic strains associated with disease.
Supplementary Material
Acknowledgments
We thank Derya Unutmaz for advice and technical support. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute for Allergy and Infectious Diseases, National Institutes of Health: human recombinant IL-2 from Maurice Gately, rgp120IIIB from ImmunoDiagnostics, Inc., gp41 hybridoma Chessie 8 from George Lewis, anti-gp120 and anti-gp41 MAbs 2G12 and 5F3, respectively, from Hermann Katinger, anti-CD4 polyclonal antiserum from Raymond Sweet, and proviral construct pYU2 from Beatrice Hahn. We also thank Eric Freed, Minoru Tobiume, Michiyuki Matsuda, Jerome Zack, Mark Feinberg, and Bruce Chesebro for proviral constructs.
This research was supported by National Institutes of Health grant AI40364. C.A.L. is supported in part by a Medical Scientist Training Program grant to Vanderbilt University.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arganaraz, E. R., M. Schindler, F. Kirchhoff, M. J. Cortes, and J. Lama. 2003. Enhanced CD4 down-modulation by late-stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 278:33912-33919. [DOI] [PubMed] [Google Scholar]
- 5.Benichou, S., M. Bomsel, M. Bodeus, H. Durand, M. Doute, F. Letourneur, J. Camonis, and R. Benarous. 1994. Physical interaction of the HIV-1 Nef protein with β-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J. Biol. Chem. 269:30073-30076. [PubMed] [Google Scholar]
- 6.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bour, S., F. Boulerice, and M. A. Wainberg. 1991. Inhibition of gp160 and CD4 maturation in U937 cells after both defective and productive infections by human immunodeficiency virus type 1. J. Virol. 65:6387-6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bour, S., U. Schubert, and K. Strebel. 1995. The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4: implications for the mechanism of degradation. J. Virol. 69:1510-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 10.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charneau, P., G. Mirambeau, P. Roux, S. Paulus, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription: a termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 12.Chen, B. K., R. T. Gandhi, and D. Baltimore. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J. Virol. 70:6044-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, M. Y., F. Maldarelli, M. K. Karczewski, R. L. Willey, and K. Strebel. 1993. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: the cytoplasmic domain of CD4 contributes to Vpu sensitivity. J. Virol. 67:3877-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesebro, B., K. Wehrly, J. Nishio, and S. Perryman. 1992. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J. Virol. 66:6547-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. S. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 17.Cortes, M. J., F. Wong-Staal, and J. Lama. 2002. Cell surface CD4 interferes with the infectivity of HIV-1 particles released from T cells. J. Biol. Chem. 277:1770-1779. [DOI] [PubMed] [Google Scholar]
- 18.Craig, H. M., M. W. Pandori, N. L. Riggs, D. D. Richman, and J. C. Guatelli. 1999. Analysis of the SH3-binding region of HIV-1 Nef: partial functional defects introduced by mutations in the polyproline helix and the hydrophobic pocket. Virology 262:55-63. [DOI] [PubMed] [Google Scholar]
- 19.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. C. Guatelli. 2000. Interactions of HIV-1 Nef with the mu subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 271:9-17. [DOI] [PubMed] [Google Scholar]
- 20.Crise, B., L. Buonocore, and J. K. Rose. 1990. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus type 1 glycoprotein precursor. J. Virol. 64:5585-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daar, E. S., X. L. Li, T. Moudgil, and D. D. Ho. 1990. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc. Natl. Acad. Sci. USA 87:6574-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalgleish, A. G., P. C. L. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 23.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, X. Wu, W. A. O'Brien, L. Ratner, J. C. Kappes, G. M. Shaw, and E. Hunter. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Ronde, A., B. Klaver, W. Keulen, L. Smit, and J. Goudsmit. 1992. Natural HIV-1 Nef accelerates virus replication in primary human lymphocytes. Virology 188:391-395. [DOI] [PubMed] [Google Scholar]
- 25.Dettenhofer, M., and X.-F. Yu. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 73:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englund, G., T. S. Theodore, E. O. Freed, A. Engleman, and M. A. Martin. 1995. Integration is required for productive infection of monocyte-derived macrophages by human immunodeficiency virus type 1. J. Virol. 69:3216-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdtmann, L., K. Janvier, G. Raposo, H. M. Craig, P. Benaroch, C. Berlioz-Torrent, J. C. Guatelli, R. Benarous, and S. Benichou. 2000. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the μ1 chain of AP1 complex. Traffic 1:871-883. [DOI] [PubMed] [Google Scholar]
- 28.Gallay, P., S. Swingler, J. Song, F. Bushman, and D. Trono. 1995. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell 83:569-576. [DOI] [PubMed] [Google Scholar]
- 29.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart, T. K., T. Kirsh, H. Ellens, R. W. Sweet, D. M. Lambert, S. R. Petteway, J. Leary, and P. Bugelski. 1991. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 88:2189-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabbar, M. A., and D. P. Nayak. 1990. Intracellular interaction of human immunodeficiency virus type 1 (ARV-2) envelope glycoprotein gp160 with CD4 blocks the movement and maturation of CD4 to the plasma membrane. J. Virol. 64:6297-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamieson, B. D., G. M. Aldrovandi, V. Planelles, J. B. M. Jowett, L. Gao, L. M. Bloch, I. S. Y. Chen, and J. A. Zack. 1994. Requirement of human immunodeficiency virus type 1 Nef for in vivo replication and pathogenicity. J. Virol. 68:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 35.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 36.Kirsh, T., T. K. Hart, H. Ellens, J. Miller, S. A. Petteway, Jr., D. M. Lambert, J. Leary, and P. J. Bugelski. 1990. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res. Hum. Retrovir. 6:1215. [DOI] [PubMed] [Google Scholar]
- 37.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J.-C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 38.Lama, J. 2003. The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr. AIDS Res. 1:167-184. [DOI] [PubMed] [Google Scholar]
- 39.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 40.Lee, B., M. Sharron, L. J. Montaner, D. Weissman, and R. W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 96:5215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 42.Lu, X., H. Yu, S.-H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 43.Lundquist, C. A., M. Tobiume, J. Zhou, D. Unutmaz, and C. Aiken. 2002. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J. Virol. 76:4625-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandic, R., O. T. Fackler, M. Geyer, T. Linnemann, Y. H. Zheng, and B. M. Peterlin. 2001. Negative factor from SIV binds to the catalytic subunit of the V-ATPase to internalize CD4 and to increase viral infectivity. Mol. Biol. Cell 12:463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall, W. L., D. C. Diamond, M. M. Kowalski, and R. W. Finberg. 1992. High level of surface CD4 prevents stable human immunodeficiency virus infection of T-cell transfectants. J. Virol. 66:5492-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69:570-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore, J. P., J. A. McKeating, Y. X. Huang, A. Ashkenazi, and D. D. Ho. 1992. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J. Virol. 66:235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 51.Munch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandori, M. W., N. J. S. Fitch, H. M. Craig, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1996. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J. Virol. 70:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papkalla, A., J. Munch, C. Otto, and F. Kirchhoff. 2002. Nef enhances human immunodeficiency virus type 1 infectivity and replication independently of viral coreceptor tropism. J. Virol. 76:8455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel, P. G., M. T. Yu Kimata, J. E. Biggins, J. M. Wilson, and J. T. Kimata. 2002. Highly pathogenic simian immunodeficiency virus mne variants that emerge during the course of infection evolve enhanced infectivity and the ability to downregulate CD4 but not class I major histocompatibility complex antigens. J. Virol. 76:6425-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J.-L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell 97:63-73. [DOI] [PubMed] [Google Scholar]
- 57.Premkumar, D. R., X. Z. Ma, R. K. Maitra, B. K. Chakrabarti, J. Salkowitz, B. Yen-Lieberman, M. S. Hirsch, and H. W. Kestler. 1996. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res. Hum. Retrovir. 12:337-345. [DOI] [PubMed] [Google Scholar]
- 58.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68:5156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith, D. H., R. A. Byrn, S. A. Marsters, T. Gregory, J. E. Groopman, and D. J. Capon. 1987. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science 238:1704-1707. [DOI] [PubMed] [Google Scholar]
- 63.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevenson, M., C. Meier, A. M. Mann, N. Chapman, and A. Wasiak. 1988. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell 53:483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terwilliger, E. F., E. Langhoff, D. Gabuzda, E. Zazopoulos, and W. A. Haseltine. 1991. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 88:10971-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tobiume, M., M. Takahoko, T. Yamada, M. Tatsumi, A. Iwamoto, and M. Matsuda. 2002. Inefficient enhancement of viral infectivity and CD4 downregulation by human immunodeficiency virus type 1 Nef from Japanese long-term nonprogressors. J. Virol. 76:5959-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Schwedler, U., R. S. Kornbluth, and D. Trono. 1994. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc. Natl. Acad. Sci. USA 91:6992-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234:1563-1566. [DOI] [PubMed] [Google Scholar]
- 70.Wehrly, K., and B. Chesebro. 1997. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 12:288-293. [DOI] [PubMed] [Google Scholar]
- 71.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiskerchen, M., and C. Cheng-Mayer. 1996. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology 224:292-301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.