Abstract
The insect-borne Bluetongue virus (BTV) is considered the prototypic Orbivirus, a member of the Reovirus family. One of the hallmarks of Orbivirus infection is the production of large numbers of intracellular tubular structures of unknown function. For BTV these structures are formed as the polymerization product of a single 64-kDa nonstructural protein, NS1, encoded by the viral double-stranded RNA genome segment 6. Although the NS1 protein is the most abundant viral protein synthesized in infected cells, its function has yet to be determined. One possibility is that NS1 tubules may be involved in the translocation of newly formed viral particles to the plasma membrane, and NS1-specific monoclonal antibodies have been shown to react with viral particles leaving infected cells. In the present study we generated a mammalian cell line that expresses a recombinant single-chain antibody fragment (scFv) derived from an NS1-specific monoclonal antibody (10B1) and analyzed the effect that this intracellular antibody has on BTV replication. Normally, BTV infection of mammalian cells in culture results in a severe cytopathic effect within 24 to 48 h postinfection manifested by cell rounding, apoptosis, and lytic release of virions into the culture medium. However, infection of scFv-expressing cells results in a marked reduction in the stability of NS1 and formation of NS1 tubules, a decrease in cytopathic effect, an increased release of infectious virus into the culture medium, and budding of virions from the plasma membrane. These results suggest that NS1 tubules play a direct role in the cellular pathogenesis and morphogenesis of BTV.
Many viruses carry genes that encode both structural proteins that make up the virion particle and nonstructural (NS) proteins that are found only in the infected cell and are not a component of the mature virion. The structural proteins provide virions with functions such as genome encapsidation and transcription, capsid formation, receptor binding, and target cell entry. Indeed, the vast majority of information available regarding the structure and function of viral proteins is in reference to the structural proteins. In comparison to the data for structural proteins, conversely, relatively little is known about the structure and function of NS proteins from double-stranded RNA (dsRNA) viruses. It is primarily thought that these proteins play supportive roles in virus replication such as acting as chaperones for molecular folding, intracellular sorting and transport, genome packaging, capsid assembly, virus release, and control of cellular responses to infection (5, 6, 13-16, 24, 25, 32, 34). In some cases, NS proteins are dispensable for infection and replication of cells in culture but are almost always required for establishment and maintenance of a productive infection in the animal host and are often involved in viral pathogenesis (2, 29). Finally, and perhaps most importantly, the genes encoding viral NS proteins on the whole tend to be the most highly conserved sequences within the viral genome, which not only underscores their essential roles in virus survival but also brands them as attractive targets for therapeutic antiviral intervention strategies.
Bluetongue virus (BTV), an Arbovirus member of the Reoviridae family, is a complex nonenveloped virus with a segmented, dsRNA genome (28). The virion particle is composed of concentric layers of four separate virally encoded structural proteins: an outer capsid shell of two proteins, VP2 and VP5, involved in virus attachment and entry and an inner core of two proteins, VP3 and VP7, that serve to encapsidate the 10 segments of dsRNA and three additional minor proteins that function primarily as (i) an RNA-dependent RNA polymerase (VP1), (ii) a guanylyltransferase (VP4), and (iii) a helicase (VP6). In addition to these structural proteins, BTV encodes four NS proteins, NS1, NS2, and NS3 and the related NS3A, whose functions in the viral life cycle are not fully understood. More is known about the functional mechanisms of NS3, the only viral-encoded cell surface glycoprotein, than about any of the other BTV NS proteins. Recent studies have shown that NS3 interacts specifically with the p11 subunit of the heterotetrameric calpactin II complex as well as with the VP2 outer capsid protein of BTV (1). Because calpactin II is involved in cellular exocytosis, it has been proposed that the interactions between p11, NS3, and VP2 provide a mechanism by which newly assembled virions exploit the exocytic pathway for nonlytic virus release. This viral maturation pathway may be particularly important during infection of insect vectors such as Culicoides species, which appear to be less pathogenic to the host than is observed during the infection of mammalian counterparts. The NS2 protein is synthesized to a high level in infected cells and is mostly present in cytoplasmic inclusion bodies. It is the only virus-specific phosphoprotein, is rich in charged amino acid residues, and has been shown to bind ssRNA but not dsRNA (30). It is believed that NS2 is involved in recruiting specific viral RNA species into inclusion bodies during the assembly of virus components (18).
The most abundantly expressed protein during BTV infection is the 64-kDa NS1 protein. One of the most striking intracellular morphological features during BTV infection is the formation of abundant tubular structures within the cytoplasm. Expression of the NS1 gene in insect cells by recombinant baculovirus results in tubule formation similar to that observed during BTV infection (31). Cryoelectron microscopic analysis revealed that BTV tubules have a helical configuration with an average diameter of 52.3 nm and a length of up to 1,000 nm (10). No function has been ascribed to these tubules to date, although it has been suggested that these may play a role in virus particle translocation to the plasma membrane because NS1-specific monoclonal antibodies have been shown to react with virus particles that are in close proximity to inclusion bodies or that are exiting from infected cells (4). Mutational analysis of the NS1 gene from the BTV-10 serotype has shown that the amino and carboxy termini, as well as a pair of internal cysteine residues at positions 337 and 340, are essential for NS1 tubule formation (22). Several independent lines of evidence suggest that the carboxy terminus of NS1 is exposed on the surface of tubules: (i) the primary antigenic site of NS1 has been localized to the carboxy terminus (23), (ii) addition of extra peptide sequences and whole proteins such as green fluorescent protein (GFP) to the carboxy terminus of NS1 has no effect on tubule formation (7, 8, 21), and finally, (iii) monoclonal antibodies raised to linear epitopes corresponding to the carboxy terminus react specifically with intact tubules analyzed by immunoelectron microscopy (23).
In an attempt to better understand the function of NS1 we decided to interrupt tubule formation and monitor the effects on BTV replication. The common approach for most viruses would be to introduce a lethal mutation in the gene of interest and generate a recombinant virus carrying this gene by reverse genetics; however, no such system exits for introducing mutations in the dsRNA genome of BTV. Therefore, we have undertaken an alternative approach for expression of an intracellular antagonist to the NS1 protein in anticipation that it would interfere with tubule formation or function. Our previous studies revealed that the carboxy terminus of NS1 was required for tubule formation and that a hybridoma cell line that we generated was able to produce a NS1 monoclonal antibody specific to this region (23). Therefore, it is reasonable to believe that introducing this antibody or its antigen-binding domain into BTV-infected cells would serve our purpose; and indeed, this was the case. The approach we settled on was the relatively well-characterized single-chain antibody expression system (29).
MATERIALS AND METHODS
Viruses and cells.
BTV type 10 was plaque purified and propagated as described elsewhere (20). BSR cells, a derivative of hamster kidney BHK cells, were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and a mixture of penicillin and streptomycin. BSR Tet-Off cells were maintained as described above with the addition of 100 μg of G418/ml. BSR-scFv-αNS1 and BSR-EGFP cells were maintained in the same manner as BSR Tet-Off cells but with the addition of 100 μg of hygromycin/ml and 1 μg of doxycycline (Dox)/ml.
Construction of a single-chain antibody fragment (scFv) and expression in mammalian cells.
The standard methods and reagents we employed for construction and selection of bacteriophage displaying a high-affinity scFv have previously been described (11). Briefly, total mRNA was isolated from hybridoma cell line 10B1 and used for PCR amplification and linkage of VH and Vκ genes. The linked V genes were then ligated into the NotI site of pHEN1, and the resulting plasmid, pHEN-sc10B1, was transformed into competent TG1 Escherichia coli by electroporation. The bacterial culture was then transduced with a M13Ko7 phage library, and recombinant sc10B1-displaying phage was rescued by panning in immunotubes coated with purified NS1 protein followed by a round of colony isolation and phage enzyme-linked immunosorbent assay as previously described (11, 29).
Construction of a double stable BSR cell line for coexpression of single-chain antibody and EGFP.
Recombinant sc10B1-displaying phage were used to reinfect TG1 E. coli and prepare DNA for excision of the sc10B1 gene with NotI. This fragment was then ligated into the NotI site of pB1-EGFP (Clonetech) and was designated pB1-EGFP-sc10B1 and used to transfect BSR Tet-Off mammalian cells (Clonetech) in combination with a second plasmid, pTK-Hyg (Clonetech), to permit selection using 200 μg of hygromycin/ml of a double stable cell line which expresses both the sc10B1 molecule and the enhanced GFP (EGFP) reporter gene (3). An additional cell line, BSR-EGFP, was generated to serve as an experimental control by cotransfecting pB1-EGFP and pTK-Hyg and selecting for Hygr.
Radioimmunoprecipitation, sucrose gradient centrifugation, and pulse chase analysis.
For detection of the scFv-αNS1 molecule, BSR-scFv-αNS1 cells were cultured in 35-mm-diameter dishes in the presence or absence of 1 μg of Dox/ml and radiolabeled with 200 μCi of [35S]Met-Cys for 1 h. The cells were then disrupted in lysis buffer containing Tris, NaCl, and EDTA (TNE) buffer and 1% NP-40, and the radiolabeled lysates were immunoprecipitated using purified NS1 tubules, rabbit antiserum to NS1 (RaαNS1), and protein A Sepharose. After the immune complexes were washed several times with NP-40 lysis buffer, the samples were denatured with β-mercaptoethanol and boiling and analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) and autoradiofluorography.
For analysis of BTV tubule formation, BSR-scFv-αNS1 cells were grown in cultures in 100-mm-diameter dishes in the presence or absence of 1 μg of Dox/ml, infected with BTV-10 at a multiplicity of infection (MOI) of 1, and radiolabeled at 24 h postinfection (p.i.) with 200 μCi of [35S]Met-Cys for 1 h. The cells were then disrupted in NP-40 lysis buffer, and the radiolabeled lysates were loaded onto 20 to 60% continuous sucrose gradients in TNE buffer in SW-41 centrifuge tubes and centrifuged for 2 h at 20,000 rpm. Fractions were collected and immunoprecipitated with RaαNS1 and then processed for SDS-PAGE as described above.
For pulse chase analysis of NS1, BSR-scFv-αNS1 cells were grown in cultures in 35-mm-diameter dishes in the presence or absence of 1 μg of Dox/ml, infected with BTV-10 at an MOI of 5, and pulse labeled at 24 h p.i. with 200 μCi of [35S]Met-Cys for 15 min. The labeling medium was replaced with normal DMEM, and the cultures were chased for 4 additional hours. Pulse and chase samples were disrupted with NP-40 lysis buffer, immunoprecipitated with RaαNS1, and processed for SDS-PAGE analysis as described above.
Electron microscopy.
Cells were grown to confluency on 35-mm-diameter dishes, infected with BTV-10 at an MOI of 5, fixed with gluteraldehyde at 24 h p.i., postfixed for 1 h at 4°C in osmium tetroxide in phosphate-buffered saline, and prepared for electron microscopy as previously described (12). Specimens were examined with a Hitachi H7000 microscope.
RESULTS
Construction and selection of a high-affinity single-chain antibody fragment (scFv) to the NS1 protein.
In short, construction of a single-chain antibody fragment involves the rescue of immunoglobulin variable-region genes (V genes), which encode the antigen binding specificity, from mouse hybridomas. The gene rescue is performed by independently PCR amplifying the V-gene regions of both the heavy and light chains from the antibody genes of interest and linking them together as a single polypeptide-encoding gene via a stretch of glycine codons. We used 10B1, the mouse hybridoma cell line, which produces a monospecific antibody that binds with high-level affinity to the carboxy terminus of the NS1 protein encoded by the BTV-10 serotype (23). Following the standard protocols described in Materials and Methods for single-chain antibody fragment construction and isolation, we identified the presence of several phage-display clones with high-level binding specificity for the BTV-10 NS1 protein and selected one for construction of the mammalian cell expression system described below.
Inducible, intracellular expression of scFv-αNS1 in mammalian cells.
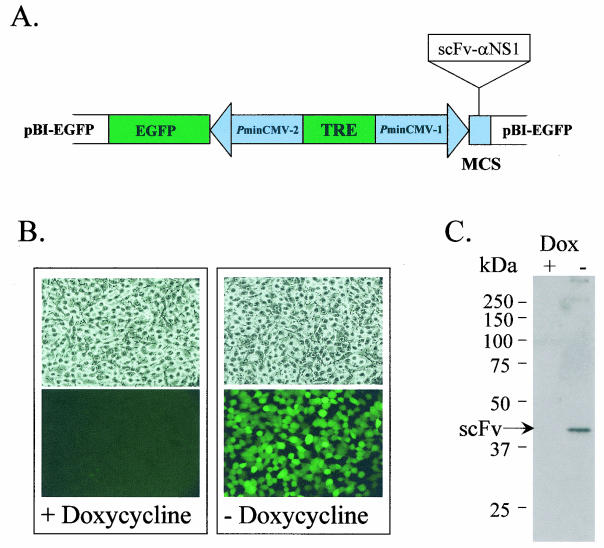
One of the major problems associated with constitutive expression of intracellular antibody molecules, often referred to as “intrabodies,” in mammalian cell lines is that they are often toxic to the cell over extended periods of time and therefore make selection and maintenance of clonal lines difficult. To overcome this obstacle we utilized an inducible-expression system called Tet-Off (Clonetech), which is a tetracycline-regulated expression system that is especially suited for ubiquitous expression of potentially toxic genes in cell cultures. With the Tet-Off system, removal of tetracycline (or of the tetracycline derivative Dox) from the culture medium results in high-level transcription initiation from a PminCMV-2 promoter under the control of a tet-responsive element (Fig. 1A).
FIG. 1.
Construction of a double stable BSR cell line for coexpression of scFv-αNS1 and EGFP. (A) The scFV-αNS1 gene fragment was rescued from phage and ligated into the Tet-Off-responsive plasmid pB1-EGFP (Clonetech) and cotransfected into BSR Tet-Off cells along with pTK-Hyg (Clonetech) carrying a selectable marker (Hygr) for isolation of clones in the presence of hygromycin. MCS, multiple cloning site. (B) One clone, designated BSR-scFv-αNS1 (shown in all four panels; light microscopy was used to produce the images shown in the top panels, and fluorescence microscopy was used to produce the images of the same clone shown in the bottom panels), was selected for its tight regulation and high-level expression of the EGFP reporter gene in the absence (−) of Dox, as determined by fluorescence microscopy (bottom right panel). There was no EGFP fluorescence observed in the presence of Dox (bottom left panel). (C) Radioimmunoprecipitation and SDS-PAGE analysis of BSR-scFv-αNS1 cells in the presence (+) and absence (−) of Dox, showing expression of scFv-αNS1 in the absence of Dox (right lane).
Following cotransfection of pB1-EGFP-sc10B1 and pTK-Hyg into BSR Tet-Off cells, a clone was selected in the presence of hygromycin that expressed high levels of EGFP (Fig. 1B) and analyzed by radioimmunoprecipitation and SDS-PAGE for coexpression of the sc10B1 intrabody, hereinafter referred to as scFv-αNS1 (Fig. 1C). Following induction of scFv-αNS1 there were no detectable changes in cell viability or host protein expression over a period of 5 days compared to the results seen with uninduced cultures (data not shown). These results show that a continuous mammalian cell line (designated BSR-scFv-αNS1) was obtained that can be efficiently regulated to express an intracellular single-chain antibody fragment specific for the NS1 protein of BTV-10.
Changes in cytopathology and infectious virus production in cells expressing scFv-αNS1.
We chose BSR cells (a derivative of the hamster kidney line BHK) for these experiments, because these cells are well known for their ability to support productive BTV infection. Once we generated a successful cell line that could be induced to express high levels of scFv-αNS1, the next step was to examine whether expression of scFv-αNS1 has any effect on BTV replication process. Therefore, BSR-scFv-αNS1 cells were infected with BTV in the absence or presence of Dox (i.e., in the presence or absence of scFv-αNS1, respectively) and both the cytopathic effects (CPE) and levels of virus replication and infectivity of these were monitored. When BSR-scFv-αNS1 cells were infected in the presence of Dox and monitored by light microscopy, a typical pattern of CPE was observed (Fig. 2, top panels) consistent with infection of control BSR cells (data not shown). In contrast, little to no CPE was observed at similar time points in BSR-scFv-αNS1 cells infected in the absence of Dox when the intrabody was being expressed (Fig. 2, bottom panels), suggesting that the scFv-αNS1 molecule was interfering with virus replication.
FIG. 2.
CPE of BTV-10 infection in the presence and absence of scFv-αNS1. Light microscopy of uninfected BSR-scFv-αNS1 cells (left panels) or infected with BTV-10 at 1 day p.i. (middle panels) and 2 days p.i. (right panels) in the presence (top panels) or absence (bottom panels) of Dox. Little-to-no CPE was observed in cells expressing the scFv-αNS1 molecule (bottom middle and bottom right panels), whereas extensive CPE (typical of BTV infection in BSR cells) was observed in the presence of Dox (top middle and top right panels).
To further characterize the effect of scFv-αNS1 expression on virus replication, we examined the levels of infectious virus produced both intracellularly and in the culture medium from cells infected with BTV in the presence or absence of Dox. To our surprise, little difference was observed in the levels of infectious virus (from 0.8 × 106 to 3 × 106 PFU/ml) found in the cytoplasm of cultures raised in the presence or absence of Dox, indicating that intracellular expression of scFv-αNS1 has little or no effect on virus maturation and infectivity (Table 1). It is interesting that this observation did not concur with the CPE results that suggested that expression of scFv-αNS1 inhibited virus replication. When we examined the extracellular culture medium from samples infected in the presence and absence of Dox, furthermore, most-unexpected results were obtained: a more than 10-fold increase in infectious virus production was observed from cultures infected in the absence of Dox (0.6 × 107 to 2 × 107 PFU/ml) compared to the results seen with cultures with Dox (3 ×105 to 5 ×105 PFU/ml) (Table 1). These results indicate that scFv-αNS1 expression does not in fact suppress virus replication as suggested by the CPE results but rather enhances virus release from the infected cell.
TABLE 1.
Infectious BTV titers produced in BSR-scFv-αNS1 cellsa
| Doxycycline | BTV titer (PFU/ml) at indicated day p.i.
|
|||
|---|---|---|---|---|
| Intracellular
|
Extracellular
|
|||
| 1 | 2 | 1 | 2 | |
| + | 1 × 106 (0.3)b | 3 × 106 (1.7) | 5 × 105 (0.1) | 3 × 105 (0.2) |
| − | 8 × 105 (0.1) | 1 × 106 (0.4) | 2 × 107 (1.5) | 6 × 106 (2.6) |
Virus titers were determined by standard plaque formation analysis of BSR cells. Intracellular samples were collected by Dounce homogenization of cells in DMEM and titration of the cytoplasmic supernatants. Extracellular sample results represent direct titration of the culture media. Intracellular and extracellular virus samples were prepared and analyzed without detergent. Values represent the averages of three separate experiments conducted at 1 or 2 days p.i. and are reported as PFU/ml.
Standard deviations are shown in parentheses as 106 PFU/ml.
Taken together, these results indicated that expression of the scFv-αNS1 intrabody leads to a reduction in BTV-induced cytopathology and a concurrent increase in infectious virus production. On the surface these two observations seem to contradict one another; however, they are reminiscent of the pattern one observes when BTV infects its insect host: efficient replication and infectivity with little to no pathogenicity.
NS1 tubule formation is inhibited in the presence of scFv-αNS1 intrabody.
As discussed above, one of the characteristic features of BTV infection is the formation of abundant tubular structures, or tubules, made up exclusively of multimeric forms of NS1 within the cytoplasm of infected cells. In a previous study it was shown that the carboxy terminus of the NS1 protein is critical in the formation of tubules and that this domain is also the antigenic determinant of monoclonal antibody 10B1 (23), the antibody used to generate the scFv-αNS1 intrabody in the present study. Therefore, it is reasonable to speculate that expression of this intrabody in cells during BTV infection may result in the inhibition of tubule formation. To examine this possibility we infected BSR-scFv-αNS1 cells in the presence and absence of Dox and analyzed tubule formation by sucrose gradient centrifugation and radioimmunoprecipitation followed by SDS-PAGE (Fig. 3). In the presence of Dox we observed high-molecular-weight, multimeric forms of the NS1 protein in the gradient consistent with the formation of tubules (Fig. 3A). When scFv-αNS1 intrabody was expressed in the absence of Dox, in contrast, these multimeric NS1 structures were absent (Fig. 3B) and only low-molecular-weight or monomeric isoforms of NS1 were detected in the upper fractions of the gradient, suggesting that the intrabody was preventing tubule formation during BTV infection. This observation is not due to interference between scFv-αNS1 and the polyclonal rabbit antiserum used for immunoprecipitation, because the same result was observed by Western blot analysis (data not shown).
FIG. 3.
Velocity gradient analysis of NS1-containing subcellular structures. BSR-scFv-αNS1 cells were infected with BTV-10 in the presence (A) or absence (B) of Dox, radiolabeled with [35S]Met-Cys, and analyzed by sucrose gradient centrifugation (20 to 60%), immunoprecipitation, and SDS-PAGE for the formation of NS1 tubules. A typical pattern of multimeric NS1 distribution about two-thirds of the way into the gradient consistent with tubule formation was observed in the presence of Dox (A). In contrast, no NS1 protein was detected in this portion of the gradient in the absence of Dox (B), indicating that tubules were not formed.
To follow up on this observation, pulse chase analysis was performed during BTV infection of BSR-scFv-αNS1 cells in the presence and absence of Dox and cell lysates were analyzed by immunoprecipitation and SDS-PAGE (Fig. 4). In the presence of Dox the NS1 protein remained stable after a 4-h chase period, whereas very little NS1 was detected after the chase in the absence of Dox. These results indicate that expression of scFv-αNS1 plays a role in the degradation of NS1 during BTV infection, most likely through a direct interaction with the carboxy-terminal epitope and subsequent targeting of the NS1-intrabody complex to the cellular degradation pathway.
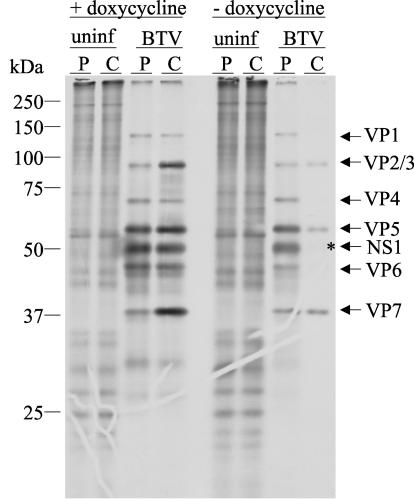
FIG. 4.
Pulse chase analysis of NS1 expression in the presence and absence of scFv-αNS1. BSR-scFv-αNS1 cells were infected with BTV-10 in the presence (+) or absence (−) of Dox, pulse labeled (P) with [35S]Met-Cys for 15 min, chased (C) with unlabeled medium for 4 h, and analyzed by immunoprecipitation with RaαNS1 antiserum and SDS-PAGE. Uninfected cells (uninf) were used as controls. NS1 protein was stable after the 4-h chase in the presence of Dox (fourth lane from the left), whereas NS1 appeared to be degraded after 4 h in the absence of Dox when scFv-αNS1 was being expressed (asterisk in lane 8). Under these conditions all of the BTV structural proteins are often coprecipitated with NS1 due to a weak interaction between virions and the NS1 protein.
Expression of scFv-αNS1 during BTV infection causes a shift from lytic virus release to budding.
In an effort to further characterize the effects of scFv-αNS1 expression on cell morphology, tubule formation, and virus release, we performed ultrastructural electron microscopic analysis on BTV-infected BSR-scFv-αNS1 cells in the presence and absence of Dox (Fig. 5). In the presence of Dox we observed the typical effects of BTV infection in mammalian cells; these effects included the presence of cytoplasmic inclusion bodies, tubules, mature virus particles, and areas of severe morphological destruction near the plasma membrane consistent with lytic release of virus (Fig. 5A). In contrast, a very different effect was seen in the absence of Dox. Although viral inclusion bodies and mature virus particles could be seen, there were no cytoplasmic tubules observed in any of the sections examined. Furthermore, individual virus particles were often associated with the plasma membrane and were observed to be budding from the surface of the cell (Fig. 5B). These results support the idea resulting from the biochemical pulse chase analysis that intracellular expression of scFv-αNS1 inhibits NS1 tubule formation and, as a result, leads to a shift from lytic release of virus to budding from the plasma membrane; they also support the hypothesis that tubules play an integral role in the mechanism of virus release from infected cells (4). We have attempted to determine whether infectious virions isolated from the medium of insect cells (C636) or from BSR-scFv-αNS1 cells in the absence of Dox possess a lipid envelope; to date, they appear to be nonenveloped (data not shown). We examined these virions by velocity gradient sedimentation (in the presence or absence of detergent) followed by electron microscopy (negative stained or embedded and sectioned) and could not detect a lipid envelope. Perhaps the BTV budding process results in a transient or unstable envelope that is readily lost during virus purification. Whether or not a lipid envelope modulates the infectivity or life cycle of BTV in the insect host has yet to be determined.
FIG. 5.
Ultrastructural analysis of BTV-10 infection in the presence and absence of scFv-αNS1. BSR-scFv-αNS1 cells were infected with BTV-10 in the presence (+) or absence (−) of Dox and prepared for electron microscopy at 24 h p.i. as described in Materials and Methods. (A) Typical effects of BTV infection in mammalian cells; these effects include the presence of cytoplasmic viral inclusion bodies (VIBs), tubules (TUBs), and mature virus particles and areas of severe morphological destruction near the plasma membrane consistent with lytic release of virus. (B) During expression of scFv-αNS1, no cytoplasmic tubules were detected and virus particles were seen exiting cells by budding from the plasma membrane. Bar, 500 nm.
All of the experiments described in Results were also carried out with additional cell controls, including BSR-EGFP, which was used to monitor the effects of EGFP on BTV infection, wild-type BSR cells, and BSR Tet-Off cells; in each case, there were no demonstrable effects on BTV replication or tubule formation (data not shown).
DISCUSSION
NS proteins play critical roles in virus replication, and elucidation of their mechanisms of action is one of the central themes in virus research today. An increasing body of evidence shows that these NS proteins control many of the key replication and assembly processes within the infected target cell and therefore may serve as suitable targets for antiviral intervention strategies. In the present study we devised a way to interfere with the intracellular function of the NS1 protein and the formation of NS1-derived tubules of BTV, resulting in a striking reduction in cellular pathogenesis and a shift in virus release from cell lysis to budding, thus confirming the hypothesis that viral NS genes do indeed encode critical functions in the viral life cycle.
BTV is an Arbovirus member of the Reoviridae family and is capable of infecting both mammalian and insect hosts. When BTV infects a vertebrate host, especially domestic ruminants such as sheep and cattle, it occasionally causes high levels of morbidity and mortality manifested by mucosal edema, hydrothorax, hydropericardium, serosal hemorrhages, hypotension, and shock (26, 27). Furthermore, infection of pregnant animals often results in fetal infection, abortion, or congenital anomalies including runting, blindness, deafness, hydranencephaly, arthrogryposis, campylognathia, and prognathia (17, 19, 26, 27, 33). In contrast, infection of insect vector hosts such as Culicoides species results in a high level of virus replication in the midgut and salivary glands, with little to no detectable viral pathogenesis on the whole. One of the major morphological differences observed during infection of insect and mammalian cells in culture is that virus appears to preferentially bud from the plasma membrane of insect cells, leaving the cells relatively intact over the course of a week or more, whereas in mammalian cell cultures a high proportion of virus remains cell associated, leading to cell death and subsequent lysis within a day or two. A recent study from our group shows that the NS protein NS3 plays an important role in BTV egress (1) and may be a key determinant of budding from insect cells due to its high level of expression in that system (9).
It is not yet possible to introduce mutations into the genome of BTV and study the effects of these changes on virus replication and pathogenesis, because a reverse genetics system has not been developed for this dsRNA virus genus. Therefore, we employed an alternative approach to knockout viral protein function, namely, intracellular expression of a single-chain antibody fragment (scFv) with specificity to the NS1 protein (scFv-αNS1). We reasoned that infection of mammalian cell cultures that ubiquitously express the scFv-αNS1 may lead to observable changes in the viral life cycle due to interference with NS1 function or tubule formation; indeed, this was the case. Four major changes were apparent straightaway: first, there was a tremendous reduction in virus-induced CPE; second, there was a more than 10-fold increase in the amount of virus released into the culture medium; third, there was a shift from lytic release of virus to budding from the plasma membrane; and fourth, NS1 tubule formation was completely inhibited by scFv-αNS1 expression. Each of these changes, except for the lack of tubule formation, is reminiscent of what occurs during BTV infection of insect cells in culture.
On the basis of these findings we propose that the NS1 protein is a major determinant of pathogenesis in the vertebrate host and that its mechanism of action is the augmentation of virus-cell association and not transport of virus to the cell surface, which ultimately leads to lysis of the infected cell. One observation, however, appears to contradict this proposal: NS1 tubules are also found in abundance in BTV-infected insect cells, and yet there is little CPE in the invertebrate system, and progeny virions are released by budding. But as mentioned earlier, the NS3 protein is overexpressed in insect cells (9) and it has been clearly demonstrated to form a molecular bridge between calpactin, a component of the cellular exocytic pathway, and the outer viral capsid protein VP2 (1). In the present study, however, no change in the level of NS3 expression was observed in BTV-infected BSR-scFv-αNS1 cultures with or without Dox (data not shown). With these findings taken together, we propose that BTV-induced cellular pathogenesis is a function of the relative ratio of NS1 tubule levels to NS3 protein levels within the cytoplasm of infected cells. When NS3 protein levels are low relative to NS1 tubule levels, as in the case of mammalian cells, progeny virus accumulates within the cytoplasm of the cell, leading to lysis and cell death. When intracellular NS3 protein levels in insect cells are high relative to NS1 tubule levels, however, NS3-directed budding overrides the intracytoplasmic accumulation effect imparted by NS1. When NS1 tubule formation was inhibited by scFv-αNS1 expression in mammalian cells in the present study, the residual NS3 protein levels may have been sufficient to promote a shift from lytic release to budding. Further studies are currently being conducted to test the hypothesis that NS1:NS3 ratios contribute to BTV pathogenesis and release from host cells.
Acknowledgments
We thank Anna Olivera for expert technical help and Leigh Millican for electron microscopy (UAB) analyses.
This work was supported partly by grants from the National Institutes of Health (United States) and the Wellcome Trust Fund, London, United Kingdom.
REFERENCES
- 1.Beaton, A. R., J. Rodriguez, Y. K. Reddy, and P. Roy. 2002. The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release. Proc. Natl. Acad. Sci. USA 99:13154-13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaney, J. E., Jr., G. G. Manipon, B. R. Murphy, and S. S. Whitehead. 2003. Temperature sensitive mutations in the genes encoding the NS1, NS2A, NS3, and NS5 nonstructural proteins of dengue virus type 4 restrict replication in the brains of mice. Arch Virol. 148:999-1006. [DOI] [PubMed] [Google Scholar]
- 3.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 4.Eaton, B. T., A. D. Hyatt, and S. M. Brookes. 1990. The replication of bluetongue virus. Curr. Top. Microbiol. Immunol. 162:89-118. [DOI] [PubMed] [Google Scholar]
- 5.Elazar, M., K. H. Cheong, P. Liu, H. B. Greenberg, C. M. Rice, and J. S. Glenn. 2003. Amphipathic helix-dependent localization of NS5A mediates hepatitis C virus RNA replication. J. Virol. 77:6055-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florese, R. H., M. Nagano-Fujii, Y. Iwanaga, R. Hidajat, and H. Hotta. 2002. Inhibition of protein synthesis by the nonstructural proteins NS4A and NS4B of hepatitis C virus. Virus Res. 90:119-131. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, M. K., M. V. Borca, and P. Roy. 2002. Virus-derived tubular structure displaying foreign sequences on the surface elicit CD4+ Th cell and protective humoral responses. Virology 302:383-392. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh, M. K., L.-L. Li, C. Fayolle, G. Dadaglio, A. Murphy, F. Lemonnier, P. Roy, and C. Leclerc. 2002. Induction of HLA-A2 restricted CTL responses by a tubular structure carrying human melanoma epitopes. Vaccine 20:2463-2473. [DOI] [PubMed] [Google Scholar]
- 9.Guirakhoo, F., J. A. Catalan, and T. P. Monath. 1995. Adaptation of bluetongue virus in mosquito cells results in overexpression of NS3 proteins and release of virus particles. Arch. Virol. 140:967-974. [DOI] [PubMed] [Google Scholar]
- 10.Hewat, E. A., T. F. Booth, and P. Roy. 1992. Structure of bluetongue virus particles by cryoelectron microscopy. J. Struct. Biol. 109:61-69. [DOI] [PubMed] [Google Scholar]
- 11.Hoogenboom, H. R., A. D. Griffiths, K. S. Johnson, D. J. Chiswell, P. Hudson, and G. Winter. 1991. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 19:4133-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, L. V., R. W. Compans, A. R. Davis, T. J. Bos, and D. P. Nayak. 1985. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol. Cell. Biol. 5:2181-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohl, A., R. F. Clayton, F. Weber, A. Bridgen, R. E. Randall, and R. M. Elliott. 2003. Bunyamwera virus nonstructural protein NSs counteracts interferon regulatory factor 3-mediated induction of early cell death. J. Virol. 77:7999-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konan, K. V., T. H. Giddings, Jr., M. Ikeda, K. Li, S. M. Lemon, and K. Kirkegaard. 2003. Nonstructural protein precursor NS4A/B from hepatitis C virus alters function and ultrastructure of host secretory apparatus. J. Virol. 77:7843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krug, R. M., W. Yuan, D. L. Noah, and A. G. Latham. 2003. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology 309:181-189. [DOI] [PubMed] [Google Scholar]
- 16.Liu, W. J., H. B. Chen, and A. A. Khromykh. 2003. Molecular and functional analyses of Kunjin virus infectious cDNA clones demonstrate the essential roles for NS2A in virus assembly and for a nonconservative residue in NS3 in RNA replication. J. Virol. 77:7804-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luedke, A. J., R. H. Jones, and T. E. Walton. 1977. Overwintering mechanism for bluetongue virus: biological recovery of latent virus from a bovine by bites of Culicoides variipennis. Am. J. Trop. Med. Hyg. 26:313-325. [DOI] [PubMed] [Google Scholar]
- 18.Lymperopoulos, K., C. Wirblich, I. Brierley, and P. Roy. 2003. Sequence specificity in the interaction of Bluetongue virus non-structural protein 2 NS2 with viral RNA. J. Biol. Chem. 278:31722-31730. [DOI] [PubMed] [Google Scholar]
- 19.MacLachlan, N. J. 1994. The pathogenesis and immunology of bluetongue virus infection of ruminants. Comp. Immunol. Microbiol. Infect. Dis. 17:197-206. [DOI] [PubMed] [Google Scholar]
- 20.Mertens, P. P., J. N. Burroughs, and J. Anderson. 1987. Purification and properties of virus particles, infectious subviral particles, and cores of bluetongue virus serotypes 1 and 4. Virology 157:375-386. [DOI] [PubMed] [Google Scholar]
- 21.Mikhailov, M., K. Monastyrskaya, T. Bakker, and P. Roy. 1996. A new form of particulate single and multiple immunogen delivery system based on recombinant bluetongue virus-derived tubules. Virology 217:323-331. [DOI] [PubMed] [Google Scholar]
- 22.Monastyrskaya, K., T. Booth, L. Nel, and P. Roy. 1994. Mutation of either of two cysteine residues or deletion of the amino or carboxy terminus of nonstructural protein NS1 of bluetongue virus abrogates virus-specified tubule formation in insect cells. J. Virol. 68:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monastyrskaya, K., E. A. Gould, and P. Roy. 1995. Characterization and modification of the carboxy-terminal sequences of bluetongue virus type 10 NS1 protein in relation to tubule formation and location of an antigenic epitope in the vicinity of the carboxy terminus of the protein. J. Virol. 69:2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nibert, M. L. 2002. Rotavirus translation control protein takes RNA to heart. Structure (Cambridge) 10:129-130. [DOI] [PubMed] [Google Scholar]
- 25.Noah, D. L., K. Y. Twu, and R. M. Krug. 2003. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 307:386-395. [DOI] [PubMed] [Google Scholar]
- 26.Osburn, B. I., R. T. Johnson, A. M. Silverstein, R. A. Prendergast, M. M. Jochim, and S. B. Levy. 1971. Experimental viral-induced congenital encephalopathies. II. The pathogenesis of bluetongue virus infection of fetal lambs. Lab. Investig. 25:206-213. [PubMed] [Google Scholar]
- 27.Parsonson, I. M. 1992. Overview of bluetongue virus infection of sheep, p. 713-724. In T. E. Walton and B. I. Osburn (ed.), Bluetongue, African horsesickness and related orbiviruses. CRC Press, Boca Raton, Fla.
- 28.Roy, P. 2000. Orbiviruses and their replication. In B. N. Fields (ed.), Fields virology, fourth ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 29.Sullivan, D. E., M. U. Mondelli, D. T. Curiel, V. Krasnykh, G. Mikheeva, P. Gaglio, C. B. Morris, S. Dash, and M. A. Gerber. 2002. Construction and characterization of an intracellular single-chain human antibody to hepatitis C virus non-structural 3 protein. J. Hepatol. 37:660-668. [DOI] [PubMed] [Google Scholar]
- 30.Thomas, C. P., T. F. Booth, and P. Roy. 1990. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J. Gen. Virol. 71:2073-2083. [DOI] [PubMed] [Google Scholar]
- 31.Urakawa, T., and P. Roy. 1988. Bluetongue virus tubules made in insect cells by recombinant baculoviruses: expression of the NS1 gene of bluetongue virus serotype 10. J. Virol. 62:3919-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varani, G., and F. Allain. 2002. How a rotavirus hijacks the human protein synthesis machinery. Nat. Struct. Biol. 9:158-160. [DOI] [PubMed] [Google Scholar]
- 33.Young, S., and D. R. Cordy. 1964. An ovine fetal encaphalopathy caused by bluetongue virus vaccine. J. Neuropathol.s Exp. Neurol. 23:635-642. [DOI] [PubMed] [Google Scholar]
- 34.Zhirnov, O. P., T. E. Konakova, T. Wolff, and H. D. Klenk. 2002. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 76:1617-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]