Abstract
Despite numerous studies showing therapeutic potential, no central dopamine D1 receptor ligand has ever been approved, because of potential limitations, such as hypotension, seizures, and tolerance. Functional selectivity has been widely recognized as providing a potential mechanism to develop novel therapeutics from existing targets, and a highly biased, functionally selective D1 ligand might overcome some of the past limitations. SKF-83959 [6-chloro-3-methyl-1-(m-tolyl)-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8-diol] is reported to be a highly biased D1 ligand, having full agonism at D1-mediated activation of phospholipase C (PLC) signaling (via GαQ) and antagonism at D1-mediated adenylate cyclase signaling (via GαOLF/S). For this reason, numerous studies have used this compound to elucidate the physiologic role of D1-PLC signaling, including a novel molecular mechanism (GαQ-PLC activation via D1-D2 heterodimers). There is, however, contradictory literature that suggests that SKF-83959 is actually a partial agonist at both D1-mediated adenylate cyclase and β-arrestin recruitment. Moreover, the D1-mediated PLC stimulation has also been questioned. This Minireview examines 30 years of relevant literature and proposes that the data strongly favor alternate hypotheses: first, that SKF-83959 is a typical D1 partial agonist; and second, that the reported activation of PLC by SKF-83959 and related benzazepines likely is due to off-target effects, not actions at D1 receptors. If these hypotheses are supported by future studies, it would suggest that caution should be used regarding the role of PLC and downstream pathways in D1 signaling.
The Potential Utility of a Functionally Selective D1 Ligand
Animal models have suggested the utility of selective D1 agonists for a variety of therapeutic targets, including Parkinson’s disease (Taylor et al., 1991; Mailman and Nichols, 1998; Mailman et al., 2001), cognition (Arnsten et al., 1994; Schneider et al., 1994; Steele et al., 1996, 1997; Goldman-Rakic et al., 2004; Toda and Abi-Dargham, 2007; Rosell et al., 2014), and other disorders. In fact, for Parkinson’s disease, the efficacy of D1 agonists in animal models has translated into clinical trials with large effect sizes similar to those seen in the preclinical models (Rascol et al., 1999, 2001). A recent preliminary clinical study (Rosell et al., 2014) also reported significant effects on working memory consistent with predictions from preclinical models (Arnsten et al., 1994; Schneider et al., 1994; Steele et al., 1996, 1997). Despite this support for the clinical efficacy of D1 agonists, there are reports of serious D1-mediated side effects that may prevent approval of a D1 agonist, including rapid tolerance (Asin and Wirtshafter, 1993; Gulwadi et al., 2001), profound hypotension (Blanchet et al., 1998), and seizures (Starr, 1996).
During the past decade, it has become clear that one way of improving a pharmacological profile is to discover functionally selective ligands for the target receptor (Kenakin, 2007; Mailman, 2007; Urban et al., 2007; Neve, 2009). Functional selectivity describes the property of some ligands to interact with a single receptor and differentially affect the signaling pathways engaged by that target. The degree to which the ligand differentially affects signaling pathways is termed the bias of the ligand. The first use of the term "functional selectivity" in the context of a single receptor was with dopamine receptor ligands (Mailman et al., 1998; Lawler et al., 1999), and the earliest therapeutic utility of this mechanism was suggested by aripiprazole, a D2-preferring compound with a mechanism of action clearly differentiated from earlier approved antipsychotic drugs (Kikuchi et al., 1995; Lawler et al., 1999; Shapiro et al., 2003; Mailman, 2007; Mailman and Murthy, 2010). Thus, if a D1 agonist with high bias for D1-mediated signaling pathways were available, it would be an excellent investigational tool, and it also might overcome some of the possible limitations sometimes thought to be obligatory with D1 agonists.
SKF-83959, the First Functionally Selective D1 Ligand?
Dopamine receptors were originally classified on the basis of their coupling to adenylate cyclase (Kebabian et al., 1972; Garau et al., 1978; Kebabian and Calne, 1979), and this canonical signaling of the D1-like receptors (D1, D5) is thought to involve coupling to the G proteins GαOLF or GαS (Neve et al., 2004; Mailman and Huang, 2007). Thus, the cAMP resulting from D1 receptor activation could initiate a host of downstream cascades, such as those engaged by the activation of cAMP/protein kinase A signaling. The importance of this pathway was challenged, however, with reports that the behavioral effects of some D1 agonists were unrelated to cAMP/protein kinase A signaling (Gnanalingham et al., 1995a,b), but rather involved non–cAMP-mediated signaling, including phospholipase C (PLC)–mediated calcium elevation (Undie and Friedman, 1990; Undie et al., 1994; Andringa et al., 1999b; O'Sullivan et al., 2004). This has led to the hypothesis that a functionally selective D1 ligand highly biased against cAMP signaling might have an improved therapeutic index.
Much of this research has focused on SKF-83959 [6-chloro-3-methyl-1-(m-tolyl)-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8-diol] because it was purported to be a highly biased D1 ligand that was a biochemical antagonist at D1-coupled adenylate cyclase, but a full agonist at D1-stimulated PLC via GαQ (Panchalingam and Undie, 2001; Jin et al., 2003; Zhen et al., 2005; Rashid et al., 2007b; Hasbi et al., 2009) (see Fig. 1 for structures of SKF-83959 and other relevant ligands). SKF-83959 is behaviorally active in rat and primate Parkinson’s disease models via its action at D1 receptors (Arnt et al., 1992; Jin et al., 2003), and it has behavioral activity in several animal species that is known to be induced by D1 full or partial agonists but not by D1 antagonists (Gnanalingham et al., 1995a).
Fig. 1.
Research ligands and drugs that have properties relevant to these issues.
The concatenation of these data led to the hypothesis that the behavioral actions of SKF-83959 were independent of D1-mediated cAMP signaling, but depended on D1 actions at PLC/calcium signaling (Downes and Waddington, 1993; Deveney and Waddington, 1995; Rashid et al., 2007a; Hasbi et al., 2009; Fujita et al., 2010; Perreault et al., 2010), possibly mediated by D1-D2 heterodimers (Rashid et al., 2007a; Hasbi et al., 2009; Perreault et al., 2010; Chun et al., 2013). This would mean that SKF-83959 is the first highly biased, functionally selective D1 ligand (Arnt et al., 1992; Undie and Friedman, 1992; Undie et al., 1994; Gnanalingham et al., 1995b) and would make it an important probe for studying mechanisms related to D1 signaling (Zhang et al., 2005, 2007, 2009; Yu et al., 2008; Liu et al., 2009a,b; Perreault et al., 2011, 2014) as is often explicitly stated: “SKF 83959 robustly stimulat[es] the D1-D2 heteromer-mediated calcium signal and [does] not activat[e] adenylyl cyclase by the D1-D1 homomer” (Perreault et al., 2014); SKF-83959 is “a selective [phosphatidylinositol]-linked D1-like receptor agonist” (Zhang et al., 2009); and there are “potential stimulant-antagonist actions, as observed with SKF-83959” (Neumeyer et al., 2003).
Some years ago, Pacheco and Jope (1997) noted some contradictions in the literature, but speculated that “variations in experimental methods likely contribute to the differing results,” although these issues were not identified. They themselves reported that “dopamine D1 receptors directly stimulate the [phosphoinositide] signaling system” in the human brain (Pacheco and Jope, 1997). In noting such inconsistencies, we had offered a skeptical view of the hypothesis that SKF-83959 was a highly biased, functionally selective ligand (Huang et al., 2001). Yet the increasing numbers of papers whose data depend in large part on the highly biased signaling of SKF-83959 have made it important to re-examine the pharmacology of this compound. In evaluating the evidence for SKF-83959 being a highly biased ligand, one must also consider the role of D1-PLC signaling. We have chosen to do this by formulating a series of hypotheses that together form the basis for accepted dogma about the direct role for D1 receptors in stimulating GαQ-PLC and, more recently, the role of D1-D2 heterodimers in affecting PLC/Ca2+-mediated signaling.
Hypothesis: SKF-83959 Is a Selective, High-Affinity D1 Ligand
There is universal agreement that SKF-83959 has nanomolar affinity for both rat and human D1 receptors, heterologously expressed or in situ (Arnt et al., 1992; Chun et al., 2013; Lee et al., 2014), and also that the ligand has micromolar affinity for D2 receptors (Arnt et al., 1992; Chun et al., 2013; Lee et al., 2014), where it is an antagonist at cAMP signaling and a low-intrinsic-activity partial agonist at β-arrestin recruitment (Lee et al., 2014). Similar D1-D2 selectivity with a wide range of D1 intrinsic activities has been shown for other ligands with similar benzazepine structures [i.e., ligands with substituents on a backbone of 1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine] (Setler et al., 1978; Iorio et al., 1983; Weinstock et al., 1985; Neumeyer et al., 2003). Because the D2 affinities of most benzazepines are in the micromolar range, this provides an obvious off-target mechanism when high concentrations or doses are used experimentally. Ligands of this family also have measurable affinity for other neuroreceptors. Andringa et al. (1999a) reported that SKF-83959 had micromolar affinity for all α-adrenoreceptors and the norepinephrine transporter, and Neumeyer et al. (2003) noted that it also had affinity for both 5-HT2A (KI = 88 nM) as well as the α2-adrenergic (KI, ∼3000 nM) receptors. More recently, Chun et al. (2013) reported the results of a broader screen, reporting micromolar affinity for dozens of G protein–coupled receptors, including KI < 10 μM for many receptors that couple directly to GαQ, such as the 5-HT2A and 5-HT2C serotonin, α1-adrenergic, H1 histamine, and M5 muscarinic receptors, among others. Thus, the literature shows that although SKF-83959 has its highest affinity for D1/D5 receptors, at micromolar concentrations it will interact both with D2-like dopamine receptors and a variety of other targets. Additionally, while the focus of this Minireview was on the numerous studies in which SKF-83959 was used at micromolar concentrations, a subset of off-target receptors (Neumeyer et al., 2003; Chun et al., 2013) will be engaged at much lower concentrations; thus, the need for proper experimental controls remains unobviated. The implications of such off-target actions are discussed below.
Hypothesis: SKF-83959 Has No Intrinsic Activity at D1-Mediated Stimulation of Adenylate Cyclase
Although purported to have no intrinsic activity at D1-mediated adenylate cyclase, we previously used SKF-83959 and found it to be a partial agonist (Ryman-Rasmussen et al., 2005). Because of the continuing use of this ligand for its “novel” properties, we recently did a rigorous evaluation of SKF-83959 pharmacology in a variety of heterologous and native D1 systems, studying both the parent ligand and a potential N-demethylated metabolite, 6-chloro-1-(m-tolyl)-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8-diol, that might contribute to pharmacological effects in vivo (Lee et al., 2014). Both in vitro and ex vivo, SKF-83959 was a partial agonist at D1-mediated adenylate cyclase in two different cell lines transfected with the human D1 receptor, as well as in rat striatal homogenates. Its potency and affinity were very similar in all of these systems. The functional effects were inhibited by SCH23390 [(R)-8-chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-ol], as well as by structurally dissimilar D1 antagonists, and SKF-83959 had no effect in untransfected cells. As noted above, while D1-selective, SKF-83959 also had micromolar affinity for D2 receptors, where it was an antagonist at cAMP signaling (Lee et al., 2014).
In some cases, the published data actually agree with the findings of Lee et al. (2014). For example, Gnanalingham et al. (1995b) reported that SKF-83959 did not cause significant stimulation of adenylate cyclase, yet their data clearly show measurable (∼50%) intrinsic activity, with some data points marked as significant. In addition to our previous study showing >40% intrinsic activity in a human D1 heterologous expression system (Ryman-Rasmussen et al., 2005), Chemel et al. (2012) reported high intrinsic activity in an overexpressed human D1 receptor system. Although Rashid et al. (2007b) showed a “flat” dose-response curve for SKF-83959, only relative activity units were provided, making it impossible to know the level of basal synthesis. The lowest SKF-83959 concentration tested was 10 nM (Rashid et al., 2007b), making it possible that maximal stimulation had already been caused. Without either basal synthesis levels or lower concentrations, the only valid conclusion from those data is that SKF-83959 was not a full agonist. There are, however, a few studies reporting no intrinsic activity for SKF-83959 for which obvious experimental or interpretational confounds are not evident (Arnt et al., 1992; Jin et al., 2003). It will be useful for other laboratories using this compound to independently determine whether SKF-83959 has measurable intrinsic activity in canonical assays.
Signaling via β-arrestin also represents an important non–G protein signaling pathway (Shenoy and Lefkowitz, 2005), one that can be evoked by D1 receptors (Urs et al., 2011). SKF-83959 also is a partial agonist at the D1-mediated β-arrestin activation, with intrinsic activity similar to the partial agonist SKF-38393 [(R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8-diol] and with similar potency as seen with adenylate cyclase activation (Lee et al., 2014). Importantly, its potency in both D1-mediated adenylate cyclase and β-arrestin assays was in the nanomolar range, similar to its affinity (Lee et al., 2014). Interestingly, at the D2 receptor, SKF-83959 had no intrinsic activity at adenylate cyclase but modest agonist activity at D2-mediated β-arrestin activation (Lee et al., 2014). At both of these D1-mediated functions, the potency of SKF-83959 was, again, similar to its affinity, a point of relevance to the following section. Thus, the weight of the evidence suggests that SKF-83959 is a typical partial D1 agonist, not a highly biased, noncyclase-preferring D1 ligand.
Hypothesis: SKF-83959 Activates PLC via a D1-GαQ Mechanism
Although SKF-83959 may have partial agonist properties at GαOLF/S-mediated stimulation of cAMP, it might still be useful as a moderately biased, functionally selective ligand if it were a full agonist in activating PLC. Some years ago, we briefly reviewed the evidence for D1 linkage to PLC (Huang et al., 2001) and suggested flaws with that hypothesis, yet there has been a continued use of this compound based on this property. We recently investigated whether D1 signaling was mediated by PLC activation. There was no significant stimulation caused by SKF-83959 in either D1-transfected or wild-type HEK293 cells. Conversely, in both the wild-type and D1-transfected cells, the positive GαQ control carbachol markedly stimulated inositol monophosphate synthesis with a potency consistent with its affinity, and the response was completely blocked by atropine.
In attempting to resolve the discrepancy between these recent data (Lee et al., 2014) and a sizable literature, we carefully reviewed the experimental protocols. Strikingly, in all prior publications reporting direct D1 stimulation of PLC activity, the concentrations of SKF-83959 or other benzazepines required to elicit these effects were in the high micromolar/low millimolar range (Felder et al., 1989a,b; Dyck, 1990; Mahan et al., 1990; Undie and Friedman, 1990, 1992, 1994; Vyas et al., 1992; Pacheco and Jope, 1997; Lee et al., 2004; Banday and Lokhandwala, 2007; Liu et al., 2009a; Zhang et al., 2009; Mizuno et al., 2012). No rationale was offered for use of SKF-83959 and chemically related benzazepines at concentrations far greater than their nanomolar KD values. For SKF-83959 in particular, this raises a conundrum. The functional potency reported for D1-mediated PLC activation is about 4 orders of magnitude greater than either its D1 affinity or its D1 potency for stimulating cAMP synthesis or activating β-arrestin.
Earlier we summarized the off-target activity of SKF-83959 and other benzazepines, noting the many receptors and transporters whose KD values are in the range of 1–10 μM. Thus, when concentrations of 100–300 μM are used, these (as well as other) targets are probably engaged unintentionally. It would seem prudent that there be tests of the obvious alternate hypothesis that activation of PLC, when found, is mediated by one or several of these off-target receptors. In fact, some data already support this latter hypothesis. Yu et al. (1996) reported that 5 μM fenoldopam (an SKF-83959 analog that has high D1 intrinsic activity) increased PLC activity, but only by 30–50%, and that this increase was mediated by cAMP, not direct PLC activation. Similarly, Jin et al. (2003) reported that the D1 receptor linked to PLC was found in striatum, hippocampus, frontal cortex, and cerebellum. Because neither D1-binding sites nor D1 mRNA are found in the cerebellum, this suggests an off-target, rather than D1, mechanism. Finally, in almost every study using pharmacological antagonism of D1-PLC effects, the sole antagonist employed was the structurally similar benzazepine SCH23390 (Felder et al., 1989a,b; Dyck, 1990; Mahan et al., 1990; Undie and Friedman, 1990, 1992, 1994; Vyas et al., 1992; Pacheco and Jope, 1997; Lee et al., 2004; Banday and Lokhandwala, 2007; Liu et al., 2009a; Zhang et al., 2009; Mizuno et al., 2012). Yet when structurally diverse antagonists were used, a very different picture emerges. For example, Jin et al. (2003) reported that the antagonist cis-flupenthixol was only half as effective as SCH23390 in blocking purported D1-PLC activation; moreover, the D1/D2 antagonist (+)-butaclamol failed to block these effects at all.
In our view, the most compelling evidence comes from the study of Friedman et al. (1997), who found that the PLC activation ex vivo was unaffected by knockout of the D1 receptor. Rather than interpreting this as evidence for a non-D1 action of the benzazepines, Friedman et al. (1997) proposed that there was a novel D1-like receptor that was responsible, despite inconsistencies with both the known expression of the D5 receptor and the likely properties of then-orphan receptors in the mouse genome. Recalling that every published study over the last three decades failed to find D1-mediated PLC activation at theoretical fractional occupancies even of 95%, there seems compelling support for the alternate hypothesis that off-target effects were the actual mechanism. The original idea, however, has morphed into a newer second hypothesis that D1-D2 heterodimers actually activate the GαQ-PLC-Ca2+ cascade. Although we feel the foundation for this novel hypothesis was flawed, it must be evaluated on its merits.
Hypothesis: D1-D2 Heterodimer–Mediated Activation of GαQ-PLC-Ca2+ by D1-Selective Ligands Is an Important Signaling Mechanism
George and coworkers have recognized the discrepancy between functional potencies of SKF-83959 and its affinity, and hypothesized that whereas D1 receptors alone (monomer and/or homodimer) could not stimulate PLC signaling, this would occur via coactivation of a D1-D2 heterodimer in a GαQ protein–dependent manner (Lee et al., 2004; Rashid et al., 2007a,b; Hasbi et al., 2009, 2011). Their view is that the limiting factor is the micromolar affinity of SKF-83959 for the D2 receptor (Rashid et al., 2007b; Lee et al., 2014). There are several lines of evidence, however, that are inconsistent with this D1-D2 hypothesis.
First, if the effects are dependent on D2 receptors, it is unclear how one can conclude that the occupancy of the D1 receptor is important as the D1 receptor is essentially saturated at the concentrations at which there is even partial fractional occupancy of the D2. In addition, the reports of PLC activation in either kidney or brain (Felder et al., 1989a,b; Dyck, 1990; Mahan et al., 1990; Undie and Friedman, 1990, 1992, 1994; Vyas et al., 1992; Pacheco and Jope, 1997; Lee et al., 2004; Banday and Lokhandwala, 2007; Liu et al., 2009a; Zhang et al., 2009; Mizuno et al., 2012) show no effect at concentrations that would essentially saturate both D1 and D2 receptors (e.g., 10 μM). The rare reports showing functional Ca2+ effects at more reasonable concentrations also have anomalies. For example, one study reported essentially a quantal-like response for SKF-83959 in which no significant effects were seen at 30 nM concentrations, but a maximal effect at 100 nM (see Fig. 1B in Hasbi et al., 2009). This type of dose-response behavior is inconsistent with a Gα-type of mechanism that almost always yields a graded response.
More recently, it has been suggested that there is a unique role of D1-D2 heterodimers in subsets of coexpressing striatal neurons (Perreault et al., 2012, 2014). Although this is a change from the original hypothesis of direct D1-GαQ-PLC activation by SKF-83959, it is important to consider its merits. One line of evidence that argues against the D1-D2 heterodimer hypothesis is that Chun et al. (2013) failed to find the predicted calcium signaling. Secondly, in HEK293 cells, SKF-83959 is an antagonist at D2-mediated adenylate cyclase and a low-intrinsic-activity partial agonist at D2-mediated β-arrestin recruitment (Lee et al., 2014), inconsistent with a dopamine-like physiologic effect. Thirdly, there was no PLC activation by SKF-83959 in HEK293 cells coexpressing D1 and D2 receptors (Lee et al., 2014). Finally, although there may be some coexpressing D1 and D2 cells (especially early in development), the vast majority of adult striatal neurons [where D1-PLC activation was first reported (Undie and Friedman, 1990, 1992; Friedman et al., 1993)] are highly segregated. Medium spiny neurons of the direct pathway are largely D1-expressing, those of the indirect pathway largely D2-expressing, and such segregation also occurs elsewhere in the brain (Gerfen et al., 1990; Harrison et al., 1990; Scibilia et al., 1992; Rappaport et al., 1993; Le Moine and Bloch, 1995; Aubert et al., 2000; Deng et al., 2006; Bateup et al., 2008; Matamales et al., 2009; Bertran-Gonzalez et al., 2010). In summary, while the D1-D2 heterodimer hypothesis is enticing, there is a significant body of evidence that suggests it may not be valid.
Hypothesis: D1-PLC Signaling of SKF-83959 Causes Novel Behavioral Effects
One purportedly novel characteristic of SKF-83959 is its D1-like behavioral activity in the absence of the ability to activate adenylate cyclase. This has been reported in both murine (Arnt et al., 1992; Downes and Waddington, 1993) and primate species (Gnanalingham et al., 1995a) and has been used as prima facie functional evidence for the importance of the D1-GαQ-PLC and/or D1-D2 heterodimer–GαQ-PLC-Ca2+ signaling pathway. There are, however, two lines of evidence that suggest this hypothesis may not be true. These relevant in vivo studies have relied solely on the benzazepine family of D1 agonists that, like SKF-83959, are behaviorally effective at quite low doses. For example, Arnt et al. (1992) reported that SKF-83959 caused maximal turning in the unilateral 6-hydroxydopamine model at a dose of 0.3 μmol/kg, whereas Deveney and Waddington (1995) found that yet lower doses were active in evoking D1 behaviors in unlesioned rats. Yet even assuming no metabolism (and these catechols are rapidly metabolized), this yields a predicted concentration at the D1 receptor far lower than required in vitro to activate either the hypothesized D1-PLC or D1-D2 heterodimer systems. More recently, Medvedev et al. (2013) concluded that PLCβ via D1 mechanisms regulated forward locomotion in mice, yet the sole experimental selective D1 agonist, SKF-81297, was used at doses of 10 mg/kg, far higher than the 0.001–0.3 mg/kg known to produce typical D1-like behavioral activity (Vermeulen et al., 1994; Cai and Arnsten, 1997; Diaz Heijtz and Castellanos, 2006). A parsimonious deduction would seem to be that D1-like behavioral effects are irrelevant to these purported PLC signaling mechanisms requiring near-millimolar concentrations.
Another important potential confound also relates to pharmacokinetic factors. For example, a classic phase I metabolic reaction is N-demethylation, and SKF-83959 has the chemical properties that suggest it would be a substrate for this type of cytochrome P450–mediated reaction (see Fig. 1). Recently, we demonstrated that the N-demethylated product of SKF-83959 actually has somewhat greater D1 intrinsic activity than the parent compound (Lee et al., 2014). Thus, even if one still posits that the behavioral effects of SKF-83959 cannot involve adenylate cyclase signaling, the possibility that all of its behavioral actions might be explained by one or more active metabolites must be addressed.
Reconciliation of the Role of D1 Receptors and GαQ-PLC Activation
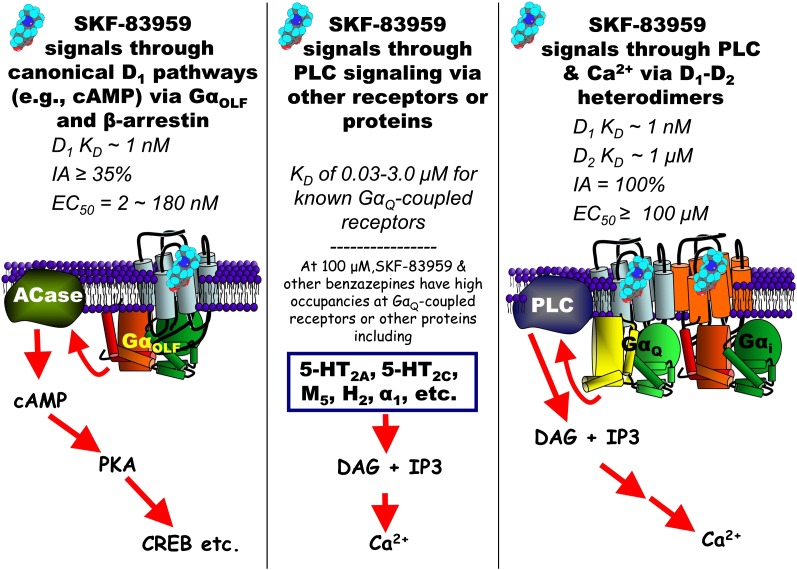
Popper (1959) has written, “Whenever the ‘classical’ system of the day is threatened by the results of new experiments which might be interpreted as falsifications…, the system will appear unshaken to the conventionalist. He will explain away the inconsistencies which may have arisen; perhaps by blaming our inadequate mastery of the system. Or he will eliminate them by suggesting ad hoc the adoption of certain auxiliary hypotheses, or perhaps of certain corrections to our measuring instruments.” It is with due irony that this seems to encapsulate the issues addressed in this Minireview. We have not sought to be provocative, but rather to follow the notion that it is appropriate, and even desirable, to be skeptical (not cynical) about hypotheses (or even theories or laws). Devising experiments to disprove a hypothesis honors the original conceptualization if those experiments fail to do so. Thus, the fundamental question is whether SKF-83959, or indeed any other D1 agonist, causes PLC activation via actions at the D1 receptor. We have encapsulated the issues at hand into three interrelated hypotheses illustrated in Fig. 2.
Fig. 2.
Schematic representation of three hypotheses related to D1 signaling by SKF-83959. ACase, adenylate cyclase; CREB, cAMP response element–binding protein; DAG, diacylglycerol; IA, intrinsic activity; IP3, inositol triphosphate; PKA, protein kinase A.
The first hypothesis (Fig. 2, left) deals with whether SKF-83959 is highly biased because of a lack of activity at canonical signaling pathways, a property used to explain why D1-PLC signaling was important for behavioral/physiologic effects of SKF-83959 and related compounds. As we have reviewed, the weight of the literature (although not unanimous) suggests that the compound is actually a partial agonist at D1 receptors in both heterologous systems and in the striatum, with activity in at least three D1-mediated pathways: adenylate cyclase, β-arrestin, and receptor internalization (Gnanalingham et al., 1995b; Ryman-Rasmussen et al., 2005; Chemel et al., 2012; Lee et al., 2014). Thus, above and beyond the hypothesis that the D1 receptor engages GαQ-PLC, SKF-83959 would, at most, have modest bias, and canonical mechanisms would have to be ruled out before assigning physiologic/behavioral effects to PLC signaling.
The second hypothesis (Fig. 2, center) posits that PLC-mediated signaling is an important mechanism for SKF-83959 and other D1 agonists. The studies in both kidney and brain that have supported a role of D1 activation of PLC have been consistent in the use of ligands only from the benzazepine family and have employed these agonists at concentrations orders of magnitude higher either than their KD or than their functional potency in canonical assays. Moreover, they generally used only the structurally similar SCH23390 as an antagonist (see references cited earlier). Unfortunately, the use of suprapharmacological concentrations of SKF-83959 and failure to consider non-D1 mechanisms is not unique to studies of PLC signaling (Yu et al., 2008; Guo et al., 2013). It is unclear why off-target actions, an obvious factor in pharmacological designs, have not generally been considered, especially as many of the likely targets are GαQ-linked receptors.
To complicate matters, numerous studies failing to show D1-mediated stimulation of phosphoinositide hydrolysis (Kelly et al., 1988; Dearry et al., 1990; Rubinstein and Hitzemann, 1990; Pedersen et al., 1994; Demchyshyn et al., 1995; Kimura et al., 1995; Lee et al., 2014) have often been ignored. Indeed, Wallace and Claro (1990) noted that dopamine (at concentrations that stimulate cAMP synthesis) actually inhibited muscarinic stimulation of phosphoinositide hydrolysis. The most telling piece of data is the fact that PLC activation by SKF-83959 and other benzazepines was absolutely preserved in tissue from mice that had the D1 receptor knocked out genetically (Friedman et al., 1997). Together, the available data suggest that the second hypothesis in Fig. 2 is false—SKF-83959 and other benzazepines do not stimulate PLC activity via the D1 receptor.
The third hypothesis (Fig. 2, right) posits a critical role for D1-D2 heterodimers, and is the one of the greatest of current interest because of the novel underlying molecular mechanism. In Popper’s words, it may be considered as a “correction” of the initial hypothesis (Fig. 2, center). Although it was based originally on the D1-PLC mechanism and the use of SKF-83959, it has now taken on a life of its own because of the hypothesized unique role of D1-D2 heterodimers in subsets of coexpressing striatal neurons (Perreault et al., 2012, 2014). We have reviewed the available data that we feel can be construed as weakening this hypothesis. Above and beyond the experimental issues [including our failure to be able to see evidence of PLC signaling in D1-D2–cotransfected HEK293 cells (Lee et al., 2014)], there is the question of the degree to which D1-D2 colocalization occurs in the mature nervous system. Thus, our view is that hypothesis 3 is also false. Some may view this conclusion as premature, yet a recent paper concluded: “the mechanism of D1 [receptor]/D2 [receptor]–mediated calcium signaling involves more than receptor-mediated Gq protein activation, may largely involve downstream signaling pathways, and may not be completely heteromer-specific. In addition, SKF-83959 may not exhibit selective activation of D1-D2 heteromers, and its significant cross-reactivity to other receptors warrants careful interpretation” (Chun et al., 2013). Unfortunately, this may be interpreted by some as simply being an experimental difference between investigators. A more skeptical view is that an extensive literature has already provided adequate evidence to falsify all of these prevailing hypotheses, much as we suggested years ago (Huang et al., 2001).
Conclusions
Although we have tried to offer a critical review of the available literature, there are several issues that we could not address. An obvious one with a pharmacological basis has to do with receptor reserve, a well known mechanism that can affect interpretation of functional studies and might be thought to be a reason for the disparate literature. Yet it is important to remember that much of the PLC hypothesis has been based on studies done ex vivo using brain or kidney tissue, where presumably all investigators were dealing with both physiologically relevant and similar receptor reserve. Thus, the huge difference in D1 potencies between canonical functional assays and PLC activation is unlikely to involve receptor reserve. Moreover, the near-millimolar concentrations of D1 agonists required to cause PLC activation ex vivo will never be achieved in vivo when administering D1 dopamine agonists to animals at behaviorally active concentrations (Taylor et al., 1991; Arnsten et al., 1994; Schneider et al., 1994; Mottola et al., 2002).
In addition to our recent work (Lee et al., 2014), there are other experiments that might be useful if one felt that the available data were not adequately conclusive. The two most obvious are those with a pharmacological basis. First, future studies should take advantage of the structurally diverse D1 agonists and antagonists that are available (e.g., Fig. 1). Second, when there is a question of off-target engagement, studies done in vivo or ex vivo should measure drug concentrations in tissue. In addition, a host of molecular approaches might also be useful. The most challenging relates to the idea that D1-D2 heterodimers are the mechanistic key. Although we feel that this hypothesis is not well supported, others may feel that further studies are required to reach firm conclusions. Techniques such as proximity ligation assays (Trifilieff et al., 2011) can be used to look for molecular proximity between D1 and D2 in brain slices, and bioluminescence resonance energy transfer can be used to determine if GαQ is activated by the D1 alone or when complexed with D2 (Urizar et al., 2011). Indeed, there are recent data using such approaches that failed to find evidence for either D1 agonist engagement of GαQ or molecular proximity of D1 and D2 in mouse brain (J. Javitch et al., personal communication). Such data, while “negative,” are compelling evidence for the perspectives we have offered.
There may be substantial impact to resolution of this controversy. The potential clinical utility of D1 agonists has slowly been receiving human validation (Rascol et al., 1999; Rosell et al., 2014), although the development of an approvable drug has been inhibited by issues including pharmacokinetics, seizures, and hypotension. A functionally selective D1 agonist could theoretically have advantages (Mailman, 2007), yet whereas some existing D1 ligands do have some degree of functional selectivity (Lewis et al., 1998; Ryman-Rasmussen et al., 2005, 2007), no compound has yet been shown to have sufficient signaling bias to translate into meaningful pharmacological differences. Importantly, we believe that none of the known D1-selective benzazepines is a highly biased D1 ligand, contrary to what has been hypothesized. It is important that the field should focus on the substantial scientific issues that remain to be elucidated about D1 function and their impact on drug discovery, and not be distracted by mechanisms that may be artificial.
Acknowledgments
The authors thank Drs. Kent Vrana and Mechelle Lewis for comments on this manuscript.
Abbreviations
- PLC
phospholipase C
- SCH23390
(R)-8-chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-ol
- SKF-38393
(R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8-diol
- SKF-83959
6-chloro-3-methyl-1-(m-tolyl)-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8-diol
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Lee, Yang, Mailman.
Footnotes
This work was supported, in part, by the National Institutes of Health National Institute of Mental Health [Grants U01-MH082441 and R01-MH040537]; and by a Pennsylvania Keystone Innovation Grant.
R.B.M. has interests in issued and pending patents related to dopamine D1 receptor mechanisms that constitute a conflict of interest for which there is university oversight. The remaining authors declare no conflicts of interest.
References
- Andringa G, Drukarch B, Leysen JE, Cools AR, Stoof JC. (1999a) The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur J Pharmacol 364:33–41 [DOI] [PubMed] [Google Scholar]
- Andringa G, Stoof JC, Cools AR. (1999b) Sub-chronic administration of the dopamine D(1) antagonist SKF 83959 in bilaterally MPTP-treated rhesus monkeys: stable therapeutic effects and wearing-off dyskinesia. Psychopharmacology (Berl) 146:328–334 [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. (1994) Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 116:143–151 [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Sánchez C. (1992) Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol 213:259–267 [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D. (1993) Effects of repeated dopamine D1 receptor stimulation on rotation and c-fos expression. Eur J Pharmacol 235:167–168 [DOI] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. (2000) Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol 418:22–32 [PubMed] [Google Scholar]
- Banday AA, Lokhandwala MF. (2007) Oxidative stress reduces renal dopamine D1 receptor-Gq/11alpha G protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol 293:F306–F315 [DOI] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. (2008) Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11:932–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. (2010) What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat 4:136 DOI: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet PJ, Fang J, Gillespie M, Sabounjian L, Locke KW, Gammans R, Mouradian MM, Chase TN. (1998) Effects of the full dopamine D1 receptor agonist dihydrexidine in Parkinson’s disease. Clin Neuropharmacol 21:339–343 [PubMed] [Google Scholar]
- Cai JX, Arnsten AF. (1997) Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther 283:183–189 [PubMed] [Google Scholar]
- Chemel BR, Bonner LA, Watts VJ, Nichols DE. (2012) Ligand-specific roles for transmembrane 5 serine residues in the binding and efficacy of dopamine D1 receptor catechol agonists. Mol Pharmacol 81:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, Sibley DR. (2013) D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol Pharmacol 84:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A, Gingrich JA, Falardeau P, Fremeau RT, Jr, Bates MD, Caron MG. (1990) Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature 347:72–76 [DOI] [PubMed] [Google Scholar]
- Demchyshyn LL, Sugamori KS, Lee FJ, Hamadanizadeh SA, Niznik HB. (1995) The dopamine D1D receptor. Cloning and characterization of three pharmacologically distinct D1-like receptors from Gallus domesticus. J Biol Chem 270:4005–4012 [DOI] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. (2006) Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat 32:101–116 [DOI] [PubMed] [Google Scholar]
- Deveney AM, Waddington JL. (1995) Pharmacological characterization of behavioural responses to SK&F 83959 in relation to ‘D1-like’ dopamine receptors not linked to adenylyl cyclase. Br J Pharmacol 116:2120–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Castellanos FX. (2006) Differential effects of a selective dopamine D1-like receptor agonist on motor activity and c-fos expression in the frontal-striatal circuitry of SHR and Wistar-Kyoto rats. Behav Brain Funct 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes RP, Waddington JL. (1993) Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine ‘D1-like’ receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur J Pharmacol 234:135–136 [DOI] [PubMed] [Google Scholar]
- Dyck LE. (1990) Effects of dopamine on phosphoinositide hydrolysis in slices of rat striatum and cortex. Neurochem Int 17:77–82 [DOI] [PubMed] [Google Scholar]
- Felder CC, Blecher M, Jose PA. (1989a) Dopamine-1-mediated stimulation of phospholipase C activity in rat renal cortical membranes. J Biol Chem 264:8739–8745 [PubMed] [Google Scholar]
- Felder CC, Jose PA, Axelrod J. (1989b) The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther 248:171–175 [PubMed] [Google Scholar]
- Friedman E, Butkerait P, Wang HY. (1993) Analysis of receptor-stimulated and basal guanine nucleotide binding to membrane G proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem 214:171–178 [DOI] [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. (1997) D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol 51:6–11 [DOI] [PubMed] [Google Scholar]
- Fujita S, Kiguchi M, Kobayashi M, Kinsella A, Koshikawa N, Waddington JL. (2010) Assessment of jaw movements by magnetic sensor in relation to topographies of orofacial behaviour in freely moving rats: studies with the dopamine D1-like receptor agonists SKF 83822 vs SKF 83959. Eur J Pharmacol 632:39–44 [DOI] [PubMed] [Google Scholar]
- Garau L, Govoni S, Stefanini E, Trabucchi M, Spano PF. (1978) Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci 23:1745–1750 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432 [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Erol DD, Hunter AJ, Smith LA, Jenner P, Marsden CD. (1995a) Differential anti-Parkinsonian effects of benzazepine D1 dopamine agonists with varying efficacies in the MPTP-treated common marmoset. Psychopharmacology (Berl) 117:275–286 [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Hunter AJ, Jenner P, Marsden CD. (1995b) Stimulation of adenylate cyclase activity by benzazepine D1 dopamine agonists with varying efficacies in the 6-hydroxydopamine lesioned rat—relationship to circling behaviour. Biochem Pharmacol 49:1185–1193 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. (2004) Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 174:3–16 [DOI] [PubMed] [Google Scholar]
- Gulwadi AG, Korpinen CD, Mailman RB, Nichols DE, Sit SY, Taber MT. (2001) Dinapsoline: characterization of a D1 dopamine receptor agonist in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 296:338–344 [PubMed] [Google Scholar]
- Guo L, Zhao J, Jin G, Zhao B, Wang G, Zhang A, Zhen X. (2013) SKF83959 is a potent allosteric modulator of sigma-1 receptor. Mol Pharmacol 83:577–586 [DOI] [PubMed] [Google Scholar]
- Harrison MB, Wiley RG, Wooten GF. (1990) Selective localization of striatal D1 receptors to striatonigral neurons. Brain Res 528:317–322 [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, George SR. (2009) Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci USA 106:21377–21382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR. (2011) Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Lawler CP, Lewis MM, Nichols DE, Mailman RB. (2001) D1 dopamine receptors. Int Rev Neurobiol 48:65–139 [DOI] [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. (1983) SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther 226:462–468 [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. (2003) SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem 85:378–386 [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. (1979) Multiple receptors for dopamine. Nature 277:93–96 [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Petzold GL, Greengard P. (1972) Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc Natl Acad Sci USA 69:2145–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Batty I, Nahorski SR. (1988) Dopamine receptor stimulation does not affect phosphoinositide hydrolysis in slices of rat striatum. J Neurochem 51:918–924 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2007) Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci 28:407–415 [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, Morita S. (1995) 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther 274:329–336 [PubMed] [Google Scholar]
- Kimura K, Sela S, Bouvier C, Grandy DK, Sidhu A. (1995) Differential coupling of D1 and D5 dopamine receptors to guanine nucleotide binding proteins in transfected GH4C1 rat somatomammotrophic cells. J Neurochem 64:2118–2124 [DOI] [PubMed] [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20:612–627 [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. (1995) D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol 355:418–426 [DOI] [PubMed] [Google Scholar]
- Lee SM, Kant A, Blake D, Murthy V, Boyd K, Wyrick SJ, Mailman RB. (2014) SKF-83959 is not a highly-biased functionally selective D1 dopamine receptor ligand with activity at phospholipase C. Neuropharmacology DOI: 10.1016/j.neuropharm.2014.05.042 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, O’Dowd BF, George SR. (2004) Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem 279:35671–35678 [DOI] [PubMed] [Google Scholar]
- Lewis MM, Watts VJ, Lawler CP, Nichols DE, Mailman RB. (1998) Homologous desensitization of the D1A dopamine receptor: efficacy in causing desensitization dissociates from both receptor occupancy and functional potency. J Pharmacol Exp Ther 286:345–353 [PubMed] [Google Scholar]
- Liu J, Wang F, Huang C, Long LH, Wu WN, Cai F, Wang JH, Ma LQ, Chen JG. (2009a) Activation of phosphatidylinositol-linked novel D1 dopamine receptor contributes to the calcium mobilization in cultured rat prefrontal cortical astrocytes. Cell Mol Neurobiol 29:317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang W, Wang F, Cai F, Hu ZL, Yang YJ, Chen J, Chen JG. (2009b) Phosphatidylinositol-linked novel D1 dopamine receptor facilitates long-term depression in rat hippocampal CA1 synapses. Neuropharmacology 57:164–171 [DOI] [PubMed] [Google Scholar]
- Mahan LC, Burch RM, Monsma FJ, Jr, Sibley DR. (1990) Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc Natl Acad Sci USA 87:2196–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB. (2007) GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB, Huang X. (2007) Dopamine receptor pharmacology, in Parkinson's Disease and Related Disorders, Part 1 (Koller WC, Melamed E. eds) pp 77–105, Elsevier, New York [Google Scholar]
- Mailman R, Huang X, Nichols DE. (2001) Parkinson’s disease and D1 dopamine receptors. Curr Opin Investig Drugs 2:1582–1591 [PubMed] [Google Scholar]
- Mailman RB, Murthy V. (2010) Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des 16:488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB, Nichols DE. (1998) Dopamine D1 receptor agonists as antiparkinson drugs. Trends Pharmacol Sci 19:255–256 [DOI] [PubMed] [Google Scholar]
- Mailman RB, Nichols DE, Lewis MM, Blake BL, Lawler CP. (1998) Functional effects of novel dopamine ligands: dihydrexidine and Parkinson's disease as a first step, in Dopamine Receptor Subtypes: From Basic Science to Clinical Application (Jenner P, Demirdemar R. eds) pp 64–83, IOS Stockton Press, Amsterdam [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Hervé D, Girault JA. (2009) Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One 4:e4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev IO, Ramsey AJ, Masoud ST, Bermejo MK, Urs N, Sotnikova TD, Beaulieu JM, Gainetdinov RR, Salahpour A. (2013) D1 dopamine receptor coupling to PLCβ regulates forward locomotion in mice. J Neurosci 33:18125–18133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Kurokawa K, Ohkuma S. (2012) Dopamine D1 receptors regulate type 1 inositol 1,4,5-trisphosphate receptor expression via both AP-1- and NFATc4-mediated transcriptional processes. J Neurochem 122:702–713 [DOI] [PubMed] [Google Scholar]
- Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, et al. (2002) Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther 301:1166–1178 [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. (2003) Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol 474:137–140 [DOI] [PubMed] [Google Scholar]
- Neve KA. (2009) Functional Selectivity of G Protein-Coupled Receptor Ligands, Humana, New York [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24:165–205 [DOI] [PubMed] [Google Scholar]
- O’Sullivan GJ, Roth BL, Kinsella A, Waddington JL. (2004) SK&F 83822 distinguishes adenylyl cyclase from phospholipase C-coupled dopamine D1-like receptors: behavioural topography. Eur J Pharmacol 486:273–280 [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Jope RS. (1997) Comparison of [3H]phosphatidylinositol and [3H]phosphatidylinositol 4,5-bisphosphate hydrolysis in postmortem human brain membranes and characterization of stimulation by dopamine D1 receptors. J Neurochem 69:639–644 [DOI] [PubMed] [Google Scholar]
- Panchalingam S, Undie AS. (2001) SKF83959 exhibits biochemical agonism by stimulating [35S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology 40:826–837 [DOI] [PubMed] [Google Scholar]
- Pedersen UB, Norby B, Jensen AA, Schiodt M, Hansen A, Suhr-Jessen P, Scheideler M, Thastrup O, Andersen PH. (1994) Characteristics of stably expressed human dopamine D1a and D1b receptors: atypical behavior of the dopamine D1b receptor. Eur J Pharmacol 267:85–93 [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. (2012) Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: regulation of BDNF, GAD67 and VGLUT1/2. PLoS One 7:e33348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O’Dowd BF, George SR. (2010) The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem 285:36625–36634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. (2011) The dopamine d1-d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. (2014) Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology 39:156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper K (1959) The Logic of Scientific Discovery, Routledge Classics (2002 Reissue), London. [Google Scholar]
- Rappaport MS, Sealfon SC, Prikhozhan A, Huntley GW, Morrison JH. (1993) Heterogeneous distribution of D1, D2 and D5 receptor mRNAs in monkey striatum. Brain Res 616:242–250 [DOI] [PubMed] [Google Scholar]
- Rascol O, Blin O, Thalamas C, Descombes S, Soubrouillard C, Azulay P, Fabre N, Viallet F, Lafnitzegger K, Wright S, et al. (1999) ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson’s disease. Ann Neurol 45:736–741 [DOI] [PubMed] [Google Scholar]
- Rascol O, Nutt JG, Blin O, Goetz CG, Trugman JM, Soubrouillard C, Carter JH, Currie LJ, Fabre N, Thalamas C, et al. (2001) Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson disease. Arch Neurol 58:249–254 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, O’Dowd BF, Verma V, George SR. (2007a) Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci 28:551–555 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. (2007b) D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA 104:654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell DR, Zaluda LC, McClure MM, Strike KS, Barch DM, Harvey PD, Girgis RR, Hazlett EA, Mailman RB, Abi-Dargham A, et al. (2014) Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JE, Hitzemann RJ. (1990) Further evidence against the coupling of dopamine receptors to phosphoinositide hydrolysis in rat striatum. Biochem Pharmacol 39:1965–1970 [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Griffith A, Oloff S, Vaidehi N, Brown JT, Goddard WA, 3rd, Mailman RB. (2007) Functional selectivity of dopamine D1 receptor agonists in regulating the fate of internalized receptors. Neuropharmacology 52:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Nichols DE, Mailman RB. (2005) Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol Pharmacol 68:1039–1048 [DOI] [PubMed] [Google Scholar]
- Schneider JS, Sun ZQ, Roeltgen DP. (1994) Effects of dihydrexidine, a full dopamine D1 receptor agonist, on delayed response performance in chronic low dose MPTP-treated monkeys. Brain Res 663:140–144 [DOI] [PubMed] [Google Scholar]
- Scibilia RJ, Lachowicz JE, Kilts CD. (1992) Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse 11:146–154 [DOI] [PubMed] [Google Scholar]
- Setler PE, Sarau HM, Zirkle CL, Saunders HL. (1978) The central effects of a novel dopamine agonist. Eur J Pharmacol 50:419–430 [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. (2003) Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28:1400–1411 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. (2005) Seven-transmembrane receptor signaling through β-arrestin. Sci STKE 2005:cm10. [DOI] [PubMed] [Google Scholar]
- Starr MS. (1996) The role of dopamine in epilepsy. Synapse 22:159–194 [DOI] [PubMed] [Google Scholar]
- Steele TD, Hodges DB, Jr, Levesque TR, Locke KW. (1997) D1 agonist dihydrexidine releases acetylcholine and improves cognitive performance in rats. Pharmacol Biochem Behav 58:477–483 [DOI] [PubMed] [Google Scholar]
- Steele TD, Hodges DB, Jr, Levesque TR, Locke KW, Sandage BW., Jr (1996) The D1 agonist dihydrexidine releases acetylcholine and improves cognition in rats. Ann N Y Acad Sci 777:427–430 [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lawrence MS, Redmond DE, Jr, Elsworth JD, Roth RH, Nichols DE, Mailman RB. (1991) Dihydrexidine, a full dopamine D1 agonist, reduces MPTP-induced parkinsonism in monkeys. Eur J Pharmacol 199:389–391 [DOI] [PubMed] [Google Scholar]
- Toda M, Abi-Dargham A. (2007) Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep 9:329–336 [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slättman M, Gullberg M, Javitch JA. (2011) Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undie AS, Friedman E. (1990) Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther 253:987–992 [PubMed] [Google Scholar]
- Undie AS, Friedman E. (1992) Selective dopaminergic mechanism of dopamine and SKF38393 stimulation of inositol phosphate formation in rat brain. Eur J Pharmacol 226:297–302 [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. (1994) Inhibition of dopamine agonist-induced phosphoinositide hydrolysis by concomitant stimulation of cyclic AMP formation in brain slices. J Neurochem 63:222–230 [DOI] [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. (1994) Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem 62:2045–2048 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Urizar E, Yano H, Kolster R, Galés C, Lambert N, Javitch JA. (2011) CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol 7:624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Daigle TL, Caron MG. (2011) A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology 36:551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen RJ, Drukarch B, Sahadat MC, Goosen C, Wolters EC, Stoof JC. (1994) The dopamine D1 agonist SKF 81297 and the dopamine D2 agonist LY 171555 act synergistically to stimulate motor behavior of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned parkinsonian rhesus monkeys. Mov Disord 9:664–672 [DOI] [PubMed] [Google Scholar]
- Vyas SJ, Eichberg J, Lokhandwala MF. (1992) Characterization of receptors involved in dopamine-induced activation of phospholipase-C in rat renal cortex. J Pharmacol Exp Ther 260:134–139 [PubMed] [Google Scholar]
- Wallace MA, Claro E. (1990) A novel role for dopamine: inhibition of muscarinic cholinergic-stimulated phosphoinositide hydrolysis in rat brain cortical membranes. Neurosci Lett 110:155–161 [DOI] [PubMed] [Google Scholar]
- Weinstock J, Hieble JP, Wilson JW. (1985) The chemistry and pharmacology of 3-benzazepine derivatives. Drugs Future 10:645–696 [Google Scholar]
- Yu PY, Eisner GM, Yamaguchi I, Mouradian MM, Felder RA, Jose PA. (1996) Dopamine D1A receptor regulation of phospholipase C isoform. J Biol Chem 271:19503–19508 [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang JR, Sun PH, Guo Y, Zhang ZJ, Jin GZ, Zhen X. (2008) Neuroprotective effects of atypical D1 receptor agonist SKF83959 are mediated via D1 receptor-dependent inhibition of glycogen synthase kinase-3 β and a receptor-independent anti-oxidative action. J Neurochem 104:946–956 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ma L, Wang F, Chen J, Zhen X. (2007) Chronic SKF83959 induced less severe dyskinesia and attenuated L-DOPA-induced dyskinesia in 6-OHDA-lesioned rat model of Parkinson’s disease. Neuropharmacology 53:125–133 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou Z, Wang D, Li A, Yin Y, Gu X, Ding F, Zhen X, Zhou J. (2009) Activation of phosphatidylinositol-linked D1-like receptor modulates FGF-2 expression in astrocytes via IP3-dependent Ca2+ signaling. J Neurosci 29:7766–7775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Jiang XL, Zhang SE, Hough CJ, Li H, Chen JG, Zhen XC. (2005) The paradoxical effects of SKF83959, a novel dopamine D1-like receptor agonist, in the rat acoustic startle reflex paradigm. Neurosci Lett 382:134–138 [DOI] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Friedman E. (2005) The role of the phosphatidyinositol-linked D1 dopamine receptor in the pharmacology of SKF83959. Pharmacol Biochem Behav 80:597–601 Footnotes [DOI] [PubMed] [Google Scholar]