Abstract
Pneumocystis jiroveci pneumonia (PJP) is an important opportunistic infection in immunosuppressed hosts. At our center, nine transplant recipients developed PJP over a 4-month period. The median time from transplant was 56 months and none of them was on cotrimoxazole prophylaxis at the time of developing the infection. Over half had been admitted to the renal transplant ward for unrelated indications and contracted the infection in-hospital. Diagnosis was based on microbiological demonstration of P. jiroveci in sputum and/or bronchoalveolar lavage in symptomatic patients. Atypical clinical and radiological signs were common with poor correlation of symptoms to computed tomography findings. Cotrimoxazole therapy was effective; however, patients with pre-existing graft dysfunction developed hyperkalemia commonly (50%). Alternative treatment with clindamycin and primaquine combination was equally effective. Early diagnosis and prompt treatment resulted in low mortality rate (11%). The outbreak was halted after universal use of cotrimoxazole prophylaxis to all patients admitted to the renal transplant ward. We report the first ever outbreak of PJP in Indian renal transplant recipients with possible inter-human transmission of infection in admitted patients.
Keywords: Epidemic, Pneumocystis jiroveciii, pneumonia, renal transplant antation
Introduction
Pneumocystis jiroveci infection causes a severe opportunistic pneumonia in renal transplant recipients (RTRs). Cotrimoxazole prophylaxis is routinely recommended after renal transplant for 6-12 months and hence, P. jiroveci pneumonia (PJP) is rarely encountered in the early transplant period.[1] It was initially believed that the activation of latent Pneumocystis fungus in the lungs was responsible for most cases. The current thinking, however, is that most cases of Pneumocystis infections are due to a de novo acquisition of infection by inter-human airborne transmission of infection.[2,3] In fact, outbreaks of PCP have been documented in renal transplant units with molecular tracing of the fungus.[4] We report a cluster of PJP in our transplant unit with some peculiarities of presentation and treatment that were noted.
Materials and Methods
Our 20-bed renal transplant ward is part of a 1200-bed multispecialty tertiary care unit. There is a renal transplant intensive care unit that cares for the immediate post-transplant patients that is isolated from the rest of the ward. The other 17 beds are distributed in three rooms. There are about 500 RTRs on follow-up. The renal transplant outpatient department (OPD) is about 100 m from the ward that houses the inpatients with no common area. However, sampling for tacrolimus levels and other specialized lab tests for nephrology patients is at a special counter that may lead to brief admixture of these patients.
The diagnosis of PJP was established by microscopic demonstration of P. jiroveci on sputum and/or bronchoalveolar lavage in patients who had respiratory symptoms and/or radiological evidence of infection. Gomori methenamine silver (Grocott) stain was used to microscopically demonstrate P. jiroveci.
Results
During the period from January to April 2013, out of the 13 RTRs suspected based on clinical and radiological grounds, nine were diagnosed to have PJP. The median age of the patients was 39 (range 28-53) years; the median time from transplant being 56 (range 13-128) months. The practice at our center is to give cotrimoxazole prophylaxis for the first 6 months; hence none of these patients were on any form of PJP prophylaxis.
In the entire year 2012, there had been only two cases of PJP hence occurrence of nine cases over 3 months in early 2013 constituted an epidemic. Our index patient was admitted on 19 January 2013 with fever and dry cough. Amongst the other eight patients, five of them were admitted for indications other than clinical suspicion of a respiratory infection such as graft dysfunction, evaluation of solitary pulmonary nodule (histoplasmosis on histopathology), weight loss for investigation (military tuberculosis), urinary tract infection and malaria. These five patients developed fever and cough in hospital starting 2-13 days after admission and investigations revealed evidence of PJP. The other three patients had recently visited nephrology OPD at least once in 2 weeks prior to admission. They, however, were admitted with symptoms of fever and cough. Surely, at least 55% of our patients had nosocomially acquired the infection. The other patients may have acquired the infection in the community or during the brief encounters at the common blood sampling stations for RTRs.
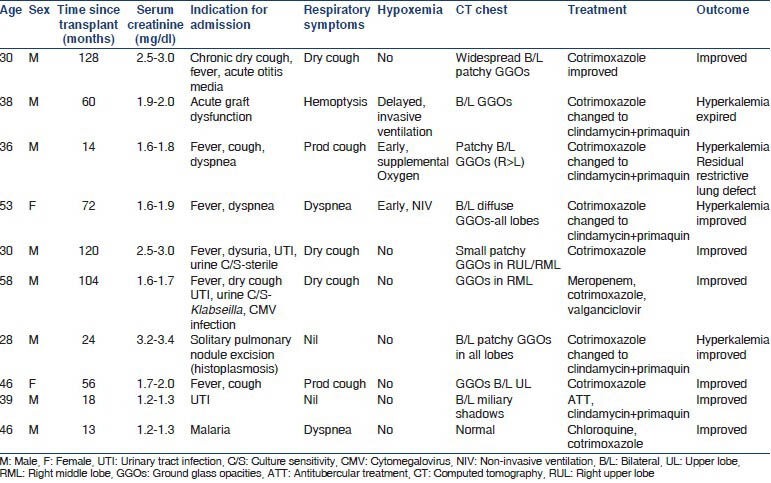
The patient details are as shown in Table 1. All patients were symptomatic with fever and cough. Only three of the nine patients had hypoxemia and only one patient required mechanical ventilation. Five out of nine (56%) of our patients had respiratory signs demonstrable in the form of crackles or wheeze. The majority (67%) did not desaturate on pulse oximetry despite a 6 min walk test although they were symptomatic with fever and cough.
Table 1.
Demographics, clinical characteristics, treatment and outcome of Pneumocystis jiroveci pneumonia patients
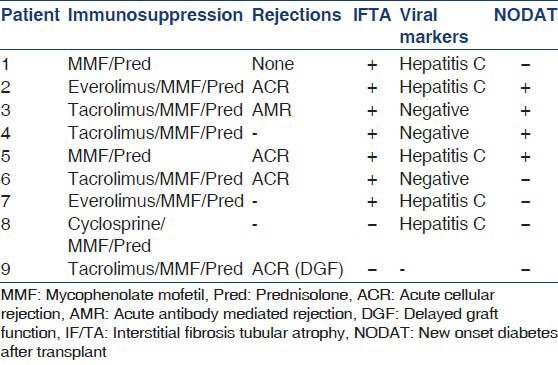
The details of immunosuppression and past rejections are as shown in Table 2. Over half the cases had a past history of acute rejection (5/9 cases) and biopsy proven interstitial fibrosis/tubular atrophy had been documented in the nine majority of patients (7/9 cases). No specific correlation could be drawn of PJP incidence with the immunosuppressive protocol used. Other associated conditions documented were hepatitis C infection (5/9 cases) and new onset diabetes after transplant (NODAT) (4/9 cases).
Table 2.
Predisposing risk factors to Pneumocystis jiroveci pneumonia
The radiological picture was highly varied resulting in delay of the diagnosis. Five out of nine (55%) of our patients had bilateral ground glass opacities classical of PJP. However, three had small localized infiltration with minimal ground glass discernable around the infiltrate. One had a completely normal computed tomography (CT) chest and another had military mottling on chest radiography as well as CT chest.
All patients were treated with cotrimoxazole (trimethoprim 15-20 mg/kg) initially, with additional use of steroids (prednisolone 1 mg/kg in two divided doses for 5 days followed by taper over a fortnight) in patients with hypoxemia (paO2 <70 mm Hg or the alveolar-arterial gradient >35 mm of Hg). Suitable renal dose modification for patients with epidermal growth factor receptor (eGFR) <30 ml/min was indicated in a third of our patients. Nearly 44% of our patients did not tolerate cotrimoxazole therapy warranting change of treatment to a combination of clindamycin and primaquine. The common causes of change of treatment were hyperkalemia and worsening of renal function with one patient requiring hemodialysis temporarily. The combination of clindamycin and primaquine was well-tolerated and proved to be effective in these patients. Majority of patients (8/9, 89%) recovered while only one patient who went on mechanical ventilation expired of ventilator associated pneumonia with sepsis.
Discussion
PJP is an opportunistic fungal infection that occurs in 6% of RTRs if no prophylaxis is given to them.[5] The risk factors for PJP in RTRs are the number and type of acute rejections, cytomegalovirus infection, other immunomodulating co-infections such as tuberculosis, hepatitis C and the use of potent immunosuppressive agents.[6] A trend toward a higher incidence with occasional failure of prophylaxis has been reported, especially when heavy immunosuppression is used.[7,8] Our study also showed an association with past acute rejection (56%), hepatitis C (56%) and NODAT in 44% cases.
We encountered nine cases of PJP in patients the majority of whom were admitted to the renal transplant ward for unrelated causes. The older school of thought was that P. jiroveci is a harmless commensal that causes opportunistic infection in the immunocompromised host. Molecular evidence for inter-human transmission of PJP in RTRs with colonized patients as human reservoirs has been demonstrated elegantly by deoxyribonucleic acid (DNA) based studies sequencing of the genotype of Pneumocystis isolated.[9] Colonization of Pneumocystis in RTRs has been recently described that can result in outbreaks of infection.[10] We have not done DNA sequencing of the Pneumocystis isolated. The circumstantial evidence of patients admitted for disparate indications developing symptoms and sign of PJP in hospital that was confirmed further by microbiological and radiological means is ample evidence for nosocomial spread. The outbreak was halted effectively by the universal use of cotrimoxazole prophylaxis to all patients admitted to the renal transplant ward irrespective of their duration post-transplant. The possibility of colonization in other asymptomatic patients that perpetuated the outbreak is a distinct possibility that cannot be ruled out.
The median time from transplantation to the development of disease was 56 months in our study. Varying time frame of occurrence (median 17 months, range 3-148 months) of infection have been documented.[11] Timing of PJP in RTRs is dependent on duration of prophylaxis used post-transplant and the hike in immunosuppression related to use of anti-rejection therapy; immunomodulatory infections playing contributory roles.[6] Our patients had widely varying time from transplant (range 13-128 months) possibly because it was related to a nosocomial outbreak largely with other factors not being important.
There are other clinical vignettes in the diagnosis and management of PJP that we learnt as the outbreak unfolded. Majority of our patients were symptomatic with fever and cough; however, dyspnea was encountered in only a fifth of our patients. The 6 min walk test was negative in the majority (77%) of our patients. This is in contrast to the reports of more fulminant clinical presentation of PJP in the non-human immunodeficiency virus setting.[12] This could be possibly because these were admitted patients who were picked up very early in their course and hence the disease had not progressed. Atypical radiological signs were the rule with a small localized patch of consolidation with surrounding ground-glassing being seen in a third of our cases. One patient had a completely normal CT scan, although he was symptomatic with breathlessness and improved after cotrimoxazole with prednisolone. Another patient admitted for evaluation of solitary pulmonary nodule underwent resection of the nodule in the right upper lobe that revealed histoplasmosis on histopathology. A repeat CT scan to assess the post-operative status revealed that he had developed extensive bilateral ground glass opacities; remarkably, the patient was completely asymptomatic. Similar widely varying radiological picture has been described.[12] A high index of suspicion, good microbiological support, early bronchoscopy coupled with high-resolution computed tomography chest are the cornerstones to pick up cases early.
We encountered hyperkalemia as a troublesome side-effect of cotrimoxazole therapy in nearly half of our patients. Hyperkalemia in these patients can be attributed to the tubular effects of trimethoprim. All these patients had pre-existing graft dysfunction with serum creatinine >1.8 mg/dl (mean eGFR 36 ml/min). Hyperkalemia has been reported with cotrimoxazole use, although, surprisingly there are few reports of this side-effect in the setting of renal transplant.[13,14] In those patients who were converted from cotrimoxazole because of hyperkalemia and/or worsening of graft dysfunction, the combination of clindamycin and primaquine was found to be effective. Mortality around 50% has been documented in many studies for PJP in RTRs, whereas we had only one fatality (1/9).[15,16] Since our patients were under direct observation when they developed symptoms in-hospital, early institution of treatment, possibly resulted in the low mortality.
Conclusions
Nephrologists need to be alert to the possibility of PJP outbreaks due to nosocomial transmission in renal transplant center. A high index of suspicion is needed to pick up cases early in view of atypical clinical presentation. Prompt radiological investigations coupled with meticulous microbiological examination can result in early diagnosis. Early and effective treatment can give gratifying results. Hyperkalemia is a common side-effect of the trimethoprim component of cotrimoxazole when used in the anti PJP doses, especially in the presence of graft dysfunction. Nosocomial outbreak can be halted by instituting universal cotrimoxazole prophylaxis to all admitted patients irrespective of the post-transplant period.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;(9 Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 2.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347–53. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 3.De Boer MG, Bruijnesteijn van Coppenraet LE, Gaasbeek A, Berger SP, Gelinck LB, van Houwelingen HC, et al. An outbreak of Pneumocystis jiroveci pneumonia with 1 predominant genotype among renal transplant recipients: Interhuman transmission or a common environmental source? Clin Infect Dis. 2007;44:1143–9. doi: 10.1086/513198. [DOI] [PubMed] [Google Scholar]
- 4.Brunot V, Pernin V, Chartier C, Garrigue V, Vetromile F, Szwarc I, et al. An epidemic of Pneumocystis jiroveci pneumonia in a renal transplantation center: Role of T-cell lymphopenia. Transplant Proc. 2012;44:2818–20. doi: 10.1016/j.transproceed.2012.09.089. [DOI] [PubMed] [Google Scholar]
- 5.Gerrard JG. Pneumocystis carinii pneumonia in HIV-negative immunocompromised adults. Med J Aust. 1995;162:233–5. doi: 10.5694/j.1326-5377.1995.tb139873.x. [DOI] [PubMed] [Google Scholar]
- 6.Radisic M, Lattes R, Chapman JF, del Carmen Rial M, Guardia O, Seu F, et al. Risk factors for Pneumocystis carinii pneumonia in kidney transplant recipients: A case-control study. Transpl Infect Dis. 2003;5:84–93. doi: 10.1034/j.1399-3062.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 7.Maini R, Henderson KL, Sheridan EA, Lamagni T, Nichols G, Delpech V, et al. Increasing Pneumocystis pneumonia, England, UK, 2000-2010. Emerg Infect Dis. 2013;19:386–92. doi: 10.3201/eid1903.121151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Date A, Krishnaswami H, John GT, Mathai E, Jacob CK, Shastry JC. The emergence of Pneumocystis carinii pneumonia in renal transplant patients in a south Indian hospital. Trans R Soc Trop Med Hyg. 1995;89:285. doi: 10.1016/0035-9203(95)90542-1. [DOI] [PubMed] [Google Scholar]
- 9.Le Gal S, Damiani C, Rouillé A, Grall A, Tréguer L, Virmaux M, et al. A cluster of Pneumocystis infections among renal transplant recipients: Molecular evidence of colonized patients as potential infectious sources of Pneumocystis jirovecii. Clin Infect Dis. 2012;54:e62–71. doi: 10.1093/cid/cir996. [DOI] [PubMed] [Google Scholar]
- 10.Fritzsche C, Riebold D, Fuehrer A, Mitzner A, Klammt S, Mueller-Hilke B, et al. Pneumocystis jirovecii colonization among renal transplant recipients. Nephrology (Carlton) 2013;18:382–7. doi: 10.1111/nep.12054. [DOI] [PubMed] [Google Scholar]
- 11.Borstnar S, Lindic J, Tomazic J, Kandus A, Pikelj A, Prah J, et al. Pneumocystis jirovecii pneumonia in renal transplant recipients: A national center experience. Transplant Proc. 2013;45:1614–7. doi: 10.1016/j.transproceed.2013.02.107. [DOI] [PubMed] [Google Scholar]
- 12.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–98. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 13.Funai N, Shimamoto Y, Matsuzaki M, Watanabe M, Tokioka T, Sueoka E, et al. Hyperkalaemia with renal tubular dysfunction by sulfamethoxazole-trimethoprim for Pneumocystis carinii pneumonia in patients with lymphoid malignancy. Haematologia (Budap) 1993;25:137–41. [PubMed] [Google Scholar]
- 14.Sheehan MT, Wen SF. Hyperkalemic renal tubular acidosis induced by trimethoprim/sulfamethoxazole in an AIDS patient. Clin Nephrol. 1998;50:188–93. [PubMed] [Google Scholar]
- 15.Arend SM, Westendorp RG, Kroon FP, van’t Wout JW, Vandenbroucke JP, van Es LA, et al. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin Infect Dis. 1996;22:920–5. doi: 10.1093/clinids/22.6.920. [DOI] [PubMed] [Google Scholar]
- 16.Elinder CG, Andersson J, Bolinder G, Tydén G. Effectiveness of low-dose cotrimoxazole prophylaxis against Pneumocystis carinii pneumonia after renal and/or pancreas transplantation. Transpl Int. 1992;5:81–4. doi: 10.1007/BF00339221. [DOI] [PubMed] [Google Scholar]


