Abstract
Fungal peritonitis (FP) is a rare, but serious complication of peritoneal dialysis. We analyzed the incidence of FP, associated risk factors and outcome of patients with FP and evaluated the role of prophylactic antifungal agent in reducing its incidence. We studied all patients with FP from January 2005 to January 2012. Study period was divided into two parts, period I (January 2005 to January 2010), when prophylactic antifungal was not used and period II (January 2010 to January 2012), when prophylactic antifungal (fluconazole) was used. A total of 142 episodes of peritonitis were documented during this period of which 20 (14%) were FP. During the study period I, 18 of 102 episodes of peritonitis (17.6%) and in the study period II (with antifungal prophylaxis), only 2 of 40 episodes of peritonitis (5%) were due to fungal infection (P = 0.04). Nine out of 20 patients (45%) had prior exposure to antibiotics. Fungal isolates were Candida albicans in 65%, non-albicans Candida in 25%, Rhizopus species in 5% and Alternaria in 5% of the patients. While 12 out of 20 patients (60%) recovered completely and were re-initiated on continuous ambulatory peritoneal dialysis (CAPD), 4 of them expired (20%) and 4 others (20%) were shifted to hemodialysis. Use of prophylactic antifungal agent significantly reduced the incidence of FP (P = 0.04). We conclude that - fluconazole when used as a prophylactic agent in the setting of bacterial peritonitis significantly reduces the incidence of subsequent FP in CAPD patients.
Keywords: Candida, fungal, peritonitis
Introduction
Fungal peritonitis (FP) is a rare but potentially fatal complication of chronic peritoneal dialysis (PD), associated with high morbidity and mortality ranging between 20% and 30%. FP accounts for 1-15% of all peritonitis episodes.[1] In those who survived peritonitis, the inflammatory process often causes irreversible damage to the peritoneal membrane leading to subsequent dropout from PD therapy in 40% of the patients.[2] The most common causative organism is Candida, predominantly Candida albicans. The most important risk factor associated with the development of FP includes previous antibiotic therapy, particularly for bacterial peritonitis.[1] The management of FP poses a difficult challenge. International society of PD guidelines for peritonitis 2010 recommend immediate catheter removal once fungus is identified by microscopy or culture.[3,4,5] The conventional antifungal regimens include fluconazole, amphotericin B and flucytosine alone or in combination, optimally based on fungal susceptibilities.[5] The newer agents such as caspofungin and voriconazole have the potential to alter treatment strategies for FP.[6,7,8] The beneficial role of prophylactic antifungal in bacterial peritonitis reducing the incidence of subsequent secondary FP is controversial, with some favoring them[9,10] and others showing no benefit.[11,12]
Subjects and Methods
In this retrospective study, we reviewed the dialysis records of all 224 end stage renal disease (ESRD) patients initiated on continuous ambulatory peritoneal dialysis (CAPD) between January 2005 and January 2012. We divided the study period into two parts: Period I (January 2005 to January 2010), when prophylactic antifungal was not used and study period II (January 2010 to January 2012), when prophylactic antifungal (fluconazole) was used with bacterial peritonitis. All patients in the study period II received oral fluconazole at a dose of 200 mg/day for 7 days as prophylaxis. During the study, demographic characteristics, cause of chronic kidney disease, details of PD prescription, presence of comorbid illness, duration on PD, peritonitis rates, prior history of fungal or bacterial peritonitis, nature of fungal isolates and clinical outcomes (including mortality, loss of PD catheter and successful reinsertion of PD catheter) were the variables which were analyzed. The risk of prior exposure to antibiotics within the 12 weeks period before the onset of peritonitis was also analyzed.
The diagnosis of FP was based on the isolation of fungi from PD fluid in the setting of classical features of peritonitis (fever, pain abdomen, cloudy peritoneal effluent containing 100 white blood cells/μl or greater with at least 50% polymorphonuclear cells).
The specimen sent for FP included CAPD fluid and CAPD catheter. Distal 2.5-5 cm of the CAPD catheter was cut from the catheter hub and dropped into a sterile container. About 50 ml of PD effluent was centrifuged at 3000 g for 15 min. The sediment was used for microscopic examination with 10% KOH and calcofluor to look for fungal elements. The CAPD catheter tip was flushed with sterile saline using a needle and syringe and this was used for microscopy as above. The sediment and fluid from the catheter tip were inoculated in two Sabouraud's dextrose agar tubes of which one was incubated at 37°C and the other at 25°C. Growth obtained was identified based on macroscopic and microscopic morphology.
Data was analyzed using Statistical Package for Social Sciences (SPSS) 18 software version. Descriptive statistics were reported as mean ± standard deviation. Chi-square test or Fisher's exact test were used to assess the significance of variables between the two groups.
Results
We had a total of 224 patients on CAPD between January 2005 and January 2012 with a cumulative follow-up of 5435.4 months. Demographic characteristics of the study population have been depicted in Table 1. Mean age of the study population was 51.9 ± 12.54 years with male to female ratio of 14:6. Twenty out of 142 episodes of peritonitis (14%) documented during the study period was due to FP. The mean duration on CAPD before development of fungal infection was 35.6 ± 6.2 months. Incidence of FP in our center was 1 episode every 271.8 months.
Table 1.
Demographic characteristics of study population
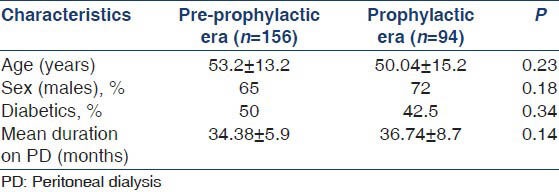
Predominant cause of ESRD in this group was diabetic nephropathy (50%). Other causes includes hypertensive nephrosclerosis (15%, n = 3), chronic glomerulonephritis (15%, n = 3), chronic interstitial nephritis (10%, n = 2) and others (10%, n = 2).
During the study period I, 18 of 102 episodes of peritonitis (17.6%) were due to fungal infection whereas during the study period II (with antifungal prophylaxis); only 2 of 40 episodes of peritonitis (5%) were due to fungal infection. The incidence of FP was significantly lower during the study period II (P = 0.04). Majority of the patients presented with pain abdomen (90%) and cloudy PD effluent (95%).
Common fungal isolate [Figure 1] in our center was C. albicans accounting for 65% of the cases (n = 12) [Figure 2]. Other fungal isolates includes non-albicans Candida 25% (n = 5), Rhizopus species 5% (n = 1) [Figure 3] and Alternaria alternata 5% (n = 1) [Figure 4]. Among non-albicans Candida species, three episodes were due to Candida tropicalis and two were due to Candida parapsilosis. Alternaria, a dematiaceous fungus is a very rare species, known to cause FP with only few case reports in the world literature. During the study period II, both episodes of FP were due to non-albicans Candida species.
Figure 1.
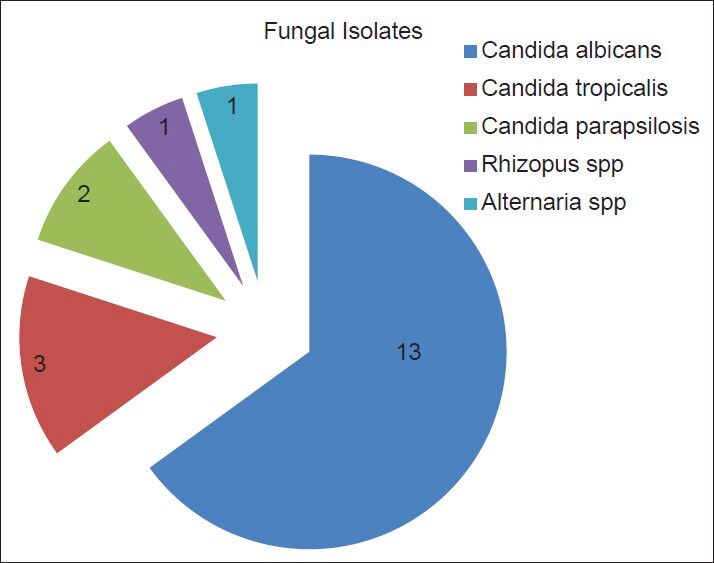
Fungal isolates in our center
Figure 2.
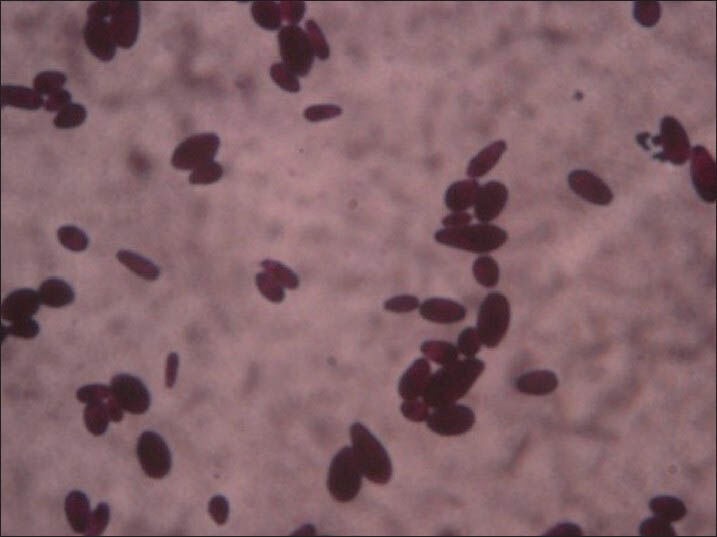
Gram stain morphology (×100) of Candida species colonies showing Gram-positive budding yeast cells
Figure 3.
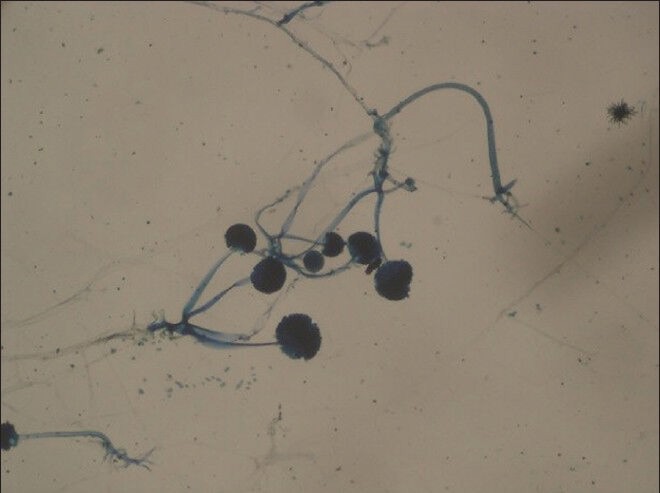
Lacto phenol cotton blue mount of Rhizopus species (×20) showing stolons connecting unbranched sporangiophores terminating in dark round sporangia with spores
Figure 4.
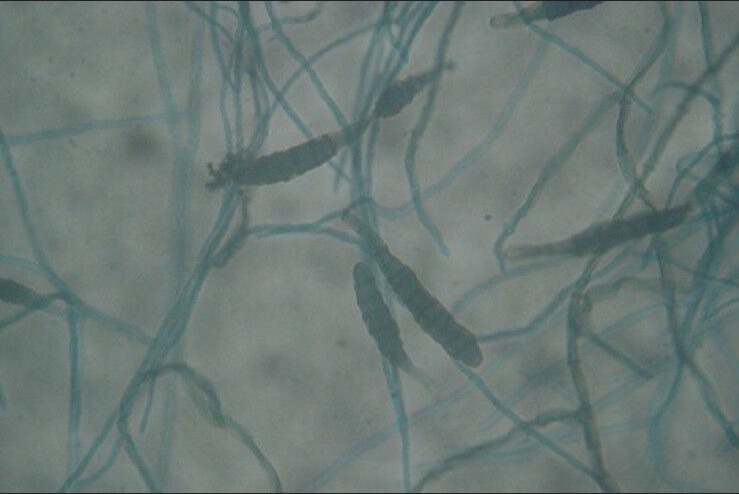
Lacto phenol cotton blue mount of Alternaria (×40) showing dark septate hyphae bearing large conidia with transverse and longitudinal septations
Prompt removal of Tenckhoff catheter was carried out in all 20 patients within 24 h of isolation of fungi either by culture or by demonstration of fungal filaments in gram's stain. All patients were treated with appropriate antifungal agents.
Four (20%) patients died of sepsis and septic shock. Out of which two had non-albicans Candida infection, one had C. albicans and the other one had Rhizopus species infection. Four (20%) patients had technique failure because of severe peritoneal adhesions and were shifted to hemodialysis. Remaining 12 (60%) patients recovered completely and they were put back on CAPD after a latent period of 6-12 weeks.
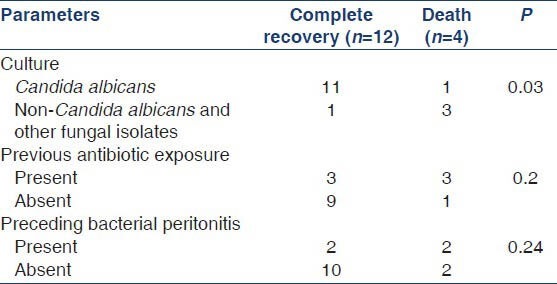
We analyzed various parameters associated with poor outcomes (mortality + technique failure) in patients with FP by logistic regression analysis. Three factors predicted poor outcome in our cohort: (a) FP by non-Candida species (P = 0.004), (b) prior exposure to antibiotics (P = 0.03) and (c) preceding history of bacterial peritonitis (P = 0.02). Though mean serum albumin was lower in patients with FP (2.4 g/dl) as compared to those with bacterial peritonitis (2.7 g/dl), the difference was not statistically significant (P = 0.09). Age, sex, diabetic status and clinical features were comparable between the two groups (FP vs. bacterial peritonitis). On subgroup analysis, infection due to non-Candida species predicted higher risk of mortality (P = 0.03) [Tables 2 and 3].
Table 2.
Risk factors for poor outcome (technique failure and mortality)
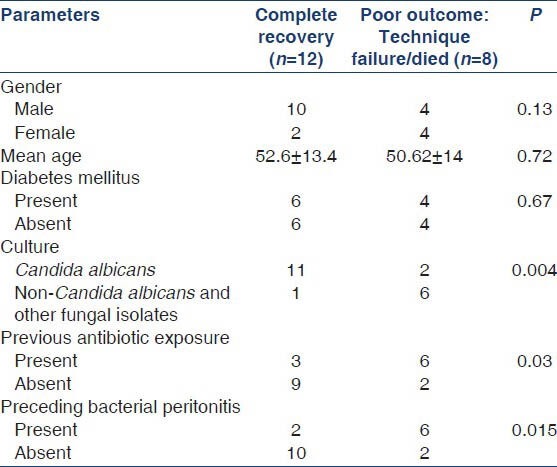
Table 3.
Risk factors for mortality
Discussion
FP accounts for 3-6% of all peritonitis episodes complicating PD in adults; however, in some centers, the numbers can be higher.[1] Indian studies have reported incidence of FP rates varying from 14.3%[13] to 23.88%, respectively.[14] In our study, FP accounted for 14% of all peritonitis episodes.
Most FP cases are caused by yeasts, with Candida species accounting for 70-90% in adults and 80-100% in the pediatric population.[1] We also noted C. albicans as the most common fungus isolated. The most important predisposing factor for the development of FP in CAPD patients is prior exposure to antibiotic therapy, especially for the treatment of bacterial peritonitis. The reported incidence of prior antibiotic exposure in CAPD patients with FP ranges from 34% to 80%, respectively.[2,13,15,16,17,18] In our study, 45% of the patients had prior exposure to antibiotics. It has been postulated that broad-spectrum antibiotics suppress the normal intestinal flora, leading to an overgrowth of intestinal fungi, which migrate across the intestinal wall to reach the peritoneal cavity and cause FP.[19]
Though, a substantial proportion of FP occurs without recent exposure to antibiotics. The causes of these de novo cases of FP vary and may include direct contamination of the dialysis catheter during the exchange procedure, underlying intestinal pathology such as diverticulosis in the host[15] and environmental contamination.[20]
The PD catheter should be removed as soon as possible after a diagnosis of FP, as recent reports have shown that leaving the catheter in situ is associated with higher rates of mortality and technique failure.[2,17] The suggestion is that FP results in formation of a biofilm around the dialysis catheter, rendering the eradication of the fungal infection difficult without removal of the catheter.[21] Isolated cases of successful continuation of CAPD without catheter removal have been reported with the use of various regimens of intraperitoneal antifungal agents;[22] however, the overall success rate of this approach is low and many patients still eventually require catheter removal. The conventional empirical treatment for FP was a combination of intravenous amphotericin B and flucytosine or oral fluconazole and flucytosine.[23] The roles of newer antifungal agents such as caspofungin and voriconazole in the treatment of CAPD-related FP remain to be determined. In our study, PD catheter was removed in all the patients and was treated with appropriate antifungal agents.
The mortality rate in CAPD-related FP ranges from 5% to 53%, respectively.[2,13,15,16,17,18,24] Consistently, many studies indicate that leaving the catheter in situ is associated with greater mortality.[2,13,16] One of the Indian study describes a mortality rate of 60.46%[14] in which PD catheter was left in situ in most of the patients. Many patients are unable to resume CAPD after FP because of peritoneal fibrosis,[2,13,15,16,17,18,24] which accounts for 40% of the cases. In our study, 60% patients were recovered completely and were reinitiated on CAPD. Nearly 20% of the patients were shifted to hemodialysis due to technique failure. Mortality rate due to FP in our center was 20%.
Given that recent antibiotic exposure is a recognized risk factor for FP in CAPD patients, administration of antifungal prophylaxis with every antibiotic prescription may help to reduce the occurrence of FP. The study of Lo et al.[25] demonstrated that 4-times a day oral nystatin 500,000 U with every antibiotic prescription significantly reduced both overall incidence and the incidence of antibiotic-related Candida peritonitis in CAPD patients; however, two subsequent studies failed to confirm a benefit for nystatin prophylaxis.[11,12] We however did not administer antifungal prophylaxis in our PD patients with every antibiotic prescription. In patients with bacterial peritonitis, administration of prophylactic oral fluconazole throughout the time they received antibiotics significantly prevented the appearance of secondary FP.[9,10] In our center, we noticed a significant reduction in the FP rates with the use of prophylactic anti-fungal agent (fluconazole) in patients with bacterial peritonitis.
The novel feature of this study is that, for the first time in our country a study has been conducted evaluating the role of antifungal prophylaxis in CAPD patients with bacterial peritonitis in Indian setting. The results favored the use of fluconazole prophylaxis. The limitation of our study is relatively small numbers. However, we also noted a change in the epidemiology of fungal isolates from C. albicans to non-C. albicans in the post prophylactic era, after usage of fluconazole, which has not been reported by others in the West.
We conclude that patients with prior antibiotic exposure are at higher risk of developing FP (odds ratio: 1.22) and C. albicans is the most common fungus isolated. Prophylactic antifungal agent in all patients with bacterial peritonitis significantly reduces the incidence of subsequent FP.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int. 2009;(29 Suppl 2):S161–5. [PubMed] [Google Scholar]
- 2.Wang AY, Yu AW, Li PK, Lam PK, Leung CB, Lai KN, et al. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: Analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis. 2000;36:1183–92. doi: 10.1053/ajkd.2000.19833. [DOI] [PubMed] [Google Scholar]
- 3.Warady BA, Bashir M, Donaldson LA. Fungal peritonitis in children receiving peritoneal dialysis: A report of the NAPRTCS. Kidney Int. 2000;58:384–9. doi: 10.1046/j.1523-1755.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 4.Raaijmakers R, Schröder C, Monnens L, Cornelissen E, Warris A. Fungal peritonitis in children on peritoneal dialysis. Pediatr Nephrol. 2007;22:288–93. doi: 10.1007/s00467-006-0289-x. [DOI] [PubMed] [Google Scholar]
- 5.Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25:107–31. [PubMed] [Google Scholar]
- 6.Madariaga MG, Tenorio A, Proia L. Trichosporon inkin peritonitis treated with caspofungin. J Clin Microbiol. 2003;41:5827–9. doi: 10.1128/JCM.41.12.5827-5829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fourtounas C, Marangos M, Kalliakmani P, Savidaki E, Goumenos DS, Vlachojannis JG. Treatment of peritoneal dialysis related fungal peritonitis with caspofungin plus amphotericin B combination therapy. Nephrol Dial Transplant. 2006;21:236–7. doi: 10.1093/ndt/gfi162. [DOI] [PubMed] [Google Scholar]
- 8.Molina P, Puchades MJ, Aparicio M, García Ramón R, Miguel A. Fungal peritonitis episodes in a peritoneal dialysis centre during a 10-year period: A report of 11 cases. Nefrologia. 2005;25:393–8. [PubMed] [Google Scholar]
- 9.Thodis E, Vas SI, Bargman JM, Singhal M, Chu M, Oreopoulos DG. Nystatin prophylaxis: Its inability to prevent fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 1998;18:583–9. [PubMed] [Google Scholar]
- 10.Williams PF, Moncrieff N, Marriott J. No benefit in using nystatin prophylaxis against fungal peritonitis in peritoneal dialysis patients. Perit Dial Int. 2000;20:352–3. [PubMed] [Google Scholar]
- 11.Restrepo C, Chacon J, Manjarres G. Fungal peritonitis in peritoneal dialysis patients: Successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int. 2010;30:619–25. doi: 10.3747/pdi.2008.00189. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa NK, Suh H, Cabralda T. Antifungal prophylaxis for secondary fungal peritonitis in peritoneal dialysis patients. Adv Perit Dial. 1996;12:189–91. [PubMed] [Google Scholar]
- 13.Prasad KN, Prasad N, Gupta A, Sharma RK, Verma AK, Ayyagari A. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: A single centre Indian experience. J Infect. 2004;48:96–101. doi: 10.1016/s0163-4453(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 14.Ram R, Swarnalatha G, Neela P, Murty KV. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: A single-centre experience in India. Nephron Clin Pract. 2008;110:c207–12. doi: 10.1159/000167867. [DOI] [PubMed] [Google Scholar]
- 15.Chan TM, Chan CY, Cheng SW, Lo WK, Lo CY, Cheng IK. Treatment of fungal peritonitis complicating continuous ambulatory peritoneal dialysis with oral fluconazole: A series of 21 patients. Nephrol Dial Transplant. 1994;9:539–42. doi: 10.1093/ndt/9.5.539. [DOI] [PubMed] [Google Scholar]
- 16.Goldie SJ, Kiernan-Tridle L, Torres C, Gorban-Brennan N, Dunne D, Kliger AS, et al. Fungal peritonitis in a large chronic peritoneal dialysis population: A report of 55 episodes. Am J Kidney Dis. 1996;28:86–91. doi: 10.1016/s0272-6386(96)90135-3. [DOI] [PubMed] [Google Scholar]
- 17.Manzano-Gayosso P, Hernández-Hernández F, Méndez-Tovar LJ, González-Monroy J, López-Martínez R. Fungal peritonitis in 15 patients on continuous ambulatory peritoneal dialysis (CAPD) Mycoses. 2003;46:425–9. doi: 10.1046/j.0933-7407.2003.00922.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen CM, Ho MW, Yu WL, Wang JH. Fungal peritonitis in peritoneal dialysis patients: Effect of fluconazole treatment and use of the twin-bag disconnect system. J Microbiol Immunol Infect. 2004;37:115–20. [PubMed] [Google Scholar]
- 19.Eisenberg ES, Leviton I, Soeiro R. Fungal peritonitis in patients receiving peritoneal dialysis: Experience with 11 patients and review of the literature. Rev Infect Dis. 1986;8:309–21. doi: 10.1093/clinids/8.3.309. [DOI] [PubMed] [Google Scholar]
- 20.Yuen KY, Seto WH, Ching TY, Cheung WC, Kwok Y, Chu YB. An outbreak of Candida tropicalis peritonitis in patients on intermittent peritoneal dialysis. J Hosp Infect. 1992;22:65–72. doi: 10.1016/0195-6701(92)90131-5. [DOI] [PubMed] [Google Scholar]
- 21.Marrie TJ, Noble MA, Costerton JW. Examination of the morphology of bacteria adhering to peritoneal dialysis catheters by scanning and transmission electron microscopy. J Clin Microbiol. 1983;18:1388–98. doi: 10.1128/jcm.18.6.1388-1398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Chiang SS, Hseih SJ, Shen HM. Successful treatment of fungal peritonitis with intracatheter antifungal retention. Adv Perit Dial. 1995;11:172–5. [PubMed] [Google Scholar]
- 23.Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, et al. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int. 2000;20:396–411. [PubMed] [Google Scholar]
- 24.Nagappan R, Collins JF, Lee WT. Fungal peritonitis in continuous ambulatory peritoneal dialysis – The Auckland experience. Am J Kidney Dis. 1992;20:492–6. doi: 10.1016/s0272-6386(12)70262-7. [DOI] [PubMed] [Google Scholar]
- 25.Lo WK, Chan CY, Cheng SW, Poon JF, Chan DT, Cheng IK. A prospective randomized control study of oral nystatin prophylaxis for Candida peritonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1996;28:549–52. doi: 10.1016/s0272-6386(96)90466-7. [DOI] [PubMed] [Google Scholar]


