Abstract
BACKGROUND
In 2002, the US National Heart, Lung, and Blood Institute (NHLBI) conducted a workshop to determine needs of the cell therapy community. A consensus emerged that improved access to cGMP facilities, regulatory assistance, and training would foster the advancement of cellular therapy.
STUDY DESIGN AND METHODS
A 2003 NHLBI request for proposals resulted in four contracts being awarded to three cell-manufacturing facilities (Baylor College of Medicine, University of Minnesota, and University of Pittsburgh) and one administrative center (The EMMES Corporation). As a result, Production Assistance for Cellular Therapies (PACT) was formed.
RESULTS
As of October 1, 2008, PACT has received 65 preliminary applications of which 45 have been approved for product manufacture. A variety of cell therapies are represented including T-regulatory cells, natural killer cells, adipose-derived stem cells, cardiac progenitor cells for cardiac disease, hematopoietic progenitor cells (HPCs) for central nervous system applications, cytotoxic T lymphocytes, and dendritic cells. A total of 169 products have been administered under 12 applications and 2 reagents were manufactured and delivered. Fourteen peer-reviewed publications and 15 abstracts have resulted from the PACT project to date. A cell therapy textbook is nearly complete. PACT technical projects have addressed assay development, rapid endotoxin testing, shipping of cell products, and CD34+ HPC isolation from low-volume marrow. Educational Web seminars and onsite training through workshops have been conducted.
CONCLUSIONS
PACT is an active and successful cell therapy manufacturing resource in the United States, addressing research and training while forging relationships among academia, industry, and participating institutions.
In 2002, to determine investigator needs in cellular therapeutics, the National Heart, Lung, and Blood Institute (NHLBI) conducted a workshop entitled “Immune reconstitution and cell-based therapy following hematopoietic stem cell (HSC) transplantation.”1 The workshop addressed the biology of immune reconstitution after transplantation, but also considered newer and more complex cell-processing methods and the changing regulatory environment in which they were emerging. The workshop participants recognized that, compared with classical HSC processing and transplantation, a much broader array of cell products and tissue types was being brought from the research laboratory to the clinic and that these activities presented both opportunities and challenges that were new and very significant. Opportunities included the emerging possibilities related to specific cell selection, stimulation, expansion, and how these and other laboratory manipulations were enabling fundamentally new treatments for a range of diseases. Challenges included the substantial difficulties often encountered when translating and scaling up preclinical research projects from their beginnings in the basic research laboratory. The workshop participants also recognized that cost and availability of highly specialized technical and regulatory expertise were significant barriers to the growth and success of human cellular therapy. Relatively few academic research institutions at the time had access to experienced cell-processing personnel, manufacturing facilities, and the regulatory expertise needed to develop a cell product internally. To address these issues and foster the growth of innovative scientific concepts in cellular therapy, the NHLBI initiated the Production Assistance for Cellular Therapies (PACT) program as a scientific, regulatory, and educational resource for the cell therapy community.2
METHODS
Participating institutions and governance
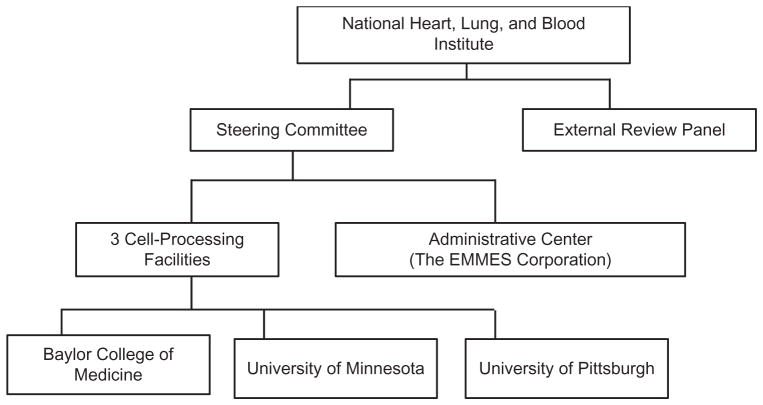
After a competitive application process described in the NHLBI Request for Proposals (NHLBI BAA HB-0306/7), four contracts were awarded in September 2003. The three participating cell-processing facilities are located at the Baylor College of Medicine in Houston, Texas; the University of Minnesota in Minneapolis, Minnesota; and the University of Pittsburgh in Pittsburgh, Pennsylvania. The EMMES Corporation in Rockville, Maryland, was awarded the contract as administrative center. A steering committee was established, consisting of representatives from each manufacturing facility, the administrative center, NHLBI, and external NHLBI-appointed co-chairs. The steering committee formulates and implements policy decisions related to the conduct of the project. An external review panel was convened by NHLBI to independently review and annually evaluate the PACT program and to advise NHLBI regarding the adequacy of progress toward its goals. The program structure is illustrated in Fig. 1.
Fig. 1.
Organization of the NHLBI program Production Assistance for Cellular Therapies (PACT).
NHLBI expectations of PACT
The NHLBI has charged the PACT facilities with manufacturing a clinical-grade product for requesting investigators. These investigators may lack access to or expertise in cGMP manufacturing or sufficient funds to manufacture their cell therapy product. Additionally, to further advance the field of cellular therapy among investigators, PACT is tasked with establishing an effective educational program. This educational initiative has fostered partnerships with transfusion medicine and hematology, which is in the purview of NHLBI’s mission for PACT.
The application process: types of application and their evaluation
The application process consists of two stages in which the steering committee reviews and votes on all applications at each stage. Applications are accepted on a rolling basis. Investigators are asked first to submit a preliminary application for product manufacturing support. The preliminary application is designed to be brief and easy for the applicant to complete but provide sufficient detail to allow the steering committee members to determine whether the application is scientifically sound. Review is based largely upon the following criteria:
Demonstration of an adequate plan and/or resources for use of the cell therapy product in a clinical trial and the availability of other funds to complete the proposed clinical trial;
Rationale for clinical benefit;
Evidence that the product can be manufactured in a clinical cell therapy laboratory;
Ability of one or more of the PACT facilities to manufacture the product;
Relevance to the NHLBI scientific mission;
Likelihood of meeting regulatory requirements;
In addition to being classified as translational or clinical, based on the amount of developmental work required to manufacture the cell therapy product for administration in a clinical trial, applications are also categorized according to their origin and the affiliation of the principal investigator. Internal applications come from a PACT investigator, non-PACT internal applications come from a non-PACT investigator at a PACT site (local collaborator), and external applications come from applicants wholly external to the PACT project; these may be academic centers, other nonprofit organizations, or commercial entities.
If the steering committee approves the preliminary application, the applicant is invited to submit a full application that provides in-depth information regarding the scientific, technical, and logistic details required to manufacture the requested product. An important part of the full application involves the clinical trial protocol, access to patients for the trial, a plan for evaluating the biologic (as well as clinical) effect of the novel cellular product, and the funding and resources for these activities. Two or more experts from the specific scientific field of the proposal are solicited to perform an independent peer review of full applications in addition to the peer review provided by the steering committee members. Outside reviewers provide a written summary evaluating the strengths and weaknesses of preliminary data, manufacturing feasibility, significance to the field, degree of innovation, and overall scientific merit of the application. The independent review is then submitted to the steering committee, to assist in the final decision to approve or reject the submission. When a full application is approved for product manufacturing, a processing facility and a designated technical liaison are assigned to work with the applicant to develop the timeline, milestones, budget, and a contract between organizations if one is needed. The staff at the appointed manufacturing facility work closely with the applicant to understand the product characteristics, methods of delivery, and other requirements. Manufacturing is performed under a formal contract between the organizations based upon a mutually agreed upon template. Contractual agreements between organizations may also include confidentiality agreements, material transfer agreements, and agreements regarding intellectual property rights.
Cell manufacturing and clinical trials
PACT provides product development and manufacturing support to its investigators, local collaborators, and outside investigators; its scientific mission is to advance the field of cellular therapy and to enable scientifically meritorious basic research related to human somatic cell therapy to reach the clinical trial stage even if the investigator lacks the internal manufacturing resources or infrastructure needed. The program provides cell therapy products produced in compliance with cGMP guidelines for research use in human clinical trials. Recently, NHLBI has further authorized PACT to develop guidelines whereby limited funding may be used to assist with approved human clinical trials involving PACT cell products. Because the project must also meet the global objectives of the NHLBI, the approved projects have been focused thus far on modulating the host immune system, aiding in the repair and regeneration of damaged/ diseased tissues, organs, and biologic systems and providing data validating procedures common to all cell applications, for example, shipping and endotoxin testing.
Technology transfer, an important part of PACT, involves the exchange of technical information and development of procedures to expedite progress of preclinical research work toward clinical-scale production. Several product request applications have involved site visits by PACT investigators to applicants’ laboratories (and vice versa) to foster the exchange of information regarding standard operating procedures (SOPs) and technical troubleshooting. These site visits have aided the establishment of effective communication between the applicant and manufacturing facility and have greatly facilitated the progress of preclinical/translational work.
Once it is established that a basic science process has a possible clinical application, the process must be further evaluated to determine whether it is feasible from a technical and regulatory perspective. This evaluation will determine if the project can progress to the scale-up production phase. The implementation of a clinical-scale manufacturing method involves the development of SOPs, quality control testing, defined steps in production, criteria for acceptable starting material, final product specifications, performing validation runs to ensure the reproducibility of the final manufacturing process, training staff, providing data to support an investigational new drug (IND) application and institutional review board submission, and final development of appropriate release criteria. PACT commits significant resources to assist investigators with manufacturing regulatory compliance efforts as part of filing an IND.
Educational objectives
PACT’s educational mission is to make beneficial and authoritative educational experiences widely available for the cell therapy community through a variety of conventional and electronic media. An education committee was formed as a subgroup of the steering committee to design and pursue specific educational initiatives to fulfill this objective. The committee’s goal is to promote interest in cellular therapy among physicians, product manufacturers, and scientists-in-training and to provide educational experiences in a variety of venues that can assist interested individuals with current or future careers in cellular therapy. The education committee performs several functions to accomplish its mission which include the following:
Planning and designing education/training programs;
Developing didactic and interactive training materials;
Conducting Web seminars and training workshops;
Conducting PACT cell-processing facility tours for training workshop attendees;
Developing and coauthoring a cell therapy book;
Providing general cell therapy resource materials for public distribution;
Providing testing tools to assess learning achievement and adequacy of teaching.
PACT technical projects
The PACT investigators and steering committee members recognize that progress in cell therapy often depends on development of new assays or methods that, once validated, can enter general use and become broadly applicable enabling technologies for a variety of projects. Standalone research proposals focused on development of validation and standardization of commonly used methods would not typically be supported by the National Institutes of Health (NIH) or other major extramural funding agencies. Therefore, the NHLBI and the PACT investigators have chosen to support carefully selected technical projects internally and collaboratively. Technical projects currently in various stages include evaluation of a rapid endotoxin detection assay (Endosafe, Charles River, Wilmington, MA), optimal conditions for interinstitutional shipping of human cellular products, and feasibility of processing small volumes of human marrow on a cell separating device (Isolex, Miltenyi Biotec, Auburn, CA).
RESULTS
Application receipt, classification, and evaluation
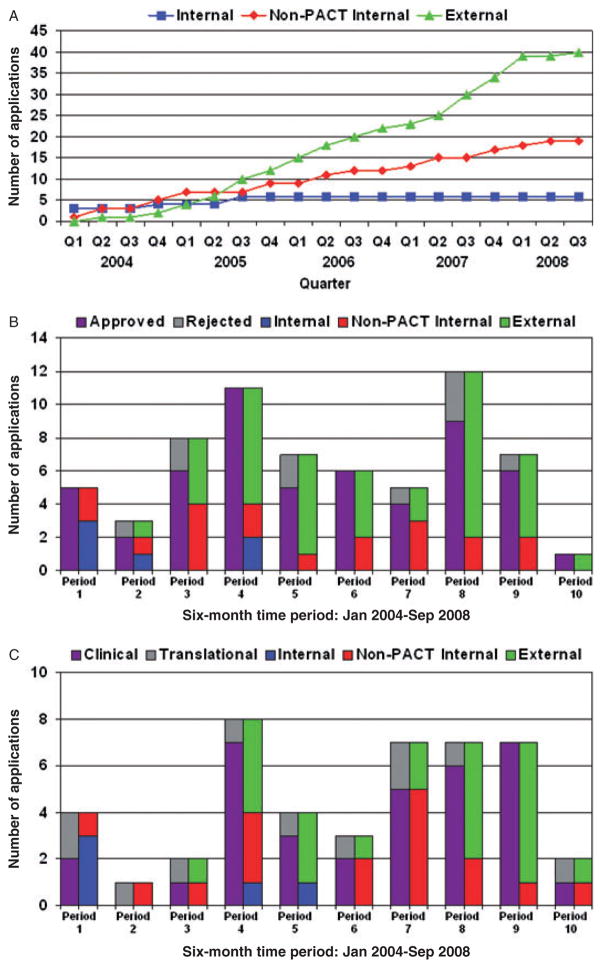
Figure 2A depicts the cumulative growth in preliminary applications according to the application’s origin (PACT investigators, local collaborators, and non-PACT investigators) since the PACT program began. Figs. 2B and 2C show, for each 6-month interval since the program began, the number of preliminary and full applications according to both applications’ origin and review status. As of October 1, 2008, 55 different investigators have submitted 65 preliminary applications requesting manufacturing support for cellular therapy products from the PACT group. Of the 65 preliminary applications received, 40 were external, 19 were from a non-PACT investigator at a PACT site (local collaborator), and 6 were internal applications from individual PACT investigators. One of the 40 external preliminary applications, a request for GMP consulting services, was reclassified and approved as an educational initiative. Six preliminary applications were rejected based on steering committee review of merit or a lack of relevance to the NHLBI mission and 2 applications were deferred pending submission of additional information.
Fig. 2.
(A) Preliminary applications by origin, on a quarterly basis, from January 2004 through September 2008. (B) Preliminary application by review status and application origin on a semiannual basis. (Period 10 for this figure only contains data from July 2008 through September 2008.) (C) Data shown are full applications by application type and origin on a semiannual basis. (Period 10 for this figure only contains data from July 2008 through September 2008.)
A total of 45 full applications were generated from the 56 preliminary applications that received steering committee approval. Four approved preliminary applications are pending submission of a full application, 3 preliminary applications were withdrawn by the submitting investigator, and 4 preliminary applications were closed due to the lack of investigator response. Forty-five full applications have been approved for product manufacturing.
At the time each of the 45 full applications was approved, 37 were considered clinical status (ready for GMP manufacture) and 8 were classified as translational (needing further development and validation before GMP manufacture). In some instances, an investigator’s application is reclassified from clinical to translational after initial conference with the PACT technical liaison. Reclassification from translational to clinical has not occurred.
The PACT Administrative Center and investigators have worked to achieve a timely turnaround at each stage of the application process and to improve these turnaround times as the project has gained infrastructure, experience, and momentum. To accomplish this responsiveness, the administrative center defined and tracked critical time-related milestones involving the application process and the resulting metrics are presented in Table 1. An improvement in turnaround time is demonstrated when the metrics from the start-up phase of the project (Years 1–2) are compared to those representing the current operational phase (Years 3 to the present). The development of a Web-based application process system has improved the submission process and facilitated tracking for preliminary and full applications.
TABLE 1.
Turnaround intervals (days) associated with the receipt and evaluation of applications to PACT: start-up period compared with post–start-up period*
| Turnaround interval | Start-up phase (days), Years 1 and 2 | Operational phase (days), Years 3–5 |
|---|---|---|
| Decision to applicant on preliminary application | 34 (3–130) | 19 (2–41) |
| Receipt of full application by PACT after approval of preliminary application | 110 (35–267) | 259 (14–1597) |
| Completion of outside peer review for full application | 24 (2–79) | 19 (4–58) |
| Decision returned to applicant on full application after its receipt | 88 (13–201) | 53 (3–145) |
Data are reported as median (range). n = 65 total initial applications received.
Cell manufacturing and clinical trials
Approved applications represent a wide variety of cell types and clinical hypotheses. Table 2 provides a partial listing of cell therapy product requests and treatment indications to date. An extensive list of cell therapy products available for manufacture by the PACT group can be found on the NHLBI PACT Web site (http://www.pactgroup.net). Table 3 illustrates the scientific pedigree for four representative PACT products by tracing their original rationale in the scientific literature forward in time to key references that document how these cellular therapy concepts made the journey from the research bench to clinical trial. While this path may not be as clear for all products, these representative examples illustrate not only the cell therapy manufacturing PACT has executed but also the strong basic science it has been based on and reflect PACT’s contribution to the advancement of cell therapy.
TABLE 2.
Partial listing of cell therapy product requests and treatment indications
| Cell product* | Treatment indication or objective |
|---|---|
| Cardiac stem/progenitor cells | Cardiac regeneration in acute myocardial infarction |
| Mesenchymal stem cells* | Repair cardiac damage in patients after myocardial infarction Sickle cell disease |
| Autologous CD34+ stem cells | Intermittent claudication |
| Autologous marrow MNCs* | Cardiac repair during left ventricular assisted device placement Stroke Left-ventricular function after acute myocardial infarction Treatment of traumatic brain injury |
| Cytomegalovirus (CMV), adenovirus, and EBV-specific cytotoxic T lymphocytes (PB-derived) | Prevent and treat viral infection (CMV, EBV, or adenovirus) Treatment of refractory posttransplant lymphoproliferative disease |
| Autologous mature apoptotic dendritic cells with human immunodeficiency virus (HIV)-1 | Therapeutic autologous vaccine for HIV-1 infected patients |
| Dendritic cells antisense and oligodeoxyribonucleotides | Preserving residual β cell mass in Type 1 diabetes |
| Allogeneic (haploidentical) natural killer cells | Control residual viral infection |
| Vaccine-human fetal lung-derived fibroblast cell line | Non–small cell lung cancer |
| Human leukemic T-cell line | Prostate cancer |
| B95-8 EBV LCL line (reagent) | Expand EBV-specific cytotoxic T lymphocytes to treat lymphoma and nasopharyngeal carcinoma |
| Human chronic myeloid leukemia–derived cell line | Treatment of persistent or relapsed B-lymphoid malignancies |
| Autologous LMP-1 and LMP-2–specific cytotoxic T lymphocytes | Treatment of EBV positive Hodgkin’s and non-Hodgkin’s lymphomas |
| Dendritic cells loaded with killed autologous chronic lymphocytic leukemia cells | Therapeutic vaccination of chronic lymphocytic leukemia patients |
| Natural killer-92 cell line | Treatment of advanced lymphomas and leukemias |
| Natural killer cells (UCB-derived) | Treatment of myeloid leukemia |
| Allogeneic (leukapheresed) natural killer cells | Postautologous stem cell transplant for patients at risk of relapse |
| Allo-depleted donor leukocytes | Enhance immune reconstitution |
| Multivalent virus-specific cytotoxic T lymphocytes (UCB-derived) | Immune reconstitution of cord blood transplant patients |
| Allogeneic T-cell-depleted progenitor cells (PB-derived) | Improve immune reconstruction posttransplant |
| Hematopoietic progenitor stem cells (UCB-derived) | Marrow reconstitution |
| CD4+/CD25+ T regulatory cells (PB-derived) | Prevent graft-vs.-host disease (GVHD) and maintain graft-versus-leukemia |
| Allogeneic EBV-LCL cell line | Natural killer cell expansion to reduce GVHD and enhance graft-versus-tumor after nonmyeloablative allogeneic hematopoietic cell transplant |
| T-regulatory cells (UCB-derived) | Decrease GVHD and enhance engraftment |
| Skeletal myoblasts | Translational development (cardiac) |
| Stem cells (adipose-derived) | Translational development (ischemia) |
| Pancreatic islets cells | Translational development to improve digestion process closed system (diabetes) |
Multiple applications are grouped by cell product.
EBV = Epstein-Barr virus; PB = peripheral blood; UCB = umbilical cord blood.
TABLE 3.
Representative NHLBI PACT cell therapy products: from key scientific rationale in the literature to clinical application in human cellular therapy
| Cell product | Clinical rationale | Scientific basis citations | Clinical application citations |
|---|---|---|---|
| CMV pp65-specific cytotoxic T0-lymphocytes | Prevention and treatment of CMV after HLA antigen–mismatched allogeneic HSC transplantation | Leen et al.;4 Sili et al.5 | Bollard et al.;6 Kennedy-Nasser and Bollard;7 Leen et al.8,9 |
| CD4+/CD25+ T-regulatory cells | Prevention of GVHD while maintaining graft-versus-tumor effect | Blazar et al.;10 Godfrey et al.11,12 | Godfrey et al.12 |
| Autologous mature apoptotic dendritic cells with HIV-1 | Improving host immune control of residual host immune HIV infection during highly active anti-retroviral therapy | Connolly et al.13 | Whiteside et al.14 |
| Allogeneic haploidentical natural killer cells (allo-NK cells) | Myeloid leukemia | Chiorean and Miller;15 Miller;16 Miller et al.17 | Miller et al.;18 McKenna et al.19 |
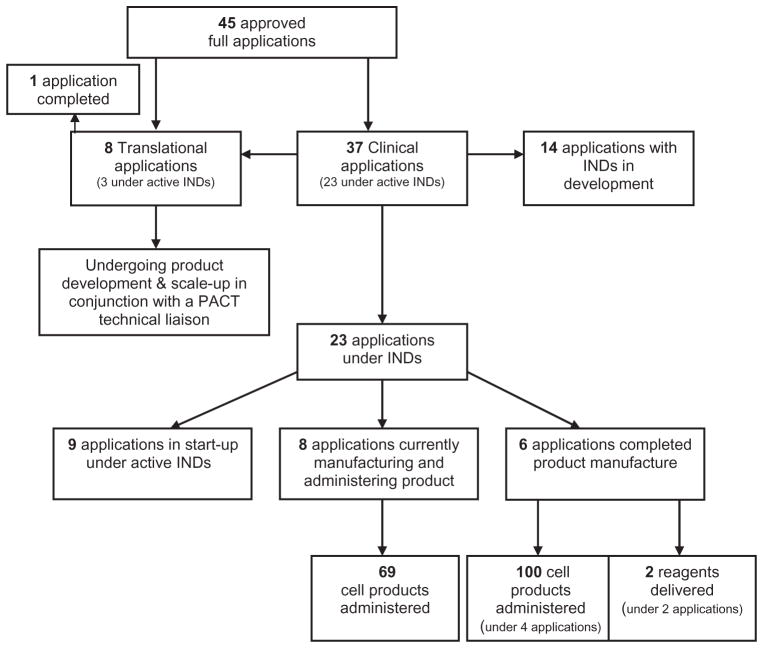
Figure 3 shows the workflow, including cell product manufacturing and product administration to patients related to the 45 currently approved full applications, 26 (23 clinical and 3 translational) of which are currently under active INDs. Six clinical projects have completed their manufacturing life cycle and all scheduled product has been delivered to the applicant for clinical use and 1 translational project has been completed. Projects classified as translational are addressing development and scale-up procedures typical to cellular processing. These include characterization of cell types, achievement of adequate yield for clinical use when applying multiple sequential cell selections, achieving culture expansion yields needed for clinical application using biochemically defined media; optimizing changes involving a closed system and modified culture conditions; deciding on precise product definition and developing assays to define the chosen characteristics; developing and validating processes related to packaging, shipping, receiving, sometimes thawing, and recharacterizing; and administering a product in the receiving institution. Besides product development, several remaining approved applications may require additional investigator funding for the clinical trial or human subjects approvals to move forward. The steering committee and the administrative center continue to work closely with these investigators to advance their projects where feasible. The steering committee may also consider a project for termination, on a case-by-case basis, if it appears that a project is unable to move forward in a timely fashion despite maximal available support from the investigator and PACT. Such difficult decisions have occasionally been required to avoid encumbering PACT funds in situations where meaningful research data are not likely to result.
Fig. 3.
Workflow, product manufacturing, and product administration associated with the current 45 approved full applications to NHLBI PACT.
Overall, as of October 1, 2008, PACT clinical projects have resulted in the administration of 169 cellular products and the manufacture of two reagents, all used within the context of a specific IND and clinical trial. Fifty-one products were shipped and administered at an institution different from the manufacturing location. Although detailed clinical outcome data concerning these specific clinical trials are not within the scope of this report, PACT has collected basic data related to product administrations. The product administrations to date have been successful in that each product met its release criteria and no product-related serious adverse events have been reported in association with their administration.
Translational scientific studies
As noted previously, not all applications to PACT are ready for cell manufacturing in a controlled environment to support a clinical trial immediately. Still, the steering committee tries to assess scientific merit and feasibility of projects, choosing some to receive substantial development work with the intention that a viable clinical product may result and that it will be able to enter a clinical trial. Examples include the development of T-regulatory cells for clinical application. The potential value of these cells was judged to have such medical significance that substantial time and resources have been committed to development of a GMP-compatible method for their isolation. Another example has been the rich stem cell biology that has derived from PACT’s work with investigators to develop a clinically useful cellular product from adipose stem cells.3 Remarkable differences in proliferative capacity and susceptibility to apoptosis were found to correlate with harvest location and subject age. Additionally, recent data have suggested that multipotent stem cells characterized by markers including CD31+ (endothelial cells), CD34+, CD90+, and CD146+ (endothelial and perivascular cells) may exist in adipose tissue as determined by immunohistochemistry and immunofluorescence analysis of frozen sections. The investigators are studying these cell populations to determine their hematopoietic potential.
Educational objectives: Web seminars, workshops, Web site, and meeting participation
Web seminars
The PACTWeb seminars have been developed to offer educational opportunities in the major areas of cell therapy. Since its inception, PACT has offered 11Web seminars at no charge to participants, covering 21 different topics.
Up to 300 registered participants per seminar (often each registration represents multiple participants at a site) have given very positive reviews of these educational events and their ideas have contributed to future topic selections. Additional seminars are in the planning stages. Topics and timing of these seminars are coordinated with new or existing Web seminars developed by professional societies in the field of cell therapy to better serve the cell therapy community at large. Table 4 shows the dates and topics of completed Web seminars.
TABLE 4.
NHLBI PACT no cost educational Web seminars by topic and date
| Date | Topic | Topic | Topic |
|---|---|---|---|
| 03/31/05 | Facility cleaning and disinfection | Cell processing, validation, and performance qualification | Equipment qualification |
| 07/14/05 | Biologic product deviation reporting | Chemistry, manufacturing, and controls writing for IND applications | Staff training and competency |
| 01/19/06 | Managing multiple specimens in a cell-processing facility | Rapid release testing | Packaging and shipping of human cell and tissue products |
| 07/20/06 | Facility master files | SOP development | Development and operation of a QA system for deviations from SOPs in a clinical cell therapy laboratory |
| 10/26/06 | Cell therapy data management | ||
| 02/22/07 | Adverse event reporting for 351 products | Adverse event reporting in IND studies | |
| 07/19/07 | Interpreting AABB cellular therapy standards | Interpreting FACT cellular therapy standards | |
| 10/11/07 | How to survive audits and inspections | FDA inspection: preparation, inspection, and follow-up | |
| 02/21/08 | Cryopreservation of cell therapy products | ||
| 06/25/08 | Training and career development grant opportunities at NIH | Overview of cell therapy in lung biology and disease | |
| 07/31/08 | Validation processes |
Workshops
Three onsite educational workshops have been held on core requirements for good manufacturing and good tissue practices (GMP/GTP). The first workshop was held at Baylor College of Medicine in April 2006, the second occurred in April 2007 at the University of Minnesota, and the third occurred in May 2008 at the University of Pitts-burgh. Speakers included PACT investigators as well as outside experts from neighboring institutions. The 2-day workshops offered GMP compliance–related topics including facility design, determining adequate environmental monitoring, managing cell product–related databases, properly documenting and maintaining product batch records, product testing and release criteria, and cryopreservation of hematopoietic progenitor cells (HPCs). Tours of each cell manufacturing facility were conducted and lab practicals in endotoxin and mycoplasma testing and CD34+ enumeration by flow cytometry were performed at the University of Pittsburgh facilities. Targeted audiences included but were not limited to physicians, fellows, residents, lab directors, and technicians. A survey was designed to collect data on the experience and current position of the attendees and the overall organization of the current workshop. Additionally, PACT obtained suggestions on how to improve future training sessions and solicited other topics of interest from the participants. In the three educational workshops, 99 percent of participants responded that the overall value of the workshop was excellent to very good. In addition, 97 percent of the participants reported that they were somewhat likely, likely, or very likely to change their practice behavior based on the information that was obtained during the PACT-hosted workshops. The feedback received from participants was also used to guide planning for future educational Web seminars and GMP training curriculum.
Continuing education credits
PACT has entered into a partnership with AABB to provide continuing medical education (CME) credits and continuing education units to attendees of PACT-sponsored Web seminars and workshops. The administrative center is responsible for providing the appropriate materials (such as content, learning objectives, speaker disclosure, and background information) to the AABB for review and CME/ continuing education units approval. AABB subsequently manages the CME credit distribution and grants attendees CE certificates through the AABB Live Learning Center.
NHLBI PACT Web site
The educational materials developed for Web seminars, workshops, and audio conferences remain available on the website at http://www.pactgroup.net under the education link. The Web site has evolved to become a significant educational resource to the cell therapy community and serves as a portal for disseminating information concerning upcoming Web seminars and workshops. Recent resource additions to the site have included a centralized listing for cell therapy material resources such as GMP-grade cytokine and antibody information and availability and PACT facility SOPs (provided upon request as informational examples). Web traffic and access to these resources continues to increase steadily, suggesting that they are indeed meeting an important educational need in the cell therapy community.
Meeting participation
PACT has actively participated in both national and international cell therapy meetings. PACT has conducted cell therapy educational sessions at the 2005 and 2007 AABB annual meetings. PACT provided speakers and entered into partnership and sponsorship agreements with AABB in support of the 2007 AABB Spring Conference and with ISCT in support of the 2007 and 2008 Annual Somatic Cell Therapy meetings respectively. PACT representatives have attended the biannual cell therapy liaison meetings with the Food and Drug Administration (FDA) for the past 3 years.
PACT technical projects
Several carefully chosen projects are undergoing collaborative implementation among the three PACT manufacturing facilities and the administrative center.
Rapid endotoxin assay evaluation
Although various FDA-approved methods are utilized to detect endotoxin in cellular therapy products, such as limulus amebocyte lysate assay, currently none has a suitable combination of simplicity, rapid turnaround time, accuracy, precision, cost, and FDA-approved status. PACT, in collaboration with the NIH Department of Transfusion Medicine, has undertaken a multisite comparison study, between the commercially developed Endosafe rapid release endotoxin test system and the traditional limulus amebocyte lysate methods for measuring endotoxin. At present, testing is complete; data have been assembled into a manuscript, which has been accepted for publication in Cytotherapy, and is currently in press.
Shipping validation for human cellular products
The objective of this project is to establish validated shipping conditions for a variety of somatic cell products. The PACT group has undertaken the task of assembling a set of procedures to guide the shipping process and the type of testing that will be required by the receiving facility. These procedures are being validated in the course of an exchange of cellular products among the three PACT facilities. By performing this formal validation study, PACT expects to establish acceptable conditions for a variety of cell types to facilitate cell product preservation and integrity during transit and to ensure that the cellular product remains stable and sterile during shipment. As of October 1, 2008, seven different types of cell product have been shipped, received, processed, and recharacterized at the receiving facilities. Data are currently being collected from the study and will be prepared for publication when it is complete.
Processing small volumes of HPCs
A number of emerging applications in human cell therapy call for rapid, reliable manipulation of a HPC source for patient use. To address this evolving key technology need, NHLBI PACT centers are developing or refining two methods. First, a two-phase marrow cell processing/ selection protocol has also been developed internally for use on the Isolex device. Ten low-volume marrow validation runs have been completed at a single facility to develop a standardized process for use across all PACT centers during Phase 2. Initial results have been reviewed and the other two centers have obtained marrow samples and completed Phase 2 of the study in May 2008. Data are currently being analyzed and a publication is anticipated. In addition, the PACT centers at Baylor College of Medicine and the University of Minnesota are providing cell-processing services to the NHLBI Cardiovascular Cell Therapy Research Network. This network consists of five clinical cardiac centers collaborating on protocols to treat cardiac diseases using regenerative cell therapy. The first protocols will use autologous marrow mononuclear cells (MNCs) enriched using the Sepax device to treat patients after acute myocardial infarction. PACT continues to advance these procedures recognizing that they may have broad future application where turnaround time is critical and a closed system is highly desirable.
Cell therapy textbook
PACT investigators, with editing and leadership from Dr Adrian Gee from Baylor College of Medicine, have developed a multiauthor authoritative contemporary textbook in cellular therapy. Contributing experts from inside and outside of PACT have participated and the book is nearing publication submission.
DISCUSSION
The NHLBI, through its PACT research contract, demonstrates its commitment to advancing the field of cellular therapy through innovative science, collaboration, and education. After an initial start-up period, NHLBI PACT has now reached an efficient operational phase, in which there are more than 30 simultaneous projects in development or manufacture and the administration of PACT-produced cell therapy products is accelerating across a growing number of clinical trials. Of considerable importance is the fact that NHLBI and the PACT group have been able to achieve these goals not only through the efforts of individual institutions and investigators, but also through close collaboration with other academic centers, other NIH-sponsored programs, nonprofit organizations, and commercial entities. In both its scientific and its educational work areas, NHLBI and PACT have been able to achieve their mission while also engaging the broader cell therapy community.
Acknowledgments
This project was supported by NHLBI Contracts N01-HB-37163, N01-HB-37164, N01-HB-37165, and N01-HB-37166 from the National Heart, Lung, and Blood Institute.
The authors express their appreciation to David Styers and Deborah Wood of EMMES for providing data tables and figures and their assistance and dedication in bringing this article to fruition. The following individuals are being acknowledged for their valued contribution of and participation in PACT group initiatives: Baylor College of Medicine—April Durett, Helen Heslop, MD, Helen Huls, Deborah Lyon, and Pamela Watson; University of Minnesota—Sheryl Adams, Mary Clay, Diane Kadidlo, Jeff Miller, MD, Therese Schierman, and Darin Sumstad; and University of Pittsburgh—Deborah Griffin, Joseph Kiss, MD, Eileen Koch, Linda Moore, Jennifer Sprague, and Joanna Stanson.
ABBREVIATIONS
- CME
continuing medical education
- HSC(s)
hematopoietic stem cell(s)
- IND(s)
investigational new drug(s)
- NHLBI
National Heart, Lung, and Blood Institute
- SOP(s)
standard operating procedure(s)
Footnotes
All authors have signed a conflict of interest form and hereby attest that they have no conflict of interests to disclose regarding the publication of this article.
References
- 1.Stroncek D, Harvath L, Barrett J. National Heart, Lung, and Blood Institute of the National Institutes of Health forum on immune reconstitution and cellular therapy following hematopoietic stem-cell transplantation. Cytotherapy. 2002;4:415–8. doi: 10.1080/146532402320776008. [DOI] [PubMed] [Google Scholar]
- 2.Mondoro TH, Thomas JW, Henslee-Downey PJ, Peterson CM. NHLBI plans for the promise of cell-based therapies. Cytotherapy. 2005;7:317–27. doi: 10.1080/14653240500237741. [DOI] [PubMed] [Google Scholar]
- 3.Schipper B, Marra K, Zhang W, Donnenberg A, Rubin J. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–44. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leen AM, Sili U, Savoldo B, Jewell AM, Piedra PA, Brenner MK, Rooney CM. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–9. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 5.Sili U, Huls MH, Davis AR, Gottschalk S, Brenner MK, Heslop HE, Rooney CM. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–56. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, Khalil M, Wu MF, Huls MH, Chang CC, Gresik MV, Gee AP, Brenner MK, Rooney CM, Heslop HE. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy-Nasser AA, Bollard CM. T cell therapies following hematopoietic stem cell transplantation: surely there must be a better way than DLI? Bone Marrow Transplant. 2007;40:93–104. doi: 10.1038/sj.bmt.1705667. [DOI] [PubMed] [Google Scholar]
- 8.Leen AM, Myers GD, Bollard CM, Huls MH, Sili U, Gee AP, Heslop HE, Rooney CM. T-cell immunotherapy for adenoviral infections of stem-cell transplant recipients. Ann N Y Acad Sci. 2005;1062:104–15. doi: 10.1196/annals.1358.013. [DOI] [PubMed] [Google Scholar]
- 9.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 10.Blazar BR, Taylor PA. Regulatory T cells. Biol Blood Marrow Transplant. 2005;11:46–9. doi: 10.1016/j.bbmt.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–61. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–8. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 13.Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease—a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–80. [PubMed] [Google Scholar]
- 14.Whiteside TL, Piazza P, Reiter A, Stanson J, Connelly NC, Rinaldo CR, Jr, Riddler SA. Production of DC-based vaccine containing inactivated autologous virus for therapy of patients with chronic HIV-1 infection. Clin Vaccine Immunol. 2008 doi: 10.1128/CVI.00066-08. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiorean EG, Miller JS. The biology of natural killer cells and implications for therapy of human disease. J Hematother Stem Cell Res. 2001;10:451–63. doi: 10.1089/15258160152509073. [DOI] [PubMed] [Google Scholar]
- 16.Miller JS. Biology of natural killer cells in cancer and infection. Cancer Invest. 2002;20:405–19. doi: 10.1081/cnv-120001185. [DOI] [PubMed] [Google Scholar]
- 17.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, Guethlein LA, Trachtenberg EA, Haagenson M, Horowitz MM, Klein JP, Weisdorf DJ. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–61. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 19.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, Dewaard R, McGlave PB, Weisdorf DJ, Wagner JE, McCullough J, Miller JS. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]