Abstract
Aims
To compare the profile of signs of neonatal abstinence syndrome (NAS) in methadone- versus buprenorphine-exposed infants.
Design, setting and participants
Secondary analysis of NAS data from a multi-site, double-blind, double-dummy, flexible-dosing, randomized clinical trial. Data from a total of 129 neonates born to opioid-dependent women who had been assigned to receive methadone or buprenorphine treatment during pregnancy were examined.
Measurements
For 10 days after delivery, neonates (methadone = 72, buprenorphine = 57) were assessed regularly using a 19-item modified Finnegan scale. Data from neonates who required pharmacological treatment (methadone = 41, buprenorphine = 27) were included up to the time treatment was initiated. The incidence and mean severity of the total NAS score and each individual sign of NAS were calculated and compared between medication conditions, as was the median time until morphine treatment initiation among treated infants in each condition.
Findings
Two NAS signs (undisturbed tremors and hyperactive Moro reflex) were observed significantly more frequently in methadone-exposed neonates and three (nasal stuffiness, sneezing, loose stools) were observed more frequently in buprenorphine-exposed neonates. Mean severity scores on the total NAS score and five individual signs (disturbed and undisturbed tremors, hyperactive Moro reflex, excessive irritability, failure to thrive) were significantly higher among methadone-exposed neonates, while sneezing was higher among buprenorphine-exposed neonates. Among treated neonates, methadone-exposed infants required treatment significantly earlier than buprenorphine-exposed infants (36 versus 59 hours postnatal, respectively).
Conclusions
The profile of neonatal abstinence syndrome differs in methadone- versus buprenorphine-exposed neonates, with significant differences in incidence, severity and treatment initiation time. Overall, methadone-exposed neonates have a more severe neonatal abstinence syndrome.
Keywords: Buprenorphine, incidence, in utero, methadone, neonatal abstinence syndrome, profile, severity, signs, treatment initiation
INTRODUCTION
Opioid dependence during pregnancy is often compounded by multiple risk factors contributing to adverse maternal and neonatal consequences [1–6]. In the context of comprehensive care, maintenance treatment with methadone, a full mu agonist, improves maternal and neonatal outcomes relative to no treatment or medication-assisted withdrawal [6–8]. However, in utero methadone exposure can result in neonatal abstinence syndrome (NAS). NAS is characterized by central nervous system (CNS) hyperirritability, gastrointestinal (GI) dysfunction, respiratory distress and autonomic nervous system (ANS) signs. High-pitched crying, hyperactive Moro reflex, increased muscle tone, sleep disturbances, tremors, excoriation due to excessive movement, excessive irritability and seizures are signs of CNS hyperirritability. Signs of GI dysfunction include poor feeding, vomiting and loose stools. Respiratory distress is indicated by nasal stuffiness and tachypnea. ANS disturbances include sweating, fever, yawning and sneezing [9]. Untreated NAS can result in significant morbidity and mortality [9]. In many instances, methadone-associated NAS requires extended medical monitoring in the early postnatal period and often necessitates pharmacological treatment with opioid agonist medications and prolonged hospitalization [8].
Buprenorphine, a partial mu agonist and kappa antagonist, was approved for the treatment for opioid dependence in non-pregnant adults in 2002 in the United States [10]. In addition to demonstrating its efficacy as a maintenance medication, studies testing the clinical utility of buprenorphine in non-pregnant adults also reported that abrupt discontinuation of this medication resulted in a milder withdrawal syndrome that had a different profile compared to withdrawal from full opioid agonists [11–14]. For example, it has been suggested that autonomic signs of withdrawal in non-pregnant adults are less pronounced after discontinuation of buprenorphine, and that buprenorphine withdrawal may be delayed in onset relative to withdrawal from a full opioid agonist [13,15].
The promising observations of a milder withdrawal with buprenorphine in adults prompted a number of studies comparing the NAS of infants exposed in utero to methadone versus buprenorphine [16–21]. Many reported results suggestive of a milder, more limited NAS among neonates prenatally exposed to buprenorphine, but methodological limitations (e.g. small sample sizes, open label, non-randomized designs, high rates of concomitant other drug use that could confound NAS results) tempered the strength of the conclusions that could be drawn from these studies.
More recently, the Maternal OpioidTreatment: Human Experimental Research (MOTHER) study was completed. The MOTHER study was a multi-site randomized clinical trial (RCT) designed to compare the NAS outcomes of neonates exposed in utero to methadone versus buprenorphine [22]. Following random assignment to methadone or buprenorphine, maternal participants received study medication in a double-blind, double-dummy manner to protect the study blind, and also received voucher-based incentives contingent upon negative urinalysis results to minimize other drug use. The results of the MOTHER study indicated that buprenorphine-exposed neonates required significantly less morphine to treat NAS and had a significantly shorter duration of hospitalization compared to methadone-exposed neonates, although there were no differences between medication conditions in the percentage of neonates who required pharmacological treatment or in peak total NAS scores. These results provided additional evidence that buprenorphine-exposed neonates experience signs of neonatal abstinence, but it remained unclear exactly how the broader profile of NAS differed between methadone- and buprenorphine-exposed neonates. Thus, the aim of the present study was to perform a secondary analysis of the MOTHER NAS data to characterize and compare more fully the profile of NAS in methadone- versus buprenorphine-exposed neonates. Understanding how other measures of NAS, such as incidence, mean severity over time and time until treatment initiation compare in neonates exposed in utero to methadone or buprenorphine could help to explain the more favorable outcomes often observed in buprenorphine-exposed neonates. In addition, as few studies in the scientific literature have analyzed and reported NAS data at the level of the individual sign, these analyses also have the potential to provide clinically important details about the NAS produced by each medication.
METHOD
A detailed description of maternal and neonatal participants, procedures and outcomes can be found in this Supplement [23] and in Jones et al. [22]. Details pertinent to the present study are described in more detail below.
Procedures
Maternal study procedures
One hundred and seventy-five maternal participants in the MOTHER study were randomized to either methadone (n = 89) or buprenorphine (n = 86) [22]. Study medications were administered to maternal participants in a double-blind, double-dummy manner. A flexible dosing schedule was used throughout the pregnancy to minimize possible bias resulting from over- or under-medication and to avoid confounding comparisons between medications due to possible differences in dose adequacy. The mean [±standard error (SE)] doses of methadone and buprenorphine at delivery were 78.2 ± 4.0 and 16.2 ± 0.9 mg, respectively. All maternal participants also provided breath and urine samples thrice weekly to assess alcohol and other drug use and earned monetary incentives contingent upon providing drug-negative urine and breath samples. Results reported by Jones et al. [22] indicate that this procedure minimized concomitant drug use successfully, with only 12% of mothers positive for illicit drugs at delivery, and no difference between the two study medication conditions.
Neonatal study procedures
One hundred and thirty-one maternal participants (73 methadone and 58 buprenorphine) delivered infants while in the study protocol. While maternal treatment attrition was markedly different between medication conditions, this difference was not statistically significant [22]. Additionally, there were no significant differences between medication conditions with respect to any baseline characteristics [22]. At delivery, neonates averaged 38 weeks gestation (14% preterm deliveries), 2972 g and 49 cm in length, and had a mean head circumference of 33 cm and mean 1- and 5-minute APGAR scores of 8 and 9, respectively. There were no significant differences between medication conditions on any of these neonatal birth outcomes [22]. Two neonates, one exposed to each medication, had serious medical complications and were not available for NAS assessment, leaving a sample size of 129 neonates (72 methadone and 57 buprenorphine) for the present study.
NAS assessment
Upon delivery, neonates were hospitalized for assessment of NAS. While hospitalized, NAS assessments were conducted every 3–4 hours; most neonates were hospitalized for at least the first 4 days postnatal (96 hours). When a neonate was discharged, assessments were conducted by trained staff at least twice daily at least 8 hours apart until at least postnatal day 10 (240 hours). All assessors were blind to the maternal medication condition.
NAS was assessed systematically using a 19-item modified Finnegan scale known as the MOTHER scale. The modifications made to the original Finnegan to create the MOTHER scale have been described by [19,24], and involved changes in the number of items administered. Some items were removed due to overlap with other items [e.g. vomiting (regurgitation) and projectile vomiting; watery stools and loose stools] or because they do not respond to treatment with opioids (e.g. myoclonic jerking, mottling). In addition, two items were added. Irritability was added to include infants who express irritability without crying (e.g. grimacing), and failure to thrive was added to include infants whose hypertonicity or excessive movements were associated with significant weight loss. Each of the 19 individual NAS signs on the modified scale is observed and assigned a numerical value based upon objective criteria (see Table 1). Higher numerical values are assigned for a more severe presentation of the NAS sign, and clinically significant NAS signs have higher possible values. After rating each NAS sign, the scores for each individual sign are added together to compute a total score. The MOTHER study protocol called for initiation of pharmacological treatment for two consecutive total scores ≥9 or one score >13. Sixty-eight neonates (41 methadone and 27 buprenorphine) required pharmacological treatment for NAS [22].
Table 1.
The 19 individual neonatal abstinence syndrome (NAS) signs that form the total score, scoring scales and objective criteria for positive scores.
| NAS sign | Scoring scale | Objective criteria for positive scoresa |
|---|---|---|
| Crying | 0, 2, 3 | 2 = inconsolable >15 seconds OR cry intermittently for <5 minutes 3 = inconsolable >15 seconds AND cry intermittently for >5 minutes |
| Sleep | 0, 1, 2, 3 | 0 = sleeps more than 3 hours after feeding 1 = sleeps 2–3 hours after feeding 2 = sleeps 1–2 hours after feeding 3 = sleeps <1 hour after feeding |
| Hyperactive Moro reflex | 0, 1, 2 | 1 = arms up 3–4 seconds, jitteriness present 2 = arms up for 5 seconds or more |
| Disturbed tremors | 0, 1, 2 | 1 = hands or feet, up to 3 seconds 2 = arms or legs >3 seconds |
| Undisturbed tremors | 0, 1, 2 | 1 = hands or feet, up to 3 seconds 2 = arms or legs >3 seconds |
| Increased muscle tone | 0, 1, 2 | 1 = flex/extend difficult, head lag present 2 = no flex/extend, no head lag |
| Excoriation | 0, 1, 2 | 1 = skin red and intact, or healing, no longer broken 2 = skin broken |
| Generalized seizure | 0, 8 | 8 = tonic-clonic, subtle staring, chewing, arching |
| Fever | 0, 1 | 1 = temp ≥ 37.3°C |
| Frequent yawning | 0, 1 | 1 = 4 or more in past 3–4-hour observation period |
| Sweating | 0, 1 | 1 = wet on forehead, upper lip |
| Nasal stuffiness | 0, 1 | 1 = any nasal noise |
| Sneezing | 0, 1 | 1 = 4 or more in past 3–4-hour observation period |
| Tachypnea | 0, 2 | 2 = respiratory rate >60/minute |
| Poor feeding | 0, 2 | 2 = uncoordinated gulping and frequent stops |
| Vomiting | 0, 2 | 2 = vomits whole feeding or 2 or more times during feed |
| Loose stools | 0, 2 | 2 = ½ liquid, ½ solid or liquid without water ring |
| Failure to thrive | 0, 2 | 2 = current weight ≥ 10% below birth weight |
| Excessive irritability | 0, 1, 2, 3 | 1 = consoling calms infant in 3–5 minutes 2 = consoling calms infant in 6–15 minutes 3 = consoling calms in >15 minutes or not at all |
Signs listed in the order they appeared on the instrument.
For every sign except sleep, a score of 0 = not present. This table is an adaptation of Fig. 2 in the Supplementary Appendix from Jones et al. [22]. Both the original item definitions [39] and the morphine medication protocol [19,24] were refined before initiation of Maternal Opioid Treatment: Human Experimental Research (MOTHER) study data collection.
An expert rater was established for the multi-site study. Each participating site’s gold standard rater was trained by the expert rater and was required to score within 2 points of the expert rater on the total NAS score. In turn, NAS raters at each site were trained by that site’s gold standard rater and were required to score within 2 points of the gold standard rater on the total NAS score. Every 6 months, the expert rater supplied videos of neonates undergoing NAS assessment to re-certify inter-rater reliability at each site. Results reported by Jones et al. [22] indicate that the smallest intraclass correlation estimate [ICC (2, 2)] of the degree of agreement between the NAS raters and the gold-standard rater exceeded 0.94, indicating excellent rater agreement.
Statistical analyses
Scores for each individual NAS sign were examined in 4-hour blocks up to 96 hours postnatal and in 12-hour blocks after 96 hours postnatal, reflecting the change in scoring frequency that often occurred if study infants were discharged from the hospital before postnatal day 10. Scores from multiple NAS assessments performed in a given time block were averaged so that each time block contributed a single score for each individual sign. Scores of neonates who required pharmacological treatment with morphine were included until the time block that treatment was initiated, as morphine treatment confounds further NAS assessments.
To address the aim of comparing the NAS profile of methadone- and buprenorphine-exposed neonates, three analyses were conducted. First, the incidence of the total NAS score and each individual NAS sign was determined by calculating the percentage of neonates who ever had a positive total score (>0) or received a positive score (>0) for each individual sign at any time during the 240-hour observation period. Potential differences in incidence between medication conditions were examined using χ2 tests. Second, mean severity for the total NAS score and each individual NAS sign was compared between medication conditions using PROC MIXED for two-way analysis of variance (ANOVA) (medication condition × time) controlling for site [25,26]. This analysis also allowed us to examine the time–course of the total NAS score and each individual sign and possible interactions between medication condition and time. These analyses were performed using SAS statistical software version 9 (SAS Institute, Cary, NC, USA). Third, among neonates treated for NAS, median time of treatment initiation was compared between medication conditions using the Wilcoxon rank sum test. Statistical significance was determined based on P < 0.05 (two-sided).
RESULTS
Incidence
All neonates in each study medication condition had at least one total NAS score greater than 0 at some point during the observation period. Regarding incidence of the individual signs of NAS, two signs were observed significantly more often in the methadone compared to the buprenorphine condition: undisturbed tremors and hyperactive Moro reflex (Ps = 0.03; Table 2). Three individual signs were observed significantly more often in the buprenorphine compared to the methadone condition: nasal stuffiness, sneezing and loose stools (Ps = 0.01; Table 2).
Table 2.
Number (%) of neonates who ever had a score >0.
| NAS sign | Methadone (n = 72) | Buprenorphine (n = 57) | χ2 P-value |
|---|---|---|---|
| Total score | 72 (100) | 57 (100) | 1.00 |
| Disturbed tremors | 72 (100) | 55 (96) | 0.11 |
| Increased muscle tone | 71 (99) | 57 (100) | 0.37 |
| Sleep | 65 (90) | 55 (96) | 0.17 |
| Tachypnea | 62 (86) | 51 (89) | 0.57 |
| Fever | 61 (85) | 53 (93) | 0.15 |
| Undisturbed tremors | 58 (81) | 36 (63) | 0.03 |
| Hyperactive Moro reflex | 55 (76) | 33 (58) | 0.03 |
| Sneezing | 55 (76) | 53 (93) | 0.01 |
| Crying | 40 (56) | 32 (56) | 0.94 |
| Excessive irritability | 39 (54) | 38 (67) | 0.15 |
| Poor feeding | 39 (54) | 28 (49) | 0.57 |
| Vomiting | 38 (53) | 33 (58) | 0.56 |
| Excoriation | 34 (47) | 32 (56) | 0.31 |
| Loose stools | 33 (46) | 40 (70) | 0.01 |
| Nasal stuffiness | 20 (28) | 29 (51) | 0.01 |
| Frequent yawning | 15 (21) | 17 (30) | 0.24 |
| Sweating | 15 (21) | 12 (21) | 0.98 |
| Failure to thrive | 12 (17) | 7 (12) | 0.49 |
| Generalized seizure | 0 | 2 (4) | 0.11 |
Entries shown in bold type indicate significant differences between medication conditions. NAS = neonatal abstinence syndrome.
Mean severity
Main effects of maternal medication exposure condition
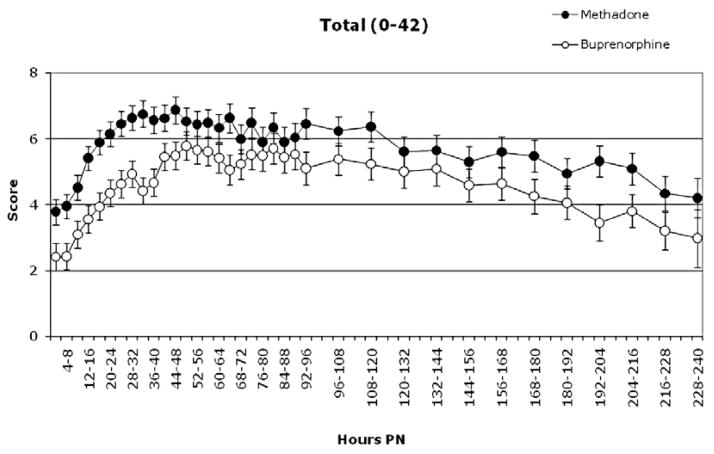
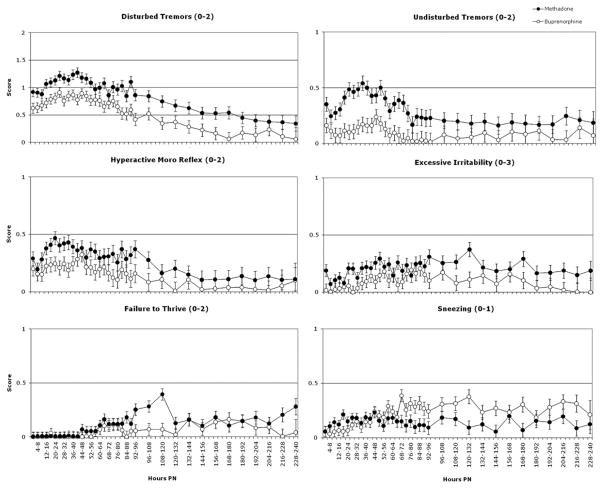
Significant differences between methadone-versus buprenorphine-exposed neonates were observed on mean severity of the total NAS score and six individual signs of NAS: disturbed tremors, undisturbed tremors, hyperactive Moro reflex, excessive irritability, failure to thrive and sneezing (Ps ≤ 0.04; Figs 1 & 2). Methadone-exposed neonates had significantly higher mean scores compared to buprenorphine-exposed neonates on the total score and each of these individual signs except for sneezing, on which buprenorphine-exposed neonates had higher mean scores.
Figure 1.
Least square (LS) means (± standard error) of the total neonatal abstinence syndrome (NAS) score in methadone- and buprenorphine-exposed neonates from birth to 240 hours postnatal (PN). The figure illustrates significant main effects of medication condition and of time (Ps < 0.05). The numerical range after the title indicates the minimum and maximum score for the total score. The y-axis is presented on a smaller scale to allow for more detailed inspection of the data
Figure 2.
Least square (LS) means (± standard error) of the total neonatal abstinence syndrome (NAS) in methadone- and buprenorphine-exposed neonates from birth to 240 hours postnatal (PN). A ‘0’ was plotted for time-points where the LS mean was negative. All figures illustrate significant main effects of medication condition and of time (Ps < 0.05). The numerical range after the name of each individual sign indicates the minimum and maximum score for that sign. The y-axis is presented on a smaller scale for most signs to allow for more detailed inspection of the data
Main effects of time
Significant time effects were observed for the total NAS score and 14 individual NAS signs: sleep, hyperactive Moro reflex, disturbed tremors, undisturbed tremors, increased muscle tone, excoriation, fever, frequent yawning, nasal stuffiness, sneezing, poor feeding, loose stools, failure to thrive and excessive irritability (Ps ≤ 0.04). All exhibited the inverted U-shaped function typical of opioid withdrawal wherein withdrawal scores increase, peak, and then decrease more gradually over time [27,28].
Medication condition × time interactions
Significant medication condition × time interactions were observed on two NAS signs: sneezing and tachypnea (Ps ≤ 0.03). For sneezing, the methadone-exposed neonates initially had slightly higher scores, but from 48 to 240 hours postnatal, scores of buprenorphine-exposed neonates were consistently higher (Fig. 2, bottom right panel). For tachypnea, no consistent differences were apparent between the two conditions prior to 156 hours postnatal, with mean (±SE) scores in both conditions averaging 0.47 ± 0.02. From 156 to 240 hours postnatal, scores among methadone-exposed neonates were consistently higher compared to buprenorphine-exposed neonates, averaging 0.66 ± 0.04 and 0.36 ± 0.03, respectively (data not shown).
Median time to morphine treatment initiation among treated infants
There was a significant difference in the median time of morphine treatment initiation among the treated infants in each medication condition. Median (inter-quartile range) time to treatment initiation was 36 (26–60) versus 59 (46–83) hours postnatal in methadone- versus buprenorphine-exposed neonates, respectively (P < 0.01).
DISCUSSION
The present study aimed to compare the profile of NAS in methadone- versus buprenorphine-exposed infants as part of a secondary analysis of data collected in a large, methodologically rigorous RCT. The findings suggest that the profile of the NAS produced by in utero exposure to the two medications prior to or in the absence of pharmacological treatment differs. Of primary interest are the observations that the mean severity of the total NAS score was significantly higher in methadone- versus buprenorphine-exposed neonates, and that this overall difference was driven by greater severity on five of 19 (26%) of the individual NAS signs that make up the total score. The majority of the individual signs on which methadone-exposed neonates had greater incidence and higher scores were signs of CNS hyperirritability (e.g. hyperactive Moro reflex, disturbed and undisturbed tremors and excessive irritability). The increased metabolic demands associated with more severe tremors and irritability may also have contributed to the higher mean score for failure to thrive among methadone-exposed neonates [29]. Regarding signs of ANS dysfunction, the only significant differences between medication conditions were a higher incidence and severity of sneezing among buprenorphine- versus methadone-exposed neonates. This contrasts with the adult literature, where autonomic signs of opioid withdrawal are generally less pronounced with buprenorphine compared to full mu-opioid agonists [13]. It is unclear at this time whether these discrepant findings are simply a function of methodological differences in the assessment of withdrawal in neonates versus adults, or whether they represent true differences in the expression of NAS in different phases of development.
Buprenorphine-exposed neonates who required pharmacological treatment did so significantly later than the methadone-exposed neonates who required treatment, on average nearly 24 hours later. This finding is consistent with the adult literature, where results suggest a later onset of withdrawal after abrupt termination of buprenorphine compared to full mu-opioid agonists [15]. Clinical guidelines have long recommended that drug-exposed neonates be monitored closely for at least the first 4 days postnatal [30]. The data from the present study provide empirical support for extending this practice to buprenorphine-exposed neonates.
The differences in the profiles of NAS among methadone- versus buprenorphine-exposed neonates may be explained by differences in the transplacental transfer and/or the pharmacokinetics of the two medications. Research using ex-situ human placental tissue has demonstrated that, compared to methadone, buprenorphine is absorbed more readily from the maternal circulation into placental tissue, but released less readily from the placental tissue into the fetal circulation, suggesting that a fetus would be exposed to less buprenorphine [31–33]. In addition, there are well-established differences in the intrinsic activity and receptor affinity of buprenorphine and full mu-opioid agonists, with buprenorphine exhibiting lower activity but higher affinity at the mu-opioid receptor [34,35]. As a result, buprenorphine that reaches the fetus probably has a less than maximal opioid effect and dissociates from the receptor more slowly than full mu-opioid agonists [34]. One or more of these mechanisms could contribute to differences in the expression of NAS in methadone- and buprenorphine-exposed neonates.
It is interesting to note that mean severity of the total NAS score differed between medication conditions in the present analyses, while peak total NAS score, examined and reported in the primary outcomes paper [22], did not. Peak total NAS score was the single highest total score each infant ever had, independent of time and whether or not the infant was currently receiving NAS medication. These findings underscore the need to characterize NAS fully, including examining more than one aspect of overall score as well as examining data at the level of the individual sign. Despite the lack of difference on peak total NAS score, the results of the present study suggest there are differences in the profile of NAS produced by in utero exposure to methadone versus buprenorphine that may help to explain the more positive clinical outcomes observed typically among buprenorphine-exposed neonates.
More generally, more detailed examinations of NAS data are likely to advance empirically the field of NAS assessment and treatment. Most NAS scales were developed initially and underwent rudimentary validation testing in the mid-1970s, driven by dramatic increases in the number of opioid-exposed neonates requiring assessment and treatment in many urban hospitals as a result of an epidemic of heroin use [36]. The scoring system was based typically on the pathological significance of each individual sign, with the signs that had the greatest potential for clinically adverse events (e.g. seizures) given the highest scores [37]. While the development and dissemination of these scales were landmark steps in advancing the assessment and treatment of NAS in this vulnerable population, there have been few studies to improve NAS scales. One topic receiving increased research attention recently is the large number of items assessed by NAS scales, which has reportedly limited their clinical use [29]. Analyses of NAS data at the level of the individual sign could provide evidence of the significance of individual signs that could contribute to efforts to modify NAS scales without compromising their clinical utility. For example, a recent study comparing NAS scores in opioid-exposed and non-exposed neonates reported that an index composed of three individual signs (hyper-active Moro reflex, undisturbed tremors and increased muscle tone) discriminated accurately between groups and could serve as a cost-effective screening mechanism for identifying opioid-exposed neonates [38].
Several limitations should be considered when interpreting the results of this study. One limitation is that while the sample size was large for a study of this type, it remains quite small from a statistical perspective, limiting power to detect additional differences between medication conditions. For example, there was a potentially alarming difference regarding the incidence of seizures among neonates exposed to the two medications, with two (4%) buprenorphine-exposed neonates receiving at least one positive score for seizures compared to none of the methadone-exposed neonates. Given the low baseline rate of occurrence, we were not powered in this secondary analysis to detect potential differences in such outcomes. Nevertheless, it is reassuring to note that the incidence of seizures among buprenorphine-exposed neonates in this study trends towards the bottom of the range reported in the literature on opioid-exposed neonates (2–11%) [29]. A second limitation is that data from neonates who required pharmacological treatment were excluded from the analyses once treatment was initiated. As a consequence, our results may underestimate measures of incidence and severity in both medication conditions. Ethical considerations preclude the study of NAS in untreated but symptomatic neonates and pharmacological treatment, by definition, alters the parameters of NAS. As a result, the next best course of action was to include the data of neonates who needed pharmacological treatment up to the initiation of treatment. Additional studies focusing on treated neonates would also be interesting and could explore how the individual signs and their weighted scores drive initiation of, maintenance on and weaning from NAS pharmacotherapy. A third limitation is that it remains unclear how comfort measures may have influenced the expression of NAS in the present study. Comfort measures are non-pharmacological interventions (e.g. dimming ambient lights, swaddling the neonate and providing the neonate with non-nutritive sucking opportunities) that are recommended to help assuage signs of NAS [9,24]. The extent to which such interventions influenced the present results is unknown. Another potential limitation is the NAS scoring tool used. While this tool measures 19 individual signs of NAS, there may be additional signs that differentiate the profile of NAS of methadone- versus buprenorphine-exposed neonates that we did not measure.
Despite these limitations, the rigorous design of the MOTHER study provided a unique opportunity to examine and compare the profiles of NAS that result from in utero exposure to these two medications. The profile of NAS differed in methadone- versus buprenorphine-exposed neonates, with significant differences in incidence, severity and treatment initiation time. Overall, methadone-exposed neonates had a more severe NAS. These results may help to explain the more favorable clinical outcomes often observed in buprenorphine-exposed neonates.
Acknowledgments
We thank Brown University (R01 DA 015778) and Drs Barry Lester, Amy Salisbury, Suzanne Caron, Jeff Michaud, and Lawrence Novo, Katheleen Hawes, Danielle Finch, Marissa Cerrone, and the staff of the Level II Nursery at St. Luke’s Hospital; Wayne State University (R01 DA 15832) and co-Investigators Drs Carl Christen-son, Virginia Delaney-Black, Robert Sokol, Charles Schuster, Eugene Cepeda, and the assistance of Darlene Tansil and Mea Ebenbichler; Johns Hopkins University (R01 DA 015764) and the staff Ave Childrey, Laetitia Lemoine, Heather Fitzsimons, Julia Shadur, Michelle Tuten, Cheryl Claire, Behavioral Pharmacology Research Pharmacy and Nursing staff, Center for Addiction and Pregnancy staff, and co-Investigators Drs Donald Jasinski, Lauren Jansson, Robert Dudas, Lorraine Milio, Martha Velez, Vickie Walters, Eric Strain, and George Bigelow; Thomas Jefferson University (R01 DA 015738), Dr Amber Holbrook and the research staff, Family Center staff, OB and Pediatric nursing staff, and co-Investigators Drs Vincenzo Berghella, Jason Baxter, Jay Greenspan, and Laura McNicholas; University of Toronto (R01 DA 015741) Toronto Centre for Substance Use in Pregnancy, co-Investigators Drs Alice Ordean and Bhushan Kapur, and Ms Alla Osadchy as research coordinator and the assistance of Ms Lydia Pantea; Vanderbilt University (R01 DA 017513 and M01RR00095) and co-Investigators Drs Karen D’Apolito (co-PI), Paul Bodea-Barothi, Nancy Chescheir, Joseph Gigante, Barbara Engelhardt, nurse practitioners Michelle Collins, Mavis Schorn, and Karen Starr, as well as the assistance of Cayce Watson and Mark Nickel; University of Vermont (R01 DA 018410 and M01RR109) co-Investigators Drs John Brooklyn, Stephen Higgins, Anne Johnston, Marjorie Meyer, and Stacey Sigmon; Medical University of Vienna (R01 DA 018417) co-Investigators Drs Kenneth Thau, Bernadette Winklbaur, Nina Ebner, Klaudia Rohrmeister, Inge Frech, Martin Langer, Manfred Weninger, and Nina Kopf; Ingrid Kügler and nurses Doris Leopoldinger and Burgi Gfrerer, and Reinhold Jagsch, Verena Metz, Katrin Klebermass, Anne Unger, Andjela Baewert, Heidi Amon, Constantin Aschauer, Alexander Hecht; and Center for Substance Abuse Research Project Director Emy Nakamura (Parham); data monitors Ben Falls and Patricia Zangrillo; data preparation and analyses staff Kimberly Caldeira, Laura Dykstra, Dr Shawn Flower, Sarah Kaspersi, Gillian Pinchevsky, Lauren Stern, Kathryn Vincent, Dr Michael Wagner, Emily Winick and Elizabeth Zarate.
Footnotes
Clinical trial registration
The clinical trial was registered with ClinicalTrials.gov (Identifier: NCT00271219; title: RCT Comparing Methadone and Buprenorphine in Pregnant Women).
Present Paper
No additional declarations of interest.
Declarations of interest
MOTHER Study
All MOTHER grants are from the National Institute on Drug Abuse (NIDA) unless noted otherwise: Brown University, R01 DA 015778; Johns Hopkins University, R01 DA 015764; Medical University of Vienna, R01 DA 018417; Thomas Jefferson University, R01 DA 015738; University of Toronto, R01 DA 015741; University of Vermont, R01 DA 018410 and M01 RR 109; Vanderbilt University, R01 DA 017513 and M01 RR 00095, and Wayne State University, R01 DA 15832.
Reckitt Benckiser Healthcare, Hull, UK supplied buprenorphine tablets (and the associated placebo) via NIDA. Neither Reckitt Benckiser nor NIDA had any involvement in study design, data collection, analysis, interpretation, or manuscript preparation.
H. J. discloses that she has received reimbursement for time and travel from Reckitt Benckiser.
G. F. discloses that she has received financial support and honoraria for presentations from Reckitt Benckiser, as well as financial support and honoraria for presentations from Schering Plough.
P. S. discloses that he has received an unrestricted educational grant from Schering Canada to provide a single training program on buprenorphine treatment in 2000. His hospital receives funds from the Government of Ontario to develop and provide a training program of which he is the course director for all Ontario physicians who wish to treat opioid dependence including in pregnant women. However, the buprenorphine mono product is not available in Canada.
K. O’G. discloses that he has received reimbursement for time from Reckitt Benckiser.
All other authors declare no competing financial interests.
No contractual constraints on publishing have been imposed by any agency from which an author has received funding.
References
- 1.Hulse GK, Milne E, English DR, Holman CDJ. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction. 1997;92:1571–9. [PubMed] [Google Scholar]
- 2.Kandall SR, Albin S, Gartner LM, Lee K-S, Eidelman A, Lowinson J. The narcotic-dependent mother: fetal and neonatal consequences. Early Hum Dev. 1977;1:159–69. doi: 10.1016/0378-3782(77)90017-2. [DOI] [PubMed] [Google Scholar]
- 3.Lester BM, Andreozzi L, Appiah L. Substance use during pregnancy: time for policy to catch up with research. Harm Reduct J. 2004;1:5–49. doi: 10.1186/1477-7517-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lifschitz MH, Wilson GS, Smith EO, Desmond MM. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics. 1985;75:269–74. [PubMed] [Google Scholar]
- 5.Messinger DS, Bauer CR, Das A, Seifer R, Lester BM, Lagasse LL, et al. The Maternal Lifestyle Study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113:1677–85. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 6.Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103:1429–40. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones HE, O’Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17:372–86. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- 8.Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. Obstet Gynecol Clin North Am. 1998;25:139–51. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- 9.Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, Weitzman ML, Wilson MH, editors. Primary Pediatric Care. 2. St Louis: Mosby; 1992. pp. 1367–78. [Google Scholar]
- 10.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990;47:525–34. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- 12.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch Gen Psychiatry. 1978;35:501–16. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:S59–77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 14.Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–9. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- 15.Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 16.Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. Eur Addict Res. 2009;15:128–34. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- 17.Binder T, Vavrinkova B. Prospective randomized comparative study of the effect of buprenorphine, methadone and heroin on the course of pregnancy, birthweight of newborns, early postpartum adaptation and course of the neonatal abstinence syndrome (NAS) in women followed up in the outpatient department. Neuro Endocrinol Lett. 2008;29:80–6. [PubMed] [Google Scholar]
- 18.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–81. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones HE, Johnson RE, Jasinski DR, O’Grady KE, Chisolm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Lejeune C, Simmar-Durand L, Gourarier L, Aubisson S Groupe d’Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug Alcohol Depend. 2006;82:250–7. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones HE, Fischer G, Heil SH, Kaltenbach K, Martin PR, Coyle MG, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)—approach, issues, and lessons learned. Addiction. 2012;107 (Suppl 1):28–35. doi: 10.1111/j.1360-0443.2012.04036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5:47–55. [PMC free article] [PubMed] [Google Scholar]
- 25.Glass GV, Peckham PD, Sanders JR. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev Educ Res. 1972;42:237–88. [Google Scholar]
- 26.Stiger TR, Kosinski AS, Barnhart HX, Kleinbaum DG. ANOVA for repeated ordinal data with small sample size—a comparison of ANOVA, MANOVA, WLS and GEE methods by simulation. Commun Stat B Simul Comput. 1998;27:357–75. [Google Scholar]
- 27.Kolb L, Himmelsbach CK. Clinical studies of drug addiction, III: a critical review of the withdrawal treatments with method of evaluating abstinence syndromes. Am J Psychiatry. 1938;94:759–99. [Google Scholar]
- 28.Himmelsbach CK. Further studies of the addiction liability of Demerol. J Pharmacol Exp Ther. 1943;79:5–9. [Google Scholar]
- 29.American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 1998;101:1079–88. [PubMed] [Google Scholar]
- 30.Substance Abuse and Mental Health Services Administration/Center for Substance Abuse Treatment (SAMHSA/CSAT). . Treatment Improvement Protocol 5: Improving Treatment for Drug-Exposed Infants. Center for Substance Abuse Treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1993. [PubMed] [Google Scholar]
- 31.Coles LD, Lee IJ, Hassan HE, Eddington ND. Distribution of saquinavir, methadone, and buprenorphine in maternal brain, placenta, and fetus during two different gestational stages of pregnancy in mice. J Pharm Sci. 2009;98:2832–46. doi: 10.1002/jps.21644. [DOI] [PubMed] [Google Scholar]
- 32.Nanovskaya T, Deshmukh S, Brooks M, Ahmed MS. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 33.Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL, Hankins GD, Ahmed MS. Bidirectional transfer of methadone across human placenta. Biochem Pharmacol. 2005;69:187–97. doi: 10.1016/j.bcp.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Boas RA, Villiger JW. Clinical actions of fentanyl and buprenorphine. Br J Anaesth. 1985;57:192–6. doi: 10.1093/bja/57.2.192. [DOI] [PubMed] [Google Scholar]
- 35.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–72. [PubMed] [Google Scholar]
- 36.Hughes PH, Rieche O. Heroin epidemics revisited. Epidemiol Rev. 1995;17:66–73. doi: 10.1093/oxfordjournals.epirev.a036186. [DOI] [PubMed] [Google Scholar]
- 37.Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int J Clin Pharmacol. 1975;12:19–32. [PubMed] [Google Scholar]
- 38.Jones HE, Harrow C, O’Grady KE, Crocetti M, Jansson LM, Kaltenbach K. Neonatal abstinence scores in opioid-exposed and nonexposed neonates: a blinded comparison. J Opioid Manag. 2010;6:409–14. doi: 10.5055/jom.2010.0038. [DOI] [PubMed] [Google Scholar]
- 39.D’Apolito K. A scoring system for assessing neonatal abstinence syndrome [video tapes and manual] Seattle: University of Washington, School of Nursing; 1994. [Google Scholar]