Abstract
Background
Fat grafting is a promising technique for soft-tissue augmentation, although graft retention is highly unpredictable and factors that affect graft survival have not been well defined. Because of their capacity for differentiation and growth factor release, adipose-derived stem cells may have a key role in graft healing. The authors’ objective was to determine whether biological properties of adipose-derived stem cells present within human fat would correlate with in vivo outcomes of graft volume retention.
Methods
Lipoaspirate from eight human subjects was processed using a standardized centrifugation technique and then injected subcutaneously into the flanks of 6-week-old athymic nude mice. Graft masses and volumes were measured, and histologic evaluation, including CD31+ staining for vessels, was performed 8 weeks after transplantation. Stromal vascular fraction isolated at the time of harvest from each subject was analyzed for surface markers by multi-parameter flow cytometry, and also assessed for proliferation, differentiation capacity, and normoxic/hypoxic vascular endothelial growth factor secretion.
Results
Wide variation in percentage of CD34+ progenitors within the stromal vascular fraction was noted among subjects and averaged 21.3 ± 15 percent (mean ± SD). Proliferation rates and adipogenic potential among stromal vascular fraction cells demonstrated moderate interpatient variability. In mouse xenograft studies, retention volumes ranged from approximately 36 to 68 percent after 8 weeks, with an overall average of 52 ± 11 percent. A strong correlation (r = 0.78, slope = 0.76, p < 0.05) existed between stromal vascular fraction percentage of CD34+ progenitors and high graft retention.
Conclusion
Inherent biological differences in adipose tissue exist between patients. In particular, concentration of CD34+ progenitor cells within the stromal vascular fraction may be one of the factors used to predict human fat graft retention. (Plast. Reconstr. Surg. 132: 845, 2013.)
Autologous fat grafting has gained tremendous momentum in clinical practice because of increasing recognition of potential applications in aesthetic and reconstructive surgery. However, although it may provide a minimally invasive means of restoring soft-tissue structure, fat grafting remains unpredictable in the hands of plastic surgeons. Specifically, graft resorption can lead to loss of more than 50 percent of injected soft-tissue volume.1 Fat supplementation with growth factors2–5 and the use of biomaterial scaffolds6–9 have been explored in animal models in an attempt to improve retention outcomes.
Understanding inherent biological properties of adipose tissue from individual patients that may predict successful outcomes will help improve clinical care and lead to the development of therapeutic strategies to make fat grafting more predictable. A key element of adipose tissue is the adipose-derived stem cell. This cell type may play a pivotal role in mediating the healing and retention of fat grafts, as studies10–13 have demonstrated that adipose-derived stem cells secrete growth factors including insulin-like growth factor, hepatocyte growth factor, transforming growth factor beta 1, and vascular endothelial growth factor (VEGF). Although the molecular mechanisms remain poorly understood, angiogenic induction and adipogenic differentiation of these progenitor cells are believed to contribute to improved long-lasting graft results observed in both animal studies14–16 and clinical settings.17 Moreover, experimental evidence demonstrates that admixing adipose-derived stem cells with fat grafts can increase retention.14–16,18
Our group has previously shown the importance of a CD34+ adipose-derived stem cell subpopulation.19–21 Evidence that fat graft enrichment with adipose-derived stem cells may improve healing, along with their impressive known biological properties, suggests that adipose-derived stem cells play an important role in graft healing. Indeed, one could speculate that age, sex, micro-environment, and anatomical depot of adipose tissue all influence adipose-derived stem cell function and differentiation potential,22–24 thus partially explaining the inconsistent animal model results reported in the literature.
In this study, we hypothesize that native adipose-derived stem cell concentration in grafted fat tissue and adipose-derived stem cell functional properties may serve as valuable predictors of transplanted fat graft survival. Specifically, we examined the following in processed fat graft material from eight human subjects: (1) prevalence of adipose-derived stem cell subpopulations as assessed by multiparameter flow cytometry of surface markers of adipose stem cell (CD34+) percentage; and (2) proliferative/adipogenic potentials and hypoxia-induced vascular endothelial growth factor (VEGF) secretion profiles of primary adipose-derived stem cell cultures. Fat grafts processed in a uniform manner were implanted into a nude mouse model with primary measured outcomes of graft volume, histologic architecture, and vascularity.
PATIENTS AND METHODS
Human Subjects
Adipose tissue was harvested from the abdominal subcutaneous region of eight human adult patients (four men and four women) undergoing elective liposuction and cosmetic contouring of the trunk. Mean ± SD age of the patients was 31.4 ± 11.8 years and mean ± SD body mass index was 30.3 ± 8.6. All patients were healthy and nondiabetic. The University of Pittsburgh Institutional Review Board approved the procedure of collecting adipose tissue samples.
Lipoaspirate Harvesting and Processing
Human lipoaspirate was processed according to the Coleman method.17 Briefly, adipose tissue was gently aspirated using two-hole blunt harvesting cannulae attached to 10-ml Luer-Lok syringes. The capped 10-ml Luer-Lok syringes were then centrifuged at 1200 g for 3 minutes. After the upper (oil) and lower (blood and infiltration liquids) layers were removed, the lower one-third of the middle layer (purified/processed lipoaspirate) was transferred through Luer-Lok connectors to 1-ml syringes. Lipoaspirate was either injected directly into animals or processed further to isolate adipose-derived stem cells (see below).
Adipose-Derived Stem Cell Isolation
Primary human adipose-derived stem cells were isolated from adipose tissue as described previously.25 Briefly, adipose tissue was digested in collagenase solution (type II; Worthington Biochemical Corp., Lakewood, N.J.) with gentle shaking in a 37°C water bath for approximately 30 minutes. The digested tissue was centrifuged at 180 g for 10 minutes and then filtered to remove large debris. Next, the cellular pellet (stromal vascular fraction) was resuspended in erythrocyte lysis buffer and recentrifuged at 180 g for 10 minutes. Flow cytometric staining and analysis was performed on the freshly isolated stromal vascular fraction.
For proliferation and differentiation studies, the stromal vascular fraction was plated and cultured on tissue culture-treated flasks in adipose-derived stem cell plating medium [Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% Fungizone (Bristol-Myers Squibb, New York, N.Y.), and 0.001% dexamethasone]. After overnight incubation, nonadherent cells were removed and the cell medium was replaced with fresh plating medium. After 24 to 48 hours, adipose-derived stem cells were maintained and expanded until near confluence (passage 0), harvested, frozen, and then stored at −80°C until use.
Flow Cytometry
Freshly isolated stromal vascular fraction was maintained on ice and stained for analytical flow cytometry as described previously.19–21 Briefly, cell suspensions were centrifuged (200 g for 7 minutes) and the cell pellets were preincubated with 5μl of neat mouse serum (Sigma, St. Louis, Mo.) to minimize nonspecific antibody binding. Cells were simultaneously stained with 2 μl each of the following monoclonal mouse anti-human fluoro-chrome-conjugated antibodies: CD3-FITC, CD146-PE, CD34-ECD, CD90-PE-Cy5, and CD117-PE-Cy7 (Beckman Coulter, Brea, Calif.); and CD31-APC and CD45-APC-Cy7 (BD Biosciences, San Jose, Calif.). Analytical samples were fixed with 2% methanol-free formaldehyde (Polysciences, Warrington, Pa.), permeabilized with 0.1% saponin (Beckman Coulter) in phosphate-buffered saline with 0.5% bovine serum albumin, and incubated with 7.7 μg/ml 4′,6-diamidino-2-phenylindole. Eight-color, 14-parameter data files were acquired on a three-laser Gallios Flow Cytometer (Beck-man Coulter), and cell sorting was performed on a Beckman-Coulter MoFlo high speed sorter. Flow cytometry was performed at the University of Pitts-burgh Cancer Institute Cytometry Facility.
Proliferation Assay
Cells (passage 1) were seeded in quintuplicate in 96-well plates at a density of 15,000 cells/well and then cultured in adipose-derived stem cell plating medium for 72 hours (at 37°C). Adipose-derived stem cell proliferation was measured using the CyQUANT Cell Proliferation Assay Kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer’s instructions. Fluorescence intensity was measured using a Tecan SpectraFluor Plus micro-plate reader (Tecan Group, Ltd., Männedorf, Switzerland). Cell number was calculated from a prepared standard curve, and percentage increase in cell proliferation was calculated using the following equation: (
Adipogenesis Assay
Cells (passage 1) were seeded as above and cultured overnight at 37°C. The next day, adipose-derived stem cell plating medium was replaced with preadipocyte differentiation medium (Zen-Bio, Inc., Research Triangle Park, N.C.). Differentiation medium was replaced every other day for 14 days. At the end of this period, adipose-derived stem cell differentiation was quantified by intra-cellular lipid accumulation using the AdipoRed Assay Reagent (Lonza Walkersville, Inc., Walkersville, Md.) and a Tecan SpectraFluor Plus micro-plate reader according to the manufacturer’s instructions. Percentage increase in adipogenesis was calculated using the following equation:
VEGF Secretion
Patient adipose-derived stem cells (passage 1 or passage 2) were seeded in duplicate onto six-well plates at a density of 125,000 cells/well and cultured for 48 hours at 37°C in 5% carbon dioxide–humidified incubators under hypoxic (approximately 10% oxygen) or normoxic (21% oxygen) conditions. Cultured medium was then collected and assayed for secreted VEGF protein by means of enzyme-linked immunosorbent assay (Millipore Corp., Billerica, Mass.).
Animals
Six-week-old female athymic nude mice (Harlan Laboratories, Inc., Indianapolis, Ind.) were kept under controlled environmental conditions with a constant laminar airflow, a temperature of 20° to 23°C, a humidity of 40 to 60 percent, and a 12-hour-light/12-hour-dark cycle in the Division of Laboratory Animal Resources facilities of the University of Pittsburgh. Mice were given access to standard laboratory chow and sterilized water ad libitum. All animal experiments were approved by the University of Pittsburgh animal care facility.
Xenograft Implantation/Explantation
Mice were anesthetized intraperitoneally with 12 mg/kg xylazine (Vedco, Inc., St. Joseph, Mo.) and 80 mg/kg ketamine (Ketathesia; Butler Schein Animal Health Supply, North Dublin, Ohio) before implantation. Following sedation, processed lipoaspirate (1 ml per injection) was bilaterally injected subcutaneously (fan-style with multiple passes) into the flank regions using a 16-gauge blunt-tip infiltration cannula. Mice were given intramuscular injections of 5 mg/kg ketoprofen (Fort Dodge Animal Health, Overland Park, Kan.) following the procedure for pain or discomfort.26 At 8 weeks after implantation, mice were killed, explants were isolated and assessed for masses on a standard laboratory balance, and volumes were calculated using an Accupyc II 1340 gas pycnometer (Micromeritics, Norcross, Ga.). Explant volumes (three independent measurements) were calculated using the following equation:
Hematoxylin and Eosin Staining
Explants were fixed in 10% nonbuffered for-malin, processed according to routine methods by Tissue-Tek VIP (Miles Scientific, Naperville, Ill.), and embedded in paraffin (Leica EG1140H; Leica Microsystems, Buffalo Grove, Ill.). Tissue samples were cryosectioned at 8-μm thickness and stained with hematoxylin and eosin using standard procedures.
Antibody CD31+ Staining
Horseradish peroxidase–based CD31+ antibody staining was conducted to confirm blood vessels identified with hematoxylin and eosin staining. For both human specific and pan-CD31+ staining, sections were initially deparaffinized with xylene and rehydrated through a graded ethanol series. Heat-mediated antigen retrieval was performed with 0.01 M (pH 6.0) citrate buffer (Sigma) at 95° to 100°C for 20 minutes. After blocking with Avidin/Biotin (Vector Laboratories, Burlingame, Calif.) and endogenous enzyme solution (Dako, Carpinteria, Calif.), slides were incubated for 10 minutes in a protein block (Dako), followed by 5% goat serum (Sigma) for human CD31+ staining or 5% rabbit serum for pan-CD31+ staining to prevent nonspecific binding. Next, slides were incubated with rabbit anti-human CD31 (1:500; Abcam, Cambridge, Mass.) or goat anti-CD31 (M-20 clone, 1:100; Santa Cruz Biotechnology, Santa Cruz, Calif.) overnight at 4°C. Slides were then incubated with biotinylated goat anti-rabbit immunoglobulin G (1:100; Abcam) or biotinylated rabbit anti-goat immunoglobulin G secondary antibody (1:200; Vector). Diaminobenzidine (Vector) was applied to detect CD31+ staining cells. Finally, slides were dehydrated, cleared in xylene, and mounted for imaging.
Tissue Imaging
Images were obtained on an Olympus Provis microscope (Olympus Optical Co., Tokyo, Japan) using brightfield microscopy. Blood vessels were quantified under high-magnification (original magnification, × 200) light microscopy by counting the average number of vessels present in six independent fields per slide (three slides per graft), as determined by three blinded reviewers (J.E.V., C.W.C., and R.E.K.).
Statistical Analysis
Values are reported as means ± SD. Data comparing experimental outcomes within patients (i.e., proliferation, differentiation, VEGF protein secretion, and CD31+ counts) were analyzed by one-way analysis of variance (IBM SPSS, Version 19; IBM Corp., Armonk, N.Y.). Data comparing experimental conditions (10% oxygen versus 21% oxygen for the same patient, VEGF protein secretion) were analyzed by paired t test (SPSS). Linear regression analysis (SPSS) was used to assess relationships between percentage graft retention and (1) CD34+ adipose-derived stem cell percentage yield, and (2) VEGF protein secretion. Values of p < 0.05 were considered statistically significant.
RESULTS
Percentage Yield of CD34+ Adipose-Derived Stem Cells
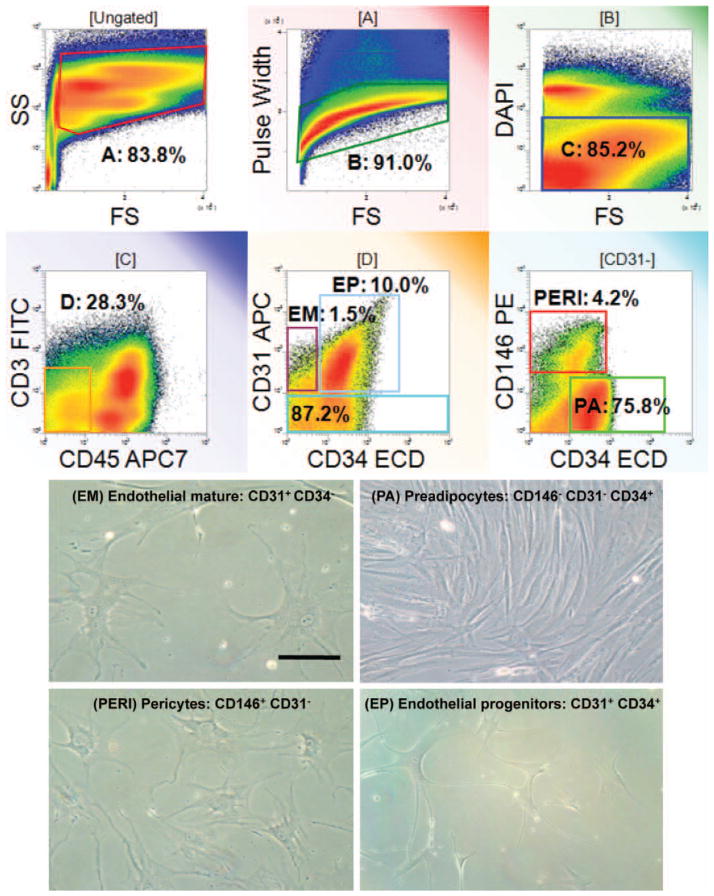
Among CD45-negative nonhematopoietic cells isolated using flow cytometry, we detected four stromal vascular fraction populations as shown in Figure 1: endothelial mature, endothelial progenitor, pericyte, and preadipocyte/supra-adventitial adipose-derived stem cell populations. These distinct populations of cells were characterized based on cell surface markers as described previously.19–21 Figure 2 illustrates the large variation in CD34+ adipose-derived stem cell percentage yield among the eight patients with a range of approximately 4 to 37 percent adipose-derived stem cell content on a per-yield basis of processed lipoaspirate. Overall, the average CD34+ adipose-derived stem cell percentage yield was 21.3 ± 15 percent (mean ± SD), with no statistically significant difference between men and women.
Fig. 1.
Analysis of four distinct subpopulations sorted from human stromal vascular fraction. Freshly isolated stromal vascular fraction from abdominal adipose tissue was stained for flow cytometric analysis using a combination of CD31, CD34, CD45, and CD146. (Above) Multiparameter flow cytometric sort strategy of human stromal vascular fraction showing four distinct subpopulations: endothelial mature (EM), endothelial progenitor (EP), pericyte (PERI), and preadipocyte/supra-adventitial adipose-derived stem cell (PA) populations. (Below) Morphologic images of stromal vascular fraction sorted subpopulations (original magniflcation, × 200). Scale bar = 10 μm for all images.
Fig. 2.
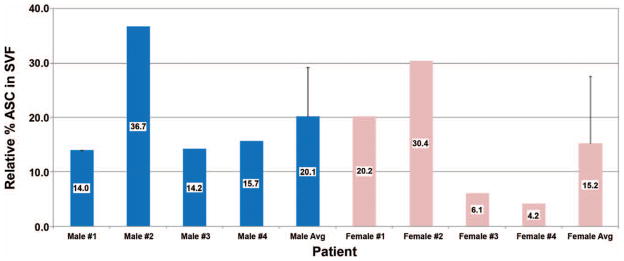
Percentage yield of adipose-derived stem cells (ASC) in stromal vascular fraction (SVF) isolated from patients. For each patient, stromal vascular fraction was processed from lipoaspirate and then subjected to flow cytometry and quantified for adipose-derived stem cell content. Mean supra-adventitial adipose-derived stem cell percentage ± SD is indicated.
Adipose-Derived Stem Cell Proliferation
Following isolation of adipose-derived stem cell patient cultures, we first examined the relative proliferation profiles of these stem cells under normal growth conditions. We observed that adipose-derived stem cell cultures from seven of the eight patients exhibited an approximate twofold increase in growth over a 72-hour time period (Fig. 3). In contrast, primary cells from male patient 4 displayed nearly a fivefold (p < 0.01) increase in growth under similar conditions. Although no significant difference in adipose-derived stem cell proliferation was observed between male and female patients, these findings suggest that variable adipose-derived stem cell growth rates could contribute to overall graft viability and retention.
Fig. 3.
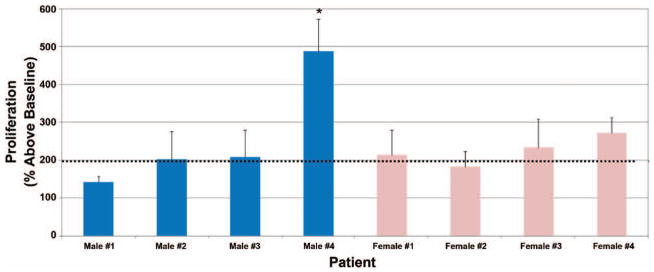
Proliferation variability among primary cultured human adipose-derived stem cells. Isolated adipose-derived stem cells were seeded and treated as described earlier under Patients and Methods. Data are representative of means ± SD. Dotted line indicates population doubling relative to baseline seeding density (*p < 0.05).
Adipose-Derived Stem Cell Differentiation
Having found moderate interpatient adipose-derived stem cell growth variability, we next examined the differentiation potential of adipose-derived stem cells isolated from the four male and four female patients. As shown in Figure 4, treatment of the eight adipose-derived stem cell cultures with differentiation medium over 14 days caused a fourfold to sixfold increase (p < 0.05) in lipid accumulation over individual baseline controls in the AdipoRed Assay. Although modest variability was evident, no significant difference regarding adipogenic potentials was observed between male and female adipose-derived stem cell cultures.
Fig. 4.
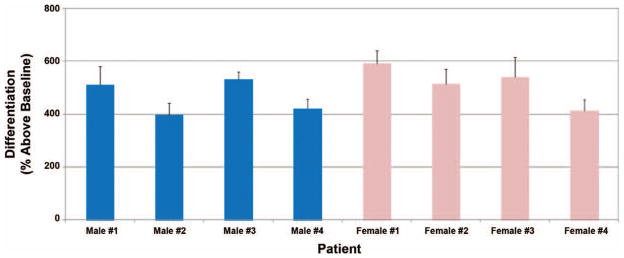
Variability in differentiation potential among primary cultured human adipose-derived stem cells. Isolated adipose-derived stem cells were seeded and treated as described earlier under Patients and Methods. Data are representative of means ± SD.
Xenografts: Volume Retention and CD34+ Adipose-Derived Stem Cell Percentage Yield
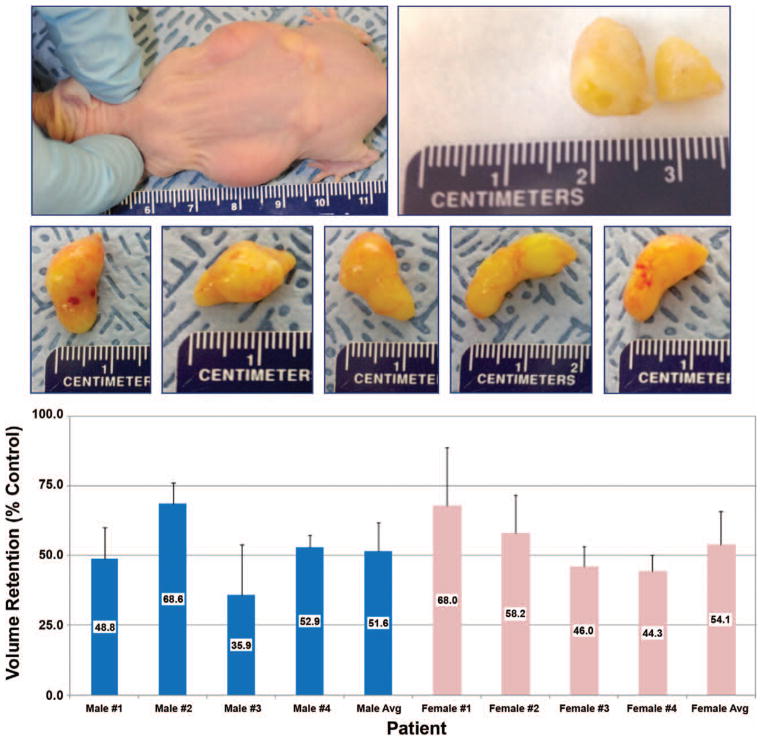
Following in vitro adipose-derived stem cell differentiation and expansion, we tested potential sex differences relating to in vivo fat xenografts (Fig. 5, above and center). To both validate our in vivo xenograft mouse model and to examine the potential graft retention variability among patient lipoaspirates, volume measurements of human graft implants were recorded 8 weeks after transplantation. Figure 5, below illustrates that fat graft retention volumes were quite variable among the eight patient samples, ranging from nearly 36 to 68 percent after 8 weeks, with an overall average of 52 ± 11 percent. A significant difference in xenograft volume retention was not calculated between male and female lipoaspirate xenografts. Also, xenograft retention volume did not correlate positively with respect to either patient age or patient body mass index. However, a strong correlation coefficient (r = 0.78, slope = 0.76, p < 0.05) was found between percentage of CD34+ supra-adventitial adipose-derived stem cell prevalence and fat graft volume retention (Fig. 6).
Fig. 5.
Volume retention of human adipose tissue transplants in athymic nude mice. (Above and center) Representative images of athymic mouse 4 weeks after implantation (above, left), dissected fat graft explant (above, right), and isolated explants 8 weeks after implantation (center). (Below) For each patient, 1 ml of processed human lipoaspirate was bilaterally injected subcutaneously into each flank of female athymic nude mice (n = 4 to 5 per patient). After 8 weeks, mice were killed, tissue implants were excised, and volumes were recorded using a gas pycnometer. Average volume retention per mouse is indicated (percent ± SD).
Fig. 6.
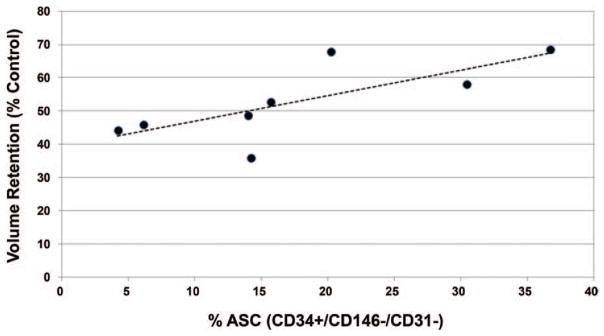
Relationship between adipose-derived stem cell percentage yield and fat graft retention in mice. Higher adipose-derived stem cell (ASC) percentage yield correlated strongly (r = 0.78, p < 0.05) with increased graft volume retention.
VEGF Secretion: Normoxic and Hypoxic Conditions
Because of the reduced oxygen levels in adipose tissue versus typical (21% oxygen) cell culture conditions,27–29 and the increased growth rates13,30,31 and growth factor secretion13,32,33 of adipose-derived stem cells under hypoxic conditions, we measured secreted VEGF protein from adipose-derived stem cells cultured under hypoxemia (approximately 10% oxygen) for 48 hours. As shown in Figure 7, VEGF protein secretion was highly variable among the eight adipose-derived stem cell cultures under normoxic (21% oxygen) conditions, ranging from approximately 40 to 250 pg/ml. Consistent with previous literature,13,32,33 all adipose-derived stem cell cultures demonstrated significantly increased (p < 0.05) VEGF protein secretion (increase of approximately 150 to 600 percent) under hypoxic (approximately 10% oxygen) versus normoxic (21% oxygen) conditions (Fig. 7). In addition, a positive correlation (r = 0.47, slope = 0.03, p > 0.05) was calculated between hypoxia-induced VEGF secretion and graft volume retention (Fig. 8).
Fig. 7.
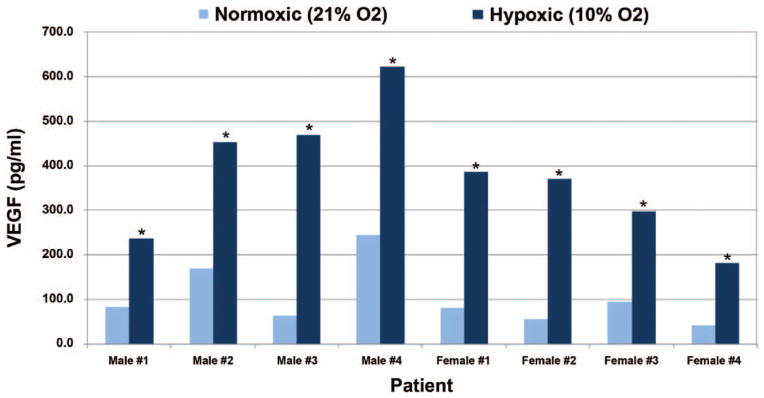
Effect of hypoxia on growth factor release from primary cultured human adipose-derived stem cells. Adipose-derived stem cells isolated from human patients were cultured under various atmospheric oxygen ( O2) conditions (approximately 10% and 21%) for 48 hours. Medium was then collected and assayed for concentrations of secreted VEGF by means of enzyme-linked immunosorbent assay (*p < 0.05 versus 21%).
Fig. 8.
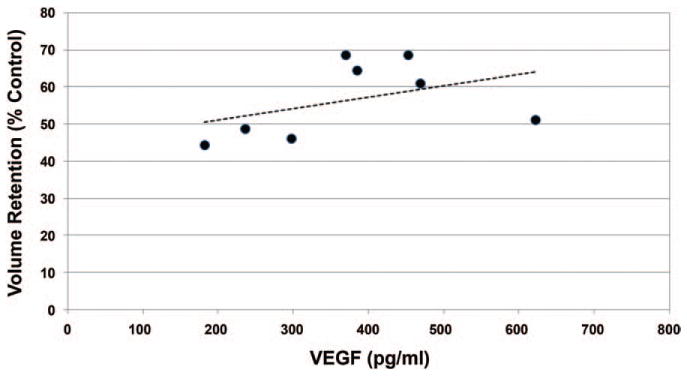
Relationship between VEGF protein secretion profiles and fat graft retention in mice. Increased VEGF protein secretion in vitro (induced by hypoxic conditions) correlated favorably (r = 0.47, p > 0.05) with increased graft retention.
Xenografts: Architecture and Vascularity
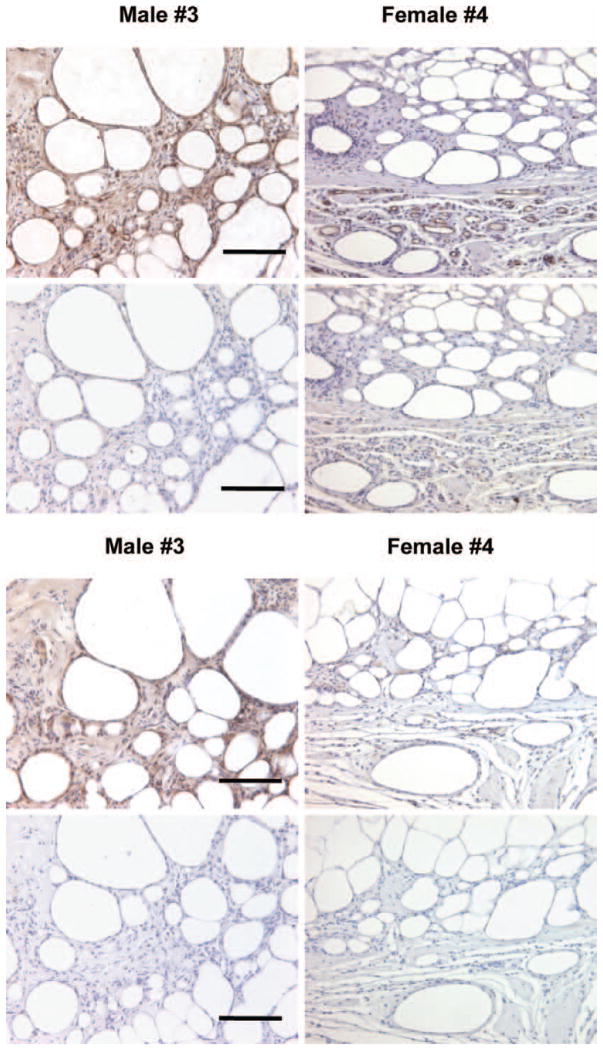
Given the importance of neovascularization, we examined whether increased blood vessel density was correlated with increased xenograft retention. After 8 weeks, all xenografts were surrounded by collagen and fibrous capsules, with the peripheral and central zones composed of viable mature adipocytes. Hematoxylin and eosin analyses indicated that overall mature fat architecture did not differ between the xenografts (Fig. 9). In addition, very limited fibrosis and cyst formation were observed among the isolated grafts.
Fig. 9.
Histologic hematoxylin and eosin staining of adipose tissue explants 8 weeks after implantation. Representative histologic staining of explants was performed with hematoxylin and eosin. Specific patients are indicated (original magnification, × 200). Scale bar = 10 μm for all images.
Analyses of graft vascularization revealed no strong correlation between blood vessel density and graft retention. Assessment of CD31+ counts from six magnified fields per explant indicated that the average number of blood vessels per field did not differ significantly between patients (Table 1). Antibody staining for CD31+ confirmed that the structures that were assessed by means of hematoxylin and eosin staining were indeed blood vessels (Figs. 10 through 12). Based on overall pan-CD31+ staining (Fig. 10, above), the predominance of positive staining was attributed to (donor) human-specific CD31 (Fig. 10, below), although minimal (host) mouse-specific CD31 staining was evident. As expected, human tonsil tissue (positive control) demonstrated endothelial staining for human-specific CD31+ (Fig. 11).
Table 1.
Xenograft Vascularity*
| Patient | Average Pan-CD31 Counts/HPF (L + R) | Average Human CD31 Counts/HPF (L + R) |
|---|---|---|
| Male | ||
| 1 | 63 | 70 |
| 2 | 79 | 53 |
| 3 | 96 | 51 |
| 4 | 137 | 65 |
| Mean ± SD | 94 ± 31.6 | 60 ± 9.4 |
| Female | ||
| 1 | 87 | 80 |
| 2 | 95 | 69 |
| 3 | 101 | 69 |
| 4 | 141 | 93 |
| Mean ± SD | 106 ± 24.3 | 78 ± 11.7 |
HPF, high-power field; L + R, left and right xenografts per mouse.
Average blood vessel count per high-power field. After 8 weeks, explants were isolated and blood vessels quantified by counting the average number of vessels present in six independent fields per slide (three slides per graft), as determined by three blinded reviewers. Average blood vessel density per group is reported as mean ± SD.
Fig. 10.
Vascularity staining of adipose tissue explants 8 weeks after implantation. Representative histologic staining of explants was performed with (above 4 images) CD31 primary (pan) antibody and (below 4 images) CD31 primary (human-specific) antibody. (Above) Primary CD31 staining; (below) primary CD31 delete staining. After 8 weeks, no significant correlation was observed between blood vessel density (per six high-power fields per explant) and graft volume retention (r < 0.1). Specific patients are indicated (original magnification, × 200). Scale bar = 10 μm for all images.
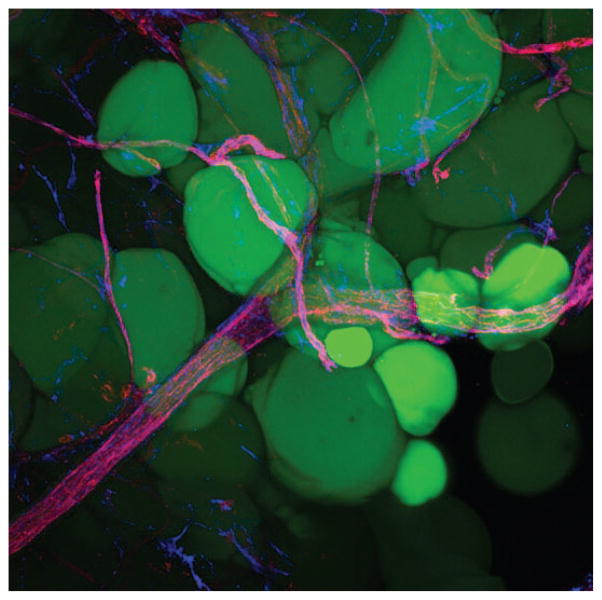
Fig. 12.
Whole mount projection (original magnification, × 20) of human subcutaneous fat stained with CD31 (red) and CD34 (blue) antibodies. Note the CD34+ perivascular cell staining along the blood vessels. Mature adipocytes are shown in green (LipidTOX; Invitrogen).
Fig. 11.
Vascularity staining of adipose tissue explants 8 weeks after implantation. Tissue from human tonsil was used as positive control for human-specific CD31 antibody. (Left) Primary CD31 staining and (right) primary CD31 delete staining (original magnification, × 200). Scale bar = 10 μm for all images.
DISCUSSION
Therapies such as adipose-derived stem cell supplementation and growth factor enrichment are being explored to maximize the survival of transplanted fat tissues, although volume retention remains highly variable. Despite numerous fat grafting animal studies to date,14–16,18,34 none (to our knowledge) has explored donor variability and adipose-derived stem cell growth/differentiation characteristics related to overall xenograft survival and retention. In this study, we demonstrate that CD34+ progenitor cell concentration can predict human fat graft survival in a mouse model. These findings may partially explain the high inconsistency regarding fat graft retention observed in both animal models and clinical settings.
In this study, we processed lipoaspirate by means of the well-known Coleman technique17 and calculated explant volumes using a highly accurate gas pycnometer, a very sensitive instrument for assessing fat graft volume. Although the Coleman technique has not necessarily been proven to provide the best outcomes, the methodology can be well standardized in experimental applications. Moreover, retention volumes were consistent among multiple mice per xenograft (Fig. 5, below). Given the number of animals used per patient sample (four to five), and the high-volume accuracy of gas pycnometer, we are confident that our mouse model can provide statistically significant results regarding xenograft survival.
Although adipose-derived stem cell proliferation and adipogenic potential did not prove to be positively correlated with increased fat graft retention in our studies, we did detect a strong correlation between CD34+ adipose-derived stem cell yield and xenograft retention (r = 0.78) (Fig. 6). Consistent with this finding, we did identify a positive correlation (r = 0.47) between xenograft retention and hypoxia-induced VEGF secretion (Fig. 8). Oxygen levels in vascularized adipose tissue are known to be much lower than the typical 21% oxygen under cell culture conditions,27–29 and adipose-derived stem cell growth rates13,30,31 and growth factor secretion13,32,33 increase under hypoxic conditions. Thus, our in vitro VEGF secretion studies were conducted to mimic the stressed tissue states that occur during and after fat grafting. Adipose-derived stem cell VEGF secretion profiles did correlate favorably (r = 0.47) with enhanced xenograft retention (Fig. 6), which indicates consistency between in vitro findings and in vivo results and suggests that enhanced xenograft retention in mice is attributable to innate biological properties of adipose-derived stem cells and not simply to adipose-derived stem cell quantity.
Inadequate revascularization following transplantation results in cellular apoptosis, cyst formation and, ultimately, graft necrosis.35–38 In our studies, we did not find a strong correlation between xenograft retention and blood vessel density. The average number of vessels present in six (random) independent fields was scored by three blinded (and experienced) reviewers and based on CD31+ staining. Histologic sections were cut from the middle one-third of each graft to obtain representative slices of each graft’s architecture and vascularity. Blood vessel counts among the three blinded reviewers were very similar, confirming high consistency and reliability. We posit that other factors such as blood vessel size, adipose-derived stem cell–to–stromal vascular fraction cell ratio, and host vasculature contributions play critical roles.
Our study is not without limitations. First, the number of patients sampled (n = 8) is fairly small. However, we were able to use grafts from both male (n = 4) and female (n = 4) patients displaying a wide range of ages and body mass indexes. Another limitation with this study is the duration between graft implantation and explantation (8 weeks). We have demonstrated that 4 to 8 weeks is sufficient for graft vasculature formation. For example, in this study, the majority of CD31+ staining was attributed to (donor) human-specific CD31+ (Table 1), although perhaps a longer period (e.g., >3 months) would favor a stronger correlation between xenograft retention and vascularity. For future studies, monitoring other potential biomarkers associated with both adipose (mature) stromal and adipose-derived stem cells would certainly prove useful.
CONCLUSIONS
We analyzed various adipose-derived stem cell characteristics (growth, differentiation, and hypoxia-induced VEGF protein secretion) and tissue graft properties (CD34+ percentage yield and vascularity), which may aid in predicting successful outcomes for fat graft survival. Our results demonstrate that CD34+ adipose-derived stem cell percentage yield may be one biological factor that can predict fat graft retention.
Acknowledgments
This work was supported by National Cancer Institute grant RO1-CA114246 (to J.P.R.) and University of Pittsburgh Cancer Institute Cytometry Facility, Cancer Center Support Grant P30CA047904. The authors acknowledge Dr. Ludo Zimmerlin and Michael Meyer for assistance with flow cytometry, Meghan McLaughlin for assistance with tissue processing and cell culture studies, and Anthony Machi for technical assistance with histologic analysis. Also, the authors acknowledge Dr. Catherine J. Baty and Jenny M. Karlsson for assistance with imaging.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
References
- 1.Ersek RA. Transplantation of purified autologous fat: A 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219–227. [PubMed] [Google Scholar]
- 2.Kawaguchi N, Toriyama K, Nicodemou-Lena E, et al. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuksel E, Weinfeld AB, Cleek R, et al. Increased free fat-graft survival with the long-term, local delivery of insulin, insulin-like growth factor-I, and basic fibroblast growth factor by PLGA/PEG microspheres. Plast Reconstr Surg. 2000;105:1712–1720. doi: 10.1097/00006534-200004050-00017. [DOI] [PubMed] [Google Scholar]
- 4.Park B, Kong JS, Kang S, Kim YW. The effect of epidermal growth factor on autogenous fat graft. Aesthetic Plast Surg. 2011;35:738–744. doi: 10.1007/s00266-011-9679-y. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Liu Y, Tan W, Hong X. Study on effect of PluronicF-127 and vascular endothelial growth factor composite delivery system for improving the survival of grafted fat (in Chinese) Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010;27:600–605. [PubMed] [Google Scholar]
- 6.Stillaert FB, Abberton KM, Keramidaris E, et al. Intrinsics and dynamics of fat grafts: An in vitro study. Plast Reconstr Surg. 2010;126:1155–1162. doi: 10.1097/PRS.0b013e3181ebe224. [DOI] [PubMed] [Google Scholar]
- 7.Mauney JR, Nguyen T, Gillen K, et al. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 9.Yuksel E, Weinfeld AB, Cleek R, et al. Augmentation of adipofascial flaps using the long-term local delivery of insulin and insulin-like growth factor-1. Plast Reconstr Surg. 2000;106:373–382. doi: 10.1097/00006534-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Yang JA, Chung HM, Won CH, Sung JH. Potential application of adipose-derived stem cells and their secretory factors to skin: Discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther. 2010;10:495–503. doi: 10.1517/14712591003610598. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Johnstone BH, Cook TG, et al. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007;25:3234–3243. doi: 10.1634/stemcells.2007-0388. [DOI] [PubMed] [Google Scholar]
- 12.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Xia Y, Kim WS, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipo-transfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 15.Masuda T, Furue M, Matsuda T. Novel strategy for soft tissue augmentation based on transplantation of fragmented omentum and preadipocytes. Tissue Eng. 2004;10:1672–1683. doi: 10.1089/ten.2004.10.1672. [DOI] [PubMed] [Google Scholar]
- 16.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118(Suppl):121S–128S. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 17.Coleman SR. Structural fat grafting: More than a permanent filler. Plast Reconstr Surg. 2006;118(Suppl):108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell sub-populations. Plast Reconstr Surg. 2011;128:663–672. doi: 10.1097/PRS.0b013e318221db33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013;83:124–140. doi: 10.1002/cyto.a.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 23.Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010;18:1875–1880. doi: 10.1038/oby.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brayfield CA, Marra KG, Rubin JP. Adipose tissue regeneration. Curr Stem Cell Res Ther. 2010;5:116–121. doi: 10.2174/157488810791268582. [DOI] [PubMed] [Google Scholar]
- 26.Haneke E. Skin rejuvenation without a scalpel. I. Fillers. J Cosmet Dermatol. 2006;5:157–167. doi: 10.1111/j.1473-2165.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 27.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 29.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 30.Carrancio S, López-Holgado N, Sánchez-Guijo FM, et al. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp Hematol. 2008;36:1014–1021. doi: 10.1016/j.exphem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Weijers EM, Van Den Broek LJ, Waaijman T, et al. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng. 2011;17:2675–2685. doi: 10.1089/ten.tea.2010.0661. [DOI] [PubMed] [Google Scholar]
- 32.Rubina K, Kalinina N, Efimenko A, et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15:2039–2050. doi: 10.1089/ten.tea.2008.0359. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen JG, Frøbert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the proangiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 34.Lu F, Li J, Gao J, et al. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg. 2009;124:1437–1446. doi: 10.1097/PRS.0b013e3181babbb6. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378–386. discussion 387. [PubMed] [Google Scholar]
- 36.Nishimura T, Hashimoto H, Nakanishi I, Furukawa M. Microvascular angiogenesis and apoptosis in the survival of free fat grafts. Laryngoscope. 2000;110:1333–1338. doi: 10.1097/00005537-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 38.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: Safety and efficacy. Plast Reconstr Surg. 2007;119:775–785. doi: 10.1097/01.prs.0000252001.59162.c9. discussion 786. [DOI] [PubMed] [Google Scholar]