Abstract
Background
Intimal hyperplasia (restenosis) is an exaggerated healing response leading to failure of half of vascular interventions. Increasing evidence suggests that circulating progenitor cells contribute to intimal pathology, and clinical studies have demonstrated a correlation between progenitor cells and the incidence of restenosis after cardiovascular interventions. The aims of this study were to characterize the temporal response of CD34+ progenitors following vascular injury in an ovine model and to evaluate an affinity pheresis approach to attenuate this response.
Methods
An ovine model underwent either operative vascular injury or a nonvascular surgery (n = 3 per group). Blood was examined perioperatively over 2 weeks by flow cytometry. Next, an affinity pheresis approach to mediate systemic depletion of CD34 progenitors was designed. Custom agarose pheresis matrix with antibody affinity toward CD34 or an isotype control was evaluated in vitro. Next, following vascular injury, sheep underwent perioperative whole blood volume pheresis toward either the progenitor cell marker CD34 (n = 3) or an isotype control (n = 4) for 14 days. Animals were monitored by physical exam as well as complete blood counts. Cells recovered by pheresis were eluted and examined by flow cytometry.
Results
Flow cytometry revealed a focal surge of circulating CD34 cells after vascular injury but not among surgical controls (P = .05). Toward the goal of an approach to attenuate the surge of CD34 progenitors, an evaluation of high-flow affinity matrix revealed efficacy in removal of progenitors from ovine blood in vitro. Next, a separate group of animals undergoing affinity pheresis after vascular injury was evaluated to mediate systemic depletion of CD34+ cells. Again, a surge of CD34+ cells was observed among isotype pheresis animals following vascular intervention but was attenuated over 20-fold by a CD34 pheresis approach (P = .029). Furthermore, an average of 77 million CD34-positive cells were eluted from the CD34 pheresis matrix. Despite multiple sessions of pheresis, complete blood counts remained essentially unchanged over 2 weeks.
Conclusions
Despite evidence suggesting a role for CD34+ circulating progenitor cells in restenotic pathology, the temporal pattern of CD34 progenitors after vascular injury has not been previously defined. We have demonstrated a surge among circulating CD34+ cells that appears confined to procedures involving vascular injury and that this event seems to occur early after vascular injury. We further conclude that CD34 affinity pheresis attenuates the surge. This approach for direct depletion of progenitors may have important implications for the study of progenitors in vascular restenosis.
Restenosis and late graft failure threaten the durability of endovascular and open vascular procedures alike. Up to half of endovascular and open lower extremity bypasses will occlude in 5 years,1,2 half of vascular access grafts in 18 months,3 and 30% angiographic stenosis among bare metal coronary stents by 1 year.4 Even after aggressive surveillance and reintervention on these recurrent lesions, longevity remains poor. A common theme of failure among these seemingly diverse vascular beds is the pathology known as intimal hyperplasia. Intimal hyperplasia is characterized by proliferation of smooth muscle cells and extracellular matrix that ultimately limits luminal diameter and leads to vessel occlusion. This pathology has increased the need for both surveillance as well as additional interventions, contributing costs to the health care system and increasing rates of limb loss and death. While drug-eluting stents have proven effective in reducing some restenosis in coronary contexts, obstacles to use of this technology in peripheral vascular beds include the longer length of lesions, greater complexity in the composition of the plaques, restrictions on use at points of anatomic flexion or bifurcation, and small but significant risk of acute thrombosis.5,6 For these reasons, further understanding of the pathology of intimal hyperplasia is essential toward improving patient outcomes after vascular interventions.
Multiple authors have suggested a role of bone marrow-derived progenitors in the pathology of intimal hyperplasia.7–14 Even some authors who have challenged a direct cellular contribution to intimal lesions have noted increases among signaling molecules known to be involved in bone marrow mobilization of progenitors.15 This would suggest some contribution of circulating progenitors, even if not at a cellular level. Most importantly, multiple groups have highlighted an increased incidence of coronary restenosis among patients with increased numbers of circulating progenitors.16,17 Several laboratories have described a vascular injury signal that mediates both circulating progenitor mobilization but also homing to sites of injury.18–20 It remains unclear, however, if mobilization of these cells is limited to surgeries involving macrovascular injury, or merely a generalized injury response to surgical stress.
In summary, there remains controversy regarding the role of circulating progenitors in restenosis. The primary aim of this study was to document the temporal pattern of circulating progenitor cells after vascular injury and to compare these results with the response after a non-vascular surgery in a large animal model. As in the human condition, the marker CD34 has been previously defined as a marker of hematopoietic progenitor cells with colony-forming activity in sheep.21 A second objective of this study was to evaluate a short-term affinity pheresis approach to mediate depletion of circulating progenitors and the impact if any on mature hematopoietic cells. Despite the apparent technical simplicity of progenitor depletion, selective affinity depletion of progenitor cells from the circulation has not been described previously in the literature.
METHODS
Affinity antibodies
8D11 antibody reactive to ovine CD34 was characterized21 and kindly provided by Dr Christopher Porada (University of Nevada, Reno, Nev). This antibody was manufactured by Aldevron (Freiburg, Germany). An isotype-matched IgG1 control antibody was purchased from MP Biomedical (Solon, Ohio).
Preparation of affinity matrix
For the creation of macro agarose affinity beads, plain agarose beads with a diameter range of 150 μm to 300 μm (ABT, Madrid, Spain) were functionalized with N,N disuccinimidyl carbonate (DSC; Sigma-Aldrich, St Louis, Mo) using some minor modifications for the protocol of Barkawi.22 Briefly, agarose beads were rinsed with graded aqueous and acetone washes until a final rinse with dry acetone (Acros Organics, Geel, Belgium). DSC was dissolved to a concentration of 0.26 M in acetone (dry) and added to the anhydrous beads. Dimethylaminopyridine (Sigma-Aldrich) was dissolved in dry acetone to a concentration of 0.65 M and then added dropwise to the DSC/antibody mixture. After 6 hours of incubation at room temperature, beads were rinsed with serial rinses of dry acetone (dry), propanol (dry), and finally, phosphate-buffered saline (PBS). Immediately following the saline rinse, 8D11 antibody or MOPC were added at a ratio of 1 mg per 15 mL of settled beads. Alternately, CD34 antibody was conjugated as previously described using commercially available N-hydroxysuccinimide (NHS) Fastflow Sepharose beads (GE Healthcare, Buckinghamshire, United Kingdom).23 As previously described, only beads filtered for size >70 μm were used for conjugation.
In vitro cell depletion assay
Specimens of 35 mL of heparinized ovine peripheral blood were incubated with conditions of no beads, NHS affinity beads, custom macro CD34 affinity beads, and isotype control macro affinity beads for 30 minutes at 37°. DSC and NHS beads were prepared with equivalent antibody mass of antibody. Beads were then removed with 70-μm mesh filters (Fisher, Pittsburgh, Pa). Leukocytes were recovered by gradient centrifugation (Histopaque 1077; Sigma-Aldrich) and evaluated by flow cytometry.
Animals
Female Dorset cross sheep between the ages of 4 and 6 months were purchased from an approved University of Pittsburgh vendor. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Sheep were monitored perioperatively for evidence of neurologic, cardiovascular, allergic, or pulmonary complications. Animals were fed Rumilab chow (PMI, St. Louis, Mo).
Vascular injury model
Sheep were allocated into two groups, including control surgery with exposure but not injury of the vessels (n = 3) and a vascular injury group that underwent placement of an arteriovenous graft and a contralateral carotid angioplasty (n = 3). For the control group, the carotid vessels were exposed but not injured on both sides. Heparin (APP Pharmaceuticals, Schaumburg, Ill) was administered at 100 units per kg to all animals. In the vascular injury group, a 6-mm polytetrafluoroethylene graft was placed between the carotid artery and jugular vein in an arteriovenous loop graft configuration using 6-0 polypropylene suture (Covidien, Mansfield, Mass). The left carotid was then exposed and underwent balloon angioplasty injury with a 6-mm balloon (Ultrathin SDS; Boston Scientific, Natick, Mass) that was inflated three times for the injury. Incisions were closed with absorbable suture in two layers.
Affinity pheresis
Pheresis groups included sheep that all underwent vascular injury and were divided into groups of isotype (n = 4) and CD34 affinity pheresis (n = 3). Under general anesthesia, a dual-lumen high-flow dialysis catheter (Covidien) was placed percutaneously in the internal jugular vein, and flow was initiated through the saline-primed pheresis circuit prior to incision. A centrifugal perfusion pump (Biomedicus; Medtronic, Minneapolis, Minn) was used to perfuse blood from the dialysis catheter to the affinity cartridge and then back to the animal. Affinity pheresis was initiated prior to the vascular injury (postoperative day 0), and a 90-minute pheresis was complete by the time of restoration of blood flow. Blood specimens were drawn before and after the initial and after each subsequent pheresis sessions. Following 2 more days of induction pheresis (days 1 and 2), 30-minute pheresis occurred at a maintenance schedule on days 4, 7, 9, 11, and 14. The 30-minute duration of pheresis was based on calculation of the time to cycle the entire blood volume over the pheresis column twice, with an extended period of 90 minutes for the first pheresis. Following each pheresis session, the pheresis circuit was rinsed with warm normal saline to return all unbound blood and cells to the animal.
Affinity column cell elution
Cells were eluted from the affinity columns following the first three pheresis sessions of each animal. Saline-rinsed beads were trypsinized (0.05% Trypsin – EDTA; Invitrogen, Grand Island, NY) for 10 minutes at 37°. Trypsin was neutralized, and the cells were centrifuged and plated in a glass dish overnight in RPMI with 10% fetal calf serum (Hyclone; Thermo-Fisher Scientific, Pittsburgh, Pa). Eluted cells were counted and examined by flow cytometry.
Flow cytometry
Flow cytometry was conducted on an initial prepheresis specimen and postpheresis specimens. Cells eluted from the affinity columns were also examined by flow cytometry. Peripheral blood mononuclear cells were isolated from 5 mL sheep blood by Ficoll density gradient (Histopaque 1077; Sigma-Aldrich). Single cells were suspended in aliquots of 3 × 106 cells in 100 μL PBS. After blocking the cells for 15 minutes with 10% mouse serum, they were incubated for 45 minutes with mouse monoclonal antibodies conjugated with fluorochromes, directed to several progenitor cell markers: antisheep CD34-PECy7 CD34 antibody manually conjugated in the lab to PECy7 using Lynx Rapid RPE-Cy7 Antibody Conjugation Kit (AbD Serotec, Raleigh, NC) and antisheep CD45-FITC (AbD Serotec); along with antihuman VEGFR2(flk-1)-PerCpCy5.5 purchased from Biolegend (San Diego, Calif), antihuman CD133/1(AC133)-APC from Miltenyi Biotec (Auburn, Calif). Appropriate matched isotype controls were used. Samples were then washed in PBS and fixed in 2% paraformaldehyde for 20 minutes at room temperature. After another wash using 0.5% BSA in PBS, the cells were permeabilized for 10 minutes using a 0.1% saponin + 0.5% BSA solution in PBS. Finally, the cells were washed and resuspended in PBS, and after adding DAPI (Invitrogen), they were analyzed using a CyAN ADP high-speed analyzer (Beckman Coulter, Inc, Fullerton, Calif). The data files were analyzed using Summit 4.3 Version Software (Beckman Coulter, Inc). For analysis of cytometric data, an approach of sequential gating was used to exclude doublets (cell clusters), CD34 negative cells, and finally red cells and debris. The backscatter of the resulting events reveal the population of high positive CD34 cells. In addition, isotype control thresholds were set at fluorescence intensity <1%. Cells eluted from the affinity column were directly stained in a similar fashion.
Complete blood counts
Complete blood counts were drawn preoperatively and then weekly to assess for changes among traditional clinical parameters including platelet count, white blood cell count, neutrophils, and hemoglobin (Marshfield Labs, Cleveland, Ohio).
Statistics
Results from in vitro were analyzed with a Student t-test on postoperative day 1. Results among animals undergoing nonvascular and vascular surgeries and those treated with pheresis by isotype or CD34 pheresis were compared using a Mann-Whitney test at postoperative day 1 on absolute cell counts of 300,000 events.
RESULTS
Balloon angioplasty mediates endothelial injury in the ovine model
The ovine model has been previously described for several contexts of vascular reconstruction and graft stenosis.24–27 While angioplasty is recognized clinically and among several animal models to cause endothelial injury, it has not been explicitly demonstrated in the ovine setting. In order to evaluate the efficacy of a balloon-based approach for endothelial injury, acute balloon-injured carotid was compared with 4-month chronic injured and noninjured carotid arteries as demonstration of this model. Scanning electron microscope examination (n = 3) confirms a denuded endothelial monolayer in the acute injured specimens with re-endothelialization noted in chronic and noninjured specimens (Fig 1).
Fig. 1.
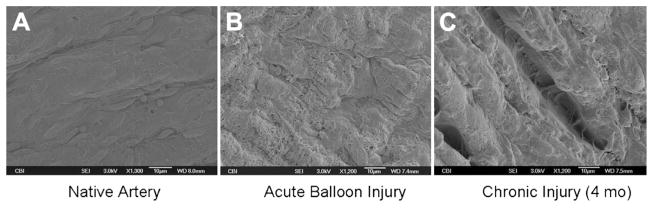
Carotid angioplasty mediates endothelial injury in an ovine model. Compared with uninjured native carotid artery (A), acute balloon injury achieves endothelial injury (B) as shown in these scanning electron microscope images of the luminal surface of the vessel (×1200). By 4 months, the area of endothelial injury has re-endothelialized (C) in these representative images (n = 3).
A surge of peripheral CD34-positive cells accompanies vascular injury but not nonvascular surgeries
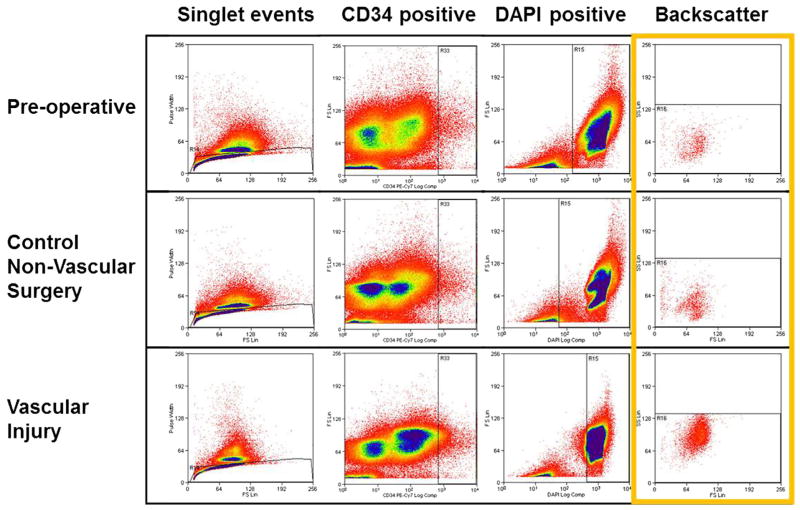
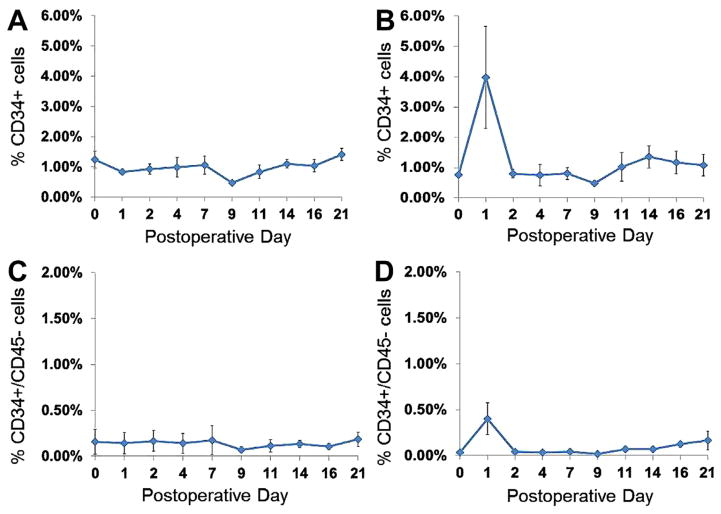
Vascular injury is known to initiate a cascade of signals that are associated with mobilization of bone marrow progenitors.20 Given the potential roles of bone marrow-derived progenitors, it is not known if mobilization of progenitors is specific to vascular injury or to surgical stress in general. For this reason, the mobilization of CD34-positive cells into the blood was compared between animals following vascular injury and control surgical procedures. Blood specimens were drawn before surgery and then on postoperative days 0, 1, 2, 4, 7, 9, 11, 14, and 16, followed by assessment by flow cytometry (Fig 2). Following vascular injury (n = 3), a fivefold surge of CD34 cells was observed on postoperative day 1 (P = .05) that was not observed among animals undergoing control vessel exposure surgery (n = 3; Fig 3). In order to exclude mature cells, we examined the marker CD45 and observed similar trends among CD34+CD45− cells. While variability among CD34 cell magnitude was observed, a surge was noted among all animals. This variation may be a reflection of the heterogeneous animals of this study.
Fig. 2.
Flow analysis of sheep for CD34-positive cells. Blood specimens drawn preoperatively, or from the first postoperative day after nonvascular or vascular surgery were compared. A total of 300,000 events from peripheral blood specimens were gated sequentially for singlet events, CD34 positivity, DAPI positivity, and finally backscattered to reveal final population of cells highly expressing CD34. Increased numbers of CD34-positive cells can be seen after vascular injury (bottom right).
Fig. 3.
Surge of total CD34 and CD34-positive, lineage negative cells is observed among animals undergoing vascular injury. Flow cytometry of sheep undergoing either control surgery without vascular injury (A and C) (n = 3) as compared with sheep undergoing vascular injury (B and D) (n = 3). Shown are percent CD34+ or CD34+/CD45− cells from all events. A and B reveal percent of CD34 cells and suggest a surge as high as sevenfold compared with baseline following vascular injury (P = .05 on postoperative day 1). C and D indicate the percent of CD34+/CD45− cells, again revealing a surge among lineage negative progenitors. Results are expressed as mean ± standard error.
Custom beads with affinity toward CD34 mediate depletion from whole blood
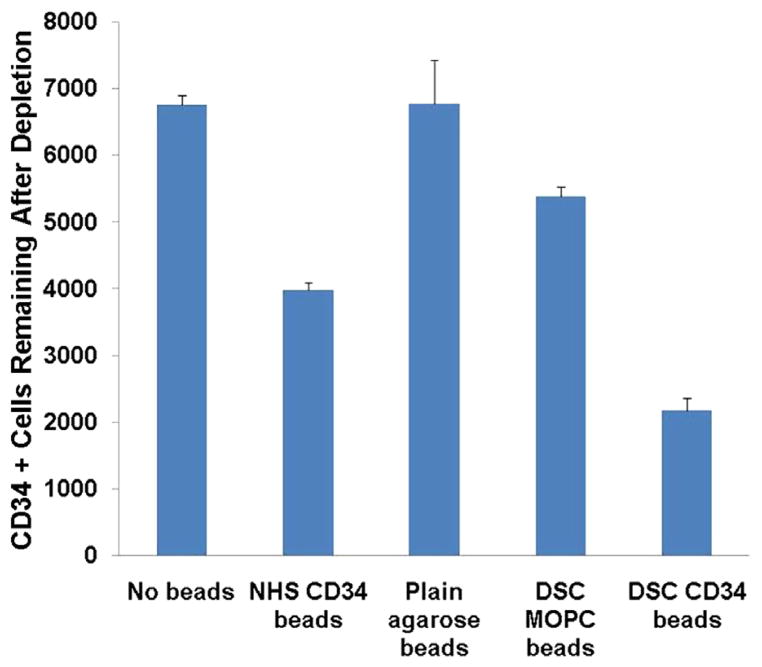
While methods exist to increase certain types of cells in the blood, such as G-CSF or erythropoietin, there are currently no approaches to mediate depletion of specific populations of circulating cells short of bone marrow ablation. Our group has previously described the use of an affinity pheresis with commercially available NHS sepharose beads for the purposes of cell recovery using only physiologic blood pressure for propulsion of the pheresis.28 In order to adapt this approach toward the demands of a circulatory depletion and pump-based propulsion, we recognized limitations on flow rates through the small (70–165 μm) tightly packed beads of our previous commercially available bead format. To facilitate improved flow rates and expected higher volumes of blood using a cardiopulmonary perfusion pump, a higher flow system based on a larger bead format (150–300 μm) was developed. To compare the efficacy of our custom-conjugated beads with our previous standard, we compared NHS beads conjugated to anti-CD34 antibody with macro beads that were unconjugated (plain), conjugated to an isotype antibody (MOPC), or conjugated to the anti-CD34 antibody by their capacity to deplete CD34 cells from isolated blood samples in vitro. As shown in Fig 4, our results suggest that CD34 affinity DSC beads deplete CD34-positive cells in a statistically significant manner relative to no beads (P = .005), isotype control affinity beads (P = .01), and even relative to commercially available NHS beads with affinity for CD34 (P = .02).
Fig. 4.
CD34 affinity agarose beads prepared with N,N′-Disuccinimidyl carbonate (DSC) reveal specific depletion of CD34 cells from ovine blood specimens. Blood specimens were incubated with no beads, commercial N-hydroxysuccinimide (NHS) sepharose beads conjugated to CD34 antibody, plain agarose beads, isotype control antibody (MOPC) DSC beads, or CD34 conjugated DSC beads (n = 3 for all conditions; standard error bars are shown). CD34-affinity DSC beads deplete CD34-positive cells in a statistically significant manner relative to isotype control affinity beads (P = .01).
A CD34 affinity column attenuates the surge of CD34-positive cells after vascular injury
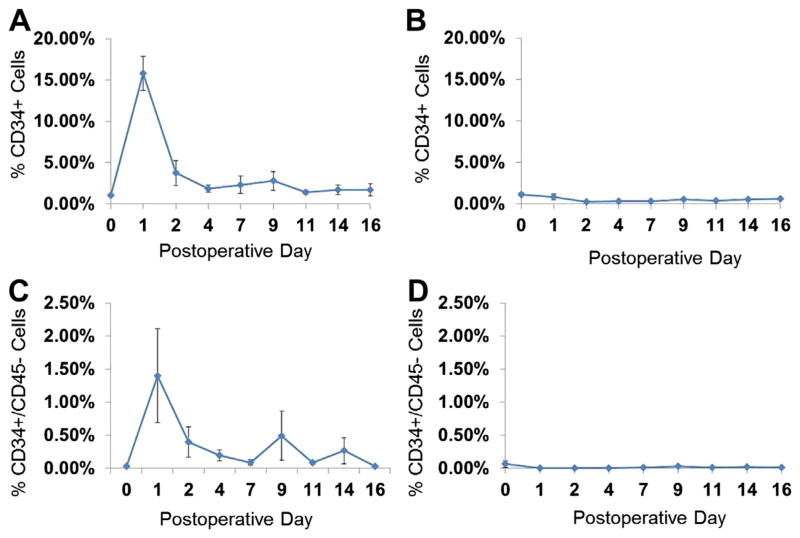
Based on the successful depletion of CD34-positive cells in vitro, we reasoned that an affinity approach could attenuate the surge among CD34 cells in the entire circulation of a large animal model. This approach would provide a model to examine the effects of progenitors in various contexts, including intimal hyperplasia. To test this hypothesis, a new group of animals was examined for the ability to achieve systemic depletion of CD34 cells as measured by flow cytometry. Immediately prior to vascular injury on the day of surgery, animals underwent pheresis using matrix with affinity to either CD34 or an isotype control. Compared with animals undergoing vascular injury with an isotype control, affinity column animals undergoing pheresis with a CD34 affinity column demonstrated a 20-fold attenuation of CD34 response after vascular injury that was statistically significant (P = .029; Fig 5). This relative depletion among animals treated with CD34 affinity pheresis was also observed when marker combinations of CD34+/CD45−, CD34+/VEGFR2+ and CD34+/CD133+ were compared with isotype-depleted animals. In our study, pheresis was well tolerated with no evidence of neurologic, pulmonary, or other observed side effects.
Fig. 5.
CD34 affinity agarose beads attenuate the surge of CD34 cells following vascular injury. Shown are percent CD34+ or CD34+/CD45− cells from all events. Following vascular injury, a surge of CD34+ cells (A) and CD34+/CD45− (C) is seen despite pheresis with an isotype affinity column (n = 4). In contrast, a CD34 affinity column (n = 3) attenuates the surge and mediates a relative state of depletion among both CD34+ (B) and CD34+/CD45− cells (D). A statistically significant difference is observed among total CD34 expressing cells between the two groups (P = .029 on postoperative day 1). Results are expressed as mean ± standard error.
Cells eluted from a CD34 affinity column reveal high numbers of CD34-positive cells
As further confirmation that our results in the peripheral blood were a result of CD34 depletion, cells were enzymatically eluted from isotype and CD34 affinity matrix after pheresis. Following enzymatic release, cells were allowed to recover receptor expression overnight. Compared with the isotype column, which yielded few eluted cells, a CD34 affinity matrix yielded between 35 and 158 million of CD34-positive cells, which appear to surge in the first pheresis days, consistent with the day of maximal surge among peripheral blood specimen analysis of isotype control-treated animals (Fig 6).
Fig. 6.
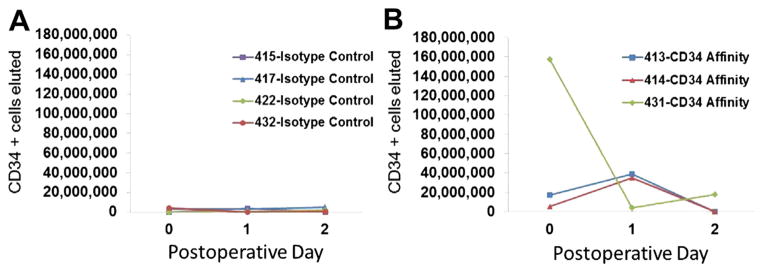
CD34 affinity column recovers large numbers of CD34-positive cells. Cells eluted following pheresis of animals undergoing vascular injury were counted and examined by flow cytometry. Relative to the rare cells recovered by an isotype column (A), CD34 affinity column (B) recovered an average of 77 million CD34-positive cells.
Cell counts following depletion of progenitor cells
Medical devices, particularly those associated with blood, have been associated with complications that include thrombocytopenia, anemia, and neutropenia. In order to examine changes among other mature circulating blood cells, a complete blood cell count was assessed at the time of surgery and then weekly for 2 weeks. Despite acute depletion of circulating progenitor cells among CD34 pheresis animals, no evidence of significant changes were noted in hemoglobin or platelet counts (Table). Interestingly, a small but statistically significant increase in neutrophils was noted among animals following CD34 pheresis, although this remained well within the normal range for the ovine model. Also, white blood cell values were slightly increased among animals treated with isotype pheresis, although again, this remained well within normal range.
Table.
Complete blood counts are minimally changed following depletion of CD34 cells by affinity pheresis
| Prepheresis
|
Post 1 week
|
P value | Post 2 weeks
|
P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Isotype | Hemoglobin | 8.8 | 0.8 | 9.0 | .9 | .9003 | 8.8 | 0.9 | .8756 |
| Plateletsa | 384 | 131.7 | 574.0 | 22.2 | .3793 | 492.3 | 118.9 | .5825 | |
| White blood cells | 4.4 | 1.1 | 5.4 | .2 | .0277 | 5.6 | 0.8 | .0204 | |
| Segmented neutrophils | 1.6 | 0.4 | 1.8 | 1.0 | .6666 | 2.0 | 0.8 | .5446 | |
| CD34 | Hemoglobin | 8.3 | 0.4 | 8.3 | .9 | .9445 | 9.0 | 1.1 | .3908 |
| Platelets | 592.3 | 131.7 | 668.0 | 22.2 | .6548 | 586.0 | 112.1 | .9152 | |
| White blood cells | 4.4 | 1.1 | 5.6 | .2 | .2570 | 6.5 | 1.5 | .0746 | |
| Segmented neutrophils | 1.6 | 0.8 | 3.1 | 1.4 | .0841 | 3.7 | 0.8 | .0289 | |
SD, Standard deviation.
Complete blood counts were drawn before vascular injury and subsequently at 1 and 2 weeks. Shown are blood counts from animals undergoing MOPC isotype pheresis (n = 4) and CD34 affinity pheresis (n = 3). Small but statistically significant increases were noted among white blood cells and segmented neutrophils. Notably, these values remain well within the range of normal for the ovine model.
Platelets could not be assessed on two timepoints for one isotype pheresis animal. As a result, this animal was excluded from platelet comparison.
DISCUSSION
Intimal hyperplasia has a profound adverse impact on the outcomes of vascular interventions as well as patient outcome. Clinically, restenosis encompasses a wide spectrum of vascular patients including disease of the coronary arteries, peripheral vasculature, and even those with vascular access for dialysis. Poor long-term outcomes underscore the need for new approaches toward prevention of this important clinical dilemma. While drug-eluting stents have proven effective in the coronary circulation, they remain discouraged from use at flexion points in the peripheral vasculature due to fracture risk,5 limitations on subsequent open bypass options, and possible increased risk of thrombosis secondary to impaired local endothelial repair.6 For these reasons, despite their utility in coronary applications, alternative approaches to drug-eluting stents are needed. Several studies have highlighted the role of bone marrow-derived progenitors, as contributing to between 20% and 66% of cells in intimal lesions.7,10–13 Although bone marrow-derived cells are widely known for their roles in hematopoiesis and, more recently, their contribution to endothelial repair,29 several authors have demonstrated that CD34-positive cells can differentiate into cells expressing smooth muscle markers.30,31 Among several animal models, increased recruitment or mobilization of progenitor cells was associated with accelerated intimal disease following cardiovascular interventions.8,32 These findings were echoed in the human condition by results of the MAGIC trial, which was terminated prematurely due to accelerated restenosis of patients treated with the progenitor mobilization cytokine G-CSF following bare metal stent placement.9
Whether progenitor cells directly contribute to the cellular mass of intimal lesions remains an area of controversy; however, even authors who have published against direct cellular contribution have reported that blockade of chemokines involved in progenitor cell mobilization and homing, such as sdf1-alpha,15 can reduce intimal pathology. Similar findings have been observed after blockade of the c-kit positive cells.11 Of interest, drug-eluting stents, the only currently available clinical therapy to reduce intimal hyperplasia, involve the use of immunosuppressive agents. It is possible that progenitors mediate an inflammatory microenvironment favorable for intimal lesions. In summary, the literature appears to support that bone marrow-derived progenitors play some role in intimal pathology, whether by a direct cellular contribution or by fostering a favorable inflammatory environment. Despite the potential role in vascular restenosis, the temporal pattern of circulatory progenitor cells after vascular injury has not, to date, been defined.
To provide insight on the pattern of circulating CD34-positive progenitors after vascular injury, we have examined a sheep model of vascular injury, consisting of an arteriovenous graft with contralateral carotid angioplasty. One objective of this study was to determine whether a mobilization of CD34 progenitor cells occurs after an open vascular procedure and whether such a response is confined to vascular interventions or rather is just a generalized response to surgical stress. Our findings suggest that there is a surge of CD34-positive cells limited to animals undergoing vascular injury. Acknowledging the overlap of CD34 expression on other cells such as mast cells and endothelial progenitor cells (EPC), we further analyzed our results and found similar CD34 surge trends even when CD45+ (mast cells) and CD133+ (EPC) events were excluded. Interestingly, our control group inherently entailed microvascular damage during surgery as well as needlestick cannulation for blood draws. Despite these minor vascular injuries, the control group still did not elicit a significant CD34 mobilization in peripheral blood as compared with animals with macrovascular injury. In some ways, the surge of CD34 progenitors is not unexpected, given the role of these cells in the repair of endothelial injury.29 With regards to intimal pathology, however, previous human studies have demonstrated that patients with increased numbers of preprocedural17 and postprocedural16,33 CD34 positive progenitors carry statistically higher risk for coronary restenosis. The short longitudinal study of Inoue et al33 among human coronary stent patients revealed both increases among CD34 cells but also an increase of mobilization cytokines such as G-CSF, suggesting these CD34+ cells originate from the bone marrow reservoir. To date, there have been no studies evaluating CD34 response after open peripheral vascular procedures or comparing these findings with nonvascular procedures. The results of this study appear to address this important question, at least in the ovine model.
A significant hurdle toward evaluating whether circulating progenitor cells are bystanders or play an active role in intimal hyperplasia is the absence of approaches to modulate progenitor cells in the circulation. While several approaches intervene at the level of circulating progenitors, they serve to increase and not decrease the numbers of cells by either cytokine mobilization or direct cell infusion. In contrast, the options for reducing cells in a specific manner is more challenging. Leukapheresis removes cells relatively nonspecifically based on cell density, often impacting other nontarget cell types. Pharmacologic therapies to reduce circulating progenitors are limited largely to chemotherapeutic agents and are limited by bystander toxicity. For these reasons, with currently available approaches, it becomes difficult to establish a depletion of specific cell types for a limited period of time.
We hypothesized that an affinity pheresis approach of the entire blood volume directed toward CD34 may attenuate the surge response of target CD34 cells in a large animal model. For this purpose, we applied a previously described affinity pheresis approach.23 In examining our results, it is noteworthy that the magnitude of the CD34 surge of vascular injury with isotype pheresis appears substantially higher than observed among animals who underwent vascular injury without pheresis, approaching fourfold when comparing means on postoperative day 1. Conceivably, this may represent a response to the components of the pheresis circuit. Alternately, the high flow and intravenous turbulence of the pheresis treatment may exacerbate the vascular injury and thereby the CD34 mobilization. While it merits further investigation, this finding is certainly intriguing, considering the exceptionally rapid stenosis pattern observed clinically with high-flow dialysis catheters and turbulent surgical arteriovenous access. Despite this more accentuated mobilization of CD34 cells in the peripheral circulation among isotype pheresis-treated animals, a CD34 affinity pheresis approach not only attenuated the CD34 surge but also mediated a relative state of depletion compared with baseline. Importantly, complete CD34 depletion was not achieved, which may be related to either the large volume of distribution, technical issues related to optimal binding under high flow conditions, or limits of detection of these rare cells. Regardless, if CD34-positive cells do indeed play a role in intimal lesions, it remains to be seen if complete depletion is even necessary. It is conceivable that prevention of the CD34 surge alone would be sufficient to prevent restenosis. One goal of our study that could not be achieved was the assessment of the effect of CD34 depletion on intimal change. Despite both published reports of rapid restenosis,24 as well as our own published experience in the sheep model,26 significant restenosis was not observed among controls at 4 months. As a result of regional availability, the breed in this study differs from either of the two published studies and may explain these findings.
Importantly, progenitors have been implicated in the physiologic roles of both angiogenesis and wound healing.34,35 If progenitors are involved in restenosis, further investigation will be essential to determine the specific conditions and timing under which progenitors may play a physiologic or pathologic role. Some medical devices may result in changes in the complete blood count, such as thrombocytopenia,36 neutropenia, and anemia.37 This would seem especially probable for a pheresis approach that removes the precursors to mature hematopoietic cells. Despite our relative depleted state, there appeared to be no deficiencies among white blood cells, platelets, or hemoglobin after pheresis. A subtle but statistically significant neutrophilia was seen only among CD34 pheresis animals but, importantly, remained well within the realm of normal values for the ovine model. The results of this study suggest a significant progenitor mobilization event at the time of vascular injury that may represent kindling for the pathology of intimal hyperplasia. We expect that the depletion approach described here may be a useful tool to continue to explore the role of CD34-circulating progenitors in the development of intimal lesions. Direct progenitor depletion may be especially important as a tool to isolate the overlap of roles between vascular smooth muscle cells and progenitor cells in this pathology. Based on reports of clinical association between restenosis in the setting of higher numbers of circulating progenitors,16,17 this approach of direct progenitor depletion may eventually have therapeutic utility, as well. To our knowledge, no other previous study has allowed intervention directly at the level of the progenitor cell. This affinity approach may also be useful in other disease contexts where depletion of specific circulating cells is desirable.
Clinical Relevance.
Restenosis is a major cause of failure after vascular inteventions. Progenitor cells in the blood are believed to contribute to this disease, and published human studies reveal an association between these cells and increased incidence of restenosis. Our ovine study characterized the pattern of CD34+ progenitor cells after vascular injury and suggests an early, focal surge event. We further demonstrate a novel pheresis approach to reduce these cells in the bloodstream. This study offers new insights on the progenitor cell changes that follow vascular injury and may provide new insights on this life- and limb-threatening disease.
Acknowledgments
This work was supported by the American Surgical Association Foundation Fellowship Research Award, the Vascular Cures Wylie Scholar Award, and the University of Pittsburgh Medical Center, Department of Surgery.
The authors thank Larry Fish, PhD, for providing statistical analysis for this project, as well as Jeremy Dann and Derek McQuade for technical support.
Footnotes
Author conflict of interest: none.
Presented at the Thirty-seventh Annual Meeting of the Southern Association for Vascular Surgery, Basic Science Plenary Session, Nassau, The Bahamas, January 23–26, 2013.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: AH, BT
Analysis and interpretation: AH, BT, AD
Data collection: AH, JK, AD, EM, BT
Writing the article: AH, BT
Critical revision of the article: BT
Final approval of the article: BT
Statistical analysis: Not applicable
Obtained funding: BT
Overall responsibility: BT
References
- 1.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. discussion: 751. [DOI] [PubMed] [Google Scholar]
- 2.Siracuse JJ, Giles KA, Pomposelli FB, Hamdan AD, Wyers MC, Chaikof EL, et al. Results for primary bypass versus primary angioplasty/stent for intermittent claudication due to superficial femoral artery occlusive disease. J Vasc Surg. 2012;55:1001–7. doi: 10.1016/j.jvs.2011.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO. Vascular access survival and incidence of revisions: a comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg. 2001;34:694–700. doi: 10.1067/mva.2001.117890. [DOI] [PubMed] [Google Scholar]
- 4.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082–9. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 5.Laird JR. Limitations of percutaneous transluminal angioplasty and stenting for the treatment of disease of the superficial femoral and popliteal arteries. J Endovasc Ther. 2006;13(Suppl 2):II30–40. doi: 10.1177/15266028060130S207. [DOI] [PubMed] [Google Scholar]
- 6.Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc Interv. 2011;4:1057–66. doi: 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 8.Rotmans JI, Heyligers JM, Verhagen HJ, Velema E, Nagtegaal MM, de Kleijn DP, et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112:12–8. doi: 10.1161/CIRCULATIONAHA.104.504407. [DOI] [PubMed] [Google Scholar]
- 9.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–6. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–72. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 11.Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, et al. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res. 2006;99:617–25. doi: 10.1161/01.RES.0000243210.79654.fd. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–90. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 13.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–9. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 14.Langwieser N, Schwarz JB, Reichenbacher C, Stemmer B, Massberg S, Langwieser NN, et al. Role of bone marrow-derived cells in the genetic control of restenosis. Arterioscler Thromb Vasc Biol. 2009;29:1551–7. doi: 10.1161/ATVBAHA.109.188326. [DOI] [PubMed] [Google Scholar]
- 15.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, et al. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–8. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schober A, Hoffmann R, Opree N, Knarren S, Iofina E, Hutschenreuter G, et al. Peripheral CD34+ cells and the risk of instent restenosis in patients with coronary heart disease. Am J Cardiol. 2005;96:1116–22. doi: 10.1016/j.amjcard.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia F, Cianfrocca C, Rosano G, Mercuro G, Speciale G, Pasceri V. Role of endothelial progenitor cells in restenosis and progression of coronary atherosclerosis after percutaneous coronary intervention: a prospective study. JACC Cardiovasc Interv. 2010;3:78–86. doi: 10.1016/j.jcin.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–91. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 19.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–60. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 20.Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009;49:502–10. doi: 10.1016/j.jvs.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porada CD, Harrison-Findik DD, Sanada C, Valiente V, Thain D, Simmons PJ, et al. Development and characterization of a novel CD34 monoclonal antibody that identifies sheep hematopoietic stem/progenitor cells. Exp Hematol. 2008;36:1739–49. doi: 10.1016/j.exphem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkawi LS, Tam YY, Tillman JA, Pederson B, Calio J, Al-Amier H, et al. A high-throughput method for the quantitative analysis of indole-3-acetic acid and other auxins from plant tissue. Anal Biochem. 2008;372:177–88. doi: 10.1016/j.ab.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Tillman BW, Yazdani SK, Geary RL, Corriere MA, Atala A, Yoo JJ. Efficient recovery of endothelial progenitors for clinical translation. Tissue Eng Part C Methods. 2009;15:213–21. doi: 10.1089/ten.tec.2008.0416. [DOI] [PubMed] [Google Scholar]
- 24.Kohler TR, Kirkman TR. Dialysis access failure: a sheep model of rapid stenosis. J Vasc Surg. 1999;30:744–51. doi: 10.1016/s0741-5214(99)70114-x. [DOI] [PubMed] [Google Scholar]
- 25.Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neo-intimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable vascular wrap paclitaxel-eluting mesh. J Vasc Surg. 2007;45:1029–37. doi: 10.1016/j.jvs.2007.01.057. discussion: 1037–8. [DOI] [PubMed] [Google Scholar]
- 26.Tillman BW, Yazdani SK, Neff LP, Corriere MA, Christ GJ, Soker S, et al. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg. 2012;56:783–93. doi: 10.1016/j.jvs.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–40. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yazdani SK, Tillman BW, Berry JL, Soker S, Geary RL. The fate of an endothelium layer after preconditioning. J Vasc Surg. 2010;51:174–83. doi: 10.1016/j.jvs.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 29.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 30.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 31.Simper D, Mayr U, Urbich C, Zampetaki A, Prokopi M, Didangelos A, et al. Comparative proteomics profiling reveals role of smooth muscle progenitors in extracellular matrix production. Arterioscler Thromb Vasc Biol. 2010;30:1325–32. doi: 10.1161/ATVBAHA.110.204651. [DOI] [PubMed] [Google Scholar]
- 32.Juthier F, Vincentelli A, Gaudric J, Corseaux D, Fouquet O, Calet C, et al. Decellularized heart valve as a scaffold for in vivo recellularization: deleterious effects of granulocyte colony-stimulating factor. J Thorac Cardiovasc Surg. 2006;131:843–52. doi: 10.1016/j.jtcvs.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Inoue T, Sata M, Hikichi Y, Sohma R, Fukuda D, Uchida T, et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation. 2007;115:553–61. doi: 10.1161/CIRCULATIONAHA.106.621714. [DOI] [PubMed] [Google Scholar]
- 34.Madeddu P, Emanueli C, Pelosi E, Salis MB, Cerio AM, Bonanno G, et al. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004;18:1737–9. doi: 10.1096/fj.04-2192fje. [DOI] [PubMed] [Google Scholar]
- 35.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–77. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 36.Konkle BA. Acquired disorders of platelet function. Hematology Am Soc Hematol Educ Program. 2011;2011:391–6. doi: 10.1182/asheducation-2011.1.391. [DOI] [PubMed] [Google Scholar]
- 37.Meyer AD, Wiles AA, Rivera O, Wong EC, Freishtat RJ, Rais-Bahrami K, et al. Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med. 2012;13:e255–61. doi: 10.1097/PCC.0b013e31823c98ef. [DOI] [PMC free article] [PubMed] [Google Scholar]