Abstract
Serial EBV load monitoring of clinically asymptomatic pediatric thoracic organ transplant patients has identified three groups of children who exhibit undetectable (<100 copies/ml), chronic low (100–16,000 copies/ml), or chronic high (>16,000 copies/ml) EBV loads in peripheral blood. Chronic high EBV load patients have a 45% rate of progression to late-onset posttransplant lympho-proliferative disorders. In this article, we report that asymptomatic patients carrying EBV loads (low and high) expressed increased frequencies of EBV-specific CD8+ T cells, as compared with patients with undetectable EBV loads. Although patients with low viral load displayed EBV-specific CD8+ T cells with moderate signs of activation (CD38+/−/CD127+/−), programmed death 1 upregulation and effective IFN-γ secretion, high EBV load carriers showed significant CD38+ upregulation, features of cellular exhaustion (programmed death 1+/CD127−) accompanied by a decline in IFN-γ release. Immunopolarization of EBV-specific CD8+ T cells was skewed from the expected type 1 (IFN-γ) toward type 0 (IFN-γ/IL-5) in patients, and Tr1 (IL-10) in high load carriers. These results indicate the importance of chronic EBV load and of the levels of antigenic pressure in shaping EBV-specific memory CD8+ T cells. Concomitant phenotypic and functional EBV monitoring is critical for identifying the complex “functional” versus “exhausted” signature of EBV-specific CD8+ T cells, with implications for immunologic monitoring in the clinic.
Epstein–Barr virus is a γ-herpes virus that infects >90% of healthy adults worldwide (1, 2). The virus persists in the B lymphocyte pool as a lifelong asymptomatic latent infection that can undergo occasional reactivations into the lytic state with production of infectious virions (viral recrudescence) (1, 2). EBV infection triggers effective memory type 1 (IFN-γ, TNF-α, cytotoxicity) T cell responses specific for both EBV-lytic and -latent Ags that maintain the virus under tight immunologic control with no detectable viral load in the peripheral circulation in most healthy individuals (3, 4). Although CD4+ T cells provide “help” during priming and memory reactivation of antiviral CD8+ T cells, the latter compartment plays the pivotal protective role in EBV immunosurveillance against episodic lytic viral reactivation, as well as for latent infection control (5–8).
In acquired immunodeficiencies, such as posttransplant (post-Tx) immunosuppression, EBV T cellular immune surveillance is impaired, resulting in increased incidence of EBV-associated posttransplant lymphomas (PTLDs) with high mortality (9–13). Pediatric patients are at greatest risk for EBV complications among Tx patients, especially when they are EBV− pre-Tx (lacking EBV memory T cells) and become EBV+ post-Tx under immunosuppression (11, 13). Serial long-term EBV monitoring by PCR in the peripheral blood of pediatric thoracic Tx patients who EBV seroconverted after transplantation has identified three groups of clinically asymptomatic children: ~30% who exhibit undetectable (<100 copies/ml) EBV loads (UVL), resembling “normal” EBV latency; approximately 50% display persistent low (100–16,000 copies/ml) viral loads (LVL), whereas ~20% show persistent, dangerously high (>16,000 copies/ml) EBV loads (HVL) in peripheral blood for months to years after primary post-Tx EBV infection, a status not encountered in immunocompetent subjects (14, 15). We have recently shown that asymptomatic HVL carriers had a 45% risk for progression to aggressive late-onset PTLD, including diffuse large B cell, Hodgkin’s, and Burkitt’s lymphomas (16). Characterization of CD8+ T cells profiles and of their dynamics in asymptomatic pediatric Tx patients should clarify, at least in part, the immunopathogenesis that associates with the shift from EBV latency to the persistent HVL carrier state, which may help clinicians develop better prophylactic strategies.
Hierarchical and progressive loss of type 1 CD8+ T cell function is a hallmark of T cell exhaustion during chronic viral infections in mice and humans, with IFN-γ production being the last function lost (17, 18). The programmed death 1 (PD-1) molecule, a member of the CD28/B7 family, has been shown to critically regulate T cell activation by delivering inhibitory signals that suppress TCR signaling and function (19). During chronic LCMV infection in mice and HIV and HCV infections in humans, PD-1 is upregulated on CD8+ T cells and is instrumental in inhibiting virus-specific CD8+ T cell proliferation, cytokine production, and cytolytic activity, leading to cellular exhaustion (20–23). However, recent studies in HIV and HCV patients have concluded that a PD-1 upregulation on CD8+ T cells can be either a sign of physiologic T cell activation during viral infection control or can associate with T cell exhaustion (24, 25). Therefore, other molecules in addition to PD-1 should be monitored to accurately identify the Ag-specific CD8+ T cell status. For example, CD127 (IL-7Rα) is an important receptor for T cell survival and memory differentiation, and is shown to significantly downmodulate during persistent HVL infections and to inversely correlate with the state of CD8+ T cell exhaustion (26–28). CTLA-4 is yet another inhibitory molecule that can be upregulated on T cells during chronic viral infections, but its contribution to CD8+ T cell exhaustion is less well understood (21, 29).
Although the expression of PD-1 has been documented on EBV-specific CD8+ T cells from subjects undergoing acute infectious mononucleosis and during resolution (30), as well as from HIV-and HCV-infected subjects (23, 31), no studies were performed on EBV-specific CD8+ T cells from asymptomatic pediatric thoracic Tx patients to clarify the significance of chronic EBV load, PD-1 upregulation, and CD8+ T cell functional state. In addition, most of the previous immunologic studies in Tx patients monitored few parameters at a time (7, 8), which may be insufficient for an accurate understanding of the complex Ag-specific CD8+ T cell differentiation state at a given time. In this study, we have used tetramer (TMR) technology to detect EBV-specific CD8+ T cells, concomitantly incorporating several memory (CD45RA, CD62L) and activation/differentiation (CD38, CD127, PD-1) markers in flow cytometry. We have also monitored the EBV-specific functional (IFN-γ, IL-5, and IL-10) parameters for a comprehensive definition of immunologic “signatures” in these asymptomatic pediatric Tx patients in relation to their EBV load levels.
Our results indicate that chronic EBV-Ag–specific stimulation (LVLs and HVLs) is associated with increased CD8+ T cell frequencies that display heterogeneous phenotypic and functional features with direct implications for immunological monitoring in the clinical setting. In addition, we have found that EBV-specific CD8+ T cells are alternatively polarized (decreased IFN-γ/IL-5 ratio) in children, a state that may contribute, in addition to immunosuppression and other yet unknown factors, to EBV reactivation and viral load accumulation in pediatric Tx patients.
Materials and Methods
Human subjects and PBMC isolation
Forty-four asymptomatic pediatric thoracic Tx patients and 11 healthy control (HC) volunteers were consented under Institutional Review Board-approved protocols at the University of Pittsburgh and analyzed in a cross-sectional study with additional longitudinal observations in 70% of patients over a period of 3 y. Patient demographics and immunosuppressive regimens are shown in Table I. All patients were EBV+ at the time of blood donation, as confirmed by serology, and were also positive for HLA-A02 and/or HLA-B08 allele. Patients were divided into three groups according to their peripheral blood EBV loads determined by PCR, regardless of their pre-Tx EBV status (see below). Maintenance immunosuppression was comparable for all three cohorts of patients, and consisted of a combination of a calcineurin inhibitor (tacrolimus or cyclosporine) and an antiproliferative agent (mycophenolate mofetil) with or without the use of low-dose prednisone. Nine patients received induction therapy with polyclonal anti-T cell Abs (Thymoglobulin or ATGAM). Five to ten milliliters of heparinized whole blood was collected from each subject and used directly in flow cytometry analysis, whereas PBMCs were isolated by density gradient centrifugation (32) and banked frozen for functional assays.
Table I.
Patient demographics
| UVL (n = 9) | Chronic LVL (n = 21) | Chronic HVL (n = 14) | HC (n = 11) | |
|---|---|---|---|---|
| Type of Tx | 9 heart | 20 heart, 1 lung | 9 heart, 4 lung, 1 heart/lung | NA |
| Mean time post-Tx ± SD, y | 5.4 ± 4.5 | 8.2 ± 3.9 | 4.2 ± 3.0 | NA |
| Mean age ± SD, y | 8.3 ± 5.2 | 13.4 ± 4.2 | 10.8 ± 5.9 | 42.8 ± 7.0 |
| Sex, M/F | 4/5 | 12/9 | 7/7 | 4/7 |
| EBV load, genome copies/ml (range) | Undetectable | 100–13,000 | 22,000–510,000 | Undetectable |
| HLA | -A02(8)–B08(2) | -A02(17)–B08(7) | -A02(14)–B08(3) | -A02(9)–B08(3) |
| Induction therapy | 11% | 5% | 50% | NA |
| EBV status pre-Txa | 89% seronegative | 52% seronegative | 86% seronegative | NA |
| IS regimen | 44% CNI | 29% CNI | 28% CNI | |
| 22% CNI/MMF | 29% CNI/MMF | 29% CNI/MMF | ||
| 33% CNI/MMF/Pred | 18% CNI/Pred | 7% CNI/Pred | ||
| 24% CNI/MMF/Pred | 36% CNI/MMF/Pred |
All patients were EBV seropositive at time of analysis.
CNI, calcineurin inhibitor; IS, immunosuppressive; MMF, mycophenolate mofetil; NA, nonapplicable; Pred, prednisone.
Definition of EBV load groups
Patients were categorized into three groups according to their peripheral whole blood EBV load levels as follows: 1) UVL carriers with no EBV load detected by PCR in >80% of determinations including the time of analysis; 2) chronic LVL carriers with EBV loads ranging between 100 and 16,000 genomic copies/ml blood, detected in >20% of measurements, including the time of analysis; and 3) chronic HVL carriers with EBV loads >16,000 genomic copies/ml blood on at least 50% of determinations, and over a period of at least 6 mo before the current immunologic analyses (33).
Synthetic peptides and HLA class I/peptide TMRs
EBV-derived peptides were synthesized at the Peptide Synthesis Facility at University of Pittsburgh: 1) BMLF1 (GLCTLVAML)-lytic cycle and LMP2a (CLGGLLTMV)-latent cycle peptides restricted by HLA-A02+; and 2) BZLF1 (RAKFKQLL)-lytic cycle and EBNA3A (FLRGRAYGL)-latent cycle peptides restricted by HLA-B08+. These peptides were used to stimulate EBV-specific CD8+ T cells in ELISPOT assays at a final concentration of 10 μg/ml. (PE)-HLA-A02+ TMRs incorporating HLA-A02–restricted peptides were purchased from Beckman Coulter (Fullerton, CA), whereas (PE)-HLA-B08+ TMRs incorporating HLA-B08–restricted peptides were generated at the National Institute of Allergy and Infectious Diseases MHC-Tetramer Core Facility (Emory University, Atlanta, GA).
Media and reagents
RPMI 1640 was supplemented with 2 mM L-glutamine, 10 mM HEPES, 100 IU/ml penicillin/streptomycin, and 10% heat-inactivated FCS (all from Life Technologies BRL, Grand Island, NJ) and referred to as complete media. BSA, PMA, and ionomycin were purchased from Sigma (St. Louis, MO).
Flow cytometry
Whole blood (100 μl/tube) was incubated with a mixture of mAbs including CD62L-FITC (Beckman Coulter), CD45RO-FITC, CD45RA-allo-phycocyanin, CD8-PerCp, CD38-PE-Cy7, CD152-allophycocyanin (BD, San Jose, CA), CD127-Pacific blue, PD-1–FITC (eBioscience, San Diego, CA), and a given EBV-specific TMR for 30 min in the dark at room temperature. Isotype controls and a negative TMR (Beckman Coulter) were used as negative control. Cells were then incubated with 2 ml/tube of 1X lysing buffer (BD) for an additional 10 min at room temperature to allow RBCs lysis. Tubes were washed twice and fixed with 1% paraformaldehyde. All events were collected using an LSR II (BD) flow cytometer and analyzed with Diva software (BD) or FlowJo (Tree Star, Ashland, OR).
IFN-γ and IL-5 ELISPOT assays
ELISPOT assays were performed as previously described (34). In brief, 96-well plates (Millipore, Bedford, MA) were precoated with anti-human IFN-γ (10 μg/ml; Mabtech, Sweden) or anti-human IL-5 (10 μg/ml; BD Pharmingen) mAbs in PBS (Cellgro, Herndon, VA) overnight at 4°C. Plates were washed and PBMCs were seeded at 3 × 105/well with correspondent EBV-peptide (10 μg/ml) at 37°C and 5% CO2 for 24 (IFN-γ) and 48 h (IL-5). PMA (5 ng/ml) plus ionomycin (100 ng/ml) was used as positive control (1 × 105cells/well). The wells were washed, and a secondary biotinylated anti-human IFN-γ mAb (2 μg/ml; Mabtech) or bio-tinylated anti-human IL-5 (2 μg/ml; BD Pharmingen) mAbs were added for an additional 2 or 4 h, respectively, at 37°C. The reaction was developed with 3-amino-9-ethylcarbazole substrate (Sigma) for IFN-γ and tetramethylbenzidine substrate (KPL Laboratories, Gaithersburg, MD) for IL-5. Spots were counted with an ELISPOT plate reader (Immunospot; Cellular Technology, Cleveland, OH).
Determination of IL-10 levels by ELISA
Culture supernatants from ELISPOT assays were collected and levels of IL-10 quantified by ELISA. Primary and secondary mAbs, and the recombinant cytokine for IL-10 ELISA were purchased from Pierce Endogen (Rockford, IL). The lower limits of detection for this assay were 60 pg/ml.
Statistical analysis
The distributions of all laboratory variables were examined, and the logarithm transformation was applied when data were approximately log-normally distributed. For analyses involving one observation per patient, two-tailed Student t tests were used to compare mean values between viral load groups. When multiple observations were available per patient, generalized estimating equations models with empirical SEs were used to estimate mean levels for Ag measures by group controlling for the correlation among measures from the same patient. The significance (p values) of the global hypothesis test for any differences among all viral load groups and the significance of each pairwise test were derived from these models. Generalized estimating equations linear regression models with empirical SE estimates were also used to analyze the association between viral load (coded as a continuous variable), lytic and latent Ag levels, and functional outcome measures controlling for within patient correlation of observations. The p values ≤0.05 were considered statistically significant.
Results
Characterization of memory CD8+ T cell phenotypes in peripheral blood of pediatric thoracic organ Tx patients
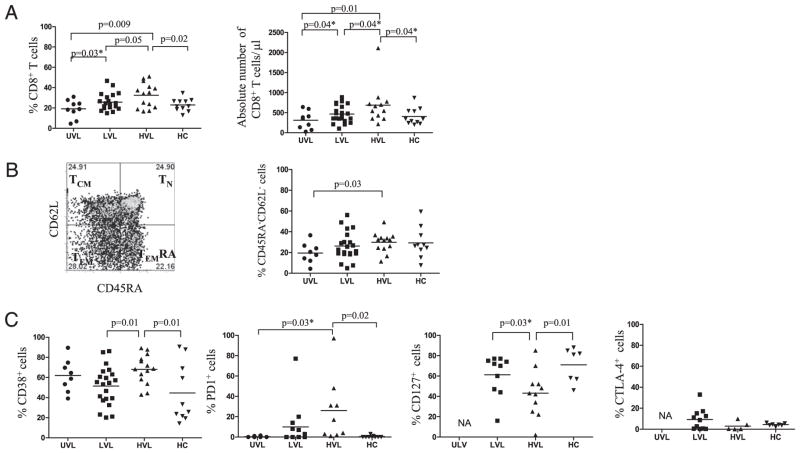
Chronic antigenic stimulation by latent viruses has profound effects on the phenotype and function of memory CD8+ T cells (35). Therefore, we decided to investigate the overall frequency, memory, and activation phenotypes of CD8+ T cells from our patients. We considered UVL patients as controls for pediatric LVL and HVL Tx patients, who were similar for sex, age, and immunosuppression, but did not carry EBV loads in peripheral blood. We also considered latently infected HC volunteers as controls, to further illustrate differences between memory responses to EBV in children and adults. Fig. 1A shows the overall frequency (%) and absolute numbers of CD8+ T cells in peripheral blood for each cohort. Patients carrying an EBV load (LVL and HVL) presented significantly higher frequencies (LVL: 26 ± 8; HVL: 33 ± 12%) and absolute numbers (LVL: 500 ± 200 cells/μl; HVL: 700 ± 500 cells/μl) of circulating CD8+ T cells as compared with UVL (frequency, 19 ± 9%; absolute number, 300 ± 200 cells/μl). HVL patients displayed the highest percentage of CD8+ T cells among groups, and presented with a significantly greater proportion of T effector memory (TEM: CD45RA−CD62L−) CD8+ T cells as compared with UVL patients (Fig. 1B). In addition, CD8+ T cells from HVL carriers also expressed the highest levels of CD38 and PD-1 expression with concomitant downregulation of CD127 (Fig. 1C). CTLA-4 was not selectively upregulated on CD8+ T cells from HVL carriers (Fig. 1C). Taken together, these results suggest that high EBV antigenic burden present in the asymptomatic HVL pediatric Tx recipients has triggered a bystander increase in the frequency of CD8+ T cells that show signs of T cell activation (CD38+/CD45RA−/CD62L−) and exhaustion (PD-1+/CD127−). Conversely, the lack of constant EBV-Ag challenge seen with UVL patients resulted in CD8+ T cells with quiescent phenotypes. LVL patients and HCs displayed intermediate signs of CD8+ T cell activation.
FIGURE 1.
Frequency and activation/memory phenotypes of CD8+ T cells in peripheral blood. A, Frequency (%) and absolute numbers of CD8+ T cells in PBLs assessed from pediatric Tx patients and HCs. B, Dot plots presenting the CD8+ TN (naive), TCM, TEMRA, and TEM, and total percentage of CD8+ T cells with CD45RA−CD62L− (TEM) phenotype analyzed in PBLs from pediatric Tx patients and HCs. C, CD8+ T cell expression of CD38, PD-1, CD127, and CTLA-4 in PBLs from pediatric Tx patients and HCs. *p ≤ 0.05, one-tailed t test.
Frequency of EBV-specific CD8+ T cells in peripheral blood of asymptomatic pediatric thoracic organ Tx patients
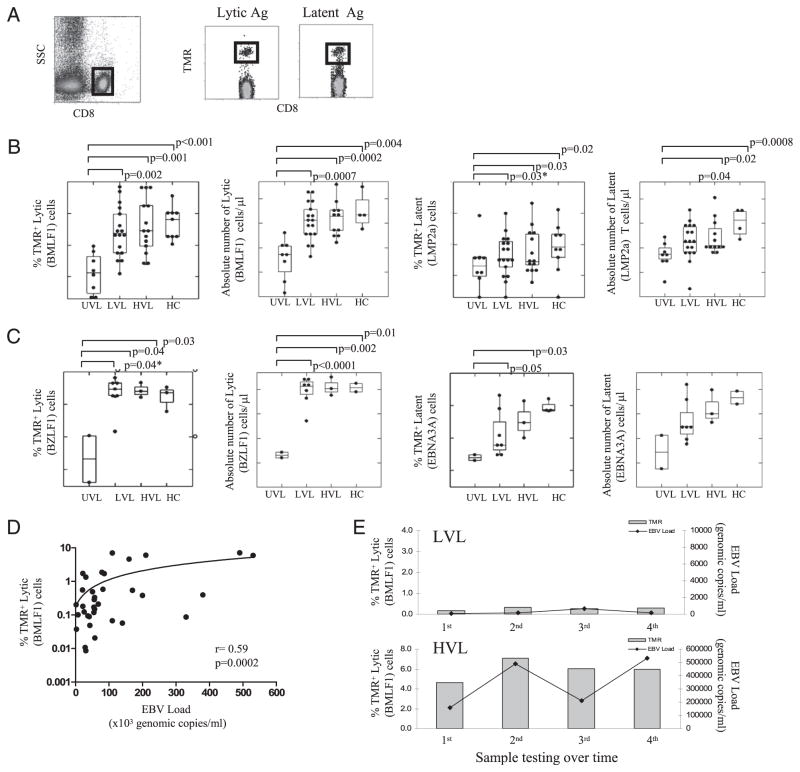
We next investigated the overall frequency of EBV-specific CD8+ T cells from peripheral blood of asymptomatic Tx patients carrying EBV loads (LVL and HVL) as compared with UVL and HCs. Fig. 2A shows the gating strategy for EBV-specific CD8+ T cell identification. We have used flow cytometry with TMR probes specific for EBV-lytic (BMLF1, BZLF1) or -latent (LMP2a, EBNA3A) epitopes presented on HLA-A02+ (Fig. 2B) or HLA-B08+ allele (Fig. 2C) to identify the frequencies (%) and absolute numbers of EBV-specific CD8+ T cells detected from each subject. Although HLA-B08+ allele is less frequent (~10%) in the general population as compared with HLA-A02 (~45%), the purpose of analyzing HLA-A02+ and HLA-B08+ patients was to demonstrate whether the patterns of lytic- or latent-specific CD8+ T cell responses are similar across MHC class I restriction elements. Overall, both LVL and HVL pediatric Tx patients displayed significantly larger populations (% and absolute numbers) of circulating EBV-lytic and -latent specific CD8+ T cells as compared with UVL carriers, in whom the TMR+ cells were barely detectable by flow cytometry (Fig. 2B, 2C). This finding supports the role of Ag stimulation in triggering the Ag-specific T cell clonal expansion. Interestingly, EBV lytic-specific TMR+ cells were more frequent than the latent-specific TMR+ cells for all groups tested, suggesting a more active lytic viral stimulation than latent antigenic challenge (Fig. 2B, 2C). A significant direct correlation between the EBV load and percentage of EBV-lytic TMR+ cells was found over time in HVL patients (Fig. 2D). In addition, LVL and HVL carriers displayed comparable high levels of EBV-lytic–specific CD8+ T cells to HCs. We speculate that HCs displayed high EBV-lytic–specific CD8+ T cell frequencies, despite undetectable circulating EBV loads at the time of analysis, because of progressive CD8+ T cell clonal inflation and accumulation in response to occasional Ag challenge throughout life (36). In addition, >70% of participants were analyzed longitudinally over the period of this study to determine the stability of EBV-specific CD8+ T cell frequencies. Results showed that TMR frequencies remained consistent for each EBV-epitope over the study period, for all asymptomatic patients carrying a stable EBV load (Fig. 2E).
FIGURE 2.
Frequency of EBV-specific CD8+ T cells in peripheral blood. A, Gating strategy to identify the EBV (TMR)-specific CD8+ T cells in peripheral blood. B, Frequency (%) and absolute counts of HLA-A02–restricted, CD8+ T cells specific for lytic (BMLF1) and latent (LMP2a) epitopes. C, Frequency (%) and absolute counts of HLA-B08–restricted, CD8+ T cells specific for lytic (BZLF1) and latent (EBNA3A) epitopes. Data in B and C are represented as Tukey box plots, where the boxes indicate median and quartile values. Each dot represents the mean value of the frequencies of all EBV-specific CD8+ T cells determinations for a given subject. D, Correlation between EBV loads and the frequency of HLA-A02–restricted EBV lytic (BMLF1)-specific CD8+ T cells. Each dot represents the value obtained from one determination in one patient at a time. E, Follow-up analysis of the EBV load and the frequency of EBV-lytic (BMLF1)-specific CD8+ T cells in peripheral blood of representative HLA-A02+ LVL (top graph) and HVL (bottom graph) pediatric Tx patients. In each case, the measurements were performed at 6 mo intervals of each other. *p ≤ 0.05, one-tailed t test.
Activation/differentiation memory phenotypes of EBV-specific CD8+ T cells in peripheral blood of asymptomatic pediatric thoracic organ Tx patients
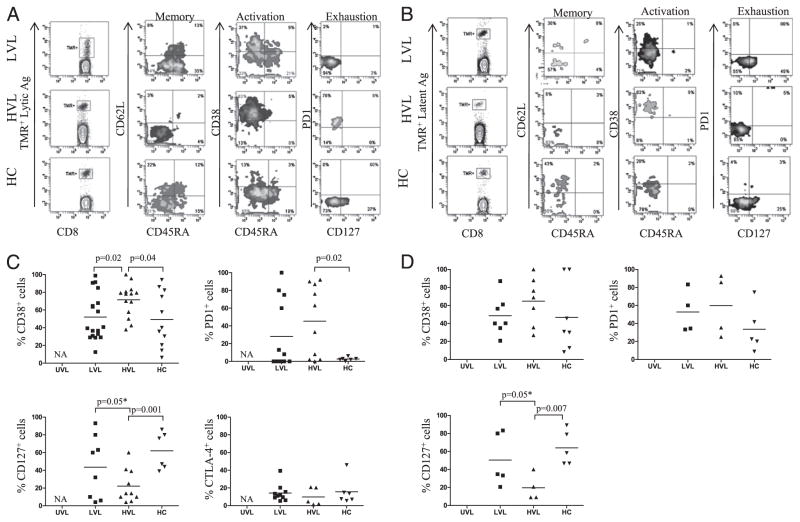
We next characterized the memory phenotypes of EBV-specific (TMR+) CD8+ T cells from different cohorts of asymptomatic Tx patients and HCs. As illustrated in Fig. 3, there were significant differences in the levels and patterns of memory subsets and of activation/differentiation status, depending on the level of EBV load (LVL versus HVL) and on the source of EBV-Ag (lytic versus latent). Representative staining patterns of EBV-specific CD8+ T cells are shown in Fig. 3A (lytic) and 3B (latent), whereas overall data are shown in Fig. 3C (lytic) and 3D (latent). For most patients, the EBV-lytic–specific CD8+ T cells were divided between TEM and terminally differentiated effector memory (TEMRA: CD45RA+CD62L−) (Fig. 3A), whereas EBV-latent–specific CD8+ T cells were divided between central memory (TCM: CD45RA−CD62L+) and TEM (Fig. 3B). These patterns were also detected in HCs (Fig. 3A, 3B), and confirm the previously described relation between EBV-epitope source and EBV-specific (TMR+) CD8+ T cell’s distinct ability to home in the periphery (TEM, TEMRA) or in the secondary lymphatic organs (TCM), and to function (TEM versus TCM) (3, 8, 34). In addition, CD38 and CD127 molecules were expressed at intermediate levels on both EBV lytic- and latent-specific CD8+ T cells from LVL patients (and HCs), a reflection of their moderate EBV-antigenic challenge and quiescent status (Fig. 3C, 3D). EBV-specific CD8+ T cells from HVL carriers expressed significant greater levels of CD38 as compared with LVL patients and HCs, significantly increased PD-1, and significantly lower levels of CD127 as compared with HCs (Fig. 3C, 3D). Moreover, some HVL pediatric Tx patients displayed uniformly EBV lytic- and latent-specific CD8+ T cells with effector (CD45RA−CD62L−) phenotypes, CD38+, PD-1+, and CD127−, indicative of acute signs of EBV-antigenic challenge (Fig. 3A, 3B). Interestingly, CTLA-4 was not selectively upregulated on EBV-specific CD8+ T cells from patients or HCs (Fig. 3C). No data could be recorded for EBV-specific CD8+ T cells from UVL patients, because the frequencies of TMR+ cells in this cohort were at the limit of detection of the technique. Taken together, these results show that asymptomatic pediatric Tx patients that carry an EBV load are phenotypically heterogeneous, and that the level of EBV antigenic pressure is important for shaping EBV-specific memory/activation and differentiation CD8+ T cell status.
FIGURE 3.
Activation/memory phenotypes of EBV-specific memory CD8+ T cells in peripheral blood. Peripheral blood from representative HLA-A02+ pediatric Tx patients and HCs was stained to detect either EBV-lytic–specific CD8+ (A) T cells or EBV-latent–specific (B) CD8+ T cells, and further analyzed for their memory phenotype distribution and for expression of CD38, CD127, and PD-1. C, Overall expression of CD38, PD-1, CD127, and CTLA-4 on EBV-lytic (BMLF1)-specific CD8+ T cells. D, Overall expression of CD38, PD-1, and CD127 on EBV-latent (LMP2a)–specific CD8+ T cells. *p ≤ 0.05, one-tailed t test.
Frequency of functional (IFN-γ+) EBV-specific memory CD8+ T cells in peripheral blood
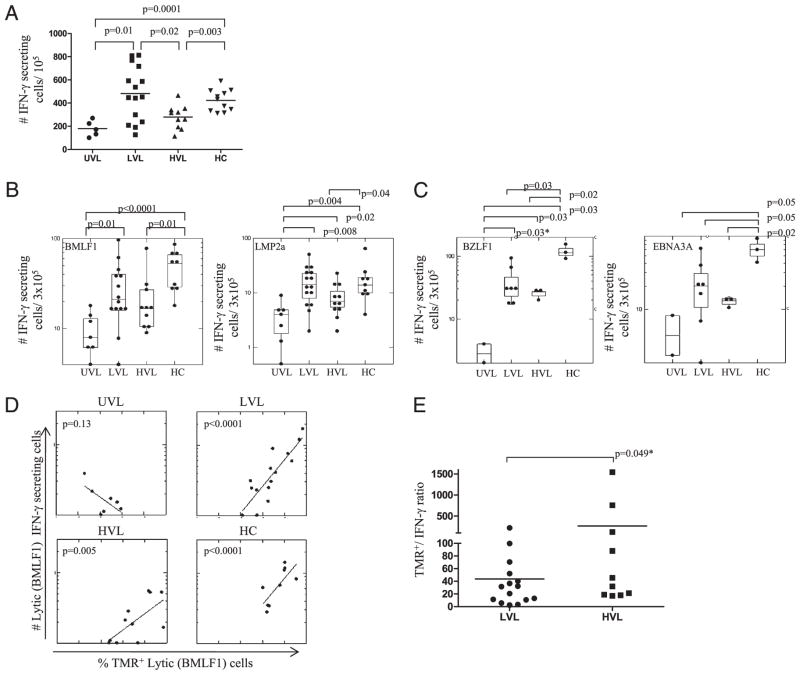
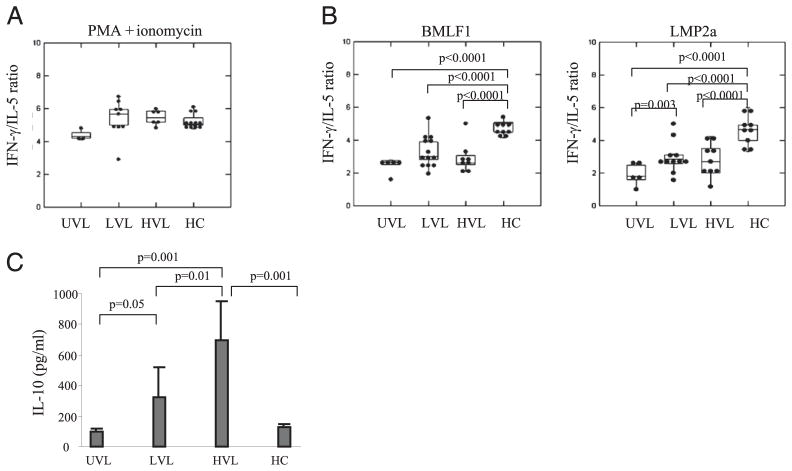
We next inquired whether the differences in the levels of EBV load have functional consequences. The overall frequency of IFN-γ–producing cells in response to nonspecific PMA+ionomycin stimulation (Fig. 4A) and to EBV-specific peptide stimulation (Fig. 4B, 4C) was significantly higher in LVL pediatric Tx patients than in UVL. Although the EBV-specific CD8+ T cell absolute numbers in LVL and HVL were comparable (Fig. 2B, 2C), HVL carriers showed significantly lower levels of IFN-γ–producing cells in response to PMA+ionomycin (Fig. 4A), and a decline in IFN-γ responses against EBV lytic- and latent-specific stimulation, as compared with LVL carriers (Fig. 4B, 4C). These data confirm the phenotypic findings and suggest CD8+ T cell functional “exhaustion” in HVL carriers. In addition, HCs displayed strong IFN-γ responses to nonspecific and to EBV-specific stimulation, comparable with LVL pediatric Tx patients, and significant higher than HVL (Fig. 4A–4C), confirming their functionality. There was a highly significant direct correlation between the percentage of EBV-lytic–specific TMR+ cells and EBV-lytic–specific IFN-γ+ cells from LVL patients and HCs, whereas the correlation was less significant for HVL carriers (Fig. 4D). No correlation was observed in UVL carriers. In addition, the ratios between the absolute numbers of lytic-specific TMR+ to absolute numbers of lytic-specific IFN-γ+ were higher for HVL carriers (266 ± 502) compared with LVL carriers (43 ± 56) (Fig. 4E), indicating less functional (IFN-γ+) cells among TMR+ CD8+ T cells for HVL carriers.
FIGURE 4.
Frequency of functional (IFN-γ+) EBV-specific memory CD8+ T cells in peripheral blood. A, The number of IFN-γ–secreting cells/105 PBMCs in response of PMA+ionomycin stimulation from pediatric Tx patients and HCs. B, The number of IFN-γ–secreting cells/3 × 105 PBMCs in response HLA-A02–restricted EBV-lytic– (BMLF1) and -latent (LMP2a)-specific peptides, or (C) HLA-B08–restricted EBV-lytic (BZLF1) and -latent (EBNA3A)-specific peptide stimulation. D, Correlation between EBV-lytic (BMLF1)-specific CD8+ T cells and the frequency of IFN-γ–producing EBV-lytic (BMLF1)-specific CD8+ T cells in response to peptide stimulation in peripheral blood of pediatric Tx patients and HCs. E, Ratio between absolute numbers of EBV-lytic (BMLF1) TMR+ and IFN-γ+. Each dot in B–E represents the mean value of the determinations for a given subject. *p ≤ 0.05, one-tailed t test.
Immunopolarization of memory EBV-specific CD8+ T cell in peripheral blood of asymptomatic pediatric thoracic organ Tx patients
It is well established that type 1 (IFN-γ) CD8+ T cells are essential for an adequate anti-EBV control, whereas type 2 (IL-4, IL-5) or Tr1 (IL-10) CD8+ T cells may be supportive of viral complications. Therefore, we assessed the immunopolarization of circulating CD8+ T cells from asymptomatic pediatric Tx patients and HCs by calculating the ratio between IFN-γ– and IL-5–producing cells, and by measuring IL-10 production. After a short in vitro exposure to PMA+ionomycin (Fig. 5A), memory CD8+ T cells from asymptomatic pediatric Tx patients displayed a true type 1 immunopolarization with a high IFN-γ/IL-5 ratio (5:1), regardless of their EBV load, and similar to the polarization of memory CD8+ T cells from HCs who are known to display type 1 polarization (7, 8). Interestingly, however, after in vitro exposure to EBV-lytic– or EBV-latent–specific peptide stimulation, memory CD8+ T cells from asymptomatic pediatric Tx patients displayed a skewed type 0 immunopolarization (IFN-γ/IL-5 ratio: 2.5:1), whereas HVL carriers also showed increased IL-10 production (Fig. 5B, 5C). These results were significantly different from those obtained with HCs who maintained a strong type 1 polarization (IFN-γ/IL-5 ratio: 5:1) and low IL-10 in response to EBV stimulation (Fig. 5B, 5C). Of note, all three cohorts of Tx patients were on similar immunosuppressive regimens (Table I). Together, these results indicate a biased immunopolarization of EBV-specific CD8+ T cells in pediatric Tx patients as compared with type 1 immunopolarization seen in HCs, which may be detrimental for effective EBV control. In addition, increased IL-10 production by EBV-specific CD8+ T cells in response to EBV-peptide stimulation seen with HVL suggests induced Tr1 as a mechanism of cellular exhaustion.
FIGURE 5.
Immunopolarization of memory EBV-specific CD8+ T cells in peripheral blood. A, The ratio between the number of IFN-γ– and of IL-5–secreting cells from PBMCs in response to PMA+ionomycin stimulation from pediatric Tx patients and HCs. B, Ratio between the numbers of IFN-γ– and IL-5–secreting cells from PBMCs in response to EBV-lytic (BMLF1) or EBV-latent (LMP2a) stimulation from HLA-A02+ pediatric Tx patients and HCs. C, IL-10 secretion measured by ELISA in the supernatants harvested from IL-5 ELISPOT in response to EBV-lytic (BMLF1) stimulation from HLA-A02+ pediatric Tx patients and HCs.
Discussion
Our results have identified critical differences in the CD8+ T cell phenotype and function among asymptomatic pediatric thoracic Tx patients. LVL and HVL patients displayed significant expansion of bystander CD8+ T cells and of EBV-specific CD8+ T cells as compared with UVL carriers. We also found that EBV-lytic–specific CD8+ T cell responses were more frequent than EBV-latent–specific responses in both LVL and HVL carriers; this was confirmed for both HLA-A02+ and HLA-B08+ patients. Moreover, EBV load directly correlated with EBV-lytic–specific CD8+ T cells in HVL patients. Although our previous published data highlighted the accumulation of EBV latently infected B cells with high viral DNA copy number in HVL carriers (37, 38), our results in this study indicate for the first time, to our knowledge, that asymptomatic chronic HVL carrier state after pediatric thoracic organ transplantation may entail active lytic viral replication (identified by TMR staining) in addition to the accumulation of EBV latently infected cells in peripheral blood. Thus, future studies are required to clarify the nature of the life cycle of the virus in immunosuppressed children. A puzzling situation was offered by the UVL patients (~30% of asymptomatic pediatric Tx patients) who carried EBV infection in its latent form (no detectable viral loads) and did not present EBV-specific TMR+ cells in the peripheral blood. The lack of the chronic EBV load in this cohort may be independent of T cell control, but possibly depend on innate immune surveillance, an inherent lack of host B cell permissiveness for increased EBV replication, or other factors (e.g., genetic, virus related).
We have further identified differences in the activation/differentiation status of memory CD8+ T cells in patients carrying a viral load (Fig. 3) (26–28). EBV-specific CD8+ T cells from both LVL and HVL carriers upregulated PD-1, and this is in good agreement with previously published data that illustrate PD-1 up-regulation on either physiologic Ag stimulation (24) or chronic antigenic stimulation leading to functional exhaustion (21). EBV-specific CD8+ T cells from LVL displayed overall intermediate levels of CD127 and CD38, and retained potent IFN-γ, whereas HVL carriers showed significant CD38 upregulation, exhausted phenotypes (PD-1+/CD127−), and a decline in IFN-γ production (Figs. 3, 4). These data confirm Lang et al.’s (27) results describing an inverse correlation between IL-7Rα expression and CD8+ T cell exhaustion during persistent Ag stimulation, and also Cellerai et al.’s (39) data showing that CD127 (re)-expression is linked with CD8+ T cells functionality (proliferative capacity). In addition, Paul et al. (40) demonstrated that CD38 upregulation is a strong predictor for virus disease progression in HIV-infected individuals, whereas diminishment in CD38 indicates effective antiretroviral therapy in viremic HIV patients (41). Because HVL carriers in our study expressed significantly high levels of CD38, future studies are required to follow-up these patients and determine the predictive role of CD38 for progression to symptomatic disease (PTLD) in our patient population. Interestingly, a recent report by Crough et al. (42) has shown that CD38 upregulation is coincident with IFN-γ decline in CMV-specific CD8+ T cells from symptomatic CMV recrudescence in solid organ Tx patients (unlike our results, which showed increased CD38 and decline in IFN-γ in asymptomatic HVL carriers), whereas IFN-γ was preserved in asymptomatic CMV Tx patients with LVLs. We believe that the differences between our studies may reflect differences in the following factors: 1) life cycles of CMV versus EBV, 2) age of the patients studied, and 3) clinical/virologic criteria of diagnosis.
Interestingly, some patients in the LVL or HVL groups did not express PD-1 on their EBV-specific CD8+ T cells (Fig. 3C, 3D). This suggests either that PD-1 expression may have been transient or that another inhibitory pathway (e.g., CTLA-4, TIM3, 2B4, LAG3) may have been involved in these patients (29, 43–47). Our results demonstrate that CTLA-4 expression is not selectively upregulated on EBV-specific CD8+ T cells from HVL carriers (Fig. 3C) and endorse future studies that focus on other inhibitory molecules.
Our analyses have further revealed that pediatric patients presented with skewed EBV-specific immunopolarization from the expected type 1 (IFN-γ) to type 0 (IFN-γ/IL-5). Our data confirm previously published results that children, although in the process of building “healthy” type 1 immunity (by continuous exposure to viruses and bacteria), display an immature T cell immunopolarization (48, 49). The biased immune status of pediatric patients, further burdened by chronic immunosuppression (50), may create a milieu permissive for impaired antiviral surveillance and, therefore, may place pediatric Tx cohorts at higher risk for persistent viral complications. We postulate that the biased EBV immunopolarization observed specifically in pediatric Tx patients under immunosuppressive therapy may represent an independent confounding factor contributing to this switch from latency to chronic infection. In addition, HVL patients, although on similar immunosuppressive regimens as UVL and LVL carriers, displayed increased IL-10 release in response to EBV stimulation, suggesting EBV-specific CD8+ regulatory T cell induction as a mechanism leading to cellular exhaustion rather than anergy (51).
Earlier publications on persistent viral infections have concluded that, although functionally competent CD8+ T cells are critical for effective long-term control of viral latency with occasional recrudescence, they rely on CD4+ T help during priming and secondary expansion of memory CD8+ T cells (5, 6, 52). Conversely, in the absence of CD4+ T help, CD8+ T cells become progressively exhausted, allowing for the viral load to accumulate (35, 53). We have recently examined the CD4+ T cell immuno-competence in the same cohorts of asymptomatic pediatric Tx patients (54). We used the Cylex assay to measure CD4+ T cell ATP release in response to PHA stimulation. This test was previously shown to predict the risk for chronic viral complications in immunocompromised patients (55–57). Our published results revealed a significant decline in the CD4+ T immunocompetence and a significant inverse correlation between EBV load and CD4+ T cell ATP release in the HVL group, indicating the importance of CD4+ T cell help for the long-term maintenance of CD8+ T cell memory (54). Our findings provide a rational for future prospective multicenter studies focusing on asymptomatic HVL carriers and their long-term CD8+ and CD4+ T cell follow-up, and to study potential correlations among viral load, immunologic parameters, and their predictive role for development of PTLD.
Acknowledgments
We thank Dr. Angus W. Thomson for valuable review of the manuscript. We also acknowledge the National Institutes of Health Tetramer Facility (Emory University, Atlanta, GA) for generating the PE-HLA-B08-BZLF1 and -EBNA3A tetramers.
This work was supported by an SCCOR grant awarded by the National Heart, Lung, and Blood Institute (5 P50 HL074732-03).
Abbreviations used in this article
- HC
healthy control
- HVL
high viral load
- LVL
low viral load
- PD-1
programmed death 1
- PTLD
posttransplant lymphoma
- TCM
central memory T cell
- TEM
effector memory T cell
- TEMRA
terminally differentiated effector memory T cell
- TMR
tetramer
- Tx
transplant
- UVL
undetectable viral load
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Murray PG, Young LS. Epstein-Barr virus infection: basis of malignancy and potential for therapy. Expert Rev Mol Med. 2001;3:1–20. doi: 10.1017/S1462399401003842. [DOI] [PubMed] [Google Scholar]
- 2.Thorley-Lawson DA. EBV the prototypical human tumor virus—just how bad is it? J Allergy Clin Immunol. 2005;116:251–261. doi: 10.1016/j.jaci.2005.05.038. quiz 262. [DOI] [PubMed] [Google Scholar]
- 3.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MF. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 6.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 7.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 8.Catalina MD, Sullivan JL, Brody RM, Luzuriaga K. Phenotypic and functional heterogeneity of EBV epitope-specific CD8+ T cells. J Immunol. 2002;168:4184–4191. doi: 10.4049/jimmunol.168.8.4184. [DOI] [PubMed] [Google Scholar]
- 9.Snow AL, Martinez OM. Epstein-Barr virus: evasive maneuvers in the development of PTLD. Am J Transplant. 2007;7:271–277. doi: 10.1111/j.1600-6143.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- 10.Nalesnik MA. Clinical and pathological features of post-transplant lymphoproliferative disorders (PTLD) Springer Semin Immunopathol. 1998;20:325–342. doi: 10.1007/BF00838047. [DOI] [PubMed] [Google Scholar]
- 11.Webber SA, Naftel DC, Fricker FJ, Olesnevich P, Blume ED, Addonizio L, Kirklin JK, Canter CE Pediatric Heart Transplant Study. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367:233–239. doi: 10.1016/S0140-6736(06)67933-6. [DOI] [PubMed] [Google Scholar]
- 12.Green M, Webber S. Posttransplantation lymphoproliferative disorders. Pediatr Clin North Am. 2003;50:1471–1491. doi: 10.1016/s0031-3955(03)00127-5. [DOI] [PubMed] [Google Scholar]
- 13.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 14.Rose C, Green M, Webber S, Ellis D, Reyes J, Rowe D. Pediatric solid-organ transplant recipients carry chronic loads of Epstein-Barr virus exclusively in the immunoglobulin D-negative B-cell compartment. J Clin Microbiol. 2001;39:1407–1415. doi: 10.1128/JCM.39.4.1407-1415.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green M, Webber SA. EBV viral load monitoring: unanswered questions. Am J Transplant. 2002;2:894–895. doi: 10.1034/j.1600-6143.2002.21003.x. [DOI] [PubMed] [Google Scholar]
- 16.Bingler MA, Feingold B, Miller SA, Quivers E, Michaels MG, Green M, Wadowsky RM, Rowe DT, Webber SA. Chronic high Epstein-Barr viral load state and risk for late-onset posttransplant lymphoproliferative disease/lymphoma in children. Am J Transplant. 2008;8:442–445. doi: 10.1111/j.1600-6143.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 17.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 19.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 21.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 22.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann DE, Walker BD. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS. 2008;3:362–367. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 25.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, et al. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174:2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 27.Lang KS, Recher M, Navarini AA, Harris NL, Löhning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 30.Greenough TC, Campellone SC, Brody R, Jain S, Sanchez-Merino V, Somasundaran M, Luzuriaga K. Programmed death-1 expression on Epstein Barr virus specific CD8+ T cells varies by stage of infection, epitope specificity, and T-cell receptor usage. PLoS ONE. 2010;5:e12926. doi: 10.1371/journal.pone.0012926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen T, Zheng J, Xu C, Liu J, Zhang W, Lu F, Zhuang H. PD-1 expression on peripheral CD8+ TEM/TEMRA subsets closely correlated with HCV viral load in chronic hepatitis C patients. Virol J. 2010;7:310. doi: 10.1186/1743-422X-7-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bøyum A, Løvhaug D, Tresland L, Nordlie EM. Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand J Immunol. 1991;34:697–712. doi: 10.1111/j.1365-3083.1991.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 33.Wadowsky RM, Laus S, Green M, Webber SA, Rowe D. Measurement of Epstein-Barr virus DNA loads in whole blood and plasma by TaqMan PCR and in peripheral blood lymphocytes by competitive PCR. J Clin Microbiol. 2003;41:5245–5249. doi: 10.1128/JCM.41.11.5245-5249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macedo C, Donnenberg A, Popescu I, Reyes J, Abu-Elmagd K, Shapiro R, Zeevi A, Fung JJ, Storkus WJ, Metes D. EBV-specific memory CD8+ T cell phenotype and function in stable solid organ transplant patients. Transpl Immunol. 2005;14:109–116. doi: 10.1016/j.trim.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 37.Schauer E, Webber S, Green M, Rowe D. Surface immunoglobulin-deficient Epstein-Barr virus-infected B cells in the peripheral blood of pediatric solid-organ transplant recipients. J Clin Microbiol. 2004;42:5802–5810. doi: 10.1128/JCM.42.12.5802-5810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schauer E, Webber S, Kingsley L, Green M, Rowe D. Increased Ig-null B lymphocytes in the peripheral blood of pediatric solid organ transplant recipients with elevated Epstein-Barr viral loads. Pediatr Transplant. 2009;13:311–318. doi: 10.1111/j.1399-3046.2008.00918.x. [DOI] [PubMed] [Google Scholar]
- 39.Cellerai C, Perreau M, Rozot V, Enders FB, Pantaleo G, Harari A. Proliferation capacity and cytotoxic activity are mediated by functionally and phenotypically distinct virus-specific CD8 T cells defined by interleukin-7Ralpha (CD127) and perforin expression. J Virol. 2010;84:3868–3878. doi: 10.1128/JVI.02565-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul ME, Shearer WT, Kozinetz CA, Lewis DE. Comparison of CD8(+) T-cell subsets in HIV-infected rapid progressor children versus non—rapid progressor children. J Allergy Clin Immunol. 2001;108:258–264. doi: 10.1067/mai.2001.117179. [DOI] [PubMed] [Google Scholar]
- 41.Kolber MA, Saenz MO, Tanner TJ, Arheart KL, Pahwa S, Liu H. Intensification of a suppressive HAART regimen increases CD4 counts and decreases CD8+ T-cell activation. Clin Immunol. 2008;126:315–321. doi: 10.1016/j.clim.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Crough T, Fazou C, Weiss J, Campbell S, Davenport MP, Bell SC, Galbraith A, McNeil K, Khanna R. Symptomatic and asymptomatic viral recrudescence in solid-organ transplant recipients and its relationship with the antigen-specific CD8(+) T-cell response. J Virol. 2007;81:11538–11542. doi: 10.1128/JVI.00581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, auto-immune disease, and persistent infections. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 47.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol. 1998;183:149–156. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 49.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popescu I, Macedo C, Abu-Elmagd K, Shapiro R, Hua Y, Thomson AW, Morelli AE, Storkus WJ, Metes D. EBV-specific CD8+ T cell reactivation in transplant patients results in expansion of CD8+ type-1 regulatory T cells. Am J Transplant. 2007;7:1215–1223. doi: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 51.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 52.Klenerman P. Commentary: T cells get by with a little help from their friends. Eur J Immunol. 2004;34:313–316. doi: 10.1002/eji.200324844. [DOI] [PubMed] [Google Scholar]
- 53.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 54.Macedo C, Zeevi A, Bentlejewski C, Popescu I, Green M, Rowe D, Smith L, Webber S, Metes D. The impact of EBV load on T-cell immunity in pediatric thoracic transplant recipients. Transplantation. 2009;88:123–128. doi: 10.1097/TP.0b013e3181aacdd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee TC, Goss JA, Rooney CM, Heslop HE, Barshes NR, Caldwell YM, Gee AP, Scott JD, Savoldo B. Quantification of a low cellular immune response to aid in identification of pediatric liver transplant recipients at high-risk for EBV infection. Clin Transplant. 2006;20:689–694. doi: 10.1111/j.1399-0012.2006.00537.x. [DOI] [PubMed] [Google Scholar]
- 56.Husain S, Raza K, Pilewski JM, Zaldonis D, Crespo M, Toyoda Y, Shutt K, Spichty K, Bentlejewski C, Pakstis DL, et al. Experience with immune monitoring in lung transplant recipients: correlation of low immune function with infection. Transplantation. 2009;87:1852–1857. doi: 10.1097/TP.0b013e3181a75ad2. [DOI] [PubMed] [Google Scholar]
- 57.Kowalski R, Woodcock J, Sottong P, Britz JA. An immune-based assay for HIV disease management. Am Clin Lab. 2001;20:39–40. [PubMed] [Google Scholar]