Abstract
Myc-interacting zinc finger protein 1 (Miz-1) is a BTB/POZ domain transcription factor that regulates complex processes such as proliferation and apoptosis. Constitutively Miz-1-deficient animals arrest embryonic development at E14.5 due to severe anemia and fetal liver cells lacking Miz-1 show a high cell death rate and a significant reduction of mature Ter119+ckit- or Ter119+CD71-/low cells. Consistently, the numbers of BFU-Es and CFU-Es were severely reduced in colony forming assays. Mice with conditional Miz-1 alleles deleted around E14.5 were born at expected ratios, but had reduced numbers of erythrocytes, and showed an increase in reticulocytes and Macro-RBCs in the peripheral blood. When challenged with the hemolytic agent phenylhydrazine (PHZ), Miz-1 deficient mice responded with a severe anemia after 4 days of treatment, but showed a delay in the recovery from this anemia with regard to RBC counts, hematocrit and hemoglobin levels compared to controls. In addition, an accumulation of immature CD71+Ter119+ cells occurred in the bone marrow and spleen of mice lacking a functional Miz-1. We conclude from our studies that Miz-1 is important for erythroid differentiation and development. Moreover, Miz-1 is necessary to maintain a peripheral red blood cell homeostasis in particular in response to hemolysis after oxidative stress.
Keywords: BTB/POZ domain, transcription factor, Miz-1, erythropoiesis, Epo, STAT5
Introduction
The continuous generation and development of red blood cells is an important process throughout the life of many organisms. The first steps in this process are called primitive erythropoiesis and take place in the yolk sac. Later during development, erythroid progenitors enter the fetal liver and initiate definitive erythropoiesis, which becomes predominant and essential for survival around mid-gestation. After birth, erythropoiesis takes place in the bone marrow and, under specific circumstances, in the spleen as well [1]. As with all hematopoietic and immune cells, red blood cells initially emerge from hematopoietic stem cells (HSCs). To form red blood cells, HSCs differentiate into common progenitors of the megakaryocyte and erythroid lineages, termed megakaryocyte-erythroid progenitor (MEPs) [2,3]. MEPs in turn produce cells that are either committed to become red blood cells or megakaryocytes. This process of lineage commitment and specification from bi-potential precursors is controlled by receptor signaling and the expression of a number of specific transcriptional regulators, notably the GATA-binding transcription factors (GATA-1 and GATA-2), ETS factors (FLI-1 and GABPα), Krüppel-containing factors (KLF1), Leukemia/lymphoma Related Factor (LRF), basic helix-loop-helix factors (SCL) and several adaptor proteins that interact with transcription factors (e.g. Friend of GATA-1) [4,5].
The main signaling pathway for differentiation of red blood cell lineage is initiated by erythropoietin (Epo) [6]. Epo is produced by kidney cells under hypoxia and stress conditions and induces terminal proliferation and controls developmental as well as survival processes of erythroid cells [7]. Binding of Epo to its receptor (EpoR) activates several intracellular signaling pathways that regulate proliferation, development and apoptosis [8]. For instance, Epo signaling regulates phosphorylation of Stat5, the PI3K pathway, and the MAPK pathway [8,9]. After phosphorylation, Stat5 enters the nucleus as a dimer and functions as a transcription factor regulating the expression of specific target genes, such as Bcl-xL, to prevent apoptosis in erythroid progenitors [10]. Blocking the phosphorylation of Stat5 leads to increased apoptosis in the erythroid progenitor population and a reduction in erythrocyte production [11].
Recent work has shown that the Myc-interacting zinc finger protein 1 (Miz-1) regulates the expression of Socs1, which is a regulator of the JAK kinases that phosphorylate and activate Stat5 [12,13], thereby affecting signaling through the IL-7 receptor (IL7R) in lymphoid progenitors. Similar to signaling through the EpoR, downstream effectors of the IL-7R are the anti-apoptotic genes Bcl-xL and Bcl-2. In addition to regulating JAK/STAT activity through Socs1, Miz-1 is also able to activate expression of Bcl-2 directly and further enhances IL-7 signaling [12,13].
The Miz-1 gene encodes an 80 kDa nuclear protein with thirteen C2H2 zinc finger domains at its carboxy-terminus and a POZ/BTB domain at its N-terminus [14,15]. Miz-1 acts as a transcriptional transactivator of RNA Pol II-dependent target gene promoters by recruiting co-activators such as the histone deacetylase p300/CBP [16-18]. The BTB/POZ domain is essential for the transactivating activity of Miz-1 but also serves as a protein-protein interaction module and is required for stable chromatin binding of Miz-1 [18]. The bHLH LZ transcription factor and oncoprotein c-Myc can be recruited by Miz-1 to certain target gene promoters, which leads to the inactivation of Miz-1 and blocks its ability to transactivate these target genes.
The complete deletion of the Miz-1 gene leads to early embryonic lethality at E7.5 due to defective processes during gastrulation [19]. To investigate the role of Miz-1 at later stages of development and in adult animals, conditionally deficient mice were generated in which exons 3 and 4, coding for the functional POZ domain, can be deleted by expression of a Cre-recombinase, in such a way that a non-functional Miz-1 allele is generated [12]. Animals expressing only the Miz-1 mutant lacking the POZ domain (Miz-1ΔPOZ/ΔPOZ) die at around day 14.5 of embryonic development. Miz-1ΔPOZ/ΔPOZ E14.5 embryos were smaller in size than WT embryos, were highly anemic and looked pale [12]. Breeding Miz-1ΔPOZ/loxp animals with a Sox2-Cre deleter strain [20] that deletes the POZ-domain only in the embryo proper, but not the surrounding epiblast did not alter the embryonic lethality (Kosan and Möröy, unpublished), which excluded a malfunction of extra-embryonic tissues and suggested that Miz-1 has essential functions in the development of the embryo proper and likely plays a role in embryonic erythropoiesis.
We show here that ablation of Miz-1 disrupts erythroid differentiation during embryonic development, and to a lesser extent adult erythropoiesis in the bone marrow. However, adult Miz-1-deficient mice show a reduced number of mature red blood cells as well as a higher ratio of immature reticulocytes in the peripheral blood and a high rate of splenic erythropoiesis, indicating that these animals are anemic. In addition, recovery from phenylhydrazine (PHZ)-induced anemia is delayed in Miz-1 deficient mice, which suggests a role of Miz-1 in adult stress-induced erythropoiesis.
Materials and methods
Ethics statement
The protocols for the in vivo experiments described here were reviewed and approved by the IRCM Animal Care Committee (ACC); protocol numbers are: #2013-01. All animal experiments were conducted according to institutional rules put in place by the IRCM ACC, which follow the regulations and requirements of the Canadian Council on Animal Care (www.ccac.ca).
Mice
The generation of conditional and constitutive Miz-1 deficient mice has been described previously [12]. All mice were housed under specific pathogen-free conditions and institutional animal ethics committees reviewed animal experimentation protocols and certified animal technicians regularly observed the mice in sign of distress. Adult mice were sacrificed by carbon dioxide inhalation whereas newborn pups were euthanized by decapitation following anesthesia by carbon dioxide inhalation as per standard operating procedure approved by the IRCM ACC and the CCAC. All efforts were made to minimize the number of animals used and to reduce their suffering. All mice were backcrossed with C57BL/6 mice for at least 8 generations.
Flow cytometry and cell sorting
Cell populations from spleen and bone marrow were analyzed by flow cytometry using an LSR (BD Biosciences) or sorted on a MoFlo (Cytomation). Cells were passed through a 23-gauge needle, filtered through a cell strainer and resuspended in PBS (1% FCS, 10 mM EDTA). Usually, 1-5 x 106 cells were stained with antibodies at a 1:200 concentration for 20 min., washed with PBS and analyzed immediately. Antibodies used were ordered from BD-Biosciences (Missisauga, ON, Canada) or Bio-Legend (San Diego, CA, USA).
Q-PCR
TRIzol (Invitrogen) was applied to isolate RNA/DNA/protein from sorted cells according to the manufacturers protocol. Quantitative RT-PCR was performed in a 20 µl reaction volume containing 900 nM of each primer, 250 nM TaqMan probe, and 1 µl TaqMan Universal PCR Master Mix (ABI, Germany) according to the manufacturer’s instructions. The relative expression of genes of interest was calculated relative to the GAPDH mRNA levels.
Immunoblot analysis
For immunoblot analysis, MACS sorted Ter119+ cells were lysed in RIPA buffer containing protease inhibitors (complete Mini; Roche Diagnostics) on ice for 20 minutes and then sonicated for 10 minutes in a water bath (Branson 5510). Immunoblotting was performed using anti-pSTAT5 (D47E7, Cell Signaling), anti-STAT5 (Cell Signaling) or anti-β-actin (AC-15, Sigma Aldrich).
Chromatin immunoprecipitation
Assays were performed on MACS sorted primary Ter119+ cells. Cells were fixed with 1% formaldehyde. Cell lysis was performed using the following buffers: total cell lysis - 5 mM PIPES pH 8, 85 mM KCl, 0.5% NP-40, 1X protease inhibitor cocktail, 1 mM PMSF, and nuclear lysis - 50 mM Tris, 10 mM EDTA, 1% SDS, 1X protease inhibitor cocktail, 1 mM PMSF. After sonication (Covaris E220 sonicator), immunoprecipitation was performed using Protein A/G Dynabeads (Life Technologies) and 10 ug of rabbit anti-Miz-1 (H-190, Santa Cruz Biotechnology), or rabbit control IgG antibodies (Santa Cruz Biotechnology).
Results
Miz-1 is required for the development and survival of erythroid cells during embryonic development
Flow cytometric analysis of fetal liver cells with antibodies against the erythroid markers glycophorin-A associated antigen (Ter119) and transferrin receptor (CD71) and with c-kit, a marker for immature hematopoietic cells, showed a severe defect in the maturation of erythroid cells in Miz-1ΔPOZ/ΔPOZ mice at E 14.5 (Figure 1A). The percentage of total c-Kit-, Ter119+ fetal liver cells of Miz-1-deficient mice was reduced at least 5-fold, the CD71low, Ter119+ and CD71+, Ter119+ cell populations were almost absent compared to controls and a new cell population with reduced expression of Ter119 and CD71 appeared (Figure 1A). Colony-forming assays with Miz-1-deficient progenitors and control cells showed that the numbers of both the erythroid colony forming unit (CFU-E) and the burst forming unit (BFU-E), the earliest known erythroid precursor cells, were severely reduced or were not detectable in the absence of a functional Miz-1 protein (Figure 1B).
Figure 1.
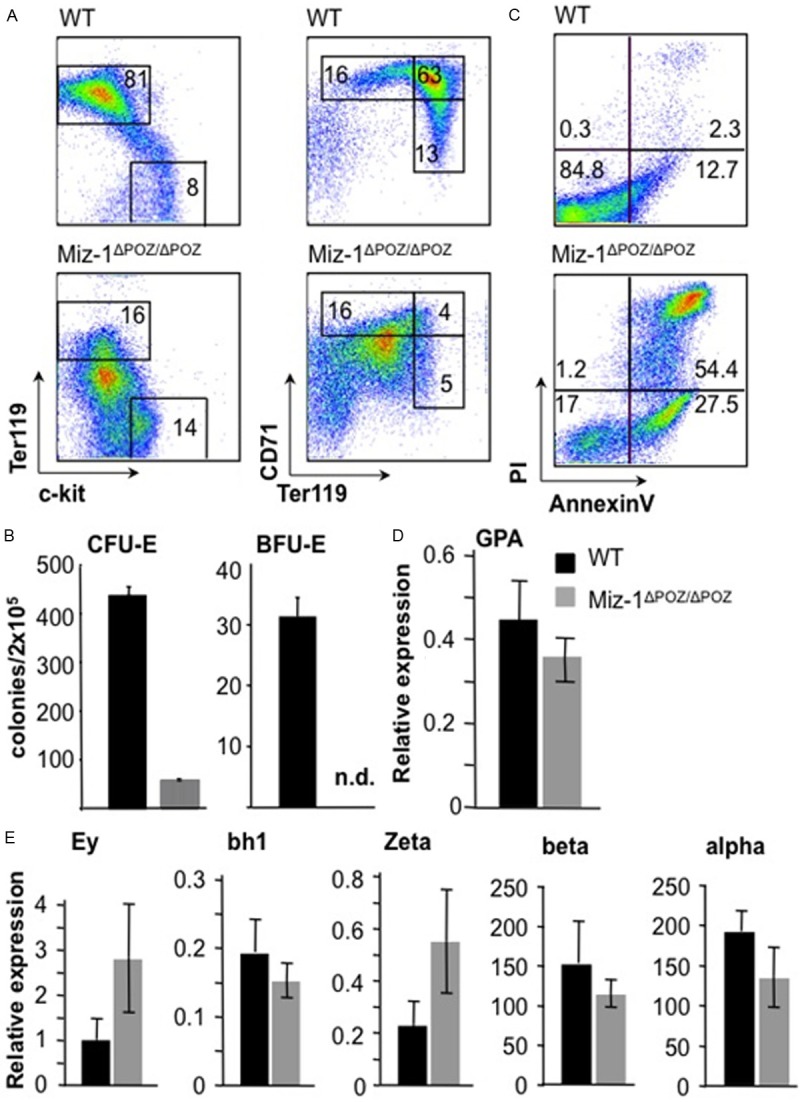
Miz-1 deficiency disrupts erythropoiesis in the fetal liver. A. Surface expression of Ter119, CD71 and c-Kit on fetal liver cells from embryos at day E14.5. Dead cells were excluded by forward/sideward scatter (FSC/SSC) gating. Numbers indicate the percentages of cells falling into the respective indicated gates. Data represent three independent experiments. B. Colony-forming unit-erythroid (CFU-E) and blast-forming unit-erythroid (BFU-E) of fetal liver cells from Miz-1ΔPOZ/ΔPOZ and WT mice were analyzed after 3 or 9 days respectively. C. Flow cytometric analysis of AnnexinV and PI staining of fetal liver cells from Miz-1ΔPOZ/ΔPOZ and WT embryos (E14.5). Data represent at least three independent experiments. D. Quantitative Real-Time PCR (QRT-PCR) was performed on cDNA from fetal liver cells from Miz-1ΔPOZ/ΔPOZ and WT mice at E13.5. Shown is the expression of the GPA gene normalized to S16. E. RT-PCR analysis to determine the expression levels of embryonic (Ey, bh1 and Zeta) and adult (beta and alpha) globin genes normalized to GPA. D, E. Data represent four independent experiments. Error bars indicate standard deviation.
Flow cytometric analysis using AnnexinV and propidium iodide (PI) staining showed that only 17% of Miz-1ΔPOZ/ΔPOZ fetal liver cells were negative for AnnexinV and PI, while 84.8% of WT cells were negative for AnnexinV and PI, indicating that almost all fetal liver cells from Miz-1ΔPOZ/ΔPOZ mice have either initiated or have already completed a cell death program (Figure 1C). Expression of glycophorin A (GPA), an erythroid-specific marker and of the adult globin genes alpha and beta was not affected by Miz-1 deficiency, while the expression of the embryonic Ey- and Zeta-globin genes were slightly up-regulated in Miz-1-deficient fetal liver cells compared to control cells (Figure 1D, 1E). These findings indicate that Miz-1 is required for the survival of erythroid cells during embryonic development, but that it does not significantly affect the expression of neither adult nor embryonic globin genes.
Miz-1 is dispensable for adult erythropoiesis but contributes to the production of RBCs
To analyze the effect of Miz-1 deficiency on adult erythropoiesis, we crossed Miz-1fl/fl mice with animals carrying a vav-Cre transgene, allowing Cre recombinase expression in all hematopoietic cells [21]. Expression of the vav-Cre transgene begins at day E14.5 and Miz-1fl/fl x vav-Cre animals reach adulthood without detectable defects in erythropoiesis during embryonic development (not shown) [12]. It is therefore likely that the defect of embryonic erythropoiesis in Miz-1ΔPOZ/ΔPOZ embryos must occur at stages of development before E14.5. A comparison of bone marrow cells by flow cytometry from Miz-1fl/fl x vav-Cre mice and WT controls showed no difference in percentages of megakaryocyte precursors (MEPs, not shown) or more committed Ter119+ erythroblasts, such as basophilic erythroblasts, polychromatophilic erythroblasts and orthochromatophilic erythroblasts (see gates I-IV, Figure 2A). AnnexinV staining on Ter119+ bone marrow cells from adult WT and Miz-1-deficient mice did not reveal significant levels of ongoing apoptosis in the absence of Miz-1 (Figure 2B). While the expression of Miz-1 target genes Socs1 and Cdkn1a were up-regulated as expected from previous published findings [12-14,22], BclxL and Bcl-2 expression levels were not significantly changed in Ter119+ cells from Miz-1fl/fl x vav-Cre mice compared to WT mice (Figure 2C).
Figure 2.
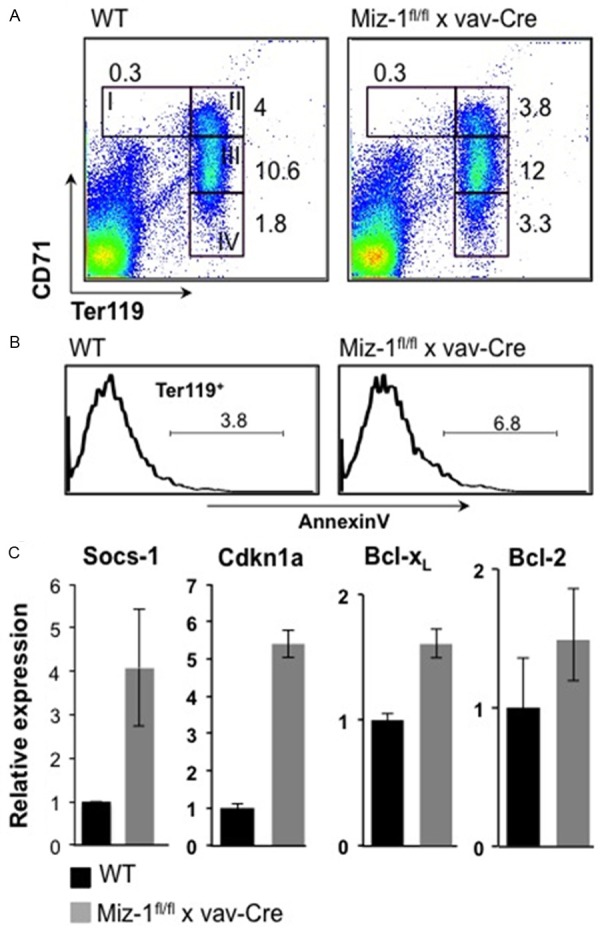
Adult erythropoiesis in the bone marrow occurs in the absence of Miz-1. A. FACS analysis was performed on bone marrow cells from adult Miz-1fl/fl x vav-Cre and WT mice. The expression of Ter119 and CD71 was analyzed and dead cells were excluded by forward/sideward scatter (FSC/SSC) gating. Numbers indicate percentages of cells falling into the following gates: CD71+Ter119low (gate I, proerythroblasts), CD71+Ter119+ (gate II, basophilic erythroblasts), CD71medTer119+ (gate III, polychromatophilic erythroblasts), CD71lowTer119+ (gate IV, orthochromatophilic erythroblasts). B. Ter119+ bone marrow cells from adult Miz-1fl/fl vav-Cre and WT mice were analyzed using AnnexinV staining. C. Ter119+ cells were isolated from adult Miz-1fl/fl vav-Cre and WT bone marrow. RNA from these cells was reverse transcribed and cDNA was used for quantitative RT-PCR (QRT-PCR). Expression of indicated genes was normalized to theexpression of Gapdh and presented as fold increase compared to WT cells. Data represent two independent experiments each done in triplicates. Error bars indicate standard deviation.
However, a significant reduction of red blood cell numbers (RBC), hemoglobin (HGB) and hematocrit (HCT) levels was observed in the peripheral blood of adult Miz-1fl/fl x vav-Cre mice (Figure 3) as well as an abnormally high rate of macrocytes (RBC-Macro; WT: 0.09±0.12%; Miz-1fl/fl x vav-Cre: 0.8±0.36%) and newly generated reticulocytes (WT: 2.96±0.6%; Miz-1fl/fl x vav-Cre: 4.05±0.88%), hypochromic red blood cells (Hypo; WT: 0.23%±0.1%; Miz-1fl/fl x vav-Cre: 0.84%±0.53%) and an increased cellular volume (MCV; WT: 50.73±1.89%; Miz-1fl/fl x vav-Cre: 53.68±1.44%) (Figure 3). Finally, Miz-1fl/fl x vav-Cre mice also showed a reduction of platelets (PLT) compared to WT mice (Figure 3). These findings indicate that although ablation of Miz-1 function does not affect adult erythroid maturation or survival of erythroid cells in the bone marrow, the lack of a functional Miz-1 protein still leads to the perturbation of major blood parameters in adult animals. This suggests that erythropoiesis in Miz-1-deficient mice is overall insufficient to generate WT levels of red blood cells.
Figure 3.
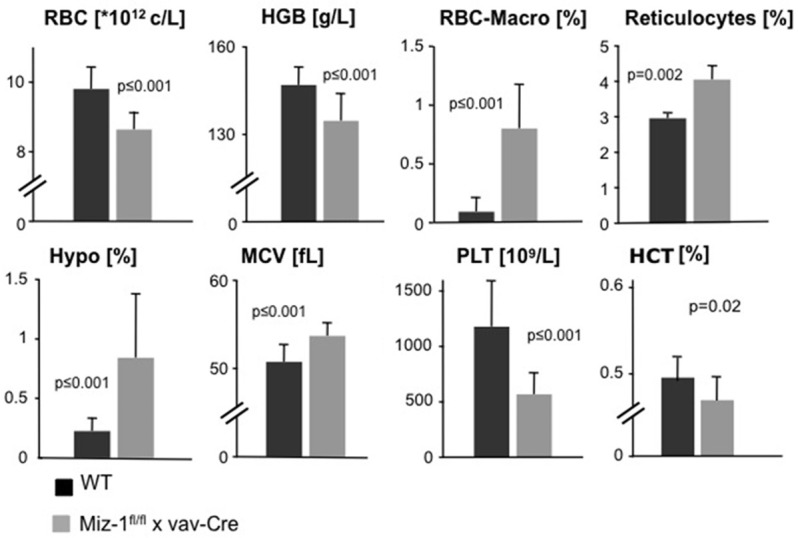
Altered blood parameters in Miz-1-deficient mice. Peripheral blood from adult Miz-1fl/fl x vav-Cre and WT mice were analyzed for red blood cell counts (RBC), hemoglobin (HGB) and hematocrit (HCT) levels, macrocytes (RBC-Macro) and newly generated reticulocytes, hypochromic red blood cells (Hypo), cellular volume (MCV) (fL: femtoliters) and platelet counts (PLT). Data represent twelve independent experiments. Error bars indicate standard deviation.
Miz-1 is required for stress-induced erythropoiesis
Further flow cytometric analysis showed that spleens of Miz-1-deficient mice show clear signs of extramedullary erythropoiesis. While WT mice showed only about 1% Ter119+ cells in the spleen, Miz-1-deficient mice accumulated up to 45% Ter119+ cells (Figure 4A). This suggested that Miz-1-deficient mice initiate a compensatory mechanism possibly to counterbalance an inefficient erythropoiesis in the bone marrow and the low red cell levels in peripheral blood. To investigate this further, we decided to analyze the effect of Miz-1 deficiency on stress-induced erythropoiesis.
Figure 4.
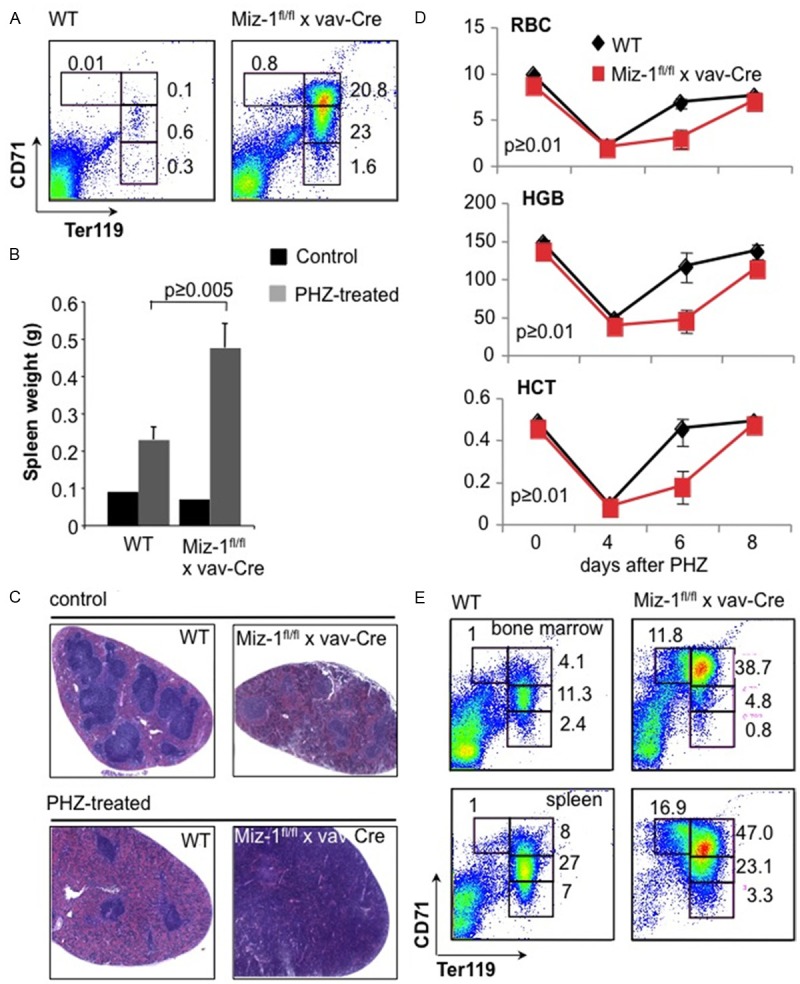
Aberrant response to stress erythropoiesis in Miz-1-deficient mice. A. FACS analysis of splenocytes from adult Miz-1fl/fl x vav-Cre and WT mice as described in Figure 2A. The data are representative of at least three independent experiments. B. Miz-1fl/fl x vav-Cre and WT mice were injected with PHZ and the weight of spleens of the indicated mice was measured before (control) and 6 days after PHZ treatment. C. Splenic sections were taken before (control) and 6 days after PHZ treatment and analyzed by hematoxylin/eosin staining (HE staining). D. Red blood cell counts (RBC), hemoglobin (HGB) and hematocrit (HGT) levels were analyzed before and 4, 6 and 8 days after PHZ injection. E. FACS analysis of bone marrow or spleen cells from adult Miz-1fl/fl x vav-Cre and WT mice 6 days after PHZ treatment. The surface expression of Ter119 and CD71 was analyzed and dead cells were excluded by forward/sideward scatter (FSC/SSC) gating. Numbers indicate percentages of cells falling into the following gates: CD71+Ter119low (gate I, proerythroblasts), CD71+Ter119+ (gate II, basophilic erythroblasts), CD71medTer119+ (gate III, polychromatophilic erythroblasts), CD71lowTer119+ (gate IV, orthochromatophilic erythroblasts).
We used the hematolytic agent phenylhydrazine (PHZ) to induce a hemolysis that leads to anemia and provokes stress-induced erythropoiesis in spleen and bone marrow. Six days after PHZ injection, spleens from Miz-1fl/fl x vav-Cre mice were found to be significantly larger than WT spleens and to have entirely lost the typical splenic architecture of red and white pulp, probably due to an exaggerated expansion of the early erythroid compartment (Figure 4B, 4C). Further, we detected a reduced level of RBC counts as well as lower HCT and HGB levels in the peripheral blood of both WT and Miz-1-deficient mice 4 days after PHZ injection (Figure 4D), indicating that the response of Miz-1-deficient mice to PHZ-induced anemia is similar to the response in WT mice. However, 6 days after PHZ injection Miz-1fl/fl x vav-Cre mice still showed a significant decrease in RBC counts and HCT and HGB levels compared to WT mice (Figure 4D). Similarly, on a cellular level, 6 days after PHZ treatment Miz-1fl/fl x vav-Cre mice showed a strong accumulation of Ter119+, CD71+ basophilic erythroblasts in the bone marrow and spleen contrary to WT mice (Figure 4E). However, 8 days after PHZ treatment Miz-1fl/fl x vav-Cre mice reached almost WT levels of RBC counts, HCT and HGB (Figures 4D, 5) as well as percentages of CD71medTer119+ polychromatophilic erythroblasts and CD71lowTer119+ orthochromatophilic erythroblasts (Figure 5).
Figure 5.
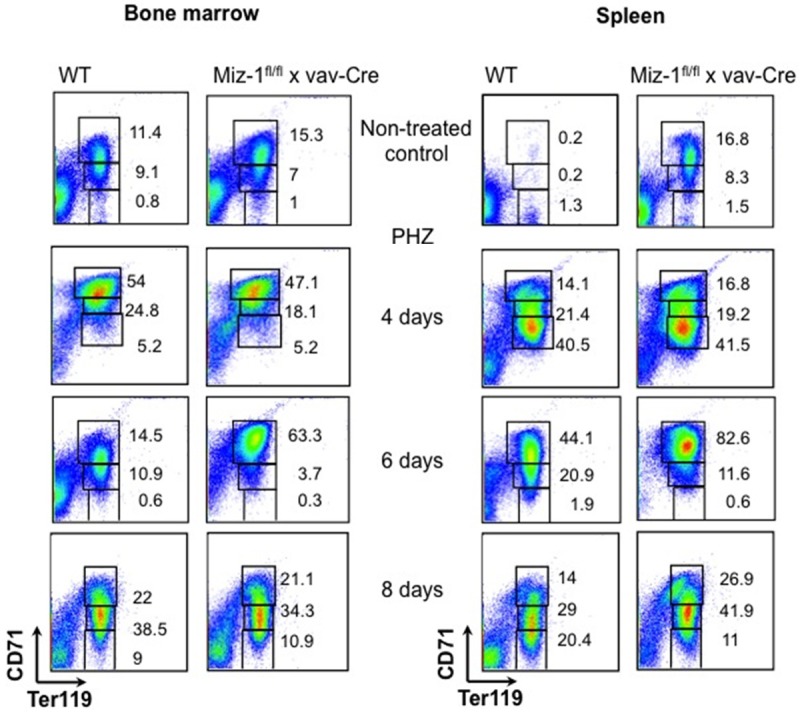
Stress erythropoiesis is delayed Miz-1-deficient mice. Flow cytometric analysis of bone marrow and spleen cells from adult Miz-1fl/fl x vav-Cre and WT mice for expression of the indicated markers before and 4, 6 and 8 days after PHZ treatment. Dead cells were excluded by forward/sideward scatter (FSC/SSC) gating. Numbers indicate percentages of cells falling into the following gates: CD71+Ter119low (gate I, proerythroblasts), CD71+Ter119+ (gate II, basophilic erythroblasts), CD71medTer119+ (gate III, polychromatophilic erythroblasts), CD71lowTer119+ (gate IV, orthochromatophilic erythroblasts).
Miz-1 is required for normal EpoR signaling
EpoR signaling through Stat5 is essential for erythrocyte development. However, conditional inactivation of Epo in adult mice leads to only a mild anemia and a slight reduction RBC counts [23], while Stat5a/b deficient mice show a severe anemia during embryonic and neonatal stages of development but only a mild anemia in adult animals [24]. As these phenotypes are similar to that of Miz-1-deficient mice, we sought to determine whether Miz-1 plays a role in the EpoR signaling pathway during erythropoiesis. Activation of Miz-1-deficient Ter119+ splenocytes with Epo led to increased phosphorylation of Stat5 compared to WT splenocytes (Figure 6A). This may be due to the fact that Miz-1-deficient Ter119+ cells were unable to activate Socs1 after Epo stimulation as compared to WT cells stimulated with Epo (Figure 6B). However, ChIP-PCR analysis indicated that Miz-1 does not occupy the Socs1 promoter (Figure 6C), suggesting the regulation of Socs1 by Miz-1 is indirect in these cells.
Figure 6.
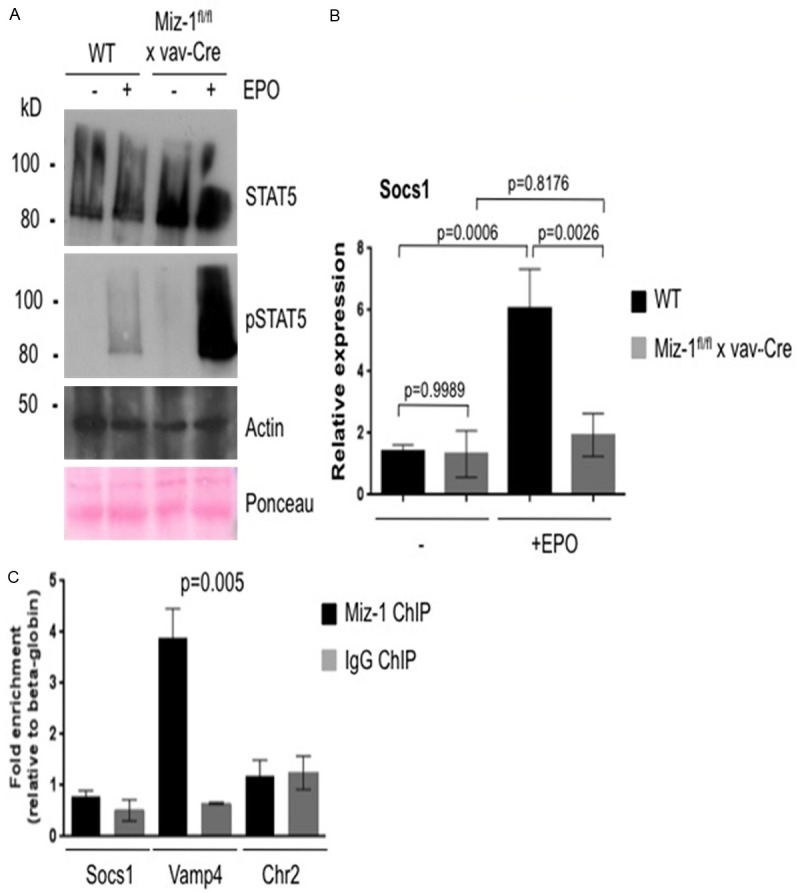
Miz-1 regulates EpoR signaling through Stat5 phosphorylation. A. Stat5/pStat5 Western Blot on lysates from Ter119+ splenocytes from WT and Miz-1-deficient mice stimulated (+Epo) or not (-Epo) with Epo. Actin and Ponceau staining are presented as loading controls. Blots are representative of at least two independent experiments. B. Ter119+ splenocytes were isolated from adult Miz-1fl/fl vav-Cre and WT animals stimulated (+Epo) or not (-Epo) with Epo. RNA from these cells was reverse transcribed and cDNA was used for quantitative RT-PCR (QRT-PCR). Expression of indicated genes was normalized to the expression of Gapdh. Data represent two independent experiments each done in triplicates. Error bars indicate standard deviation. C. Miz-1 and IgG control ChIP in Ter119+ splenocytes. Data are presented as fold enrichment at indicated regions relative to beta-globin promoter and are representative of three independent experiments. Vamp4 promoter is the positive control [33] and Chr2 (intergenic region in chromosome 2) is the negative control.
Discussion
Here we present evidence that implicates the transcription factor and BTB/POZ domain protein Miz-1 in the regulation of erythropoiesis during embryonic development, probably at the early stage of definitive erythropoiesis and very likely leads to embryonic death at E14.5 due to perturbed erythropoiesis. Our data also show that Miz-1 is important for an efficient production of red blood cells in adult mice at steady state but in particular under stress conditions, for instance in response to an acutely induced anemia. In addition to its regulatory role in early lymphoid cells, Miz-1 has also been described as an important co-factor of the proto-oncogene c-Myc. A number of studies indicate that Miz-1 participates through this interaction in the repression of the negative cell-cycle regulators p21 (Cdkn1a) and p15 (Cdkn2b) [14,22]. Interestingly, similar to what we describe here for Miz-1-deficient mice, c-Myc ablation during erythroid development using an EpoR-Cre deleter leads to severe anemia and embryonic lethality at E12.5 [25]. However, in contrast to Miz-1-deficient mice, these animals showed a perturbed α- and β-globin gene expression pattern [25]. Since globin expression levels are not significantly affected, it is likely that the role of Miz-1 during embryonic erythroid development is c-Myc independent, similar to its role in lymphocyte development [12,13]. This is also supported by the fact that a Myc knock-in mouse mutant (MycV394D KI-mutant [12,13]), which expresses a form of c-Myc that is unable to interact with Miz-1, does not show any obvious defects in fetal or adult erythropoiesis (Kosan and Möröy, unpublished).
A role of Miz-1 in early developmental stages of erythropoiesis, possibly at the transition from primitive to definitive erythropoiesis, was first suggested by our previously reported finding that a constitutive inactivation of Miz-1 leads to anemia and embryonic lethality at E14.5 [12]. The block in differentiation of erythroid cells and their massive apoptosis in constitutively Miz-1-deficient embryos as described here lends further support to this hypothesis. In addition, the absence or severe reduction of precursor cells in these Miz-1-deficient mice that can give rise to BFU-E or CFU-E in response to erythropoietin (Epo) is also in agreement with this notion and would also be compatible with a function of Miz-1 in erythropoiesis that is linked to the Epo-initiated signaling pathway.
Epo signaling has been described to be indispensible for fetal erythropoiesis and knockout mice of Epo or the Epo receptor (EpoR) succumb to severe anemia [7] at the same time point during embryonic development as Miz-1-deficient mice. The transcription factor Stat5 is activated following EpoR stimulation during fetal development and Stat5a-/-5b-/- embryos are also severely anemic [10]. Although the formation of BFU-Es and CFU-Es is dependent on Epo signaling [7], fetal liver cells of Stat5a-/-5b-/- mice give rise to almost normal numbers of BFU-Es but show severely reduced CFU-E colony numbers with an increased rate of apoptosis [10]. This is most likely due to an elevated expression and a compensatory function of Stat1 observed in Stat5a-/-5b-/- mice [26]. Stat1 can also be activated by Epo signaling and binds directly to the Bcl-xL promoter [27]. The elevated expression levels of Socs1 and Cdkn1a (p21) that we describe here in Miz-1-deficient Ter119+ bone marrow cells may explain the reduction or absence of both CFU-E and BFU-E formation from bone marrow precursors in the absence of Miz-1, since Socs1 binds to Jak2 and thus blocks the activation of both Stat5 and Stat1, which are both targets of Jak2 [28-30]. In addition, previous studies have shown that ectopic expression of Cdkn1a leads to reduced BFU-E and CFU-E colony formation from erythroid progenitors [31]. It is thus likely that the combination of elevated levels of Socs1 and Cdkn1a in the bone marrow dampen Epo/EpoR signaling in such a way that colony formation is no longer effective.
The situation of Miz-1 deficient erythroid cells isolated from spleen is different, but is also consistent with a role of Miz-1 in Epo signaling. In splenic erythroid cells, Socs1 mRNA levels are not higher in the absence of Miz-1 and, in contrast to WT cells, cannot be up-regulated in response to Epo. Since the Socs1 promoter is not occupied by Miz-1 in these cells, the regulation of Socs1 expression by Epo cannot be directly mediated by Miz-1. However, the lack of Socs1 up-regulation by Epo offers an explanation for the high levels of Stat5 phosphorylation seen in erythroid cells from Miz-1 deficient mice upon Epo treatment. It is thus conceivable that an overshooting Stat5 activity upon Epo stimulation is at least in part responsible for the strong accumulation of Ter119+, CD71+ basophilic erythroblasts in spleens of Miz-1 deficient mice under PHZ treatment, and explains the role of Miz-1 in the Epo-mediated stress response in erythroid cells.
These findings and the similarities between Epo and Stat5 knockout mice and Miz-1-deficient animals support a role of Miz-1 in regulating the Epo/Stat5 pathway in erythrocytes. Both Epo-deficient [23] and Stat5a/b-deficient [24] mice develop only a mild anemia as adult mice. Moreover, the phenotype in Stat5 deficient mice can be explained by the different requirements of Stat5 signaling during fetal and adult erythropoiesis. It has been show that the erythropoietic rate during fetal development or stress response is at least 10 times higher than steady-state erythropoiesis in adult mice [24]. A recent study showing a low Stat5 activation prevails during steady state conditions and that stress induces high Stat5 activation [32] supports this as well. Our studies show that Miz-1 plays critical roles in both erythroid maturation and adult stress mediated erythropoiesis likely by modulating the Epo/Stat5 signaling cascade in erythroid cells.
Acknowledgements
We are indebted to Mathieu Lapointe for technical assistance and Marie-Claude Lavallée, Mélanie St-Germain and Jade Dussureault for excellent animal care, Eric Massicotte, Julie Lord for FACS and cell sorting. Tarik Möröy holds a Canada Research Chair (Tier 1). Grants from the CIHR (MOP-115202) to T.M. and from the Lady Tata Memorial Trust to C.K. supported this work.
Disclosure of conflict of interest
None.
References
- 1.Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–114. doi: 10.1016/s0268-960x(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 2.Debili N, Coulombel L, Croisille L, Katz A, Guichard J, Breton-Gorius J, Vainchenker W. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood. 1996;88:1284–1296. [PubMed] [Google Scholar]
- 3.Vannucchi AM, Paoletti F, Linari S, Cellai C, Caporale R, Ferrini PR, Sanchez M, Migliaccio G, Migliaccio AR. Identification and characterization of a bipotent (erythroid and megakaryocytic) cell precursor from the spleen of phenylhydrazine-treated mice. Blood. 2000;95:2559–2568. [PubMed] [Google Scholar]
- 4.Dore LC, Crispino JD. Transcription factor networks in erythroid cell and megakaryocyte development. Blood. 2011;118:231–239. doi: 10.1182/blood-2011-04-285981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilber A, Nienhuis AW, Persons DA. Transcriptional regulation of fetal to adult hemoglobin switching: new therapeutic opportunities. Blood. 2011;117:3945–3953. doi: 10.1182/blood-2010-11-316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- 7.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 8.Constantinescu SN, Ghaffari S, Lodish HF. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 9.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 11.Chida D, Miura O, Yoshimura A, Miyajima A. Role of cytokine signaling molecules in erythroid differentiation of mouse fetal liver hematopoietic cells: functional analysis of signaling molecules by retrovirus-mediated expression. Blood. 1999;93:1567–1578. [PubMed] [Google Scholar]
- 12.Kosan C, Saba I, Godmann M, Herold S, Herkert B, Eilers M, Moroy T. Transcription factor miz-1 is required to regulate interleukin-7 receptor signaling at early commitment stages of B cell differentiation. Immunity. 2010;33:917–928. doi: 10.1016/j.immuni.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Saba I, Kosan C, Vassen L, Moroy T. IL-7R-dependent survival and differentiation of early T-lineage progenitors is regulated by the BTB/POZ domain transcription factor Miz-1. Blood. 2011;117:3370–3381. doi: 10.1182/blood-2010-09-310680. [DOI] [PubMed] [Google Scholar]
- 14.Peukert K, Staller P, Schneider A, Carmichael G, Hanel F, Eilers M. An alternative pathway for gene regulation by Myc. EMBO J. 1997;16:5672–5686. doi: 10.1093/emboj/16.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider A, Peukert K, Eilers M, Hanel F. Association of Myc with the zinc-finger protein Miz-1 defines a novel pathway for gene regulation by Myc. Curr Top Microbiol Immunol. 1997;224:137–146. doi: 10.1007/978-3-642-60801-8_14. [DOI] [PubMed] [Google Scholar]
- 16.Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, Bernard S, Quarto M, Capra M, Goettig S, Kogel U, Scheffner M, Helin K, Eilers M. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell. 2005;123:409–421. doi: 10.1016/j.cell.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Herold S, Wanzel M, Beuger V, Frohme C, Beul D, Hillukkala T, Syvaoja J, Saluz HP, Haenel F, Eilers M. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol Cell. 2002;10:509–521. doi: 10.1016/s1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 18.Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Moroy T, Bartek J, Massague J, Hanel F, Eilers M. Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- 19.Adhikary S, Peukert K, Karsunky H, Beuger V, Lutz W, Elsasser HP, Moroy T, Eilers M. Miz1 is required for early embryonic development during gastrulation. Mol Cell Biol. 2003;23:7648–7657. doi: 10.1128/MCB.23.21.7648-7657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 21.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 22.Wanzel M, Herold S, Eilers M. Transcriptional repression by Myc. Trends Cell Biol. 2003;13:146–150. doi: 10.1016/s0962-8924(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 23.Zeigler BM, Vajdos J, Qin W, Loverro L, Niss K. A mouse model for an erythropoietin-deficiency anemia. Dis Model Mech. 2010;3:763–772. doi: 10.1242/dmm.004788. [DOI] [PubMed] [Google Scholar]
- 24.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 25.Pang CJ, Lemsaddek W, Alhashem YN, Bondzi C, Redmond LC, Ah-Son N, Dumur CI, Archer KJ, Haar JL, Lloyd JA, Trudel M. Kruppel-like factor 1 (KLF1), KLF2, and Myc control a regulatory network essential for embryonic erythropoiesis. Mol Cell Biol. 2012;32:2628–2644. doi: 10.1128/MCB.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 27.Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K, Kishimoto T. Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Invest. 1997;99:2898–2905. doi: 10.1172/JCI119484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 29.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 30.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 31.Albanese P, Chagraoui J, Charon M, Cocault L, Dusanter-Fourt I, Romeo PH, Uzan G. Forced expression of p21 in GPIIb-p21 transgenic mice induces abnormalities in the proliferation of erythroid and megakaryocyte progenitors and primitive hematopoietic cells. Exp Hematol. 2002;30:1263–1272. doi: 10.1016/s0301-472x(02)00933-5. [DOI] [PubMed] [Google Scholar]
- 32.Porpiglia E, Hidalgo D, Koulnis M, Tzafriri AR, Socolovsky M. Stat5 signaling specifies basal versus stress erythropoietic responses through distinct binary and graded dynamic modalities. PLoS Biol. 2012;10:e1001383. doi: 10.1371/journal.pbio.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf E, Gebhardt A, Kawauchi D, Walz S, von Eyss B, Wagner N, Renninger C, Krohne G, Asan E, Roussel MF, Eilers M. Miz1 is required to maintain autophagic flux. Nat Commun. 2013;4:2535. doi: 10.1038/ncomms3535. [DOI] [PMC free article] [PubMed] [Google Scholar]


